Abstract
Background
Oncology clinical trials are complex, and the COVID-19 pandemic caused major disruptions in 2020.
Methods
Using its networking and sharing of best practices, the Association of American Cancer Institutes, comprising 105 cancer centers, solicited a longitudinal series of voluntary surveys from members to assess how clinical trial office operations were affected. The surveys showed that centers were able to keep oncology trials available to patients while maintaining safety. Data were collected regarding interventional clinical trial accruals for the calendar years 2019, 2020, and 2021.
Results
Data demonstrated a sizeable decrease in interventional treatment trial accruals in both 2020 and 2021 compared with prepandemic figures in 2019. No cancer center reported an increase in interventional treatment trial accruals in 2020 compared with 2019, with most centers reporting a moderate decrease. In mid-2022, 15% of respondents reported an increasing trend, 31% reported no significant change, and 54% continued to report a decrease.
Conclusions
The pandemic necessitated rapid adoption of trial operations, with the emergence of several best practices, including remote monitoring, remote consenting, electronic research charts, and work-from-home strategies for staff. The national infrastructure to conduct trials was significantly affected by the pandemic, with noteworthy resiliency, evidenced by improvements in efficiencies and patient-centered care delivery but with residual capacity challenges that will be evident for the foreseeable future.
In January 2020, the United States documented its first case of COVID-19. In the weeks that followed, the SARS-CoV-2 virus spread, necessitating substantial changes in public policy. The World Health Organization declared a pandemic on March 11, 2020, and government officials reacted with mandatory quarantines and the suspension of nonessential medical services. Cancer clinical services were among those disrupted (1). Cancer clinical trials, many of which are essential treatment options for patients whose standard treatment options are limited, were not immune to the effects of such unprecedented orders. Cancer centers needed to learn quickly how to sustain the conduct of these oncology trials while keeping staff and patients safe (2,3).
Clinical trial activation and execution across key stakeholders, including cancer centers, health-care systems, government, and industry, were also affected across the globe (4). Activating and conducting oncology clinical trials are complex tasks, requiring many moving parts working together, and maintenance of clinical trial activity proved challenging during a global pandemic. Initially, cancer centers had to determine which trials they could keep open, balancing cancer patients’ safety with their desire to access or continue their care on a trial. Centers had to pivot rapidly from relying heavily on clinical and research staff working on site and in person to a model with the many staff working remotely and incorporating new aspects of remote research services (5,6). Early in the pandemic, the National Cancer Institute (NCI) published guidance to reduce regulatory and procedural barriers affecting the conduct of cancer clinical trials, helping usher in a new era of clinical trial modernization (7,8).
Given that backdrop, we sought to determine how the pandemic affected clinical trial activation, operations, and accrual at cancer centers and how the centers adapted to new workflows and practices during the pandemic. The Association of American Cancer Institutes (AACI) Clinical Research Innovation (CRI) Steering Committee solicited a series of electronic and voluntary surveys to member sites in a longitudinal manner during the pandemic to assess how clinical trial office operations were affected, including changes to clinical trial operations, accruals, resource prioritization, remote monitoring, work-from-home or flex schedules, and staffing.
Methods
Participants
The AACI comprises 105 of the leading academic and freestanding cancer research centers in North America, representing a large component of the US cancer clinical research infrastructure. AACI advances the objectives of cancer centers by facilitating interaction among the centers, educating policy makers, and fostering partnerships between cancer centers and other cancer organizations to improve cancer care. The AACI CRI was established as an AACI initiative in 2009 to address shared administrative challenges given the complexity of cancer clinical trial conduct. CRI is guided by a member-elected steering committee that includes cancer center clinical trial office medical directors, administrators, and senior staff who represent the variety of expertise associated with cancer clinical trial conduct.
During the period of the longitudinal surveys, all but the last survey were distributed using the AACI CRI listserv, which consisted of 85 cancer centers that conduct clinical research trials. Eight AACI member centers are basic science centers that do not provide clinical care. The final survey was conducted during the AACI CRI annual meeting in July 2022. The CRI listserv and survey participation are voluntary and available to all cancer center members. There was no compensation for participation.
Survey 1 requested accrual and operational information to determine the impact that COVID-19 had had on cancer center clinical trial operations in the first 2 quarters (January 1–June 30) of calendar year 2020 compared with calendar year 2019 (prepandemic). Survey 1 was distributed on September 9, 2020. As a follow-up, 2 reminders were sent asking members to complete the survey. Survey 1 closed on September 18, 2020. Survey 2 was distributed on May 17, 2021, in the same manner as survey 1. The purpose was to follow up on the continuing impact of COVID-19 on cancer center clinical trial office operations during the latter part of 2020—specifically, July 1 through December 30. Three reminders and a copy of the survey were sent before the survey closed on June 4, 2021. A third data request was included in the follow-up to survey 2 and involved a brief invitation in early 2022 to update the remainder of the calendar year 2021 accrual data. This request was made by email and sent only to survey 2 respondents. Two reminder emails were sent before the survey closed on May 1, 2022. Final data collection was through a poll of attendees taken during the ACCI CRI annual meeting in July 2022 (survey 3). In-person and virtual attendees answered the survey questions in the meeting app during the meeting’s opening session.
Survey design
The surveys developed and reported on in this study were developed by AACI in conjunction with CRI Steering Committee members. All surveys were designed by the same group and underwent iterative reassessment throughout the survey period. All surveys included in this report were conducted in a prospective manner but subject to retrospective analysis. Given that AACI staff and survey team members did not anticipate the longevity of the pandemic at the beginning of survey development, individual survey content did change some over time to collect data necessary to inform AACI policies and programs.
The study team solicited topics from the member listserv and developed the first survey based on these priority thematic areas. The initial survey was developed to collect data quickly on the impact that COVID-19 had had on clinical trials and clinical trial office operations in preparation for an informational session at the AACI/Cancer Center Administrators Forum annual meeting in October 2020, to be attended by NCI leadership and cancer center directors. Survey 1 had 17 questions designed by the CRI Steering Committee and was administered through SurveyMonkey after distribution to the CRI listserv (see Supplementary Methods: Survey 1, available online). Members were given 10 days to respond.
Survey 2 was designed to provide an update to survey 1 data for the AACI CRI annual meeting in July 2021. The group kept the initial 17 questions, with only administrative updates to wording for interpretive clarification, and added 12 questions to gauge some of the adaptations centers were employing and staffing impacts noted a year into the pandemic (see Supplementary Methods: Survey 2, available online). Given the dynamic impact of the pandemic, a clarification email was sent to survey 2 respondents asking for updates to their data, for a total of 2021 enrollments. This update occurred through 4 questions emailed to the respondents of survey 2 (see Supplementary Methods: Survey 2, available online).
In the final survey data collected at the 2022 AACI CRI annual meeting (survey 3), 7 multiple-choice questions were distributed through the in-meeting app. The questions focused on updates to enrollment information, ongoing operational challenges, accruals, staffing, remote monitoring, and work-from-home policies (see Supplementary Methods: Survey 3, available online).
No specific questions about observational or other noninterventional research activities were posed. Institutional review board approval was not deemed necessary given the survey objectives and lack of human subject data collected.
To standardize survey answers, respondents were instructed to complete the questions based on the cancer center’s accrual reporting per NCI Office of Cancer Centers P30 Data Table 4 of the cancer center support grant. A link was provided to the cancer.gov Data Table 4 guide. Interventional treatment trials were defined using the following NCI definition: “trials designed to evaluate one or more interventions for treating a disease, syndrome, or condition.” A standardized definition was used to enhance the validity of responses; the NCI definition was chosen given respondents’ familiarity with this definition for reporting to ClinicalTrials.gov and NCI funding opportunities.
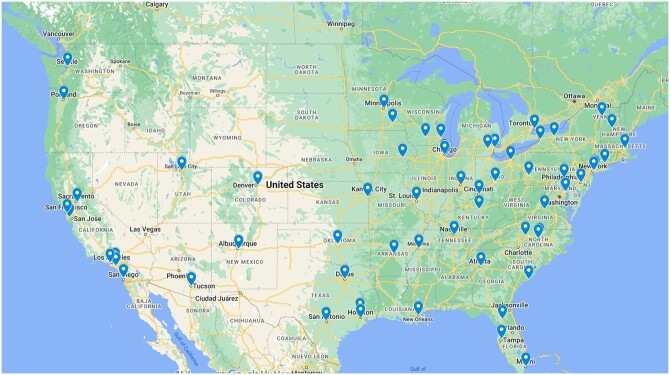
Results were collated by AACI staff, analyzed by descriptive statistics, and presented in aggregate to maintain the confidentiality of participating centers, consistent with AACI survey policy. Except where indicated, any change over time is in reference to the 2019 (prepandemic) base year. Figure 1 shows the geographic distribution of AACI member centers that participated in at least 1 survey, and Figure 2 demonstrates the evolution of survey participation by these centers.
Figure 1.
Distribution and locations of participating centers.
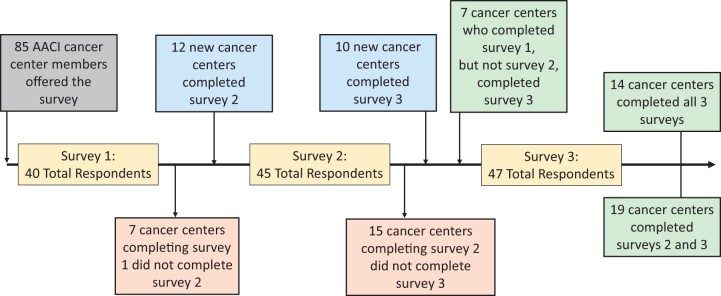
Figure 2.
Survey evolution and participation among respondents. AACI = Association of American Cancer Institutions.
Results
Forty of 85 AACI listserv-participating cancer centers completed survey 1 (47%). The majority were NCI-designated cancer centers (n = 34), with 31 having comprehensive status. Survey 2 was completed by 45 centers (53%). Of those, 36 were NCI-designated cancer centers. Survey 3 was completed by 47 centers (55%).
Impact on clinical trial accruals
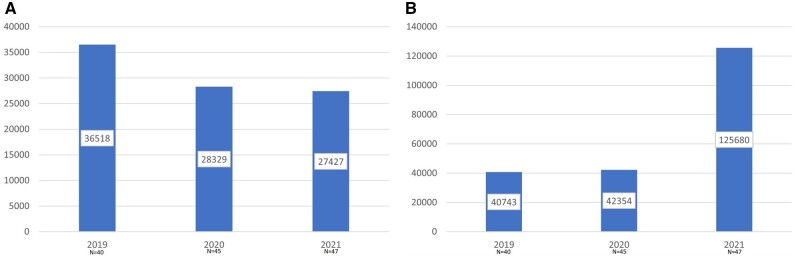
Data regarding clinical trial accruals were collected from all survey components for the calendar years 2019, 2020, and 2021, with change in accruals over the years analyzed. There were no obvious differences when considering centers by NCI designation status (NCI designated, NCI comprehensive designated, non–NCI designated) or by geographic region (Northeast, Southern, Midwest, Western). As shown in Figure 3, A, there was a 25% decrease in interventional treatment trial accruals in both 2020 and 2021 compared with prepandemic figures in 2019. No cancer center reported an increase in interventional treatment trial accruals in 2020 compared with 2019, with the majority of centers reporting a moderate decrease. At the 2022 AACI CRI annual meeting, 15% of respondents reported an increase, 31% reported no significant change, and 54% continued to report a decrease at the midpoint of 2022, despite having more survey respondents.
Figure 3.
A) Interventional treatment trial accruals (national aggregate) per year, by survey participants. B) Interventional nontreatment trial accruals (national aggregate) per year, by survey participants.
Interestingly, the trend in accruals to interventional nontreatment trials increased over the same period (Figure 3, B). Accruals increased slightly (4%) in 2020 over 2019, with a much larger increase (nearly 3-fold) from 2020 to 2021. Although there was a slight increase in the number of survey respondents year over year, there remained more than a doubling of interventional nontreatment enrollments, even when significantly high-enrolling centers were removed from analysis as outliers (data not shown). For nontreatment interventional trials, approximately half the attendees at the 2022 AACI CRI annual meeting reported a decrease in accruals to these trial types relative to prepandemic levels, while the remainder represented the source of these steady or increased accruals. Annual meeting attendees reported staffing challenges as the most common reason for decreased interventional accruals.
Impact on clinical trial monitoring
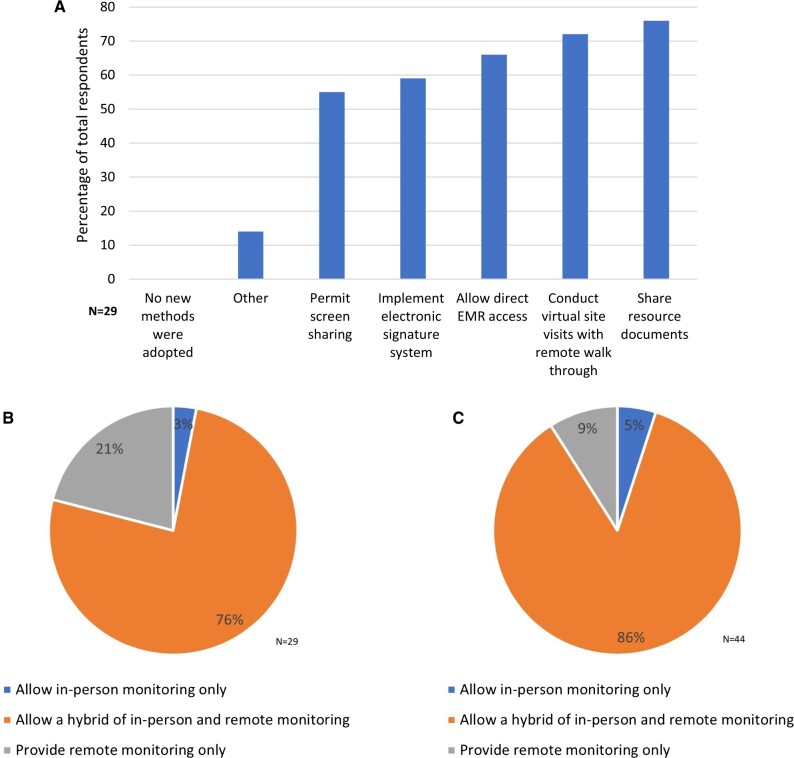
The COVID-19 public health emergency necessitated rapid adoption of remote work for cancer centers and industry trial sponsors. Before the pandemic, most centers allowed only on-site monitoring visits, but strategies to enable remote monitoring were required as cancer centers limited visitors and restrictions on travel were in place for most trial sponsors. Centers adopted a variety of methods to allow remote monitoring (Figure 4, A; n = 29 responses), including screen sharing, an electronic monitoring system, direct electronic health record access, virtual site visits conducted with remote walk-throughs, and electronic sharing of resource documents, with most centers (>70%; data not shown) implementing more than 1 solution. At the time of the initial survey in 2020 (Figure 4, B; n = 29 responses), most centers expected to maintain both remote and on-site monitoring. Only 1 center expected to return to only in-person monitoring; 6 centers intended to allow remote monitoring only. Updated impressions from the 2022 poll at the AACI CRI annual meeting similarly found that the vast majority of responding centers (n = 40) planned to continue with a hybrid monitoring plan (Figure 4, C; n = 44).
Figure 4.
A) New methods adopted to facilitate remote monitoring as a result of the COVID-19 pandemic. B) Trial monitoring options that centers offered in the early phase of the pandemic (2020). C) Trial monitoring options that cancer centers offered moving forward (2022). EMR = electronic medical record.
Impact on clinical trial office staffing
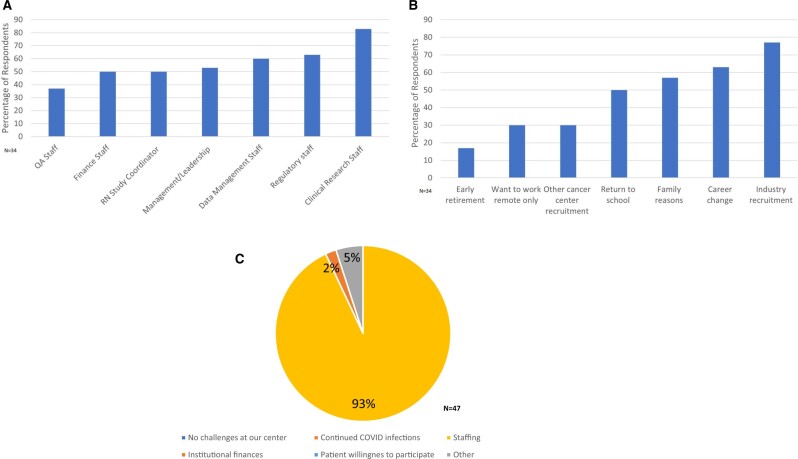
Clinical trial office staff turnover is always a challenge, given the limited pool of experienced applicants and the time required to fully onboard a clinical trial office team member (up to 6 months). The “great resignation” that hit the hospitality, health-care, and service industries particularly hard during the pandemic was also acutely felt across AACI member clinical trial offices. All centers reported some degree of staffing impact on operations. Thirty-four centers reported staff turnover in all departments, including quality assurance, finance, nurse study coordinators, clinical trial office management and leadership, data management, regulatory, and clinical coordinators (Figure 5, A). Reported reasons for staff departure included early retirement, preference for fully remote work, transition to another institutional department, and career change (Figure 5, B). Only a minority of centers had had a remote work option before the pandemic. By 2022, attendees reported that 81% allowed remote work options (depending on job-specific responsibilities), 18% reported that all clinical trial office positions allowed for some remote work. Only 1 center did not allow remote work for any staff member. All participants had continued challenges with the follow-up in-person survey in July 2022, with staffing identified as the greatest challenge to clinical research operations (Figure 5, C). Few identified finances as a problem, and no center stated that patient willingness to participate or continued COVID-19 infections were affected enrollment recovery.
Figure 5.
A) Clinical research office departments affected by departures. B) Reasons for clinical research staff departures. C) Greatest ongoing challenge to clinical research operations at cancer centers (2022). QA = quality assurance; RN = registered nurse.
Discussion
The COVID-19 pandemic’s impact on cancer center clinical research operations in the United States cannot be overstated. This study is the first to attempt to characterize and quantify the longitudinal impact of the pandemic on cancer center clinical research operations, staffing, and potential outlook. Survey participants represent a broad and geographically diverse segment of AACI membership, including “emerging” centers and those with NCI designation (both with and without comprehensive status). Importantly, our findings are consistent with enrollment trend data generated exclusively from NCI-designated cancer centers, suggesting that the impact on national cancer clinical research capacity is not restricted to designated centers (9).
The abrupt disruption of normal clinical trial and health-care operations required rapid adaptation by cancer centers, patients, and trial sponsors. These survey data highlight the innovative solutions that most centers implemented to continue to meet patient care needs and maintain patient and staff safety. Some of these changes had previously been proposed or piloted but quickly became the norm, accelerated through the sharing of best practices and innovative pilot projects through AACI CRI listservs, discussion forums, and working groups. These practices included implementing remote site-monitoring visits, hybrid work models for clinical trial office staff, decentralized study conduct, and allowing patients to submit consent electronically (10,11). Many changes were reinforced by the NCI through a policy shift, enabling provision of remote consent and care. Our survey demonstrates the continued acceptance of such widespread enhancements, which most trial sites confirm will remain in place, establishing a new norm.
Clinical trial offices at most major cancer centers continue to struggle to a larger degree with interventional treatment trial enrollments compared with interventional nontreatment studies (eg, screening, diagnostic, and supportive care). The national trend in cancer trial enrollments is of interest both from a historical perspective and as a cautionary tale moving forward. Early in the pandemic, there was an acute national reduction in interventional nontreatment study enrollments; numbers have since rebounded to a level much higher than before the pandemic. The pandemic’s initial impact on enrollment patterns (reduced nontreatment but maintained treatment) has been reported by others both at the individual institutional and network center levels (3,12). Our study, however, is one of the first to report the continued timeline of this story, now with the opposite impact on enrollments being demonstrated (reduced treatment with rebounding nontreatment). The survey did not gather data regarding the reasons for this second phase of impact, but often nontreatment trials are less labor intensive and allow more flexibility in scheduling or can be conducted remotely, including at community network sites, which may explain why these accruals increased despite pandemic-related operational and staffing changes (13,14). Many centers opened COVID-19–related studies in patients with cancer, as well, which could further contribute to the more than doubling of nontreatment accruals during the pandemic. Such trials as a category also typically have less patient and research office burden for per-patient participation and can be designed with broad eligibility criteria (eg, breast cancer survivors having received cytotoxic chemotherapy), thus benefitting from postpandemic operational modifications. Through discussions conducted among AACI member clinical trial office medical and administrative director forums, it should be noted that our data are representative of more than just a few centers representing outliers and suggest that these trial types have the potential to dominate many centers’ enrollment metrics for the foreseeable future. These studies, however, also offer an opportunity to provide impactful health-care improvement interventions to relatively large patient populations, despite situational challenges.
Importantly, enrollment in interventional treatment trials decreased during the pandemic, is currently lower than prepandemic levels, and is projected by the vast majority of survey respondents to continue to remain lower than before the pandemic. This, too, is the result of multiple factors—namely, the complex nature of cancer treatment clinical trial design, delivery, monitoring, and oversight. As a whole, these studies are less amenable to off-site treatment, remote care delivery, and broad eligibility criteria, with many targeting relatively infrequent or uncommon patient subsets or requiring evidence of biomarkers. That said, we join with our NCI leaders and peers across the globe in seeing this as an opportune time for postpandemic cancer clinical trial reform (15-17). Such innovations extend beyond eligibility criteria revisions and should focus on eliminating barriers for decentralized trial conduct, remote monitoring of and data trial quality, telemedicine resources, and other opportunities to keep patients closer to home, with minimal burden added to participate in clinical trials. Adopting this approach could have the added benefit of reducing health disparities (18,19).
Compounding the challenges for all trials but treatment trials in particular is clinical trial office staff turnover. Many centers lost senior and experienced staff and the attendant generational knowledge base. New staff are still onboarding, managing fewer patients and fewer trials than their more seasoned predecessors. As a result, most centers are having difficulty administering complex cancer treatment trials or are abandoning rare-disease or under-resourced studies, thus limiting the number of new trials being opened. It is unclear how long the staffing impact will continue to affect center operations, given that some market pressures affecting job selections have not yet stabilized. Many centers are accelerating staff feeder programs, assessing alternative academic payment models, and focusing resources on retention. Cancer centers find themselves in a new reality relative to the prioritization of increasingly limited resources that have dwindled further during the pandemic. This situation has important implications for the national capacity to conduct interventional cancer treatment clinical trials using prepandemic regulatory, infrastructure, and reimbursement models that have yet to adjust to the current situation.
Our study benefitted from the diversity of participating AACI trial sites and the regions represented (Figure 1), the longitudinal data collection, and the anonymity of the results that encouraged accurate data reporting. The study is not without limitations, including 1) the impact of recall bias on data reporting; 2) the voluntary nature of participation, which may not be wholly representative of all cancer center clinical trial offices, may have resulted in disproportionate underreporting from centers with the least staff reserve to complete the survey, or may have resulted overreporting from those centers affected the most; 3) the modification of some survey questions over time; 4) the lack of details on trial types and participating patient demographics; and 5) some variability in the centers that participated at different survey time points (Figure 2). For centers that completed 2 or more surveys, their results match the overall group trends (data not shown), suggesting minimal impact on the overall findings. Despite these limitations, the data these surveys provided represent one of the best overall perspectives on where the nation’s cancer center clinical trial infrastructure and operational model now stand and where they are headed in the foreseeable future. To that end, AACI is launching a longitudinal and annual benchmarking survey of all cancer centers to maintain a real-time assessment of the nation’s cancer clinical trial infrastructure and capacity.
Cancer clinical trials are a critical component of both patient care and research progress. COVID-19 significantly affected the national infrastructure to conduct these trials, but that infrastructure demonstrated noteworthy resiliency. Through improvements in efficiencies and adaptation of operational models, patient-centered care continued to be delivered. Nonetheless, the pandemic imposed major resource limitations, including unprecedented staff turnover and loss of institutional knowledge. Pandemic-related limitations also favor interventional nontreatment trials more than treatment trials, with centers experiencing reduced capacity and resource restrictions for the latter. This shift will likely have significant consequences for the nation’s cancer clinical trial capacity for the foreseeable future.
Supplementary Material
Contributor Information
Thomas J George, University of Florida Health Cancer Center, Gainesville, FL, USA.
Tara L Lin, University of Kansas Cancer Center, Kansas City, KS, USA.
Tricia Adrales Bentz, Hollings Cancer Center, Medical University of South Carolina, Charleston, SC, USA.
Stefan Grant, Atrium Health Wake Forest Baptist Comprehensive Cancer Center, Winston-Salem, NC, USA.
Collette M Houston, Memorial Sloan Kettering Cancer Center, New York City, NY, USA.
Melissa A Nashawati, Mays Cancer Center at UT Health San Antonio MD Anderson Cancer Center, San Antonio, TX, USA.
Bhanu Pappu, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas, TX, USA.
Helen Peck, Wilmot Cancer Institute, University of Rochester Medicine, Rochester, NY, USA.
Alex Zafirovski, Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, IL, USA.
Kimberly Kerstann, Winship Cancer Institute of Emory University, Atlanta, GA, USA.
Patricia LoRusso, Yale Cancer Center, Yale School of Medicine, New Haven, CT, USA.
Anne Schnatterly, West Virginia University Cancer Institute, Morgantown, WV, USA.
Janie Hofacker, Association of American Cancer Institutes, Pittsburgh, PA, USA.
Kendra Cameron, Association of American Cancer Institutes, Pittsburgh, PA, USA.
Hailey Honeycutt, Association of American Cancer Institutes, Pittsburgh, PA, USA.
Theresa L Werner, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Data Availability
The data underlying this article are the property of the AACI and was collected from member institutions in accordance with organizational privacy policies given the confidential nature of center clinical research operations, contracts, and budgets. The data provided for this analysis are organized at a summary level, with AACI staff serving as honest brokers to deidentify and manage the confidential nature of individual cancer center institutional data. Requests to associate the data provided here with center identifiers can be submitted in writing to the AACI Board of Directors for review.
Author contributions
Thomas J. George, MD, FACP (Conceptualization; Formal analysis; Methodology; Writing—original draft; Writing—review & editing), Tara L. Lin, MD (Conceptualization; Formal analysis; Methodology; Writing—original draft; Writing—review & editing), Tricia Adrales Bentz, MHA, CCRP (Methodology; Writing—review & editing), Stefan Grant, MD, JD, MBA (Methodology; Writing—review & editing), Collette Houston, BA (Methodology; Writing—review & editing), Melissa A. Nashawati, MPA (Methodology; Writing—review & editing), Bhanu Pappu, PhD, MHA (Methodology; Writing—review & editing), Helen Peck, RN, MA, OCN, CCRP (Methodology; Writing—review & editing), Alex Zafirovski, MBA, RT(T)(ARRT) (Methodology; Writing—review & editing), Kimberly Kerstann, PhD (Methodology; Writing—review & editing), Patricia LoRusso, DO, PhD (Methodology; Writing—review & editing), Anne Schnatterly, MBA, BSN, RN, CCRP (Methodology; Writing—review & editing), Janie Hofacker, RN, BSN, MS (Conceptualization; Methodology; Supervision; Writing—original draft; Writing—review & editing), Kendra Cameron, MBA (Data curation; Formal analysis; Methodology; Project administration; Visualization; Writing—original draft; Writing—review & editing), Hailey Honeycutt, BS (Data curation; Formal analysis; Methodology; Project administration; Visualization; Writing—original draft; Writing—review & editing), Theresa L. Werner, MD (Conceptualization; Formal analysis; Methodology; Writing—original draft; Writing—review & editing).
Funding
This project was supported by the AACI and its more than 100 member cancer centers. Nine of the 16 authors reported P30 grant funds supporting their role within their institutions for this project. Any additional funding agencies mentioned were not involved with the writing of the manuscript or the decision to submit it for publication.
Conflicts of interest
Dr George has disclosed consulting or holding an advisory role for Tempus and Pfizer. Dr Stefan Grant disclosed holding equity for TheraBionic, Inc; is an advisory board member for Florence Healthcare; and receives research support from Guardant Health. Dr Patricia LoRusso disclosed being on the following advisory boards for the following: AbbVie; Agios; GenMab; Roche-Genentech; CytomX; Takeda; Cybrexa; Agenus; IQVIA; TRIGR; Pfizer; ImmunoMet; Black Diamond; GlaxoSmithKline; QED Therapeutics; AstraZeneca; EMD Serono; Shattuck; Astellas; Salarius Silverback; MacroGenics; Kyowa Kirin Pharmaceutical Development; Kineta, Inc.; Zentalis Pharmaceuticals; Molecular Templates; ABL Bio; SK Life Science; STCube Pharmaceuticals; Bayer; I-Mab; Seagen; imCheck; Relay Therapeutics; Stemline; Compass BADX; Mekanist; Mersana Therapeutics; BAKX Therapeutics; Scenic Biotech; Qualigen; Roivant Sciences; and NeuroTrials. The remaining authors have disclosed that they have not received any financial consideration from any person or organization to support the preparation, analysis, results, or discussion of this article.
References
- 1. Yabroff KR, Wu XC, Negoita S, et al. Association of the COVID-19 pandemic with patterns of statewide cancer services. J Natl Cancer Inst. 2022;114(6):907-909. doi: 10.1093/jnci/djab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Broom A, Kenny K, Page A, et al. The paradoxical effects of COVID-19 on cancer care: current context and potential lasting impacts. Clin Cancer Res. 2020;26(22):5809-5813. doi: 10.1158/1078-0432.CCR-20-2989. [DOI] [PubMed] [Google Scholar]
- 3. Tolaney SM, Lydon CA, Li T, et al. The impact of COVID-19 on clinical trial execution at the Dana-Farber Cancer Institute. J Natl Cancer Inst. 2021;113(11):1453-1459. doi: 10.1093/jnci/djaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Upadhaya S, Yu JX, Oliva C, Hooton M, Hodge J, Hubbard-Lucey VM.. Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov. 2020;19(6):376-377. doi: 10.1038/d41573-020-00093-1. [DOI] [PubMed] [Google Scholar]
- 5. Boughey JC, Snyder RA, Kantor O, et al. Impact of the COVID-19 pandemic on cancer clinical trials. Ann Surg Oncol. 2021;28(12):7311-7316. doi: 10.1245/s10434-021-10406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waterhouse DM, Harvey RD, Hurley P, et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American Society of Clinical Oncology survey. JCO Oncol Pract. 2020;16(7):417-421. doi: 10.1200/OP.20.00275. [DOI] [PubMed] [Google Scholar]
- 7. National Cancer Institute. Interim Guidance for Patients on Clinical Trials Supported by the NCI Cancer Therapy Evaluation Program and the NCI Community Oncology Research Program (NCORP). https://ctep.cancer.gov/content/docs/Memorandum_on_Interim_Guidance_for_Clinical_Trial_Activities_Affected_by_the_Novel_Coronavirus-3-13-2020.pdf. Published March 13, 2020. Accessed March 2, 2023.
- 8. National Cancer Institute. Additional Guidance Regarding Alternative Procedures for Clinical Trials Supported by the NCI Cancer Therapy Evaluation Program (CTEP) and NCI Community Oncology Research Program (NCORP) Affected by the Spread of the Novel Coronavirus. https://ctep.cancer.gov/investigatorResources/docs/Memorandum_on_Additional_Guidance_for_Clinical_Trial_Activities_Affected_by_the_Novel_Coronavirus_3-23-2020.pdf. Published March 23, 2020. Accessed March 2, 2023.
- 9. Prindiville SA, Sarosy GA, Loose D, Ciolino H, Doroshow JH.. Patterns of enrollment in cancer treatment trials during the COVID-19 pandemic at national cancer institute-designated cancer centers. Cancer J. 2022;28(2):111-117. doi: 10.1097/PPO.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sessa C, Cortes J, Conte P, et al. The impact of COVID-19 on cancer care and oncology clinical research: an experts' perspective. ESMO Open. 2022;7(1):100339. doi: 10.1016/j.esmoop.2021.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Curtis A, Qu Y.. Impact of using a mixed data collection modality on statistical inferences in decentralized clinical trials. Ther Innov Regul Sci. 2022;56(5):744-752. doi: 10.1007/s43441-022-00416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Unger JM, Xiao H, LeBlanc M, Hershman DL, Blanke CD.. Cancer clinical trial participation at the 1-year anniversary of the outbreak of the COVID-19 pandemic. JAMA Netw Open. 2021;14(7):e2118433. doi: 10.1001/jamanetworkopen.2021.18433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugalski JM, Franco T, Shulman LN, et al. COVID-19 and cancer center operations: lessons learned from the NCCN best practices committee. J Natl Compr Canc Netw. 2022;20(13). doi: 10.6004/jnccn.2021.7102. [DOI] [PubMed] [Google Scholar]
- 14. Suman A, van Es J, Gardarsdottir H, et al. ; Trials@Home Consortium. A cross-sectional survey on the early impact of COVID-19 on the uptake of decentralised trial methods in the conduct of clinical trials. Trials. 2022;23(1):856. doi: 10.1186/s13063-022-06706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karzai F, Dahut WL.. Lessons from the impact of the COVID-19 pandemic at the National Cancer Institute: cancer research and care. Cancer J. 2022;28(2):118-120. doi: 10.1097/PPO.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samimi G, House M, Benante K, et al. Lessons learned from the impact of COVID-19 on NCI-sponsored cancer prevention clinical trials: moving toward participant-centric study designs. Cancer Prev Res (Phila). 2022;15(5):279-284. doi: 10.1158/1940-6207.CAPR-21-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morton C, Sullivan R, Sarker D, Posner J, Spicer J.. Revitalising cancer trials post-pandemic: time for reform. Br J Cancer. 2023;128(8):1409-1414. doi: 10.1038/s41416-023-02224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu S, Gerber DE, Beg MS.. Decentralized clinical trials in oncology: are we ready for a virtual-first paradigm? J Clin Oncol. 2023;41(2):181-185. doi: 10.1200/JCO.22.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adesoye T, Katz MHG, Offodile AC.. 2nd meeting trial participants where they are: decentralized clinical trials as a patient-centered paradigm for enhancing accrual and diversity in surgical and multidisciplinary trials in oncology. JCO Oncol Pract. 2023;19(6):317-321. doi: 10.1200/OP.22.00702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are the property of the AACI and was collected from member institutions in accordance with organizational privacy policies given the confidential nature of center clinical research operations, contracts, and budgets. The data provided for this analysis are organized at a summary level, with AACI staff serving as honest brokers to deidentify and manage the confidential nature of individual cancer center institutional data. Requests to associate the data provided here with center identifiers can be submitted in writing to the AACI Board of Directors for review.