Abstract
To determine the optimal media for optochin susceptibility testing of Streptococcus pneumoniae, we measured inhibition zones for 72 S. pneumoniae and 22 Streptococcus viridans isolates on three blood-containing media. Because 15.3, 0, and 22.2% of S. pneumoniae organisms were misidentified on Columbia agar, Trypticase soy agar (TSA), and Mueller-Hinton agar, respectively, each containing sheep blood, we recommend that TSA-sheep blood agar be used.
Optochin (ethylhydrocupreine hydrochloride) is currently widely used as an inexpensive and reliable means to presumptively identify Streptococcus pneumoniae. It has been known, since the turn of the century, when it was first synthesized, to inhibit the growth of S. pneumoniae. The early studies of Moore in 1915 showed that while optochin was ineffective as a therapeutic agent, it did inhibit the growth of S. pneumoniae in broth culture (5). In 1955, Bowers and Jeffries showed that optochin-saturated filter paper placed on the surface of unspecified horse blood agar medium onto which S. pneumoniae had been plated reliably produced a zone of inhibition. This simple test made it possible to distinguish S. pneumoniae from Streptococcus viridans, which was consistently optochin resistant (3). Bowen et al. subsequently showed that optochin inhibition was independent of capsular type when inhibition tests were performed on Trypticase soy agar (TSA) supplemented with human blood (2).
The current National Committee for Clinical Laboratory Standards (NCCLS) guidelines regarding optochin inhibition recommend using a “blood agar plate,” specifying neither the type of blood nor the type of agar (4). There are currently several choices of sheep blood agar media which support the growth of S. pneumoniae. We questioned whether the recommended optochin inhibition zone criteria (“zones”) are applicable to three popular types of agar plates, TSA, Columbia agar (COL), and Mueller-Hinton agar (MH), supplemented with sheep blood. As the current guidelines also recommend incubation in 5% CO2, and this has been previously shown to improve recovery of the organism (1) and result in smaller zones when TSA-sheep blood agar plates are used (6), we studied optochin zones in air and CO2 by using the same three types of media.
(Part of this research was presented in poster format at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, 28 September to 1 October 1997, Toronto, Ontario, Canada.)
Seventy-two clinical isolates of S. pneumoniae and 22 isolates of S. viridans previously isolated from either blood or cerebrospinal fluid were removed from storage at −70°C and incubated overnight at 35°C in 5% CO2 on Columbia agar with 5% sheep blood. The identification of all S. pneumoniae isolates had been confirmed prior to the study by the Quebec Public Health Laboratory by means of bile solubility testing and capsular antigen serotyping, whereas S. viridans isolates were confirmed via bile solubility and carbohydrate fermentation.
A pilot study of nine S. pneumoniae isolates showed that zone size was slightly affected by inoculum. We found a mean decrease in zone size of 1.0 mm for large inocula (2.0 MacFarland) versus small inocula (0.5 MacFarland), and hence all subsequent studies were performed with a standardized inoculum (from 24-h colonies) prepared in nutrient broth corresponding to 0.5 MacFarland unit. For each isolate, two agar plates of each medium type were swabbed in three directions and two 6-mm optochin disks (Taxo-P; BBL, Becton Dickinson Microbiology Systems, Cockeysville, Md.) were placed on each plate with flamed forceps. TSA- and COL-sheep blood agar were purchased from Becton Dickinson, while the MH-sheep blood agar was made in the laboratory from premixed powder (Becton Dickinson) with 5% defibrinated sheep blood added (Quélab, Montréal, Québec, Canada). One plate of each agar type was incubated overnight at 35°C in 5% CO2; the other was incubated in air. Zones were measured at 20 to 22 h by one investigator (M.A.G.) with the same calipers throughout the study, which were recalibrated before each plate was read.
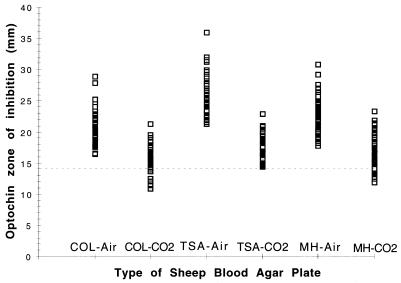
Three clinical isolates of S. pneumoniae failed to grow in air on all three media, and one isolate failed to grow on TSA in air. All isolates of S. viridans grew in air and CO2. Incubation in air produced significantly larger zones for each S. pneumoniae isolate that grew (Fig. 1). The mean zones for the S. pneumoniae isolates plated onto each medium incubated in CO2 and air are presented in Table 1. All of the S. pneumoniae isolates produced a zone around the optochin disk in both air and CO2, while none of the S. viridans isolates produced a zone, regardless of the incubation atmosphere.
FIG. 1.
Optochin zones of inhibition for 72 isolates of S. pneumoniae on COL-, TSA-, and MH-sheep blood agar incubated in air and CO2.
TABLE 1.
Optochin inhibition zones for 72 isolates of S. pneumoniae on COL-, TSA-, and MH-sheep blood agar incubated in air and in CO2
| Medium and conditions | Mean zone of inhibition (mm) | SD | No. (%) of isolates with a zone of <14 mm | P valuea |
|---|---|---|---|---|
| COL and air | 20.7 | 2.29 | 0 | <10−8 |
| COL and CO2 | 15.4 | 1.77 | 11 (15) | <10−7 |
| TSA and air | 25.1 | 2.87 | 0 | <10−8 |
| TSA and CO2 | 17.4 | 1.68 | 0 | NAb |
| MH and air | 22.4 | 2.92 | 0 | <10−8 |
| MH and CO2 | 15.9 | 2.50 | 16 (22) | <10−8 |
Compared to results obtained with TSA and CO2 by using a two-tailed matched t test.
NA, not applicable.
The current NCCLS criteria for the presumptive identification of S. pneumoniae include a zone of >14 mm with a 6-mm optochin disk incubated overnight in 5% CO2. For zones that are >6 mm but ≤14 mm, additional testing such as bile solubility must be performed. No zone is consistent with an alpha streptococcus other than S. pneumoniae (4). Applying these criteria, the following number of S. pneumoniae isolates would have been misidentified by this procedure: COL, 11 of 72 (15.3%); TSA, 0 of 72; and MH, 16 of 72 (22.2%). Also, we would have required additional procedures to confirm the identification. In laboratories that identify S. pneumoniae only by the optochin test, these isolates would have been wrongly reported as S. viridans.
Our study has shown that optochin sensitivity tests performed on different sheep blood agar media yield significantly disparate results. Using media other than TSA-sheep blood agar will result in a substantial number of isolates with indeterminate zones which will require further testing before the organisms can be identified as S. pneumoniae. This is both labor-intensive and costly and may also slow down reporting.
We also demonstrated a small inoculum effect. The practical significance of this effect is likely minimal if one uses TSA-sheep blood agar, as none of the isolates produced optochin zones of <14 mm in spite of large inocula (2.0 MacFarland). One of the isolates plated onto COL did produce a zone that was <14 mm when plated at 2.0 MacFarland.
It has been previously reported that incubation in CO2 is suggested for S. pneumoniae, as approximately 8% of isolates either will not grow or will grow poorly in air (1). In our study, 4% of isolates did not grow in air. Of those that did, all produced significantly larger optochin zones than when grown in CO2 on all three media. These results concur with a previous study using TSA-sheep blood agar plates (6). While incubation in air allows for an even greater discrimination between optochin zones for S. pneumoniae and S. viridans, it cannot be recommended, as a significant number of S. pneumoniae isolates will not grow, making the test results uninterpretable.
We conclude that if current NCCLS recommendations are followed, optochin sensitivity testing for S. pneumoniae should be performed in 5% CO2 on TSA-sheep blood agar media to avoid initial misidentification, further identification tests, and delays in reporting identifications.
REFERENCES
- 1.Austrian R, Collins P. Importance of carbon dioxide in the isolation of pneumococci. J Bacteriol. 1966;92:1281–1284. doi: 10.1128/jb.92.5.1281-1284.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowen M K, Thiele L C, Stearman B D, Schaub I G. The optochin sensitivity test: a reliable method for identification of pneumococci. J Lab Clin Med. 1957;49:641–642. [PubMed] [Google Scholar]
- 3.Bowers E F, Jeffries L R. Optochin in the identification of Str. pneumoniae. J Clin Pathol. 1955;8:58–60. doi: 10.1136/jcp.8.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isenberg H D, editor. Clinical microbiology procedures handbook. Washington, D.C: American Society for Microbiology; 1992. pp. 1.20.25–1.20.26. [Google Scholar]
- 5.Moore H F. The action of ethylhydrocupreine (optochin) on type strains of pneumococci in vitro and in vivo, and on some other microorganisms in vitro. J Exp Med. 1915;22:269–285. doi: 10.1084/jem.22.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ragsdale A R, Sanford J P. Interfering effect of incubation in carbon dioxide on the identification of pneumococci by optochin discs. Appl Microbiol. 1971;22:854–855. doi: 10.1128/am.22.5.854-855.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]