Abstract
Background
Despite the significant progress made in South Africa in getting millions of individuals living with HIV into care, many patients still present or re-enter care with Advanced HIV Disease (AHD). We aimed to estimate the prevalence of AHD among ART-naive and ART-experienced patients in South Africa using studies published between January 2010 and May 2022.
Methods
We searched for relevant data on PubMed, CINAHL, Scopus and other sources, with a geographical filters limited to South Africa, up to May 31, 2022. Two reviewers conducted all screening, eligibility assessment, data extraction, and critical appraisal. We synthesized the data using the inverse-variance heterogeneity model and Freeman-Tukey transformation. We assessed heterogeneity using the I2 statistic and publication bias using the Egger and Begg’s test.
Results
We identified 2,496 records, of which 53 met the eligibility criteria, involving 11,545,460 individuals. The pooled prevalence of AHD among ART-naive and ART-experienced patients was 43.45% (95% CI 40.1–46.8%, n = 53 studies) and 58.6% (95% CI 55.7 to 61.5%, n = 2) respectively. The time trend analysis showed a decline of 2% in the prevalence of AHD among ART-naive patients per year. However, given the high heterogeneity between studies, the pooled prevalence should be interpreted with caution.
Conclusion
Despite HIV’s evolution to a chronic disease, our findings show that the burden of AHD remains high among both ART-naive and ART-experienced patients in South Africa. This emphasizes the importance of regular measurement of CD4 cell count as an essential component of HIV care. In addition, providing innovative adherence support and interventions to retain ART patients in effective care is a crucial priority for those on ART.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-023-08521-4.
Keywords: Burden, Advanced HIV disease, ART-naïve, ART-experienced, South Africa
Background
Efforts to quickly expand access to human immunodeficiency virus (HIV) care and treatment have led to a significant number of individuals starting antiretroviral therapy (ART). The most notable increase has occurred in South Africa, which is home to 7.5 million of the estimated 37.7 million people living with HIV (PLHIV) [1]. Access to ART in South Africa has grown from 103,300 to 2005 to 5,500,000 in 2021. Despite significant progress in expanding access to ART, the burden of Advanced HIV Disease (AHD) in South Africa remains high [1–4]. AHD is defined as a CD4 count of fewer than 200 cells/mm3 or a WHO stage 3 or 4 event in adults and adolescents, and all children with HIV younger than five years old are considered to have AHD [5, 6]. The proportion of people presenting with AHD has remained relatively unchanged in the past five years, despite the number of PLHIV receiving ART in low- and middle-income countries (LMIC) more than doubling over this period [4, 7, 8]. These patients are at high risk of death mainly due to Tuberculosis and cryptococcal meningitis, even after starting ART (which can increase the inflammatory response) and the risk increases with decreasing CD4 cell count [9].
AHD is prevalent in Sub-Saharan Africa (SSA), with studies reporting between 32% and 71% of patients initiating care with AHD, and up to 60% of patients presenting for care with AHD after disengagement [10, 11]. A large multi-country cohort study using data from HIV treatment programs in 6 SSA countries during 2005–2018 showed that South Africa had the highest percentage of adults with AHD (59.7%) starting ART. However, the study also noted that the percentage of adults starting ART with AHD steadily declined over time [4].
Contrary to the expectation that PLHIV will progress from testing and early ART initiation to consistent drug adherence and viral suppression, many PLHIV instead need assistance re-engaging in care with AHD following treatment failure or interruption [12]. This is a concerning trend observed in countries with established HIV programs, where an increasing number of patients present for care with AHD after a period of treatment interruption or ART-experience [13]. In a study by Osler et al. [2] in South Africa, the proportion of ART-experienced patients with a CD4 count less than 50 cells/mm3 increased from 14.3% to 2008 to 56.7% in 2017 and this group seeks hospital care following ART interruption or virological failure.
Previous meta-analysis studies have primarily focused on analysing temporal trends of CD4 count at presentation to care and treatment initiation, globally [14], in developed countries [15] and in low and middle-income countries [16]. It is crucial to estimate the prevalence of AHD among ART-naive and ART-experienced patients to understand the outcomes of ART programs, guide HIV prevention efforts, and predict the need for adjunctive therapies [17–19]. The true estimates of AHD among ART-naïve and ART-experienced patients in South Africa are not well known. This systematic review and meta-analysis aimed to estimate the prevalence of AHD among ART-naive and ART-experienced patients in South Africa, using data from studies published between January 1, 2010 and May 31, 2022. Furthermore, we sought to examine trends in the prevalence of AHD throughout this period.
Methods
This study employed a systematic review and meta-analysis design and the reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement [20]. The protocol for this systematic review was registered and published on the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number: CRD42021245429.
Criteria for considering studies for review
Type of studies
We included both published and unpublished observational studies (such as cross-sectional and cohort studies), as well as HIV program data. Additionally, we included population-based cross-sectional studies or surveys. Studies had to be published between January 1, 2010 and May 31, 2022 to be eligible. However, we excluded experimental studies, case studies, case series, duplicate reports, commentaries, and reviews from our analysis.
Condition
Published and unpublished needs Number of patients with AHD., We defined AHD as any CD4 < 200 cells/mm3 or a WHO stage 3 or 4 events. All children with HIV younger than five years old should be considered as having AHD. We calculated the prevalence by dividing the number of observed AHD by the total number of observed PLHIV on ART and expressed it as a percentage.
Context
We included studies conducted in South Africa between January 1, 2010 and May 31, 2022.
Population
ART-naïve and ART-experienced (all age groups) patients were considered for this review.
Search strategy and data source
We conducted a comprehensive search of studies in various databases including PubMed, CINAHL, Scopus, Scielo, and Africa Wide, with a geographical limited to South Africa. A hand search of citations from selected studies was conducted to identify additional studies missing from the original electronic searches and sought input from experts for any additional studies. Conference proceedings of the Conference on Retrovirus and Opportunistic Infections (CROI) and International Aids Society Conference (IAS) were screened to retrieve information from any further study that may not have been included in the electronic databases. Our search was conducted on July 16, 2021 and updated on May 31, 2022, and we limited the search to studies published between January 1, 2010 and May 31, 2022 and no language restriction was applied. The specific electronic search strategy is outlined in supplementary appendix 1.
Screening of studies for inclusion
The records of identified studies from the search databases were imported into COVIDENCE [https://app.covidence.org/], a software program used for managing systematic reviews. We removed duplicate abstracts before screening. Two reviewers, Marcel Kitenge (MK) and Omololu Aluko (OA), screened studies for initial inclusion using titles and abstracts. Both MK and OA independently evaluated the full text of studies that met the criteria for full-text screening, using a predefined eligibility checklist. Any disagreements about study eligibility were resolved through discussion and consensus.
Data extraction
After identifying eligible studies, two reviewers (MK and OA) independently extracted data from each study using a standardised pre-piloted data extraction form in COVIDENCE. We also extracted information on the characteristics of included studies, such as authors, citations, year of publication, study period, provinces, populations (whether inpatient or outpatient), study design used, and median CD4 count at enrolment. In case of missing information, we clarified the conducted study or the studies that had relevant data, which were not reported in the published manuscript, we contacted the authors for additional information.
Assessment of study quality and risk of bias
We evaluated the methodological quality of the included studies by assessing their internal validity, and generalizability of results. A ten-item rating system developed by Hoy et al. [21] was used to evaluate aspects such as sampling, sample size, outcome measurement, outcome assessment, and statistical reporting. Each item was assigned a score of 1 (yes) or 0 (no), and the scores were summed to generate an overall quality score ranging from 0 to 10. Each study was classified as having a low, moderate, or high risk of bias based on the number of questions answered as “yes”. Studies with scores higher than 8 were classified as low risk, 6–8 as moderate risk, and 5 or lower as high risk. Two reviewers (MK and OA) independently assessed study quality, and any discrepancies in rating were resolved through discussions and consensus. Eligibility assessment, data extraction, and assessment of the risk of bias were performed using COVIDENCE.
Data analysis
We pooled data on the prevalence of AHD using STATA version 17 [22] and the metaprop package [23]. We employed a random-effects meta-analysis framework as we anticipated variability in the prevalence estimates from different studies. The package models the prevalence estimates using the exact binomial distribution and then applies the Freeman-Turkey double arcsine variance stabilizing transformations, normalizing the estimates before pooling and then back-transforming the estimates. The pooled estimates were then computed using the method described by DeSimonian and Kacker [24].
We assessed heterogeneity between studies using Cochran’s Q statistic [25] (expressed as X2 and p-values) and the I2 statistic. We also explored sources of heterogeneity through subgroup analyses and meta-regression analysis. The following study characteristics were evaluated using univariate meta-regression models: study design (cross-sectional or non-cross-sectional), sample size (continuous), median CD4 count, methodology quality score, and publication year. Given included studies had substantial heterogeneity, we conducted a sensitivity analysis to investigate if the results were influenced by a single study. Due to high degree of heterogeneity, we presented pooled estimates with corresponding prediction intervals as prediction intervals are a more conservative way to incorporate uncertainty in the analysis.
We also estimated trend or change in prevalence of AHD among ART-naive patients overtime by fitting meta-regression model, modelling it as a weighted linear function of calendar year. We also assessed the presence of publication bias by examining funnel plots, and formally using statistical testing such as the Egger test [26] and the Begg’s test [27]. A p-value < 0.1 was considered as indicating significant publication bias.
Results
Search results
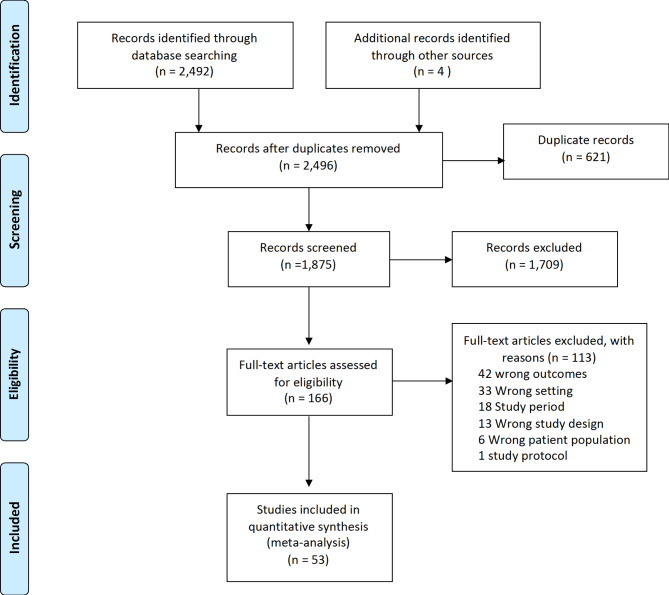
We identified 2,496 records from searches. After removing duplicate and excluding 1,709 irrelevant records, the full texts of 166 studies were assessed for eligibility; 53 of these were included in this systematic review (Fig. 1). The reasons for exclusion are listed in Fig. 1.
Fig. 1.
PRISMA flowchart showing search, selection of and final included studies
Characteristics of included studies
The 53 studies published between January 2010 to January 2022 consisted of a total of 11,545,460 individuals who were HIV positive. Twenty-four of the studies were cross-sectional [13, 28–50]; two were cross-sectional population surveys [51, 52], thirteen were longitudinal analyses using routine electronic HIV programmatic data [2–4, 53–62]; ten were prospective cohort studies [63–72] and the remaining 4 were retrospective cohort studies [73–76]. The study population for 48 studies was among adults: two studies enrolled adolescents [50, 62] and three enrolled children [30, 38, 39].
Five studies enrolled inpatients while the remaining enrolled outpatients. We provided detailed information about the included studies and summarised key features in Table 1.
Table 1.
Characteristics of included studies
| Study authors | Year | Province | Study design | Study period | Total sample | Number of AHD | Assessment method | Population | ART status* | Median CD4 | Setting | ART-experienced Total sample |
Number of AHD (ART-experienced) | Summary score | Risk of bias | Refence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adeniyi | 2018 | Eastern Cape | Cohort (P)** | 2015–2016 | 594 | 307 | CD4 | Adults | Naive | Outpatients | 8 | Moderate | [69] | |||
| Bock | 2018 | Western Cape | Cohort (P)** | 2014–2015 | 2423 | 631 | CD4 | Adults | Naive | 328 | Outpatients | 8 | Moderate | [70] | ||
| Boulle | 2014 | National | Cohort (R)** | 2005–2010 | 30,467 | 24,193 | CD4 | Adults | Naive | 102 | Outpatients | 7 | Moderate | [54] | ||
| Carmona | 2018 | National | Cohort (R)** | 2005–2016 | 8,036,919 | 2,973,032 | CD4 | Adults | Naive | Outpatients | 7 | Moderate | [3] | |||
| Cassidy | 2022 | Western Cape | Cohort (R)** | 2008–2018 | 5738 | 1349 | CD4 | Adolescents | Naive | 318 | Outpatients | 9 | Low | [62] | ||
| Chihana | 2019 | KwaZulu-Natal | Cross-sectional | 2013 | 1400 | 130 | CD4 | Adults | Both | Outpatients | 10 | Low | [52] | |||
| Cholera | 2017 | Gauteng | Cross-sectional | 2013 | 301 | 159 | CD4 | Adults | Naive | 188 | Outpatients | 10 | Low | [41] | ||
| Clouse | 2013 | Gauteng | Cohort (P) | 2010 | 1430 | 743 | CD4 | Adults | Naive | 268 | Outpatients | 10 | Low | [65] | ||
| Conan | 2019 | KwaZulu-Natal | Cross-sectional | 2018 | 834 | 38 | CD4 | Adults | Naive | 483 | Outpatients | 10 | Low | [51] | ||
| Dorward | 2020 | KwaZulu-Natal | Cross-sectional | 2017 | 390 | 93 | CD4 | Adults | Naive | 366 | Outpatients | 7 | Moderate | [48] | ||
| DrainPaul | 2013 | KwaZulu-Natal | Cross-sectional | 2011 | 830 | 279 | CD4 | Adults | Naive | 186 | Outpatients | 8 | Moderate | [36] | ||
| Dramowski | 2011 | Gauteng | Cross-sectional | 2007 | 510 | 408 | WHO stage | Children | Both | 700 | Inpatients | 5 | High | [30] | ||
| Feucht | 2016 | KwaZulu-Natal | Cross-sectional | 2010 | 250 | 205 | WHO stage | Children | Naive | 822 | Inpatients | 8 | Moderate | [39] | ||
| Fomundam | 2017 | Gauteng | Cross-sectional | 2015 | 12,413 | 3973 | CD4 | Adults | Naive | Outpatients | 8 | Moderate | [43] | |||
| Glencross | 2020 | Limpopo | Cohort (R)** | 2005–2015 | 57,490 | 19,854 | CD4 | Adults | Naive | 283 | Outpatients | 8 | Moderate | [61] | ||
| Haas | 2020 | Western Cape | Cohort (R)** | 2004–2017 | 58,664 | 24,693 | CD4 | Adults | Naive | 186 | Outpatients | 10 | Low | [60] | ||
| Haddow | 2012 | KwaZulu-Natal | Cross-sectional | 2006 | 498 | 343 | WHO stage | Adults | Naive | 106 | Outpatients | 8 | Moderate | [34] | ||
| Hunt | 2017 | KwaZulu-Natal | Cross-sectional | 2013 | 1299 | 687 | CD4 | Adults | Naive | 230 | Outpatients | 7 | Moderate | [40] | ||
| Kamkuemah | 2015 | Western Cape | Cohort (R)** | 2006 | 1091 | 621 | CD4 | Adults | Naive | 154 | Outpatients | 8 | Moderate | [55] | ||
| Kranzer | 2012 | Western Cape | Cross-sectional | 2010 | 164 | 20 | CD4 | Adults | Naive | 385 | Outpatients | 7 | Moderate | [33] | ||
| Larsen | 2019 | Gauteng | Cross-sectional | 2015 | 6826 | 2594 | CD4 | Adults | Naive | Outpatients | 10 | Low | [45] | |||
| Larson | 2010 | Gauteng | Cohort (R)** | 2009 | 9399 | 3293 | WHO stage | Adults | Naive | 73 | Outpatients | 7 | Moderate | [53] | ||
| Lawn | 2011 | Western Cape | Cohort (P) | 2011 | 235 | 125 | CD4 | Adults | Naive | 171 | Outpatients | 10 | Low | [64] | ||
| Lewis | 2012 | KwaZulu-Natal | Cohort (R)** | 2016–2018 | 2575 | 210 | CD4 | Adults | Naive | 106 | Outpatients | 10 | Low | [76] | ||
| Lilian | 2019 | Gauteng | Cohort (R)** | 2017–2018 | 32,290 | 3737 | WHO stage | Adults | Naive | Outpatients | 10 | Low | [58] | |||
| Lurie | 2014 | Western Cape | Cohort (P) | 2009–2011 | 1465 | 991 | CD4 | Adults | Naive | Outpatients | 10 | Low | [68] | |||
| Maduna | 2015 | National | Cohort (R)** | 2008 | 5976 | 3431 | CD4 | Adults | Naive | 207 | Outpatients | 7 | Moderate | [75] | ||
| Magidson | 2019 | Western Cape | Cohort (P) | 2010 | 190 | 65 | CD4 | Adults | Naive | 418 | Outpatients | 8 | Moderate | [72] | ||
| Manicklal | 2014 | Western Cape | Cross-sectional | 2012 | 748 | 126 | CD4 | Adults | Naive | Outpatients | 5 | High | [37] | |||
| Maskew | 2011 | Western Cape | Cross-sectional | 2008–2009 | 404 | 309 | CD4 | Adults | Naive | 100 | Outpatients | 8 | Moderate | [29] | ||
| Meintjes | 2015 | Western Cape | Cross-sectional | 2012–2013 | 209 | 137 | CD4 | Adults | Both | 134 | Inpatients | 376 | 234 | 8 | Moderate | [13] |
| Mnyani | 2017 | Gauteng | Cross-sectional | 2005–2015 | 198 | 142 | CD4 | Adults | Naive | 236 | Inpatients | 8 | Moderate | [42] | ||
| Naidoo | 2014 | KwaZulu-Natal | Cohort (R)** | 2007–2010 | 969 | 722 | CD4 | Adults | Naive | 128 | Outpatients | 10 | Low | [74] | ||
| Ndlovu | 2014 | Gauteng | Cohort (P) | 2004–2010 | 2489 | 1322 | WHO stage | Adults | Naive | 83 | Outpatients | 8 | Moderate | [67] | ||
| Nglazi | 2012 | Western Cape | Cohort (R)** | 2008–2010 | 893 | 102 | CD4 | Adults | Naive | 382 | Outpatients | 7 | Moderate | [73] | ||
| Nir | 2021 | Western Cape | Cross-sectional | 2014–2019 | 181 | 95 | CD4 | Adults | Naive | 225 | Outpatients | 9 | Low | [49] | ||
| Nyakato | 2022 | National | Cross-sectional | 2004–2017 | 2733 | 472 | CD4 | Adolescents | Naive | Outpatients | 10 | Low | [50] | |||
| Oni | 2011 | Western Cape | Cross-sectional | 2008–2010 | 228 | 45 | CD4 | Adults | Naive | 312 | Outpatients | 10 | Low | [31] | ||
| Osler | 2018 | Western Cape | Cohort (R)** | 2008–2017 | 1,771,201 | 397,214 | CD4 | Adults | Both | Outpatients | 10 | Low | [2] | |||
| Otwombe | 2013 | Gauteng | Cohort (P) | 2006–2009 | 891 | 616 | WHO stage | Adults | Naive | 67 | Outpatients | 10 | Low | [66] | ||
| Patel | 2010 | KwaZulu-Natal | Cross-sectional | 2009 | 81 | 62 | CD4 | Adults | Naive | 132 | Outpatients | 7 | Moderate | [28] | ||
| Patten | 2020 | National | Cohort (R)** | 2005–2018 | 283,134 | 158,825 | CD4 | Adults | Naive | 140 | Outpatients | 8 | Moderate | [59] | ||
| Peter | 2012 | Western Cape | Cross-sectional | 2009–2010 | 281 | 78 | CD4 | Adults | Naive | 86 | Inpatients | 8 | Moderate | [35] | ||
| Rane | 2018 | KwaZulu-Natal | Cohort (P) | 2013–2016 | 1271 | 372 | CD4 | Adults | Naive | 335 | Outpatients | 8 | Moderate | [71] | ||
| Rossouw | 2015 | KwaZulu-Natal | Cross-sectional | 2008–2012 | 65 | 54 | WHO stage | Children | Naive | 558 | Outpatients | 9 | Low | [38] | ||
| Shigayeva | 2019 | KwaZulu-Natal | Cohort (R)** | 2008–2018 | 27,929 | 10,125 | CD4 | Adults | Both | Outpatients | 726 | 411 | 10 | Low | [57] | |
| Sogbanmu | 2019 | Eastern Cape | Cross-sectional | 2016–2017 | 335 | 153 | WHO stage | Adults | Naive | 350 | Outpatients | 10 | Low | [46] | ||
| Tendesayi | 2016 | National | Cohort (R)** | 2010–2014 | 1,070,900 | 498,785 | CD4 | Adults | Naive | 213 | Outpatients | 10 | Low | [56] | ||
| Theron | 2011 | Western Cape | Cross-sectional | 2007–2010 | 123 | 66 | CD4 | Adults | Naive | 182 | Outpatients | 10 | Low | [32] | ||
| VanRie | 2011 | Gauteng | Cohort (P) | 2004–2007 | 7536 | 6073 | CD4 | Adults | Naive | 99 | Outpatients | 7 | Moderate | [63] | ||
| Venter | 2010 | Gauteng | Cross-sectional | 2017 | 553 | 266 | CD4 | Adults | Naive | 199 | Outpatients | 5 | High | [44] | ||
| Zaniewski | 2020 | National | Cohort (R)** | 2005–2018 | 94,167 | 56,228 | CD4 | Adults | Naive | Outpatients | 8 | Moderate | [4] | |||
| VanSchalkwyk | 2020 | Gauteng | Cross-sectional | 2015–2016 | 5280 | 637 | CD4 | Adults | Naive | 32 | Outpatients | 8 | Moderate | [47] |
Footnotes: *ART status = ART naïve, ART experienced, or both; **Cohort (P) = prospective; Cohort (R) = retrospective analysis of HIV program data,
Risk of bias assessment of included studies
In the assessment of the methodological quality, three studies were deemed to be of poor methodological quality. Of the remaining 50 records, 28 studies were deemed to be of moderate quality and 22 studies of high methodological quality (supplementary, appendix 2).
Prevalence of AHD among ART-naïve patients in South Africa
The 53 studies had a combined total of 11,545,460 PLHIV with 4,204,835 patients having AHD at ART enrolment. The prevalence of AHD in South Africa ranged between 5 and 82%. The pooled overall prevalence of AHD among ART-naïve patients was 43.4% (95% CI 40.1–46.8%, n = 53 studies). There was substantial heterogeneity among included studies (I2 = 99.9, P < 0.001) as shown in Fig. 2. Our estimates in prediction intervals analysis showed that the prevalence of AHD among ART-naïve patients was 44.3% (95% CI 38.0 to 50.0).
Fig. 2.
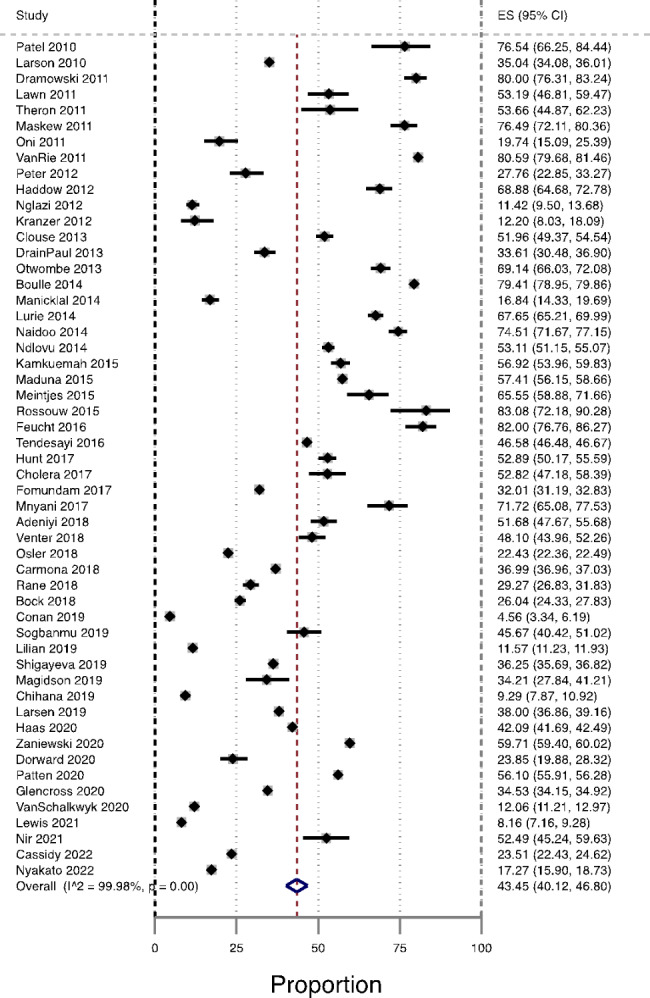
Forest plot of prevalence of AHD among ART-naïve in South Africa, from studies published from 2010–2022
Prevalence of AHD among ART-naïve patients by age group
The lowest pooled prevalence of AHD among ART-naïve was observed among adolescents 21% (95% CI 20.55–22.30%, n = 2 studies) and adults’ group 42.2% (95% CI 38.7–45.7%, n = 48 studies). The highest pooled prevalence of AHD among ART-naïve was observed among children 80.9% (95% CI 78.2–83.6%, n = 3 studies). The test for subgroups differences indicated that there was a statistically significant subgroup effect for age (P < 0.001), Fig. 3.
Fig. 3.
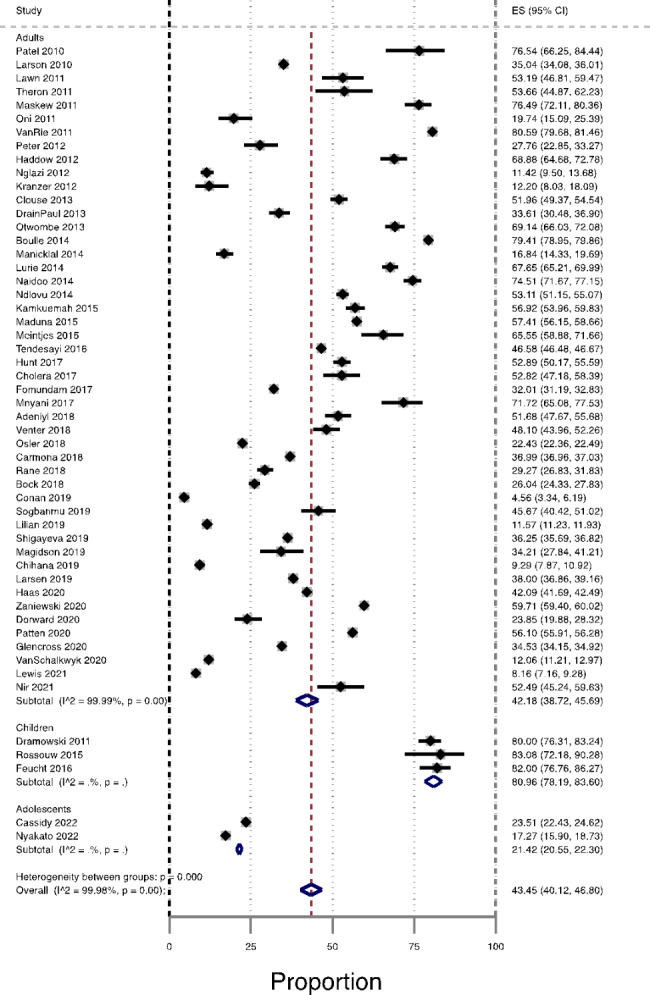
Prevalence of AHD among ART-naïve patients in South Africa by age group, from studies published during the period 2010–2022
Prevalence of AHD among ART-native patients by assessment methods: CD4 versus WHO clinical staging system
The pooled prevalence of ADH among ART-naïve patients was significantly higher among studies that used the WHO clinical staging system to assess AHD, 58.8% (95% CI 58.8–76.0%, n = 9), compared with a pooled prevalence of AHD estimate in 44 studies that used CD4 count criteria; 40.5% (95% CI 37.0% to 44.1, n = 44), Fig. 4.
Fig. 4.
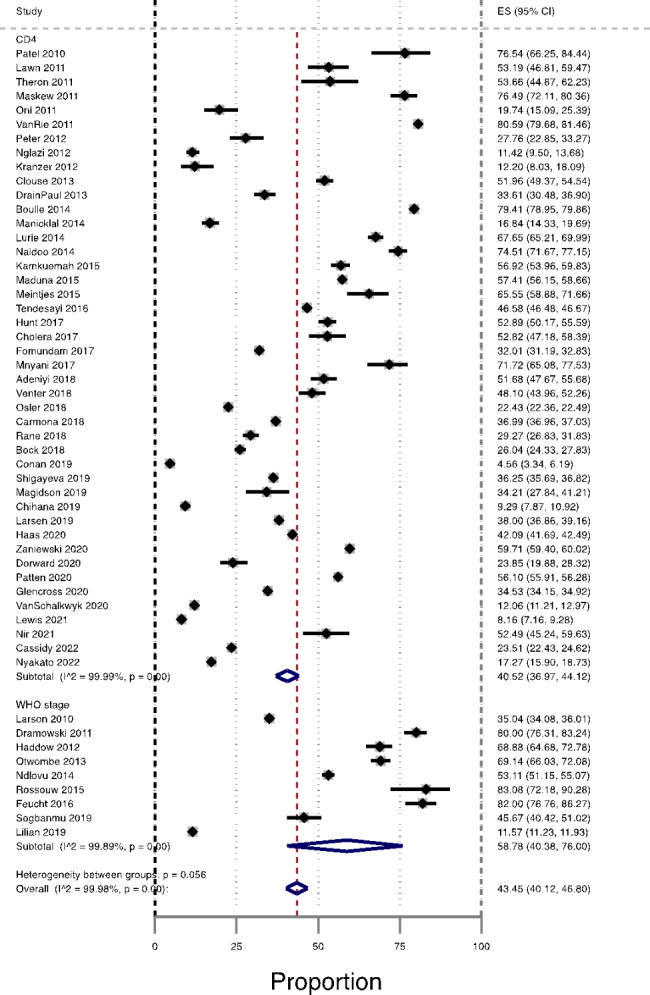
Prevalence of AHD among ART-naïve patients in South Africa by Assessment methods (CD4 count and WHO clinical staging system), from studies published during the period 2010–2022
Additionally, the prevalence of AHD among ART-naïve patients was 66.1% (95% CI 45.2–84.2%, n = 5) in studies conducted in hospital settings (inpatients) compared with 41.1% (95% CI 37.7–44.6%, n = 48) in studies conducted in ambulatory studies or outpatients (Appendix 3, Fig. 1) p < 0.001. We further conducted a subgroup analysis evaluating the prevalence of AHD among ART-naïve patients across study designs, the highest prevalence of AHD was observed among prospective cohort studies with 51.8% (95% CI 36.7–66.8%, n = 10), followed by cross sectional studies, retrospective analysis of HIV program data, retrospective cohort studies and cross-sectional survey with 46.9%, 41.1%, 35.1% and 7.3%, respectively, (Appendix 3, Fig. 2). Moreover, from sub-analyses, we observed that ADH among ART-naïve patients was more common among studies analysing national HIV cohort, followed by studies conducted in Eastern Cape province, Gauteng, KwaZulu-Natal, Western Cape, and Limpopo (Appendix 3, Fig. 3).
Sensitivity analysis
We performed one study leave out at a time sensitivity analysis and its result showed that the polled estimated prevalence of AHD among ART-naïve patients obtained when each of the included studies was left out from the analysis at a time was within the 95% confidence limit of the pooled estimate of AHD when all studies were pooled together. The average prevalence of AHD among ART-naïve patients when one of the 53 studies was left out from the analysis ranges between 44.0% (95% CI 38.0–50.0%) and 45.0% (95% CI 39.0–51.0%) (Appendix 3, Fig. 4).
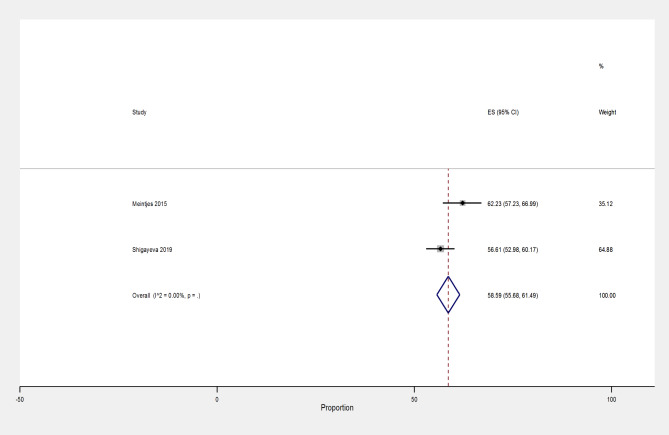
Prevalence of AHD among ART-experienced patients
We identified two studies assessing the prevalence of AHD among ART-experienced patients, including one cross-sectional and a retrospective analysis of HIV programmatic data which were included in the pairwise meta-analysis. The pooled prevalence of AHD among ART-experienced patients provided by two studies with 1,102 participants was 58.6% (95% CI 55.7 to 61.5%, n = 2), Fig. 5.
Fig. 5.
Prevalence of AHD among ART-experienced patients in South Africa, from studies published during the period 2010–2022
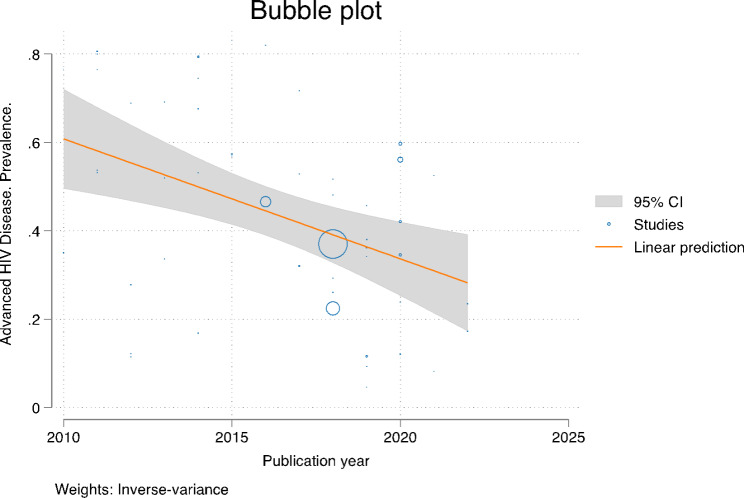
Temporal trend
In this study, we also attempted to assess the time trend of the prevalence of AHD among ART-naïve patients in South Africa from studies published from 2010 to 2022. The result indicated that there was a general linear trend of AHD declining in each successive year, (coefficient: -0.02, 95% CI -0.03 to 0.01), Fig. 6. Similarly, cumulative meta-analysis showed that the pooled prevalence of AHD among ART-naïve declined over time.
Fig. 6.
The time trend of advanced HIV disease from studies published during the period 2010–2022
Meta-regression
All meta-analyses in this review exhibited a substantial heterogeneity in pooled estimates, with subgroup and time trend analysis demonstrating potential associations with age, year of publication, study setting, and the AHD assessment method. In the adjusted meta-regression analysis, the period in which the selected studies were published was the only factor that remained associated with the prevalence of AHD (crude meta-regression coefficient: -0.02, 95% CI -0.03 to 0.01), meaning the prevalence of ADH among ART-naïve patients declined in each successive year by 2%, Table 2.
Table 2.
Crude and adjusted meta-regression analysis of the prevalence of AHD among ART-naïve patients
| Crude Coefficient | 95% CI | p-value | Adjusted Coefficient | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Publication year* | − 0.02 | -0.04 to -0.01 | 0.010 | -0.02 | -0.03 to 0.01 | 0.048 |
|
Data collection (Cross-sectional vs. Other) |
0.04 | -0.07 to 0.17 | 0.450 | -0.02 | -0.14 to 0.10 | 0.756 |
|
Setting (Inpatient vs. Outpatient) |
-0.23 | -0.44 to -0.03 | 0.026 | -0.18 | -0.40 to 0.04 | 0.109 |
| Methodological Quality Score* | -0.02 | -0.07 to 0.01 | 0.224 | -0.01 | -0.05 to 0.04 | 0.788 |
|
Assessment method (WHO Stage vs. CD4 count) |
0.16 | 0.01 to 0.32 | 0.048 | 0.10 | -0.05 to 0.26 | 0.202 |
*These explanatory variables were treated as continuous in the meta-regression model and the remaining variables were treated as categorical variables.
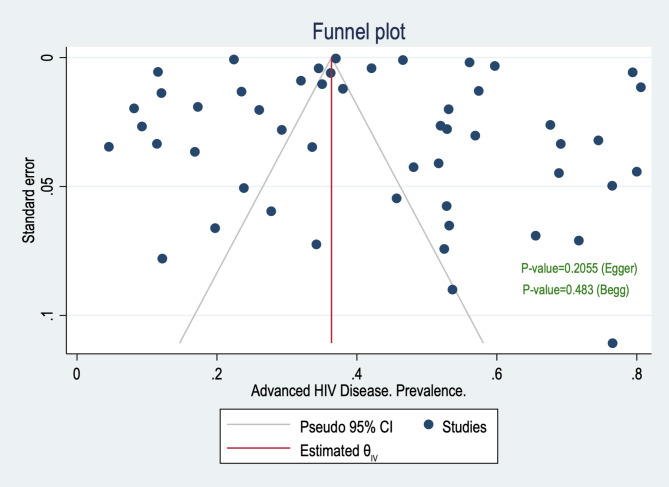
Publication bias
Conventional funnel plots suggested that there was no evidence of publication bias in the meta-analysis. Additionally, the results of Egger and Begg’s tests were in accordance with the funnel plots, which suggested no publication bias (Begg’s p-value = 0.483 and Egger’s p-value = 0.2055), Fig. 7.
Fig. 7.
Publication bias prevalence estimate, from studies published during the period 2010–2022
Trim and fill method was also used to assess publication bias for the prevalence of AHD among ART-naïve patients. Under the random-effects model, the point estimate for the pooled prevalence of AHD was 43.4% (95% CI 40.1–46.8%). Using Trim and Fill, the imputed point estimate was marginally different from the pooled prevalence with 41.4% (95% CI 35.2–47.6%), the method suggests a total of 2 studies were missing from this review for the prevalence estimate.
Discussion
This systematic review analysed 53 studies from 5 provinces in South Africa, involving 11,545,460 patients who received ART services between 2010 and 2022. The majority of the studies were cross-sectional analyses (24 studies), followed by longitudinal analysis of HIV programmatic data (13 studies), cross-sectional surveys (2 studies), prospective cohort studies (10 studies), and retrospective studies (4 studies). The overall prevalence of AHD among patients presenting to HIV services between 2010 and 2021 was 43.4%, however, there was substantial heterogeneity with this ranging between 4.5 and 83.1%. Due to the high heterogeneity between included studies, the pooled prevalence estimate should be interpreted with caution. Estimation of prediction intervals showed narrow range of expected prevalence of AHD among ART-naïve patients between 38.0% and 50.0% in a future, new systematic review and meta-analysis.
Subgroup analyses related to AHD diagnostic methods (CD4 versus WHO) and age did not explain the observed heterogeneity. Among the studies (n = 2) reporting on the presentation of ART-experienced patients, AHD was 58.6%. A time trend analysis revealed a decline of approximately 9.0% in the prevalence of AHD over the 10 years, with a prevalence of 52.4% in 2010 and 43.4% in 2022.
Since the start of the HIV programme, CD4 counts have been used to guide treatment decisions and identify those at risk for morbidity, mortality, and increased medical and psychosocial needs [5]. During the review period ART initiation guidelines were revised several times (patients were deemed eligible for ART if their CD4 count was < 200 cells/mm3 prior to 1 May 2010 or < 350 cells/mm3 after 1 May 2010 and 500 cells/mm3 between January 2015 and 31 August 2015) to expand form ART eligibility based on CD4 count and other co-morbidity, to all PLHIV becoming eligible for ART irrespective of CD4 count by 2016. As such, prior to and in 2010 the majority of PLHIV were at risk of starting ART with AHD whereas after 2010 this decreases due to guideline changes [5, 6].
Our study found that the proportion of patients presenting with AHD remains high but has decreased over time due to increased HIV testing and access to ART. Time trend analysis showed a 2% yearly decline in AHD among ART-naive patients. Our findings align with previous studies from both high-income and low- and middle-income settings [11, 14–16, 28, 29], which also reported either a high proportion or a modest decline in patients with AHD. Given the moderate decline in the proportion of AHD among ART-naïve patients, these results emphasize the importance of expanding HIV testing and timely linkage to care, to promote early ART initiation.
A meta-analysis data, which reported a mean CD4 count at ART initiation in 27 countries of 186 cells/mm3, suggest at least one in four ART patients in SSA initiated ART with advanced disease in 2015 and CD4 count at presentation to care and at ART initiation have not increased over the past decade [16]. When restricted analysis to studies conducted in South Africa, the meta-analysis found a statistically significant increase in CD4 counts at presentation but no change at ART initiation. To reduce the prevalence of AHD among ART-naïve patients in South Africa, continued attention to programmatic strategies facilitating earlier HIV testing and linkage to care are needed, including referral to primary health care and post-discharge support for adherence and retention, as well as implementing WHO-recommended universal ART eligibility (“treat-all policy”) guidelines for PLHIV [30, 31]. Furthermore, community awareness of AHD needs to be increased, as it is still limited [31].
Our study also found that 58.6% of patients with AHD were ART-experienced, this is a newly alarming trend observed in countries with long-standing HIV treatment programs and even in high ART coverage settings. Among ART-experienced, progress to AIDS either because they either interrupt treatment or because of treatment failure. The decline in the number of patients presenting for the first time with AHD could be offset by an increasing number of patients on long-term ART who have interrupted, stopped, or failed therapy [32–34]. Only two studies contributed to this outcome, therefore we cannot draw a firm conclusion using this finding. This evidence is consistent with findings from a multi-country cohort study conducted in 2 SSA countries evaluating characteristics and hospital outcomes of HIV-infected patients which showed that 65.1% of patients were admitted with AHD. Among inpatients with AHD, 71.7% were ART-experienced at admission with a median of 55.9 months on treatment (2). This means ongoing transmission due to ongoing viraemia among those who are out of care [36]. This supports a need for strategies to reduce gaps in HIV care and encourage return (re-engagement interventions such as welcome back services) before marked deterioration [37, 38], Given that more and more people who are in HIV programs are treatment experienced. This work supports the current reconceptualized non-linear HIV care cascade, which accounts for the cycling of patients in and out of care and the mobile populations served by ART programs in many SSA countries [12].
Furthermore, our subgroup analyses revealed a statistically significant difference in the prevalence of AHD among ART-naive patients between studies that used the WHO clinical staging system and those that used CD4 count criteria to identify patients with AHD, with 58.78% and 40.52% respectively. The higher prevalence among studies using the WHO clinical staging system should be interpreted with caution due to the poor accuracy associated with the WHO clinical staging system [39]. A systematic review and meta-analysis assessing the accuracy of WHO clinical staging system compared with CD4 count found that the WHO clinical stage 3 or 4 had a sensitivity of 60% and specificity of 73% for a CD4 threshold of ≤ 200 cells/mm3, and that sensitivity and specificity decreased as the CD4 count threshold increased [40]. Therefore, relying on the WHO clinical staging system alone risks missing a substantial number of PLHIV with severe immune suppression. Additionally, a multi-country study from Kenya, Malawi, Uganda, and Zimbabwe found that almost half of the people with CD4 count < 100 cells/mm3 were classified as having WHO clinical stage 1 or 2 disease [41].
The role of the CD4 count in HIV management is changing, with a shift away from using CD4 count to decide when to start ART or monitor treatment efficacy. Nevertheless, CD4 count remains the best measurement of patients’ immune and clinical status, the risk of opportunistic infections, and diagnosis decision-making, particularly for patients with AHD [30, 42].
Hence, identifying people with AHD who are eligible for elements of a package of care requires CD4 cell count testing. In addition, determining the immune status of PLHIV whose treatment is failing by virological criteria can help in guiding clinical management decisions (providing a package of care to reduce mortality and morbidity among people with AHD, which package includes screening, treatment, and/or prophylaxis for major opportunistic infections, rapid ART initiation and intensified adherence support interventions) [43].
Furthermore, the shift from monitoring CD4 cell count to monitoring viral load among individuals on ART has contributed to the de-prioritisation of testing CD4 cell count. Monitoring of viral load and CD4 cell count complement one another and should not be placed in competition. Monitoring VL supports viral suppression and triage into differentiated models of care, whereas CD4 cell count identifies people for whom an additional intervention is most urgently needed [44]. For these reasons, WHO recommends that HIV programs should retain the capacity to perform CD4 cell count at baseline and in the case of virological failure.
Strengths and limitations
To the best of our knowledge, this is one of the first reviews to investigate the prevalence of AHD among ART-naïve and ART-experienced patients in South Africa. Of the 53 studies included in our meta-analysis, 13 were longitudinal analyses of HIV programmatic data, estimating the burden of AHD in real-world conditions at public health facilities, therefore maximizing generalizability. A further strength of this review is that we comprehensively searched for all eligible studies in all major databases and rigorously carried out the study following the PRISMA guidelines.
This review has several limitations that warrant discussion. Firstly, caution should be exercised when evaluating the current review results as there was substantial heterogeneity among included studies; this is not uncommon when evaluating systematic reviews of this nature. The impact of study quality on pooled prevalence was assessed by conducting meta-regression. Meta-regression demonstrated no evidence of data collection method or methodological quality score or assessment method or setting (inpatients versus outpatients).
A further limitation of this review is that gender-stratified data are not reported in most of the studies, making them unusable in the current meta-analysis. Although programs described patients as ART-naïve, many people who present to HIV service as ART-naïve patients may in fact be ART-experienced but have been out of care and are re-presenting as though they have never been in care before. Meta-analysis assumes that the data from contributing studies are independent of each other. However, there may be overlap in the patients summarized in the included studies, especially for those longitudinal studies using HIV program data, thus violating the assumption of data independence required for meta-analysis, introducing bias to the overall estimate, and that results would be biased towards the results of those patients included more than once.
Implications for practice and future research
The current evidence suggests that the proportion of AHD among ART-naïve patients has declined over time (during the observed period) but remains consistently high. Therefore, CD4 count measurements remain important to identify people with AHD despite the introduction of the treat-all guidelines in 2016. These findings highlight the ongoing need for baseline CD4 count measurement to identify a large number of patients with AHD among ART-naïve patients, in order to provide differentiated models of care for this group of patients [5], guide prophylaxis against opportunistic infections (severe bacterial infections, TB and cryptococcal disease), and reduce mortality [41, 45–48]. Our findings also emphasize the importance of maintaining CD4 count testing capacity and infrastructure if donor funding for the CD4 measurement is reduced as global efforts prioritize the scale-up of VL monitoring in LMICs.
Capacity building and training for healthcare workers are also needed to quickly identify and timeously treat patients who are critically ill to save lives. This includes addressing health care workers’ attitude to be welcoming and understanding to patients re-engaging back into care.
Further research priorities should focus to assess the impact of universal test and treat guidelines on CD4 count testing at ART initiation among adults. Further research is needed on evaluating long-term outcomes among ART-naïve patients with advanced HIV disease pre- and post-universal test and treat guidelines under field conditions.
Conclusion
Despite progress in making ART available to millions of PLHIV in South Africa, the burden AHD among ART-naive and ART-experienced patients remains consistently high. This is despite South Africa having a well-established ART program with excellent population-level coverage. Our findings highlight that CD4 cell count measurement remains an essential component of HIV care and is critically important in identifying individuals with low CD4 counts and enabling differentiated care focused on reducing the high morbidity and mortality in this vulnerable patient group. These results emphasize the importance of expanding HIV testing and timely linkage to care, to promote early ART initiation. There is a need to programmatically improve and decentralize screening for AHD at primary health clinics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary files: Appendix 1: Search Strategy. Appendix 2: Risk of bias and quality of included studies. Appendix 3: Supplementary figures. Figure 1 Prevalence of AHD among ART-naïve by study setting (hospital versus outpatients). Figure 2. Prevalence of AHD among ART-naïve patients by study design. Figure 3. Prevalence of AHD among ART-naïve patients by province. Figure 4. A sensitivity analysis of the prevalence of AHD among ART-naïve patients in South Africa when each indicated studies are removed at a time with its 957% confidence interval.
Acknowledgements
The authors are grateful to the information specialists Dilshaad Brey and Gill Morgan for designing the electronic search strategy, both librarian at University of cape Town, Health Sciences library.
List of abbreviations
- AHD
Advanced HIV Disease
- AIDS
Acquired Immune Deficiency Syndrome
- ART
Anti-Retroviral Therapy
- CD4
Cluster of Differentiation 4
- CrAg
Cryptococcal antigen
- EPTB
Extra-pulmonary tuberculosis
- GXP
GeneXpert
- HIV
Human Immunodeficiency Virus
- LMIC
Low- and middle-income countries
- PLWHIV
People living with HIV
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PROSPERO
Prospective Register of Systematic Reviews
- SSA
Sub-Saharan Africa
- TB
Tuberculosis
- TB LAM
Tuberculosis lipoarabinomannan antigen
- VL
Viral Load
- WHO
Woold Health Organization
Authors’ contributions
MKK, GF, OA, IEW and PN contributed to the conceptualisation of this systematic review.MKK and OA were involved in the study selection process, assessment of risk of bias and dataextraction. MKK, GF, OA, IEW and PN drafted the manuscript and contributed to criticallyreviewing the manuscript. All the authors provided the final approval of the manuscript forpublication.
Funding
This work is based on the research supported in part by the National Research Foundation of South Africa (Grant Numbers 132218). The content is solely the responsibility of the authors and does not necessarily represent the officials of National Research Foundation of South Africa.
Data availability
Data will be available upon reasonable request of the corresponding.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.HIV/AIDS JUNPo. Confronting inequalities: lessons for pandemic responses from 40 years of AIDS. Report No: UNAIDS/JC3020E Global AIDS update Geneva. Joint United Nations Programme on HIV/AIDS (UNAIDS; 2021.
- 2.Osler M, Hilderbrand K, Goemaere E, Ford N, Smith M, Meintjes G, et al. The Continuing Burden of Advanced HIV Disease over 10 years of increasing antiretroviral Therapy Coverage in South Africa. Clin Infect Dis. 2018;66(suppl2):118–s25. doi: 10.1093/cid/cix1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmona S, Bor J, Nattey C, Maughan-Brown B, Maskew M, Fox MP, et al. Persistent high Burden of Advanced HIV Disease among Patients seeking care in South Africa’s National HIV Program: Data from a Nationwide Laboratory Cohort. Clin Infect Dis. 2018;66(suppl2):111–s7. doi: 10.1093/cid/ciy045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaniewski E, Dao Ostinelli CH, Chammartin F, Maxwell N, Davies MA, Euvrard J, et al. Trends in CD4 and viral load testing 2005 to 2018: multi-cohort study of people living with HIV in Southern Africa. J Int AIDS Soc. 2020;23(7):e25546. doi: 10.1002/jia2.25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization WH. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. 2017. [PubMed]
- 6.Jamil MS, Eshun-Wilson I, Witzel TC, Siegfried N, Figueroa C, Chitembo L, et al. Examining the effects of HIV self-testing compared to standard HIV testing services in the general population: a systematic review and meta-analysis. EClinicalMedicine. 2021;38:100991. doi: 10.1016/j.eclinm.2021.100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNAIDS. Lessons for pandemic responses from 40 years of AIDS. 2021.
- 8.Benzekri NA, Sambou JF, Ndong S, Tamba IT, Faye D, Diallo MB, et al. Prevalence, predictors, and management of advanced HIV disease among individuals initiating ART in Senegal, West Africa. BMC Infect Dis. 2019;19(1):261. doi: 10.1186/s12879-019-3826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May M, Boulle A, Phiri S, Messou E, Myer L, Wood R, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-saharan Africa: a collaborative analysis of scale-up programmes. The Lancet. 2010;376(9739):449–57. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndlovu Z, Burton R, Stewart R, Bygrave H, Roberts T, Fajardo E, et al. Framework for the implementation of advanced HIV disease diagnostics in sub-saharan Africa: programmatic perspectives. The Lancet HIV. 2020;7(7):e514–e20. doi: 10.1016/S2352-3018(20)30101-6. [DOI] [PubMed] [Google Scholar]
- 11.Auld AFSR, Oboho I, Ross C, Bateganya M, Pelletier V. Trends in Prevalence of Advanced HIV Disease at Antiretroviral Therapy. Morb Mortal Wkly Rep. 2017;66(21):2–7. doi: 10.15585/mmwr.mm6621a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrenkranz P, Rosen S, Boulle A, Eaton JW, Ford N, Fox MP, et al. The revolving door of HIV care: revising the service delivery cascade to achieve the UNAIDS 95-95-95 goals. PLoS Med. 2021;18(5):e1003651. doi: 10.1371/journal.pmed.1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meintjes G, Kerkhoff AD, Burton R, Schutz C, Boulle A, Van Wyk G, et al. HIV-Related Medical admissions to a south african District Hospital remain frequent despite effective antiretroviral therapy Scale-Up. Med (Baltim) 2015;94(50):e2269. doi: 10.1097/MD.0000000000002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IeDea, Collaborations CC. Global Trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of Treatment Programs. Clin Infect Dis. 2018;66(6):893–903. doi: 10.1093/cid/cix915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A systematic review and meta-regression of temporal trends in adult CD4(+) cell count at presentation to HIV care, 1992–2011. Clin Infect Dis. 2013;57(7):1027–37. doi: 10.1093/cid/cit421. [DOI] [PubMed] [Google Scholar]
- 16.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. 2015;60(7):1120–7. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Organization WH. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: December 2011. World Health Organization; 2011. [PubMed]
- 18.Organization WH. The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV: policy guidance. World Health Organization; 2015. p. 9241509635. Report No.
- 19.Organization WH. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults in resource-limited settings: recommendations for a public health approach. 2006.
- 20.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–9. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Press S. Stata Statistical Software. College Station: Stata Corporation; 2016. [Google Scholar]
- 23.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Archives of Public Health. 2014;72(1):1–10. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis: John Wiley & Sons; 2021.
- 26.Egger M, Smith GD. Meta-analysis: potentials and promise. BMJ. 1997;315(7119):1371–4. doi: 10.1136/bmj.315.7119.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088 – 101. [PubMed]
- 28.Patel VB, Singh R, Connolly C, Kasprowicz V, Zumla A, Ndungu T, et al. Comparison of a clinical prediction rule and a LAM antigen-detection assay for the rapid diagnosis of TBM in a high HIV prevalence setting. PLoS ONE. 2010;5(12):e15664. doi: 10.1371/journal.pone.0015664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maskew M, Macphail AP, Whitby D, Egger M, Wallis CL, Fox MP. Prevalence and predictors of kaposi sarcoma herpes virus seropositivity: a cross-sectional analysis of HIV-infected adults initiating ART in Johannesburg, South Africa. Infect Agent Cancer. 2011;6:22. doi: 10.1186/1750-9378-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dramowski A, Coovadia A, Meyers T, Goga A. Identifying missed opportunities for early intervention among HIV-infected paediatric admissions at Chris Hani Baragwanath hospital, Soweto, South Africa. 2011. p. 16–23.
- 31.Oni T, Burke R, Tsekela R, Bangani N, Seldon R, Gideon HP, et al. High prevalence of subclinical tuberculosis in HIV-1-infected persons without advanced immunodeficiency: implications for TB screening. Thorax. 2011;66(8):669–73. doi: 10.1136/thx.2011.160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, Murray S, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011;184(1):132–40. doi: 10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 33.Kranzer K, Govindasamy D, van Schaik N, Thebus E, Davies N, Zimmermann M, et al. Incentivized recruitment of a population sample to a mobile HIV testing service increases the yield of newly diagnosed cases, including those in need of antiretroviral therapy. HIV Med. 2012;13(2):132–7. doi: 10.1111/j.1468-1293.2011.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haddow LJ, Moosa MY, Mosam A, Moodley P, Parboosing R, Easterbrook PJ. Incidence, clinical spectrum, risk factors and impact of HIV-associated immune reconstitution inflammatory syndrome in South Africa. PLoS ONE. 2012;7(11):e40623. doi: 10.1371/journal.pone.0040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peter JG, Theron G, Muchinga TE, Govender U, Dheda K. The diagnostic accuracy of urine-based Xpert MTB/RIF in HIV-infected hospitalized patients who are smear-negative or sputum scarce. PLoS ONE. 2012;7(7):e39966. doi: 10.1371/journal.pone.0039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DrainPaul K, Losina E, Parker G, Giddy J, Ross D, Katz JN, et al. Risk factors for late-stage HIV disease presentation at initial HIV diagnosis in Durban, South Africa. PLoS ONE. 2013;8(1):55305. doi: 10.1371/journal.pone.0055305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manicklal S, van Niekerk AM, Kroon SM, Hutto C, Novak Z, Pati SK, et al. Birth prevalence of congenital cytomegalovirus among infants of HIV-infected women on prenatal antiretroviral prophylaxis in South Africa. Clin Infect Dis. 2014;58(10):1467–72. doi: 10.1093/cid/ciu096. [DOI] [PubMed] [Google Scholar]
- 38.Rossouw TM, Feucht UD, Melikian G, van Dyk G, Thomas W, du Plessis NM, et al. Factors Associated with the development of Drug Resistance mutations in HIV-1 infected children failing protease inhibitor-based antiretroviral therapy in South Africa. PLoS ONE. 2015;10(7):e0133452. doi: 10.1371/journal.pone.0133452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feucht UD, Meyer A, Thomas WN, Forsyth BW, Kruger M. Early diagnosis is critical to ensure good outcomes in HIV-infected children: outlining barriers to care. AIDS Care. 2016;28(1):32–42. doi: 10.1080/09540121.2015.1066748. [DOI] [PubMed] [Google Scholar]
- 40.Hunt GM, Dokubo EK, Takuva S, de Oliveira T, Ledwaba J, Dube N, et al. Rates of virological suppression and drug resistance in adult HIV-1-positive patients attending primary healthcare facilities in KwaZulu-Natal, South Africa. J Antimicrob Chemother. 2017;72(11):3141–8. doi: 10.1093/jac/dkx252. [DOI] [PubMed] [Google Scholar]
- 41.Cholera R, Pence BW, Gaynes BN, Bassett J, Qangule N, Pettifor A, et al. Depression and Engagement in Care among newly diagnosed HIV-Infected adults in Johannesburg, South Africa. AIDS Behav. 2017;21(6):1632–40. doi: 10.1007/s10461-016-1442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mnyani CN, Buchmann EJ, Chersich MF, Frank KA, McIntyre JA. Trends in maternal deaths in HIV-infected women, on a background of changing HIV management guidelines in South Africa: 1997 to 2015. J Int AIDS Soc. 2017;20(3). [DOI] [PMC free article] [PubMed]
- 43.Fomundam HN, Tesfay AR, Mushipe SA, Mosina MB, Boshielo CT, Nyambi HT, et al. Prevalence and predictors of late presentation for HIV care in South Africa. S Afr Med J. 2017;107(12):1058–64. doi: 10.7196/SAMJ.2017.v107i12.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venter DF, Chersich W, Majam MF, Akpomiemie M, Arulappan G, Moorhouse N et al. M,. CD4 cell count variability with repeat testing in South Africa: Should reporting include both absolute counts and ranges of plausible values? International Journal of S T D & AIDS. 2018;29(11):1048-56. [DOI] [PubMed]
- 45.Larsen A, Cheyip M, Tesfay A, Vranken P, Fomundam H, Wutoh A, et al. Timing and predictors of initiation on antiretroviral therapy among newly-diagnosed HIV-Infected persons in South Africa. AIDS Behav. 2019;23(2):375–85. doi: 10.1007/s10461-018-2222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sogbanmu OO, Goon DT, Obi LC, Iweriebor BC, Nwodo UN, Ajayi AI, et al. Socio-demographic and clinical determinants of late presentation among patients newly diagnosed with HIV in the Eastern Cape, South Africa. Med (Baltim) 2019;98(8):e14664. doi: 10.1097/MD.0000000000014664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Schalkwyk E, Mhlanga M, Maphanga TG, Mpembe RS, Shillubane A, Iyaloo S, et al. Screening for invasive fungal disease using non-culture-based assays among inpatients with advanced HIV disease at a large academic hospital in South Africa. Mycoses. 2020;63(5):478–87. doi: 10.1111/myc.13071. [DOI] [PubMed] [Google Scholar]
- 48.Dorward J, Drain PK, Osman F, Sookrajh Y, Pillay M, Moodley P, et al. Short communication: early antiretroviral therapy is Associated with Better viral suppression and less HIV Drug Resistance after implementation of Universal Treatment in South Africa. AIDS Res Hum Retroviruses. 2020;36(4):297–9. doi: 10.1089/aid.2019.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nir TM, Fouche JP, Ananworanich J, Ances BM, Boban J, Brew BJ, et al. Association of Immunosuppression and viral load with subcortical brain volume in an International Sample of People living with HIV. JAMA Netw Open. 2021;4(1):e2031190. doi: 10.1001/jamanetworkopen.2020.31190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyakato P, Schomaker M, Fatti G, Tanser F, Euvrard J, Sipambo N, et al. Virologic non-suppression and early loss to follow up among pregnant and non-pregnant adolescents aged 15–19 years initiating antiretroviral therapy in South Africa: a retrospective cohort study. J Int AIDS Soc. 2022;25(1):e25870. doi: 10.1002/jia2.25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conan N, Simons E, Chihana ML, Ohler L, FordKamara E, Mbatha M, et al. Increase in HIV viral suppression in KwaZulu-Natal, South Africa: community-based cross sectional surveys 2018 and 2013. What remains to be done? PLoS ONE. 2022;17(3):e0265488. doi: 10.1371/journal.pone.0265488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chihana ML, Huerga H, Van Cutsem G, Ellman T, Goemaere E, Wanjala S, et al. Distribution of advanced HIV disease from three high HIV prevalence settings in Sub-Saharan Africa: a secondary analysis data from three population-based cross-sectional surveys in Eshowe (South Africa), ndhiwa (Kenya) and Chiradzulu (Malawi) Glob Health Action. 2019;12(1):1679472. doi: 10.1080/16549716.2019.1679472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larson BA, Brennan A, McNamara L, Long L, Rosen S, Sanne I, et al. Lost opportunities to complete CD4 + lymphocyte testing among patients who tested positive for HIV in South Africa. World Health Organization Bulletin. 2010;88(9):675–80. doi: 10.2471/BLT.09.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boulle A, Schomaker M, May MT, Hogg RS, Shepherd BE, Monge S, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11(9):e1001718. doi: 10.1371/journal.pmed.1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamkuemah M, Kaplan R, Bekker LG, Little F, Myer L. Renal impairment in HIV-infected patients initiating tenofovir-containing antiretroviral therapy regimens in a primary Healthcare setting in South Africa. Trop Med Int Health. 2015;20(4):518–26. doi: 10.1111/tmi.12446. [DOI] [PubMed] [Google Scholar]
- 56.Kufa T, Shubber Z, MacLeod W, Takuva S, Carmona S, Bor J, et al. CD4 count recovery and associated factors among individuals enrolled in the south african antiretroviral therapy programme: an analysis of national laboratory based data. PLoS ONE. 2019;14(5):e0217742. doi: 10.1371/journal.pone.0217742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shigayeva A, Gcwensa N, Ndlovu CD, Ntumase N, Sabela S, Ohler L, et al. Retention on ART and viral suppression among patients in alternative models of differentiated HIV service delivery in KwaZulu-Natal, South Africa. PLOS Glob Public Health. 2022;2(12):e0000336. doi: 10.1371/journal.pgph.0000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lilian RR, Rees K, McIntyre JA, Struthers HE, Peters RPH. Same-day antiretroviral therapy initiation for HIV-infected adults in South Africa: analysis of routine data. PLoS ONE. 2020;15(1):e0227572. doi: 10.1371/journal.pone.0227572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patten GE, Euvrard J, Anderegg N, Boulle A, von der Arendse KD, et al. Advanced HIV disease and engagement in care among patients on antiretroviral therapy in South Africa: results from a multi-state model. AIDS. 2023;37(3):513–22. doi: 10.1097/QAD.0000000000003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haas AD, van den Ruffieux Y, Lund C, Boulle A, Euvrard J, et al. Excess mortality associated with mental illness in people living with HIV in Cape Town, South Africa: a cohort study using linked electronic health records. Lancet Glob Health. 2020;8(10):e1326–e34. doi: 10.1016/S2214-109X(20)30279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glencross DK, Cassim N, Coetzee LM. Documented higher burden of advanced and very advanced HIV disease among patients, especially men, accessing healthcare in a rapidly growing economic and industrial hub in South Africa: a call to action. S Afr Med J. 2020;110(6):505–13. doi: 10.7196/SAMJ.2020.v110i6.14352. [DOI] [PubMed] [Google Scholar]
- 62.Cassidy T, Cornell M, Makeleni B, Horsburgh CR, Duran LT, de Azevedo V, et al. Attrition from Care among Men initiating ART in male-only clinics compared with men in General Primary Healthcare Clinics in Khayelitsha, South Africa: a matched propensity score analysis. AIDS Behav. 2023;27(1):358–69. doi: 10.1007/s10461-022-03772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Rie A, Westreich D, Sanne I. Tuberculosis in patients receiving antiretroviral treatment: incidence, risk factors, and prevention strategies. J Acquir Immune Defic Syndr. 2011;56(4):349–55. doi: 10.1097/QAI.0b013e3181f9fb39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8(7):e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clouse K, Pettifor A, Maskew M, Bassett J, Van Rie A, Gay C, et al. Initiating antiretroviral therapy when presenting with higher CD4 cell counts results in reduced loss to follow-up in a resource-limited setting. Aids. 2013;27(4):645–50. doi: 10.1097/QAD.0b013e32835c12f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Otwombe KN, Variava E, Holmes CB, Chaisson RE, Martinson N. Predictors of delay in the diagnosis and treatment of suspected tuberculosis in HIV co-infected patients in South Africa. Int J Tuberc Lung Dis. 2013;17(9):1199–205. doi: 10.5588/ijtld.12.0891. [DOI] [PubMed] [Google Scholar]
- 67.Ndlovu Z, Chirwa T, Takuva S. Incidence and predictors of recovery from anaemia within an HIV-infected south african cohort, 2004–2010. Pan Afr Med J. 2014;19:114. doi: 10.11604/pamj.2014.19.114.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lurie MN, Kirwa K, Daniels J, Berteler M, Kalichman SC, Mathews C. High burden of STIs among HIV-infected adults prior to initiation of ART in South Africa: a retrospective cohort study. Sex Transm Infect. 2014;90(8):615–9. doi: 10.1136/sextrans-2013-051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adeniyi OV, Ajayi AI, Moyaki MG, Goon DT, Avramovic G, Lambert J. High rate of unplanned pregnancy in the context of integrated family planning and HIV care services in South Africa. BMC Health Serv Res. 2018;18(1):140. doi: 10.1186/s12913-018-2942-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bock P, Fatti G, Ford N, Jennings K, Kruger J, Gunst C, et al. Attrition when providing antiretroviral treatment at CD4 counts > 500cells/µL at three government clinics included in the HPTN 071 (PopART) trial in South Africa. PLoS ONE. 2018;13(4):e0195127. doi: 10.1371/journal.pone.0195127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rane MS, Hong T, Govere S, Thulare H, Moosa MY, Celum C, et al. Depression and anxiety as risk factors for delayed care-seeking behavior in human immunodeficiency virus-infected individuals in South Africa. Clin Infect Dis. 2018;67(9):1411–8. doi: 10.1093/cid/ciy309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magidson JF, Fatch R, Orrell C, Amanyire G, Haberer JE, Hahn JA. Biomarker-measured unhealthy alcohol use in relation to CD4 count among individuals starting ART in Sub-Saharan Africa. AIDS Behav. 2019;23(6):1656–67. doi: 10.1007/s10461-018-2364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nglazi MD, van Schaik N, Kranzer K, Lawn SD, Wood R, Bekker LG. An incentivized HIV counseling and testing program targeting hard-to-reach unemployed men in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2012;59(3):e28–34. doi: 10.1097/QAI.0b013e31824445f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naidoo K, Karim QA, Bhushan A, Yende-Zuma N, McHunu PK, Frohlich J, et al. High rates of tuberculosis in patients accessing HAART in rural South Africa. J Acquir Immune Defic Syndr. 2014;65(4):438–46. doi: 10.1097/QAI.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maduna PH, Dolan M, Kondlo L, Mabuza H, Dlamini JN, Polis M, et al. Morbidity and mortality according to latest CD4 + cell count among HIV positive individuals in South Africa who enrolled in project Phidisa. PLoS ONE. 2015;10(4):e0121843. doi: 10.1371/journal.pone.0121843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis L, Sookrajh Y, Gate K, Khubone T, Maraj M, Mkhize S, et al. Differentiated service delivery for people using second-line antiretroviral therapy: clinical outcomes from a retrospective cohort study in KwaZulu-Natal, South Africa. J Int AIDS Soc. 2021;24(Suppl 6):e25802. doi: 10.1002/jia2.25802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary files: Appendix 1: Search Strategy. Appendix 2: Risk of bias and quality of included studies. Appendix 3: Supplementary figures. Figure 1 Prevalence of AHD among ART-naïve by study setting (hospital versus outpatients). Figure 2. Prevalence of AHD among ART-naïve patients by study design. Figure 3. Prevalence of AHD among ART-naïve patients by province. Figure 4. A sensitivity analysis of the prevalence of AHD among ART-naïve patients in South Africa when each indicated studies are removed at a time with its 957% confidence interval.
Data Availability Statement
Data will be available upon reasonable request of the corresponding.