Abstract
Background
Multiple Sclerosis (MS) is a chronic debilitating disease that targets the central nervous system. Globally it is estimated that 2.8 million people live with MS (2018) and as there is no known cure; therefore, identifying methods to increase a patient’s quality of life (QoL) is of considerable importance. Non-pharmacological interventions are a viable and effective option to increase QoL in patients with MS, however, to date, the literature lacks a complete systematic review of these interventions.
Methods
A literature search was conducted for studies published up until March 4th 2022 in Scopus, Web of Science, CINAHL Plus, The Cochrane Library, Medline, and Embase. Studies were included if they were randomized control trials (RCTs) assessing a non-pharmacological intervention in adults with MS and measured QoL using the MSQOL-54, SF-36 or MSQLI tools for at least two time points. Quality assessment of each study was completed as well as a review of publication bias. Where possible, meta-analysis was conducted using a random effects model and for other studies a qualitative synthesis was presented.
Results
Thirty studies were included in the meta-analysis and eleven studies were summarized qualitatively. The pooled effects across all non-pharmacological interventions showed a modest improvement in both the physical and mental components of QoL, with a standardized mean difference (SMD) of 0.44 (95% CI 0.26–0.61) and 0.42 (95% CI 0.24–0.60), respectively. Non-pharmacological interventions based around a physical activity were found to be particularly effective in improving both the physical composite score (PCS) and mental composite score (MCS), with an SMD of 0.40 (95% CI 0.14–0.66) and 0.31 (95% CI 0.08–0.55), respectively. Interventions incorporating balance exercises presented a significant advantageous solution for improving QoL, with an SMD of 1.71 (95% CI 1.22, 2.20) and 1.63(95% CI 1.15–2.12) for PCS and MCS respectively.
Conclusions
This systematic review and meta-analysis identified that non-pharmacological interventions can be an effective method of improving QoL in patients with MS, especially modalities with a physical activity component and balance interventions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-023-01185-5.
Keywords: Multiple sclerosis, Quality of life, Non-pharmacological therapies, Physical activity, Behavioral interventions, Psychological interventions, Systematic review, Meta-analysis
Background
Multiple Sclerosis (MS) is a chronic and debilitating neurological disease characterized by an individual’s immune cells attacking conductive myelin sheaths in the central nervous system (CNS) [1]. This leads to impairment in electrical nerve signaling, causing varying degrees of disability and neurodegeneration [1]. The risk of development and progression of MS can be decreased by modifying certain lifestyle factors including ultraviolet light exposure, vitamin D intake, weight loss, fish oil consumption and smoking cessation [2]. Globally, the mean age of diagnosis is 32 years old and there is no cure, meaning it is a lifelong condition affecting an individual’s peak productive years [3]. For this reason, treatment for MS is focused on ensuring that patients live a life of relatively good quality and maintain health in aspects important to them [4–6].
Health-related quality of life (HRQoL) is a concept used to represent a person’s perception of their health status [7]. It is a broad and holistic term that considers the physical, mental, social, and functional aspects of an individual's health at a point in time [7]. HR-QoL is measured through standardized tools and instruments, offering a quantitative method to monitor an individual’s health status in response to intervention changes over time. The MSQOL-54, SF-36 or MSQLI are widely validated and utilized tools for measuring HRQoL in MS [8–10]. MS patients find physical functioning, role limitation, vitality, general health, and the presence of bodily pain, predominant contributors to their QoL [10]. Furthermore, a patient’s perception of their own QoL is predictive of future disease progression and disability [3, 11].
Global MS prevalence has increased from 2.3 million in 2013 to 2.8 million in 2020, likely in part due to improved survival [12]. As MS prevalence increases, it is of critical importance to identify interventions that can increase the QoL of affected individuals [3]. This study aims to synthesize the available evidence and determine, quantitatively, the effect that non-pharmacological interventions have on QoL; with the goal of informing clinical practice and improving QoL in adults living with MS.
Methods
Search strategy
This systematic review and meta-analysis utilized the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [13]. A systematic search reviewed all peer reviewed articles in English from inception of the database until March 4, 2022. Scopus, Web of Science, Cumulative Index of Nursing and Allied Health Literature (CINAHL Plus), The Cochrane Library, Medline and Embase were searched. Articles were selected based on broad keywords identified within the title and abstract of the publications. The following keywords and Boolean search criteria were implemented (‘multiple sclerosis’ OR ‘encephalomyelitis disseminate’ OR ‘demyelinating’) AND (‘Health Status Questionnaire’ OR ‘SF-36’ OR ‘Multiple Sclerosis Quality of Life-54’ OR ‘MSQOL-54’ OR ‘Multiple sclerosis quality of life inventory’ OR ‘MSQLI’). The full search strings are available in Additional file 1 for each database. The search was limited to three validated QoL measurement tools, the Multiple Sclerosis quality of life inventory (MSQLI), MSQOL-54 and SF-36. The search was not expanded to other validated tools as they were judged to differ widely in aspects such as their complexity, aspects of QoL measured, completion time and recall period. Moreover, some of the commonly used MS specific QoL tools measure aspects that are not covered in others making them not directly comparable instruments [9].
Inclusion criteria
All articles were independently evaluated by two reviewers to assess eligibility and disagreements were discussed, as required, with a third reviewer to decide. We only included articles which performed studies on adult patients with MS and did not focus on those with additional comorbidities (Table 1). If patients within the study had other comorbidities the study was still included unless the particular co-morbidity was an inclusion criterion to participate. Studies evaluating the impact of acute clinical care, such as the evaluation of nursing practices, were not included. We define ‘non-pharmacological’ as any intervention used to improve quality of life without a pharmaceutical or surgical modality (dietary supplements, vitamins and nutraceuticals were excluded from the definition of a pharmaceutical agent). We categorized non-pharmacological interventions into five broad categories; physical activity, behavioral or psychological, tissue manipulation, nutraceuticals/supplement, diet, and other non-pharmacological interventions.
Table 1.
Summary of inclusion and exclusion criteria
| Inclusion | Exclusion |
|---|---|
| English language | Not in English or partial content in English |
| Randomized control study published in peer-reviewed journals in any region of the world | Case studies, cross-sectional designs, cohort studies, quasi-experimental designs or cross-over designs |
| Non-pharmacological interventions | Pharmacological, acute clinical care, or Surgical interventions |
| Adults (≥ 18 y.o) | Children (< 18 y.o) |
| Patients with a clinical diagnosis of multiple sclerosis—all types and severities | Patients with additional comorbidities as specified in the inclusion criteria of the study |
| Studies measuring of Quality of Life at baseline and at least one time point after, for both arms utilizing MSQOL-54, SF-36 or the MSQLI | Studies not providing sufficient statistical data on pre- and post-QoL test scores such as SD, SE or P-values |
| Full text manuscript/publication | Abstracts, reviews, conference presentations |
For the meta-analysis, we only included studies with a comparator arm including no intervention, usual/standard care (such as the continuation of a previous intervention), placebo, or a minor non-active intervention (such as education materials). Studies with multiple arms not containing a control group were reviewed and synthesized separately from the meta-analysis portion.
This review only included randomized control studies. For both the intervention and control arm, studies had to measure QoL at baseline and at another time point. Data on pre- and post-QoL scores such as mean, standard deviation (SD), standard error (SE), confidence intervals, or P-values had to be provided. Cross-over designs were not included due to (i) the potential issue of a carry-over effect which could confound the result and (ii) the difficulty in accounting for these long-term effects [14].
Data extraction
The following information was extracted from the studies: study author, year of publication, country-region of the study, whether the study was conducted in a single center or multicenter, sample size, participants’ MS type, inclusion criteria for the Kurtzke Expanded Disability Status Scale (EDSS), mean age and range of participants, percentage of female participants, a description of the intervention and control, frequency and duration of the intervention, QoL measurement tool used, a statement summarizing the main results related to QoL changes, and metrics associated with the results (pre- and post-scores, mean difference, SD, SE, etc.). If QoL was measured at multiple time points after baseline only the last reading was extracted. The mean difference between two timepoints was computed as the arithmetic difference, and a pooled SD was calculated using the initial and follow-up time points assuming normal distribution. The pooled SD was calculated by summing the variances of each time point and taking the square root.
There were two main outcomes of interest related to the measurement tool post intervention (SF-36 and MSQOL-54); the reported change in the physical composite score (PCS) and the reported change in the mental composite score (MCS). The PCS is a representation of role limitations due to physical health, including bodily pain, energy/fatigue, sexual function, social function, and health distress, and the MCS is a representation of role limitations due to mental health, including overall quality of life, emotional well-being, social function and vitality [15].
Data synthesis
Studies included in the meta-analysis portion of the review were assessed using the standardized mean difference between the intervention and control arm as the chosen effect size. For those studies not reporting a PCS score, the physical functioning score was used as it contributes the most weight to the scoring of the PCS [15, 16]. Similarly for the MCS score, the mental health score or emotional well-being subscale was used in cases where it was not available [15, 16].
A random effect model was selected to accommodate the likely heterogeneity in the populations included in the studies. The meta-analysis was stratified by the type of non-pharmacological intervention and done for both the PCS and MCS separately utilizing RevMan 5.4.1 [17]. A pooled effect for each non-pharmacological subgroup was calculated with 95% confidence intervals and a corresponding overall effect across all studies was also performed. An I2 statistic was calculated for each non-pharmacological intervention subgroup as well as overall to assess heterogeneity. I2 statistics of 0–40%, 30–60%, 50–90% and 75–100% were considered as; might not be important, moderate heterogeneity, substantial heterogeneity and considerable heterogeneity, respectively [18]. A sensitivity analysis was incorporated by excluding a study from each subgroup and examining its effect on the I2 statistic and SMD. Publication bias was analyzed and discussed by visual inspection of funnel plots. For those studies for which the mean was not within the 95% CI of the overall effect as per the forest plot, a t-test was performed using STATA 15.1, with a null hypothesis that the SMD in that study was equal to the overall/pooled standardized mean difference observed across all studies [19].
Quality assessment
Studies included were evaluated using the Joanna Briggs Institute (JBI) critical appraisal checklist for randomized control trials (RCTs), a tool used to measure the methodological quality and risk of bias of a study [20, 21]. The completed JBI checklist is available in Additional file 2. Studies were deemed of insufficient quality and excluded if greater than 6 questions on the checklist were answered ‘No’.
Results
Literature search results
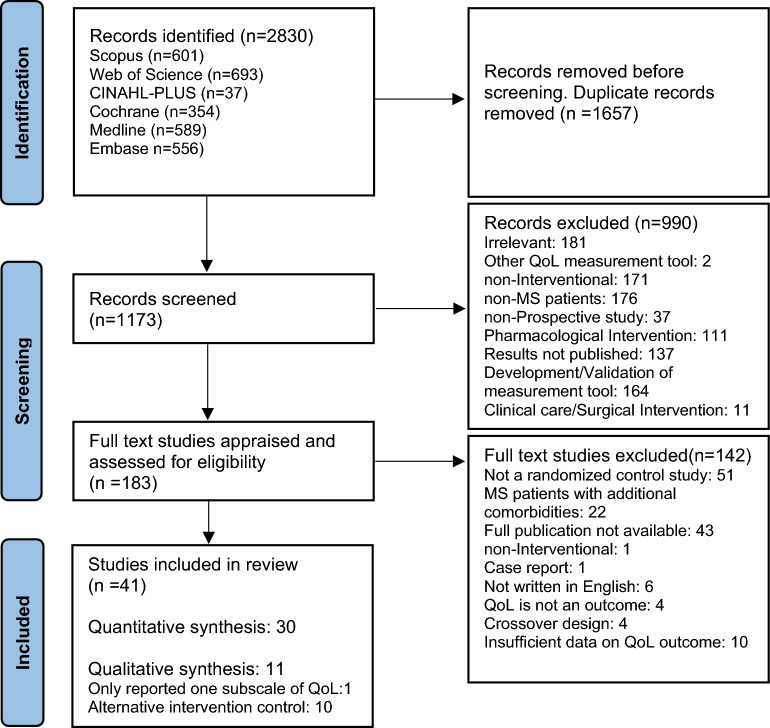
A total of 2830 articles were identified from Scopus, Web of Science, CINAHL Plus, The Cochrane Library, Medline and Embase. Of these 1657 were identified as duplicate reports via Mendeley’s duplicate identification tool and through manual review. An additional 990 studies were excluded by two reviewers after reviewing the abstracts and titles. Full text review was conducted for the remaining 183 studies and of these 41 were deemed eligible to include in the current review (Fig. 1).
Fig. 1.
PRISMA flow diagram detailing the selection process for review and inclusion of studies into the systematic review and meta-analysis
Study characteristics
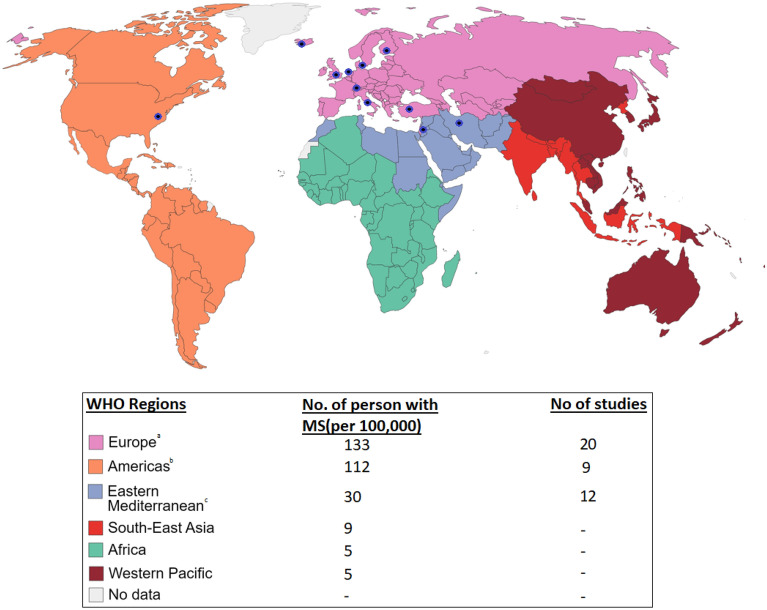
For the quantitative review, there were 30 applicable studies identified published between 2002 and 2021. The duration of therapy ranged from 3 days to 6 months with 80% of studies being exposed to the non-pharmacological intervention for greater than 2 months. Sample sizes ranged from 11 to 169 participants and the total number of participants across all studies included was 2089. The most common study setting was Iran, 11, then there were 6 studies from America, 3 from the United Kingdom, 3 from Italy and the remaining 7 studies were done in Germany, Iceland, Netherlands, Denmark, Finland and Turkey (Fig. 2). 66% of studies used MSQOL-54, an MS-specific instrument to measure HRQoL and the rest utilized SF-36, a generic HRQoL measurement tool. The average age of participants was 42.1 years old and the average percentage of females in a study was 78% (range 54–100%). Most studies, 77%, either did not preclude participation based on their EDSS score or had inclusion criteria less than 5.5 indicating the patient’s ability to walk without aid or rest for 100 m [22]. The remaining 23% of studies had inclusion criteria with EDSS scores > 5.5 indicating the needs for assistance and a more severe disability [22]. Further details of each included study as well as a summary of their main results related to the QoL outcome are presented in Table 2.
Fig. 2.
World map showing the six WHO regions [71] and the corresponding prevalence of MS [12] and number of studies included from each. aThere were 8 studies from Italy, 3 studies from the United Kingdom, 2 studies from Germany, 2 studies from Denmark, 1 study from Iceland, 1 study from The Netherlands, 1 study from Finland, 1 study from Switzerland, and 1 study from Turkey. bThere were 9 studies from the United States of America. cThere were 11 studies from Iran and 1 study from Jordan
Table 2.
Summary of the characteristics of studies included in the meta-analysis investigating the effects of non-pharmacological interventions on health-related quality of life (HRQoL)—categorized by type of non-pharmacological intervention
| Author/year | Region | Sample size (Ix/Cx)a | Participants | Mean age (range) | % Female | Intervention group | Control group | Duration | HRQoL tool | Summary of main outcome related to HRQoL |
|---|---|---|---|---|---|---|---|---|---|---|
| Physical activity interventions | ||||||||||
| Ahadi 2013 [23] | Iran—Single Center | 31 (10: Treadmill, 11: Yoga, 10: Control) | MS EDSS Score 1–4 | 34 (19–54) | 100 | Treatment 1: treadmill training consisted of 24 sessions of treadmill training (30 min), at 40–75% of age-predicted maximum heart rate. Treatment 2: Yoga group subjects participated in a thrice weekly 60–70-min sessions of Hatha yoga intervention | Followed own routine treatment program | 8 weeks | MSQOL-54 | The treadmill training program subjects showed a significant increase in the PCS. The yoga group also showed a significant increase in PCS (P = 0.02) and MCS (P = 0.00) |
| Ahmadi, Arastoo 2010 [24] | Iran—Single Center | 20 (10, 10) | MS EDSS Score 1–4 | 37 (19–54) | 100 | Treadmill training (30 min), at 40–75% of age-predicted maximum heart rate—3 times per week | Wait-list control | 8 weeks | MSQOL-54 | In the control group, there was no significant change in any of the MSQOL-54 scale scores. Differences between the treadmill training group and the control group were statistically significant in 5 items of the MSQOL-54 scale scores (physical function, pain, energy, health perception and physical health composite) |
| Ahmadi 2010 [45] | Iran—Single Center | 21 (11i, 10c) | MS EDSS Score 1–4 | 34 (19–54) | 100 | Hatha yoga classes 60–70 min 3 sessions per week | Wait-list control | 8 weeks | MSQOL-54 | Significant increase in some of MSQOL-54 scale scores in the yoga group (P ≤ 0.05). No changes for the control group. There was a significant difference found in both the PCS and MCS mean change between the intervention and control groups |
| Backus 2020 [25] | America—Atlanta—Single Center | 12 (6, 6) | MS EDSS Score 7–8.5 | 55 | 58 | Participants cycled volitionally with assistance from the electrical stimulation (Functional electrical stimulation: FES) as needed and with oversight for safety by the exercise staff. The goal was for participants to train three times a week | Wait list control | 3 months | MSQOL-54 | Significant increase in the physical health, health perception, health distress, and PCS in the training group |
| Barclay 2019 [26] | UK—Glasgow-Single Center | 24 (15i, 9c) | MS EDSS Score 6–8.5 | 54 | 63 | 30 min of lower limb cycling programme using active passive trainers (2 min passive warm up, 26 min active cycling, 2 min passive cool down), 5 days per week | Usual care | 5 weeks | MSQOL-54 | Significant increase in both groups in both PCS and MCS. A larger effect size was demonstrated for the intervention group (0.93) and medium effect size in the control group (0.46) |
| Bjarnadottir 2007 [27] | Iceland | 16 (6i, 10c) | MS EDSS Score < 4 | 37 | 63 | Outpatient aerobic and strength exercise program (60 min) three times a week | Usual care—no change from previous | 5 weeks | SF-36 | Significant increase in vitality and a trend toward improved QoL in 5 of 8 parameters of SF-36 |
| Carter 2013 [46] | UK—Sheffield—Single Center | 30 (16i, 14c) | MS EDSS Score ≤ 5.5 | 40 (24–49) | 87 | Pragmatic exercise intervention (2 × supervised and 1 × home-based session per week) | Usual care | 10 weeks | MSQOL-54 | Significant increase in QoL which was sustained for up to 3 months after the intervention |
| Dalgas 2010 [33] | Denmark—Single Center | 31 (16i, 15c) | MS EDSS Score 3–5.5 | 48 | 65 | Progressive resistance training of the lower extremities performed twice weekly | Usual activity | 3 months | SF-36 | PCS QoL was increase significantly more in the intervention arm and this was maintained at follow-up after further 12 weeks |
| Hebert 2018 [35] | America—Colorado—Single Center | 88 (44, 44) | MS | 45 | 85 | Balance and Eye-Movement Exercises for People with Multiple Sclerosis (BEEMS) was administered twice weekly with supervision and daily home exercise (phase 1) and in 1 supervised session weekly with daily home exercise (phase 2) | No treatment | 4 months | SF-36 | The BEEMS group showed a statistically greater increase in MCS compared to controls at 6 and 14 weeks |
| Jeong 2021 [32] | America-New York | 45 (29i, 16c) | MS EDSS Score 5.5–7.5 | 57 | 73 | Custom home exercise program with additional assistance by the telerehabilitation system | Usual care—custom daily home exercise plan | 3 months | MSQOL-54 | Patients in the telerehabilitation group showed significant improvement in pain and cognitive function symptoms in comparison with the control group |
| Kargarfard 2012 [47] | Iran—Isfahan | 21 (10i, 11c) | RRMS (relapse remitting MS) EDSS Score ≤ 3.5 | 33 | 100 | Supervised aquatic exercise in a swimming pool (3 times a week, each session lasting 60 min) | Usual care | 8 weeks | MSQOL-54 | Patients in the aquatic exercise group showed significant increases in QoL at 4 and 8 weeks compared with the control group |
| Langeskov-Christensen [34] | Denmark-multicentre | 86 (43, 43) | MS EDSS score 0–6 | 45 | 60 | Supervised progressive aerobic exercise (PAE) sessions with one continuous and one interval exercise session performed each week | Habitual lifestyle | 24 weeks | SF-36 | No differences in the SF-36 were found |
| Oken 2004 [36] | America—Oregon | 57 (15: Exercise, 22: Yoga, 20: control) | MS EDSS Score ≤ 6 | 49 | 93 | Group 1: weekly exercise class using a stationary bicycle along with home exercise Group 2: Weekly 90-min Iyengar yoga class along with home practice | Wait-list control | 6 months | SF-36 | Exercise and Yoga both significantly increase vitality on the SF-36 more as compared to the control group |
| Pappalardo 2016 [43] | Italy—Catania—Single Center | 146 (49: outpatient, 49: inpatient, 48: control) | MS EDSS Score 4–8 | 46 (25–74) | 64 | Group A—Outpatient rehabilitation: once daily, 6 days per week, each session 60 min Group B—Inpatient rehabilitation: twice-daily, 6 day per week, each session 60 min | Wait-list control | 5 weeks | SF-36 | Outpatient rehabilitation significantly improved all sub scales of the SF-36 and was found to be more effective at improving QoL than inpatient rehabilitation |
| Patti 2002 [44] | Italy—Catania—Single Center | 111 (58i, 53c) | MS EDSS Score 4–8 | 46 (25–60) | 58 | Outpatient rehabilitation program, 6 days a week | Wait-list control | 6 weeks | SF-36 | QoL increase significantly in the intervention group and this was seen at 6 and 12 weeks. The difference was significant between the intervention and control for all 8 subscales |
| Romberg 2005 [28] | Finland—Single Center | 95 (47i, 48c) | MS EDSS Score 1–5.5 | 44 | 64 | Progressive exercise program, mainly consisting of resistance training, it combined resistance training (3–4 times a week) with aerobic endurance training (once a week) | No treatment | 6 months | MSQOL-54 | “There was no effect seen in the MSQOL-54. The scores on the PCS and MCS of the MSQOL-54 were stable with no differences between groups” |
| Behavioral and psychological interventions | ||||||||||
| De Giglio 2015 [37] | Italy—Rome—Single Center | 35 (18i, 17c) | RRMS | 44 | 74 | Dr. Kawashima’s Brain Training (DKBT): How Old Is Your Brain video game 30 min/day, 5d/wk | No treatment | 8 weeks | MSQOL-54 | DBKT improved QoL significantly in the MCS, role limitations due to emotional problems, emotional wellbeing, and cognitive function when compared to the control |
| Jongen 2019 [48] | Netherlands—Single Center | 158 (79, 79) | RRMS EDSS score ≤ 4 | 40 | 88 | Intensive 3-day social cognitive treatment (can do treatment) | No treatment | 3 days | MSQOL-54 | In the intervention arm PCS and MCS were improved significantly at 1 month but this was not sustained at 3 and 6 months |
| Momenabadi 2019 [49] | Iran—Kermin—Single Center | 80 (40, 40) | RRMS EDSS score ≤ 5 | 30 (20–35) | 85 | 18 training sessions based on the main constructs of the health-promoting self-care behaviors system. Training class twice a week in 45–60 min sessions. In addition to holding in person training sessions, patients in the intervention group were followed up by phone calls and texts during the training period | No treatment | 3 months | MSQOL-54 | There was a significant increase in 14 subscales of QoL, MCS and PCS in the intervention arm and this was not observed in the control arm |
| O'Hara 2002 [38] | UK—Greater London | 169 (73i, 96c) | MS | 51 (28–81) | 70 | Self-care programme primarily comprised a discussion of self care strategies supported by an information booklet developed for the study in line with consumer priorities—2 discussion lasting 1–2 h over the month | No treatment | 1 month | SF-36 | There was a significant improvement in MCS in the intervention arm as compared to the control arm |
| Stuijbergen 2003 [39] | America—Community setting southwestern US | 113 (56i, 57c) | MS | 46 (25–60) | 100 | Lifestyle-change classes weekly for 90 min, then telephone follow-up | No treatment | 8 weeks and then telephone follow-up for 3 months | SF-36 | A significant improvement in the intervention arm was seen in mental health on the SF36 when compared to the control. There was no significant difference between the intervention and control for vitality, physical function, role–physical or role–emotional, social functioning, or general health |
| Nutraceutical/supplement interventions | ||||||||||
| Ashtari 2016 [29] | Iran | 94 (47, 47) | RRMS EDSS score ≤ 4 | 33 | 85 | 50,000 IU vitamin D3 every 5 days | Placebo | 3 months | MSQOL-54 | A significant increase in Mental Health QoL was seen in the intervention arm and this was not seen in the control arm |
| Etemadifar 2013 [50] | Iran—Isfahan—Single Center | 54 (26, 26) | RRMS EDSS score < 5 | 34 | 100 | 250-mg Korean ginseng tablets twice daily after breakfast and evening meal | Placebo | 3 months | MSQOL-54 | A significant increase in most of the domains of the MSQOL were seen in the intervention arm as compared to the control arm |
| Namjooyan 2019 [40] | Iran—Khuzestan | 51 (26i, 25c) | MS EDSS score 2–5.5 | 47 | 63 | Two parts of ajwain and one part of Iranian borago were soaked in water for 24 h and one part of cinnamon was soaked in water for 72 h then were distilled—patients given 15 cc total per day (split in four capsules per day) | Placebo | 3 months | MSQOL-54 | A significant increase was seen in the physical and mental components of QoL in the intervention arm as compared to the control arm and this was sustained at 3 months |
| Nozari 2019 [30] | Iran—single centre | 50 (25, 25) | RRMS | 30 | 54 | 5 mg daily folic acid tablets and three divided doses of 1 mg injective vitamin B12 | Placebo | 2 months | MSQOL-54 | A significant difference was seen in the physical and mental components of QoL in the intervention arm as compared to the control arm |
| Siahpoosh 2018 [51] | Iran—single centre | 66 (33, 33) | MS | 32 | 68 | Capsules with powder of grape seed extract (plus excipients 50 mg) twice a day | Placebo | 1 month | MSQOL-54 | A significant difference was seen in the physical and mental components of QoL in the intervention arm as compared to the control arm |
| Diet interventions | ||||||||||
| Moravejolahkami 2020 [41] | Iran—single centre | 147 (68i, 79c) | RRMS EDSS score ≤ 3 | 39 | 83 | A modified version of Mediterranean Diet (mMeD), based on higher intake of fresh fruits and vegetables, whole grains, monounsaturated fatty acids, fish, and low to moderate consumption of dairy products, meat, and poultry | Traditional Iranian diet | 6 months | MSQOL-54 | A significant increase was seen in the physical components of QoL in the intervention arm, however, this was not seen in the MCS |
| Tissue manipulation | ||||||||||
| Doğan 2021 [52] | Turkey-multi center | 66 (33, 33) | MS EDSS score < 5.5 | 38 | NA | Reflexology was applied on each patient in the intervention group for 3 sessions a week | No treatment | 3 months | MSQOL-54 | A significant increase in the combined physical and mental health scores were found in the intervention group |
| Other interventions | ||||||||||
| Shinto 2008 [31] | America—Oregon—Single Center | 45 (15, 15, 15) | RRMS EDSS score ≤ 6 | 44 | 87 | Intervention 1: naturopathic medicine which entailed 8 visits with a naturopath including daily supplements, Vitamins, diet intervention and counseling Intervention 2: MS education which entailed 8 visits with a nurse trained in MS care | Usual care | 6 months | SF-36 | “There were no significant differences between groups on any outcome measure” |
| Vermöhlen 2017 [42] | Germany—Multi Center | 56 (25i, 31c) | MS EDSS 4–6.5 | 51 | 81 | Hippotherapy once a week | Usual care | 3 months | MSQOL-54 | A significant increase was seen in the PCS and MCS in the intervention arm |
aIn the sample size column ‘i’ refers to the intervention arm and ‘c’ is in reference to the control arm
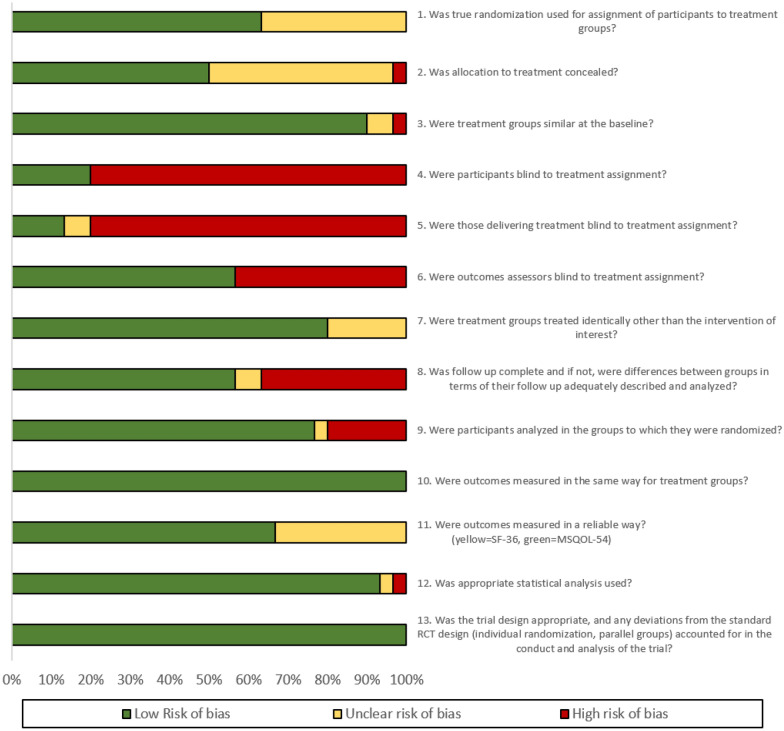
Methodological quality and risk of bias
A full review of the methodological quality and risk of bias for the 30 RCTs included in the meta-analysis is summarized in Fig. 3, further details are available in Additional file 2. Although all studies are RCTs, it was unclear if true random assignment was utilized for 11 of the studies [23–32]. The control arms and intervention arms were similar at baseline for 27 out of 30 studies. Differences between the intervention arm and control arm were in the majority (80%) of studies only attributable to the intervention assignment and not other factors such as baseline characteristics or follow-up frequency. For 11 studies there were differences between arms in the number of withdrawals and incomplete outcome data, and these differences were not sufficiently described [27, 33–42]. However, 23 studies did conduct an intention-to-treat analysis which examined the effects based on the initial allocation of a participant. Namjooyan et al. [40] did not calculate SD correctly and this was recalculated based on the confidence interval presented. No studies were excluded on the basis of low methodological quality or a high risk of bias.
Fig. 3.
Summary of methodological quality and risk of bias based on the JBI critical appraisal checklist for RCTs across all studies included in the meta-analysis
Effects of non-pharmacological intervention included in the meta-analysis
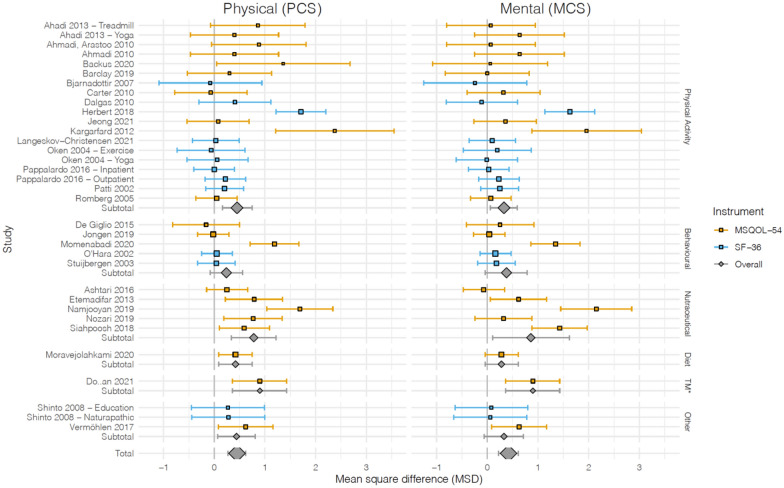
There were 16 physical activity intervention studies in the meta-analysis, and activities involved rehabilitations programs [43, 44], yoga [23, 36, 45], aerobic and strength exercises [26–28, 32–34, 46], aquatic exercise [47], balance and eye-movement exercises [35], treadmill training and cycling programs [24, 25, 36] (Fig. 4). The physical component of QoL saw an overall positive effect with an SMD of 0.40 (95% CI 0.14, 0.66), however, substantial heterogeneity was observed (I2 = 69%, P < 0.001). Hebert et al. [35] which utilized a specifically created regiment of balance and eye-movement exercises for people with MS (BEEMS) for 4 months showed a larger SMD of 1.71 (95% CI 1.22, 2.20). Kargarfard et al. [47] which utilized a supervised aquatic exercise program for 8 weeks also showed a larger SMD of 2.38 (1.21, 3.55). Performing a sensitivity analysis by excluding Hebert et al. still showed a significant pooled effect of physical activity interventions on the PCS, SMD 0.24 (95% CI 0.06, 0.42) with an (I2 = 32%, P = 0.09). An overall positive effect was also seen on the mental component of QoL with an SMD of 0.31 (95% CI 0.08, 0.55), however, substantial heterogeneity was observed (I2 = 62%, P < 0.001). Hebert et al. [35] and Kargarfard et al. [47] showed a larger SMD of 1.63 (95% CI 1.15, 2.12) and 1.96 (95% CI 0.88, 3.04) respectively. A sensitivity analysis excluding Hebert et al. still showed a significant pooled effect of physical activity interventions on the MCS, SMD to 0.18 (95% CI 0.04, 0.32) with an (I2 = 0%, P = 0.52). The changes in the I2 statistics indicate that most of the heterogeneity was likely due to Hebert et al. for both PCS and MCS.
Fig. 4.
Forest plots of non-pharmacological intervention effect on the physical health (left column) and mental health (right column) component of health-related quality of life domains. Point estimates indicate the mean and error bars indicate the 95% confidence interval. Blue and orange indicate that the study utilized the SF-36 and MSQOL-54 measurement tool, respectively. The mean value pointer size is scaled according to the sample size of each study
There were 5 behavioral and psychological interventions studies in the meta-analysis and activities included brain training programs [37], a short social cognitive treatment [48], self-care programs and lifestyle change classes [38, 39, 49]. The physical component of QoL did not see a significant overall effect with an SMD of 0.22 (95% CI − 0.19, 0.62) with substantial heterogeneity observed (I2 = 81%, P < 0.001). Momenabadi et al. [49] which investigated training sessions educating participants on health-promoting self-care behaviors lasting 4 months showed a significant effect on PCS, SMD of 1.19 (95% CI 0.71, 1.67). A sensitivity analysis excluding Momenabadi et al. still showed no significant pooled effect of behavioral/psychological interventions on the PCS, SMD 0.01 (95% CI − 0.17, 0.19) with an (I2 = 0%, P = 0.94). A significant overall effect was also not seen on the mental component of QoL with an SMD of 0.38 (95% CI − 0.04, 0.79) with substantial heterogeneity observed (I2 = 81%, P ≤ 0.001). Momenabadi et al. also showed a large significant effect on MCS, SMD of 1.35 (95% CI 0.86, 1.83). A sensitivity analysis excluding Momenabadi et al. still showed no significant pooled effect of behavioral/psychological interventions on the MCS, SMD 0.13 (95% CI − 0.05, 0.31) with an (I2 = 0%, P = 0.91). The changes in the I2 statistics indicate that almost all of the heterogeneity was likely due to Momenabadi et al. for both PCS and MCS.
There were 5 nutraceutical and supplements studies in the meta-analysis and included vitamin D [29], Korean ginseng tablets [50], folic acid tablets & vitamin B12 injections [30], grape seed extract capsules [51] and a traditional formulation containing cinnamon, ajwain and Iranian boragom [40]. The interventions ranged in duration from 1 to 3 months. An overall positive effect was seen on the physical component of QoL with an SMD of 0.78 (95% CI 0.34, 1.22), however, substantial heterogeneity was observed (I2 = 71%, P = 0.007). Ashtari et al. [29] was the only study within the subgroup that did not show a significant effect on PCS, SMD of 0.25 (95% CI − 0.15, 0.66). Performing a sensitivity analysis by excluding Namjooyan et al. [40] still showed a significant pooled effect of nutraceutical/supplement interventions on the PCS, SMD 0.54 (95% CI 0.28, 0.80) with an (I2 = 9%, P < 0.001). The change in the I2 statistic indicates that most of the heterogeneity was likely due to Namjooyan et al. An overall positive effect was also seen on the mental component of QoL with an SMD of 0.86 (95% CI 0.11, 1.62), however, considerable heterogeneity was observed (I2 = 90%, P < 0.001). A sensitivity analysis was done but no single study contributed significantly to the heterogeneity in MCS. Korean ginseng, the traditional formulation containing cinnamon, ajwain and Iranian boragom, and grape seed extract capsules all showed a significant effect on the mental health component of QoL; SMD 0.62 (95% CI 0.062, 1.17), SMD 2.15 (95% CI 1.45, 2.85), and SMD 1.45 (95% CI 0.88, 1.97), respectively.
For interventions involving a change in diet, only one study was included in the meta-analysis and involved a modified Mediterranean diet, lasting 6 months [41]. A positive effect was seen on the physical component of QoL with an SMD of 0.42 (95% CI 0.09, 0.75). A significant effect was not seen on the mental component of QoL with an SMD of 0.28 (95% CI − 0.04, 0.61).
There was one study included as part of the tissue manipulation category and involved reflexology, lasting 3 months [52]. A positive effect was seen on both the physical component of QoL with an SMD of 0.79 (95% CI 0.26, 1.32) and the mental component of QoL with an SMD of 0.90 (95% CI 0.36, 1.43).
Other interventions included a naturopathic medicine regimen [31], MS education [31], and hippotherapy [42]. A positive effect was seen on the physical component of QoL with an SMD of 0.44 (95% CI 0.07, 0.81). Only the Vermohlen et al. study [42], which utilized hippotherapy once a week for 3 months, showed a significant improvement in PCS, SMD of 0.62 (95% CI 0.08, 1.16). A significant overall effect was not seen on the mental component QoL with an SMD of 0.33 (95% CI − 0.06, 0.71). However, the Vermohlen et al. study showed an improvement in MCS, SMD of 0.63 (95% CI 0.09, 1.17).
t-Tests comparing individual study effects and the overall pooled effect estimate
For the effect of non-pharmacological intervention on the physical health component of QoL there were 4 studies for which the SMD was outside the 95% CI of the pooled overall effect (Fig. 4) [35, 40, 47, 49]. For each of these studies a t-test was performed with a null hypothesis that the SMD was no different than the overall pooled SMD of 0.44 (SD: 4.08). Hebert et al. and Kargarfard et al. both showed strong evidence against the null hypothesis (P-value = 0.0042 and 0.0439 respectively). Namjooyan et al. and Momenabadi et al. showed weak evidence against the null hypothesis and it is possible that the difference in SMD was due to chance (P-value = 0.0692 and 0.4323, respectively).
For the effect of non-pharmacological intervention on the mental health component of QoL there were 5 studies for which the SMD was outside the 95% CI of the pooled overall effect (Fig. 4) [35, 40, 47, 49, 51]. For each of these studies, a t-test was performed with a null hypothesis that the SMD was no different than the overall pooled SMD of 0.42 (SD: 4.20). Hebert et al. showed strong evidence against the null hypothesis (P-value = 0.0085). Siahpoosh et al. (P-value = 0.0955), Namjooyan et al. (P-value = 0.0525), Momenabadi et al. (P-value = 0.3718) and Kargarfard et al. (P-value = 0.1455) showed weaker evidence against the null hypothesis and it is possible that the differences in SMD were due to chance.
Summary of studies not included in meta-analysis—qualitative synthesis: there were 11 studies not included in the quantitative synthesis which are described in detail in Additional file 3. Seven of these studies compared physical regiments [53–59], two studies compared behavioral/psychological regimens [60, 61], one study (Weinstock-Guttman et al.) compared supplements [62] and one study examined the effects of transcranial random noise stimulation (tRNS) (Salemi et al.) [63]. Nine studies showed an improvement in some aspects of QoL, with the exceptions of Pilutti et al. [58] and Plow et al. [61]. Of these, five studies did not show a statistically significant difference in the effect between the groups. Of note Khalil et al. [55] considered the impact of a virtual reality exercise program two times per week and 1 balance exercise session at home or traditional balance exercises at home without virtual reality over a period of 6 weeks. The results showed that the arm incorporating virtual reality had a significantly larger effect on both PCS and MCS than traditional balance exercises (P-value < 0.05)[62]. Solari et al. [59] considered the impact of an inpatient rehabilitation program consisting of daily exercises two times per day lasting 45 min or a home exercise program for 3 weeks. The inpatient rehabilitation group improved significantly more in the MCS and this was sustained at 3 and 9 weeks (P-value = 0.001) [66]. Impellizzeri et al. [60] considered the impact of a conventional cognitive rehabilitation (CCR) and neurologic music therapy (NMT) or just CCR 6 times per week for 8 weeks. Results showed a significantly greater improvement in mental health in the CCR and NMT group as compared to the only CCR group (P-value < 0.001) [67]. Salemi et al. [63] considered the impact of tRNS applied 15 min daily for 2 weeks or a sham control group [70]. The results showed a significant increase in the tRNS group in both the ‘change in health’ and role limitation due to physical problems’ subscales, P-value = 0.006 and 0.001, respectively [63].
Publication bias
The funnel plot of the studies reporting a PCS outcome is presented in Additional file 4. There is asymmetry in the plot implying that there is the possibility of publication bias [71]. The gap in the bottom left corner of the plot indicates that studies with a larger SE which found no significant effect on PCS may have gone unpublished [64]. The funnel plot of the studies reporting an MCS outcome is also presented in Additional file 4. This plot is more symmetrical around the mean SMD compared to the PCS outcome funnel plot, implying that there may have been some studies which found a strong negative effect but went unpublished [64].
Discussion
This is the first systematic review incorporating a meta-analysis to examine the effects of non-pharmacological measures on improving HRQoL in adults with MS. This study found that overall, there was a modest improvement in both the PCS and MCS of QoL measures across all studies incorporating non-pharmacological measures (Fig. 4). Important heterogeneity was observed, however, when stratified by type of non-pharmacological measure and performing sensitivity analysis the source of heterogeneity was elucidated. The results of this study build on previous work on this topic which qualitatively summarized the benefits of psychological and behavioral interventions for improving QoL [10].
Principal findings
Across the 30 studies included in the meta-analysis there was a moderate effect for both the PCS and MCS on QoL for physical interventions. Although these results had a high risk of heterogeneity, most was contributed by a single study [Hebert et al.] and when this was removed a moderate effect was still observed for both PCS and MCS. Aquatic exercise [47] and balance and eye movement exercises (BEEMS) [35] showed a larger effect on PCS and MCS than the pooled effect within the physical intervention category (Fig. 4). When a t-test was performed comparing the SMD of individual studies and the overall pooled SMD in PCS, BEEMS and aquatic exercise both showed a statistically significant result indicating high probability that the difference was not due to chance. BEEMS also showed a significantly different effect on the MCS as compared to the overall pooled effect. BEEMS is a promising finding as previous studies have also shown a positive effect on patients with MS in aspects such as fatigue, posture control and disability [65–68]. Dizziness and instability are significant symptoms in patients with MS [69] and perhaps improving these over the course of the 4-month BEEMS program contributed greatly to the larger increase in MCS/PCS observed as compared to physical interventions which focused exclusively on either aerobic or resistance training.
Behavioral and psychological interventions did not show an overall pooled effect on PCS or MCS. Although there was a substantial amount of heterogeneity, it was mostly contributed by one study, Momenabadi et al. [49]. Conversely, the results of a review conducted by Gil-Gonzalez et al. [10] did comment on an effect of these types of interventions on QoL. This was a surprising finding, however, although some of the studies did report a significant effect on MCS in the intervention arm, not all studies included in the review by Gil-Gonzalez [10] included a control arm and this may have contributed to this apparent discrepancy.
Nutraceuticals and supplements overall showed an improvement in the PCS and MCS even after accounting for heterogeneity between studies, although the source of heterogeneity was unable to be determined for the effects on MCS. Therefore, it is likely that this subgroup is a good option to increase PCS and a possible one to improve MCS in patients with MS. Caution is recommended when generalizing these findings as all studies in this subgroup were done in a single country, Iran. The results in the diet intervention group, involving a modified Mediterranean diet, showed a moderate effect on PCS and no effect on MCS. This result needs to be interpreted with caution as the control arm was a traditional Iranian diet [41] which may differ substantially from other diets around the world such as the typical western diet. The tissue manipulation category involving reflexology demonstrated a benefit to both PCS and MCS. However, the tissue manipulation subgroup only included one single country study with a small sample size [52]. Thus, the conclusions of the study may not be widely generalizable and require further investigation.
The remaining studies not falling into any other categories were naturopathic medicine regimen [31], MS education [31], and hippotherapy [42]. The results were pooled, however, the interventions varied substantially and thus do not warrant discussion as a summary estimate. Hippotherapy showed a positive improvement in PCS, SMD of 0.62 (95% CI 0.08, 1.16) and although this is only one study a previous non-RCT also showed a positive effect of hippotherapy on aspects such as pain, muscle tension and balance [70]. Thus, it would be worthwhile for future studies to further explore the efficacy of hippotherapy on MS and QoL.
The results of the qualitative review, including 11 studies, were complimentary to that of the meta-analysis and quantitative synthesis. The Gandolfi et al. [54] study which incorporated SIBT in one of the arms provides further evidence for the effectiveness and importance of balance training for people with MS. Khalil et al. and Munari et al. both incorporated virtual reality (VR) into the physical intervention and had positive outcomes on QoL [55–57]. Pilutti et al. which investigated a stepper training program in one arm and a treadmill training in the other arm found no significant effects on any QoL outcome for either of these interventions [58]. It is possible, however, that no effect was observed due to the fact that all patients had progressive MS and were significantly disabled (EDSS score 6–8). This may indicate that perhaps exercise interventions may diminish in effectiveness in those with a more disabling disease status. Impellizzeri et al. added neurologic music therapy (NMT) to conventional cognitive rehabilitation (CCR) and this showed a greater improvement in mental health indicating the need to further explore NMT [60]. Another unique intervention was transcranial random noise stimulation (tRNS), a form of non-invasive brain stimulation, that showed statistically significant improvements in the ‘change in health’ and ‘role limitation due to physical problems’ subscales [63]. tRNS also needs to be further studied in MS patients with a larger sample size to more concretely understand its effects on QoL.
Quality of the evidence
The systematic review was successful in identifying 1173 studies for screening which speaks to the wide and broad search criteria. This led to 41 RCTs of similar design to be included in this review which allowed for the ability to make robust and useful discoveries. There was minimal selection bias introduced by restricting the review to SF-36, MSQLI, MSQoL-54 as these are the most widely used and comparable measure of HR-QoL in MS. Non-pharmacological studies were of similar duration, with 77% being administered for 2 months or greater which allowed for a fair comparison to be made across interventions and provided further support to pool the results. Also, the studies included in the meta-analysis were overall representative of the global MS population, with studies covering 10 diverse countries (Fig. 2) [71] and being majority female in representation (range 54–100% female in studies compared to 69% globally). The mean age of a participant in this review at 42.1 years old with a SD of 7.7 was comparable to the global mean age of MS diagnosis of 32 years old [3]. There was likely some selection bias within the studies; this is because for 11 of the studies we are not certain if true randomization was used and for 15 studies it was unclear if allocation was concealed. For those studies administering a nutraceutical/supplement sufficient blinding of participants was maintained using a placebo [29, 40, 50, 51]. However, in 13 studies those assessing QoL were not blind to assignment, and this may have introduced detection bias overestimating the results [23, 25, 28, 33, 34, 39, 45, 48, 49, 51, 52, 72]. In addition, for many studies it was not feasible to blind participants to the interventions and this may have contributed significant bias and led to an overestimation of the true benefit. Confounding was not deemed to be an issue in this study as potential confounders such as age or EDSS score were controlled for via randomization. Finally, it is also possible based on visual inspection of the funnel plots that publication bias was present and thus studies that showed a negative effect of non-pharmacological interventions went unpublished. However, this may have been due to heterogeneity in the interventions, for example, the majority of nutraceutical/supplement studies showed a statistically significant effect on PCS whereas the majority of behavioral/psychological studies showed no statistically significant effect on PCS. Overall, the quality of the studies was good as all were RCTs with similar patient characteristics at baseline and the majority, 23, conducted an intention-to-treat analysis. No studies were excluded from this review on the basis of quality.
Limitations
Studies were limited in size with a mean sample size of 67 patients, and this can affect the power of the studies and uncertainty of the effect size. Additionally, most studies were conducted at a single center making it difficult to generalize the outcomes to a wider region or country. There was also some heterogeneity in the disability status of patients across studies. This is noteworthy, as those with more disabling disease may be less likely to derive benefit from certain interventions and conversely those with less disabling disease may be less likely to derive benefit from interventions designed for those with a higher EDSS score. The duration of therapy and follow-up was overall quite short and given this is a chronic condition one cannot make claims that the benefits seen will be maintained over longer periods. Lastly, it should be noted that this review was not prospectively registered.
Conclusion
This systematic review and meta-analysis found that overall, non-pharmacological interventions improve QoL in persons with MS and that physical activity is particularly important. Specifically, balance exercises present a significant advantageous solution for improving both the mental and physical components of QoL. Other modalities such as nutraceuticals and supplements, diet and hippotherapy were found to improve either the PCS or MCS and behavioral and psychological interventions did not show an improvement in either the PCS or MCS. Comparing the pooled estimates of non-pharmacological intervention types needs to be done with caution as each subgroup did not contain an equal number of studies. Thus, we cannot draw definitive conclusions with regards to comparing the pooled effects. Further, this review looked only at changes in composite scores, this does not preclude that a significant change in overall QoL may have been seen or a change in one subscale may have been observed. Future studies on this topic should examine; all subscales of the QoL tools as well as the PCS and MCS, only include studies for which the inclusion criteria specified an EDSS score of < 5.5, and examine interventions over a longer duration of time. Also, additional studies need to be done to provide more evidence and clarify the true effects of nutraceuticals and supplements, diet, hippotherapy and behavioral and psychological interventions on QoL. Although there are still gaps in the existing literature to draw definite conclusions, this work demonstrates that non-pharmacological interventions improve QoL in persons with MS and that physical activity is likely to be very important. Moreover, many of the interventions discussed have few risks to implementation and thus represent a real and viable solution for improving QoL in persons with MS.
Supplementary Information
Additional file 1. Detailed search strategy.
Additional file 2. Complete JBI critical appraisal checklist for all studies included in the meta-analysis.
Additional file 3. Qualitative synthesis of studies not included in the meta-analysis.
Additional file 4. Funnel plots of studies included in the meta-analysis.
Acknowledgements
None.
Abbreviations
- BEEMS
Balance and eye-movement exercises for people with MS
- CCR
Conventional cognitive rehabilitation
- CI
Confidence interval
- CINAHL
Cumulative Index of Nursing and Allied Health Literature
- CNS
Central nervous system
- DKBT
Dr. Kawashima’s Brain Training
- EDSS
Kurtzke Expanded Disability Status Scale
- FES
Functional electrical stimulation
- HRQoL
Health-related quality of life
- JBI
Joanna Briggs Institute
- LSHTM
London School of Hygiene and Tropical Medicine
- MCS
Mental composite score
- mMeD
Modified Version of Mediterranean Diet
- MS
Multiple sclerosis
- MSIS-29
Multiple sclerosis impact scale
- MSQLI
Multiple sclerosis quality of life inventory
- MSQOL-54
Multiple sclerosis quality of life-54
- MusiQoL
Multiple Sclerosis International Quality of Life questionnaire
- NMT
Neurologic music therapy
- PCS
Physical composite score
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QoL
Quality of life
- RAGT
Robot assisted gait training
- RCT
Randomized control trial
- RRMS
Relapse remitting multiple sclerosis
- SD
Standard deviation
- SE
Standard error
- SF-36
Short form health survey
- SIBT
Sensory integration balance training
- SPMS
Secondary progressive multiple sclerosis
- SMD
Standardized mean difference
- tRNS
Transcranial random noise stimulation
Author contributions
VG developed the study idea and design. VG and KM conducted the literature search and data collection. VG and DH created the figures. KM completed the secondary manuscript reviews. VG conducted data analysis and data interpretation and took the lead in writing of the manuscript. KM and DH assisted with drafting and revising the manuscript. DH supervised the work and provided methodological and statistical guidance. All authors provided critical feedback and helped shape the research, analysis and manuscript. All authors read and approved the final manuscript.
Funding
The publication costs for this work were provided by the Faculty of Epidemiology and Public Health at London School of Hygiene and Tropical Medicine
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional files.
Declarations
Ethics approval and consent to participate
This study was exempt from the need for approval by the London School of Hygiene and Tropical Medicine—Research Governance & Integrity Office.
Consent for publication
Not applicable.
Competing interests
VG is an employee and shareholder of Hoffmann-La Roche. No specific funding was received for the conduct of this project from Hoffmann-La Roche and the work and ideas presented are the authors alone. KM and DH have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brück W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005;252(SUPPL. 5):3–9. doi: 10.1007/s00415-005-5002-7. [DOI] [PubMed] [Google Scholar]
- 2.Grant WB, Riise T. Multiple sclerosis: a lifestyle disease? Neurology. 2016;86:1275–1276. doi: 10.1212/WNL.0000000000002487. [DOI] [PubMed] [Google Scholar]
- 3.Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler J. 2020;26(14):1816–1821. doi: 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amtmann D, Bamer AM, Kim J, Chung H, Salem R. People with multiple sclerosis report significantly worse symptoms and health related quality of life than the US general population as measured by PROMIS and NeuroQoL outcome measures. Disabil Health J. 2018;11(1):99–107. doi: 10.1016/j.dhjo.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 5.McCabe MP, McKern S. Quality of life and multiple sclerosis: comparison between people with multiple sclerosis and people from the general population. J Clin Psychol Med Settings. 2002;9(4):287–295. [Google Scholar]
- 6.Pittock SJ, Mayr WT, McClelland RL, Jorgensen NW, Weigand SD, Noseworthy JH, et al. Quality of life is favorable for most patients with multiple sclerosis: a population-based cohort study. Arch Neurol. 2004;61(5):679–686. doi: 10.1001/archneur.61.5.679. [DOI] [PubMed] [Google Scholar]
- 7.Post MWM. Definitions of quality of life: what has happened and how to move on. Top Spinal Cord Inj Rehabil. 2014;20(3):167–180. doi: 10.1310/sci2003-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyatt GH, Ferrans CE, Halyard MY, Revicki DA, Symonds TL, Varricchio CG, et al. Exploration of the value of health-related quality-of-life information from clinical research and into clinical practice. Mayo Clin Proc. 2007;82(10):1229–1239. doi: 10.4065/82.10.1229. [DOI] [PubMed] [Google Scholar]
- 9.Bandari DS, Vollmer TL, Khatri BO, Tyry T. Assessing quality of life in patients with multiple sclerosis. Int J MS Care. 2010;12(1):34–41. doi: 10.7224/1537-2073-12.1.34. [DOI] [Google Scholar]
- 10.Gil-González I, Martín-Rodríguez A, Conrad R, Pérez-San-Gregorio MÁ. Quality of life in adults with multiple sclerosis: a systematic review. BMJ Open. 2020;10(11):e041249. doi: 10.1136/bmjopen-2020-041249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visschedijk MAJ, Uitdehaag BMJ, Klein M, van der Ploeg E, Collette EH, Vleugels L, et al. Value of health-related quality of life to predict disability course in multiple sclerosis. Neurology. 2004;63(11):2046–2050. doi: 10.1212/01.wnl.0000145769.51420.ed. [DOI] [PubMed] [Google Scholar]
- 12.MSIF TMSIF. Atlas of MS 3rd edition. 2020. p. 1–36. www.atlasofms.org.
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibbald B, Roberts C. Understanding controlled trials. Crossover trials. BMJ. 1998;316(7146):1719. doi: 10.1136/bmj.316.7146.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware JEJ. SF-36 health survey update. Spine. 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Vickrey BG. Multiple sclerosis quality of life (MSQOL)-54 instrument. Mult Scler. 1995. http://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/MSQOL54_995.pdf.
- 17.Revman @ training.cochrane.org. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman.
- 18.Chapter-10 @ training.cochrane.org. https://training.cochrane.org/handbook/current/chapter-10.
- 19.Index @ www.stata.com. https://www.stata.com/.
- 20.Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):1–11. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Critical-appraisal-tools @ jbi.global. https://jbi.global/critical-appraisal-tools.
- 22.Expanded-disability-status-scale-edss @ mstrust.org.uk. https://mstrust.org.uk/a-z/expanded-disability-status-scale-edss.
- 23.Ahadi F, Tabatabaee SM, Rajabpour M, Ghadamgahi A, Kaljahi MP. Effect of 8-week aerobic exercise and yoga training on depression, anxiety, and quality of life among multiple sclerosis patients. Iran Rehabil J. 2013;11(17):75–80. [Google Scholar]
- 24.Ahmadi A, Arastoo AA, Nikbakht M. The effects of a treadmill training programme on balance, speed and endurance walking, fatigue and quality of life in people with multiple sclerosis. Int Sport J. 2010;11(4):389–397. [Google Scholar]
- 25.Backus D, Moldavskiy M, Sweatman WM. Effects of functional electrical stimulation cycling on fatigue and quality of life in people with multiple sclerosis who are nonambulatory. Int J MS Care. 2020;22(4):193–200. doi: 10.7224/1537-2073.2019-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barclay A, Paul L, MacFarlane N, McFadyen AK. The effect of cycling using active-passive trainers on spasticity, cardiovascular fitness, function and quality of life in people with moderate to severe multiple sclerosis (MS); a feasibility study. Mult Scler Relat Disord. 2019;34:128–134. doi: 10.1016/j.msard.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Bjarnadottir OH, Konradsdottir AD, Reynisdotir K, Olafsson E. Multiple sclerosis and brief moderate exercise. A randomised study. Mult Scler. 2007;13(6):776–782. doi: 10.1177/1352458506073780. [DOI] [PubMed] [Google Scholar]
- 28.Romberg A, Virtanen A, Ruutiainen R. Long-term exercise improves functional impairment but not quality of life in multiple sclerosis. J Neurol. 2005;252(7):839–845. doi: 10.1007/s00415-005-0759-2. [DOI] [PubMed] [Google Scholar]
- 29.Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, Mansourian M. High dose vitamin D intake and quality of life in relapsing-remitting multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. Neurol Res. 2016;38(10):888–892. doi: 10.1080/01616412.2016.1227913. [DOI] [PubMed] [Google Scholar]
- 30.Nozari E, Ghavamzadeh S, Razazian N. The effect of vitamin B12 and folic acid supplementation on serum homocysteine, anemia status and quality of life of patients with multiple sclerosis. Clin Nutr Res. 2019;8(1):36–45. doi: 10.1002/central/CN-02251587/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinto L, Calabrese C, Morris C, Yadav V, Griffith D, Frank R, et al. A randomized pilot study of naturopathic medicine in multiple sclerosis J Altern Complement Med. 2008;14(6):793. J Altern Complement Med. 2008;14(5 CC-Complementary Medicine CC-Multiple Sclerosis and Rare Diseases of the CNS):489‐496. 10.1002/central/CN-00763850/full. [DOI] [PMC free article] [PubMed]
- 32.Jeong IC, Karpatkin H, Finkelstein J. Physical telerehabilitation improves quality of life in patients with multiple sclerosis. Stud Health Technol Inform. 2021;284:384–388. doi: 10.3233/SHTI210752. [DOI] [PubMed] [Google Scholar]
- 33.Dalgas U, Stenager E, Jakobsen J, Petersen T, Hansen HJ, Knudsen C, et al. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler. 2010;16(4):480–490. doi: 10.1177/1352458509360040. [DOI] [PubMed] [Google Scholar]
- 34.Langeskov-Christensen M, Hvid LG, Jensen HB, Nielsen HH, Petersen T, Stenager E, et al. Efficacy of high-intensity aerobic exercise on common multiple sclerosis symptoms. Acta Neurol Scand. 2022;145(2):229–238. doi: 10.1111/ane.13540. [DOI] [PubMed] [Google Scholar]
- 35.Hebert JR, Corboy JR, Vollmer T, Forster JE, Schenkman M. efficacy of balance and eye-movement exercises for persons with multiple sclerosis (BEEMS) Neurology. 2018;90(9):e797–e807. doi: 10.1212/WNL.0000000000005013. [DOI] [PubMed] [Google Scholar]
- 36.Oken BS, Kishiyama S, Zajdel D, Bourdette D, Carlsen J, Haas M, et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology. 2004;62(11):2058–2064. doi: 10.1212/01.wnl.0000129534.88602.5c. [DOI] [PubMed] [Google Scholar]
- 37.De Giglio L, De Luca F, Prosperini L, Borriello G, Bianchi V, Pantano P, et al. A low-cost cognitive rehabilitation with a commercial video game improves sustained attention and executive functions in multiple sclerosis: a pilot study. Neurorehabil Neural Repair. 2015;29(5):453–461. doi: 10.1177/1545968314554623. [DOI] [PubMed] [Google Scholar]
- 38.O’Hara L, Cadbury H, De Souza L, Ide L, O’Hara L, Cadbury H, et al. Evaluation of the effectiveness of professionally guided self-care for people with multiple sclerosis living in the community: a randomized controlled trial. Clin Rehabil. 2002;16(2):119–128. doi: 10.1191/0269215502cr478oa. [DOI] [PubMed] [Google Scholar]
- 39.Stuijbergen AK, Becker H, Blozis S, Timmerman G, Kullberg V, Stuifbergen AK, et al. A randomized clinical trial of a wellness intervention for women with multiple sclerosis. Arch Phys Med Rehabil. 2003;84(4):467–476. doi: 10.1053/apmr.2003.50028. [DOI] [PubMed] [Google Scholar]
- 40.Namjooyan F, Ghanavati R, Majdinasab N, Zadeh HR. The efficacy of traditional formulation on quality of life and fatigue in multiple sclerosis patients: a randomized double-blind placebo-control clinical trial. J Contemp Med Sci. 2019;5(2):96–100. [Google Scholar]
- 41.Moravejolahkami AR, Paknahad Z, Chitsaz A, HojjatiKermani MA, Borzoo-Isfahani M. Potential of modified Mediterranean diet to improve quality of life and fatigue severity in multiple sclerosis patients: a single-center randomized controlled trial. Int J Food Prop. 2020;23(1):1993–2004. [Google Scholar]
- 42.Vermohlen V, Schiller P, Schickendantz S, Drache M, Hussack S, Gerber-Grote A, et al. Hippotherapy for patients with multiple sclerosis: a multicenter randomized controlled trial (MS-HIPPO) Mult Scler J. 2018;24(10):1375–1382. doi: 10.1177/1352458517721354. [DOI] [PubMed] [Google Scholar]
- 43.Pappalardo A, D’Amico E, Leone C, Messina S, Chisari C, Rampello L, et al. Inpatient versus outpatient rehabilitation for multiple sclerosis patients: effects on disability and quality of life. Mult Scler Demyelinating Disord. 2016;1(1):1–7. doi: 10.1002/central/CN-01298747/full. [DOI] [Google Scholar]
- 44.Patti F, Ciancio MR, Reggio E, Lopes R, Palermo F, Cacopardo M, et al. The impact of outpatient rehabilitation on quality of life in multiple sclerosis. J Neurol. 2002;249(8):1027–1033. doi: 10.1007/s00415-002-0778-1. [DOI] [PubMed] [Google Scholar]
- 45.Ahmadi A, Nikbakh M, Arastoo A, Habibi A-H. The effects of a yoga intervention on balance, speed and endurance of walking, fatigue and quality of life in people with multiple sclerosis. J Hum Kinet. 2010;23(1):71–78. [Google Scholar]
- 46.Carter AM, Daley AJ, Kesterton SW, Woodroofe NM, Saxton JM, Sharrack B. Pragmatic exercise intervention in people with mild to moderate multiple sclerosis: a randomised controlled feasibility study. Contemp Clin Trials. 2013;35(2):40–47. doi: 10.1016/j.cct.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Kargarfard M, Etemadifar M, Baker P, Mehrabi M, Hayatbakhsh R. Effect of aquatic exercise training on fatigue and health-related quality of life in patients with multiple sclerosis. Arch Phys Med Rehabil. 2012;93(10):1701–1708. doi: 10.1016/j.apmr.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Jongen PJ, van Mastrigt GA, Heerings M, Visser LH, Ruimschotel RP, Hussaarts A, et al. Effect of an intensive 3-day social cognitive treatment (can do treatment) on control self-efficacy in patients with relapsing remitting multiple sclerosis and low disability: a single-centre randomized controlled trial. PLoS ONE. 2019;14(10):e0223482. doi: 10.1371/journal.pone.0223482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Momenabadi V, Kaveh MH, Nakhaee N, Shirazi KK, Dastoorpoor M, Sedighi B. Effect of educational intervention based on health-promoting self-care behaviors model on quality of life, resilience, and sense of coherence in patients with multiple sclerosis: a randomized controlled trial. Iran Red Crescent Med J. 2019;21(12):e97240. [Google Scholar]
- 50.Etemadifar M, Sayahi F, Abtahi S-H, Shemshaki H, Dorooshi G-A, Goodarzi M, et al. Ginseng in the treatment of fatigue in multiple sclerosis: a randomized, placebo-controlled, double-blind pilot study. Int J Neurosci. 2013;123(7):480–486. doi: 10.3109/00207454.2013.764499. [DOI] [PubMed] [Google Scholar]
- 51.Siahpoosh A, Majdinasab N, Derakhshannezhad N, Khalili HR, Malayeri A. Effect of grape seed on quality of life in multiple sclerosis patients. J Contemp Med Sci. 2018;4(3):148–152. [Google Scholar]
- 52.DilekDoğan H, Tan M. Effects of reflexology on pain, fatigue, and quality of life in multiple sclerosis patients: a clinical study. Altern Ther Health Med. 2021;27(5):14–22. [PubMed] [Google Scholar]
- 53.Bansi J, Bloch W, Gamper U, Riedel S, Kesselring J. Endurance training in MS: short-term immune responses and their relation to cardiorespiratory fitness, health-related quality of life, and fatigue. J Neurol. 2013;260(12):2993–3001. doi: 10.1007/s00415-013-7091-z. [DOI] [PubMed] [Google Scholar]
- 54.Gandolfi M, Geroin C, Picelli A, Munari D, Waldner A, Tamburin S, et al. Robot-assisted vs. sensory integration training in treating gait and balance dysfunctions in patients with multiple sclerosis: a randomized controlled trial. Front Hum Neurosci. 2014;8(May):318. doi: 10.3389/fnhum.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khalil H, Al-Sharman A, El-Salem K, Alghwiri AAA, Al-Shorafat D, Khazaaleh S, et al. The development and pilot evaluation of virtual reality balance scenarios in people with multiple sclerosis (MS): a feasibility study. NeuroRehabilitation. 2018;43(4):473–482. doi: 10.3233/NRE-182471. [DOI] [PubMed] [Google Scholar]
- 56.Kerling A, Keweloh K, Tegtbur U, Kück M, Grams L, Horstmann H, et al. Effects of a short physical exercise intervention on patients with multiple sclerosis (MS) Int J Mol Sci. 2015;16(7):15761–15775. doi: 10.3390/ijms160715761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munari D, Fonte C, Varalta V, Battistuzzi E, Cassini S, Montagnoli AP, et al. Effects of robot-assisted gait training combined with virtual reality on motor and cognitive functions in patients with multiple sclerosis: a pilot, single-blind, randomized controlled trial. Restor Neurol Neurosci. 2020;38(2):151–154. doi: 10.3233/RNN-190974. [DOI] [PubMed] [Google Scholar]
- 58.Pilutti LA, Paulseth JE, Dove C, Jiang S, Rathbone MP, Hicks AL. Exercise training in progressive multiple sclerosis: a comparison of recumbent stepping and body weight-supported treadmill training. Int J MS Care. 2016;18(5):221–229. doi: 10.7224/1537-2073.2015-067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solari A, Filippini G, Gasco P, Colla L, Salmaggi A, La Mantia L, et al. Physical rehabilitation has a positive effect on disability in multiple sclerosis patients. Neurology. 1999;52(1):57–62. doi: 10.1212/wnl.52.1.57. [DOI] [PubMed] [Google Scholar]
- 60.Impellizzeri F, Leonardi S, Latella D, Maggio MG, FotiCuzzola M, Russo M, et al. An integrative cognitive rehabilitation using neurologic music therapy in multiple sclerosis: a pilot study. Medicine. 2020;99(4):e18866. doi: 10.1097/MD.0000000000018866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plow MA, Mathiowetz V, Lowe DA. Comparing individualized rehabilitation to a group wellness intervention for persons with multiple sclerosis. Am J Health Promot. 2009;24(1):23–26. doi: 10.4278/ajhp.071211128. [DOI] [PubMed] [Google Scholar]
- 62.Weinstock-Guttman B, Baier M, Park Y, Feichter J, Lee-Kwen P, Gallagher E, et al. Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins Leukot Essent Fat Acids. 2005;73(5):397–404. doi: 10.1016/j.plefa.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 63.Salemi G, Vazzoler G, Ragonese P, Bianchi A, Cosentino G, Croce G, et al. Application of tRNS to improve multiple sclerosis fatigue: a pilot, single-blind, sham-controlled study. J Neural Transm. 2019;126(6):795–799. doi: 10.1007/s00702-019-02006-y. [DOI] [PubMed] [Google Scholar]
- 64.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 65.Ozgen G, Karapolat H, Akkoc Y, Yuceyar N. Is customized vestibular rehabilitation effective in patients with multiple sclerosis? A randomized controlled trial. Eur J Phys Rehabil Med. 2016;52(4):466–478. [PubMed] [Google Scholar]
- 66.Brichetto G, Piccardo E, Pedullà L, Battaglia MA, Tacchino A. Tailored balance exercises on people with multiple sclerosis: a pilot randomized, controlled study. Mult Scler. 2015;21(8):1055–1063. doi: 10.1177/1352458514557985. [DOI] [PubMed] [Google Scholar]
- 67.Kasser SL, Jacobs JV, Ford M, Tourville TW. Effects of balance-specific exercises on balance, physical activity and quality of life in adults with multiple sclerosis: a pilot investigation. Disabil Rehabil. 2015;37(24):2238–2249. doi: 10.3109/09638288.2015.1019008. [DOI] [PubMed] [Google Scholar]
- 68.Hebert JR, Corboy JR, Manago MM, Schenkman M. Effects of vestibular rehabilitation on multiple sclerosis-related fatigue and upright postural control: a randomized controlled trial. Phys Ther. 2011;91(8):1166–1183. doi: 10.2522/ptj.20100399. [DOI] [PubMed] [Google Scholar]
- 69.Dizziness-and-Vertigo @ www.nationalmssociety.org. https://www.nationalmssociety.org/Symptoms-Diagnosis/MS-Symptoms/Dizziness-and-Vertigo.
- 70.Hammer A, Nilsagård Y, Forsberg A, Pepa H, Skargren E, Öberg B. Evaluation of therapeutic riding (Sweden)/hippotherapy (United States). A single-subject experimental design study replicated in eleven patients with multiple sclerosis. Physiother Theory Pract. 2005;21(1):51–77. doi: 10.1080/09593980590911525. [DOI] [PubMed] [Google Scholar]
- 71.Who-regions @ ourworldindata.org. https://ourworldindata.org/grapher/who-regions.
- 72.Saxton JM, Carter A, Daley A, Humphreys L, Snowdon N, Woodroofe N, et al. Pragmatic exercise for people with MS: a randomized controlled trial. Mult Scler. 2013;19(10):1402. doi: 10.1002/central/CN-01053273/full. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Detailed search strategy.
Additional file 2. Complete JBI critical appraisal checklist for all studies included in the meta-analysis.
Additional file 3. Qualitative synthesis of studies not included in the meta-analysis.
Additional file 4. Funnel plots of studies included in the meta-analysis.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional files.