Abstract
Introduction:
We explored the association of respiratory and cardiometabolic comorbidities with NSCLC overall survival (OS) and lung cancer-specific survival (LCSS), by stage, in a large, multicontinent NSCLC pooled data set.
Methods:
On the basis of patients pooled from 11 International Lung Cancer Consortium studies with available respiratory and cardiometabolic comorbidity data, adjusted hazard ratios (aHRs) were estimated using Cox models for OS. LCSS was evaluated using competing risk Grey and Fine models and cumulative incidence functions. Logistic regression (adjusted OR [aOR]) was applied to assess factors associated with surgical resection.
Results:
OS analyses used patients with NSCLC with respiratory health or cardiometabolic health data (N = 16,354); a subset (n = 11,614) contributed to LCSS analyses. In stages I to IIIA NSCLC, patients with respiratory comorbidities had worse LCCS (stage IA aHR = 1.51, confidence interval [CI]: 1.17–1.95; stages IB–IIIA aHR = 1.20, CI: 1.06–1.036). In contrast, patients with stages I to IIIA NSCLC with cardiometabolic comorbidities had a higher risk of death from competing (non-NSCLC) causes (stage IA aHR = 1.34, CI: 1.12–1.69). The presence of respiratory comorbidities was inversely associated with having surgical resection (stage IA aOR = 0.54, CI: 0.35–0.83; stages IB–IIIA aOR = 0.57, CI: 0.46–0.70).
Conclusions:
The presence of either cardiometabolic or respiratory comorbidities is associated with worse OS in stages I to III NSCLC. Patients with respiratory comorbidities were less likely to undergo surgery and had worse LCSS, whereas patients with cardiometabolic comorbidities had a higher risk of death from competing causes. As more treatment options for stages I to III NSCLC are introduced into the practice, accounting for cardiometabolic and respiratory comorbidities becomes essential in trial interpretation and clinical management.
Keywords: Comorbidity, NSCLC, COPD, Early-stage
Introduction
Lung cancer remains the leading cause of cancer death worldwide.1 In the past decade, major improvements have been achieved in the treatment of the major subtype of lung cancer, NSCLC, with the development of targeted therapies and immunotherapy. These new therapies have increased progression-free and overall survival (OS) time in the metastatic setting, especially in patients with targeted molecular alterations or good response to immune checkpoint inhibitors.2–4 Consequently, lung cancer mortality is decreasing faster than its incidence, and patients are living longer.5,6 Early stage NSCLC continues to be associated with a high risk of relapse despite apparent curative-intent treatment. The role of immunotherapy and targeted therapies is being explored in early stage NSCLC, with encouraging results.7–9
The prevalence of comorbidity in patients with lung cancer is substantial,10 mainly owing to common risk factors such as smoking history and advanced age.11,12 Comorbidity has previously been found to affect available treatment options and survival in patients with lung cancer.13,14 Nevertheless, the magnitude of association between comorbidity and lung cancer survival remains unclear. Prior research is limited to observational studies in homogeneous populations, including patients from the same geographic area, abstracted from regional or national registries, and including patients of European ancestry only.11,15–17 In addition, the best method to assess or report comorbidity in patients with lung cancer is not yet established. Existing comorbidity indexes such as the Charlson comorbidity index18 may be suboptimal for application in lung cancer.19 Furthermore, comorbidity can affect survival in different ways, such as by interfering with some diagnostic procedures and treatments.12 The association between comorbidity and cause of death (i.e., lung cancer versus other causes) in patients with lung cancer needs to be explored. We consider it to be particularly relevant to explore the association between comorbidity and survival in patients with lung cancer by disease stage. It can be anticipated that comorbidities may have limited impact on survival in stage IV NSCLC because of the high morbidity and mortality from the metastatic NSCLC itself. In patients with early stage NSCLC, comorbidity can hamper curative-intent treatments, such as surgery, especially because medically inoperable lung cancer has a high rate of recurrence and mortality.20 In addition, cancer treatments given in the curative setting such as chemotherapy and radiation therapy can affect long-term survival owing to long-term side effects.21 Overall, a better understanding of the reasons why comorbidity negatively affects survival could improve management and outcomes of patients with lung cancer. This will become more relevant as more treatment options become available, and patients live longer.
Our main objective is to evaluate the association between cardiometabolic and respiratory comorbidities and NSCLC survival by stage, including cause-specific survival, in a pooled analysis of comparable studies from multiple continents, with a focus on resectable stages I to IIIA NSCLC, while taking into account potential confounders such age, sex, histology subtype, education, ethnicity, smoking history, and individual study.
Materials and Methods
Data Collection
The International Lung Cancer Consortium (ILCCO) shares and harmonizes compatible data from various epidemiologic studies worldwide to facilitate lung cancer epidemiology research in large combined data sets. Requirements for inclusion of studies in ILCCO and other details are available on the Consortium’s website (http://ilcco.iarc.fr).
A total of 27 studies collected and participated in the survival analyses, among which 12 ILCCO studies collected and contributed data on comorbidities for this pooled analysis. Nine studies were conducted in North America, one in Europe, one in Brazil, and one in the People’s Republic of China; the enrollment years of the studies ranged from 1992 to 2020. Sample sizes for OS analysis by study and overall are available in Supplementary Table 1. Sample sizes by study and overall for cause-specific and surgical resection data are summarized in Supplementary Tables 2 and 3, respectively.
Written informed consent was obtained from all study subjects, and ethics review boards at each study center approved the study protocols. Consort diagram is available in Figure 1. The full data set from the 11 sites with comorbidity data included 19,493 patients. The data submitted from all 11 studies were checked for missing values, inadmissible values, aberrant distributions, and inconsistencies. Queries were sent to the investigators to resolve all potential discrepancies.
Figure 1.
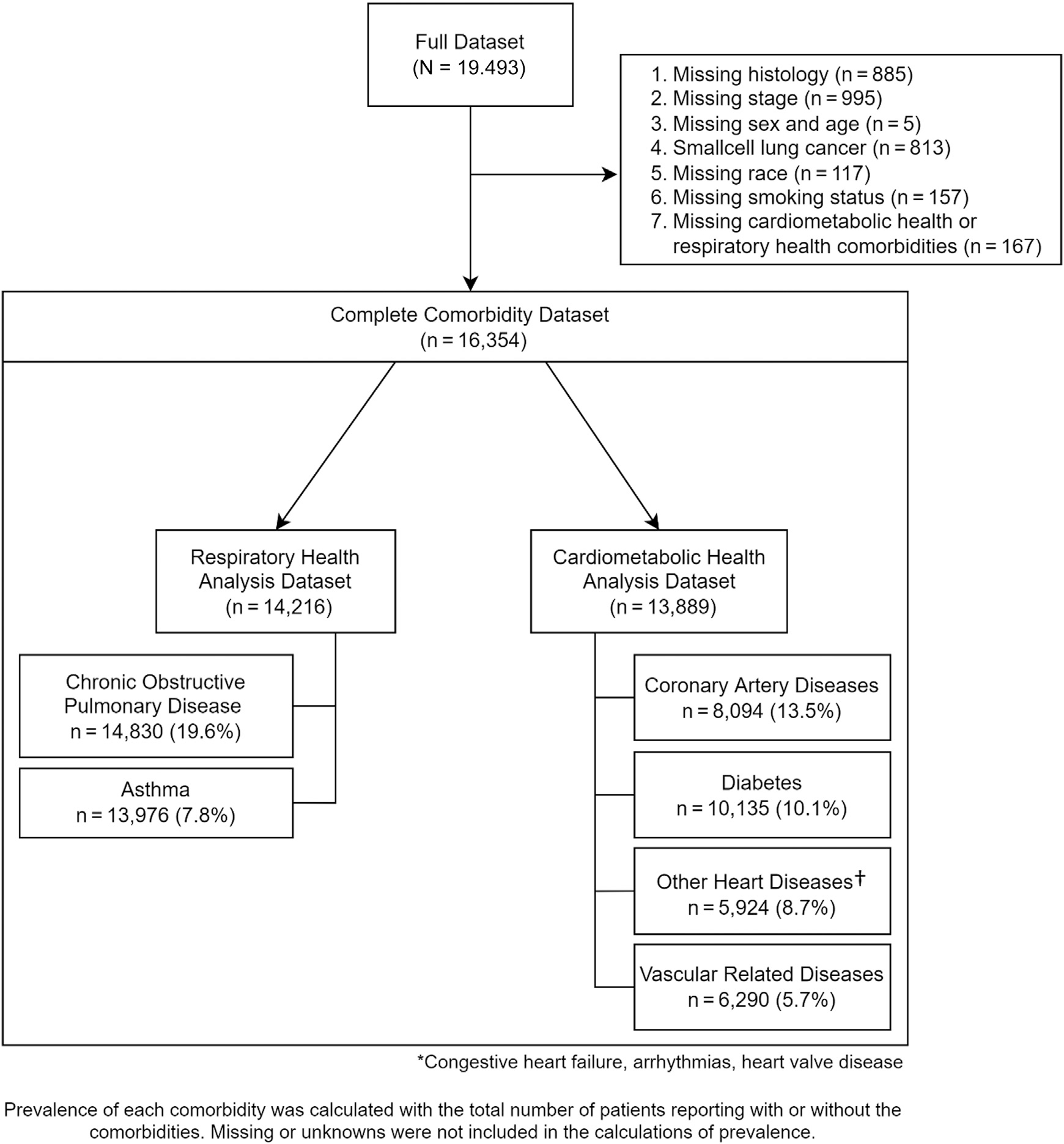
Consort diagram describing how the respiratory health and cardiometabolic health data sets were created with the prevalence* of comorbidities that were included in the analyses. *Prevalence of each comorbidity was calculated with the total number of patients reporting with or without the comorbidity, excluding those with missing or unknown comorbidity status. †Other heart diseases include congestive heart failure, arrhythmias, and heart valve disease.
Subjects with SCLC (n = 813) and those with missing respiratory and cardiometabolic comorbidity data (n = 275) or missing relevant baseline characteristics such as histology (n = 1747), stage (n = 995), sex and age (n = 5), race (n = 117), and smoking status (n = 149) were excluded. A total of 16,354 patients were available for the present investigation.
Comorbidity
Comorbidity data were based on self-reported status of being previously diagnosed with a concomitant disease in addition to lung cancer. To develop a pragmatic comorbidity index focusing on respiratory and cardiometabolic diseases, we harmonized comorbidity data from all studies by developing two main categories: “respiratory health” and “cardiometabolic” (Fig. 1). We classified all respiratory- or cardiometabolic-related comorbidities in these two groups. All the other comorbidities not directly related to respiratory or cardiometabolic health were not considered (i.e., rheumatologic, gastrointestinal, renal disorders, malignancies, among others). Comorbidities with a prevalence less than 5% were also not considered.
Respiratory health was composed of two main variables: asthma and chronic obstructive pulmonary disease (COPD). The COPD variable included “bronchitis,” “COPD,” and “emphysema.” Other respiratory conditions reported were tuberculosis, asbestosis/silicosis, pulmonary infections, and pulmonary fibrosis; these were not considered, however, owing to low prevalence (<5%) and statistical nonsignificance in the univariate survival analysis using the Cox proportional hazards (PH) model.
Cardiometabolic health was composed of the following four main variables: heart disease (including “congestive heart failure,” “arrhythmia,” “heart valve disease”), coronary artery disease (CAD) (“angina,” “CAD,” “angioplasty,” “by-pass,” “catheterization,” “cardiac arrest,” and “myocardial infarction”), diabetes mellitus (DM) (“diabetes mellitus,” “diabetic retinopathy,” “diabetic nephropathy”), and vascular disease (“peripheral vascular disease,” “cerebrovascular disease,” “transient ischemic attack,” and “subarachnoid hemorrhage”).
Heterogeneity of selected main comorbidity variables was evaluated individually across studies on the basis of the Cochrane Q statistics test. Boujat plots were used to identify studies that contributed to heterogeneity for each specific comorbidity. Sensitivity analysis was performed for these identified studies. Studies that contributed to significant heterogeneity were excluded for the analysis of the respective comorbidity variable. Two studies were removed from diabetes, and one study was removed from COPD and vascular diseases.
For each study site, Kaplan-Meier (KM) survival curves were plotted for all stages and stages I to IV individually to evaluate the survival rate by cardiometabolic or respiratory health comorbidities. Sensitivity analysis was performed for two studies, but none were removed.
Statistical Methods
Summary statistics were provided. Continuous variables were compared using Kruskal-Wallis tests, whereas categorical variables were compared using chisquare tests. On the basis of pooled data from 11 ILCCO studies with available respiratory and cardiometabolic comorbidity data, we estimated adjusted hazard ratios (aHRs) and corresponding 95% confidence interval (CI) using Cox PH models for OS, accounting for age, sex, education, race, stage, smoking status, and study. Results were presented for all stages and by individual stages. Unadjusted KM survival curves were also plotted for each stage. Lung cancer-specific survival (LCSS) was evaluated using Fine-Grey’s competing risk models and cumulative incidence functions for all stages and by individual stages, adjusted for age, sex, education, race, stage, smoking status, and study. Cox PH models were used to estimate OS by increasing number of comorbidities present (COPD, asthma, CAD, DM, other heart diseases, and vascular disease).
Subgroup-specific analysis for patients with data on surgical status was performed. We analyzed the relationship between comorbidity and surgical resection to explore potential reasons for the associations found in cause-specific survival in patients with resectable NSCLC (stages I–IIIA). Seven studies were included for the treatment analyses with surgical data.
A logistic regression model was fitted for the OR of surgical resection by cardiometabolic health-related and respiratory health-related comorbidities, adjusted for age, sex, education, race, stage, smoking status, and study. In addition, Cox PH models and the Fine-Grey’s competing risk models were also fitted to this subgroup with data on surgery for the aHRs of OS and LCSS, adjusted for age, sex, education, race, stage, smoking status, and study. Statistical analyses were conducted using R version 3.6.3 (R Foundation). The p values less than 0.05 were considered statistically significant.
Results
Baseline Characteristics and Comorbidity Variables
The demographic distribution of the pooled data set for comorbidity is displayed in Table 1. A total of 16,354 patients with NSCLC were included in the survival analyses for either respiratory health or cardiometabolic health data. Most of the patients were of European ancestry (80%). Median age was 66 (range: 21–96) years, and 52% were women. Furthermore, 73% of the patients had nonmetastatic, stages I to III NSCLC.
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Variables | Category | All Patients, N (%) | Patients With Cardiometabolic Health Data, n (%) | Patients With Respiratory Health Data, n (%) |
|---|---|---|---|---|
|
| ||||
| Total | Total | 16,354 (100) | 13,889 (100) | 14,216 (100) |
| Comorbidity | Absent | 11,432 (70) | 11,580 (83) | 10,919 (77) |
| Present | 4922 (30) | 2309 (17) | 3297 (23) | |
| Age, y | Median [min-max] | 66 [21–96] | 66 [21–96] | 66 [21–96] |
| Sex | Male | 7928 (48) | 6382 (46) | 6824 (48) |
| Female | 8426 (52) | 7507 (54) | 7392 (52) | |
| Ethnicity | White | 13,014 (80) | 10,610 (76) | 11,254 (79) |
| Asian | 2139 (13) | 2129 (15) | 1871 (13) | |
| Black | 842 (5) | 807 (6) | 799 (6) | |
| Other | 359 (2) | 343 (2) | 292 (2) | |
| Education | Elementary | 2697 (16) | 2352 (17) | 2358 (17) |
| High school/vocational | 5937 (36) | 4971 (36) | 4976 (35) | |
| Post-secondary | 4875 (30) | 3845 (28) | 4120 (29) | |
| Missing | 2845 (17) | 2721 (20) | 2762 (19) | |
| Stage | Stage I | 5952 (36) | 5129 (37) | 5247 (37) |
| Stage II | 2532 (15) | 2271 (16) | 2333 (16) | |
| Stage III | 3394 (21) | 2671 (19) | 2839 (20) | |
| Stage IV | 4476 (27) | 3818 (27) | 3797 (27) | |
| Smoking status | Never smoker | 3049 (19) | 2879 (21) | 2655 (19) |
| Ever smoker | 13,305 (81) | 11,010 (79) | 11,561 (81) | |
Max, maximum; Min, minimum.
The consort diagram (Fig. 1) reveals the breakdown of the full data set into the two comorbidity data sets that were ultimately analyzed along with the constituent comorbidities comprising each of the data sets. Respiratory health data set included 14,216 patients with lung cancer and consisted of two main respiratory comorbidities, asthma and COPD. Prevalence was 19.6% for COPD and 7.8% for asthma.
Cardiometabolic health data set included 13,889 patients and consisted of four main variables. Prevalence of each comorbidity was 13.5% for CAD, 10.1% for DM, 8.7% for other heart diseases (congestive heart failure, arrhythmias, and heart valve disease), and 5.7% for vascular disease.
Comorbidity and Overall Survival, by Disease Stage at Diagnosis
Our first aim was to investigate the association of either respiratory or cardiometabolic comorbidities with OS in patients with NSCLC, by stage. Sample sizes for OS analysis by study and overall are available in Supplementary Table 1.
Unadjusted KM survival curves by stage are illustrated in Figure 2A. For patients with a cardiometabolic comorbidity, median OS for stages I, II and III, and IV was 6.7 years, 2.5 years, and 0.7 year versus 8.6 years, 3.4 years, and 1 year, respectively, if absent. For patients with respiratory comorbidity present, median OS in stages I, II and III, and IV was 6.1 years, 2.2 years, and 1 year versus 8.9 years, 3.0 years, and 0.8 year, respectively, when absent.
Figure 2.
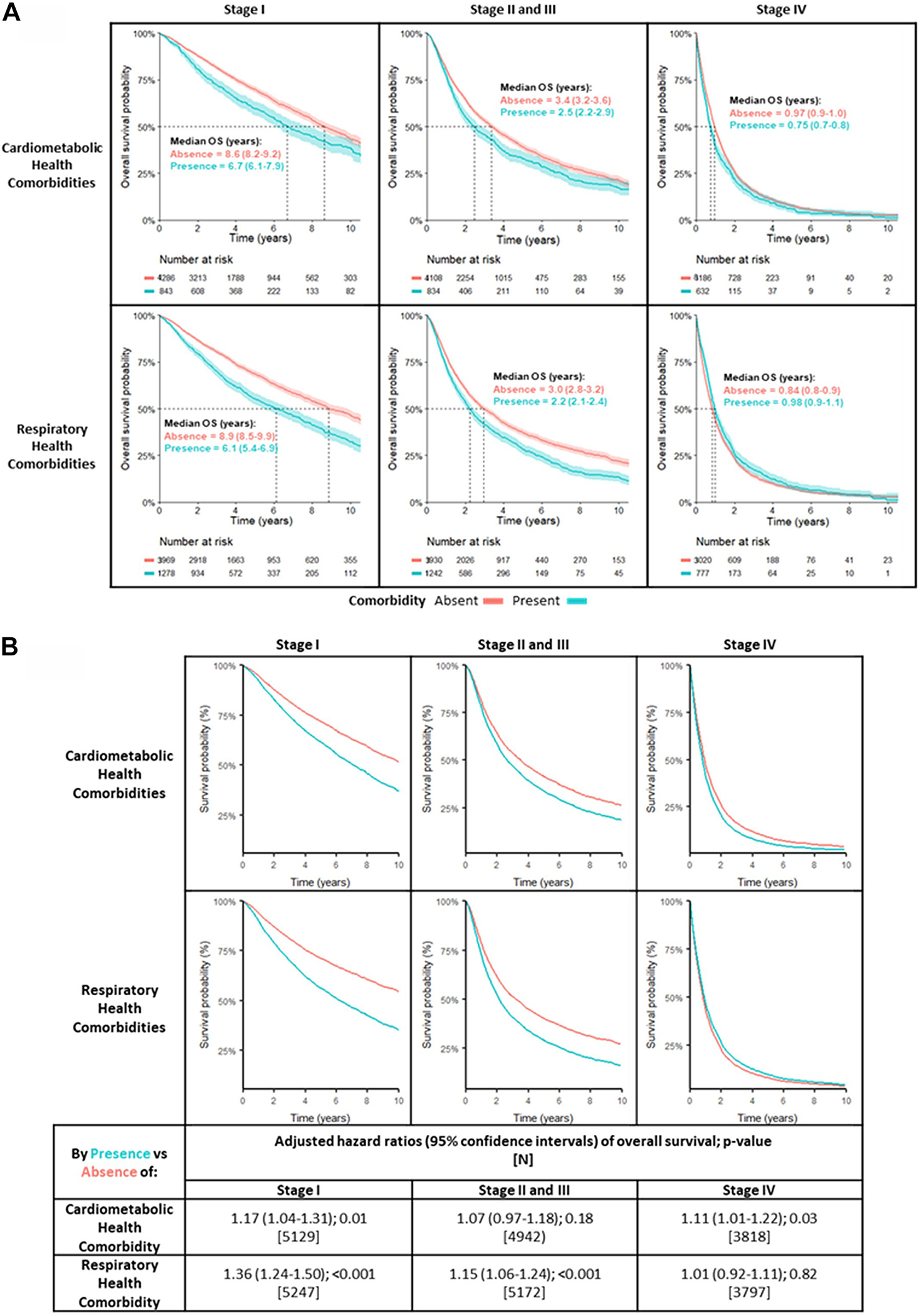
Survival curves and adjusted Cox proportional hazard models for comorbidities. (A) Unadjusted Kaplan-Meier OS curves by the presence (blue lines) and absence (red lines) of either cardiometabolic health comorbidity (top row) or respiratory health comorbidity (bottom row). Median OS in years (95% CI, shading) is presented. (B) Adjusted Kaplan-Meier overall survival curves (top graph) and adjusted Cox proportional hazard models of overall survival (bottom table) for the presence (blue line) versus absence (red line) of either cardiometabolic comorbidity or respiratory health comorbidity, separately analyzed. Separate analyses were performed for individual disease stages at the time of diagnosis. Each analysis was adjusted for age, sex, race, education, smoking status, stage (for all stages and stages II and III only), and study sites. CI, confidence interval; OS, overall survival.
Figure 2B illustrates the adjusted survival curves and adjusted Cox PH ratio by stage. For patients with cardiometabolic comorbidity, HR for death in stage I was 1.17 (95% CI: 1.04–1.31, p = 0.01) and 1.08 (95% CI: 0.97–1.18, p = 0.18) for stages II and III. For stage IV, HR was 1.11 (95% CI: 1.01–1.22, p = 0.031). For respiratory comorbidity, HR for death in stage I was 1.36 (95% CI: 1.24–1.50, p < 0.01). For stages II and III, HR was 1.15 (95% CI: 1.06–1,24, p < 0.001). No association was observed for patients at stage IV (HR = 1.01, 95% CI: 0.02–0.11).
When using a comorbidity count, HR for death increased along with the number of comorbidities, as found in the Cox PH models of OS (Table 2 and Supplementary Table 4). HRs were 1.12 (95% CI: 1.07–1.18), 1.19 (95% CI: 1.09–1.29), and 1.30 (95% CI: 1.11–1.51) for 1, 2, and 3 or more comorbidities, respectively, all statistically significant (p < 0.001).
Table 2.
Cox Proportional Hazards Models of Overall Survival by Increasing Number of Comorbidities
| Adjusted Hazard Ratios (95% Confidence Intervals) of Overall Survival by Increasing Number of Comorbidities; p Value | |||
|---|---|---|---|
|
|
|||
| Number of Comorbidities | Stage I (n = 5952) | Stages II and III (n = 5926) | Stage IV (n = 4476) |
|
| |||
| 0 | Reference | Reference | Reference |
| 1 | 1.28 (1.17–1.41), p < 0.001 | 1.09 (1.01–1.18), p = 0.04 | 1.08 (0.99–1.17), p = 0.07 |
| 2 | 1.42 (1.21–1.68), p < 0.001 | 1.18 (1.03–1.36), p = 0.02 | 1.11 (0.96–1.27), p = 0.15 |
| 3+ | 1.63 (1.22–2.18), p < 0.001 | 1.40 (1.09–1.80), p = 0.008 | 1.05 (0.81–1.37), p = 0.72 |
Note: Separate analyses were performed for all stages and for individual disease stages at the time of diagnosis. Each analysis was adjusted for age, sex, race, education, smoking status, stage (for all stages and stages II and III only), and study sites.
The influence of comorbidities on survival was greater in early stage (stages I–III) than in the stage IV patients (stage-comorbidity interaction term, crude p = 0.015; adjusted for age, sex, race, education, smoking, stage, and study site, p = 0.0402).
Comorbidity and Cause-Specific Survival in Stages I to IIIA NSCLC
Our second aim was to explore cause-specific survival for each comorbidity group in potentially resectable NSCLC (stages I–IIIA). No associations with cause-specific survival were observed in patients with stage IV NSCLC. A subset of 11,614 patients contributed to lung cancer-specific survival analyses; study sample sizes overall, for cardiometabolic, and respiratory data set is available in Supplementary Table 2.
Overall and by stage-adjusted mortality HR of LCSS and other competing causes (non–NSCLC) for cardiometabolic and respiratory health are displayed in Table 3.
Table 3.
Mortality Hazard Ratios (With 95% CI and p Values) of Lung Cancer-Specific Survival and Nonlung Cancer Competing Causes of Death, by the Presence or Absence of Either Cardiometabolic Health Comorbidity or Respiratory Health Comorbidity
| Adjusted Hazard Ratios (95% CI) of Cause-Specific Death by Presence vs. Absence of Comorbidity; p Value | |||
|---|---|---|---|
|
|
|||
| Comorbidity | Cause of Death | Stage IA | Stages IB-IIIA |
|
| |||
| Cardiometabolic health comorbidity | [n] | [2409] | [4598] |
| Death from lung cancer | 0.84 (0.63–1.11), p = 0.22 | 0.99 (0.86–1.14), p = 0.85 | |
| Death from other causes | 1.37 (1.06–1.77), p = 0.018 | 1.32 (1.03–1.71), p = 0.029 | |
| Respiratory health comorbidity | [n] | [2087] | [4200] |
| Death from lung cancer | 1.51 (1.17–1.95), p = 0.002 | 1.20 (1.06–1.36), p = 0.004 | |
| Death from other causes | 1.14 (0.85–1.53), p = 0.39 | 1.11 (0.86–1.45), p = 0.43 | |
Note: Separate analyses were performed for all stages and for individual disease stages at the time of diagnosis. Each analysis was adjusted for age, sex, race, education, smoking status, stage (except for stage IA), and study sites.
CI, confidence interval.
In patients with stages I to IIIA NSCLC, the presence of cardiometabolic comorbidity was associated with a higher risk of death from competing causes, with a HR for death from nonlung cancer causes of 1.37 for stage IA (95% CI: 1.06–1.77, p = 0.018) and HR of 1.32 for stages IB to IIIA (95% CI: 1.03–1.71, p = 0.029) if cardiometabolic comorbidity was present. Cardiometabolic comorbidity did not significantly affect LCSS in any stage.
In contrary, respiratory comorbidity had a major impact on LCSS in stages I to IIIA NSCLC. HR for death from lung cancer was 1.51 for stage IA (95% CI: 1.17–1.95, p = 0.002) and 1.20 for stages IB to IIIA (95% CI: 1.06–1.36, p = 0.004) if respiratory comorbidity was present. We did not observe any association between respiratory comorbidity and death from other causes. We differentiated between stage IA and stages IB to IIIA as there is no indication for adjuvant treatment after surgery for stage IA, as compared with stages IB to IIIA where consideration for adjuvant therapy is given.
Comorbidity and Cause-Specific Survival by Stage and Relationship Between Comorbidity and Surgical Resection
We performed an additional analysis to explore the relationship between comorbidity, cause-specific survival, and surgical resection by stage (Tables 4 and 5). Our hypothesis was that respiratory comorbidity affects LCSS in patients with resectable NSCLC (stages I–IIIA) by interfering with operability. A subset of 11,323 patients contributed to surgery analyses (Supplementary Table 3). As 89% of the patients with stage IV NSCLC did not receive any surgical intervention, we excluded them from this analysis.
Table 4.
ORs (95% CI and p Value) of Having Received Versus Not Received Lung Cancer Surgical Resection by the Presence Versus Absence of Either Cardiometabolic Health Comorbidity or Respiratory Health Comorbidity
| Adjusted OR (95% CI) of Receiving vs. Not Receiving Lung Cancer Surgical Resection; p Value [N] | ||
|---|---|---|
|
|
||
| Comorbidity | Stage IA | Stages IB-IIIA |
|
| ||
| Cardiometabolic health comorbidity | 0.73 (0.48–1.15), p = 0.167 | 0.73 (0.56–0.96), p = 0.025 |
| [1566] | [3206] | |
| Respiratory health comorbidity | 0.54 (0.35–0.83), p = 0.004 | 0.57 (0.46–0.70), p < 0.001 |
| [1405] | [3608] | |
Note: Separate analyses were performed for all stages and for individual disease stages at the time of diagnosis. Each analysis was adjusted for age, sex, race, education, smoking status, stage (except for stage IA), and study sites.
CI, confidence interval.
Table 5.
Mortality Hazard Ratios (With 95% CI and p Values) of Lung Cancer-Specific Survival and Nonlung Cancer Competing Causes of Death, by the Presence or Absence of Lung Cancer Resection
| Adjusted Hazard Ratios (95% CI) of Cause-Specific Death by Resected vs. Unresected; p Value | ||
|---|---|---|
|
|
||
| Cause of Death | Stage IA | Stages IB-IIIA |
|
| ||
| [n] | [1260] | [2646] |
| Death from lung cancer | 0.38 (0.28–0.52), p < 0.001 | 0.37 (0.32–0.42), p < 0.001 |
| Death from other causes | 1.73 (1.07–1.78), p = 0.02 | 1.18 (0.86–1.63), p = 0.31 |
Note: Separate analyses were performed for all stages and for individual disease stages at the time of diagnosis. Each analysis was adjusted for age, sex, race, education, smoking status, stage (except for stage IA), and study sites.
CI, confidence interval.
In our study, the presence of respiratory comorbidities was inversely associated with having surgical resection; the adjusted OR of having surgery in the presence of respiratory comorbidity was 0.54 (0.35–0.83, p = 0.004) for stage IA and 0.57 (0.46–0.70, p < 0.001) for stages IB to IIIA (Table 4).
In contrast, the presence of cardiometabolic comorbidity had less impact on operability in patients with stages I to III NSCLC. The adjusted OR of having surgery in the presence of any cardiometabolic comorbidity was not significant for stage IA, with a OR of 0.73 (95% CI: 0.48–1.15, p = 0.167), and the impact was less in patients with stages IB to IIIA with a OR for having surgery of 0.73 (95% CI: 0.56–0.96, p = 0.025) (Table 4).
When exploring the association between cause-specific survival and surgery for lung cancer, LCSS was significantly better in patients undergoing lung cancer surgery for all stages IA to IIIA (aHR = 0.41, 95% CI: 0.37–0.45, p < 0.001) and for stage IA (aHR = 0.38, 95% CI: 0.28–0.52, p < 0.001) and stages IB to IIIA (aHR = 0.37, 95% CI: 0.32–0.42, p < 0.001). In contrast, there was no association between the presence of cardiometabolic comorbidity and death from other causes in any stage (Table 5).
Discussion
In our study, the presence of either cardiometabolic or respiratory comorbidities was associated with significantly worse OS in stages I to III (nonmetastatic) NSCLC. The reason for each of these associations was different: in our study, patients with respiratory comorbidities were less likely to undergo surgical resection and had worse LCSS, but there were no differences by respiratory comorbidities in death from non–NSCLC causes. In contrast, patients with cardiometabolic comorbidities had a higher risk of death from competing causes, but there were no differences in LCSS. Our findings highlight the relevance of proper management of comorbidities in patients with NSCLC, especially in the early stage setting.
Prior data have revealed that certain comorbid conditions may be independent prognostic factors for lung cancer survival,11,15–17 but results are inconsistent. For example, DM or CAD was found to be predictors of survival in some16 but not all studies.11 Established indexes, such as the Charlson comorbidity index or a comorbidity count, are not consistently prognostic in lung cancer.11,19
As opposed to previous studies, we explored the impact of respiratory and cardiometabolic comorbidities on cause-specific survival, by stage, in a large, international, multicontinent NSCLC pooled data set and adjusting by confounding factors such as age, sex, race, education, and smoking status.
We found that patients with respiratory comorbidity (COPD or asthma) and nonmetastatic NSCLC were less likely to undergo surgery and had worse LCSS compared with those patients with no respiratory comorbidities. Despite surgical resection is the standard procedure for patients with early stage NSCLC, those who also have respiratory comorbidities such as COPD are frequently deemed inoperable owing to low cardiopulmonary reserve.22 Our results suggest that, even in patients with respiratory comorbidities, curative-intent surgery should be considered when possible; preoperative pulmonary rehabilitation programs before surgery or similar strategies should be pursued and reinforced.23,24 For patients with nonmetastatic, inoperable NSCLC (i.e., severe COPD), the role of nonsurgical but curative-intent approaches such as stereotactic body radiation therapy, plus or minus systemic therapy, is being explored in clinical trials.20,25
Nevertheless, we found that patients with stages I to IIIA NSCLC and cardiometabolic comorbidities were not less likely to undergo surgical resection and had similar LCSS compared with those patients without cardiometabolic comorbidity. Previous studies have revealed that cardiovascular comorbidities do not influence the long-term outcomes of patients after pulmonary resection for NSCLC.26 In our pooled data set, however, patients with cardiometabolic comorbidity were at higher risk of death from non-NSCLC causes compared with those with no cardiometabolic comorbidities. Chemotherapy and radiation therapy have a broad range of effects on the cardiovascular system and can increase the risk of developing cardiac toxicity in patients with NSCLC, leading to cardiac dysfunction.27,28 Cardiac events are even more common after high-dose thoracic radiotherapy (RT), which is usually given for unresectable stage III NSCLC.29 Before the publication of the Lung ART study results, postoperative RT (PORT) was frequently recommended in patients with mediastinal nodal involvement (stage IIIA, N2).30 Interestingly, this randomized phase 3 trial revealed an excess of deaths related to cardiopulmonary diseases in patients treated with postoperative RT. Proper management of cardiometabolic comorbidity, by starting medical therapy or lifestyle interventions sooner, may improve survivorship outcomes in patients with early stage NSCLC receiving systemic therapy and/or thoracic RT.
Our results are limited by the retrospective nature of the study. Second, this is a pooled data set including lung cancer and comorbidity data from 12 different hospitals across the world; there were differences in how the comorbidity variables were collected and registered by each study, with a risk of misclassification of comorbidity. Third, we lacked information on systemic therapy and radiation treatment, which would have enabled us to explore whether worse non-NSCLC–specific survival in patients with cardiometabolic comorbidity were associated with systemic therapy and/or radiation. Fourth, the attribution of the cause of death in patients with lung cancer can be challenging; patients with COPD and lung cancer, dying from cardiorespiratory failure, are harder to classify when attributing cause of death, compared with those patients with cardiac events or other non-NSCLC death cause. Finally, our study pooled data from 1992 to 2020, a time period overarching different treatment possibilities with relevant advances in both surgical techniques and oncological treatment and changes in TNM classification systems.
In conclusion, cardiometabolic and respiratory comorbidities are associated with significantly worse OS in stages I to III NSCLC. As more novel treatment options are introduced into practice for early stage NSCLC, accounting for cardiometabolic and respiratory comorbidities becomes relevant in trial interpretation and clinical decision making.
Supplementary Material
Acknowledgments
This study was partially supported by the Public Ministry of Labor Campinas (Research, Prevention, and Education of Occupational Cancer), FINEP - CT-INFRA (02/2010). The authors thank all members of the GTOP group (Translational Group of Pulmonary Oncology—Barretos Cancer Hospital, Brazil).
Footnotes
Disclosure: Ms. Brown is supported by the Alan B. Brown Chair. Dr. Christiani has received funding through grant U01 CA209414. Prof. Reis and Dr. Leal declare funding from the Public Ministry of Labor Campinas (Research, Prevention, and Education of Occupational Cancer, Brazil). Prof. Chen declares funding from the National Cancer Institute, National Institutes of Health through grants U01-CA063673, UM1-CA167462, and U01-CA167462. Dr. Liu was supported by Alan B. Brown Chair and the Lusi Wong Family Fund, Princess Margaret Cancer Foundation. The remaining authors declare no conflict of interest.
CRediT Authorship Contribution Statement
Miguel García Pardo: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review and editing.
Amy Chang: Data curation, Formal analysis, Investigation, Methodology, Formal analysis, Validation, Visualization, Writing—review and editing.
Sabine Schmid: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—review and editing.
Mei Dong: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—review and editing.
M. Catherine Brown: Data curation, Investigation, Methodology, Visualization, Resources, Project administration, Writing—review and editing.
David Christiani: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Hilary Aurora Tindel: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Paul Brennan: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Chu Chen: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Jie Zhang: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Brid M. Ryan: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
David Zaridze: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Matthew B. Schabath: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Leticia Ferro Leal: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Rui Manuel Reis: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Adonina Tardon: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Guillermo Fernández-Tardon: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Sanjay S. Shete: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Angeline Andrew: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Hermann Brenner: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Wei Xu: Data curation, Resources, Methodology, Visualization, Writing—original draft, Writing—review and editing.
Rayjean J. Hung: Supervision, Conceptualization, Data curation, Resources, Formal analysis, Methodology, Validation, Visualization, Writing—review and editing.
Geoffrey Liu: Supervision, Conceptualization, Data curation, Resources, Formal analysis, Methodology, Validation, Visualization, Writing—review and editing.
Consent for Publication
The authors consent to publish this work.
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at https://doi.org/10.1016/j.jtho.2022.10.020.
Availability of Data and Materials
Data and materials are available on request to the corresponding author.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. [DOI] [PubMed] [Google Scholar]
- 4.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 2017;377:829–838. [DOI] [PubMed] [Google Scholar]
- 5.Ward EM, Sherman RL, Henley SJ, et al. Annual report to the nation on the status of cancer, featuring cancer in men and women age 20–49 years. J Natl Cancer Inst. 2019;111:1279–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383:1711–1723. [DOI] [PubMed] [Google Scholar]
- 8.Wakelee HA, Altorki NK, Zhou C, et al. IMpower010: primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). J Clin Oncol. 2021;39(Suppl 15):8500–8500. [Google Scholar]
- 9.Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386:1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grose D, Morrison DS, Devereux G, et al. Comorbidities in lung cancer: prevalence, severity and links with socioeconomic status and treatment. Postgrad Med J. 2014;90:305–310. [DOI] [PubMed] [Google Scholar]
- 11.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003;103:792–802. [DOI] [PubMed] [Google Scholar]
- 12.Leduc C, Antoni D, Charloux A, Falcoz PE, Quoix E. Comorbidities in the management of patients with lung cancer. Eur Respir J. 2017;49:1601721. [DOI] [PubMed] [Google Scholar]
- 13.Birim O, Kappetein AP, Bogers AJ. Charlson comorbidity index as a predictor of long-term outcome after surgery for nonsmall cell lung cancer. Eur J Cardiothorac Surg. 2005;28:759–762. [DOI] [PubMed] [Google Scholar]
- 14.Asmis TR, Ding K, Seymour L, et al. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol. 2008;26:54–59. [DOI] [PubMed] [Google Scholar]
- 15.Islam KM, Jiang X, Anggondowati T, Lin G, Ganti AK. Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2015;24:1079–1085. [DOI] [PubMed] [Google Scholar]
- 16.Iachina M, Jakobsen E, Møller H, et al. The effect of different comorbidities on survival of non-small cells lung cancer patients. Lung. 2015;193:291–297. [DOI] [PubMed] [Google Scholar]
- 17.Deleuran T, Thomsen RW, Nørgaard M, Jacobsen JB, Rasmussen TR, Søgaard M. Comorbidity and survival of Danish lung cancer patients from 2000–2011: a population-based cohort study. Clin Epidemiol. 2013;5(suppl 1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The updated Charlson comorbidity index is a useful predictor of mortality in patients with Staphylococcus aureus bacteraemia | Epidemiology & infection. Cambridge Core. https://www.cambridge.org/core/journals/epidemiology-and-infection/article/updated-charlson-comorbidity-index-is-a-useful-predictor-of-mortality-in-patients-with-staphylococcus-aureus-bacteraemia/EB5C7C12D4FAACE25B38B1E7B9A79FEF. Accessed July 8, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganti AK, Siedlik E, Marr AS, Loberiza FR Jr, Kessinger A. Predictive ability of Charlson comorbidity index on outcomes from lung cancer. Am J Clin Oncol. 2011;34:593–596. [DOI] [PubMed] [Google Scholar]
- 20.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. [DOI] [PubMed] [Google Scholar]
- 22.Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer. 2002;37:95–101. [DOI] [PubMed] [Google Scholar]
- 23.Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33:95–98. [DOI] [PubMed] [Google Scholar]
- 24.Templeton R, Greenhalgh D. Preoperative rehabilitation for thoracic surgery. Curr Opin Anaesthesiol. 2019;32:23–28. [DOI] [PubMed] [Google Scholar]
- 25.Daly ME. Inoperable early-stage non–small-cell lung cancer: stereotactic ablative radiotherapy and rationale for systemic therapy. J Clin Oncol. 2022;40:539–545. [DOI] [PubMed] [Google Scholar]
- 26.Takenaka T, Katsura M, Shikada Y, Tsukamoto S, Takeo S. The impact of cardiovascular comorbidities on the outcome of surgery for non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. 2013;16:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardy D, Liu CC, Cormier JN, Xia R, Du XL. Cardiac toxicity in association with chemotherapy and radiation therapy in a large cohort of older patients with non-small-cell lung cancer. Ann Oncol. 2010;21:1825–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belzile-Dugas E, Eisenberg MJ. Radiation-induced cardiovascular disease: review of an underrecognized pathology. J Am Heart Assoc. 2021;10:e021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III non–small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35:1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Pechoux C, Pourel N, Barlesi F, et al. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3. Lancet Oncol. 2022;23:104–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials are available on request to the corresponding author.


