Abstract
Introduction
Mycobacterium abscessus is a rapidly growing mycobacterium commonly identified in adults with underlying pulmonary diseases but is rarely observed in children. A better understanding of this pathogen in children is essential.
Case presentation
We report the case of a 49-month-old female child without previous underlying pulmonary diseases but with acute lymphoblastic leukemia (ALL). The patient was complicated with pneumonia during chemotherapy, which was primarily characterized by spontaneous pneumomediastinum and subcutaneous emphysema on chest computed tomography (CT). M. abscessus sequences were detected by metagenomic next-generation sequencing in bronchoalveolar lavage fluid. With mechanical ventilation, closed thoracic drainage, and anti-infective therapy for 6 months, the patient’s infection was controlled. The patient completed 2.5 years of treatment for ALL, and the drugs were discontinued. The patient currently remains in complete hematologic remission.
Discussion
We reviewed the literature on 33 children with M. abscessus pulmonary disease. These children mostly had underlying immunodeficiency. Chest CT most often showed nodular shadows, consolidation, and bronchiectasis. Spontaneous pneumomediastinum and subcutaneous emphysema were not reported as major manifestations.
Conclusion
Spontaneous pneumomediastinum and subcutaneous emphysema were our patient's main characteristics on chest CT, and this study enriches the knowledge regarding possible imaging changes in M. abscessus pulmonary disease in children. This case report reflects good clinical experience in maintaining the balance between chemotherapy and anti-infective therapy in childhood ALL.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-023-04199-4.
Keywords: Mycobacterium abscessus, Pneumonia, Children, Acute lymphoblastic leukemia, Spontaneous pneumomediastinum, Subcutaneous emphysema
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy in childhood globally, and the cure rate has been constantly increasing with technological advancements and medical science improvements [1, 2]. As with other malignancies, immunodeficiency leads to enhanced susceptibility to infection. Mycobacterium abscessus is a rapidly growing nontuberculous mycobacterium (NTM) that is ubiquitous in the environment, including in water, soil, and dust [3]. Post surgery/post trauma skin and soft tissue infection, lymphadenitis, pulmonary infection (usually with underlying pulmonary chronic diseases, such as cystic fibrosis), and disseminated infection (usually associated with immunodeficiency) are common childhood infections caused by M. abscessus [4]. Reports on childhood ALL complicated with M. abscessus infection are limited; thus, a better understanding of this pathogen in children is essential.
We describe a child with ALL but without previous underlying pulmonary diseases. The patient developed M. abscessus pulmonary disease during chemotherapy, which was primarily characterized by spontaneous pneumomediastinum and subcutaneous emphysema. Her pulmonary infection was cured by a combination of antibiotics that were taken for 6 months after diagnosis, and she is still in complete hematologic remission from ALL at 2.5 years after the initial diagnosis.
Case presentation
A 49-month-old female child was admitted to our hospital in December 2019 due to pallor for the prior 3 months. She had a normal growth and development history. She was a full-term normal delivery. She had no family history of hematological malignancies. She had suffered from pallor 3 months prior, which was treated using oral iron supplementation, but her symptoms did not improve and were accompanied by fatigue and anorexia. She had experienced intermittent low-grade fevers during the previous month and gingival bleeding 3 days prior and therefore visited a local hospital. Blood tests indicated a white blood cell count of 75.3×109/L(3.5-9.5×109/L), a hemoglobin level of 41 g/L (115-150g/L) and a platelet count of 14×109/L(125-350×109/L). She was then transferred to our department for further diagnosis and treatment.
Physical examinations on admission showed an anemic appearance; scattered bleeding spots on the skin over the entire body; palpable enlarged lymph nodes in the neck, armpit, and groin; the liver was located 4 cm below the ribs, which had a moderate texture; and the spleen was located 7 cm below the ribs, which had a tough texture. Routine blood tests indicated a white blood cell count of 67.19×109/L(3.5-9.5×109/L), a lymphocyte ratio of 89.4% (20-50%), a hemoglobin level of 41 g/L (115-150g/L), and a platelet count of 19×109/L(125-350×109/L). LDH was 577U/L(120-250U/L), HS-CRP was 7.1mg/L(0-3.3mg/L), and PCT was normal. Abdominal B-ultrasound revealed hepatosplenomegaly. No obvious abnormalities were found on head MRI, lung computed tomography (CT), electrocardiogram, color Doppler echocardiography, or urinary B-ultrasound. The patient was diagnosed with B-cell ALL (B-ALL) based on her bone marrow cell morphology, immunology, cytogenetics, and molecular biology (MICM) typing. The Chinese Children’s Cancer Group study ALL-2015 (CCCG-ALL-2015) intermediate-risk group regimen was initiated.
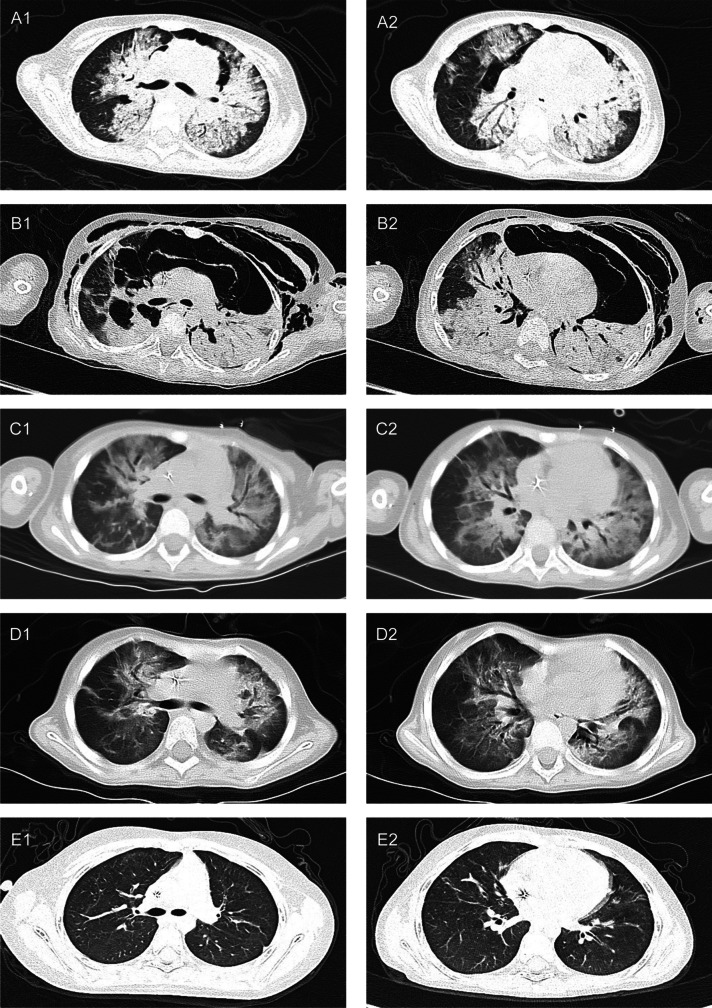
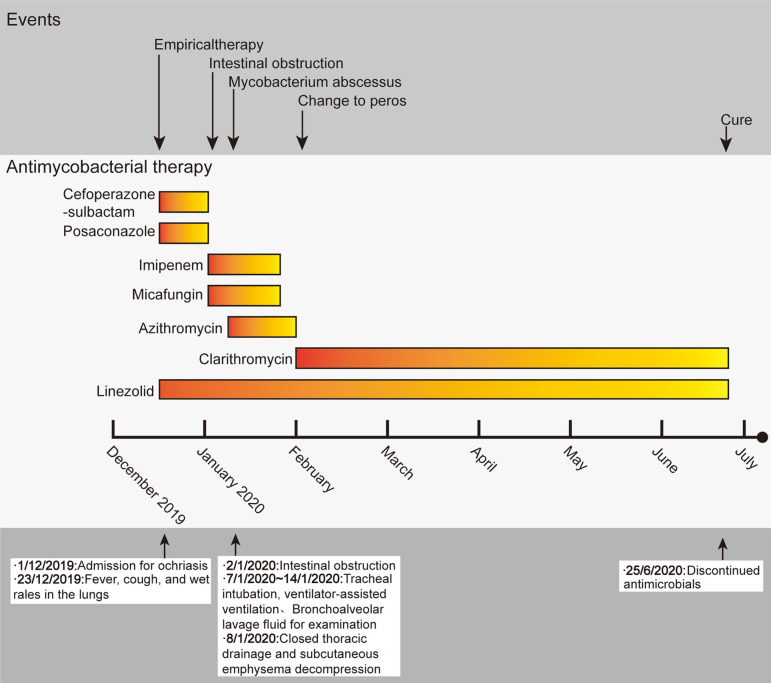
On day 8 of vincristine + daunorubicin + L-asparaginase + prednisone (VDLP) chemotherapy (December 23, 2019), the patient developed fever, cough, and moist rales in her lungs. Sputum bacterial and fungal cultures, bilateral blood cultures, and tuberculosis tests were negative. Empirically, she was intravenously injected with cefoperazone sulbactam (160 mg/kg/d) and orally given posaconazole (12 mg/kg/d) and linezolid (30 mg/kg/d). On day 18 of VDLP chemotherapy (January 2, 2020), she developed another fever, abdominal distension, abdominal pain, and weak bowel sounds, and a vertical abdominal X-ray indicated an intestinal obstruction. Sputum culture and blood culture were still negative, and a stool culture was normal. The anti-infective regimen was switched to intravenous injection of imipenem and cilastatin sodium (60 mg/kg/d), linezolid (30 mg/kg/d), and micafungin (4 mg/kg/d). She was also prescribed fasting gastrointestinal decompression and maintenance of water and electrolyte balances. On day 23 of VDLP chemotherapy (January 7, 2020), she suddenly had shortness of breath. Arterial blood gas analysis revealed a pH of 7.475, a partial pressure of oxygen (PO2) of 40.2 mmHg, a partial pressure of carbon dioxide (PCO2) of 38.5 mmHg, and a ratio of arterial oxygen pressure (PaO2)/fraction of inspired oxygen (FiO2) of 135 mmHg. A plain CT scan showed bilateral lung inflammation, partial consolidation and pneumomediastinum (Fig. 1-A1 and A2). She was endotracheally intubated for invasive mechanical ventilation (VC-AC mode) with the following parameters: FiO2 40%, peak inspiratory pressure 24 cmH2O, positive end-expiratory pressure 5 cmH2O, respiratory rate 25/minute, and inspiratory time 0.8 s. She also underwent fiberoptic bronchoscopy. Bronchoalveolar lavage fluid (BALF) was collected and sent for metagenomic next-generation sequencing (mNGS), which was performed by BGI (Shenzhen, China) using the BGISEQ-500 (China). BALF was also sent for bacterial culture, fungal culture, antacid staining, ink staining, hexamine silver staining, and a galactomannan (GM) test, and the results were negative. On day 24 of VDLP chemotherapy (January 8, 2020), a new chest CT scan showed subcutaneous emphysema, pneumomediastinum, and atelectatic lung tissues under compression (Fig. 1-B1 and B2). Closed thoracic drainage and subcutaneous emphysema cutting for decompression were performed. mNGS detected M. abscessus with 515 sequence reads and Mycobacteroides franklin with 4 sequence reads in the BALF sample (the sequencing files were deposited into the NCBI SRA database and can be retrieved at https://www.ncbi.nlm.nih.gov/ with accession number PRJNA882796). The patient was additionally given intravenous azithromycin (10 mg/kg/day, 3 days a week). On day 30 of VDLP chemotherapy (January 14, 2020), invasive mechanical ventilation was discontinued. On day 33 of VDLP chemotherapy (January 17, 2020), a new chest CT scan revealed that both pneumatosis and inflammation had improved (Fig. 1-C1 and C2), and the patient was transferred back to the general ward for continued treatment.
Fig. 1.
Chest CT results during the course of the disease. Chest CT performed on January 7, 2020(VDLP D23) (A1, A2), on January 8, 2020(VDLP D24) (B1, B2), on January 17, 2020(VDLP D33) (C1, C2),on February 1, 2020(D1, D2), and on June 28, 2020(E1, E2)
On January 24, 2020, the patient’s routine blood test, C-reactive protein, and procalcitonin results were all normal; therefore, cyclophosphamide + cytarabine + mercaptopurine (CAT) chemotherapy was started, and the anti-infective regimen was switched to oral linezolid (30 mg/kg/d) and clarithromycin (15 mg/kg/d) (Fig. 2). On day 7 of CAT (February 1, 2020), chest CT revealed that inflammation had been absorbed and had subsided more than before (Fig. 1-D1 and D2). At around 4 (June 28, 2020) of interphase treatment (mercaptopurine + dexamethasone + daunorubicin + vincristine + pegaspargase), chest CT revealed that the inflammation in both lungs had been absorbed (Fig. 1-E1 and E2). Therefore, the anti-infective therapy that had been given for 6 months was discontinued (Fig. 2). Chemotherapy was continued for ALL.
Fig. 2.
Timeline of events in the case
Fortunately, the 2.5-year chemotherapy course was completed in June 2022, and the patient currently remains in hematologic remission. During this period, the patient has had no symptoms such as cough and fever.
Discussion
M. abscessus is a rapidly growing NTM of Runyon group IV that is ubiquitous in nature [5]. It is usually nonpathogenic in immunocompetent populations but often attacks immunodeficient, trauma, and postsurgical patients as an opportunistic pathogen [6, 7]. Organ infections, such as pulmonary infection [8], lymphadenitis [9], and disseminated infection [10], can be caused by M. abscessus. According to a recent whole-genome sequencing analysis of M. abscessus strains in patients with pulmonary cystic fibrosis, M. abscessus strains have high homology, suggesting that M. abscessus can be transmitted between people [11].
Searching PubMed with the keywords “Mycobacterium abscessus” and “Children” from January 2012 to June 2022 yielded a total of 195 articles. We applied the following inclusion criteria to the papers: 1) studies published in English, including case reports and case series, 2) studies involving pulmonary infection caused by M. abscessus in patients aged below 18 years, and 3) studies with clear basic information. Eighteen articles were included (Table 1). Pulmonary infection caused by M. abscessus was more common in patients with underlying pulmonary diseases, especially cystic fibrosis. In these cases, nodular shadows, consolidation, and bronchiectasis were the major CT manifestations of pulmonary infection in children. The primary characteristics observed in the lung CT of the present case encompassed bilateral pulmonary inflammation, pneumomediastinum, and subcutaneous emphysema. Spontaneous pneumomediastinum refers to mediastinal emphysema that occurs in circumstances other than exogenous trauma or iatrogenic injury [29]. The patient’s pneumomediastinum was found on chest CT before mechanical ventilation was initiated, which excludes the possibility of ventilator-associated mediastinal emphysema. We consider that it is related to lung infection. Spontaneous pneumomediastinum in patients with pneumonia has been reported in both adults and children [30, 31], but spontaneous pneumomediastinum in patients with Mycobacterium abscessus pulmonary disease is first reported in children. This is the first report in a pediatric case. The sudden worsening of respiratory symptoms and the decrease in PO2 caused by M. abscessus pulmonary disease indicate an exacerbation of pulmonary lesions. The patient’s lung CT scan revealed the presence of pneumomediastinum, which further enriches knowledge about the imaging presentation of M. abscessus pulmonary infections in children.
Table 1.
Summary of mycobacterium abscessus complex lung infections
|
Age (years) |
Sex | Medical history | Type of infection | Antibiotics | Total duration of treatment | Iconography | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Do et al. (2013) [12] | 8 | - | CF | Lung | RIF,CFX,AMK,EMB,CAM,CIP | 12 months | CT: Bronchiectasis with centrilobular nodularities, no tree-in-bud. | Failure |
| 13 | - | Primary ciliary dyskinesia | Lung | AMK, MER, AZM | 12 months | CT:RLL bronchiectasis with scattered tiny nodular densities, no tree-in-bud. | Cure | |
| 0.5 | - | No prior history | Lung | CFX,AMK, MER, CAM | 3 months | CT: Extensive right lung consolidation with scattered nodular densities, no tree-in-bud. | Cure | |
| Iwanaga et al, (2014) [13] | 4 | F | Bronchopulmonary dysplasia | Lung | CAM, AMK, LZD, TGC | 13 months | CT: Chest demonstrated diffuse nodular opacities and areas of Consolidation | Cure |
| Jamal et al, (2014) [14] | 1.33 | - | CHD, Hypothyroidism, Chronic lung disease | Lung | AMK, CIP, CAM | - | New chest radiograph infiltrate | Cure |
| 12 | - | HCE, Spastic quadriplegia,Recurrent chest infections, Chronic lung disease | Lung | AMK, CIP, CAM | - | New chest radiograph infiltrate | Cure | |
| 1 | - | CCHD, Congenital cystic lung,RLL lobectomy,Chronic lung disease | Lung | AMK, CIP, CAM | - | New chest radiograph infiltrate | Cure | |
| 15 | - | Spinomuscular dystrophy, Trachea-esophageal fistula, Scoliosis | Lung | AMK, CIP, CAM | - | New chest radiograph infiltrate | Failure | |
| Apiwattankul et al,(2015) [15] | 9 | F | RMS | Blood, Lung | CAM, AMK, | 6 months | - | Cure |
| 13 | F | WT | Lung | CAM, LZD, AMK, MER | 42 months | - | Failure | |
| Iroh Tam et al,(2015) [16] | 6 | M | CF | Lung | Not treated | - | - | - |
| 12 | M | CF | Lung | LVFX,TOB | - | - | - | |
| 16 | F | CF | Lung | Not treated | - | - | - | |
| Emiralioğlu et al,(2016) [17] | 11 | M | Triple A Syndrome | Lung | IPM,AMK,CAM, DOX,CIP | 24 months |
X-RAY:Bilateral extensive nodular infiltrates, which were coalescing to form an area of consolidation in both lungs; CT:Multiple pulmonary nodules and consolidation in both lungs and an appearance of tree-in-bud. |
Cure |
| Campos-Herrero et al, (2016) [18] | 9 | F | CF | Lung | CAM,AMK,LZD,TGC,MER,MOC,MXF | 11 months | - | Failure |
| 9 | F | CF | Lung | 7 months | - | Improvement | ||
| 12 | M | CF | Lung | 41 months | - | Failure | ||
| 5 | F | CF | Lung | Not treated | - | - | - | |
| 11 | M | CF | Lung | - | - | - | ||
| 12 | F | CF | Lung | - | - | - | ||
| Anisowicz et al,(2016) [19] | 8 | F | Idiopathic short stature,allergic rhinitis,asthma | Lung | TGC,AZM,TOB,AMX,CVA,TZP,CLI | 5 months | CT:Multifocal tree-in-bud opacities with associated diffuse bronchiectasis and ground glass opacities, wedgeshaped process in the right middle lobe concerning | Cure |
| Scott et al, (2018) [20] | 10 | F | CF | Lung | AMK,CFX,LZD | 3.5 years | - | Cure |
| Ruffles et al, (2018) [21] | 16 | M | CF | Lung | AZM,EMB,MXF, AMK | 7 months | CT: Progressive bronchiectasis | Cure |
| Liu et al, (2019) [22] | 0.33 | F | Born prematurely | Lung | CAM,IPM,LZD,RIF,EMB,CFX,AZM,MXF,CZ | 16 months |
X-RAY: Patchy shadows localized to the right lung and lower left lung; CT:Multiple masses and small nodules across both lungs with mediastinal lymph node involvement |
Cure |
| Jones et al, (2019) [23] | 16 | F | CF | Lung | CFX,LZD,CAM,TGC,AMK,IMP/CS,MOC,TED | At least 5.5 years | Improvement | |
| 13 | M | CF | Lung | AMK,CFX,IMP/CS,LZD,MOC,TED,AZM | At least 5.6 years | Improvement | ||
| Mauch et al, (2020) [24] | 13.3 | F | Tracheobronchitis,CF | Lung | AMK,CAM | 3 months | - | Cure |
| 15.8 | M | Tracheobronchitis,CF | Lung | Not treated | - | - | Improvement | |
| Alramadhan et al,(2021) [25] | 0.33 | F | Failure to thrive | Lung | AMK,CAM,IPM | 14 months | - | Cure |
| Deniz et al, (2021) [26] | 11 | M | Autism, Pneumonia | Lung | LZD,AMK,CAM,RIF,INH,TMP-SMX | 2 years |
X-RAY: Lobar consolidation and interstitial infiltrates in the right lung; CT: Showed large, consolidated infiltration areas containing air bronchograms in the upper and middle lobes of the right lung and diffuse ground-glass opacities in the both lower lobes |
Cure |
| Chawla et al, (2022) [27] | 7 | F | CF ,Failure to thrive, Meconium ileus,Volvulus | Lung | CLOF,LZD,CFX, AMK,TGC | Estimate 24 months | X-RAY:Left lower lung focal consolidation; | Improvement |
| Weerakoon et al,(2022) [28] | 0.42 | M | CF,PFIC | Lung,Cutaneous | GEN,MER,RIF,EMB,CAM,AZM,AMK, LVFX | 5 months | X-RAY:Hyperinflated lungs, peribronchial wall thickening and bilateral lower lobe consolidations; CT:Bilateral cystic bronchiectasis and nodules | Failure |
| Xuereb et al, (2022) [8] | 8 | F | CF | Lung | AMK,MER,CFX, AZM,TOB | 3 weeks of intravenous antibiotics and three therapeutic bronchoscopies | X-RAY: The collapse of the left upper lobe | Improvement |
CAM Clarithromycin, AMK Amikacin, AZM Azithromycin, CLOF Clofazimine, EMB Ethambutol, IPM Imipenem, LZD Linezolid, TGC Tigecycline, EMB Ethambutol, IMP/CS Imlpenem/cilastatin; CFX Cefoxitin, SCF Cefoperazone-sulbactam, MCFG Micafungin, MER Meropenem, TOB Tobramycin, GEN Gentamicin, RIF Rifampicin, INH Isoniazid, PZA Pyrazinamide, LVFX Levofloxacin, MOC Minocycline, MXF Moxifloxacin, CZ Cefprozil, AMX Amoxycillin, CLI Clindamycin, TZP Piperacilllin-tazobactam, DOX Doxycycline, CIP Ciprofloxacin, CVA Clavulanate; TZD Tedizolid, RMS Rhabdomyosarcoma, EP Ependymoma, RB Retinoblastoma, WT Wilm’s tumor, RLL Right lower lobe, CHD Congenital heart disease, HCE Hypoxicis chemic encephalopathy, CCHD Congenital cyanotic heart disease, CF Cystic Fibrosis, CT Contrast-enhanced computed tomography, X-RAY Chest radiography
M. abscessus infection is difficult to diagnose in children. In clinical practice, NTM infection should be identified first, and then the species of pathogen should be further determined from clinical specimens. Currently, mycobacteria are often identified through a combination of pathogen culture and biochemical test technology in clinical practice [32]. Considering some reports of false-positive results from pathogen culture [33], an alternative to traditional laboratory microbial culture combined with biochemical test technology is difficult to identify [32]. mNGS has rapidly emerged as an advanced technique for pathogen detection that can be performed directly on clinical specimens, which is characterized by high speed, high specificity, and high throughput [34]. It is also less affected by previous antibiotic exposure [35]. With the development of NGS technology, differentiating between colonization and infection has also become possible [36]. NGS technology has been used to detect a variety of pathogens, such as Streptococcus pneumoniae and M. abscessus [37, 38]. In the reported case, we performed several cultures for bacteria and fungi, which were negative. The patient’s clinical symptoms did not improve within 2 weeks of empirical antibiotic treatment, and she developed a dramatic exacerbation of respiratory symptoms. Finally, we detected M. abscessus by mNGS of BALF, which showed 515 sequences with significantly higher sequence numbers, and in combination with the presence of immunodeficiency, clinical symptoms, and pulmonary imaging manifestations, the patient was diagnosed with M. abscessus pulmonary disease according to the diagnostic criteria of the American Thoracic Society [39]. Treatment for M. abscessus was then administered; 1 week after treatment, her clinical symptoms were alleviated; and bacterial and fungal cultures of BALF remained negative.
M. abscessus is also a special NTM due to its resistance to several antibiotics, resulting in tremendous challenges in the treatment of M. abscessus [40]. At the same time, antibiotics' toxic effects and side effects [41] and increasing treatment costs [42] during long-term treatment lead to drug discontinuation or treatment failure. With underlying pulmonary diseases, the course of antibiotic administration may be prolonged, and most patients with pulmonary infection caused by M. abscessus undergo treatment for at least 12 months [43]. Combination therapy with at least 3 antibiotics is recommended by the 2020 ATS/ERS/ESCMID/IDSA clinical practice guidelines [44]. In this case report, azithromycin and clarithromycin plus imipenem and cilastatin sodium and linezolid were used successively as anti-infective therapy. The patient had an underlying medical condition of ALL, and during chemotherapy, we treated the pulmonary infections concurrently. Considering the potential pulmonary toxicity of various medications and the risk of lung complications caused by infections, we repeated chest CT scans during the treatment. After six months of treatment, the patient no longer had a fever or cough, and lung auscultation was normal. The chest CT scans showed that the infection had been cured. No drug toxicity or side effects occurred. These findings suggest that patients without underlying pulmonary diseases can undergo treatment for less than 12 months, similar to previous findings [45]. As noted in case reports, balancing between the treatment of the primary disease and infection control creates a dilemma, i.e., the risk of disease recurrence may rise due to early termination of chemotherapy without continuing consolidation therapy, while disseminated NTM infection may occur with the continuation of high-dose chemotherapy [46]. Fortunately, ALL treatment was continued throughout anti-infective therapy, and no further acute deterioration of pulmonary function or spreading of the infection was found. After 2.5 years of treatment, the drugs for the patient’s underlying disease (ALL) were discontinued, and she currently remains in hematologic remission.
Conclusion
Spontaneous pneumomediastinum and subcutaneous emphysema was our patient's main characteristic of chest CT, which is the first such report in a child, thus enriching knowledge regarding imaging changes in M. abscessus pulmonary disease in children. This case report reflects good clinical experience in maintaining the balance between chemotherapy and anti-infective therapy for childhood ALL.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- ALL
Acute lymphoblastic leukemia
- CT
Computed tomography
- NTM
Nontuberculous mycobacterium
- CCCG-ALL-2015
The Chinese Children’s Cancer Group study ALL-2015
- VDLP
Vincristine + daunorubicin + L-asparaginase + prednisone
- PO2
Pressure of oxygen
- PCO2
Pressure of carbon dioxide
- PaO2
Arterial oxygen pressure
- FiO2
Fraction of inspired oxygen
- BALF
Bronchoalveolar lavage fluid
- mNGS
Metagenomic next-generation sequencing
- GM
Galactomannan
Authors' contributions
All authors were involved in drafting and critical revision of the manuscript and approved the final version to be published. Conception and design of the study: WL, JC, ZX, and LY. Acquisition, analysis, and interpretation of the data: LH, ST, and HC. Drafting of the manuscript: WL, AW, and CL. Revision of the manuscript: WL, YC, KZ, and NW.
Funding
This work was supported in part by the Discipline Co-construction Project of Pediatrics and Physiology (No. 2020LCXK017); the Discipline Co-construction Project of Pediatrics and Immunology (No. 2021LCXK030); the Beijing Bethune Medical Science Research Foundation (No. SCE128EN); the Clinical Research and Cultivation Program of the Second Affiliated Hospital of Anhui Medical University (No. 2020LCYSOCS304); and the Scientific Research Foundation of Anhui Medical University (No. 2021xkj168).
Availability of data and materials
Sequencing files were deposited into the NCBI SRA database and can be retrieved at https://www.ncbi.nlm.nih.gov/ with accession number PRJNA882796.
Declarations
Ethics approval and consent to participate
All procedures performed in this case report involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration. Informed consent was obtained from the patient’s parents for this study. This study was approved by the Ethics Committee of The Second Affiliated Hospital of Anhui Medical University.
Consent for publication
Written informed consent was obtained for publication from the patient’s parents in this case report, including informed consent for this study’s publication of their child’s personal or clinical information as well as any identifiable images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenyuan Liu, Jinhua Chu, Zhiwei Xie and Linhai Yang contributed equally to this work.
Contributor Information
Yan Cheng, Email: 2414791109@qq.com.
Kunlong Zhang, Email: 80148229@qq.com.
Ningling Wang, Email: zwnltt@126.com.
References
- 1.Hochberg J, Khaled S, Forman SJ, Cairo MS. Criteria for and outcomes of allogeneic hematopoietic stem cell transplant in children, adolescents and young adults with acute lymphoblastic leukemia in first complete remission. Br J Haematol. 2013;161(1):27–42. doi: 10.1111/bjh.12239. [DOI] [PubMed] [Google Scholar]
- 2.Neaga A, Jimbu L, Mesaros O, Bota M, Lazar D, Cainap S, Blag C, Zdrenghea M. Why Do Children with Acute Lymphoblastic Leukemia Fare Better Than Adults? Cancers (Basel). 2021;13(15):3886. [DOI] [PMC free article] [PubMed]
- 3.Falkinham JO., 3rd Environmental sources of nontuberculous mycobacteria. Clin Chest Med. 2015;36(1):35–41. doi: 10.1016/j.ccm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Hatzenbuehler LA, Starke JR. Common presentations of nontuberculous mycobacterial infections. Pediatr Infect Dis J. 2014;33(1):89–91. doi: 10.1097/INF.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 5.Koh WJ, Kwon OJ, Lee KS. Nontuberculous mycobacterial pulmonary diseases in immunocompetent patients. Korean J Radiol. 2002;3(3):145–157. doi: 10.3348/kjr.2002.3.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Varela E, García-Basteiro AL, Santiago B, Wagner D, van Ingen J, Kampmann B. Non-tuberculous mycobacteria in children: muddying the waters of tuberculosis diagnosis. The Lancet Respiratory Medicine. 2015;3(3):244–256. doi: 10.1016/S2213-2600(15)00062-4. [DOI] [PubMed] [Google Scholar]
- 7.Cusumano LR, Tran V, Tlamsa A, Chung P, Grossberg R, Weston G, Sarwar UN. Rapidly growing Mycobacterium infections after cosmetic surgery in medical tourists: the Bronx experience and a review of the literature. Int J Infect Dis. 2017;63:1–6. doi: 10.1016/j.ijid.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Xuereb J, Sammut P, Vella C, Balfour-Lynn IM. Resolution of lobe collapse in a child with cystic fibrosis with Mycobacterium abscessus using serial intrabronchial rhDNase. Pediatr Pulmonol. 2022;57(6):1549–1551. doi: 10.1002/ppul.25902. [DOI] [PubMed] [Google Scholar]
- 9.Chetchotisakd P, Mootsikapun P, Anunnatsiri S, Jirarattanapochai K, Choonhakarn C, Chaiprasert A, Ubol PN, Wheat LJ, Davis TE. Disseminated infection due to rapidly growing mycobacteria in immunocompetent hosts presenting with chronic lymphadenopathy: a previously unrecognized clinical entity. Clin Infect Dis. 2000;30(1):29–34. doi: 10.1086/313589. [DOI] [PubMed] [Google Scholar]
- 10.Hoyos-Bachiloglu R, Chou J, Sodroski CN, Beano A, Bainter W, Angelova M, Al Idrissi E, Habazi MK, Alghamdi HA, Almanjomi F, et al. A digenic human immunodeficiency characterized by IFNAR1 and IFNGR2 mutations. J Clin Invest. 2017;127(12):4415–4420. doi: 10.1172/JCI93486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. The Lancet. 2013;381(9877):1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do PC, Nussbaum E, Moua J, Chin T, Randhawa I. Clinical significance of respiratory isolates for Mycobacterium abscessus complex from pediatric patients. Pediatr Pulmonol. 2013;48(5):470–480. doi: 10.1002/ppul.22638. [DOI] [PubMed] [Google Scholar]
- 13.Iwanaga K, Carter ER. Mycobacterium abscessus complex lung infection in a toddler with a tracheostomy. Pediatr Pulmonol. 2014;49(3):296–298. doi: 10.1002/ppul.22789. [DOI] [PubMed] [Google Scholar]
- 14.Martin JS, Zagzag D, Egan M, Milla S, Harter D, Lighter-Fisher J. Intracranial Mycobacterium abscessus infection in a healthy toddler. Pediatr Infect Dis J. 2015;34(2):223–224. doi: 10.1097/INF.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 15.Apiwattankul N, Flynn PM, Hayden RT, Adderson EE. Infections Caused by Rapidly Growing Mycobacteria spp in Children and Adolescents With Cancer. J Pediatric Infect Dis Soc. 2015;4(2):104–113. doi: 10.1093/jpids/piu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iroh Tam PY, Kline S, Ward G, Ferrieri P. Non-tuberculous mycobacterial infection in hospitalized children: a case series. Epidemiol Infect. 2015;143(15):3173–3181. doi: 10.1017/S0950268815000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emiralioglu N, Ersoz DD, Oguz B, Saribas Z, Yalcin E, Ozcelik U, Ozsurekci Y, Cengiz AB, Kiper N. Pulmonary Mycobacterium abscessus Infection in a Patient with Triple A Syndrome. J Trop Pediatr. 2016;62(4):324–327. doi: 10.1093/tropej/fmv104. [DOI] [PubMed] [Google Scholar]
- 18.Campos-Herrero MI, Chamizo FJ, Caminero JA, Gilarranz R, Cabrera G, Cuyas J. Nontuberculous mycobacteria in cystic fibrosis patients on the Island of Gran Canaria. A population study. J Infect Chemother. 2016;22(8):526–531. doi: 10.1016/j.jiac.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Anisowicz SK, Welsh SK, Gross JE. Eradication of Mycobacterium abscessus Pulmonary Infection in a Child With Idiopathic Bronchiectasis. Glob Pediatr Health. 2016;3:2333794X16670985. doi: 10.1177/2333794X16670985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JP, Ji Y, Kannan M, Wylam ME. Inhaled granulocyte-macrophage colony-stimulating factor for Mycobacterium abscessus in cystic fibrosis. Eur Respir J. 2018;51(4):1702127. [DOI] [PubMed]
- 21.Ruffles TJC, Black R, Nicholls W, Laing B, Isles A. Osteogenic Sarcoma in an Adolescent With Cystic Fibrosis: Successful Treatment Despite Significant Obstacles. Front Pediatr. 2018;6:245. doi: 10.3389/fped.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Dong F, Liu J, Liu J, Pang Y, Zhao S, Lu J, Li H. Successful management of Mycobacterium abscessus complex lung disease in an otherwise healthy infant. Infect Drug Resist. 2019;12:1277–1283. doi: 10.2147/IDR.S198461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones LA, Doucette L, Dellon EP, Esther CR, McKinzie CJ. Use of inhaled imipenem/cilastatin in pediatric patients with cystic fibrosis: A case series. J Cyst Fibros. 2019;18(4):e42–e44. doi: 10.1016/j.jcf.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauch RM, Mansinho AAS, Rocha PMO, Zaccariotto TR, Levy CE. Nolasco da Silva MT: Nontuberculous mycobacterial infections in a Brazilian pediatric population: a seven-year survey. Pathog Glob Health. 2020;114(2):104–108. doi: 10.1080/20477724.2020.1725330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alramadhan MM, Murphy JR, Chang ML. Extensive Mycobacterium abscessus Pneumonia in an Immunocompetent Infant with No Underlying Lung Pathology. Case Rep Infect Dis. 2021;2021:6615722. doi: 10.1155/2021/6615722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deniz M, Ramasli Gursoy T, Tapisiz A, Tezer H, Aslan AT. Pulmonary Mycobacterium abscessus Infection in an 11-Year-Old Child, Successfully Treated with Inhaled/Parenteral Amikacin: A Case Report and Review of Literature. J Trop Pediatr. 2021;67(2):fmab031. [DOI] [PubMed]
- 27.Chawla R, von Bredow B, Deville J, Yang S. Reinfection or relapse? A case study of whole genome sequencing guided genomic characterization of Mycobacterium abscessus chronic infection in a cystic fibrosis patient. IDCases. 2022;28:e01491. doi: 10.1016/j.idcr.2022.e01491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weerakoon SA, Al Salti M, Mohsin J, Al Hashami H, Al Lawati T, Mohsin H. Early Disseminated Mycobacterium Abscessus Complex Infection in an Infant with Coexisting Cystic Fibrosis and Progressive Familial Intrahepatic Cholestasis: Case report and literature review. Sultan Qaboos Univ Med J. 2022;22(2):295–299. doi: 10.18295/squmj.6.2021.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahni S, Verma S, Grullon J, Esquire A, Patel P, Talwar A. Spontaneous pneumomediastinum: time for consensus. N Am J Med Sci. 2013;5(8):460–4. doi: 10.4103/1947-2714.117296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhuang Y, Zou JL, Huang YF, Hu DX, Shen X, Mao XY. Spontaneous pneumorrhachis with pneumomediastinum, scrotal emphysema, and extensive subcutaneous emphysema in a patient with pneumonia: A case report and literature review. Pediatr Pulmonol. 2023;58(4):1257–65. [DOI] [PubMed]
- 31.Orera Pérez Á, Barber Ansón M, Erice Azparren E. Pneumomediastinum and subcutaneous emphysema in COVID-19's spontaneous ventilation. Med Clin (Barc). 2022;158(8):400. doi: 10.1016/j.medcli.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabin AP, Ferrieri P, Kline S. Mycobacterium abscessus Complex Infections in Children: A Review. Curr Infect Dis Rep. 2017;19(11):46. doi: 10.1007/s11908-017-0597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirolikar S, Pandrowala A, Joshi S, Misra R, Mushrif S. False-positive blood cultures: The need for follow-up. Indian J Med Microbiol. 2020;38(3 & 4):469–471. doi: 10.4103/ijmm.IJMM_20_402. [DOI] [PubMed] [Google Scholar]
- 34.Diao Z, Han D, Zhang R, Li J. Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. Journal of Advanced Research. 2022;38:201–212. doi: 10.1016/j.jare.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin W, Yao Y, Su Y, Huang Y, Wang M, et al. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi: 10.1093/cid/ciy693. [DOI] [PubMed] [Google Scholar]
- 36.Simner PJ, Miller S, Carroll KC. Understanding the Promises and Hurdles of Metagenomic Next-Generation Sequencing as a Diagnostic Tool for Infectious Diseases. Clin Infect Dis. 2018;66(5):778–788. doi: 10.1093/cid/cix881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Han Y, Feng J. Metagenomic next-generation sequencing for mixed pulmonary infection diagnosis. BMC Pulm Med. 2019;19(1):252. doi: 10.1186/s12890-019-1022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie D, Xian Y, You J, Xu W, Fan M, Bi X, Zhang K. Co-Infection Pneumonia with Mycobacterium abscessus and Pneumocystis jiroveci in a Patient without HIV Infection Diagnosed by Metagenomic Next-Generation Sequencing. Infect Drug Resist. 2021;14:879–888. doi: 10.2147/IDR.S292768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 40.Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol. 2020;18(7):392–407. doi: 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- 41.Griffith DE. Mycobacterium abscessus and Antibiotic Resistance: Same As It Ever Was. Clin Infect Dis. 2019;69(10):1687–1689. doi: 10.1093/cid/ciz071. [DOI] [PubMed] [Google Scholar]
- 42.Leber A, Marras TK. The cost of medical management of pulmonary nontuberculous mycobacterial disease in Ontario. Canada. Eur Respir J. 2011;37(5):1158–1165. doi: 10.1183/09031936.00055010. [DOI] [PubMed] [Google Scholar]
- 43.Diel R, Ringshausen F, Richter E, Welker L, Schmitz J, Nienhaus A. Microbiological and Clinical Outcomes of Treating Non-Mycobacterium Avium Complex Nontuberculous Mycobacterial Pulmonary Disease: A Systematic Review and Meta-Analysis. Chest. 2017;152(1):120–142. doi: 10.1016/j.chest.2017.04.166. [DOI] [PubMed] [Google Scholar]
- 44.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Jr., Andrejak C, Bottger EC, Brozek J, Griffith DE, Guglielmetti L, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535. [DOI] [PMC free article] [PubMed]
- 45.Varghese B, Shajan SE, Al MO, Al-Hajoj SA. First case report of chronic pulmonary lung disease caused by Mycobacterium abscessus in two immunocompetent patients in Saudi Arabia. Ann Saudi Med. 2012;32(3):312–314. doi: 10.5144/0256-4947.2012.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan WY, Ho PL, To KK, Lam AY, Ho KW, Lau TW, So NL, Ha SY. A child with acute myeloid leukemia complicated by calcaneal osteomyelitis due to Mycobacterium abscessus infection after induction chemotherapy successfully salvaged with bedaquiline and clofazimine. Int J Infect Dis. 2021;103:9–12. doi: 10.1016/j.ijid.2020.10.102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing files were deposited into the NCBI SRA database and can be retrieved at https://www.ncbi.nlm.nih.gov/ with accession number PRJNA882796.