Abstract
Background
Limited data exists on herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) infections in migrant populations. This study investigated HSV-1 and HSV-2 seroprevalences and associations among craft and manual workers (CMWs) in Qatar who constitute 60% of Qatar’s population.
Methods
A national population-based cross-sectional seroprevalence survey was conducted on the CMW population, all men, between July 26 and September 9, 2020. 2,612 sera were tested for anti-HSV-1 IgG antibodies using HerpeSelect 1 ELISA IgG kits and for anti-HSV-2 IgG antibodies using HerpeSelect 2 ELISA IgG kits (Focus Diagnostics, USA). Univariable and multivariable logistic regression analyses were conducted to identify associations with HSV-1 and HSV-2 infections.
Results
Serological testing identified 2,171 sera as positive, 403 as negative, and 38 as equivocal for HSV-1 antibodies, and 300 sera as positive, 2,250 as negative, and 62 as equivocal for HSV-2 antibodies. HSV-1 and HSV-2 seroprevalences among CMWs were estimated at 84.2% (95% CI 82.8–85.6%) and 11.4% (95% CI 10.1–12.6%), respectively. HSV-1 infection was associated with nationality, educational attainment, and occupation. HSV-2 infection was associated with age, nationality, and educational attainment.
Conclusions
Over 80% of CMWs are infected with HSV-1 and over 10% are infected with HSV-2. The findings highlight the need for sexual health programs to tackle sexually transmitted infections among the CMW population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12985-023-02157-1.
Keyword: HSV-1, HSV-2, Prevalence, Genital herpes, Genital ulcer disease, Survey, Cross sectional study, Migrants, Qatar
Background
Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) infections are life-long and common worldwide [1]. Infections with these viruses are typically latent and asymptomatic, but with frequent reactivations, subclinical shedding and sporadic symptomatic events [2–4]. HSV-1 infection in rare cases causes severe neurological, corneal, or mucocutaneous complications [5, 6]. HSV-2 infection causes genital ulcer disease and genital herpes [7, 8], and its vertical mother-to-child transmission can cause neonatal herpes, a severe and sometimes fatal disease in newborns [9, 10]. While HSV-2 is predominantly sexually transmitted [7, 8], HSV-1 is typically orally acquired [11, 12], but with increasing role as a sexually transmitted infection, mainly through oral sex [11, 12]. HSV-2 infection is believed to increase acquisition and transmission of HIV [13], perhaps resulting in a synergy between these two infections [14–16]. The World Health Organization (WHO) and global partners are leading initiatives to enhance our understanding of the epidemiology of these two viruses, and to develop an HSV vaccine to tackle the global disease and economic burden of HSV-1 and HSV-2 infections [1, 17, 18].
Qatar, a country in the Arabian Peninsula, has unusually young, diverse demographics, in that only 9% of its residents are ≥ 50 years of age, and 89% are expatriates from over 150 countries [19]. Nearly 60% of the population comprises expatriate craft and manual workers (CMWs) who are typically single men aged 20–49 years and recruited to work in large development projects, such as those for the World Cup 2022 [20]. The objective of this study was to assess the antibody prevalence (seroprevalence) of HSV-1 and HSV-2 infections among this majority segment of Qatar’s population and to inform national health policy planning.
Methods
Study design and sampling
This study was conducted on blood sera specimens that were originally collected for a national severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seroprevalence survey among CMWs in Qatar [20]. The blood specimens were drawn between July 26, 2020 and September 09, 2020, to assess exposure to SARS-CoV-2 infection in this population, the most affected population by the first SARS-CoV-2 wave in Qatar [19, 21].
To optimize sample representativeness of the wider CMW population, a sampling strategy was devised based on analysis of the registered users’ database of the Qatar Red Crescent Society (QRCS), the main provider of primary healthcare for CMWs in the country [20]. QRCS operates four geographically distributed centers that were purposively designed to cater to the CMW population across the country. These centers operate long working hours, are located in regions where workers live, and provide services that are free of charge or heavily subsidized for accessibility and affordability.
The probability distribution of CMWs by age and nationality in the QRCS database was cross-checked and found similar to that of the Ministry of Interior database of expatriate residents [22]. Sex was not considered in the sampling strategy because the vast majority of CMWs (> 99%) are men [23]. Systematic sampling was implemented for the recruitment of the CMWs attending these centers during the study [20]. By factoring the average number of attendees per day at each of these centers, every 4th attendee visiting each center was approached to participate in this study until the sample size by age and nationality at each center has been fulfilled. There was difficulty in recruiting participants in the small age-nationality strata (such as among younger persons for specific nationalities), and thus towards the end of the study all attendees in these strata (not only every 4th attendee) were invited to participate. Written informed consent was collected from all study participants.
Sample collection and handling
An interview schedule including socio-demographic variables was administered by trained interviewers in the participant’s language of preference [20]. The study instrument was based on a protocol for SARS-CoV-2 sero-epidemiological surveys developed by the WHO [24]. Both informed consent and interview schedule were provided and collected in nine languages (Arabic, Bengali, English, Hindi, Nepali, Sinhala, Tagalog, Tamil, and Urdu) to cater to the main language groups of CMWs. Blood (10 ml) was drawn for serological testing by certified nurses and stored in an ice box before being transported to the Qatar Biobank for storage and subsequent testing.
Laboratory methods
Portion of sera (50 µL) were aliquoted from stored sera samples at the Qatar Biobank and transported to the virology laboratory at Qatar University for the serological testing. The sera at both Qatar Biobank and Qatar University were stored at − 80 °C until used for serology testing. Sera were tested for the presence of anti-HSV-1 IgG using HerpeSelect 1 enzyme linked immunosorbent assay (ELISA) kits (Cat. No. EL0910G-5, Focus Diagnostics, USA). Sera were also tested for the presence of anti-HSV-2 IgG using HerpeSelect 2 ELISA kits (Cat. No. EL0910G-5, Focus Diagnostics, USA). Both kits are approved for laboratory diagnosis of anti-HSV-1 IgG and anti-HSV-2 IgG by the United States Food and Drug Administration.
For both kits, analysis and results were interpreted according to the manufacturer’s instructions: sera with optical density index values (cut-off) < 0.90 were considered negative, values > 1.10 were considered positive, and values ranging between 0.90 and 1.10 were considered equivocal. All equivocal specimens were retested for final result. Those that remained equivocal were reported as equivocal.
Oversight
Hamad Medical Corporation and Weill Cornell Medicine-Qatar Institutional Review Boards approved this retrospective study with a waiver of informed consent. The study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Additional file 1: Table S1).
Statistical analysis
Frequency distributions were used to characterize study participants. Equivocal specimens were excluded from analysis. Probability weights were calculated based on CMW population distribution by age, nationality, and QRCS center per the QRCS registered-user database [20]. The probability weights were applied in the analyses to adjust for participants’ unequal selection.
Associations with antibody positivity for each of HSV-1 and HSV-2 were explored using Chi-square test and univariable logistic regression analyses. Any variable with p value ≤ 0.2 in the univariable regression analysis was included in the multivariable model. A p value ≤ 0.05 in the multivariable analysis was considered to provide evidence for a statistically significant association. Unadjusted and adjusted odds ratios (ORs and AORs, respectively) along with their respective 95% confidence intervals (CIs) and p values were reported. 95% CIs were not adjusted for multiplicity; thus, they should not be used to infer definitive differences between groups. Interactions were not considered. All analyses were conducted using SPSS version 27.0 (Armonk, NY, USA).
Results
Characteristics of the study population are summarized in Table 1. A total of 2,641 male CMWs consented to participate in the original SARS-CoV-2 survey, but only 2,612 (98.9%) sera specimens were available to be tested for HSV-1 and HSV-2 antibodies and included in the study. The majority of study participants were 30–39 years of age (37.1%) with a median age of 35.0 years (interquartile range 29.0–42.0 years). Of study participants, 42.1% completed intermediate or lower educational attainment, and 43.3% attended high school or vocational training. Of study participants, 27.5% were Indians, 24.0% Bangladeshis, and 21.6% Nepalese, representative of the wider CMW population in Qatar [22]. Each other nationality contributed < 10% of the total sample. Over half of the sample consisted of technical and construction workers such as carpenters, crane operators, electricians, masons, mechanics, painters, plumbers, and welders. About 5% of participants held higher professional positions such as architects, designers, engineers, operation managers, and supervisors.
Table 1.
Characteristics of study participants and outcome of HSV-1 and HSV-2 serological testing
| Characteristics | Tested | HSV-1 antibody positive | HSV-2 antibody positive | ||||
|---|---|---|---|---|---|---|---|
| N (%) | N | %a (95% CI)a | Chi-square p value | N | %a (95% CI)a | Chi-square p value | |
| Age (years) | |||||||
| ≤ 29 | 746 (28.6) | 598 | 81.8 (78.8–84.6) | 0.228 | 65 | 9.0 (7.0–11.3) | 0.017 |
| 30–39 | 968 (37.1) | 808 | 84.3 (82.0–86.4) | 107 | 11.2 (9.4–13.3) | ||
| 40–49 | 547 (20.9) | 467 | 86.5 (83.4–89.3) | 73 | 12.9 (10.2–16.0) | ||
| 50–59 | 260 (10.0) | 219 | 85.0 (79.1–89.7) | 38 | 14.1 (9.5–19.9) | ||
| 60+ | 91 (3.5) | 79 | 87.0 (73.7–95.1) | 17 | 22.2 (11.2–37.1) | ||
| Nationality | |||||||
| All other nationalitiesb | 231 (8.8) | 217 | 95.4 (91.5–97.9) | < 0.001 | 52 | 23.2 (17.5–29.9) | < 0.001 |
| Bangladeshi | 626 (24.0) | 568 | 91.4 (89.1–93.4) | 73 | 11.2 (8.9–13.9) | ||
| Egyptian | 90 (3.4) | 85 | 96.3 (89.7–99.2) | 7 | 7.2 (2.7–15.1) | ||
| Filipino | 102 (3.9) | 78 | 75.7 (64.0–85.2) | 9 | 8.6 (3.2–17.7) | ||
| Indian | 717 (27.5) | 510 | 72.6 (69.2–75.7) | 87 | 12.7 (10.4–15.2) | ||
| Nepalese | 565 (21.6) | 505 | 90.2 (87.5–92.5) | 44 | 7.6 (5.5–10.1) | ||
| Pakistani | 136 (5.2) | 125 | 92.1 (86.0–96.2) | 12 | 8.8 (4.5–15.2) | ||
| Sri Lankan | 145 (5.6) | 83 | 58.8 (49.4–67.8) | 16 | 10.1 (5.3–17.0) | ||
| QRCS center (catchment area within Qatar) | |||||||
| Fereej Abdel Aziz (Doha-East) | 612 (23.4) | 496 | 82.0 (78.7–85.0) | 0.354 | 58 | 9.6 (7.4–12.2) | 0.358 |
| Hemaila (South-West; “Industrial Area”) | 966 (37.0) | 813 | 84.9 (82.6–97.0) | 113 | 11.8 (10.0–13.9) | ||
| Mesaimeer (Doha-South) | 800 (30.6) | 672 | 85.0 (82.4–87.4) | 93 | 11.7 (9.6–14.1) | ||
| Zekreet (North-West) | 234 (9.0) | 190 | 82.8 (70.6–91.4) | 36 | 15.5 (7.4–27.4) | ||
| Educational attainment | |||||||
| Primary or lower | 628 (25.0) | 561 | 89.5 (86.8–91.8) | < 0.001 | 88 | 13.5 (10.9–16.5) | 0.128 |
| Intermediate | 429 (17.1) | 377 | 90.0 (86.8–92.7) | 48 | 10.2 (7.5–13.4) | ||
| Secondary/High school/Vocational | 1088 (43.3) | 880 | 82.5 (80.1–84.7) | 118 | 11.5 (9.6–13.5) | ||
| University | 369 (14.7) | 265 | 71.3 (66.0–76.1) | 35 | 8.8 (6.0–12.3) | ||
| Occupation | |||||||
| Professional workersc | 136 (5.3) | 92 | 67.2 (58.1–75.4) | < 0.001 | 11 | 8.1 (3.9–14.3) | 0.113 |
| Food & beverage workers | 91 (3.6) | 71 | 80.0 (69.6–88.1) | 10 | 11.3 (5.3–20.3) | ||
| Administration workers | 82 (3.2) | 61 | 71.8 (60.5–81.4) | 2 | 2.6 (0.3–9.2) | ||
| Retail workers | 171 (6.7) | 142 | 87.2 (81.1–91.9) | 19 | 11.3 (7.0–17.1) | ||
| Transport workers | 430 (16.8) | 348 | 80.9 (76.8–84.6) | 58 | 12.3 (9.3–15.9) | ||
| Cleaning workers | 104 (4.1) | 91 | 90.0 (82.4–95.1) | 11 | 8.2 (3.6–15.5) | ||
| Technical and construction workersd | 1314 (51.3) | 1123 | 86.5 (84.5–88.2) | 151 | 11.6 (10.0–13.5) | ||
| Security workers | 60 (2.3) | 56 | 94.9 (85.9–98.9) | 6 | 10.2 (3.8–20.8) | ||
| Other workerse | 175 (6.8) | 148 | 86.2 (80.2–91.0) | 23 | 13.4 (8.7–19.4) | ||
| Total (%, 95% CI) | 2612 (100.0) | 2171 | 84.2 (82.8–85.6) | NA | 300 | 11.4 (10.1–12.6) | NA |
CI, confidence interval; HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2; NA, not applicable; QRCS, Qatar Red Crescent Society
aPercentage of positive out of those tested weighted by age, nationality, and QRCS center. Equivocal specimens were excluded from the analysis
bIncludes all other nationalities of craft and manual workers residing in Qatar
cIncludes architects, designers, engineers, operation managers, and supervisors among other professions
dIncludes carpenters, construction workers, crane operators, electricians, foremen, maintenance/air conditioning/cable technicians, masons, mechanics, painters, pipe-fitters, plumbers, and welders among other professions
eIncludes barbers, firefighters, gardeners, farmers, fishermen, and physical fitness trainers among other professions
Herpes simplex virus type 1
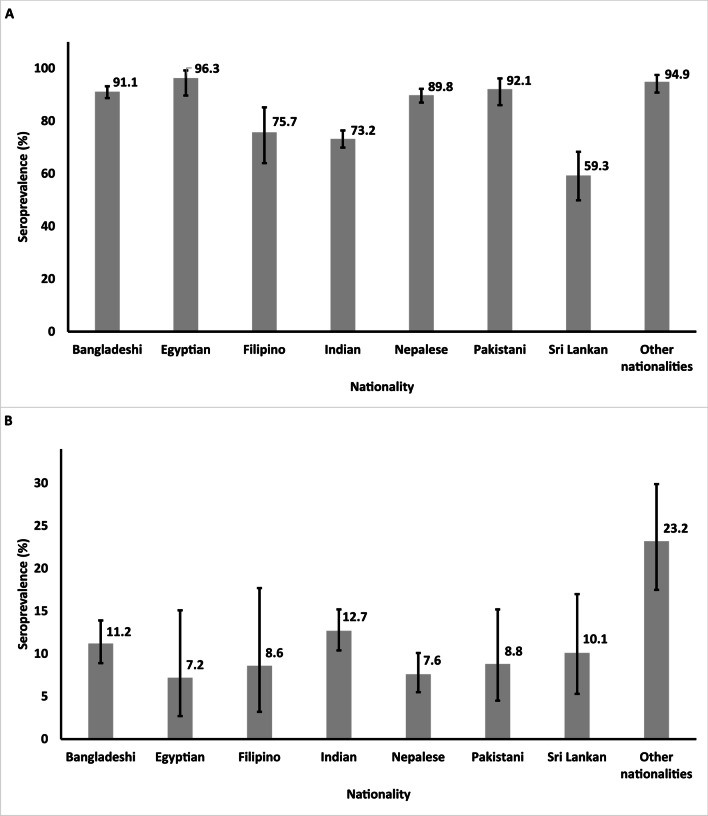
In total, 2,612 serum specimens were tested for HSV-1 antibodies (Table 1). The serological testing identified 2,171 sera as positive, 403 as negative, and 38 as equivocal. HSV-1 seroprevalence among the CMW population was estimated at 84.2% (95% CI 82.8–85.6%), after excluding equivocal specimens from both numerator and denominator of the ratio defining seroprevalence. Seroprevalence was highest among Egyptians at 96.3% (95% CI 89.7–99.2%) and lowest among Sri Lankans at 58.8% (95% CI 49.4–67.8%) (Fig. 1A).
Fig. 1.
HSV-1 (A) and HSV-2 (B) seroprevalence by nationality among the craft and manual worker population in Qatar. HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2
HSV-1 seropositivity was independently associated with each of nationality, educational attainment, and occupation (Table 2). Compared to “other” nationalities, significant differences in seropositivity were observed among Bangladeshis (AOR 0.36; 95% CI 0.17–0.77), Filipinos (AOR 0.20; 95% CI 0.08–0.49), Nepalese (AOR 0.31; 95% CI 0.14–0.66), Indians (AOR 0.11; 95% CI 0.05–0.23), Pakistanis (AOR 0.37; 95% CI 0.14–0.96), and Sri Lankans (AOR 0.06; 95% CI 0.03–0.12). Compared to workers with primary or lower education, AOR was 0.68 (95% CI 0.49–0.94) for workers with secondary/high school/vocational level education and 0.34 (95% CI 0.22–0.52) for workers with university level education. Compared to professional workers, AOR was 2.03 (95% CI 1.06–3.90) for retail workers. No association was found for age and QRCS center (proxy of catchment area/geographic location).
Table 2.
Associations with HSV-1 and HSV-2 seropositivity
| Characteristics | Anti-HSV-1 positive | Anti-HSV-2 positive | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariable regression analysis | Multivariable regression analysis | Univariable regression analysis | Multivariable regression analysis | |||||||
| ORa (95% CIa) | p value | F test p valueb |
AORa (95% CIa) | p valuec | ORa (95% CIa) | p value | F test p valueb |
AORa (95% CIa) | p valuec | |
| Age (years) | ||||||||||
| ≤ 29 | 1.00 | 0.218 | – | – | 1.00 | 0.027 | 1.00 | |||
| 30–39 | 1.19 (0.92–1.53) | 0.210 | – | – | 1.29 (0.94–1.78) | 0.120 | 1.36 (0.97–1.89) | 0.076 | ||
| 40–49 | 1.43 (0.99–1.95) | 0.053 | – | – | 1.52 (1.06–2.17) | 0.023 | 1.55 (1.06–2.26) | 0.020 | ||
| 50–59 | 1.25 (0.80–1.96) | 0.317 | – | – | 1.71 (1.06–2.76) | 0.029 | 1.77 (1.07–2.94) | 0.028 | ||
| 60+ | 1.59 (0.64–3.96) | 0.363 | – | – | 2.87 (1.35–6.11) | 0.006 | 2.79 (1.25–6.24) | 0.012 | ||
| Nationality | ||||||||||
| All other nationalitiesd | 1.00 | < 0.001 | 1.00 | 1.00 | < 0.001 | 1.00 | ||||
| Bangladeshi | 0.51 (0.25–1.05) | 0.066 | 0.36 (0.17–0.77) | 0.009 | 0.41 (0.27–0.62) | < 0.001 | 0.35 (0.22–0.54) | < 0.001 | ||
| Egyptian | 1.36 (0.35–5.39) | 0.659 | 1.66 (0.41–6.74) | 0.475 | 0.25 (0.10–0.61) | 0.002 | 0.21 (0.08–0.57) | 0.002 | ||
| Filipino | 0.15 (0.06–0.35) | < 0.001 | 0.20 (0.08–0.49) | < 0.001 | 0.29 (0.12–0.73) | 0.009 | 0.33 (0.13–0.84) | 0.021 | ||
| Indian | 0.13 (0.06–0.25) | < 0.001 | 0.11 (0.05–0.23) | < 0.001 | 0.47 (0.32–0.70) | < 0.001 | 0.41 (0.27–0.62) | < 0.001 | ||
| Nepalese | 0.44 (0.21–0.91) | 0.026 | 0.31 (0.14–0.66) | 0.003 | 0.26 (0.17–0.42) | < 0.001 | 0.23 (0.14–0.37) | < 0.001 | ||
| Pakistani | 0.54 (0.21–1.36)) | 0.188 | 0.37 (0.14–0.96) | 0.041 | 0.32 (0.16–0.64) | 0.001 | 0.26 (0.13–0.55) | 0.002 | ||
| Sri Lankan | 0.07 (0.03–0.15) | < 0.001 | 0.06 (0.03–0.12) | < 0.001 | 0.37 (0.18–0.72) | 0.004 | 0.32 (0.16–0.64) | < 0.001 | ||
| QRCS center (catchment area within Qatar) | ||||||||||
| Fereej Abdel Aziz (Doha-East) | 1.00 | 0.377 | – | – | 1.00 | 0.239 | – | – | ||
| Hemaila (South-West; “Industrial Area”) | 1.24 (0.95–1.61) | 0.118 | – | – | 1.27 (0.91–1.76) | 0.160 | – | – | ||
| Mesaimeer (Doha-South) | 1.05 (0.52–2.14) | 0.892 | – | – | 1.26 (0.89–1.78) | 0.185 | – | – | ||
| Zekreet (North-West) | 1.24 (0.94–1.65) | 0.113 | – | – | 1.76 (0.83–3.74) | 0.142 | – | – | ||
| Educational attainment | ||||||||||
| Primary or lower | 1.00 | < 0.001 | 1.00 | 1.00 | 0.054 | 1.00 | ||||
| Intermediate | 0.98 (0.66–1.46) | 0.906 | 1.09 (0.71–1.65) | 701 | 0.72 (0.49–1.06) | 0.100 | 0.74 (0.49–1.09) | 0.833 | ||
| Secondary/High school/Vocational | 0.54 (0.40–0.73) | < 0.001 | 0.68 (0.49–0.94) | 0.018 | 0.83 (0.62–1.12) | 0.219 | 0.77 (0.57–0.92) | 0.012 | ||
| University | 0.30 (0.21–0.43) | < 0.001 | 0.34 (0.22–0.52) | < 0.001 | 0.61 (0.39–0.96) | 0.032 | 0.43 (0.28–0.76) | < 0.001 | ||
| Occupation | ||||||||||
| Professional workerse | 1.00 | < 0.001 | 1.00 | 1.00 | 0.251 | – | – | |||
| Food & beverage workers | 2.02 (0.98–3.93) | 0.056 | 0.89 (0.42–1.90) | 0.770 | 1.43 (0.55–3.73) | 0.469 | – | – | ||
| Administration workers | 1.27 (0.68–2.37) | 0.452 | 0.83 (0.42–1.66) | 0.602 | 0.29 (0.06–1.44) | 0.129 | – | – | ||
| Retail workers | 3.33 (1.84–6.02) | < 0.001 | 2.03 (1.06–3.90) | 0.034 | 1.47 (0.65–3.33) | 0.353 | – | – | ||
| Transport workers | 2.10 (1.34–3.30) | 0.001 | 1.21 (0.71–3.70) | 0.483 | 1.63 (0.80–3.34) | 0.182 | – | – | ||
| Cleaning workers | 4.28 (2.03–9.01) | < 0.001 | 1.61 (0.70–3.67) | 0.261 | 1.05 (0.40–2.78) | 0.920 | – | – | ||
| Technical and construction workersf | 3.15 (3.09–4.73) | < 0.001 | 1.47 (0.90–2.40) | 0.123 | 1.54 (0.78–3.02) | 0.212 | – | – | ||
| Security workers | 8.05 (2.56–25.43) | < 0.001 | 2.05 (0.61–6.91) | 0.245 | 1.40 (0.49–4.00) | 0.534 | – | – | ||
| Other workersg | 3.15 (1.77–5.59) | < 0.001 | 1.45 (0.76–2.79) | 0.259 | 1.83 (0.84–4.01) | 0.131 | – | – | ||
AOR, adjusted odds ratio; CI, confidence interval; HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2; OR, odds ratio; QRCS, Qatar Red Crescent Society
aEstimates weighted by age, nationality, and QRCS center
bCovariates with p value ≤ 0.2 in the univariable analysis were included in the multivariable analysis
cCovariates with p value ≤ 0.05 in the multivariable analysis were considered to provide statistically significant evidence for an association with antibody positivity
dIncludes all other nationalities of craft and manual workers residing in Qatar
eIncludes architects, designers, engineers, operation managers, and supervisors among other professions
fIncludes carpenters, construction workers, crane operators, electricians, foremen, maintenance/air conditioning/cable technicians, masons, mechanics, painters, pipe-fitters, plumbers, and welders among other professions
gIncludes barbers, firefighters, gardeners, farmers, fishermen, and physical fitness trainers among other professions
Herpes simplex virus type 2
In total, 2,612 serum specimens were tested for HSV-2 antibodies (Table 1). The serological testing identified 300 sera as positive, 2,250 as negative, and 62 as equivocal. HSV-2 seroprevalence among the CMW population was estimated at 11.4% (95% CI 10.1–12.6%), after excluding equivocal specimens from both numerator and denominator of the ratio defining seroprevalence. Seroprevalence was highest among “other” nationalities at 23.2% (95% CI 17.5–29.9%) and lowest among Egyptians at 7.2% (95% CI 2.7–15.1%) (Fig. 1B).
HSV-2 seropositivity was independently associated with each of age, nationality, and educational attainment (Table 2). The AOR increased with age. Compared to those aged ≤ 29 years, AOR was 1.55 (95% CI 1.06–2.26) for those aged 40–49 years and 2.79 (95% CI 1.25–6.24) for those aged over 60 years. Compared to “other” nationalities, significant differences in seropositivity were observed among all nationalities. Compared to workers with primary or lower education, AOR was 0.77 (95% CI 0.57–0.92) for workers with secondary/high school/vocational level education and 0.43 (95% CI 0.28–0.76) for workers with university level education. No association was found for QRCS center and occupation.
Discussion
HSV-1 seroprevalence was overall high in the CMW population of Qatar at a level that exceeded 80%. However, there was substantial variation in seroprevalence by nationality ranging from 58.8% among Sri Lankans to 96.3% among Egyptians. Seroprevalence in each nationality group was similar to the seroprevalence observed in the country of origin [25–27], but not to that of Qataris [28]. This may be explained by these workers arriving to Qatar only within the last decade and not being permanent residents of Qatar. Since HSV-1 is mostly acquired orally during childhood [1, 25, 26, 29–33], these workers have likely acquired the infection before coming to Qatar. Seroprevalence did not vary by age, further supporting that HSV-1 acquisition occurred in childhood. Against paucity of HSV-1 seroprevalence data in South Asia [25], this study provides HSV-1 seroprevalence data for different South Asian nationalities, including what appears to be the first one for Nepalese in the literature.
Remarkably, this study confirms an unexplained anomaly in global HSV-1 seroprevalence data [27, 28], the low HSV-1 seroprevalence in Sri Lanka and India compared to what is expected for countries of similar socio-economic status [25, 26, 28–30]. HSV-1 seroprevalence among Sri Lankans and Indians was lowest of all nationalities, supporting the relatively low HSV-1 seroprevalence reported previously in this part of the world [25, 34, 35].
HSV-2 seroprevalence varied by nationality but was overall at about 10%, in the intermediate range compared to global data on HSV-2 seroprevalence among men [1, 36–42]. HSV-2 seroprevalence increased with age, consistent with continuing acquisition of this infection in adulthood. These findings suggest presence of genital herpes and genital ulcer disease burden in the CMW population, but this burden remains untackled and poorly documented in context of limited programs for sexual health and sexually transmitted infections.
HSV-2 seroprevalence in each nationality was similar to the seroprevalence in the country of origin, and higher than that observed among Qataris and in other countries of the Middle East and North Africa [41, 43, 44]. This is also explained by these workers arriving only within the last decade to Qatar. Against paucity of HSV-2 seroprevalence data in South Asia [36], this study provides HSV-2 seroprevalence data for different South Asian nationalities, including what appears to be the first one for Nepalese in the literature.
HSV-1 seroprevalence as well as HSV-2 seroprevalence were lower with higher socio-economic status as measured by education and occupation. This finding supports the inverse association observed in different countries between HSV-1 and HSV-2 infections and socio-economic status [28, 45, 46].
This study has limitations. While the study design was intended to be based on probability-based sampling of the total CMW population in Qatar, operational challenges forced instead a systematic sampling of QRCS attendees supplemented with probability-based weights to generate an estimate that is representative of the wider CMW population. To ensure representation of small age-nationality strata, all attendees in these strata (not only every 4th attendee) were approached to participate towards the end of the study.
Operational challenges made it also difficult to track and maintain consistent logs of the response rate by the nurses in these QRCS centers. Therefore, an exact estimate of the response rate could not be ascertained, though it was estimated based on the interviewers’ experience at > 90%. While it is possible that the recruitment scheme may have affected the generalizability of study findings, this is less likely considering that CMWs attend these centers at a high volume that exceeds 5,000 patients per day and for a range of services beyond illness such as periodic health certifications, vaccinations, and pre-travel SARS-CoV-2 testing.
Only socio-demographic variables were collected, not including sexual behavior data. Collecting sexual behavior data is difficult in this culturally conservative setting, but this difficulty highlights the value of using HSV-2 seroprevalence as a proxy biomarker of population sexual risk behavior, as demonstrated previously [44, 47]. We used a unified statistical analysis plan for both infections that employed logistic regression analyses with the associations estimated in terms of ORs. However, for highly prevalent infections such as HSV-1, the ORs are too sensitive to even small changes in seroprevalence. Too large or too small ORs may not necessarily imply large differences in actual seroprevalence.
Since the sample consisted exclusively of men, it was not possible to investigate differences in seroprevalence by sex. However, existing evidence from systematic reviews and meta-regressions suggests that HSV-2 seroprevalence is approximately 30–40% lower among men compared to women [36–41]. On the other hand, HSV-1 seroprevalence does not typically show differences by sex, as it is primarily acquired orally during childhood [25, 26, 29–33].
Though we used quality, validated, and widely used FDA-approved commercial assays, existing data suggests potential population variation in assay sensitivity and specificity [48], which may affect the estimated seroprevalence. A number of specimens tested equivocal even after repeat testing and were excluded from analysis. However, the number of equivocal specimens was relatively small to appreciably affect study findings.
Conclusions
In conclusion, over 80% of CMWs in Qatar are infected with HSV-1, and over 10% are infected with HSV-2. These findings highlight the need for monitoring of trends in HSV-1 and HSV-2 seroprevalence and etiological surveillance of genital ulcer disease, genital herpes, and other HSV-related morbidities. The findings stress the need for programs to tackle sexually transmitted infections and address broader sexual health needs of this population. The findings also support the relevance of HSV prophylactic and therapeutic vaccine development [49], as well as further epidemiological research on HSV infection in this population to address its healthcare needs.
Supplementary Information
Additional file 1: Table S1. STROBE checklist for cross-sectional studies.
Acknowledgements
We thank all participants for their willingness to be part of this study. We thank all members of the Craft and Manual Workers Seroprevalence Study Group for their efforts in supporting the collection of the samples of this study. We also thank Dr. Nahla Afifi, Director of Qatar Biobank (QBB), Ms. Tasneem Al-Hamad, Ms. Eiman Al-Khayat, and the rest of the QBB team for their unwavering support in retrieving and analyzing samples. We also acknowledge the dedicated efforts of the Surveillance Team at the Ministry of Public Health for their support in sample collection.
Author contributions
GKN and LJA co-conceived, co-designed, and co-led the study. DWA, FHA, FMS, PBN conducted the lab testing of the specimens. SRD and HC developed the study design and managed the databases. SRD performed the data analyses and wrote the first draft of the article. LJA led the statistical analyses and drafting of the article. All authors contributed to development of study protocol, data collection and acquisition, database development, discussion and interpretation of the results, and to the writing of the manuscript. All authors have read and approved the final manuscript.
Funding
The authors are grateful for support from the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine-Qatar, as well as for support provided by the Ministry of Public Health and Hamad Medical Corporation. HHA acknowledges the support of Qatar University internal grant QUCG-CAS-23/24-114. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article. Statements made herein are solely the responsibility of the authors.
Availability of data and materials
All data are available in aggregate form within the manuscript.
Declarations
Ethics approval and consent to participate
Hamad Medical Corporation and Weill Cornell Medicine-Qatar Institutional Review Boards approved this retrospective study with a waiver of informed consent.
Consent for publication
Not applicable.
Competing interests
We declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gheyath K. Nasrallah, Email: gheyath.nasrallah@qu.edu.qa
Laith J. Abu-Raddad, Email: lja2002@qatar-med.cornell.edu
References
- 1.James C, Harfouche M, Welton NJ, Turner KM, Abu-Raddad LJ, Gottlieb SL, Looker KJ. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98:315–329. doi: 10.2471/BLT.19.237149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang ML, Corey L. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198:1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Magaret A, Wald A, Corey L. Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Sci Transl Med. 2009;1:7ra16. doi: 10.1126/scitranslmed.3000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramchandani M, Kong M, Tronstein E, Selke S, Mikhaylova A, Magaret A, Huang ML, Johnston C, Corey L, Wald A. Herpes simplex virus type 1 shedding in tears and nasal and oral mucosa of healthy adults. Sex Transm Dis. 2016;43:756–760. doi: 10.1097/OLQ.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady RC, Bernstein DI. Treatment of herpes simplex virus infections. Antiviral Res. 2004;61:73–81. doi: 10.1016/j.antiviral.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol 2007;57:737–763; quiz 764–736. [DOI] [PubMed]
- 7.Garland SM, Steben M. Genital herpes. Best Pract Res Clin Obstet Gynaecol. 2014;28:1098–1110. doi: 10.1016/j.bpobgyn.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370:2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 9.Looker KJ, Magaret AS, May MT, Turner KME, Vickerman P, Newman LM, Gottlieb SL. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health. 2017;5:e300–e309. doi: 10.1016/S2214-109X(16)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinninti SG, Kimberlin DW. Neonatal herpes simplex virus infections. Semin Perinatol. 2018;42:168–175. doi: 10.1053/j.semperi.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Ayoub HH, Chemaitelly H, Abu-Raddad LJ. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: model-based predictions. BMC Med. 2019;17:57. doi: 10.1186/s12916-019-1285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein DI, Bellamy AR, Hook EW, 3rd, Levin MJ, Wald A, Ewell MG, Wolff PA, Deal CD, Heineman TC, Dubin G, Belshe RB. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56:344–351. doi: 10.1093/cid/cis891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Looker KJ, Elmes JAR, Gottlieb SL, Schiffer JT, Vickerman P, Turner KME, Boily MC. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303–1316. doi: 10.1016/S1473-3099(17)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Raddad LJ, Magaret AS, Celum C, Wald A, Longini IM, Jr, Self SG, Corey L. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS ONE. 2008;3:e2230. doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Looker KJ, Welton NJ, Sabin KM, Dalal S, Vickerman P, Turner KM, Boily M-C, Gottlieb SL. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: A population attributable fraction analysis using published epidemiological data. Lancet Infect Dis. 2020;20:240–249. doi: 10.1016/S1473-3099(19)30470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omori R, Nagelkerke N, Abu-Raddad LJ. HIV and herpes simplex virus type 2 epidemiological synergy: misguided observational evidence? A modelling study. Sex Transm Infect. 2018;94:372–376. doi: 10.1136/sextrans-2017-053336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottlieb SL, Deal CD, Giersing B, Rees H, Bolan G, Johnston C, Timms P, Gray-Owen SD, Jerse AE, Cameron CE, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: Update and next steps. Vaccine. 2016;34:2939–2947. doi: 10.1016/j.vaccine.2016.03.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva S, Ayoub HH, Johnston C, Atun R, Abu-Raddad LJ. Estimated economic burden of genital herpes and HIV attributable to herpes simplex virus type 2 infections in 90 low- and middle-income countries: A modeling study. PLoS Med. 2022;19:e1003938. doi: 10.1371/journal.pmed.1003938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Al Kanaani Z, Al Khal A, Al Kuwari E, Butt AA, Coyle P, Jeremijenko A, Kaleeckal AH, et al. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci Rep. 2021;11:6233. doi: 10.1038/s41598-021-85428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Thani MH, Farag E, Bertollini R, Al Romaihi HE, Abdeen S, Abdelkarim A, Daraan F, Elhaj Ismail AIH, Mostafa N, Sahl M, et al. SARS-CoV-2 infection is at herd immunity in the majority segment of the population of Qatar. Open Forum Infect Dis. 2021;8:ofab221. doi: 10.1093/ofid/ofab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeremijenko A, Chemaitelly H, Ayoub HH, Alishaq M, Abou-Samra AB, Al Ajmi J, Al Ansari NAA, Al Kanaani Z, Al Khal A, Al Kuwari E, et al. Herd Immunity against severe acute respiratory syndrome coronavirus 2 infection in 10 communities. Qatar Emerg Infect Dis. 2021;27:1343–1352. doi: 10.3201/eid2705.204365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministry of Interior-State of Qatar: Population distribution by sex, age, and nationality: results of Kashef database. 2020.
- 23.Planning and Statistics Authority-State of Qatar: Labor force sample survey. Available from: https://www.psa.gov.qa/en/statistics/Statistical%20Releases/Social/LaborForce/2017/statistical_analysis_labor_force_2017_En.pdf. Accessed on May 23, 2022; 2017.
- 24.World Health Organization: Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection. Available from: https://apps.who.int/iris/handle/10665/331656. Accessed on: April 15, 2020. 2020.
- 25.Khadr L, Harfouche M, Omori R, Schwarzer G, Chemaitelly H, Abu-Raddad LJ. The epidemiology of herpes simplex virus type 1 in Asia: systematic review, meta-analyses, and meta-regressions. Clin Infect Dis. 2019;68:757–772. doi: 10.1093/cid/ciy562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaabane S, Harfouche M, Chemaitelly H, Schwarzer G, Abu-Raddad LJ. Herpes simplex virus type 1 epidemiology in the Middle East and North Africa: systematic review, meta-analyses, and meta-regressions. Sci Rep. 2019;9:1136. doi: 10.1038/s41598-018-37833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasrallah G, Dargham S, Harfouche M, Abu-Raddad L. Seroprevalence of Herpes simplex virus types 1 and 2 in Indian and Filipino migrant populations in Qatar: a cross-sectional survey. East Mediterr Health J. 2020;26:609–615. doi: 10.26719/2020.26.5.609. [DOI] [PubMed] [Google Scholar]
- 28.Nasrallah GK, Dargham SR, Mohammed LI, Abu-Raddad LJ. Estimating seroprevalence of herpes simplex virus type 1 among different Middle East and North African male populations residing in Qatar. J Med Virol. 2018;90:184–190. doi: 10.1002/jmv.24916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harfouche M, Chemaitelly H, Abu-Raddad LJ. Herpes simplex virus type 1 epidemiology in Africa: systematic review, meta-analyses, and meta-regressions. J Infect. 2019;79:289–299. doi: 10.1016/j.jinf.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Sukik L, Alyafei M, Harfouche M, Abu-Raddad LJ. Herpes simplex virus type 1 epidemiology in Latin America and the Caribbean: Systematic review and meta-analytics. PLoS ONE. 2019;14:e0215487. doi: 10.1371/journal.pone.0215487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yousuf W, Ibrahim H, Harfouche M, Abu Hijleh F, Abu-Raddad L. Herpes simplex virus type 1 in Europe: systematic review, meta-analyses and meta-regressions. BMJ Glob Health. 2020;5:e002388. doi: 10.1136/bmjgh-2020-002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AlMukdad S, Harfouche M, Farooqui US, Aldos L, Abu-Raddad LJ. Epidemiology of herpes simplex virus type 1 and genital herpes in Australia and New Zealand: systematic review, meta-analyses and meta-regressions. Epidemiol Infect. 2023;151:e33. doi: 10.1017/S0950268823000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AlMukdad S, Harfouche M, Farooqui US, Aldos L, Abu-Raddad LJ. Epidemiology of herpes simplex virus type 1 in Canada: systematic review, meta-analyses, and meta-regressions. Front Public Health. 2023;11:1118249. doi: 10.3389/fpubh.2023.1118249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patwardhan V, Bhalla P. Role of type-specific herpes simplex virus-1 and 2 serology as a diagnostic modality in patients with clinically suspected genital herpes: A comparative study in Indian population from a tertiary care hospital. Indian J Pathol Microbiol. 2016;59:318–321. doi: 10.4103/0377-4929.188104. [DOI] [PubMed] [Google Scholar]
- 35.Kaur R, Gupta N, Baveja UK. Seroprevalence of HSV1 and HSV2 infections in family planning clinic attenders. J Commun Dis. 2005;37:307–309. [PubMed] [Google Scholar]
- 36.AlMukdad S, Harfouche M, Wettstein A, Abu-Raddad LJ. Epidemiology of herpes simplex virus type 2 in Asia: a systematic review, meta-analysis, and meta-regression. Lancet Regional Health Western Pacific. 2021;12:100176. doi: 10.1016/j.lanwpc.2021.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harfouche M, Abu-Hijleh FM, James C, Looker KJ, Abu-Raddad LJ. Epidemiology of herpes simplex virus type 2 in sub-Saharan Africa: Systematic review, meta-analyses, and meta-regressions. EClinicalMedicine. 2021;35:100876. doi: 10.1016/j.eclinm.2021.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harfouche M, Maalmi H, Abu-Raddad LJ. Epidemiology of herpes simplex virus type 2 in Latin America and the Caribbean: systematic review, meta-analyses and metaregressions. Sex Transm Infect 2021. [DOI] [PMC free article] [PubMed]
- 39.Alareeki A, Osman AMM, Khandakji MN, Looker KJ, Harfouche M, Abu-Raddad LJ. Epidemiology of herpes simplex virus type 2 in Europe: systematic review, meta-analyses, and meta-regressions. Lancet Reg Health Eur. 2023;25:100558. doi: 10.1016/j.lanepe.2022.100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.AlMukdad S, Farooqui US, Harfouche M, Aldos L, Abu-Raddad LJ. Epidemiology of herpes simplex virus type 2 in Canada, Australia, and New Zealand: systematic review, meta-analyses, and meta-regressions. Sex Transm Dis. 2022;49:403–413. doi: 10.1097/OLQ.0000000000001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harfouche M, Alareeki A, Osman AMM, Alaama AS, Hermez JG, Abu-Raddad LJ. Epidemiology of herpes simplex virus type 2 in the Middle East and North Africa: systematic review, meta-analyses, and meta-regressions. J Med Virol 2023. [DOI] [PubMed]
- 42.Ayoub HH, Amara I, Awad SF, Omori R, Chemaitelly H, Abu-Raddad LJ. Analytic Characterization of the Herpes Simplex Virus Type 2 Epidemic in the United States, 1950–2050. Open Forum Infect Dis. 2021;8:ofab218. doi: 10.1093/ofid/ofab218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dargham SR, Nasrallah GK, Al-Absi ES, Mohammed LI, Al-Disi RS, Nofal MY, Abu-Raddad LJ. Herpes simplex virus type 2 seroprevalence among different national populations of middle east and North African men. Sex Transm Dis. 2018;45:482–487. doi: 10.1097/OLQ.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abu-Raddad LJ, Schiffer JT, Ashley R, Mumtaz G, Alsallaq RA, Akala FA, Semini I, Riedner G, Wilson D. HSV-2 serology can be predictive of HIV epidemic potential and hidden sexual risk behavior in the Middle East and North Africa. Epidemics. 2010;2:173–182. doi: 10.1016/j.epidem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999–2010. J Infect Dis. 2014;209:325–333. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 46.Smith J, Robinson N. Age-SPecific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186:S3–S28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 47.Omori R, Abu-Raddad LJ. Sexual network drivers of HIV and herpes simplex virus type 2 transmission. AIDS. 2017;31:1721–1732. doi: 10.1097/QAD.0000000000001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashley-Morrow R, Nollkamper J, Robinson NJ, Bishop N, Smith J. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect. 2004;10:530–536. doi: 10.1111/j.1469-0691.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 49.Ayoub HH, Chemaitelly H, Abu-Raddad LJ. Epidemiological impact of novel preventive and therapeutic HSV-2 vaccination in the united states: mathematical modeling analyses. Vaccines (Basel) 2020;8:366. doi: 10.3390/vaccines8030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. STROBE checklist for cross-sectional studies.
Data Availability Statement
All data are available in aggregate form within the manuscript.