Abstract
Background
Beta-blockers are not recommended for heart failure with preserved ejection fraction (HFpEF). However, treatment effect may be more pronounced in high-risk subgroups, and patients with HFpEF and heart rate ≥70 beats/minute have emerged as such as a high-risk subset. We examined the association of high-dose beta-blocker use with outcomes in these patients.
Methods
Of the 8462 hospitalized patients with HFpEF (ejection fraction ≥50%) in the Medicare-linked OPTIMIZE-HF registry, 5422 had discharge heart rate ≥70 beats/minute. Of the 4537 patients who had no contraindications to beta-blocker use, 2797 received a prescription for a beta-blocker and 1740 did not receive one. Of the 2592 patients who had data on beta-blocker dosage, 730 received high-dose beta-blockers, defined as atenolol ≥100 mg/day, carvedilol ≥50 mg/day, metoprolol tartrate or succinate ≥200 mg/day, or bisoprolol ≥10 mg/day. Using propensity scores for the receipt of high-dose beta-blockers, we assembled a cohort of 1280 matched patients, balanced on 58 characteristics.
Results
All-cause mortality occurred in 63% and 68% of matched patients receiving high-dose beta-blocker versus no beta-blocker during 6 years (median, 2.8) of follow-up, respectively (hazard ratio {HR}, 0.86; 95% confidence interval {CI}, 0.75–0.98; p=0.027). HRs (95% CIs) for all-cause readmission and the combined endpoint of all-cause readmission or all-cause mortality associated with high-dose beta-blocker use were 0.90 (0.81–1.02) and 0.89 (0.80–1.00), respectively.
Conclusions
In patients with HFpEF and heart rate ≥70 beats/minute, high-dose beta-blocker use was associated with a significantly lower risk of total mortality. Future randomized controlled trials are needed to examine this association.
Keywords: Heart Failure with Preserved Ejection Fraction, High-Dose Beta-Blocker, Heart Rate, All-Cause Mortality
Heart failure with preserved ejection fraction (HFpEF) accounts for about half of all patients admitted for HF and has emerged as a prominent cause of significant morbidity and mortality.1, 2 Evidence-based pharmacotherapy effective in the treatment of patients with heart failure with reduced ejection fraction (HFrEF) so far has not been shown to be effective in HFpEF.3 High-dose beta-blockers are recommended for patients with heart failure with reduced ejection fraction (HFrEF), but no such evidence exists for HFpEF.3–8 Considering that the negative chronotropic properties of beta-blockers would be expected to be greater at higher doses and that treatment effects are often greater in subgroups of high-risk patients,9–11 we hypothesized that high-dose beta-blocker use would be associated with improved outcomes in the high-risk subset of patients with HFpEF and elevated heart rate. We have recently demonstrated that older patients with HFpEF and heart rate of ≥70 beats/minute have a higher risk of all-cause mortality.1 The objective of our study was to examine the association of high-dose beta-blocker use and outcomes in a propensity score-matched cohort of patients with HFpEF and a discharge heart rate ≥70 beats/minute.
Methods
Data Source and Study Population
The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) is a registry based on 48,612 heart failure hospitalizations in 259 hospitals in 48 states between March 1, 2003 and December 31, 2004. Charts were abstracted based on ICD-9 code for a principal discharge diagnosis of heart failure. Extensive data on demographics, patient and hospital characteristics, quality of care, and outcomes were collected using a Web-based registry. Detailed descriptions of the OPTIMIZE-HF have been described elsewhere.2, 12 For the current analysis, we used the Medicare-linked OPTIMIZE-HF data that included 26,376 unique patients, of which 8873 had HFpEF, defined as ejection fraction ≥50%.2, 13 We excluded 95 patients with heart rate <40 or >150 beats/minute and 3356 patients with heart rate <70 beats/minute, thus assembling a cohort of 5422 patients with heart rate ≥70 beats/minute.
Discharge Use of Beta-Blockers
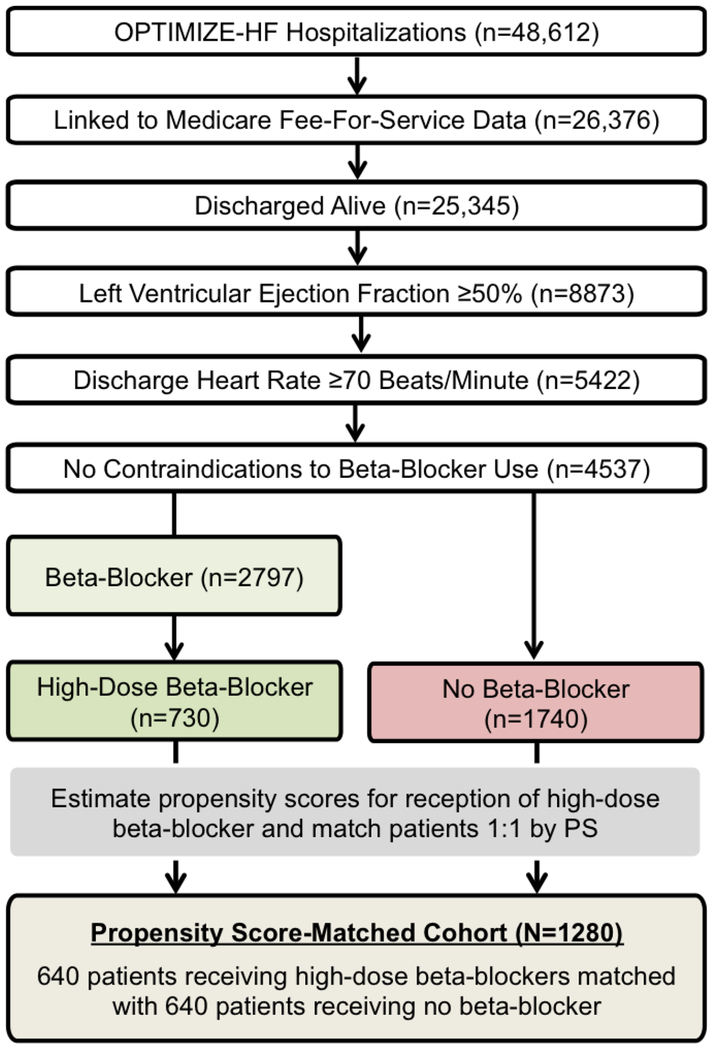
Of the 5422 patients with HFpEF and heart rate ≥70 beats/minute, information on discharge beta-blocker use and dosages was available in 5366 patients, of whom 4537 had no contraindications to beta-blocker use. Of the 4537, 2797 received a prescription for a beta-blocker and 1740 did not receive one. Of the 2797 patients, 2592 had data on beta-blocker dosage, of whom 549 (21%) received carvedilol, 893 (35%) received metoprolol succinate, 662 (26%) received metoprolol tartrate, 23 (1%) received bisoprolol, and 465 (18%) received atenolol. Overall, 730 patients received prescriptions for high-dose beta-blockers, which were defined as a daily dose of carvedilol ≥50 mg (28%; 155/549), metoprolol succinate ≥200 mg (15%; 133/893), metoprolol tartrate ≥200 mg (49%; 318/652), bisoprolol ≥10 mg (26%; 6/23), and atenolol ≥100 mg (25%; 118/465). Thus, our high-dose beta-blocker cohort consisted of 2470 patients, of whom 730 received high-dose beta-blockers, and 1740 did not receive a beta-blocker (Figure 1).
Figure 1.
Flow chart displaying assembly of propensity score (PS) matched cohort of patients with heart failure with preserved ejection fraction and discharge heart rate ≥70 beats/minute, by discharge prescriptions for high-dose beta-blocker versus no beta-blocker
Assembly of a Balanced Study Cohort
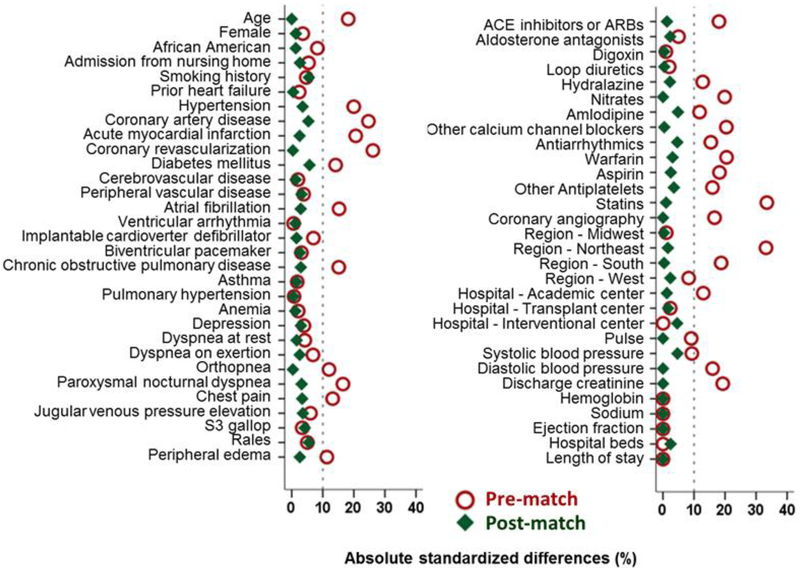
In randomized controlled trials, patients have a 50% probability of receiving a treatment, which is equal for patients receiving and not receiving treatment. This equal probability between the two treatment groups ensures that patients are balanced on baseline characteristics. Because patients in the real world are not randomly given a prescription for medications, the probability varies between 0 and 100%. This probability is a propensity score and can be estimated using measured baseline characteristics in a multivariable logistic regression model.14–16 We separately calculated propensity scores for use of a high-dose beta-blocker based on 58 baseline characteristics displayed in Figure 2. We then used a greedy matching algorithm to match patients based on their propensity scores.17 We matched 88% of the 730 patients receiving high-dose beta-blockers with 730 patients not receiving beta-blockers, thus assembling a matched cohort of 1280 patients (Figure 1).
Figure 2.
Love plot displaying absolute standardized differences comparing 58 baseline characteristics (the 4 hospital regions are used as a single variable) between 640 pairs of patients with heart failure with preserved ejection fraction (≥50%) and discharge heart rate ≥70 beats/minute, by discharge prescriptions for high-dose beta-blocker versus no beta-blocker, before and after propensity score matching (ACE=angiotensin-converting enzyme; ARB=angiotensin receptor blockers; S3=third heart sound
Outcomes Data
The primary outcome of the current analysis was all-cause mortality during overall follow-up of 6 (median, 2.8) years up to December 31, 2008. Secondary outcomes included all-cause readmission, heart failure readmission, the combined endpoint of all-cause readmission or all-cause mortality, and the combined endpoint of heart failure readmission or all-cause mortality. Medicare 100% Medicare Provider Analysis and Review (MEDPAR) and 100% Beneficiary Summary files were used to obtain data on events and time to events.13 These files contain information for 100% of Medicare beneficiaries using hospital inpatient services and dates of death.
Statistical Analyses
Between-group baseline characteristics were compared using Pearson’s Chi-square and Wilcoxon rank-sum tests, as appropriate. All outcome analyses were conducted using matched data. Kaplan-Meier survival analysis was used to generate plots for all-cause mortality by discharge prescription for high-dose beta-blocker versus no beta-blocker, and Cox regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for outcomes associated with high-dose beta-blocker versus no beta-blocker. To examine if significant associations observed in our matched data could be explained away by an unmeasured baseline characteristic, we conducted formal sensitivity analyses. We used Rosenbaum’s approach which uses a sign-score test to calculate “sensitivity bounds” for how much an unmeasured confounder would need to increase the odds of exposure (here, receiving a discharge prescription for high-dose beta-blockers) in order to explain away any significant associations between the exposure and outcome.18 In order to apply the method to time-to-event data, survival times within each matched pair are compared to determine whether one member of the pair can be clearly determined to have had a longer survival time (or event-free survival time for non-mortality outcomes) than the other member. Of note, for some pairs, it may not be possible to declare a clear “winner” due to censoring of one or both members. For the subset of pairs where a clear winner can be declared, we then test whether, in the absence of a hidden bias, if subjects in one group are significantly more likely to have longer survival times than their counterparts in the other group. Subgroup analyses were conducted to determine the homogeneity of the association of high-dose beta-blocker use and all-cause mortality in our primary matched cohort. Finally, we used a multivariable-adjusted logistic regression model to identify significant clinical predictors for use of high-dose in 2592 patients receiving beta-blockers. We set our statistical significance level at two-tailed alpha of 0.05. We used IBM SPSS Statistics for Windows software, version 24 (IBM Corp., Armonk, NY, USA) and SAS software, version 8 for Windows (SAS Institute Inc., Cary, NC, USA) for statistical analysis.
Results
Baseline Characteristics
The 1280 matched patients had a mean (±SD) age of 76 (±11) years, 66% were women, and 15% were African American, and had a mean (±SD) ejection fraction of 59 (±7) % and discharge heart rate of 82 (±10) beats/minute. Before matching, patients discharged on a high-dose beta-blocker had a lower mean age, and a greater proportion of these patients had hypertension, coronary artery disease, diabetes, and atrial fibrillation (Table 1). These and other measured baseline characteristics were balanced after matching, and absolute standardized difference for all 58 baseline characteristics were <10%, suggesting inconsequential between-group differences (Table 1 and Figure 2).
Table 1.
Baseline Characteristics in Patients with Heart Failure with Preserved Ejection Fraction ≥50% and Heart Rate ≥70 Beats/Minute, by High-Dose Beta-Blocker Versus No Beta-Blocker Use
| n (%) or mean (±SD) | Before propensity score matching (n=2470) | After propensity score matching (n=1280) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| No beta-blocker (n=1740) | High-dose beta-blocker (n=730) | P value | No beta-blocker (n=640) | High-dose beta-blocker (n=640) | P value | |
|
|
||||||
| Age (years) | 78 (11) | 76 (11) | <0.001 | 76 (12) | 76 (11) | 0.993 |
| Female | 1171 (66%) | 479 (67%) | 0.418 | 427 (67%) | 423 (66%) | 0.813 |
| African American | 226 (13%) | 116 (16%) | 0.057 | 98 (15%) | 101 (16%) | 0.817 |
| Admission from nursing home | 39 (2%) | 11 (2%) | 0.237 | 8 (1%) | 10 (2%) | 0.635 |
| Smoking history | 189 (11%) | 69 (10%) | 0.296 | 75 (12%) | 64 (10%) | 0.323 |
| Past medical history | ||||||
| Prior heart failure | 1495 (86%) | 621 (85%) | 0.582 | 546 (85%) | 547 (86%) | 0.937 |
| Hypertension | 1260 (72%) | 590 (81%) | <0.001 | 517 (81%) | 508 (79%) | 0.529 |
| Coronary artery disease | 592 (34%) | 336 (46%) | <0.001 | 293 (46%) | 276 (43%) | 0.339 |
| Acute myocardial infarction | 193 (11%) | 134 (18%) | <0.001 | 107 (17%) | 101 (16%) | 0.649 |
| Coronary revascularization | 268 (15%) | 189 (26%) | <0.001 | 147 (23%) | 146 (23%) | 0.947 |
| Diabetes mellitus | 661 (38%) | 328 (45%) | 0.001 | 256 (40%) | 274 (43%) | 0.307 |
| Cerebrovascular disease | 299 (17%) | 120 (16%) | 0.652 | 101 (16%) | 104 (16%) | 0.819 |
| Peripheral vascular disease | 216 (12%) | 100 (14%) | 0.383 | 83 (13%) | 90 (14%) | 0.567 |
| Atrial fibrillation | 562 (32%) | 289 (40%) | 0.001 | 237 (37%) | 246 (38%) | 0.604 |
| Ventricular arrhythmia | 32 (2%) | 14 (2%) | 0.895 | 12 (2%) | 13 (2%) | 0.840 |
| Implantable cardioverter defibrillator | 10 (1%) | 9 (1%) | 0.088 | 6 (1%) | 7 (1%) | 0.780 |
| Biventricular pacemaker | 20 (1%) | 11 (2%) | 0.467 | 8 (1%) | 10 (2%) | 0.635 |
| Chronic obstructive pulmonary disease | 529 (30%) | 173 (24%) | 0.001 | 151 (24%) | 159 (25%) | 0.602 |
| Asthma | 77 (4%) | 35 (5%) | 0.687 | 28 (4%) | 26 (4%) | 0.781 |
| Pulmonary hypertension | 130 (8%) | 56 (8%) | 0.864 | 51 (8%) | 50 (8%) | 0.917 |
| Anemia | 343 (20%) | 150 (21%) | 0.636 | 132 (21%) | 135 (21%) | 0.836 |
| Depression | 200 (12%) | 75 (10%) | 0.379 | 75 (12%) | 69 (11%) | 0.596 |
| Signs and Symptoms on Admission | ||||||
| Dyspnea at rest | 768 (44%) | 307 (42%) | 0.341 | 276 (43%) | 271 (42%) | 0.778 |
| Dyspnea on exertion | 1041 (60%) | 461 (63%) | 0.123 | 403 (63%) | 395 (62%) | 0.644 |
| Orthopnea | 369 (21%) | 192 (26%) | 0.006 | 159 (25%) | 160 (25%) | 0.948 |
| Paroxysmal nocturnal dyspnea | 178 (10%) | 115 (16%) | <0.001 | 84 (13%) | 91 (14%) | 0.569 |
| Chest pain | 317 (18%) | 172 (24%) | 0.002 | 151 (24%) | 142 (22%) | 0.549 |
| JVP elevation | 410 (24%) | 191 (26%) | 0.169 | 168 (26%) | 158 (25%) | 0.521 |
| Third heart sound | 99 (6%) | 36 (5%) | 0.449 | 37 (6%) | 31 (5%) | 0.455 |
| Pulmonary rales | 1054 (61%) | 460 (63%) | 0.256 | 413 (65%) | 396 (62%) | 0.324 |
| Peripheral edema | 1192 (69%) | 461 (63%) | 0.010 | 414 (65%) | 406 (63%) | 0.641 |
| Discharge medications | ||||||
| ACE inhibitors or ARBs | 913 (53%) | 448 (61%) | <0.001 | 391 (61%) | 387 (61%) | 0.819 |
| Aldosterone antagonists | 109 (6%) | 55 (8%) | 0.247 | 52 (8%) | 48 (8%) | 0.677 |
| Digoxin | 358 (21%) | 153 (21%) | 0.830 | 132 (21%) | 133 (21%) | 0.945 |
| Loop diuretics | 1373 (79%) | 582 (80%) | 0.648 | 506 (79%) | 507 (79%) | 0.945 |
| Hydralazine | 49 (3%) | 39 (5%) | 0.002 | 28 (4%) | 31 (5%) | 0.689 |
| Nitrates | 342 (20%) | 205 (28%) | <0.001 | 168 (26%) | 168 (26%) | 1.000 |
| Amlodipine | 164 (9%) | 96 (13%) | 0.006 | 71 (11%) | 81 (13%) | 0.388 |
| Other calcium channel blockers | 426 (25%) | 119 (16%) | <0.001 | 112 (18%) | 113 (18%) | 0.941 |
| Antiarrhythmics | 184 (11%) | 46 (6%) | 0.001 | 35 (6%) | 42 (7%) | 0.411 |
| Warfarin | 406 (23%) | 237 (33%) | <0.001 | 185 (29%) | 194 (30%) | 0.582 |
| Aspirin | 657 (38%) | 341 (47%) | <0.001 | 292 (46%) | 284 (44%) | 0.653 |
| Other antiplatelet drugs | 191 (11%) | 120 (16%) | <0.001 | 99 (16%) | 91 (14%) | 0.529 |
| Statins | 390 (22%) | 274 (38%) | <0.001 | 216 (34%) | 219 (34%) | 0.859 |
| In-hospital events | ||||||
| Coronary angiography | 77 (4%) | 62 (9%) | <0.001 | 45 (7%) | 45 (7%) | 1.000 |
| Clinical findings | ||||||
| Discharge heart rate (bpm) | 83 (11) | 82 (11) | 0.018 | 82 (10) | 82 (11) | 0.892 |
| Discharge systolic BP (mm Hg) | 129 (21) | 131 (22) | 0.040 | 132 (21) | 131 (22) | 0.666 |
| Discharge diastolic BP (mm Hg) | 67 (12) | 69 (13) | <0.001 | 69 (12) | 69 (13) | 0.687 |
| Discharge creatinine (mg/dL) | 1.6 (1.4) | 1.9 (1.7) | <0.001 | 1.8 (1.8) | 1.8 (1.6) | 0.913 |
| Admission hemoglobin (g/dL) | 12.0 (4) | 11.6 (2) | 0.046 | 11.7 (2) | 11.7 (2) | 0.642 |
| Admission sodium (mEq/L) | 137 (11) | 137 (10) | 0.509 | 137 (10) | 137 (10) | 0.934 |
| Ejection fraction (%) | 59.2 (8) | 58.6 (7) | 0.028 | 59.0 (7) | 58.7 (7) | 0.481 |
| Length of stay (days) | 6 (5) | 6 (5) | 0.305 | 6 (5) | 6 (5) | 0.103 |
| Hospital capacity (beds) | 395 (251) | 395 (231) | 0.967 | 408 (261) | 402 (231) | 0.640 |
| Hospital characteristics | ||||||
| Region | ||||||
| Midwest | 552 (32%) | 228 (31%) | <0.001 | 208 (33%) | 207 (32%) | 0.976 |
| Northeast | 196 (11%) | 173 (24%) | 129 (20%) | 125 (20%) | ||
| South | 615 (35%) | 195 (27%) | 181 (28%) | 180 (28%) | ||
| West | 377 (22%) | 134 (18%) | 122 (19%) | 128 (20%) | ||
| Academic center | 734 (42%) | 355 (49%) | 0.003 | 303 (47%) | 307 (48%) | 0.823 |
| Transplant center | 294 (17%) | 117 (16%) | 0.597 | 112 (18%) | 108 (17%) | 0.589 |
| Interventional center | 1328 (76%) | 557 (76%) | 0.991 | 508 (79%) | 496 (78%) | 0.415 |
ACE=angiotensin-converting enzyme; ARB=angiotensin receptor blocker; bpm=beats per minute; BP=blood pressure
High-Dose Beta-Blocker Use and Outcomes
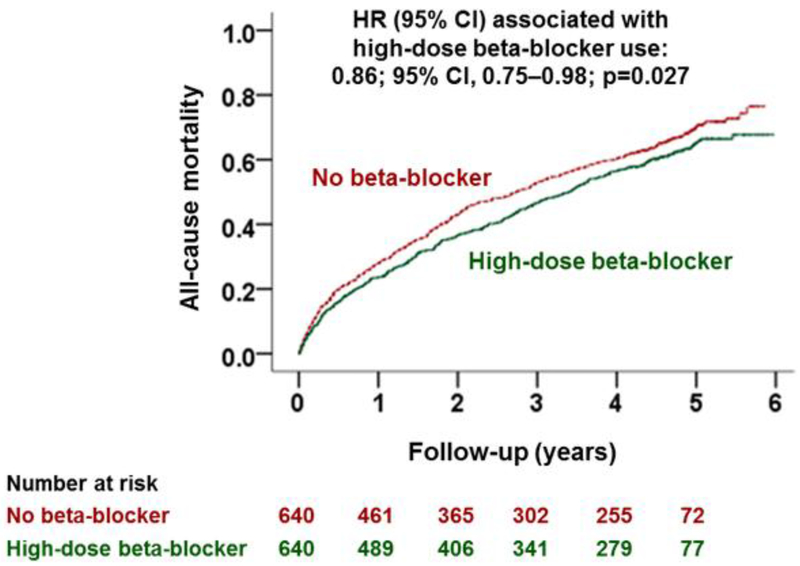
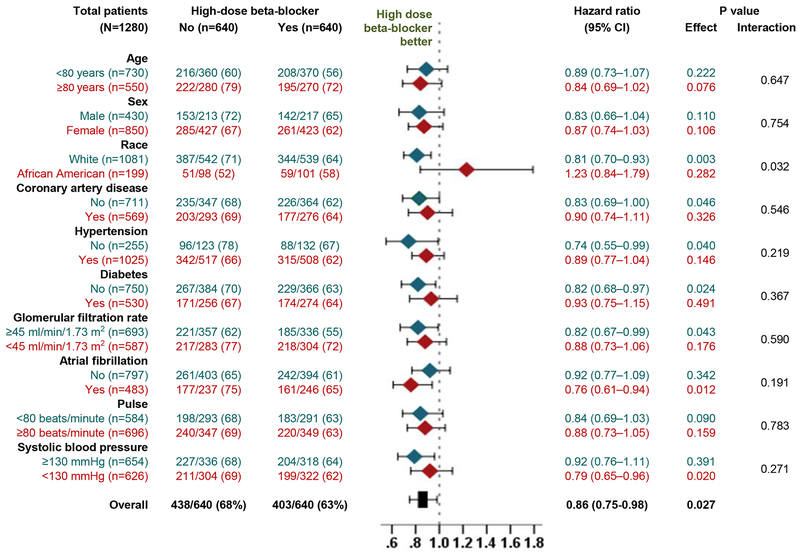
Among the 1280 matched patients, all-cause mortality occurred in 63% and 68% of those receiving a discharge prescription for high-dose beta-blocker versus no beta-blocker, respectively, during 6 (median 2.8) years of follow-up (HR, 0.86; 95% CI, 0.75–0.98; p=0.027; Table 2 and Figure 3). Of the 640 matched pairs, for 551 pairs we were able to determine a clear “winner”, of which in 311 (56.4%) pairs, patients who received a discharge prescription for high-dose beta-blockers had longer survival time than their matched counterparts who did not receive those drugs. In the absence of a hidden bias, a sign-score test for matched data with censoring demonstrates that this difference was statistically significant (p=0.003). There was no evidence that the beneficial association of high-dose beta-blocker with all-cause mortality varied by heart rate (Figure 4). The association of high-dose beta-blocker with mortality was also homogenous across various clinically relevant subgroups, except by race (Figure 4). The use of high-dose beta-blocker had no significant association with hospital readmission, but there was trend toward a lower risk for the combined endpoint of all-cause readmission and all-cause mortality (Table 2).
Table 2.
Outcomes Patients with Heart Failure with Preserved Ejection Fraction ≥50% and Heart Rate ≥70 Beats/Minute, by High-Dose Beta-Blocker Versus No Beta-Blocker Use
| Events (%) |
|||
|---|---|---|---|
| No beta-blocker (n=640) | High-dose beta-blocker (n=640) | Hazard ratio* (95% confidence interval) | |
| All-cause mortality | 438 (68%) | 403 (63%) | 0.86 (0.75–0.98); p=0.027 |
| All-cause readmission | 566 (88%) | 565 (88%) | 0.90 (0.81–1.02); p=0.100 |
| Heart failure readmission | 294 (46%) | 287 (45%) | 0.93 (0.79–1.09); p=0.362 |
| All-cause readmission or all-cause mortality | 619 (97%) | 607 (95%) | 0.89 (0.80–1.00); p=0.044 |
| Heart failure readmission or all-cause mortality | 522 (82%) | 496 (78%) | 0.90 (0.80–1.02); p=0.091 |
Associated with high-dose beta-blocker use
Figure 3.
Kaplan Meier plots for all-cause mortality in propensity score-matched patients with heart failure and preserved ejection fraction (≥50%) and discharge heart rate ≥70 beats/minute, by discharge prescriptions for high-dose beta-blocker versus no beta-blocker
Figure 4.
Forest plots displaying hazard ratios and 95% confidence intervals (CI) for all-cause mortality in subgroups of propensity score-matched patients with heart failure with preserved ejection fraction (≥50%) and discharge heart rate ≥70 beats/minute, by discharge prescriptions for high-dose beta-blocker versus no beta-blocker
Predictors of High-Dose Beta-Blocker Use
Among the 2592 pre-match patients with HFpEF who were eligible for beta-blockers, older age and chronic obstructive pulmonary disease were associated with lower odds of high-dose beta-blocker use, while atrial fibrillation and anti-hypertensive drug use were associated with higher odds of high-dose beta-blocker use (Table 3).
Table 3.
Predictors of High-Dose Beta-Blocker Use Among 2592 Pre-Match Patients with Heart Failure with Ejection Fraction ≥50% and Heart Rate ≥70 Beats/Minute Receiving Beta-Blockers with Dosage Data
| Multivariable-adjusted odds ratio* (95% confidence interval) | |
|---|---|
| Age ≥80 years | 0.67 (0.56–0.81); p<0.001 |
| Female | 0.99 (0.82–1.19); p=0.893 |
| African American | 1.31 (1.00–1.72); p=0.048 |
| Atrial fibrillation | 1.26 (1.04–1.52); p=0.016 |
| Chronic obstructive pulmonary disease | 0.75 (0.61–0.91); p=0.005 |
| Discharge prescription for ACE inhibitor or ARBs** | 1.23 (1.02–1.48); p=0.029 |
| Discharge prescription for hydralazine | 1.93 (1.22–3.05); p=0.005 |
| Discharge prescription for calcium channel blockers | 1.32 (1.08–1.61); p=0.008 |
| Serum creatinine (increments of 0.1 mg/dL) | 1.10 (1.03–1.17); p=0.008 |
| Hospital, Northeast region | 1.33 (1.07–1.65); p=0.009 |
| Hospital, Academic | 1.18 (0.98–1.41); p=0.079 |
Also adjusted for hypertension, coronary artery disease, diabetes mellitus, cerebrovascular disease, peripheral vascular disease, discharge systolic blood pressure ≥120 mm Hg, discharge heart rate ≥80 beats per minute, left ventricular ejection fraction, and discharge prescription for diuretics
ACE=Angiotensin-converting enzyme; ARB=angiotensin receptor blocker
Discussion
Findings from our study demonstrate that among hospitalized patients with HFpEF and a discharge heart rate ≥70 beats/minute, high-dose beta-blocker use was associated with a significantly lower risk of all-cause mortality. To the best of our knowledge, this is the first study to demonstrate a beneficial association between high-dose beta-blocker use and long-term outcomes in a propensity score-matched cohort of a high-risk subset of patients with HFpEF and heart rate ≥70 beats/minute.
Beta-blockers in high target doses have been shown to improve clinical outcomes in HFrEF.19–21 However, there is no randomized controlled trial evidence of efficacy of beta-blocker in HFpEF (ejection fraction ≥50%) and findings from observational studies have been variable.22–25 Treatment effects are known to be more pronounced in high-risk subsets of patients9–11 and patients with HFpEF and elevated heart rate have emerged as such a high-risk subset.1 An elevated heart rate is a marker of increased sympathetic activity, arrhythmogenicity and premature atherogenesis.26–29 If high-dose beta-blockers exert a greater negative chronotropic effect in these high-risk HFpEF patients with elevated heart rate, then that would at least in part explain the lower mortality observed in our study. In the Cardiac Insufficiency Bisoprolol Study in Elderly (CIBIS-ELD) trial, in older patients with both HFpEF and HFrEF, beta-blockers (~ 30% received high dose) reduced heart rate by 5–8 bpm at 3 months.30 The effect of beta-blockers on heart rate would be expected to be greater at higher doses during longer follow-up.4–6
Several studies have examined the association of beta-blocker use with outcomes in patients with HFpEF in general.23–25 However, to the best of our knowledge, this is the first study to report a significant beneficial association between the use of high-dose beta-blockers and improved clinical outcomes in a high-risk subset of patients with HFpEF. Our study is also distinguished by the use of ejection fraction cutoff of 50% to define HFpEF, the use of propensity score matching to assemble a balanced cohort, the use of subgroup analyses to demonstrate homogeneity, and the use of formal sensitivity analyses to assess bias by a potential unmeasured confounder.
Currently there is no evidence-based therapy for HFpEF that can improve survival.22 If the hypothesis-generating findings from our study are confirmed in a prospective randomized controlled trial, then high-dose beta-blockers may emerge as a potential therapy to improve outcomes in a large subset of high-risk of patients with HFpEF. About two-thirds of patients with HFpEF have heart rate >70 beats/minute.1 However, the impact of such therapy may be limited by patients’ ability to tolerate a high dose of beta-blocker. In our study, about a third of the patients who were taking beta-blockers were on a high dose, which is similar to that observed in the CIBIS-ELD trial.30 One of the main reasons for intolerance of high-dose beta-blocker is hypotension. Systolic blood pressure <120 or 130 mm Hg has been shown to be associated with poor outcomes in HFpEF.31 Future studies may examine high-dose beta-1 selective blockers may be better tolerated in HFpEF.32
There are several limitations to our study. Despite propensity score matching, confounding due to residual or hidden bias is possible. A hidden covariate that would increase the odds of receiving a discharge prescription for high-dose beta-blockers by 9.5% could potentially explain away this association. For such an imaginary unmeasured binary covariate to become a confounder, it would also need to be a near perfect predictor of all-cause readmission and could not be strongly correlated to any of the variables used in our propensity score model. We had no data on adherence, titration of beta-blockers, and heart rate during follow-up. Finally, our analysis was restricted to older hospitalized fee-for-service Medicare beneficiaries, which may limit generalizability.
In conclusion, in hospitalized older patients with HFpEF and a discharge heart rate ≥70 beats/minute, high-dose beta-blocker use is associated with a lower risk of all-cause mortality and the combined endpoint of all-cause readmission or all-cause mortality. If these findings can be confirmed in prospective randomized controlled trials, the beneficial role of high-dose beta-blockers, well known in HFrEF, may be extended to HFpEF, albeit to a large high-risk subset who can tolerate these drugs in high doses.
Acknowledgments
Funding: Dr. Ali Ahmed was in part supported by the National Institutes of Health through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the National Heart, Lung, and Blood Institute. Dr. Gregg Fonarow was the Principle Investigator of OPTIMIZE-HF, which was sponsored by GlaxoSmithKline.
Disclosures
Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda.Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott); Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda.
References:
- 1.Lam PH, Dooley DJ, Deedwania P, et al. Heart rate and outcomes in hospitalized patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;70:1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 4.Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 6.MERIT-HF Investigators. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 7.van Veldhuisen DJ, Cohen-Solal A, Bohm M, et al. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: Data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure). J Am Coll Cardiol. 2009;53:2150–2158. [DOI] [PubMed] [Google Scholar]
- 8.Pal N, Sivaswamy N, Mahmod M, et al. Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation. 2015;132:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Tonkin AM, Shepherd J, et al. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: the Prospective Pravastatin Pooling Project. Circulation. 2000;102:1893–1900. [DOI] [PubMed] [Google Scholar]
- 12.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Kilgore ML, Arora T, et al. Design and rationale of studies of neurohormonal blockade and outcomes in diastolic heart failure using OPTIMIZE-HF registry linked to Medicare data. Int J Cardiol. 2013;166:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbaum PRRD. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 15.Rubin DB. Using propensity scores to help design observational studies: Application to the tobacco litigation. Health Services & Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 16.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed MI, White M, Ekundayo OJ, et al. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30:2029–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, ed. Observational Studies. Vol 1. New York: Springer-Verlag; 2002:105–170. [Google Scholar]
- 19.Bristow MR, Gilbert EM, Abraham WT, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996;94:2807–2816. [DOI] [PubMed] [Google Scholar]
- 20.Fiuzat M, Wojdyla D, Kitzman D, et al. Relationship of beta-blocker dose with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial. J Am Coll Cardiol. 2012;60:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiuzat M, Wojdyla D, Pina I, Adams K, Whellan D, O’Connor CM. Heart rate or beta-blocker dose? Association with outcomes in ambulatory heart failure patients with systolic dysfunction: Results from the HF-ACTION trial. JACC Heart Fail. 2016;4:109–115. [DOI] [PubMed] [Google Scholar]
- 22.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 23.Lund LH, Benson L, Dahlstrom U, Edner M, Friberg L. Association between use of beta-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA. 2014;312:2008–2018. [DOI] [PubMed] [Google Scholar]
- 24.Fukuta H, Goto T, Wakami K, Ohte N. The effect of beta-blockers on mortality in heart failure with preserved ejection fraction: A meta-analysis of observational cohort and randomized controlled studies. Int J Cardiol. 2017;228:4–10. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Chen Y, Feng X, Teng Z, Yuan Y, Bin J. Effects of beta-blockers on heart failure with preserved ejection fraction: a meta-analysis. PLoS One. 2014;9:e90555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science. 1984;226:180–182. [DOI] [PubMed] [Google Scholar]
- 27.Kannel WB, Kannel C, Paffenbarger RS Jr., Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. [DOI] [PubMed] [Google Scholar]
- 28.Failla M, Grappiolo A, Emanuelli G, et al. Sympathetic tone restrains arterial distensibility of healthy and atherosclerotic subjects. J Hypertens. 1999;17:1117–1123. [DOI] [PubMed] [Google Scholar]
- 29.Giannoglou GD, Chatzizisis YS, Zamboulis C, Parcharidis GE, Mikhailidis DP, Louridas GE. Elevated heart rate and atherosclerosis: an overview of the pathogenetic mechanisms. Int J Cardiol. 2008;126:302–312. [DOI] [PubMed] [Google Scholar]
- 30.Edelmann F, Musial-Bright L, Gelbrich G, et al. Tolerability and feasibility of beta-blocker titration in HFpEF versus HFrEF: Insights from the CIBIS-ELD trial. JACC Heart Fail. 2016;4:140–149. [DOI] [PubMed] [Google Scholar]
- 31.Tsimploulis A, Lam PH, Arundel C, et al. Systolic Blood Pressure and Outcomes in Patients With Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2018;3:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. [DOI] [PubMed] [Google Scholar]