Abstract
Background
Headache is one of the most common neurological symptoms. Many previous studies have indicated a relationship between primary headaches and alcohol. Drinking has been associated with increased risk of tension-type headache (TTH) and migraine. However, recently published studies have not confirmed this relationship. The existing literature is inconclusive; however, migraine patients avoid alcohol. Therefore, the primary objective was to provide a reliable assessment of alcohol intake in people with primary headaches; the secondary objective was to identify any potential relationship between alcohol consumption and headache risk.
Methods
This study was based on PubMed, Embase and Web of Science database searches performed on 11 July 2023. This systematic review was registered in PROSPERO (CRD42023412926). Risk of bias for the included studies was assessed using the Joanna Briggs Institute critical appraisal tools. Meta-analyses were performed using Statistica software. The Risk Ratio (RR) was adopted as the measure of the final effect. Analyses were based on a dichotomous division of the respondents into "non-drinkers" and "drinkers" for headache patients and matched non-headache groups.
Results
From a total of 1892 articles, 22 were included in the meta-analysis. The majority demonstrated a moderate or high risk of bias. The first part of the meta-analysis was performed on data obtained from 19 migraine studies with 126 173 participants. The risk of migraine in alcohol drinkers is approximately 1.5 times lower than in the group of non-drinkers (RR = 0.71; 95% CI: 0.57–0.89). The second part involved 9 TTH studies with 28 715 participants. No relationship was found between TTH diagnosis and alcohol consumption (RR = 1.09; 95% CI: 0.93–1.27). Two of the included cluster-headache articles had inconclusive results.
Conclusions
Alcohol consumption and migraine are inversely correlated. The exact mechanism behind this observation may indicate that migraine leads to alcohol-avoidance, rather than alcohol having any protective role against migraine. There was no relationship between TTH and drinking. However, further studies related to primary headaches and alcohol consumption with low risk of bias are required. Additionally, patients and physicians should consider the latest medical data, in order to avoid the myths about alcohol consumption and primary headaches.
Keywords: Migraine, Tension-type headache, TTH, Cluster headache, Drinking, Pain, Alcohol
Introduction
Headaches are one of the most common neurological symptoms related to the sensation of pain [1] and cause a decrease in patients’ quality of life [2]. Their global prevalence is estimated at 52% of the population [3]. Headache disorders are classified according to the third edition of the International Classification of Headache Disorders (ICHD) [4, 5] as either primary headaches, secondary headaches or neuropathies and facial pains [4, 6].
The most prevalent primary headache disorder is tension-type headache (TTH) with a prevalence of 40%, followed by migraine (> 10%); while cluster headache (CH) occurs in only 0.12% of the general population [7, 8]. Furthermore, TTH is the most common neurological disorder in the world [9] and presents significantly more frequently in women of all ages, races and socioeconomic status than in men [10–12]. The peak TTH incidence occurs in people 30–39 years old. The symptoms include bilateral, pressing or tightening pain in the forehead, occiput or neck regions [13, 14]. Migraine is most often diagnosed between the 25th and 55th year of life, especially in women [5, 15]. Migraine attacks last 4–72 h and are characterized by a unilateral, throbbing headache with vomiting, nausea and photophobia or are preceded by aura. CH is considered a rare disorder and commonly affects men aged 20–30 years [16]. Its attacks appear 1–8 times a day during the active phase. These headaches are severe, located around the orbit with cranial autonomic symptoms including tearing, miosis, ptosis and anxiety [17].
Alcohol is a psychoactive substance that leads to many health problems such as cancers and traffic accidents; it directly causes impairment in attention, cognition and dexterity, and aggressiveness and loss of control [18–20]. In the USA, 51% of adults consumed alcohol in the last year; additionally, 11% of those over 50 years old and 6% over 65 age reported the symptoms of alcohol abuse or dependence [21]. In Europe, 60% of adults over 60 years of age are current drinkers, and 20% of these had higher levels of consumption than the general population [22]. Statistically, males drink more alcohol than women and have more alcohol-related behavioral disorders [23]. Drinking problems occur in every age, but in the 25–49 age group, alcohol has the highest impact on mortality caused by cancer deaths and also life disability [24, 25].
Many previous studies have proved the relationship between primary headaches and alcohol. Alcohol consumption is associated with increased risk of TTH and migraine [26–30], or as a trigger of headache attacks [31–33]; indeed, there may be increased mortality in patients with migraine [32, 34]. However, recently published studies have not confirmed the relationship between alcohol and headaches [34–36]. Data related to this area is inconclusive; however, migraine patients avoid alcohol drinking [37, 38] unlike their young peers, who often drink alcohol to have fun, cope with problems, relax and maintain friendships [39–41]. Therefore, in order to provide the most objective clues for a normal lifestyle among headache patients, based on existing single studies, our systematic review collates the data about alcohol consumption and primary headaches. The primary objective was to reliably and objectively assess alcohol intake in people with primary headaches, and the secondary objective was to identify any potential relationship between alcohol consumption and headache risk.
Methods
The systematic review presented in this paper was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA 2020) guidelines [42]. The systematic review was registered on PROSPERO (protocols in the International Register of Systematic Reviews) [43]—CRD42023412926.
Data sources and search terms
In order to perform a systematic review, articles were searched in three databases: PubMed, Embase, Web of Science on July 11, 2023. There were no limitations regarding the time frame for the data search. To satisfy the aim of this paper, the below key terms were used: ("primary headache" OR "migraine" OR "tension-type headache" OR "trigeminal autonomic cephalgia" OR "cluster headache" OR "paroxysmal hemicrania" OR "short-lasting unilateral neuralgiform headache attacks" OR "SUNCT" OR "hemicrania continua" OR "primary cough headache" OR "primary exercise headache" OR "primary headache associated with sexual activity" OR "primary thunderclap headache" OR "cold-stimulus headache" OR "primary stabbing headache" OR "nummular headache" OR "hypnic headache" OR "new daily persistent headache") AND ("alcohol" OR "alcohol drinking" OR "alcoholic beverage" OR "alcoholic consumption" OR "alcohol use" OR "alcohol intake" OR "wine" OR "beer" OR "vodka" OR "gin" OR “drinking”) AND ("correlation" OR "relationship" OR "effect" OR "influence" OR "interaction" OR "trigger" OR "associated" OR "association" OR "connection" OR "factor" OR "relation" OR "impact" OR "cause" OR "induce" OR "risk factor"). In PubMed and Web of Science, the above key terms were used in all fields; in Embase, the search was performed in titles, abstracts and keywords.
Literature search
After creating and using search terms in databases, the results were searched by three authors (BB, PN and MS1) independently. Then, the results were compared by researchers and duplicates were removed. Any remaining articles were screened by title or abstract randomly by the authors (BB, PN and MS1) with the below presented inclusion/exclusion criteria and PRISMA 2020 guidelines. Hence, papers that did not meet the inclusion criteria were excluded. In the final step, to assess the exact number of included articles, the authors (BB, PN and MS1) read the appropriate full-text papers and confirmed their relevance to the primary objective. In cases of conflict between authors in terms of the inclusion of a particular paper, the fourth researcher (MWP) decided upon a solution to the problem following discussion.
Inclusion and exclusion criteria
Studies were taken into consideration if they met the following criteria: English studies available in full-text, original papers, articles containing data about alcohol intake in patients suffering from primary headaches. Primary headaches had to be diagnosed using the appropriate criteria (IHS, ICHD). Alcohol consumption was considered in all patients, ages, populations, with all comorbidity diseases and with any primary headaches. The studies had to contain the exact information, in which way were assessed the alcohol consumption e.g. daily drinking, consumption in the last week, drinking habits during the last 2 months period etc. However, from this data, there clearly had to be an extracted division on “drinkers” and “non-drinkers”. The overall results had to be presented in a clear way with the exact numbers of drinking patients and abstainers, and these had to be assigned to the particular type of primary headaches. Additionally, results of alcohol consumption had to be compared with other groups of people who do not suffer from a particular headache (the article had to include a control/healthy population to compare data). In rare cases where a paper lacked a healthy group but where the focus was on the assessment of primary headaches, the control group was made up of another type of primary headache, whereby larger groups of patients with headache were compared to smaller groups with other headaches.
Exclusion criteria included non-English studies; non-original studies as case reports, case series, reviews, conference abstracts, book chapters; animal studies; assessments of alcohol reaction on primary headaches in the molecular pathway; primary headaches diagnosed by self-report and ICD scale, lack of presented techniques to assessments of alcohol intake habits, lack of description of alcohol intake and lack of assigned patients to a detailed type of headache and alcohol intake, lack of control/healthy group for comparison.
Data extraction
From each included paper, three authors (BB, PN and MS1) extracted the following data: study authors, country where study was conducted, criteria used to diagnose headache, number of drinkers and non-drinkers in primary headache and matched control groups, type of headache, type of control group to compare data and methods for assessment of alcohol intake. This data are presented in Table 1.
Table 1.
Features of the studies included in this paper. Abbreviations: ICHD – International Classification of Headache Disease, second and third versions; IHS – International Headache Society; TTH – tension-type headache
| No | Author | Country | Criteria to diagnose headache | Type of headache | Drinkers | Non-drinkers | Overall number of headache patients | Control group | Drinkers | Non-drinkers | Overall number of control patients | Methods used to assessment drinking | Criteria to recognize habits for alcohol consumption |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Lisicki M et al. [44] | International | ICHD-3 | Migraine | 20 | 39 | 59 | Non-headache | 30 | 47 | 77 | Self-designed questionnaire | Frequency divided on: never, ≥ once a month, ≥ once a week and every day for wine, beer and other alcoholic beverages |
| 2. | Schramm S et al. [45] | Germany | ICHD not reported the edition | Migraine | 49 | 367 | 416 | Non-headache | 96 | 367 | 463 | Questionnaire | Alcohol was assessed as the average consumption of different beverages (beer, red wine, white wine, spirits, and cocktails) within the last 4 weeks. The proportion of pure alcohol per beverage was calculated multiply by frequency of drinking. All beverages drinked by each person were summed up and presented as the total consumption of pure alcohol g/day |
| 3. | Aamodt AH et al. [46] | Norway | IHS from 1988 | Migraine | 4012 | 2197 | 6209 | Non-headache | 30,421 | 14,749 | 45,170 | Questionnaire | Abstinence, number of times per month and the number of glasses of beer, wine or liquor during the last 2 weeks |
| 4. | Hagen K et al. [36] | Norway | ICHD-2 | Migraine | 24 | 65 | 89 | Non-headache | 967 | 1086 | 2053 | Self-designed questionnaire | The participants were divided into four groups: no use, less than four times per month, four to seven times per month, or at least eight times per month |
| Tension type headache | 749 | 52 | 801 | Non-headache | 11,680 | 1086 | 12,766 | ||||||
| 5. | Misakian AL et al. [47] | International |
Modified version of the IHS |
Migraine | 149 | 922 | 1071 | Non-headache | 1717 | 6839 | 8556 | Self-designed questionnaire | Alcohol use was divided into 4 groups: never/rarely, 1–3 times/month, 1–6 times/week, daily |
| 6. | Luo J [48] | United States of America | ICHD-3 | Migraine | 51 | 458 | 509 | Non-headache | 359 | 2278 | 2637 | Questionnaire | Alcohol was divided into 2 groups: alcohol drinker (had any alcohol in the last 24 h) or non-drinker (lack of alcohol within last 24 h) |
| 7. | Özcan RK et al. [49] | Turkey | ICHD-3 beta | Migraine | 1 | 141 | 142 | Non-headache | 1 | 141 | 142 | Questionnaire | Alcohol use was defined by identifying the quantity consumed in the last month |
| 8. | Sarker MA et al. [50] | Bangladesh | IHS-2 | Migraine | 19 | 119 | 138 | Non-headache | 4 | 272 | 276 | Questionnaire | The patients were asked about consuming any alcoholic drink in the last 2 weeks |
| 9. | Le H et al. [51] | Denmark | IHS | Migraine | 5744 | 2200 | 7944 | Non-headache | 19,073 | 4431 | 23,504 | Questionnaire | Alcohol consumption was divided into 3 groups: never/seldom, monthly, weekly |
| 10. | Schramm SH et al. [52] | Germany | ICHD-2 | Migraine | 2 | 722 | 724 | Non-headache | 6 | 1068 | 1074 | Questionnaire | 2 groups were distinguished: drinking (yes) was defined as daily or almost daily drinking of alcoholic beverages and drinking (no) was defined as no or casual drinking of alcoholic beverages |
| Tension type headache | 11 | 1544 | 1555 | 6 | 1023 | 1029 | |||||||
| 11. | Kim BS et al. [53] | Korea | ICHD-3 | Migraine | 3 | 48 | 51 | Non-headache | 23 | 79 | 102 | Questionnaire + interview | Alcohol drinking was positive if regular drinking were once a week |
| 12. | Gür-Özmen S et al. [54] | Turkey | ICHD-2 | Migraine | 4 | 166 | 170 | Non-headache | 1 | 169 | 170 | Questionnaire | The mean daily alcohol intake was calculated using the beverage-specific quantity-frequency measure: number of days with alcohol consumption (beer, wine, and spirits)mean daily alcohol consumption over the past week. Amount of wine and beer in liters and spirits in glasses was assessed and calculated in grams per day |
| Tension type headache | 0 | 170 | 170 | 1 | 169 | 170 | |||||||
| 13. | Yoon MS et al. [55] | Germany | ICHD-2 | Migraine | 320 | 4244 | 4564 | Non-headache | 555 | 3086 | 3641 | Questionnaire | Alcohol was assessed by drinking daily and not daily alcohol consumption |
| Tension type headache | 126 | 1107 | 1233 | 555 | 3086 | 3641 | |||||||
| 14. | Kaltseis K et al. [26] | International | ICHD-3 | Migraine | 154 | 31 | 185 | Non-headache | 729 | 222 | 951 | Interview | Alcohol intake was divided into 2 groups: drinkers and non-drinkers. Drinkers positively answer the question whether drinked alcohol last week and disclose the type, amount, and frequency of the consumed beverages. Then, the alcohol consumption was calculated using the formula: amount of alcohol beverage in milliliters Vol% 100 0:8 ¼ alcohol in grams: |
| Tension type headache | 479 | 78 | 557 | 729 | 222 | 951 | |||||||
| 15. | Pellegrino Baena C et al. [56] | Brasil | IHS | Migraine | 179 | 1060 | 1239 | Non-headache | 1096 | 131 | 1227 | Questionnaire | Alcohol use was defined as never, former or present. Whereby never was assumed as non-drinkers, the rest of participants (former, present) were assumed as drinkers |
| 16. | McMurtray AM et al. [57] | United States of America | ICHD-2 | Migraine | 2 | 22 | 24 | Non-headache | 7 | 7 | 14 | Questionnaire | Alcohol use was defined as none, past or current. Whereby none was assumed as non-drinkers, the rest of participants (past, current) were assumed as drinkers |
| Tension type headache | 2 | 19 | 21 | Non-headache | 7 | 7 | 14 | ||||||
| 17. | Lebedeva ER et al. [27] | International | ICHD-3 | Migraine | 129 | 367 | 496 | Non-headache | 282 | 732 | 1014 | Interview | Alcohol intake was divided into consumption of light alcoholic beverages (at least 0.5 L per week) and strong alcoholic beverages (150 g per week) |
| Tension type headache | 639 | 1196 | 1835 | Non-headache | 282 | 732 | 1014 | ||||||
| 18. | Schramm SH et al. [58] | German | ICHD-2 | Migraine | 114 | 1564 | 1678 | Non-headache | 555 | 3086 | 3641 | Questionnaire | Alcohol was assessed by drinking daily and not daily alcohol consumption |
| Tension type headache | 11 | 1587 | 1598 | 555 | 3086 | 3641 | |||||||
| 19. | Scher AI et al. [59] | Netherland | IHS | Migraine | 304 | 316 | 620 | Non-headache | 3235 | 1900 | 5135 | Questionnaire |
Alcohol consumption was categorized as 0, < 1, 1 to 3 and + 4 drinks per day |
| 20. | Rasmussen BK [60] | Denmark | IHS | Tension type headache | 53 | 114 | 167 | Non-tension type headache (migraine) | 38 | 81 | 119 | Interview | Alcohol intake was recorded as number of drinks (beers. glasses of wine. and glasses of strong alcohol) per week (1 drink = 10 g of alcohol) |
| 21. | Lund N et al. [61] | Denmark | ICHD-2 | Cluster headache | 242 | 157 | 397 | Non-headache | 179 | 21 | 200 | Questionnaire + interview | Alcohol intake was positive if participants had reported any intake of alcohol per week during the past year |
| 22. | Lambru G et al. [62] | Italy | ICHD-2 | Cluster headache | 149 | 51 | 200 | Non-cluster headache (migraine) | 113 | 87 | 200 | Questionnaire | Alcohol consumption, measured in alcohol units per day and if there was positive intake the division on: mild drinkers, < 4 units/day; moderate drinkers, 4–8 units/day; heavy drinkers, > 8 units/day was conducted |
Assessment of risk bias
Due to the inclusion of many study designs, the risk of bias was evaluated using tools adjusted to the type of study. The Joanna Briggs Institute (JBI) critical appraisal tools were used for cross-sectional, cohort and case control studies [63]. According to the appropriate JBI checklist, cross-sectional studies had to be conducted on the basis of eight questions, case-controls had ten questions, while cohort studies contained 11 questions. Possible answers were “Yes”, “No”, “Unclear” or “Not applicable”. If a cross-sectional study received seven or more positive answers, a case–control eight and a cohort study nine, ten or 11, their assessments were described as having a low risk of bias. A high risk of bias was reported when a cross-sectional study received five or fewer “yes” responses, a case–control fewer than six and a cohort study below seven. A moderate risk of bias was assigned when the paper received positive answers between mentioned ranges. The assessments were conducted by three researchers (BB, PN and MS1) separately, then the fourth author (MWP) compared this data and made a final decision.
Statistical analysis
Meta-analyses were performed using Statistica v.13.3 software (Tibco Software Inc.). Due to the type of the available data (2 × 2 tables), the relative-risk ratio (RR) risk was adopted as the measure of the effect. Heterogeneity analyses were carried out using the Q statistic based on ✗2 and the corresponding p value. To determine the proportion of heterogeneity between the study estimates, the I2 statistic was used. Since the result of the heterogeneity test proved to be highly significant (p < 0.001), a random effect model was used for the meta-analysis.
In order to detect publication bias, the symmetry of funnel plots was analyzed using the Trim and Fill method, and the Egger test as well as the Begg and Mazumdar test were used. In order to assess the extent to which the assumptions of the meta-analysis and the studies included therein influenced the overall results, a sensitivity analysis was also performed. In all statistical tests, p < 0.05 was considered significant. Dichotomous division of the respondents into "non-drinkers" and "drinkers" was used.
Results
Study selection
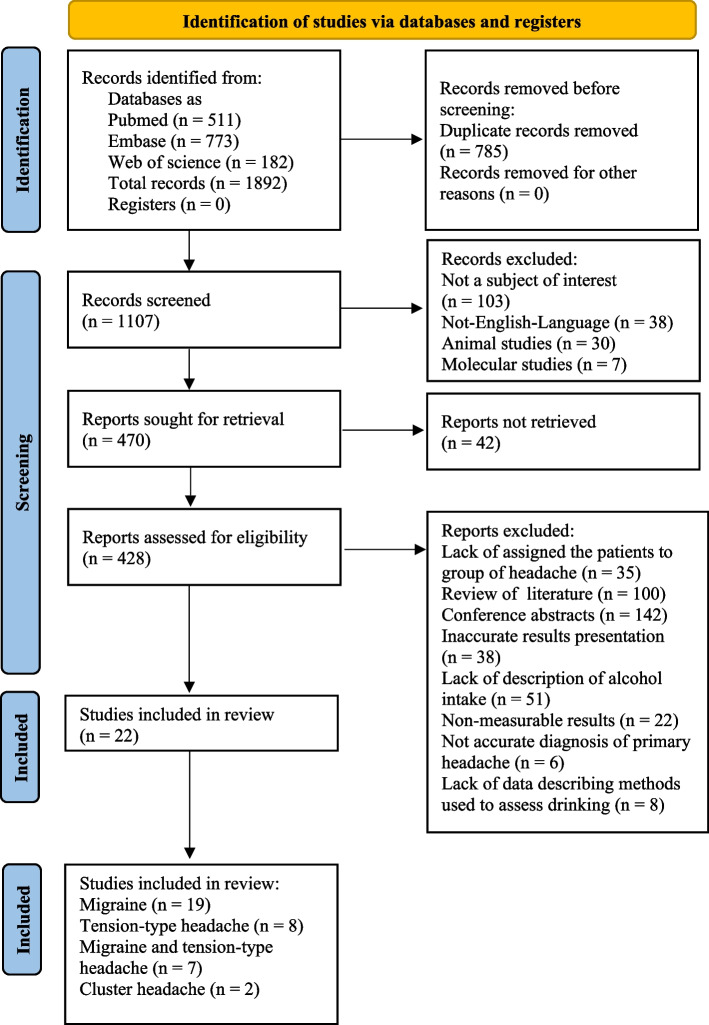
After using the above key terms, 1,892 articles were identified in the three databases. 511 papers were found in PubMed, 773 in Embase and 608 in Web of Science. At the outset, 785 duplicates were excluded. Subsequently, 38 non-English articles, 30 animal studies, seven studies concentrating on molecular pathways to alcohol intake and 562 papers not related to our topic were removed from the remaining records. Then, 142 conference abstracts, 100 reviews, four book chapters and 42 unretrieved studies were not taken into further consideration. Among the full-text articles, 35 had not assigned patients to a specific headache-type or to alcohol intake; 51 studies lacked a description of alcohol intake; 38 papers presented results in an inaccurate way; 22 studies lacked a control group; in 6 articles diagnosis of primary headaches were not based on appropriate criteria and 8 articles do not contain data about methods to define alcohol consumption. Finally, 22 articles [26, 27, 36, 44–62] were retrieved for further analysis. A detailed description of the steps performed during study selection is presented in Fig. 1.
Fig. 1.
PRISMA 2020 flow diagram
Study characteristics
The 22 included articles [26, 27, 36, 44–61, 64] came from 10 individual countries, while four papers were international [26, 27, 44, 47]. The majority of these were from Europe: three from Denmark [51, 60, 61], two from Norway [36, 46], four studies conducted in Germany [45, 52, 55, 58] and single papers the Netherlands [59] and Italy [62]. From around the world, one Korean [53], two Turkish [49, 54], one Bangladeshi [50], two United States of America (USA) [48, 57], and one Brazilian [56] were identified.
Most studies were performed with migraine cohorts – these made up 19 out of the 22 papers [26, 27, 36, 45–59, 65]. 11 studies were focused on migraine without any other primary headache [44–50, 53, 56, 58, 59]. All migraine studies [26, 27, 36, 45–59, 65] had a healthy control group without any primary headache. We identified 26 327 migraine participants, including 11 280 alcohol drinkers and 15 047 non-drinkers. The combined control groups represented 99 846 individuals: 59 157 drinkers and 40 689 non-drinkers. In total, all the migraine studies combined included 126 173 participants.
Nine studies [26, 27, 36, 52, 54, 55, 57, 58, 60] analyzed people with tension-type headache (in eight out of the 9 migraine was also evaluated [26, 27, 36, 52, 54, 55, 57, 58]). Only one study was focused only on tension-type headaches [60]. Also, the majority of the studies (eight out of the 9) had a control group of healthy people [26, 27, 36, 52, 54, 55, 57, 58]. In one study [60], migraine was a comparator instead of healthy controls. There were 7937 TTH (2070 drinkers and 5867 non-drinkers). The control group consisted of 13 304 drinkers and 7474 non-drinkers. In total, 28 715 people were included in the 9 TTH studies. We found only two articles on cluster-headache cohorts relevant to our criteria [61, 62]. One study [61] had a control group of healthy people, the second [62] had a non–cluster-headache control group (i.e., migraine). For meta-analysis purposes, there were 391 drinkers and 208 non-drinkers in the cluster-headache category; 292 drinkers and 108 non-drinkers represented the control group.
In 15 studies, headaches were diagnosed based on criteria developed by the International Classification of Headache Disorders (ICHD) [26, 27, 36, 44, 45, 48, 49, 52–55, 57, 58, 61, 62] and its various versions (six out of the 15 employed the latest third edition [26, 27, 44, 48, 49, 53]). Seven articles used International Headache Society (IHS) criteria from 1988 [46, 47, 50, 51, 56, 59, 60].
Criteria to recognize habits for alcohol consumption was various in almost each study. Some of them assessed the drinking by daily alcohol intake [44, 45, 47, 48, 55, 58, 59, 62], part of them measured drinking within one week [44, 47, 51, 53, 60, 61] or month [36, 44, 47, 49, 51]. Additionally, there were cases [56, 57] where division was based on never, current or past drinking. More accurate calculation with amount and various types of alcohol was also conducted in studies [26, 27, 45, 54]. Only few studies [46, 48–50, 52] provided the data about the period in which alcohol drinking was considered and measured. Nineteen studies used questionnaire methods to assess drinking [36, 44–59, 61, 62]. In two cases, questionnaires were supplemented by medical interviews [53, 61]. The rest of the studies were based on information obtained during a medical interview [26, 27, 44].
Analysis of alcohol consumption
Migraine
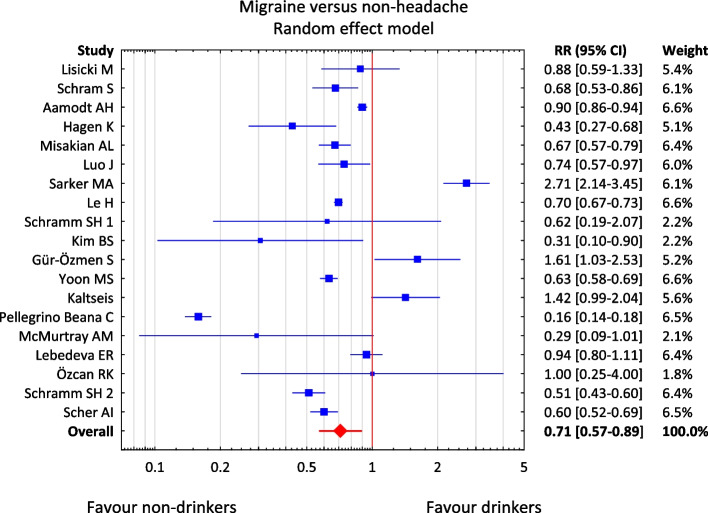
The meta-analysis included 19 studies [26, 27, 36, 45–59, 65] presenting data on the presence or absence of migraine pain and assessment of alcohol consumption status (Table 2). Due to the nature of the available data on the status of alcohol consumption, a dichotomous division of the respondents into "non-drinkers" and "drinkers" was used. The total effect obtained in the model is RR = 0.71, and the 95% confidence interval (95% CI) was in the range 0.57–0.89. The results presented in forest graphs (Fig. 2) indicate a significantly lower risk of migraine in people who consume alcohol. In the group of drinkers, the risk of migraine is approximately 1.5 times lower than in the group of non-drinkers (RR = 0.71).
Table 2.
19 migraine studies included in the meta-analysis with p-value and RR. 11 studies are highlighted in red: p is statistically significant (p < 0.05). Abbreviations: RR – relative risk; LL – lower limit for RR; UL – upper limit for RR; SE – standard error for RR; p-value –– probability value
| Study | Study | LLRR | ULRR | p-value | Weight | |
|---|---|---|---|---|---|---|
| 1 | Lisicki M [44] | 0,88 | 0,59 | 1,33 | 0,549 | 5,37% |
| 2 | Schram S [45] | 0,68 | 0,53 | 0,86 | 0,001 | 6,14% |
| 3 | Aamodt AH [46] | 0,90 | 0,86 | 0,94 | 0,000 | 6,61% |
| 4 | Hagen K [36] | 0,43 | 0,27 | 0,68 | 0,000 | 5,12% |
| 5 | Misakian AL [47] | 0,67 | 0,57 | 0,79 | 0,000 | 6,39% |
| 6 | Luo J [48] | 0,74 | 0,57 | 0,97 | 0,031 | 6,02% |
| 7 | Sarker MA [50] | 2,71 | 2,14 | 3,45 | 0,000 | 6,14% |
| 8 | Le H [51] | 0,70 | 0,67 | 0,73 | 0,000 | 6,61% |
| 9 | Schramm SH 1 [52] | 0,62 | 0,19 | 2,07 | 0,436 | 2,19% |
| 10 | Kim BS [53] | 0,31 | 0,10 | 0,90 | 0,032 | 2,51% |
| 11 | Gür-Özmen S [54] | 1,61 | 1,03 | 2,53 | 0,038 | 5,16% |
| 12 | Yoon MS [55] | 0,63 | 0,58 | 0,69 | 0,000 | 6,56% |
| 13 | Kaltseis K [26] | 1,42 | 0,99 | 2,04 | 0,055 | 5,62% |
| 14 | Pellegrino Beana C [56] | 0,16 | 0,14 | 0,18 | 0,000 | 6,46% |
| 15 | McMurtray AM [57] | 0,29 | 0,09 | 1,01 | 0,052 | 2,11% |
| 16 | Lebedeva ER [27] | 0,94 | 0,80 | 1,11 | 0,466 | 6,38% |
| 17 | Özcan RK [49] | 1,00 | 0,25 | 4,00 | 1,000 | 1,80% |
| 18 | Schramm SH 2 [58] | 0,51 | 0,43 | 0,60 | 0,000 | 6,37% |
| 19 | Scher AI [59] | 0,60 | 0,52 | 0,69 | 0,000 | 6,45% |
| Overall | 0,71 | 0,57 | 0,89 | 0,002 | 100,00% |
Fig. 2.
The result of the meta-analysis of the risk of migraine pain in patients who differ in terms of alcohol consumption. Abbreviations: RR – relative risk; CI – confidence interval
When analyzing the data in Table 2 in detail, it is worth noting that in the case of six studies [26, 27, 44, 49, 52, 57], the results are not statistically significant (p > 0.05); the results of two studies [50, 54] differ diametrically from the others; and the total result is confirmed by the 11 studies highlighted in red [36, 44–48, 51, 53, 55, 56, 58, 59].
The chi-square test and the I2 statistic were used to assess the non-compliance, i.e., heterogeneity of the studies. The test results—Q = 758, df = 18, p < 0.001, T2 = 0.186, I2 = 97.6%—indicate a significant heterogeneity of the studies included in the meta-analysis. For this reason, the random effect model was used in the meta-analysis. The observed heterogeneity may result from, among others, different status criteria, for example drinker/non-drinker, in individual studies. However, the statistical results did not change when each study was omitted from the sensitivity analysis, indicating that the overall conclusion can be considered reliable.
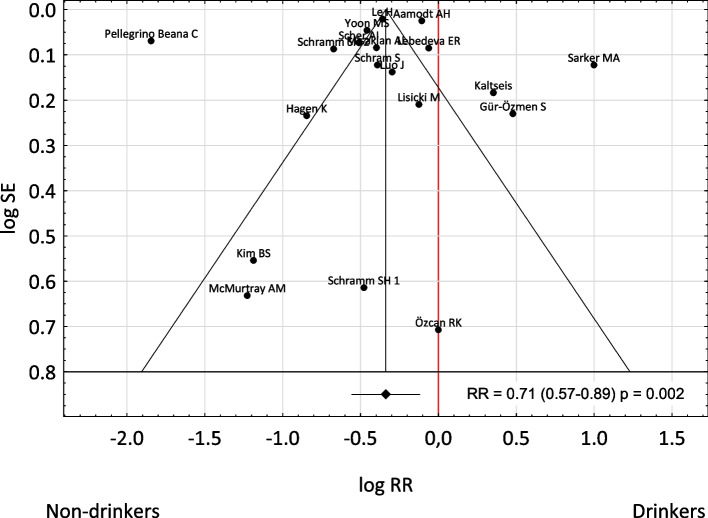
The analysis of the tunnel graph (Fig. 3) and the result of the Trin and Fill procedure, as well as the results of the Begg and Mazumdar test (p = 0.243) and the Egger test (p = 0.769) indicate the lack of statistically significant publication bias.
Fig. 3.
A tunnel graph to assessment the risk of bias of studies in included in meta-analysis. Abbreviations: RR – relative risk; CI – confidence interval
Tension-type headache
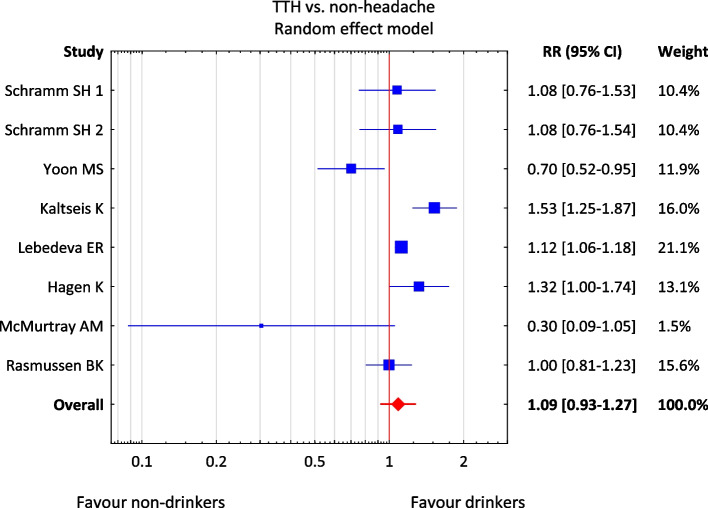
The meta-analysis included 8 out of the 9 studies [26, 27, 36, 52, 55, 57, 58, 60] with data on the incidence of tension-type headache and the assessment of alcohol consumption status, because RR for the study by Gür-Özmen et al. [54] was 0. Due to the nature of the available data on the status of alcohol consumption, a dichotomous division of the respondents into "non-drinkers" and "drinkers" was used. The test results—Q = 24.6, df = 7, p = 0.001, T2 = 0.030, I2 = 71.6%—indicate a significant heterogeneity of the studies included in the meta-analysis. Therefore, a variable effects model was used. In the group of non-drinkers, the risk of migraine attack is higher than in the group of drinkers (RR = 1.09), but the 95% CI (0.93–1.27) contains the value of 1—RR is not significantly different from 1—none of the compared groups differing in alcohol consumption is more exposed to TTH (Fig. 4). The results presented in the form of forest graphs indicate the lack of a statistically significant relationship between the risk of TTH and alcohol consumption. The control groups as a non–tension-type headache in one case [60] do not have any influence on the final results.
Fig. 4.
Result of the meta-analysis of the risk of tension headache in patients who differ in alcohol consumption. Abbreviations: RR – relative risk; CI – confidence interval; TTH – tension-type headache
Cluster headache
The two articles on cluster headaches draw contrasting conclusions. In Lund et al. [61], the risk of cluster headache is significantly lower in non-drinkers (RR = 0.65) while in Lambru et al. [62], the opposite is true: the risk of CH in non-drinkers is higher (RR = 1.54). A synthesis of both papers does not provide any meaningful answer about the relationship between alcohol consumption and cluster headache.
Risk of bias
Analysis of the 22 included studies revealed 5 cohort studies [36, 45, 52, 55, 58], 11 cross-sectional [26, 27, 44, 46–48, 51, 56, 57, 59, 60] and six case-controls [49, 50, 53, 54, 61, 62]. Of the cohort studies, two [36, 66] received fewer than 8 “yes” answers, therefore according to the assessment criteria from the Methods section above, these were assessed as having moderate risk of bias. The majority of cohort studies were within the range of 3–7 points, thus receiving a high risk of bias [52, 55, 58]. None of the cohort studies had low bias-risk. A detailed description of risk of bias assessment for the cohort studies is presented in Table 3. In cross-sectional studies, six out of the 11 had a high risk of bias [46–48, 51, 56, 60], because they received fewer than 6 positive answers. Three studies were evaluated as moderate risk, with 6 “yes” answers [44, 57, 59]. Two of the remaining cross-sectional papers achieved seven or eight points and therefore were low bias-risk [26, 27]. Table 4 summarizes the assessment of the cross-sectional risk of bias. One of the six case–control studies was assessed as having a high risk of bias [49], two a low risk of bias [50, 62] and three a moderate bias risk [53, 54, 61]. The steps for case–control assessment are presented in Table 5.
Table 3.
Assessment of risk of bias for cohort studies according to the Joanna Briggs Institute (JBI) checklist
| Study authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Overall risk of bias assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schramm S et al. [45] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Moderate |
| Hagen K et al. [36] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Moderate |
| Schramm SH et al. [52] | Yes | Yes | No | Unclear | Yes | No | No | Yes | Yes | No | Yes | High |
| Yoon MS et al. [55] | Yes | Yes | No | Yes | Yes | No | No | Unclear | Yes | Yes | Yes | High |
| Schramm SH et al. [58] | Yes | Yes | No | Yes | No | No | Yes | Unclear | Unclear | Unclear | Yes | High |
Q1—Were the two groups similar and recruited from the same population?
Q2—Were the exposures measured similarly to assign people to both exposed and unexposed groups?
Q3—Was the exposure measured in a valid and reliable way?
Q4—Were confounding factors identified?
Q5—Were strategies to deal with confounding factors stated?
Q6—Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)?
Q7—Were the outcomes measured in a valid and reliable way?
Q8—Was the follow-up time reported and sufficiently long for outcomes to occur?
Q9—Was follow-up complete, and if not, were the reasons for this incomplete follow-up described and explored?
Q10—Were strategies to address incomplete follow-up utilized?
Q11—Was appropriate statistical analysis used?
Table 4.
Assessment of risk of bias for cross-sectional studies according to the Joanna Briggs Institute (JBI) checklist
| Study authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Overall risk of bias assessment |
|---|---|---|---|---|---|---|---|---|---|
| Lisicki M et al. [44] | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Moderate |
| Aamodt AH et al. [46] | Yes | Yes | No | Yes | No | No | Yes | Yes | High |
| Lebedeva ER et al. [27] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Misakian AL et al. [47] | Yes | Yes | No | Yes | No | No | Yes | Yes | High |
| Le H et al. [51] | No | Unclear | No | Yes | Yes | Yes | Yes | Yes | High |
| Kaltseis K et al. [26] | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Low |
| Pellegrino Baena C et al. [56] | Yes | Yes | No | Yes | Unclear | No | Yes | Yes | High |
| McMurtray AM et al. [57] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Moderate |
| Rasmussen BK [60] | Yes | Yes | No | Yes | No | No | Yes | Yes | High |
| Scher AI et al. [71] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Moderate |
| Luo J [48] | Yes | Yes | No | No | Yes | Unclear | Yes | Yes | High |
Q1—Were the criteria for inclusion in the sample clearly defined?
Q2—Were the study subjects and the setting described in detail?
Q3—Was the exposure measured in a valid and reliable way?
Q4—Were objective, standard criteria used for measurement of the condition?
Q5—Were confounding factors identified?
Q6—Were strategies to deal with confounding factors stated?
Q7—Were the outcomes measured in a valid and reliable way?
Q8—Was appropriate statistical analysis used?
Table 5.
Assessment of risk of bias for case–control studies according to the Joanna Briggs Institute (JBI) checklist
| Study authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Overall risk of bias assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lambru G et al. [62] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Lund N et al. [61] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Unclear | Yes | Moderate |
| Özcan RK et al. [49] | Yes | No | Yes | Yes | Yes | Unclear | Unclear | Yes | No | Yes | High |
| Sarker MA et al. [50] | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Low |
| Kim BS et al. [53] | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes | Moderate |
| Gür-Özmen S et al. [54] | Yes | Unclear | Yes | No | Yes | Yes | Yes | Yes | Unclear | Yes | Moderate |
Q1—Were the groups comparable other than the presence of disease in cases or the absence of disease in controls?
Q2—Were cases and controls matched appropriately?
Q3—Were the same criteria used for identification of cases and controls?
Q4—Was exposure measured in a standard, valid and reliable way?
Q5—Was exposure measured in the same way for cases and controls?
Q6—Were confounding factors identified?
Q7—Were strategies to deal with confounding factors stated?
Q8—Were outcomes assessed in a standard, valid and reliable way for cases and controls?
Q9—Was the exposure period of interest long enough to be meaningful?
Q10—Was appropriate statistical analysis used?
Discussion
The primary objective of our systematic review was to reliably assess alcohol intake in patients suffering from primary headaches, and the secondary objective was to identify a potential answer to the question of whether there is any relationship between alcohol consumption and headache risk. Out of the approximately 1,900 initially selected articles, 22 met the inclusion criteria; however, the majority of these had moderate or high risk of bias. But from our review certain conclusions could be drawn. Alcohol consumption was often considered a trigger or risk factor for migraine or tension-type headache, which was supported by previous studies [31, 67]. The mechanism by which alcohol induces the particular type of headache is unknown [68]. It seems that the theory of the vasodilation of brain vessels after alcohol consumption is insufficient; more probable is pathogenesis involving receptors in the cortex or brainstem [69]. However, some studies did not confirm alcohol influence on primary headaches [51, 70]. The results of our meta-analysis of studies on over 100 000 people indicate a 1.5-lower risk of migraine in people who consume alcohol. To the best of our knowledge, few studies in the literature present similar results—that alcohol decreases the frequency of migraine attacks [38, 46]—but rarely was there any indication of the exact number of potential risks for headache. Despite previous inconclusive results for studies focusing on the relationship between alcohol and headaches, especially in migraine, about one in five headache sufferers believe that alcohol accelerates their particular headache attacks [71]. Due to this stereotype, non-drinking behavior among migraine patients is widespread, which has previously been confirmed [72, 73]. However, our systematic review only considered the simple division of drinkers and non-drinkers, because the majority studies do not distinguish the exact amount of alcohol consumed.
The exact amount of consumed alcohol may have varied effects on headache, e.g., Mostofsky et al. [74] indicate that 1–2 servings of alcohol do not correlate with headache, but five or more servings are associated with increased risk. Therefore, it should be remembered that alcohol consumption may be related to different headache results associated with drinking patterns. Studies show that moderate drinking may reduce the disease burden of mortality in comparison to abstainers [75]. Moreover, low consumption is associated with reduced risk of diabetes and heart attack [76]. However, it is an established fact that heavy drinking leads to serious diseases such as liver cirrhosis, pancreatitis, dementia and malignant neoplasms [77]. In addition, these results may differ between among different age, gender and work-status cohorts [78]. However, due to methodological issues, our meta-analysis could not consider these confounders. We also were not able to recalculate consumption descriptors to countable units. Even if some studies provide such estimations, the amount is different in each article. Additionally, there is no standardized alcohol assessment method in these publications. The included studies used units, grams, glasses, drinks, pints and milliliters, which makes it impossible to recalculate to a unified amount.
Whereas the World Health Organization (WHO) states that there is no safe alcohol dose [19], Panconesi et al. conclude that low consumption is not a contraindication for headache patients [79]. However, each patient makes individual decisions based on their own experience. Headache after a certain amount of alcohol is likely to induce behavioral reactions (i.e., alcohol-intake adjustment). Similarly, common beliefs may influence patients habits, e.g., the conviction that “red wine causes migraine”, even if studies present conflicting evidence [80, 81]. Consequently, it seems likely that people with migraine to some extent avoid alcohol, which would be one interpretation of our results. For this reason, people with migraine may gain unforeseen healthcare benefits, e.g., avoiding negative effects of alcohol consumption such as gastrointestinal cancers [82], which can be partially confirmed by Elser et al. [83].
A second explanation for the results presented in our meta-analysis might encompass a certain protective role of alcohol with regards to migraine. However, according to this idea, populations with higher migraine prevalence should have lower alcohol consumption. For example, due to religious requirements, people in Iran consume considerably less alcohol than Europeans [22, 84]; nevertheless, migraine prevalence in Iran is 15.1% [85] while in Europe it is 35% [86]. In Europe, alcohol consumption is higher than in Asian countries, but in Europe alcohol as a trigger is reported more frequently than it is in Asia [87]. Therefore, this hypothesis seems a less likely explanation for our results.
According to our results, the relationship between alcohol and headache is more pronounced in migraine than in tension-type headache [32, 88]. However, it is worth noting that more studies concentrate on migraine than TTH (19 of the included studies vs 9); moreover, the prevalence of TTH is greater than that of migraine [89]. The result from our meta-analysis was that there is a lack of a relationship between the risk of TTH and alcohol consumption. Similar results are also reported in the literature [36, 60]. Again, there are also studies where alcohol is reported as a TTH trigger [27]. Similar to our migraine meta-analysis, some confounders could not be considered, e.g., quantity and type of alcohol, gender, and episodic/chronic form of TTH. In the literature, cluster headaches are associated with alcohol and often with nitrates [81]. However, data about this topic is also inconclusive [80]. Unfortunately, the studies included in our analysis did not allow unequivocal answers in this area. Only two articles satisfied the inclusion criteria, and in Lund et al. [61] cluster headache is significantly less prevalent in non-drinkers, but in the second study—Lambru et al. [62]—this risk is higher in non-drinkers.
Assessment of alcohol consumption is challenging, because the results are dependent on the patient's honesty. Patients sometimes have a tendency not to admit their drinking habits [90]. It has been proved that self-reported alcohol consumption by patients can be underestimated; therefore, more reliable methods such as toxicological hair analysis may help to provide stronger evidence [91]. Of the studies included in our analysis, 19 were based only on questionnaires while five included interviews with patients. However, these limitations are to some extent discounted by the number of studies included and the cultural diversity of participants.
This study has some limitations. First of all, the existing studies present data in a heterogeneous way, which may have led to inaccurate results, and do not provide an exhaustive array of information. Information on the gender of participants was unavailable for analysis. So, the question of who is drinking more with a primary headache is still to be addressed. Additionally, only a few of the studies divided participants into migraine with and without aura. Therefore, there was insufficient data to analyze the relationship between alcohol and aura, and the data that does exist is inconsistent [65, 86]. As mentioned in the discussion above, alcohol consumption assessment is strongly based on patients’ honesty. If there is misleading data in questionnaires or during medical interviews, their overall subsequent analysis is also distorted. Therefore, this meta-analysis was not able to assess particular variables of alcohol in primary headaches, e.g., gender, division into type of migraine, TTH, cluster headache or type of alcohol drinking, which could be key to various previously reported results. The relatively low number of cluster-headache studies also does not allow an assessment of any correlation with alcohol drinking. Moreover, some of the studies included in our review do not present results in an accurate way or do so without assigning patients to specific headaches. Therefore, it was not possible for our meta-analysis to contain all those studies where drinking was described with primary headache. The ways describing alcohol consumption habits were variously presented in almost each study, therefore could develop the observed heterogeneity among migraine analysis. Also, the majority of the studies had high or moderate risk of bias. Thus, our results should be interpreted with care.
Conclusions
Alcohol consumption and migraine are inversely correlated. The exact mechanism behind this observation may indicate that migraine leads to alcohol-avoidance rather than alcohol having a protective role against migraine. There is no relationship between TTH and drinking. However, there is a need to conduct further studies related to primary headaches and alcohol consumption with low risk of bias. Additionally, patients and physicians should consider the latest medical knowledge to avoid perpetuating the myths about alcohol consumption and primary headaches. Additionally, it would be useful to check whether migraine patients enjoy the advantages or disadvantages of less drinking.
Key points
The meta-analysis showed a 1.5-lower risk of migraine in people who consume alcohol. However, migraine patients consume less alcohol for various reasons. Consequently, migraine patients can avoid the negative effects of alcohol consumption but also positive aspects of drinking such as protection from heart attack or diabetes, a sociable life or they may deny themselves possibilities for enjoyment. Therefore, patients with primary headache need to determine for themselves the association between alcohol and headache without any myths and influences. The results of our meta-analysis are that there is a lack of a relationship between the risk of TTH and alcohol consumption. Further studies should present exact levels of alcohol intake in standardized units, clearly state the division of migraine, TTH and cluster headache into subtypes, distinguish drinkers and non-drinkers in terms of gender and include the type of alcohol. More cluster headache studies should be conducted.
Authors’ contributions
MWP and BB was involved in the conception and visualization of the study. BB, PN, MS1, MWP collected the data and prepared a manuscript. BB was involved in data analysis. MWP, SB, MW, MS2 supervised the study. MWP, MW, MS2 and SB revised the final version of the manuscript. All authors have read and agreed to the published version of the manuscript..
Funding
Not applicable.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nieswand V, Richter M, Gossrau G. Epidemiology of Headache in Children and Adolescents—Another Type of Pandemia. Curr Pain Headache Rep. 2020;24:62. doi: 10.1007/s11916-020-00892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onofri A, Pensato U, Rosignoli C, Wells-Gatnik W, Stanyer E, Ornello R, et al. Primary headache epidemiology in children and adolescents: a systematic review and meta-analysis. J Headache Pain. 2023;24:8. doi: 10.1186/s10194-023-01541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The Global Burden of Headache: A Documentation of Headache Prevalence and Disability Worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 4.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. 10.1177/0333102413485658 [DOI] [PubMed]
- 5.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. 10.1177/0333102417738202 [DOI] [PubMed]
- 6.Berk T, Ashina S, Martin V, Newman L, Vij B. Diagnosis and Treatment of Primary Headache Disorders in Older Adults. J Am Geriatr Soc. 2018;66:2408–2416. doi: 10.1111/jgs.15586. [DOI] [PubMed] [Google Scholar]
- 7.Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia. 2008;28:614–618. doi: 10.1111/j.1468-2982.2008.01592.x. [DOI] [PubMed] [Google Scholar]
- 8.Stovner LJ, Hagen K, Linde M, Steiner TJ. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. 2022;23:34. doi: 10.1186/s10194-022-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, Alahdab F, Amit AML, Bärnighausen TW, et al. Burden of Neurological Disorders Across the US From 1990–2017: A Global Burden of Disease Study. JAMA Neurol 2021;78:165–76. 10.1001/jamaneurol.2020.4152 [DOI] [PMC free article] [PubMed]
- 10.Yu S, Liu R, Zhao G, Yang X, Qiao X, Feng J, et al. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache. 2012;52:582–591. doi: 10.1111/j.1526-4610.2011.02061.x. [DOI] [PubMed] [Google Scholar]
- 11.Lyngberg AC, Rasmussen BK, Jørgensen T, Jensen R. Has the prevalence of migraine and tension-type headache changed over a 12-year period? A Danish population survey. Eur J Epidemiol. 2005;20:243–249. doi: 10.1007/s10654-004-6519-2. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz BS, Stewart WF, Simon D, Lipton RB. Epidemiology of tension-type headache. JAMA. 1998;279:381–383. doi: 10.1001/jama.279.5.381. [DOI] [PubMed] [Google Scholar]
- 13.Crystal SC, Robbins MS. Epidemiology of tension-type headache. Curr Pain Headache Rep. 2010;14:449–454. doi: 10.1007/s11916-010-0146-2. [DOI] [PubMed] [Google Scholar]
- 14.Burch R. Migraine and Tension-Type Headache: Diagnosis and Treatment. Med Clin North Am. 2019;103:215–233. doi: 10.1016/j.mcna.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Dodick DW. Migraine Lancet. 2018;391:1315–1330. doi: 10.1016/S0140-6736(18)30478-1. [DOI] [PubMed] [Google Scholar]
- 16.Al-Karagholi MA, Peng K-P, Petersen AS, De Boer I, Terwindt GM, Ashina M. Debate: Are cluster headache and migraine distinct headache disorders? J Headache Pain. 2022;23:151. doi: 10.1186/s10194-022-01504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund NLT, Snoer AH, Jensen RH. The influence of lifestyle and gender on cluster headache. Curr Opin Neurol. 2019;32:443–448. doi: 10.1097/WCO.0000000000000680. [DOI] [PubMed] [Google Scholar]
- 18.Rehm J, Shield KD. Alcohol Use and Cancer in the European Union. Eur Addict Res. 2021;27:1–8. doi: 10.1159/000507017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global status report on alcohol and health 2018 n.d. https://www.who.int/publications-detail-redirect/9789241565639 (accessed July 22, 2023)
- 20.Ramstedt M. Alcohol and fatal accidents in the United States–a time series analysis for 1950–2002. Accid Anal Prev. 2008;40:1273–1281. doi: 10.1016/j.aap.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blazer DG, Wu L-T. The epidemiology of alcohol use disorders and subthreshold dependence in a middle-aged and elderly community sample. Am J Geriatr Psychiatry. 2011;19:685–694. doi: 10.1097/JGP.0b013e3182006a96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muñoz M, Ausín B, Santos-Olmo AB, Härter M, Volkert J, Schulz H. Alcohol use, abuse and dependence in an older European population: Results from the MentDis_ICF65+ study. PLoS One. 2018;13:e0196574. doi: 10.1371/journal.pone.0196574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilsnack RW, Wilsnack SC, Kristjanson AF, Vogeltanz-Holm ND, Gmel G. Gender and alcohol consumption: patterns from the multinational GENACIS project. Addiction. 2009;104:1487–1500. doi: 10.1111/j.1360-0443.2009.02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray K, Murphy C, Herlihy A, McCaffrey J, Codd M, Murray FE. Harmful alcohol consumption in elite sports players in Ireland. Ir J Med Sci. 2022;191:2091–2098. doi: 10.1007/s11845-021-02819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safiri S, Nejadghaderi SA, Karamzad N, Carson-Chahhoud K, Bragazzi NL, Sullman MJM, et al. Global, regional, and national cancer deaths and disability-adjusted life-years (DALYs) attributable to alcohol consumption in 204 countries and territories, 1990–2019. Cancer. 2022;128:1840–1852. doi: 10.1002/cncr.34111. [DOI] [PubMed] [Google Scholar]
- 26.Kaltseis K, Frank F, Bernar B, Kiechl S, Winder B, Kiechl-Kohlendorfer U, et al. Primary headache disorders in adolescents in North- and South-Tyrol: Findings of the EVA-Tyrol-Study. Cephalalgia. 2022;42:993–1004. doi: 10.1177/03331024221088997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebedeva ER, Kobzeva NR, Gilev DV, Olesen J. Factors Associated with Primary Headache According to Diagnosis, Sex, and Social Group. Headache. 2016;56:341–356. doi: 10.1111/head.12757. [DOI] [PubMed] [Google Scholar]
- 28.Jeyagurunathan A, Abdin E, Vaingankar JA, Chua BY, Shafie S, Chang SHS, et al. Prevalence and comorbidity of migraine headache: results from the Singapore Mental Health Study 2016. Soc Psychiatry Psychiatr Epidemiol. 2020;55:33–43. doi: 10.1007/s00127-019-01755-1. [DOI] [PubMed] [Google Scholar]
- 29.Roy R, Sánchez-Rodríguez E, Galán S, Racine M, Castarlenas E, Jensen MP, et al. Factors Associated with Migraine in the General Population of Spain: Results from the European Health Survey 2014. Pain Med. 2019;20:555–563. doi: 10.1093/pm/pny093. [DOI] [PubMed] [Google Scholar]
- 30.Milde-Busch A, Blaschek A, Borggräfe I, Heinen F, Straube A, von Kries R. Associations of diet and lifestyle with headache in high-school students: results from a cross-sectional study. Headache. 2010;50:1104–1114. doi: 10.1111/j.1526-4610.2010.01706.x. [DOI] [PubMed] [Google Scholar]
- 31.Hindiyeh NA, Zhang N, Farrar M, Banerjee P, Lombard L, Aurora SK. The Role of Diet and Nutrition in Migraine Triggers and Treatment: A Systematic Literature Review. Headache. 2020;60:1300–1316. doi: 10.1111/head.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Huang Q, Li N, Tan G, Chen L, Zhou J. Triggers of migraine and tension-type headache in China: a clinic-based survey. Eur J Neurol. 2013;20:689–696. doi: 10.1111/ene.12039. [DOI] [PubMed] [Google Scholar]
- 33.Schürks M, Kurth T, de Jesus J, Jonjic M, Rosskopf D, Diener H-C. Cluster headache: clinical presentation, lifestyle features, and medical treatment. Headache. 2006;46:1246–1254. doi: 10.1111/j.1526-4610.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 34.Harnod T, Lin C-L, Kao C-H. Survival outcome and mortality rate in patients with migraine: a population-based cohort study. J Headache Pain. 2018;19:57. doi: 10.1186/s10194-018-0889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vives-Mestres M, Casanova A, Puig X, Ginebra J, Rosen N. Alcohol as a trigger of migraine attacks in people with migraine. Results from a large prospective cohort study in English-speaking countries. Headache. 2022;62:1329–38. doi: 10.1111/head.14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinberg A, Fourier C, Ran C, Waldenlind E, Sjöstrand C, Belin AC. Cluster headache - clinical pattern and a new severity scale in a Swedish cohort. Cephalalgia. 2018;38:1286–1295. doi: 10.1177/0333102417731773. [DOI] [PubMed] [Google Scholar]
- 37.Hagen K, Åsberg AN, Stovner L, Linde M, Zwart JA, Winsvold BS, et al. Lifestyle factors and risk of migraine and tension-type headache. Follow-up data from the Nord-Trøndelag Health Surveys 1995–1997 and 2006–2008. Cephalalgia. 2018;38:1919–26. doi: 10.1177/0333102418764888. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama M, Suzuki N, Yokoyama T, Yokoyama A, Funazu K, Shimizu T, et al. Interactions between migraine and tension-type headache and alcohol drinking, alcohol flushing, and hangover in Japanese. J Headache Pain. 2012;13:137–145. doi: 10.1007/s10194-011-0413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casanova A, Vives-Mestres M, Donoghue S, Mian A, Wöber C. The role of avoiding known triggers, embracing protectors, and adhering to healthy lifestyle recommendations in migraine prophylaxis: Insights from a prospective cohort of 1125 people with episodic migraine. Headache. 2023;63:51–61. doi: 10.1111/head.14451. [DOI] [PubMed] [Google Scholar]
- 40.Niland P, Lyons AC, Goodwin I, Hutton F. “Everyone can loosen up and get a bit of a buzz on”: young adults, alcohol and friendship practices. Int J Drug Policy. 2013;24:530–537. doi: 10.1016/j.drugpo.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Kilibarda B, Mladenović I, Rakić JG. Attitudes on alcohol and drinking patterns among youth in Serbia. Srp Arh Celok Lek. 2013;141:66–71. doi: 10.2298/sarh1302066k. [DOI] [PubMed] [Google Scholar]
- 42.Lee CM, Maggs JL, Neighbors C, Patrick ME. Positive and negative alcohol-related consequences: associations with past drinking. J Adolesc. 2011;34:87–94. doi: 10.1016/j.adolescence.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLOS Medicine. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moola S, Munn Z, Sears K, Sfetcu R, Currie M, Lisy K, et al. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. Int J Evid Based Healthc. 2015;13:163–169. doi: 10.1097/XEB.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 45.Lisicki M, Schoenen J. Old Habits Die Hard: Dietary Habits of Migraine Patients Challenge our Understanding of Dietary Triggers. Front Neurol. 2021;12:748419. doi: 10.3389/fneur.2021.748419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YH, Lee JW, Kim Y, Bae JS, Kim YJ, Min C, et al. Bidirectional association between migraine and rheumatoid arthritis: two longitudinal follow-up studies with a national sample cohort. BMJ Open. 2021;11:e046283. doi: 10.1136/bmjopen-2020-046283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SJ, Park SM, Cho HJ, Park JW. Primary headaches increase the risk of dementias: An 8-year nationwide cohort study. PLoS One. 2022;17:e0273220. doi: 10.1371/journal.pone.0273220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schramm S, Tenhagen I, Schmidt B, Holle-Lee D, Naegel S, Katsarava Z, et al. Prevalence and risk factors of migraine and non-migraine headache in older people - results of the Heinz Nixdorf Recall study. Cephalalgia. 2021;41:649–664. doi: 10.1177/0333102420977183. [DOI] [PubMed] [Google Scholar]
- 49.Aamodt AH, Stovner LJ, Hagen K, Bråthen G, Zwart J. Headache prevalence related to smoking and alcohol use. The Head-HUNT Study Eur J Neurol. 2006;13:1233–1238. doi: 10.1111/j.1468-1331.2006.01492.x. [DOI] [PubMed] [Google Scholar]
- 50.Kjaergaard M, Eggen AE, Mathiesen EB, Jorde R. Association between headache and serum 25-hydroxyvitamin D: the Tromsø Study: Tromsø 6. Headache. 2012;52:1499–1505. doi: 10.1111/j.1526-4610.2012.02250.x. [DOI] [PubMed] [Google Scholar]
- 51.Misakian AL, Langer RD, Bensenor IM, Cook NR, Manson JE, Buring JE, et al. Postmenopausal hormone therapy and migraine headache. J Womens Health (Larchmt) 2003;12:1027–1036. doi: 10.1089/154099903322643956. [DOI] [PubMed] [Google Scholar]
- 52.Luo J. Association between migraine and anxiety symptoms: Results from the study of women’s health across the nation. J Affect Disord. 2021;295:1229–1233. doi: 10.1016/j.jad.2021.09.036. [DOI] [PubMed] [Google Scholar]
- 53.Cheon DY, Han K, Yang YS, Kim Y, Lee S-H, Kim C, et al. Associations between migraine and major cardiovascular events in type 2 diabetes mellitus. Cardiovasc Diabetol. 2022;21:275. doi: 10.1186/s12933-022-01705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Özcan RK, Özmen SG. The Association Between Migraine, Metabolic Syndrome, Insulin Resistance, and Obesity in Women: A Case-Control Study. Sisli Etfal Hastan Tip Bul. 2019;53:395–402. doi: 10.14744/SEMB.2018.09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarker MAB, Rahman M, Harun-Or-Rashid M, Hossain S, Kasuya H, Sakamoto J, et al. Association of smoked and smokeless tobacco use with migraine: a hospital-based case-control study in Dhaka. Bangladesh Tob Induc Dis. 2013;11:15. doi: 10.1186/1617-9625-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le H, Tfelt-Hansen P, Skytthe A, Kyvik KO, Olesen J. Association between migraine, lifestyle and socioeconomic factors: a population-based cross-sectional study. J Headache Pain. 2011;12:157–172. doi: 10.1007/s10194-011-0321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schramm SH, Moebus S, Lehmann N, Galli U, Obermann M, Bock E, et al. The association between stress and headache: A longitudinal population-based study. Cephalalgia. 2015;35:853–863. doi: 10.1177/0333102414563087. [DOI] [PubMed] [Google Scholar]
- 58.Kim B-S, Chung C-S, Lee C-B, Rhee P-L. Migraineurs Initially Visiting the Gastroenterology Department. Headache. 2016;56:555–563. doi: 10.1111/head.12775. [DOI] [PubMed] [Google Scholar]
- 59.Ohn K, Han K, Moon JI, Jung Y. Presence and severity of migraine is associated with development of primary open angle glaucoma: A population-based longitudinal cohort study. PLoS One. 2023;18:e0283495. doi: 10.1371/journal.pone.0283495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gür-Özmen S, Karahan-Özcan R. Iron Deficiency Anemia Is Associated with Menstrual Migraine: A Case-Control Study. Pain Med. 2016;17:596–605. doi: 10.1093/pm/pnv029. [DOI] [PubMed] [Google Scholar]
- 61.Wienholtz NKF, Christensen CE, Haugaard JH, Zhang DG, Ashina M, Thyssen JP, et al. Cohort profile: COpenhagen ROsacea COhort (COROCO) and COpenhagen MIgraine COhort (COMICO) BMJ Open. 2020;10:e039445. doi: 10.1136/bmjopen-2020-039445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin Q-F, Xia Q-Q, Zeng Y-L, Wu X-Y, Ye L-F, Yao L-T, et al. Prevalence of migraine in Han Chinese of Fujian province: An epidemiological study. Medicine (Baltimore) 2018;97:e13500. 10.1097/MD.0000000000013500 [DOI] [PMC free article] [PubMed]
- 63.Schiavo JH. PROSPERO: An International Register of Systematic Review Protocols. Med Ref Serv Q. 2019;38:171–180. doi: 10.1080/02763869.2019.1588072. [DOI] [PubMed] [Google Scholar]
- 64.Yoon M-S, Manack A, Schramm S, Fritsche G, Obermann M, Diener H-C, et al. Chronic migraine and chronic tension-type headache are associated with concomitant low back pain: results of the German Headache Consortium study. Pain. 2013;154:484–492. doi: 10.1016/j.pain.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 65.Ulrich V, Olesen J, Gervil M, Russell MB. Possible risk factors and precipitants for migraine with aura in discordant twin-pairs: a population-based study. Cephalalgia. 2000;20:821–825. doi: 10.1046/j.1468-2982.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- 66.Pellegrino Baena C, Goulart AC, Santos I de S, Suemoto CK, Lotufo PA, Bensenor IJ. Migraine and cognitive function: Baseline findings from the Brazilian Longitudinal Study of Adult Health: ELSA-Brasil. Cephalalgia 2018;38:1525–34. 10.1177/0333102417737784 [DOI] [PubMed]
- 67.Hinnell C, Williams J, Metcalfe A, Patten SB, Parker R, Wiebe S, et al. Health status and health-related behaviors in epilepsy compared to other chronic conditions–a national population-based study. Epilepsia. 2010;51:853–861. doi: 10.1111/j.1528-1167.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 68.McMurtray AM, Saito EK, Diaz N, Mehta B, Nakamoto B. Greater frequency of depression associated with chronic primary headaches than chronic post-traumatic headaches. Int J Psychiatry Med. 2013;45:227–236. doi: 10.2190/PM.45.3.b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schramm SH, Obermann M, Katsarava Z, Diener H-C, Moebus S, Yoon M-S. Epidemiological profiles of patients with chronic migraine and chronic tension-type headache. J Headache Pain. 2013;14:40. doi: 10.1186/1129-2377-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wöber C, Holzhammer J, Zeitlhofer J, Wessely P, Wöber-Bingöl C. Trigger factors of migraine and tension-type headache: experience and knowledge of the patients. J Headache Pain. 2006;7:188–195. doi: 10.1007/s10194-006-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scher AI, Terwindt GM, Picavet HSJ, Verschuren WMM, Ferrari MD, Launer LJ. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology. 2005;64:614–620. doi: 10.1212/01.WNL.0000151857.43225.49. [DOI] [PubMed] [Google Scholar]
- 72.Rafi A, Islam S, Hasan MT, Hossain G. Prevalence and impact of migraine among university students in Bangladesh: findings from a cross-sectional survey. BMC Neurol. 2022;22:68. doi: 10.1186/s12883-022-02594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liampas I, Papathanasiou S, Tsikritsis N, Roka V, Roustanis A, Ntontos T, et al. Nutrient Status in Patients with Frequent Episodic Tension-Type Headache: A Case-Control Study. Rev Neurol (Paris) 2021;177:1283–1293. doi: 10.1016/j.neurol.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Rasmussen BK. Migraine and tension-type headache in a general population: precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain. 1993;53:65–72. doi: 10.1016/0304-3959(93)90057-V. [DOI] [PubMed] [Google Scholar]
- 75.Hirata K, Sano H, Kondo H, Shibasaki Y, Koga N. Clinical characteristics, medication use, and impact of primary headache on daily activities: an observational study using linked online survey and medical claims data in Japan. BMC Neurol. 2023;23:80. doi: 10.1186/s12883-023-03122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lund N, Petersen A, Snoer A, Jensen RH, Barloese M. Cluster headache is associated with unhealthy lifestyle and lifestyle-related comorbid diseases: Results from the Danish Cluster Headache Survey. Cephalalgia. 2019;39:254–263. doi: 10.1177/0333102418784751. [DOI] [PubMed] [Google Scholar]
- 77.Lambru G, Castellini P, Manzoni GC, Torelli P. Mode of occurrence of traumatic head injuries in male patients with cluster headache or migraine: Is there a connection with lifestyle? Cephalalgia. 2010;30:1502–1508. doi: 10.1177/0333102409359710. [DOI] [PubMed] [Google Scholar]
- 78.Dueland AN. Headache and Alcohol. Headache. 2015;55:1045–1049. doi: 10.1111/head.12621. [DOI] [PubMed] [Google Scholar]
- 79.Panconesi A, Bartolozzi ML, Mugnai S, Guidi L. Alcohol as a dietary trigger of primary headaches: what triggering site could be compatible? Neurol Sci. 2012;33(Suppl 1):S203–205. doi: 10.1007/s10072-012-1068-z. [DOI] [PubMed] [Google Scholar]
- 80.Panconesi A. Alcohol-induced headaches: Evidence for a central mechanism? J Neurosci Rural Pract. 2016;7:269–275. doi: 10.4103/0976-3147.178654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Panconesi A, Franchini M, Bartolozzi ML, Mugnai S, Guidi L. Alcoholic drinks as triggers in primary headaches. Pain Med. 2013;14:1254–1259. doi: 10.1111/pme.12127. [DOI] [PubMed] [Google Scholar]
- 82.Krymchantowski AV, da Cunha JC. Wine and headache. Headache. 2014;54:967–975. doi: 10.1111/head.12365. [DOI] [PubMed] [Google Scholar]
- 83.Domingues RB, Domingues SA, Lacerda CB, Machado TVC, Duarte H, Teixeira AL. Alcohol use problems in migraine and tension-type headache. Arq Neuropsiquiatr. 2014;72:24–27. doi: 10.1590/0004-282X20130186. [DOI] [PubMed] [Google Scholar]
- 84.Domingues RB, Domingues SA. Headache is associated with lower alcohol consumption among medical students. Arq Neuropsiquiatr. 2011;69:620–623. doi: 10.1590/s0004-282x2011000500009. [DOI] [PubMed] [Google Scholar]
- 85.Mostofsky E, Bertisch SM, Vgontzas A, Buettner C, Li W, Rueschman M, et al. Prospective cohort study of daily alcoholic beverage intake as a potential trigger of headaches among adults with episodic migraine. Ann Med. 2020;52:386–392. doi: 10.1080/07853890.2020.1758340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferreira MP, Willoughby D. Alcohol consumption: the good, the bad, and the indifferent. Appl Physiol Nutr Metab. 2008;33:12–20. doi: 10.1139/H07-175. [DOI] [PubMed] [Google Scholar]
- 87.Masip J, Germà Lluch JR. Alcohol, health and cardiovascular disease. Rev Clin Esp (Barc) 2021;221:359–368. doi: 10.1016/j.rceng.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Hendriks HFJ. Alcohol and Human Health: What Is the Evidence? Annu Rev Food Sci Technol. 2020;11:1–21. doi: 10.1146/annurev-food-032519-051827. [DOI] [PubMed] [Google Scholar]
- 89.Stephens AN, Bishop CA, Liu S, Fitzharris M. Alcohol consumption patterns and attitudes toward drink-drive behaviours and road safety enforcement strategies. Accid Anal Prev. 2017;98:241–251. doi: 10.1016/j.aap.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 90.Panconesi A, Bartolozzi ML, Guidi L. Alcohol and migraine: what should we tell patients? Curr Pain Headache Rep. 2011;15:177–184. doi: 10.1007/s11916-011-0184-4. [DOI] [PubMed] [Google Scholar]
- 91.Malu OO, Bailey J, Hawks MK. Cluster Headache: Rapid Evidence Review. Am Fam Physician. 2022;105:24–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.