Abstract
Triple-negative breast cancer (TNBC), a highly aggressive subtype of breast cancer, negatively expresses estrogen receptor, progesterone receptor, and the human epidermal growth factor receptor 2 (HER2). Although chemotherapy is the main form of treatment for patients with TNBC, the effectiveness of chemotherapy for TNBC is still limited. The search for more effective therapies is urgent. Multiple targeted therapeutic strategies have emerged according to the specific molecules and signaling pathways expressed in TNBC. These include PI3K/AKT/mTOR inhibitors, epidermal growth factor receptor inhibitors, Notch inhibitors, poly ADP-ribose polymerase inhibitors, and antibody–drug conjugates. Moreover, immune checkpoint inhibitors, for example, pembrolizumab, atezolizumab, and durvalumab, are widely explored in the clinic. We summarize recent advances in targeted therapy and immunotherapy in TNBC, with the aim of serving as a reference for the development of individualized treatment of patients with TNBC in the future.
Keywords: Triple-negative breast cancer, Molecular subtype, Targeted therapy, Immunotherapy
Introduction
Based on the American Cancer Society, breast cancer (BC) has emerged as the second leading cause of cancer death in women, and the incidence of BC is increasing annually [1, 2]. According to the expression of biomarkers, including estrogen receptors, progesterone receptors, human epidermal growth factor receptor 2 (HER2), and Ki67, BC mainly consists of luminal A, luminal B, HER-2 overexpression, and triple-negative breast cancer (TNBC) subtypes [3]. TNBC is a specific subtype of BC, representing 15—20% of BC, and lacks expression of the estrogen receptor, progesterone receptor, and HER2 receptor on the cell surface [4, 5]. Analysis of gene expression profiles showed that TNBC was classified as a basal-like BC subtype [6]. Compared to other BC subtypes, TNBC commonly occurs in young women and is associated with increased malignancy and mortality [7, 8]. Approximately 45% of patients with TNBC have distant metastases in the brain or elsewhere, and median survival decreases from 13.3 months to 18 months [9]. Several reports have confirmed that up to 25% of patients with TNBC can recover. The Food and Drug Administration (FDA) has approved anti-metabolites, paclitaxel, and anthracyclines as adjuvant and neoadjuvant chemotherapy regimens for patients with TNBC [10, 11]. Conventional chemotherapy has shown some effectiveness in patients with TNBC. However, the toxicity of chemotherapy is harmful for patients and some patients still do not receive clinical benefit. Therefore, finding effective targets for accurate TNBC therapy is a challenging and important clinical problem to be solved [12–17].
Whole-genome sequencing studies demonstrated that TNBC is highly heterogeneous and has contributed to the classification of TNBC subtypes [18]. In recent years, "Fudan typing" has refined TNBC into various subtypes, shedding light on the accurate treatment of patients with TNBC [19, 20]. With the increasing development of histological research and the advance of bioinformatics analysis technology, cancer research is gradually developing towards large samples, multi-omics, and refinement. In recent years, potential therapeutic targets drawn from genomics, transcriptomics, metabolomics, and proteomics have emerged, and a considerable number of these research results have strong clinical translation value and have attracted widespread attention [21]. Therefore, it is necessary to develop appropriate therapeutic plans according to the unique and complex molecular characteristics and biological properties of the tumors in each TNBC patient.
Given the continuing advances in TNBC research, we summarize the fundamental characteristics and classification of TNBC and review the progress made in targeted therapy for TNBC in recent years.
Molecular typing of TNBC
It is instructive to distinguish specific molecular typing for the treatment and prognosis determination of patients with BC. For example, TNBC patients are sensitive to chemotherapeutic agents but not endocrine therapy and TNBC patients are generally highly heterogeneous, tend to metastasize, and have a poor prognosis [22]. Therefore, clarifying the molecular typing of TNBC is important to guide individualized treatment and may further improve the treatment success rate [23].
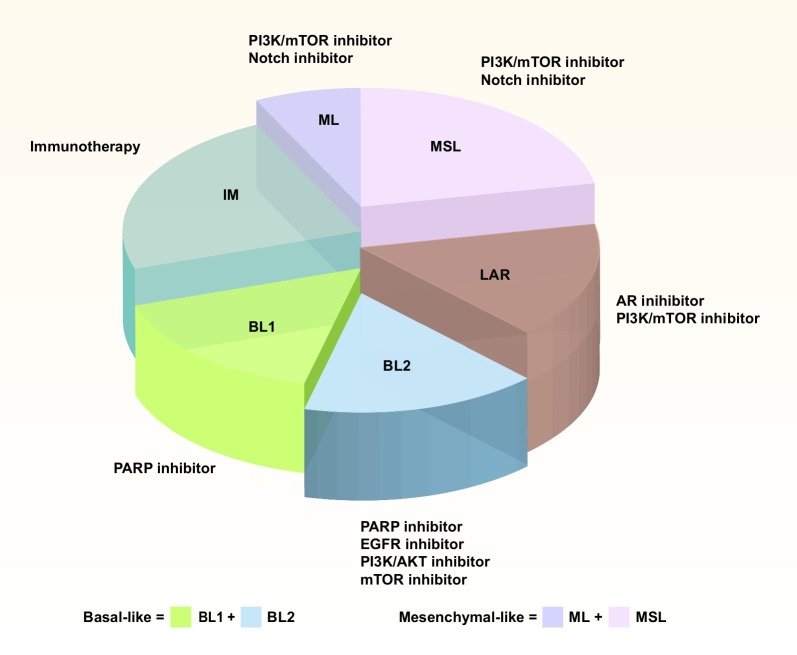
Lehmann's team divided TNBC into the following subtypes by gene expression profile of tumor samples from 587 patients with TNBC, including basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal-like (MES), mesenchymal/stem-like (MSL), immunomodulatory (IM), and luminal androgen receptor (LAR) [24]. However, this typing methodology is very homogeneous and no longer reflects the genomic characteristics of each tumor.
Currently, the most widely used is the TNBC molecular typing published by Prof. Shao Zhimin at Fudan University, known as "Fudan typing" [19, 25]. Shao's team divided 465 TNBC samples into four different subgroups by multi-omics sequencing. Namely, the LAR type, which signals through androgen receptor signaling, the MES type, which has an enrichment in growth factor signaling pathways, the IM type, which overexpresses the related signaling genes of immune cells and cytokine, and the BL type, which activates cell cycle and DNA repair with the help of reduced immune response genes [19]. This typology is similar to the results reported by Lehmann et al., but it is helpful for researchers to explore more effective individualized treatment strategies for patients with TNBC.
In 2020, Shao's group identified androgen receptor (AR), CD8, FOXC1, and DCLK1 as immunohistochemistry (IHC) biomarkers. According to the results of IHC staining, TNBC is divided into five subtypes, including IHC-based IM (IHC-IM; AR−CD8+), IHC-based LAR (IHC-LAR; AR+), IHC-based basal-like immunosuppression (IHC-BLIS; AR−CD8−FOXC1+), immune factor-based mesenchymal (IHC-MES; AR−CD8−FOXC1−DCLK1+) and IHC-based unclassifiable (AR−CD8−FOXC1−DCLK1−). The IHC-LAR subtype demonstrates the HER2 signaling pathway activation, and the IHC-IM subtype presents an immunoinflammatory phenotype, which is characterized by the infiltration of CD8+ T cells into the cancer parenchyma. Moreover, the IHC-BLIS subtype exhibits a signature overexpression of vascular endothelial growth factor (VEGF). The IHC-MES subtype shows stimulation of the JAK/STAT3 (signal transducer and activator of transcription 3) signaling pathway. IHC-based subclassification offers additional information for the prognostic assessment of patients with TNBC. This makes it easier for TNBC patients to be subtyped in clinical trials and to evaluate the effectiveness of targeted therapy for selected subtypes, which would promote treating TNBC patients in a subtype-specific manner [26]. The "FUTURE typing" was first demonstrated in the FUTURE clinical trial, and the team is currently conducting a representative series of clinical trials with "FUTURESUPER", which strives to bring the treatment regime from the FUTURE study to the front line and provide more TNBC patients with new options for early individualized treatment." The development of the "FUTURESUPER" clinical trials series has greatly promoted the accurate treatment of patients with TNBC and has a broad prospective in clinical practice [27] (Fig. 1).
Fig. 1.
The molecular subtype of TNBC. At present, TNBC is mainly divided into the following categories, including BL1/2, IM, ML, MSL, and LAR. (The molecular subtype of TNBC was adapted from Fig. 2 in [23]) BL1: basal-like 1, IM: immunomodulatory, ML: mesenchymal-like, MSL: mesenchymal stem-like, LAR: luminal androgen receptor
TNBC-related targeted therapy
Poly (ADP-ribose) polymerase (PARP) inhibitors
Malignant tumor cells are susceptible to the occurrence of mutations in the BRCA gene, such as the existence of mutations in the BRCA1/2 gene in patients with TNBC. BRCA1/2 plays a role in the homologous recombination repair of double-stranded DNA, and tumor cells containing mutations in the BRCA1/2 gene have defective DNA repair due to a deficiency in homologous recombination repair [28, 29]
PARP is a key enzyme for repairing DNA single-strand damage, and based on BRCA functional defects, PARP inhibitors are used to suppress its activity and block DNA damage repair, leading to excessive accumulation of DNA damage and ultimately to tumor cell death. Thus, PARP inhibitors could cause 'synthetic death' in BRCA1/2-deficient cancers [23, 30] (Fig. 2B). Currently, PARP inhibitors such as olaparib and talazoparib are already formally approved by the FDA for clinical therapy of patients with HER2−advanced or metastatic BC with BRCA mutations [24, 25] (Table 1).
Fig. 2.
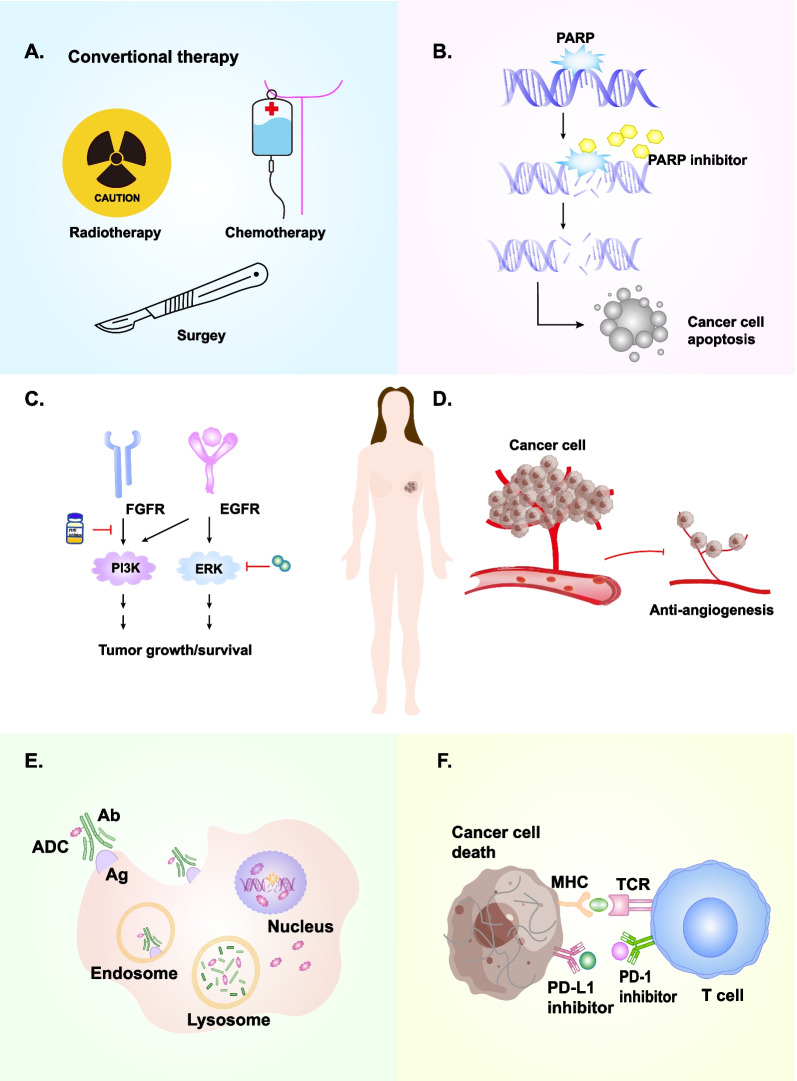
The therapeutic strategies in TNBC. There are several therapeutic strategies in TNBC. A. Traditional treatments for TNBC, including chemotherapy, radiotherapy, and surgery; B. PARP inhibitor; C. Signaling pathway-related inhibitors; D. VEGF/VEGFR inhibitors; E. ADC; F. Immune checkpoint inhibitors
Table 1.
The clinical trials of PARP inhibitors in TNBC
| Drug name | Com | Num | Trial Name | Regiment | Phase | Status | Object | POM | NCT number |
|---|---|---|---|---|---|---|---|---|---|
| Olaparib (AZD2281) | 1836 | OlympiA | 300 mg bid po | III | Active, not recruiting | Early-stage gBRCA, adjuvant therapy | iDFS | NCT02032823 | |
| Olaparib | 99 |
400 mg bid po (capsules) Or 300 mg bid po (tablets) |
II | Completed | Advanced TNBC | ORR | NCT00679783 | ||
| Olaparib | 54 | 100 mg/400 mg bid | II | Completed | gBRCA1/2 m and advanced TNBC | ORR | NCT00494234 | ||
| Olaparib | 30 | 300 mg bid for 4 weeks of each cycle | II | Not yet recruiting | mTNBC | ORR | NCT05522491 | ||
| Olaparib | Ceralasertib/ Adavosertib | 273 | VIOLETTE |
Olaparib: 300 mg bid 28-day cycle Ceralasertib: 160 mg/d Adavosertib: 150 mg bid |
II | Active, not recruiting | mTNBC | PFS | NCT03330847 |
| Olaparib | Cediranib | 155 |
Olaparib: 100–400 mg po bid on days 1–28 Cediranib: po |
I/II | Active, not recruiting | mTNBC | PFS | NCT01116648 | |
| Olaparib | Durvalumab | 45 | DORA |
Olaparib: 300 mg bid Durvalumab: iv every 28 days |
II | Completed | Advanced TNBC | PFS | NCT03167619 |
| Olaparib | Durvalumab (MEDI4736) | 264 | MEDIOLA |
Olaparib: 300 mg bid for 4 weeks Durvalumab: q4w starting on day 1 |
I/II | Active, not recruiting | gBRCAm HER2-mBC/TNBC | PFS | NCT02734004 |
| Olaparib | Durvalumab | 3 |
Olaparib: po bid for 28 days Durvalumab: iv over 1 h on day 1 |
I | Completed | mTNBC | CLIA | NCT03544125 | |
| Olaparib | Durvalumab | 132 |
Olaparib: po bid on days 1–28 Durvalumab: iv over 1 h on day 1 |
II | Recruiting | mTNBC | ORR | NCT03801369 | |
| Olaparib | Physician's choice chemotherapy | 302 | OlympiAD |
Olaparib: 300 mg bid po Capecitabine: 2500 mg/m2 day 1-14 or Vinorelbine 30 mg/m2 day 1,8 or Eribulin 1.4 mg/m2 day 1,8 |
III | Active, not recruiting | Advanced/Metastatic gBRCA, ≤ 2 prior lines | PFS | NCT02000622 |
| Olaparib | Paclitaxel | 19 |
Olaparib: 200 mg bid Paclitaxel: iv over 1 h |
I/II | Completed | mTNBC | AEs | NCT00707707 | |
| Olaparib | Radiation therapy | 24 |
Olaparib: five levels of dose, po bid each day IMRT |
I | Unknown | Advanced or mTNBC | MTD | NCT03109080 | |
|
Veliparib (ABT-888) |
Temozolomide | 294 |
Veliparib: 40 mg bid days 1–7 Temozolomide: 150 to 200 mg/m2 qd days 1–5 in each 28-day cycle |
II | Completed | Metastatic gBRCA, ≤ 0–2 prior lines | PFS | NCT01506609 | |
| Veliparib | Carboplatin and Paclitaxel | 294 |
Veliparib: 80 mg bid day 1–7 Carboplatin: day 3 of each 21-day cycle Paclitaxel: 175 mg/m2 on day 3 of each 21-day cycle |
II | Completed | Metastatic gBRCA, ≤ 0–2 prior lines | PFS | NCT01506609 | |
| Veliparib | Carboplatin and Paclitaxel | 509 | BROCADE3 |
Veliparib: 120 mg bid on day 2–5 of a 21-day cycle Carboplatin: iv AUC 6 mg/ml/min on day 1 of every cycle Paclitaxel: 80 mg/m2 iv on day 1, 8, and 15 of every cycle |
III | Active, not recruiting | Metastatic or advanced gBRCA1/2 m HER2- BC/TNBC | mPFS | NCT02163694 |
| Veliparib | Carboplatin and Paclitaxel | 634 | BrighTNess |
Veliparib: 50 mg po bid Paclitaxel: 80 mg/m2 iv weekly for 12 doses Carboplatin: AUC 6 mg/mL/min iv q3w for 4 cycles |
II | Completed | Stage II or III TNBC Neoadjuvant | pCR | NCT02032277 |
| Veliparib | Carboplatin and Paclitaxel | 116 | I-SPY 2 | NA | II | Recruiting | Stage II or III TNBC Neoadjuvant | pCR | NCT01042379 |
| Veliparib | Cyclophosphamide | 124 |
Veliparib: 60 mg po by mouth Cyclophosphamide: 50 mg po by mouth for 21d |
II | Completed | Advanced TNBC | ORR, PFS | NCT01306032 | |
|
Talazoparib (BMN 673) |
431 | EMBRACA | 1.0 mg/d po for 21 continuous days | III | Completed | Advanced/Metastatic gBRCA, ≤ 3 prior lines | PFS | NCT01945775 | |
| Talazoparib | 84 | ABRAZO | 1 mg qd | II | Terminated | gBRCA1/2 m advanced TNBC | ORR | NCT02034916 | |
| Talazoparib | 36 | 1 mg/d po for 6 months | II | Completed | gBRCA1/2 m and operable HER2- BC/TNBC | pCR | NCT02282345 | ||
| Niraparib | 216 | BRAVO | 300 mg (3 × 100 mg capsules) /d po for 21 continuous days | III | Terminated | Advanced/Metastatic gBRCA, ≤ 2 prior lines | PFS | NCT01905592 | |
| Niraparib | Pembrolizumab | 122 | TOPACIO |
Niraparib: 300 mg/d po on Day 1-21 Pembrolizumab: 200 mg iv on Day 1 of each 21-day cycle |
I/II | Completed | Advanced TNBC | DLTs, ORR | NCT02657889 |
iDFS: invasive disease-free survival; mTNBC: metastatic triple negative breast cancer; HER2-: HER2 negative; mBC: metastatic breast cancer; gBRCA1/2 m: germline BRCA1/2 mutated; ORR: overall response rate; PFS: progression-free survival; CLIA: Clinical Laboratory Improvement Act; AEs: adverse events; IMRT: intensity modulated radiotherapy; MTD: maximum tolerated dose; DLTs: dose-limiting toxicity; Com: Combination; Num: Number; POM: Primary outcome measures
Olaparib is ineffective in metastatic TNBC (mTNBC) patients and wild type BRCA1/2, but a clinical trial revealed higher objective remission rates with olaparib monotherapy in untreated TNBC [31]. The OlympiAD trial compared the progression-free survival (PFS) of patients with HER2-negative metastatic BC who received olaparib monotherapy or standard therapy. The results indicated that compared with standard therapy, median PFS with olaparib monotherapy lasted 2.8 months longer and reduced disease progression or risk of death by 42% [32]. Analysis of follow-up results showed that overall survival (OS) was prolonged for BC patients given first-line olaparib compared to the standard group. In patients with TNBC, although the olaparib group prolonged OS, the difference was not statistically significant [33]. Interestingly, response to olaparib was correlated with low RAD51 scores, high TIL or high PD-L1 expression.
Another trial, OlympiA, evaluated the effectiveness and side effects of olaparib compared to placebo in the adjunctive therapy of patients with early-stage HER2-negative BC who carry the BRCA1/2 germline mutation. Results revealed that olaparib significantly improved patient OS compared to the placebo group, with 3-year invasive disease-free survival (iDFS) of 85.9% compared to 77.1% in the placebo group, and distant disease-free survival (DDFS) was 87.5% compared to 80.4% in the placebo group. Furthermore, olaparib had no serious adverse effects [34].
Talazoparib was taken by patients with advanced BC carrying BRCA1/2 germline mutation in the EMBRACA trial, showing notably longer PFS (8.6 months versus 5.6 months) in the talazoparib arm versus the chemotherapy arm and objective response rate (ORR) was improved (62.6% versus 27.2%) [35]. In addition, the NEOTALA trial explored the effectiveness of talazoparib alone in the neoadjuvant therapy of patients with HER2-negative BC who have BRCA1/2 germline mutations. It demonstrated significantly higher pathologic complete response (pCR) rates in the evaluable and intent-to-treat populations (all TNBC patients) (45.8% and 49.2%), respectively, with a well-tolerated safety profile [36]. Sequential combination therapy with talazoparib and carboplatin suppressed primary cancer cell growth and distant metastases in patients with TNBC, laying the foundation for treating early-stage TNBC [37].
Although PARP inhibitors are effective for TNBC, clinical resistance cannot be ignored, hence need to explore resistance mechanisms further and find better and more effective treatment strategies [38].
AR inhibitors
The LAR type is driven via the AR signaling pathway, and the level of AR expression in the LAR is negatively correlated with PFS and OS in TNBC patients [39, 40]. Currently, researchers have explored many AR inhibitors for TNBC therapy [41] (Table 2). Although clinical trials have demonstrated that AR inhibitors have been effective in the therapy of TNBC patients, the exact mechanism is unclear.
Table 2.
The clinical trials of AR inhibitors in TNBC
| Drug name | Com | Num | Regiments | Ph | State | Object | POM | NCT number |
|---|---|---|---|---|---|---|---|---|
| Bicalutamide | 60 | 150 mg/d po | II | Unknown | AR+TNBC | CBR, PFS | NCT02353988 | |
| Bicalutamide | 36 | 150 mg/d po | III | Terminated | AR+TNBC | CBR | NCT03055312 | |
| Bicalutamide | 1 | 150 mg/d po | II | Terminated | AR+TNBC | CBR | NCT02348281 | |
| Bicalutamide | Ribociclib | 37 |
Bicalutamide: 150 mg/d po Ribociclib: 400 mg/d po |
I/II | Recruiting | AR+TNBC | MTD, CBR | NCT03090165 |
| Enzalutamide | 50 | 160 mg/d po for 52 weeks | II | Active, not recruiting | AR+TNBC | feasibility | NCT02750358 | |
| Enzalutamide | Paclitaxel | 37 |
Enzalutamide: PO daily on days 1–7 Paclitaxel: iv over 2 h on day 1. Treatments repeat every 7 days for up to 12 cycles |
II | Recruiting | AR+TNBC | pCR | NCT02689427 |
| GTx-024 | 32 | 18 mg/d po | II | Terminated | AR+TNBC | CBR | NCT02368691 | |
| GTx-024 | Pembrolizumab | 18 |
GTx-024: 18 mg/d po Pembrolizumab: iv over 30 min on day 1 |
II | Active, not recruiting | AR+TNBC | RR | NCT02971761 |
AR+TNBC: androgen receptor positive triple negative breast cancer; CBR: clinical benefit rate; PFS: progression-free survival; MTD: maximum tolerated dose; pCR: pathologic complete response; RR: response rate
AR+ expression was confirmed in approximately 12% of ER−PR− BC patients. Patients received bicalutamide and showed a clinical benefit rate (CBR) of 19% and 3 months mPFS, and the patients were well tolerated [42]. Enzalutamide demonstrated favorable clinical effectiveness and tolerance in patients with AR+ TNBC, mPFS and mOS were 3.3 and 17.6 months, and serious adverse events in patients were 2%. Thus, it is recommended that enzalutamide may be used to treat patients with AR+ TNBC [43]. UCBG 12–1 is a trial on the effectiveness of abiraterone plus prednisolone in AR+ advanced TNBC patients. The results indicated that patients treated with abiraterone had an mPFS of 7.5 months, an ORR of 8.22%, a CBR in 20% of patients, and manageable adverse events [44].
Additionally, researchers performed a series of studies combining AR inhibitors with other TNBC treatment regimens. Min et al. discovered that a combination of the AR inhibitor AZD3514 and olaparib played a synergetic effect role in BC cells by modulating the DNA damage response [45]. Likewise, combining an AR inhibitor with a PARP inhibitor repressed the progression of TNBC cells [46]. The above preclinical trials suggested that AR inhibitors combined with PARP inhibitors may have favorable CBR in treating TNBC patients.
Subsequently, researchers designed clinical trials related to AR inhibitors and other agents. TBCRC032 is a multicenter clinical trial, which investigates the effectiveness of enzalutamide and taselisibin AR+TNBC patients. The study demonstrated that combination therapy effectively increased the CBR of patients with TNBC (35.7%), and the mPFS was 3.4 months [47]. Moreover, Choupani et al. found that enzalutamide combination with cyclin-dependent kinase (CDK) 4/6 inhibitor ribociclib had synergistic tumor-inhibiting effects on TNBC cells [48]. Although preclinical data of AR inhibitors in combination with CDK4/6 inhibitors have shown promising antitumor effects, relevant clinical trials are still ongoing, and data are not yet available (Table 2).
Currently, most studies on the AR inhibitor in treating TNBC patients are I/II clinical trials, and there is a lack of large specimen data in phase III/IV to further explore the effectiveness of AR inhibitor in TNBC patients. Likewise, it is worth exploring whether AR inhibitors combined with other drugs such as PARP inhibitors and immunotherapy will bring about better clinical effects.
CDK inhibitors
CDK is a key enzyme that regulates transition in the various phases of the cell cycle, and continued activation can result in tumor cell proliferation [49]. DK4/6 inhibitors primarily inhibit the G1-S phase, thereby inhibiting the cellular DNA replication process [50]. The LAR subtype is highly sensitive to CDK4/6 inhibitors. Thus, using CDK4/6 inhibitors may be a potential therapeutic approach for the LAR subtype [51]. The FDA has already approved CDK4/6 inhibitors to treat TNBC patients, concluding palbociclib and ribociclib [52] (Table 3).
Table 3.
The clinical trials of CDK inhibitors in TNBC
| Drug name | Com | Num | Regiments | Ph | State | Object | POM | NCT number |
|---|---|---|---|---|---|---|---|---|
| Palbociclib | Binimetinib | 24 |
Palbociclib: 100 mg/d po, 21 days on/7 days off Binimetinib: 45 mg bid po, 21 days on/7 days off |
I/II | Active, not recruiting | mTNBC | PFS | NCT04494958 |
| Palbociclib | Paclitaxel/ carboplatin | 126 |
Palbociclib: 125 mg/d po on days 1–14 Paclitaxel: 80 mg/m2 iv on day 1, 8, 15 and 22 Carboplatin: AUC 2 iv on day 1, 8, 15 and 22 |
II | Not yet recruiting | TNBC | Early metabolic response | NCT05067530 |
| Palbociclib | Avelumab | 45 | NA | I | Recruiting | AR+TNBC | MTD | NCT04360941 |
| Trilaciclib | Gemcitabine/ Carboplatin | 102 |
Trilaciclib: 240 mg/m2 iv on day 1, 8 Gemcitabine: 1000 mg/m2 on day 1, 8 Carboplatin: AUC 2 on day 1, 8 of each 21-cycle |
II | Terminated | mTNBC | DSN | NCT02978716 |
| Trilaciclib | Gemcitabine/ Carboplatin | 194 |
Trilaciclib: 240 mg/m2 iv on day 1, 8 Gemcitabine: 1000 mg/m2 on day 1, 8 Carboplatin: AUC 2 on day 1, 8 of each 21-cycle |
III | Active, not recruiting | mTNBC | OS | NCT04799249 |
| Trilaciclib | Sacituzumab Govitecan | 30 | Trilaciclib: 240 mg/m2 iv on day 1, 8 Sacituzumab Govitecan: 10 mg/kg | II | Active, not recruiting | TNBC | PFS | NCT05113966 |
| Trilaciclib | Doxorubicin/ Cyclophosphamide/ Pembrolizumab | 24 |
Trilaciclib: 240 mg/m2 Doxorubicin: 60 mg/m2 q2w for the first 4 cycles Cyclophosphamide: 600 mg/m2 for cycles 5–16 Pembrolizumab: 400 mg iv q6w for cycles 1, 4, 9, 15 |
II | Completed | TNBC | pCR | NCT05112536 |
| Trilaciclib | Epirubicin/ Cyclophosphamide/ Pembrolizumab | 150 |
Trilaciclib: 240 mg/m2 iv on day 1 Epirubicin: 100 mg/m2 iv on day 1, q2w/q3w Cyclophosphamide:600 mg/m2 on day 1, q2w/q3w Paclitaxel:100 mg/m2 iv on day 1,8,15, q3w, 4 cycles |
II | Not yet recruiting | TNBC | The incidence of CIN | NCT05862610 |
| Etoposide | Anlotinib | 100 | Etoposide: 75 mg/d po on day 1-10, 21 days/cycle Anlotinib: 12 mg/d po on day 1-14, 21 days/cycle | II | Recruiting | Advanced TNBC | ORR | NCT04452370 |
| Abemaciclib | Surgery | 200 |
Abemaciclib: PO BID on days 1–14 or days 1–21 Surgery: no later than 12 weeks after the last dose of neoadjuvant chemotherapy |
II | Recruiting | Refractory TNBC | Incidence of AEs | NCT03979508 |
| Prexasertib | 111 | 105 mg/m2 iv once every 14 days, 28 days/cycle | II | Terminated | TNBC | Objective Response | NCT02203513 | |
| Prexasertib | LY3023414 | 10 |
Prexasertib: 80 mg/m2 iv q2w LY3023414: 150 mg bid |
II | Active, not recruiting | mTNBC | ORR | NCT04032080 |
mTNBC: metastatic triple negative breast cancer; PFS: progression-free survival; AUC: area under the curve; AR+TNBC: androgen receptor-positive triple negative breast cancer; MTD: maximum tolerated dose; DSN: duration of Severe (Grade 4) Neutropenia; OS: overall survival; pCR: pathologic complete response; ORR: overall response rate; AEs: adverse events; NA: not acquire
Several preclinical trials have shown the combination of CDK4/6 with other targeted drugs plays a favorable antitumor role in TNBC cells. Sequential combination therapy with palbociclib and paclitaxel could more effectively suppress TNBC cell proliferation [53]. Shao's team suggested that palbociclib combination with olaparib indicated synergistic antitumor effects in TNBC cells [54]. Similarly, this phenomenon is also present in other CDK and PARP inhibitors [55]. Moreover, ribociclib and PI3K inhibitor BYL719 can significantly promote G1 phase arrest in TNBC cells. Furthermore, ribociclib and BYL719 with an immune checkpoint inhibitor (ICI) resulted in complete tumor regression in TNBC xenograft models [56, 57]. Circular RNA has been related to prognosis in TNBC patients, and downregulation of circEIF3M inhibit CND1, which interacts with CDK4 to cause G1 phase arrest in TNBC cells [58].
In the PALOMA-2 trial, palbociclib and letrozole notably improved PFS in ER+/HER2− BC patients in both the general and Asian populations [59, 60]. The PALOMA-3 trial assessed the efficacy of combination therapy with palbociclib and fulvestrant in ER+/HER2− BC patients. Patients receiving palbociclib and fulvestrant extended PFS and OS compared with controls [61, 62]. Nevertheless, the PALLAS trial demonstrated that combining endocrine therapy and palbociclib failed to improve PFS compared to endocrine therapy alone in ER+/HER2− BC patients [63–65]. Additionally, other studies have shown that palbociclib is ineffective in combination with chemotherapy [66].
Similar to palbociclib, ribociclib combined with fulvestrant significantly improved OS in ER+/HER2− BC patients [67]. Compared to palbociclib, abemaciclib combined with endocrine therapy may prolong iDFS in patients with ER+/HER2− BC and has favorable safety [68]. Moreover, abemaciclib combined with fulvestrant in treating ER+/HER2− BC patients significantly improved PFS and ORR [69]. Several clinical trials related to CDK4/6 inhibitors are underway and we expect good results.
PI3K/AKT/mTOR signaling pathway inhibitors
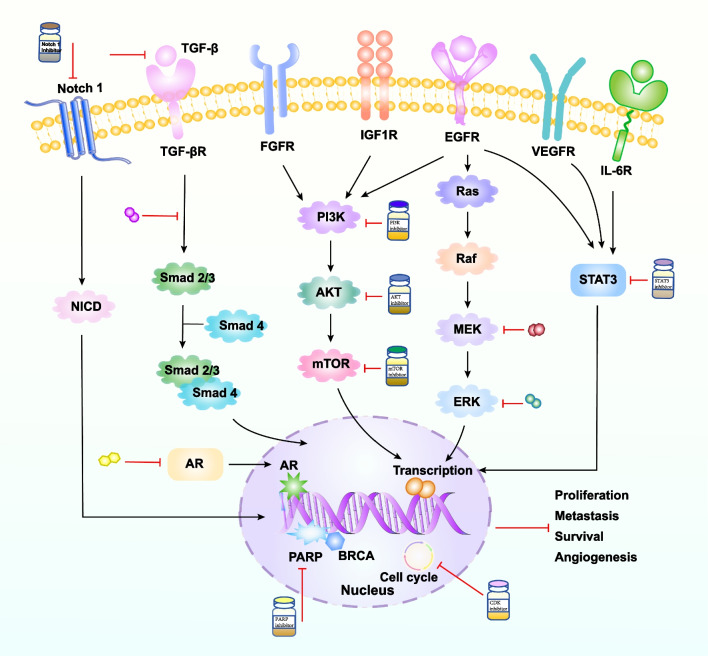
The PI3K/AKT/mTOR signaling pathway, the most prevalent cancer activation pathway, results in proliferation and a host of other malignant biological behaviors in tumor cells [70, 71]. PI3K, a critical protein in this signaling pathway, mediates tumor cell growth, proliferation, and metabolism. In addition, PI3K/AKT pathway is an important intracellular signaling pathway, which participates in the expression of genes linked to proliferation and apoptosis in cancer cells. For example, activation of AKT can regulate the expression of its downstream proteins such as cyclin A1, cyclin D1, Bax, Bcl-2, and others. Thus mediate the malignant biological behavior of various tumors [72]. Multiple genomic alterations resulted in activated PI3K pathways, such as PIK3CA and AKT [73], and act as oncogenic drivers promoting tumor cell transformation, tumor initiation, progression, and apoptosis [74]. Mutations in PIK3CA lead to tumorigenesis [75, 76]. A study has shown that PIK3CA was mutated in 20% to 40% of BC and was associated with increased resistance to chemotherapy [77]. PI3KCA mutations have been reported in approximately 10% of TNBC, but are more common in the LAR and MES subtypes. Therefore, inhibiting the PI3K/AKT/mTOR signaling pathway might be a prospective approach for treating breast cancer [19, 78, 79] (Fig. 3).
Fig. 3.
The signaling pathway and its inhibitors of TNBC. Presentation of TNBC-related signaling pathways and their inhibitors. Excitatory regulation is symbolized by black arrows, red arrows stand for inhibitory effects. (The TNBC signaling pathway and its inhibitors were adapted from Fig. 2 in [79])
Currently, relevant studies are exploring the PI3K/AKT/mTOR signaling pathway inhibitors, including capivasertib and ipatasertib. Several inhibitors have been considered in preclinical studies or clinical trials [80] (Table 4).
Table 4.
The clinical trials of signaling pathway inhibitors
| Target | Drug name | Com | Num | Regiments | Phase | Status | Object | POM | NCT number |
|---|---|---|---|---|---|---|---|---|---|
| PI3K inhibitor | Alpelisib (BYL719) | nab-paclitaxel | 137 |
Alpelisib: 300 mg qd po nab-paclitaxel: 100 mg/m2 iv on Days 1, 8 and 15 of a 28-day cycle |
III | Active, not recruiting | TNBC | PFS, ORR | NCT04251533 |
| Alpelisib (BYL719) | nab-paclitaxel | 8 |
Alpelisib: qd po on days 1–21 Nab-paclitaxel: iv over 30 min on days 1, 8, and 15 |
II | Active, not recruiting | TNBC | pCR | NCT04216472 | |
| AZD8186 | 147 | A single dose on day 1 followed by ongoing multiple dosing | I | Completed | TNBC | Safety and tolerability | NCT01884285 | ||
| BKM120 (Buparlisib) | 50 | 100 mg/d po in cycles of 28 days | II | Completed | TNBC | CBR | NCT01790932 | ||
| BKM120 | 50 | 100 mg/d po in cycles of 28 days, until disease progression | II | Completed | TNBC | CBR | NCT01629615 | ||
| BKM120 | Capecitabine | 10 |
BKM120: 100 mg/d po Capecitabine: 500 mg bid po, 14 days on and 7 days off |
II | Completed | mBC | CBR | NCT02000882 | |
| BKM120/BYL719 | Olaparib | 118 |
BKM120: 40 mg/d po Olaparib: 50 mg bid po BYL719: 250 mg/d po |
I | Completed | TNBC | MTD | NCT01623349 | |
| CUDC-907 | 43 | 60 mg/d po 5 days on 2 days off until disease progression | I | Completed | TNBC | Safety and tolerability | NCT02307240 | ||
| GDC-0941 | Cisplatin | 11 |
GDC-0941: 260 mg po, days 2–6, 9–13, 16–20, 23–27. 28 days cycle Cisplatin: 25 mg/m2 iv day 1, 8, 15 |
I/II | Terminated | TNBC | Safety and tolerability, ORR | NCT01918306 | |
| SF1126 | 44 | Dose Escalating with 3 + patients in each cohort | I | Completed | Solid cancer | DLTs | NCT00907205 | ||
|
Akt inhibitor |
GSK2141795 |
Trametinib (GSK1120212) |
37 |
GSK2141795: po qd on days 1–28 Trametinib: po qd on days 1–28 |
II | Completed | TNBC | ORR | NCT01964924 |
| GSK2141795 | Trametinib | 240 | Po qd | I | Completed | TNBC | Safety and tolerability | NCT01138085 | |
| GSK2141795 | Trametinib | 37 | Po qd on days 1–28 | II | Completed | TNBC | ORR | NCT01964924 | |
|
ONC201 (TIC10) |
4 | Po on days 3, 10, and 17 | II | Terminated | TNBC | ORR | NCT03733119 | ||
| Ipatasertib (GDC-0068) | Paclitaxel | 151 |
Ipatasertib: 400 mg/d po on days 1–21 of each 28-day cycle for 3 cycles Paclitaxel: 80 mg/m2 iv q1w |
II | Completed | TNBC | pCR | NCT02301988 | |
| Ipatasertib | Paclitaxel | 124 |
Ipatasertib: 400 mg/d po days 1–21 in each cycle of 28 days Paclitaxel: 80 mg/m2 iv on days 1, 8, and 15 |
II | Completed | TNBC | PFS | NCT02162719 | |
| PI3K /mTOR inhibitor | PF-05212384 (Gedatolisib) | Docetaxel/ Cisplatin | 110 |
90 mg/m2 iv as a 3 weeks cycle Docetaxel/Cisplatin: 75 mg/m2 iv once q3w |
I | Completed | TNBC | DLTs, ORR | NCT01920061 |
| BEZ235 | MEK162 | 29 | NA | I | Completed | TNBC | DLTs | NCT01337765 | |
| PQR309 | Eribulin (Halaven®) | 41 |
PQR309: after eribulin Eribulin: 1.4 mg/m2 iv on day 1, 8 in a period of 21 days |
I | Completed | TNBC | AEs, SAEs | NCT02723877 | |
| EGFR inhibitor | Cetuximab (Erbitux) | Ixabepilone (Ixempra ®) | 40 |
Cetuximab: 400 mg/m2 iv over 120 min on day 1 of the first of four 21 days cycles Ixabepilone: 40 mg/m2 iv over 180 min on day 1 of each of four 21 days cycles |
II | Completed | TNBC | CRR | NCT01097642 |
| Lapatinib (Tykerb ®) | Veliparib (ABT-888) | 23 |
Lapatinib:1250 mg/d for 28 days Veliparib: 200 mg/bid for 28 days |
NA | Active, not recruiting | TNBC | Safety and toxicity | NCT02158507 | |
| Lapatinib | Everolimus (mTOR inhibitor) | 5 |
Lapatinib: 1250 mg by mouth daily Everolimus: 5 mg by mouth daily |
II | Terminated | TNBC | Safety and toxicity, ORR | NCT01272141 | |
| Erlotinib (Tarceva ®) | Cisplatin plus temsirolimus | 9 |
Erlotinib: 100 mg by mouth daily Cisplatin: 30 mg/m2 iv weekly on days 1 and 8 of a 3 weeks cycle Temsirolimus: dosing level, 15 mg, 25 mg |
I | Completed | TNBC | MTD | NCT00998036 | |
| Erlotinib | Metformin | 8 |
Erlotinib: 150 mg/d Metformin: 850 mg bid |
I | Completed | TNBC | MTD | NCT01650506 | |
| Erlotinib | Bendamustine | 11 |
Erlotinib: 100 or 150 mg po on days 5—21 of each 28 days cycle Bendamustine: 100 or 120 mg/m2 iv on days 1 and 2 |
I/II | Completed | Breast Cancer | MTD, DLTs, PFS | NCT00834678 | |
| Gefitinib (Irresa ®) | 50 | 250 mg/d by mouth until disease progression | II | Unknown | TNBC | CBR | NCT01732276 | ||
| Notch inhibitor | AL101 | 67 | NA | II | Active, not recruiting | TNBC | ORR | NCT04461600 | |
| RO4929097 (R4733) | 6 | Po qd on days 1–3, 8–10, and 15–17 | II | Terminated | TNBC | ORR, PFS | NCT01151449 | ||
| RO4929097 | Carboplatin plus Paclitaxel | 14 |
RO4929097: po qd on days 1–3, 8–10, and 15–17 Paclitaxel: iv over 60 min on days 1, 8, and 15 Carboplatin: iv over 60 min on day 1 |
I | Terminated | TNBC | AEs, MTD | NCT01238133 | |
| TGF-β inhibitor | Bintrafusp Alfa (M7824) | 11 | 1200 mg once q2w | II | Completed | TNBC | ORR | NCT04489940 |
PIK3: phosphoinositide-3-kinase; TNBC: triple negative breast cancer; PFS: progression-free survival; pCR: pathologic complete response; CBR: clinical benefit rate; MTD: maximum tolerated dose; AKT: serine/threonine kinase; mTOR: mammalian target of rapamycin; EGFR: epidermal growth factor receptor; DLT: dose-limiting toxicity; AEs: adverse events; SAEs: serious adverse events; TGF-β: transforming growth factor Beta; mTNBC: metastatic triple negative breast cancer; HER2-: HER2 negative; mBC: metastatic breast cancer; gBRCA1/2 m: germline BRCA1/2 mutated; ORR: overall response rate; CRR: complete response rate
Dey et al. summarized the PI3K inhibitors currently used in TNBC clinical trials [18]. LY294002, the first synthetic PI3K inhibitor, was used to explore the mechanism of AKT inhibitor induced-apoptosis [81]. SF1126 is a chemically modified form of LY294002, shown to inhibit tumor initiation and angiogenesis in vivo [82, 83]. A phase I trial showed that SF1126 had no dose-limiting toxicity or hepatotoxicity and showed comparable efficacy against several solid tumors [84]. However, SF1126 was a potential cancer treatment, and its target mechanism in TNBC was unclear. Deng et al. found that SF1126, in combination with gefitinib, induced apoptosis of TNBC cells by blocking the EGFR-PI3K-AKT-mTOR pathway [85]. Furthermore, SF1126 combination with sorafenib showed a favorable antitumor effect of hepatocellular carcinoma in vivo [86]. Therefore, it is important to further explore the mechanism of PI3K inhibitors targeting TNBC.
In addition to PI3K inhibitors, there are several AKT inhibitors in clinical trials [87]. AZD5363 has been administered as monotherapy to treat patients with BC, gastric and prostate cancers [88]. The AKT inhibitor ipatasertib has been used as monotherapy for TNBC patients [89]. Results from the EAY131-Y subgroup of the NCI-MATCH study showed that capivasertib had antitumor activity in a range of metastatic tumors with AKT1/E17K mutations [90]. The LOTUS and PAKT studies showed that adding the AKT inhibitors ipatasertib or capivasertib to first-line paclitaxel therapy in mTNBC prolonged PFS in patients, with more apparent CBR in patients carrying PIK3CA/AKT1/PTEN mutations [91, 92]. The effectiveness of neoadjuvant ipatasertib combined with paclitaxel in early TNBC was also evaluated in the FAIRLANE study. The result demonstrated that the pCR was higher in the ipatasertib arm than in the placebo arm in patients with mutations in the PI3K/AKT/mTOR signaling pathway [93]. Currently, the first-generation mTOR inhibitors, including everolimus and sirolimus, are approved for treating BC. However, PI3K inhibitors targeting TNBC are still in phase I clinical trials [70].
Dual PI3K/mTOR inhibitor therapy is reported to be more efficient than single inhibitors [94]. Dual PI3K/mTOR inhibitors, such as apitolisib, suppressed human glioblastoma cell growth and induced apoptosis [95]. B7-H3 can promote resistance to traditional cancer therapy in a variety of tumors, and knocking out B7-H3 has been shown to increase the sensitivity of TNBC cells to everolimus [96, 97]. Dual PI3K/mTOR inhibitors are considered critical in cancer therapy, and many dual PI3K/mTOR inhibitors are available and in use, such as dactolysisib, sarmotolysisib, and voltaricoxib [94].
PI3K/AKT/mTOR oncogenic signaling pathways often induce cancer progression and are associated with resistance to targeted anticancer therapies, and more research is still needed on the effectiveness of related inhibitors [98].
Epidermal growth factor receptor (EGFR) signaling pathway inhibitors
EGFR is a tyrosine kinase receptor. It's reported that EGFR was an efficient therapeutic target in 89% of TNBC patients, especially for BL2 subtype tumors with overexpression of EGFR [99]. Moreover, EGFR predicts recurrence-free survival and OS in BC patients [100].
EGFR targeting has been approved for treating cancer patients, including tyrosine kinase inhibitors (TKIs) gefitinib and monoclonal antibodies [101] (Fig. 2C, Table 4). Gefitinib inhibits BC cell proliferation and increases the cytotoxicity of carboplatin and docetaxel [102]. In addition, combining three inhibitors, gefitinib, carboplatin, and docetaxel, may synergistically increase cytotoxicity in TNBC cells [103]. However, the reported failure of combination therapy with EGFR TKIs and monoclonal antibodies led to combination therapy with monoclonal antibodies and chemotherapeutic agents, which was a more effective therapeutic strategy. For example, in a clinical trial, cetuximab combination with carboplatin or cetuximab with cisplatin doubled pCR and prolonged PFS and OS in metastatic TNBC patients [104, 105]. Moreover, HOMER3 promoted β-catenin activation through growth factor stimulation, which in turn facilitated the progression of TNBC cells [106]. Sustained activating EGFR/KRAS/SIAH pathway has contributed to chemoresistance in TNBC, and further exploration of chemoresistance will provide new insight for future treatment of TNBC [107].
It has been shown that targeting gefitinib and everolimus can inhibit the activation of the PI3K/AKT/mTOR signaling pathway, thereby blocking cancer cell cycle progression and promoting apoptosis in TNBC cells [108].
Fibroblast growth factor receptor (FGFR)
Fibroblast growth factor receptors are activated by binding to various fibroblast growth factors and regulate numerous cellular processes. Over-activation of FGFR signaling is observed in some cancers and FGFR has interaction with hormone receptor signaling [109, 110]. There is the amplification of FGFR1 or FGFR2 in TNBC, and FGFR1 activation has been linked to OS prognosis [111–113]. Turner et al. found that FGFR1 or FGFR2-amplified TNBC cell lines were highly sensitive to the FGFR inhibitor PD173074 [114]. Dovitinib, an FGFR1/2 inhibitor, restrained the proliferation of FGFR-amplified BC cell lines [115]. A clinical conversion trial displayed gastric cancer patients with high FGFR2-amplified had higher pCR than the selective FGFR inhibitor AZD4547 [116]. But a study demonstrated that only 1 in 8 breast cancer patients with FGFR1 amplification responded to AZD4547 treatment. These data suggest that FGFR targeting has shown promising results in breast cancer, especially when FGFR is amplified [117] (Fig. 3).
Currently, some clinical trials are ongoing and enrolled patients must undergo molecular pre-screening to ensure the inclusion of patients associated with FGFR pathway activation.
Vascular endothelial growth factor receptor (VEGFR)
The continuous formation of tumor blood vessels provides sufficient nutrients for tumorigenesis and progression of TNBC [118–120]. Therefore, anti-VEGF treatment can inhibit tumor growth (Fig. 2D, Table 5).
Table 5.
The clinical trials of VEGF/VEGFR inhibitors in TNBC
| Drug name | Com | Num | Regiments | Ph | State | Object | POM | NCT number |
|---|---|---|---|---|---|---|---|---|
| Bevacizumab | 54 | 15 mg/ kg | II | Completed | mTNBC | PFS | NCT03577743 | |
| Bevacizumab | 2591 | 5 mg/kg q1w | III | Completed | TNBC | IDFS | NCT00528567 | |
| Bevacizumab | Albumin-bound paclitaxel | 128 |
Bevacizumab: 7.5 mg/kg iv q3w Albumin-bound paclitaxel: 260 mg/m2 iv q3w |
II | Recruiting | mTNBC | PFS | NCT05192798 |
| Bevacizumab | Tirelizumab | 15 |
Bevacizumab: 7.5 mg/kg iv q3w Tirelizumab: 200 mg iv q3w |
II | Recruiting | mTNBC | ORR | NCT05303038 |
| Bevacizumab | Liposomal doxorubicin hydrochloride/ Everolimus | 17 |
Bevacizumab: iv over 90 min on day 1 Liposomal doxorubicin hydrochloride: iv over about 3 h on day 1 Everolimus: po qd on days 1–21 |
II | Active, not recruiting | Advanced TNBC | pCR | NCT02456857 |
| Bevacizumab | Paclitaxel/ Docetaxel | 49 |
Bevacizumab: 10 mg/kg iv q2w Paclitaxel q1w or docetaxel q3w |
IV | Completed | TNBC | PFS | NCT01094184 |
| Bevacizumab | Docetaxel, Carboplatin | 45 |
Bevacizumab: 7.5 mg/kg iv q3w Docetaxel iv and carboplatin iv |
II | Completed | TNBC | pCR | NCT01208480 |
| Bevacizumab | Nab-paclitaxel, erlotinib | 59 |
Bevacizumab: iv over 30–90 min on days 1,15 Nab-paclitaxel: iv on days 1, 8, and 15 erlotinib hydrochloride: po qd |
II | Completed | TNBC | PFS | NCT00733408 |
| Bevacizumab | Abraxane, Carboplatin | 41 |
Bevacizumab: 10 mg/kg iv on days 1,15 Abraxane: 100 mg/m2 iv over 30 min on days 1,8,15 Carboplatin: AUC = 2 iv over 15 min on days 1,8,15 |
II | Completed | mTNBC | CBR | NCT00479674 |
| Apatinib | Camrelizumab | 58 |
Apatinib:250 mg po qd Camrelizumab: 200 mg iv on day 1 |
II | Recruiting | TNBC | pCR | NCT05556200 |
| Apatinib | Camrelizumab and nab-paclitaxel | 35 |
Apatinib:250 mg po qd Camrelizumab: 200 mg iv q2w Nab-paclitaxel: 125 mg/m2 iv q1w |
II | Not yet recruiting | TNBC | pCR | NCT05447702 |
| Apatinib | Paclitaxel and Carboplatin | 29 |
Apatinib: 250 mg po qd on day 1-14 Paclitaxel: 175 mg/m2 on day 1 Carboplatin: AUC = 4 on day 1,14 |
II | Unknown | TNBC | pCR | NCT03735082 |
| Apatinib | Capecitabine | 80 |
Apatinib: 425 mg on day 1-21 Capecitabine: 1000 mg/m2 bid on day 1-14 |
II | Recruiting | Advanced TNBC | PFS | NCT03775928 |
| Apatinib | Paclitaxel | 20 |
Apatinib: 500 mg po qd 12 weeks Paclitaxel: 80 mg/m2 on day 1 q1w |
II | Recruiting | Advanced TNBC | ORR | NCT03348098 |
| Apatinib | Albumin paclitaxel and carboplatin | 60 |
Apatinib: 250 mg po on day 1–21 Albumin paclitaxel: 260 mg/m2 iv on day 1 Carboplatin: AUC = 5–6 iv on day 1 |
II | Recruiting | TNBC | pCR | NCT03650738 |
| Apatinib | Camrelizumab and Eribulin | 46 |
Apatinib: 250 mg/d po on day 1-21 Camrelizumab: 200 mg iv q3w Eribulin: 1.4 mg/m2 iv on day 1, 8 q3w |
II | Active, not recruiting | Advanced TNBC | ORR | NCT04303741 |
mTNBC: metastatic triple negative breast cancer; PFS: progression-free survival; IDFS: invasive disease-free survival; ORR: overall response rate; pCR: pathologic complete response; CBR: clinical benefit rate
Currently, the commonly used anti-VEGF drug is bevacizumab. A Phase III trial, RIBBON 1, demonstrated that combining bevacizumab with conventional capecitabine, anthracycline, or taxane improved PFS in mTNBC patients [121]. Subsequently, the trial further analyzed the effectiveness of bevacizumab in mTNBC patients. Results have prolonged mPFS (6.0 m vs 2.7 m) in TNBC patients with bevacizumab arm versus placebo arm, and a trend toward improvement in patient OS [122].
The GeparSixto and GeparQunito trials combining bevacizumab with neoadjuvant chemotherapy for treating TNBC patients showed significant improvement in pCR in TNBC patients [123–125]. However, the results from the BEATRICE trial demonstrated bevacizumab failed to improve OS in early TNBC patients [126]. The FDA withdrew bevacizumab for treating BC because of inconsistencies in treating TNBC patients.
Apatinib has shown antitumor effects by inhibiting VEGFR signaling in TNBC cells [127]. The LANCET trial, administrating apatinib and neoadjuvant chemotherapy (apatinib and docetaxel in combination with epirubicin and cyclophosphamide) in TNBC patients showed excellent efficacy and controlled toxicity [128]. Furthermore, the NAN trial suggested that adding apatinib to advanced TNBC patients who had failed first/second-line therapy improved their PFS with good safety [129]. Liu et al. verified that the combination of camrelizumab and apatinib could effectively improve ORR in patients with advanced TNBC [130]. The above trials suggested that apatinib was effective in the treatment of some patients with TNBC.
Notch signaling pathway inhibitors
Morgan et al. described the family of transmembrane ligands and receptors named Notch. The pathway includes four Notch receptors, namely Notch-1, 2, 3, and 4 receptors, and five ligands, namely Jagged-1, Jagged-2, Delta-1, Delta-3, and Delta-4 [131, 132]. It has been reported that Delta-1 and Jagged-1 are overexpressed in BC, while Notch-1 is also important for tumorigenesis of BC in the form of oncogenic Ras downstream effectors [133, 134]. Many transcription factors encode genes that are associated with tumorigeneses in Notch signaling, including the HES family and HEY family [131]. Notch signaling pathway was essential in the progression of many types of cancer, such as hematological malignancies, BC, lung cancer, hepatocellular carcinoma, pancreatic cancer, and colorectal cancers [131, 135]. Several studies have shown that Notch-3 and Notch-4 have been associated with tumor initiation and proliferation [136]. However, overexpression of Notch-2 appears to be a protective factor in TNBC cell lines [137]. Moreover, the Notch pathway plays a relevant role in BC stem cell maintenance and expansion, and Notch receptor expression and activation are closely associated with the aggressiveness, clinicopathology, and biological phenotype (e.g., invasiveness and chemotherapy resistance) of TNBC [138].
Since the Notch receptor is overexpressed in TNBC, researchers suggest that monoclonal antibodies (mAb) target the receptor as a prospective way to treat TNBC [139]. Current studies on mAb inhibition of Notch-1 signaling indicated that it could effectively reduce the expression of HES and HEY-L families in TNBC cells, inhibit cell proliferation, and promote treatment induced-apoptosis [140]. In addition, treatment with DLL4 (delta-like ligand 4) monoclonal antibody was effective in TNBC [141]. Drugs that interfere with the Notch signaling pathway act by blocking the level of hydrolytic cleavage of the multimeric γ-secretase complex in the cytoplasm and these agents are therefore referred to as γ-secretase inhibitors [142]. Unfortunately, many agents that block the Notch pathway are not approved by FDA.
In summary, abnormal activating of the Notch signaling pathway is associated with malignant biological behavior and prognosis in TNBC. Therefore, an in-depth exploration of the role played by TNBC in this signaling pathway will further improve the understanding of TNBC pathogenesis and thereby explore new targeted therapeutic strategies (Table 4).
STAT3 signaling pathway inhibitors
STAT3 plays an oncogenic effect by participating in the regulation of the expression of genes connected to the malignant biological behavior of tumors [143]. Its constitutive activation is mainly due to the dysregulation of upstream signaling, usually mediated by several cytokines and growth factors, such as IL-6 and EGF [144, 145]. STAT3 is important in BC stem cell progression, maintaining gene expression associated with stem cell phenotype [146] (Fig. 3).
The activation of STAT3 or inhibition of ROS promotes radio-resistance in TNBC, while clonidine plays an effective sensitizer by inhibiting STAT3 and increasing ROS expression in vitro from TNBC. These results showed clonidine combined with irradiation can be an effective approach to ameliorate radiation-resistance in TNBC cells to improve therapeutic efficacy [147]. WZ-2–033, a novel STAT3 inhibitor, inhibits pY705-STAT3 phosphorylation, thereby reducing STAT3-dependent transcriptional activity and suppressing STAT3 expression from downstream genes. WZ-2–033 significantly suppressed the proliferation and tumorigenicity of TNBC in vivo and in vitro via blocking STAT3 activation [148].
Transforming growth factor (TGF) -β inhibitors
TGF-β1 is a member of the TGF-β superfamily [149]. It has been clarified that TGF-β is negatively associated with the prognosis of TNBC patients [150]. Xu et al. proposed that TGF-β was crucial in TNBC drug resistance, regulating tumor cell stemness, epithelial-mesenchymal transition, and apoptosis [149]. TGF-β inhibited the initiation and proliferation of chemotherapy-resistant tumor-initiating cells. This lays the groundwork for the adoption of combination chemotherapy in TNBC patients [151]. TGF-β overexpressed in TNBC cells, which leads to tumor metastasis. It’s suggested that TGF-β inhibitors were essential for patients with metastases [152]. Besides, TGF-β also causes immune evasion and immunotherapy resistance of TNBC [153–155]. In the tumor microenvironment, regulatory T cells, macrophages, MDSC, and fibroblasts co-express TGF-β1 and PD-L1. Bi-functional fusion protein Bintrafusp alfa was designed for simultaneous inhibition of two immunosuppressive pathways in the tumor microenvironment. The study by Lan demonstrated that Bintrafusp alfa more effectively blocked TGF-β and showed superior antitumor response compared to single-agent therapy [156]. Moreover, Yi et al. constructed an anti-TGF-β/PD-L1 bispecific antibody YM101, which promoted T-cell infiltration and exhibited stronger inhibitory tumor activity in TNBC [157–160]. In view of the role of TGF-β in TNBC, TGF-β inhibitors may be an effective treatment for TNBC (Fig. 3, Table 4).
Epigenetic modifications
Epigenetic modifications, such as DNA methylation and histone modification, are involved in the development of various cancers, and it has also been hypothesized that this may be a therapeutic strategy for TNBC [161, 162].
The ER is present in TNBC but is silenced due to the demethylation of ER CpG islands and reduced histone activity. Reactivation of the ER may be a therapeutic strategy for TNBC. Histone deacetylase (HDAC) inhibitors and demethylation inhibitors have been reported to reactivate ER [163]. Tan et al. found that the RNA N6 -methyladenosine reader YTHDC1 promotes metastasis in TNBC cells; therefore, targeting the YTHDC1/m6A/SMAD3 axis could be a potential therapeutic strategy for TNBC [164]. Decitabine induces DNA hypomethylation and has been approved by the FDA for treating myelodysplastic syndromes. It also plays a role in the treatment of patients with BC. The related clinical trial is ongoing, with results to be announced. In addition, Jiang et al. found that compound A6, which targets both HDAC and G-quadruplex (G4), significantly inhibited the proliferation of TNBC cells and demonstrated a favorable safety profile in a mouse model [165]. Moreover, the combination of HDAC inhibitors and ionizing radiation may benefit patients with TNBC [166]. The combination of HDAC inhibitors and letrozole showed favorable efficacy in patients with mBC [167]. There are several other HDAC inhibitors currently in clinical trials, such as belinostat, chidamide, romidepsin, and entinostat [168, 169].
Overall, using HDAC inhibitors or DNA methylation inhibitors may be a promising therapeutic strategy for patients with TNBC.
Immunotherapy
The 2023 ASCO conference unveiled the results of the TORCHLIGHT clinical trial, which demonstrated that the combination of toripalimab and nab-paclitaxel can significantly extend the PFS of patients with stage IV breast cancer or recurrent and metastatic TNBC [170, 171]. Furthermore, the findings from the 'FUTURESUPER' clinical trial indicated that immunotherapy based on molecular subtypes, like IM of TNBC, can improve the outcome of patients [172].
Strategies for TNBC immunotherapy include increasing the antigen-presenting capacity of dendritic cells and activating effector T lymphocyte function, suppression of regulatory T lymphocytes and myeloid-derived suppressor cells, upregulating relevant cytokines to reverse the tumor suppressive microenvironment, and promoting antitumor immune responses to kill tumor cells [18, 173–175]. For example, ICIs, CAR-T, and tumor vaccines (Fig. 2F).
The programmed death receptor (PD-1) and its ligand PD-L1 are the current topics in targeted therapies, which lead to sustained clinical relief in various types of cancer, including non-small cell lung cancer, hepatocellular carcinoma, renal cell carcinoma, and others [176–182]. Compared with other types of BC, TNBC shows a higher tumor mutation burden, higher levels of PD-L1 expression, and more immune cell infiltration into the tumor microenvironment. Hence, TNBC is the most immunogenic subtype capable of benefiting from immunotherapy. The IM type represents about 24% of TNBC and is more sensitive to immunotherapy due to its characteristic activation of immune regulatory pathways [183]. At present, there are many ongoing clinical trials for TNBC patients (Table 6).
Table 6.
The clinical trials of PD-1/PD-L1 inhibitors for TNBC
| Drug name | Com | Name | Num | Regiments | Phase | Status | Object | POM | NCT number |
|---|---|---|---|---|---|---|---|---|---|
| Pembrolizumab (MK-3475, KEYTRUDA®) | KEYNOTE-086 | 254 | 200 mg iv on day 1 of q3w for up to 35 cycles | II | Completed | mTNBC | ORR, AEs | NCT02447003 | |
| Pembrolizumab | KEYNOTE-012 | 297 | 10 mg/kg iv q3w | I | Completed | mTNBC | ORR, AEs | NCT01848834 | |
| Pembrolizumab | TAPUR | 28 | 2 mg/kg or 200 mg iv of q3w | II | Recruiting | mTNBC | ORR | NCT02693535 | |
| Pembrolizumab | SWOG 1418 | 1155 | Iv over 30 min on days 1 and 22. Cycles repeat every 42 days for 52 weeks | III | Active, not recruiting | TNBC | iDFS | NCT02954874 | |
| Pembrolizumab | Chemotherapy (Capecitabine/Eribulin/Gemcitabine/Vinorelbine) | KEYNOTE-119 | 622 |
Pembrolizumab: 200 mg iv q3w for up to 35 administrations Chemotherapy: as TPC in accordance with local regulations and guidelines |
III | Completed | Advanced or mTNBC | OS | NCT02555657 |
| Pembrolizumab | Chemotherapy (Nab-paclitaxel /Doxorubicin/ Cyclophosphamide) | KEYNOTE-173 | 60 |
Pembrolizumab: 200 mg iv q3w Nab-paclitaxel: 125 or 100 mg/m2 iv q3w doxorubicin: 60 mg/m2 iv q3w Cyclophosphamide: 600 mg/m2 q3w |
I | Completed | TNBC | DLTs, AEs | NCT02622074 |
| Pembrolizumab | Gemcitabine/carboplatin | KEYNOTE 355 | 882 |
Pembrolizumab: 200 mg iv on day 1 of each 21-day cycle Gemcitabine/carboplatin: 1000 mg/m2 (gemcitabine) and an AUC 2 (carboplatin) on days 1 and 8 of each 21-day cycle |
III | Active, not recruiting | mTNBC | AEs, PFS, OS | NCT02819518 |
| Pembrolizumab | Paclitaxel plus carboplatin | KEYNOTE-522 | 1174 |
Pembrolizumab: 200 mg iv q3w Paclitaxel + carboplatin: q3w × 4 cycle Each cycle is 21 days |
III | Active, not recruiting | TNBC | pCR, EFS | NCT03036488 |
| Pembrolizumab | Carboplatin and gemcitabine/ olaparib | KEYLYNK-009 | 460 |
Pembrolizumab: 200 mg iv on day 1 of each 21-day cycle Carboplatin: AUC 2 with gemcitabine 1000 mg/m2 iv on days 1 and 8 of each 21-day cycle Olaparib: 300 mg qd po |
II | Active, not recruiting | mTNBC | PFS, OS | NCT04191135 |
| Pembrolizumab | Eribulin | ENHANCE 1 | 258 |
Pembrolizumab: 200 mg iv on day 1 of each 21-day cycle Eribulin: 1.4 mg/m2 iv on day 1 and 8 of each 21-day cycle |
I/II | Completed | Advanced or mTNBC | ORR | NCT02513472 |
| Pembrolizumab | Ladiratuzumab vedotin | SGNLVA-002 | 211 |
Pembrolizumab: iv q3w ladiratuzumab vedotin: iv |
I/II | Recruiting | Advanced or mTNBC | DLTs, AEs, ORR | NCT03310957 |
| Pembrolizumab | Dinaciclib | 32 |
Pembrolizumab: 200 mg iv on day 1 q3w Dinaciclib: 12 mg/m2 day 1 and 8 of a 21 days cycle by 2-h iv |
I | Completed | Advanced or metastatic TNBC | MTD, DLTs | NCT01676753 | |
| Pembrolizumab | Enobosarm | 18 |
Pembrolizumab: 200 mg iv over 30 min on day 1 Enobosarm: po qd on days 1–21 |
II | Active, not recruiting | mTNBC | pCR | NCT02971761 | |
| pembrolizumab | Imprime | IMPRIME 1 | 64 |
Pembrolizumab: 200 mg iv over 30 min on Day 1 of q3w Imprime: 4 mg/kg iv over a 2-h infusion time on days 1, 8 and 15 of q3w treatment cycle |
I | Completed | Advanced or mTNBC | ORR | NCT02981303 |
| pembrolizumab | Paclitaxel/ Doxorubicin/ Cyclophosphamide | ISPY-2 |
Pembrolizumab: 200 mg iv cycles 1,4,7,10 Paclitaxel: 80 mg/m2 iv cycles 1–12 Doxorubicin: 60 mg/m2 iv every 2 or 3 weeks for 4 cycles Cyclophosphamide: 600 mg/m2 iv every 2 or 3 weeks for 4 cycles |
III | Recruiting | Stage II–III TNBC | pCR | NCT01042379 | |
| Atezolizumab | 661 | 0.01 mg/kg iv q3w | I | Completed | Advanced or mTNBC | DLTs, MTD | NCT01375842 | ||
| Atezolizumab | JAVELIN | 168 | 1.0 mg/kg once q2w | I | Completed | Advanced or mTNBC | DLTs | NCT01772004 | |
| Atezolizumab | Nab-paclitaxel/ Doxorubicin/ Cyclophosphamide | IMpassion031 | 333 |
Atezolizumab: 840 mg iv q2w Nab-paclitaxel: 125 mg/m2 iv every week for 12 weeks Doxorubicin: 60 mg/m2 iv Cyclophosphamide: 600 mg/m2 iv q2w |
III | Completed | TNBC | pCR | NCT03197935 |
| Atezolizumab | Nab-paclitaxel | IMpassion130 | 902 |
Atezolizumab: 840 mg iv on days 1 and 15 of each 28-day cycle Nab-Paclitaxel: 100 mg/m2 iv on days 1, 8, and 15 of each 28-day cycle |
III | Completed | Advanced or mTNBC | PFS, OS | NCT02425891 |
| Atezolizumab | Paclitaxel | IMpassion131 | 653 |
Atezolizumab: 840 mg iv on days 1 and 15 (± 3 days) of every 28-day cycle Paclitaxel: 90 mg/m2 iv on days 1, 8, and 15 of every 28-day cycle |
III | Completed | Advanced or mTNBC | PFS | NCT03125902 |
| Atezolizumab | Capecitabine or gemcitabine/carboplatin | IMpassion132 | 572 |
Atezolizumab: 1200 mg iv Gemcitabine: 1000 mg/m2 on days 1 and 8 of q3w Capecitabine: 1000 mg/m2 po bid on days 1 to 14 q3w |
III | Recruiting | Advanced or mTNBC | OS | NCT03371017 |
| Atezolizum | Nab-paclitaxel plus cobimetinib + | COLET | 169 |
Atezolizumab: 840 mg iv q2w on days 1 and 15 Paclitaxel: 80 mg/m2 iv on day 1, 8, 15 Cobimetinib: 60 mg/d on day 3–23 Each 28-day treatment cycle |
II | Completed | Advanced or mTNBC | PFS, ORR | NCT02322814 |
| Ipatasertib/ Paclitaxel | 140 |
Atezolizumab: 840 mg iv q2w on days 1 and 15 Ipatasertib: 400 mg/d po on days 1–21 Paclitaxel: 80 mg/m2 iv on day 1, 8, 15 |
I | Completed | Advanced or mTNBC | pCR | NCT03800836 | ||
| Atezolizumab | Paclitaxel /Doxorubicin | IMpassion 030 | 2300 |
Atezolizumab: 840 mg iv q2w Paclitaxel: 80 mg/m2 qw for 12 weeks Doxorubicin: 60 mg/m2 iv q2w |
III | Active, not recruiting | Stage II-III TNBC | iDFS | NCT03498716 |
| Atezolizumab | Capecitabine | MIRINAE | 284 |
Atezolizumab: 1200 mg iv q3w Capecitabine: 2500 mg/m2/d day 1–14, q3w for 8 cycles |
II | Recruiting | TNBC | iDFS | NCT03756298 |
| Avelumab | A-BRAVE | 474 | 10 mg/kg iv q2w for 1 year | III | Active, not recruiting | TNBC | DFS | NCT02926196 | |
| Durvalumab (MEDI4736) | Nab-paclitaxel | GeparNuevo | 174 |
MEDI4736: 1.5 g iv q4w Nab-Paclitaxel 125 mg/m2 qw for 12 weeks |
II | Completed | TNBC | pCR | NCT02685059 |
| Durvalumab | Olaparib | MEDIOLA | 264 |
Olaparib: bid starting on week 1 day 1 MEDI4736: q4w starting on week 5 day 1 |
I/II | Active, not recruiting | gBRCA-mBC | safety and tolerability; ORR | NCT02734004 |
| Nivolumab | TONIC | 84 | Nivolumab: 3 mg/kg q2w | II | Active, not recruiting | Advanced or mTNBC | PFS | NCT02499367 | |
| Nivolumab | Pembrolizumab | TOPACIO/KEYNOTE-162 | 122 |
Niraparib: 300 mg/d PO on days 1–21 Pembrolizumab: 200 mg iv on day 1 of each 21-day cycle |
I/II | Completed | Advanced or mTNBC | DLTs, ORR | NCT02657889 |
| Camrelizumab (SHR-1210) | Apatinib | 40 |
SHR-1210: 3 mg/kg iv q2w Apatinib: 250 mg/d po day 1–14 |
II | Completed | mTNBC | ORR | NCT03394287 |
mTNBC: metastatic triple negative breast cancer; TNBC: triple negative breast cancer; HER2-: HER2 negative; mBC: metastatic breast cancer; gBRCA1/2 m: germline BRCA1/2 mutated; ORR: overall response rate; AEs: adverse events; iDFS: invasive disease-free survival; OS: overall survival; DLTs: dose-limiting toxicity; PFS: progression-free survival; EFS: event-free survival; MTD: maximum tolerated dose; pCR: pathologic complete response; DFS: disease-free survival
PD-L1 links to PD-1 on the surface of tumor-infiltrating lymphocytes and inhibits lymphocyte function and cytokine release, causing the immune escape from cancer cells [184–186]. Ali et al. detected PD-L1 expression in BC at about 6.3% in 3,916 tumor samples, increasing to 19% in TNBC [187]. Mittendorf et al. also obtained the same results as Ali et al. by using the cancer genome atlas (TCGA) RNA sequencing [188]. The above results suggested that inhibition of PD-1 binding to PD-L1 might be a promising approach for TNBC.
ICIs
Currently, ICIs include PD-1 and PD-L1 inhibitors, which are widely utilized in the clinic, such as pembrolizumab, atezolizumab, durvalumab, and nivolumab [189]. Moreover, drugs related to new immunotherapeutic targets, for example, LAG3, TIM3, and ICOS are under development [190, 191].
Pembrolizumab, a PD-1 inhibitor, has demonstrated antitumor activity and manageable safety in KEYNOTE-012 and KEYNOTE-086 for pembrolizumab monotherapy in mTNBC [192–194]. The KEYNOTE-119 trial displayed that administration of pembrolizumab monotherapy didn’t prolong the comparison of OS with chemotherapy in patients with mTNBC, but in the pembrolizumab group, drug efficacy increased with increasing PD-L1 expression, demonstrating that high PD-L1 expression may be related to the CBR of pembrolizumab [195]. KEYNOTE-355 was launched to assess the effectiveness of pembrolizumab plus neoadjuvant chemotherapy as a first-line therapy for patients with early-stage TNBC. It demonstrated that pembrolizumab combined with chemotherapy resulted in a higher percentage of pCR in patients in the PD-L1 overexpression group compared to neoadjuvant chemotherapy [196, 197]. The above studies suggested that it is worth exploring the value of combining conventional treatment with immunotherapy for patients with TNBC.
The subsequent KEYNOTE-173 trial, Phase II trial I-SPY2, and Phase III trial KEYNOTE-522 combination with chemotherapy resulted in better antitumor activity, significantly improved pCR rates, and extended event-free survival in TNBC patients [198–200]. These trials confirmed the value of pembrolizumab in treating TNBC with neoadjuvant therapy [196, 201]. The AGO-B-041 trial demonstrated combined pembrolizumab with nab-paclitaxel in TNBC patients with a pCR of 59.3% [202].
The FDA has approved pembrolizumab for postoperative adjunctive therapy in TNBC patients or further chemotherapy in patients with locally recurrent, unresectable, or mTNBC with high PD-L1 expression [203].
The IMpassion031 trial displayed that combining atezolizumab with a standard chemotherapy regimen meaningfully increased pCR in TNBC patients with a good safety profile [204]. The FDA has approved neoadjuvant therapy of atezolizumab monotherapy or plus nab-paclitaxel to treat patients with metastatic or locally advanced TNBC expressing PD-L1 [205]. Another trial assessed the effectiveness of atezolizumab added to nabilone paclitaxel in TNBC patients. It clarified notably longer PFS in patients treated with the combination and a more pronounced OS benefit in the high PD-L1 expression group [206–208]. In a similar trial, IMpassion131, atezolizumab in combination with paclitaxel didn’t improve PFS and OS of TNBC patients [209]. The impact of the difference between the two assays deserves further exploration.
Durvalumab is a PD-L1 monoclonal antibody. The GeparNUEVO trial used durvalumab in the neoadjuvant setting for TNBC and observed an increase in pCR, improvement in iDFS and DDFS, and a favorable trend in OS [210, 211]. Another trial, SAFIRO2-BREAST IMMUNO, which examined the efficacy of durvalumab in metastatic BC patients, showed that durvalumab didn’t prolong PFS and OS in BC patients, but significantly prolonged OS in TNBC patients [211].
Other methods of immunotherapy
Chimeric antigen receptor (CAR) T cells therapy utilizes genetic engineering to modify a patient's peripheral T-cells, giving them the characteristics to target and identify tumor cells. After in vitro expansion and culture, cells were transfused into patients to precisely kill tumors [212–214]. CAR-T therapy is known to be effective in hematologic tumors, but its efficacy in solid tumors is still being explored [215–217]. Currently, CAR-T therapy targeting ROR1 and MUC1 are promising therapeutic targets in TNBC [218]. Harrasser et al. designed CAR-T targeting ROR1, which demonstrated favorable antitumor activity in vivo models of TNBC with a good safety profile [219]. The related clinical trials are ongoing. CAR-natural killer (NK) cells targeting EGFRvIII are available for treating BC, and preclinical studies with tissue factor-targeted CAR-NK cells as monotherapy in TNBC have shown promising efficacy [220, 221]. Moreover, EGFR-targeted CAR-T showed potential antitumor effects in TNBC. It may be a prospective immunotherapy strategy for TNBC [222]. The promising effect of CAR-T therapy in TNBC deserves further studies (Table 7).
Table 7.
The clinical trials of tumor vaccine and CAR-T in TNBC
| Drug name | Com | Num | Regiments | Phase | Status | Object | POM | NCT number |
|---|---|---|---|---|---|---|---|---|
| Neoantigen vaccine | Nab-paclitaxel plus Durvalumab | 70 |
Vaccine and poly-ICLC SC: on days 1, 4, 8, 15, 22, 50, and 78 Nab-paclitaxel: iv on days 1, 8, and 15 of each cycle Durvalumab: iv on day 1 of each cycle |
II | Recruiting | Advanced or mTNBC | PFS | NCT03606967 |
| anti-meso CAR-T cells | 20 | a standard 3 + 3 dose escalation approach | I | Unknown | TNBC | AEs | NCT02580747 | |
| cMet RNA CAR T cells | 6 | 3 × 10^7 or 3 × 10^8 cells | I | Completed | mTNBC | AEs | NCT01837602 | |
| CART-TnMUC1 cells | 112 | Single iv administration | I | Active, not recruiting |
HER2- TNBC |
DLTs | NCT04025216 | |
| EGFR/B7H3 CAR-T cells | 30 | 2 × 10^6/kg CAR-T cells | I | Recruiting | TNBC | AEs | NCT05341492 | |
| NKG2DL-targeting CAR-grafted γδ-T Cells | 10 | "3 + 3" dose escalation study design ranging from 3 × 10^8—3 × 10^9 CAR-γδ-T cells. Each cycle of therapy will consist of 4 iv, given 7 days apart | I | Unknown | TNBC | DLTs | NCT04107142 | |
| ROR1-targeted CAR T cells (LYL797) | 54 | NA | I | Recruiting | TNBC | DLTs, TEAEs | NCT05274451 | |
| ROR1-CAR-T cell | 21 | ROR1 CAR-specific autologous T-lymphocytes IV over 20–30 min | I | Terminated | TNBC | AEs | NCT02706392 | |
| PD-1+ TILs | 20 | 5 to 10 mg/mL/min | I/II | Not yet recruiting | PD1+ TNBC | AEs, PFS | NCT05451784 | |
| TC-510 | 115 | NA | I/II | Recruiting | TNBC | DLTs, ORR | NCT05451849 | |
| BT-001 | Pembrolizumab | 48 |
BT-001: administered at different dose Pembrolizumab: 200 mg iv q3w |
I/II | Recruiting | TNBC | AEs | NCT04725331 |
CAR-T: chimeric antigen receptor-T cell; mTNBC: metastatic triple negative breast cancer; HER2-: HER2 negative; mBC: metastatic breast cancer; gBRCA1/2 m: germline BRCA1/2 mutated; ORR: overall response rate; AEs: adverse events; PFS: progression-free survival; DLTs: dose-limiting toxicity; TEAEs: treatment emergent adverse events; ROR1: receptor tyrosine kinase like orphan receptor 1; PD-1: programmed cell death protein 1; EGFR: epidermal growth factor receptor; NKG2DL: natural killer group 2, member D
A tumor vaccine is an emerging immunotherapy strategy that works by introducing a tumor antigen into a patient’s body, activating or enhancing the body's immune system, and producing a valid antitumor immune response that kills or eliminates tumor cells [223]. At present, TNBC vaccines in development mainly include dendritic cell vaccines, peptide vaccines, and modified exosome vaccines [224–228]. GM-CSF is a tumor vaccine adjuvant in ongoing clinical trials for immunotherapy of BC [229]. BT-001 is an ongoing TNBC-related clinical trial as a lysovirus vaccine expressing cytotoxic T lymphocyte-associated antigen-4 antibodies and GM-CSF [230, 231] (Table 7).
In addition to this, considerable data have demonstrated that targeting nucleotide metabolism could enhance the antitumor immune response [232–234]. The efficacy of targeting nucleotide metabolism in combination with immunotherapy versus immunotherapy monotherapy for TNBC will be compared in ongoing clinical trials [235].
Combination therapy
In addition to the above treatment strategies of combining immunotherapy with chemotherapy, there are some combination approaches to maximize the benefits of cancer immunotherapy to enhance the efficacy of ICIs [236–238] (Table 7).
Combining ICIs with DNA damage repair inhibitors, including PARP inhibitors, is a promising strategy for BC patients with BRCA mutations [239]. In the TNBC tumor model, niraparib activated interferon signaling and enhanced the anti-tumor activity of the anti-PD-1 antibody BioXCell RMP1-14 in TNBC. A synergistic suppressed tumor effect was revealed when nirapanib was administered with BioXCell RMP1-14 [240]. Intriguingly, KEYNOTE-162 evaluated the efficacy of combining niraparib with pembrolizumab in advanced or mTNBC patients. Combining niraparib with pembrolizumab has displayed favorable efficacy and safety in TNBC patients with BRCA mutations [241]. Another phase II trial, I-SPY2, indicated that adding durvalumab in combination with olaparib to the standard chemotherapy raised pCR to 20% in TNBC patients [242]. TNBC patients who received nab-paclitaxel plus atezolizumab were able to extend PFS, but in the IMpassion131 clinical trial, it was disappointing that the combination of paclitaxel and atezolizumab did not improve PFS or OS in TNBC patients [209].
According to the favorable results of the above clinical trials or related studies, immunotherapy is expected to bring benefits to TNBC patients.
Antibody–drug conjugate (ADC)
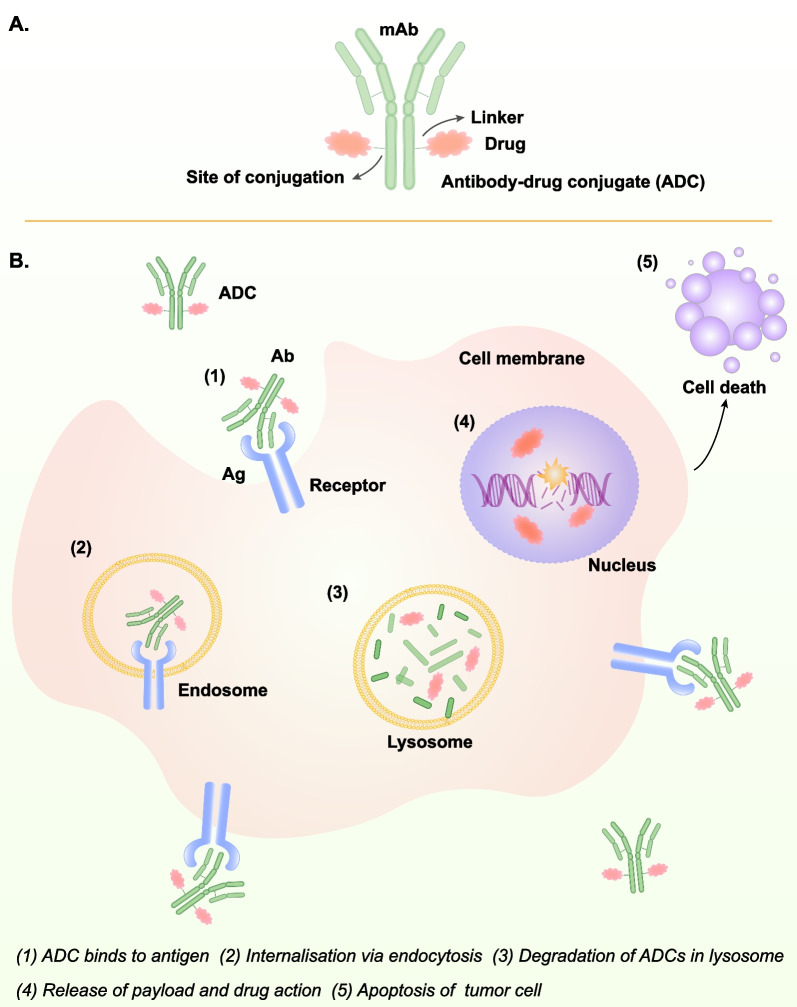
In recent years, research on ADC is in full swing. ADC mainly uses antibodies as carriers to deliver cytotoxic drugs into tumor cells, breaking double-stranded DNA and further leading to tumor cell death, thus achieving high tolerance and enhanced cytotoxic effects [243] (Figs. 2E, 4). Due to its remarkable clinical efficacy, it provides a new option for tumor patients and further prolongs their survival [244, 245].
Fig. 4.
The structure and mechanism of ADC. A. The structure of ADC, B. The mechanism of ADC: (1)-(5). (The action mechanism of ADC was adapted from Fig. 2 in [243])
Trophoblast cell-surface antigen 2 (Trop-2) is a transmembrane glycoprotein, which is encoded via the TACSTD2 gene, and is highly expressed on TNBC. Overexpression Trop-2 is often predictive of a more aggressive and worse prognosis [246, 247]. Gosartumumab (Sacituzumab govitecan, SG), also known as Trodelvy, consists of SN-38 coupled with humanized Ig G antibody that targets Trop-2. SN-38 is the active metabolite of irinotecan (CPT-11) and functions as a topoisomerase I inhibitor [248]. The IMMU-132–01 trial administrated at least second-line therapy followed by SG therapy in mTNBC patients. The SG group had an ORR of 33.3%, mPFS of 5.5 months, and OS of 13.0 months [249]. The ASCENT trial confirmed that SG significantly improved ORR (35% vs. 5%), PFS (5.6 months vs. 1.7 months), and OS (12.1 months vs. 6.7 months) in mTNBC patients compared to standard chemotherapy regimens and that patients with high TROP-2 expression were more likely to benefit from SG treatment [250]. Moreover, the serial ASCENT trial proved that the PFS and OS of patients with mTNBC who did not respond to chemotherapy significantly improved after SG treatment [251]. The success of the ASCENT study made SG the world's first approved ADC drug for mTNBC by targeting Trop-2 [252, 253].
TROPiCS-02 is an open-label, randomized, multicenter phase III study that evaluates the efficacy and safety of SG versus single-agent chemotherapy in patients with HR + /HER2- who received at least two but no more than four prior chemotherapy regimens for their metastatic disease [254]. Rugo et al. presented the results of the TROPiCS-02 and concluded that SG significantly improved PFS over chemotherapy [255]. The SASCIA trial will determine whether SG can prolong recurrence-free survival in patients with early-stage breast cancer after surgery [256].
Several studies investigating the efficacy of SG for patients with TNBC are underway. These studies assess the efficacy of the agent as neoadjuvant therapy in early TNBC and metastatic cancer in combination with immunotherapy-based regimens or with a PARP inhibitor (Table 8). Collectively, these results suggest that SG is significantly superior to chemotherapy in improving PFS and OS in recurrent and refractory TNBC and heavily pretreated and endocrine-resistant HR+/HER2−, BC subtypes with limited treatment options and poor prognosis.
Table 8.
The clinical trials of ADC in TNBC
| Drug name | Com | Num | Regiments | Phase | Status | Object | POM | NCT number |
|---|---|---|---|---|---|---|---|---|
|
PTK7-ADC (PF-06647020) |
Gedatolisib | 18 |
PTK7-ADC: 1.4 mg/kg or 2.8 mg/kg on day 1 of every 21 days cycle Gedatolisb: 110 mg or 180 mg iv on day 1, 8, 15 of every 21 days cycle |
I | Completed | TNBC | Safety and toxicity | NCT03243331 |
| Sacituzumab Govitecan (SG, Trodelvy, IMMU-132) | 80 | 10 mg/kg iv on day 1 and 8 of a 21-day cycle | II | Active, not recruiting | TNBC | ORR | NCT04454437 | |
| SG | 52 | 6 mg/kg iv on day 1 and day 8 of a 21-day cycle | I/II | Active, not recruiting | TNBC | TEAEs, DLTs, ORR | NCT05101096 | |
| SG | 540 | SG: 10 mg/kg on days 1 and 8 of a 21-day cycle | III | Recruiting |
TNBC PD-L1 negative |
PFS | NCT05382299 | |
| SG | Trilaciclib (CDK4/6 inhibitor) | 30 |
SG: 10 mg/kg reconstituted to a concentration of 1.1 mg/mL to 3.4 mg/mL in normal saline Trilaciclib: solution as a 30-min iv to be completed within 4 h prior to the start of SG |
II | Active, not recruiting | TNBC | PFS | NCT05113966 |
| SG | Talazoparib (PARP inhibitor) | 75 |
SG: 10 mg/kg iv on days 1 and 8 of a 21 day cycle Talazoparib: qd |
I/II | Recruiting | mTNBC | DLTs | NCT04039230 |
| SG | Pembrolizumab | 1514 |
SG: 10 mg/kg iv on days 1 and 8 of 21-day cycles Pembrolizumab: 200 mg iv on day 1 of 21-day cycles for 8 cycles |
III | Recruiting | TNBC | iDFS | NCT05633654 |
| SG | Pembrolizumab | 110 |
SG: given on days 1 and 8 of the 21 days cycle Pembrolizumab: given on day 1 of the 21 days cycle |
II | Recruiting | TNBC | PFS | NCT04468061 |
| SG | Pembrolizumab | 260 |
SG: 10 mg/kg iv, two days per 21-day cycle Pembrolizumab: 200 mg iv on day 1 of 21-day cycles |
II | Recruiting | TNBC | pCR | NCT04230109 |
| SG | Pembrolizumab | 440 |
SG: 10 mg/kg iv on days 1 and 8 of 21-day cycles Pembrolizumab: 200 mg iv on day 1 of 21-day cycles |
III | Recruiting |
TNBC PD-L1 positive |
PFS | NCT05382286 |
| TH1902 | 70 | 300 mg/m2 iv | I | Active, not recruiting | TNBC | Safety and Tolerability | NCT04706962 | |
| Datopotamab Deruxtecan (Dato-DXd, DS-1062a) | 118 |
NA multiple cohorts |
I/II | Recruiting | TNBC | ORR | NCT05460273 | |
| Dato-DXd | 770 | All participants enrolled in the dose escalation part | I | Recruiting | TNBC | DLTs, AEs | NCT03401385 | |
| Dato-DXd | 600 | 100 mg iv | III | Recruiting | TNBC | PFS | NCT05374512 | |
| Dato-DXd | Durvalumab | 1075 |
Dato-DXd: 6 mg/kg iv q3w × 8 cycles Durvalumab: 1120 mg iv q3w × 9 cycles |
III | Recruiting | TNBC | iDFS | NCT05629585 |
| T-DXd | Capecitabine | 139 |
T-DXd: 5.4 mg/kg iv q3w Capecitabine: 1000 mg/m2 po bid days 1–14 q3w |
I | Active, not recruiting | HER2-Low BC | AEs, SAEs | NCT04556773 |
| T-DXd | Durvalumab plus Paclitaxel | 139 |
T-DXd: 5.4 mg/kg iv q3w Durvalumab: 1120 mg iv q3w Paclitaxel: 80 mg/m2 iv qw in 3-week cycles |
I | Active, not recruiting | HER2-Low BC | AEs, SAEs | NCT04556773 |
| T-DXd | Capivasertib | 139 |
T-DXd: 5.4 mg/kg iv q3w Capivasertib: 400 mg po bid |
I | Active, not recruiting | HER2-Low BC | AEs, SAEs | NCT04556773 |
| T-DXd | Fulvestrant | 139 |
T-DXd: 5.4 mg/kg iv q3w Fulvestrant: 500 mg im q4w |
I | Active, not recruiting | HER2-Low BC | AEs, SAEs | NCT04556773 |
| T-DXd | Anastrozole | 88 |
T-DXd: 5.4 mg/kg iv q3w Anastrozole: 1 mg/d po |
II | Recruiting | HER2-Low BC | pCR | NCT04553770 |
| U3-1402 (Patritumab Deruxtecan) | 120 | 5.6 mg/kg iv on day 1 of q3w | II | Recruiting | mBC | ORR, PFS-6 | NCT04699630 | |
| CAB-ROR2- ADC (BA3021) | PD-1 inhibitor | 420 | NA | I/II | Recruiting | TNBC | Safety, ORR | NCT03504488 |
| Vobramitamab duocarmazine (MGC018) | 143 | 3.0 mg/kg iv q3w | I/II | Active, not recruiting | Advanced Solid Tumor | AEs, SAEs, DLTs | NCT03729596 |
ADC: antibody–drug conjugate; TNBC: triple negative breast cancer; BC: breast cancer; HER2-: HER2 negative; mBC: metastatic breast cancer; gBRCA1/2 m: germline BRCA1/2 mutated; ORR: overall response rate; PFS: progression-free survival; AEs: adverse events; SAEs: serious adverse events; TEAEs: treatment emergent adverse events; DLTs: dose-limiting toxicity; iDFS: invasive disease-free survival; CDK4/6: cyclin-dependent kinases 4/6; pCR: pathologic complete response
TH1902 is a peptide-docetaxel conjugate with a payload of two docetaxel molecules ester-linked to a peptide (TH19P01) designed to recognize sortilin (SORT1). TH1902 is internalized in cancer cells through SORT1 [257]. TH1902 exerts a superior anticancer activity than unconjugated docetaxel in human SORT1-positive ovarian and triple-negative breast cancer xenograft models [257–259]. TH1902 is currently being evaluated in a phase I clinical trial (Table 8).
Datopotamab deruxtecan (Dato-DXd) consists of a monoclonal antibody targeting Trop-2, DXd, a topoisomerase I inhibitor, and a cleavable tetrapeptide junction [260]. Dato-DXd has shown favorable results in mTNBC patients [261]. DS-8201a (T-DXd), is a HER2-targeted ADC, composed of an anti-HER2 antibody and a derivative from the topoisomerase I inhibitor DX-8951 (DXd) [262]. Phase II/III clinical trials exhibited that DS-8201a displayed reliable tumor inhibitory activity in HER2+ metastatic BC patients and was approved for treating metastatic HER2+ BC [263, 264]. Intriguingly, DS-8201a also presented a meaningful antitumor activity in tumors with low HER2 expression [265, 266]. In another clinical trial, DS-8201a presented good antitumor activity in patients with low HER2 expression of BC [267]. The recently published DESTINY-Breast 04 trial indicated that in patients with advanced low HER2 expression BC, DS-8201a prolonged patient PFS and OS versus chemotherapy. Patients in the BC hormone receptor-negative subgroup had a 5.6 months mPFS and a 54% reduction in disease progression or death risk in the DS-8201a group, compared to the chemotherapy group. The mOS was extended to 9.9 months and the death risk decreased to 52% [268].
Nectin-4, a type I transmembrane cell adhesion molecule, is involved in the formation and maintenance of adherens junctions in cooperation with cadherins. Rabet et al. showed that nectin-4 is a cell surface biomarker frequently overexpressed in TNBC. They developed anti-nectin 4 ADC, N41mab-vcMMAE, which induced a complete and durable response in vitro and in vivo on nectin-4-positive samples [269]. In addition, Guo et al. developed a well-designed ADC, IC1-MMAE, as a potent targeted therapeutic agent for treating refractory TNBC in vivo. They provided experimental evidence of using ICAM1 as an effective ADC target for TNBC [270].
Based on the excellent efficacy of Trop-2-based ADCs in TNBC, more ADCs are in clinical trials, and we expect to see more benefits for patients. Moreover, the extension of DS-8201a therapy into the field of HER2 low expression is innovative and may cause new therapeutic options for breast cancer.
Targeting regulated cell death (RCD)
In recent years, regulated cell death is associated with cancer progression and treatment, and ferroptosis is an iron-dependent type of RCD, which is not dependent on caspase cascade reaction [271–273]. In addition, ferroptosis has accumulated lipid peroxidation products and lethal reactive oxygen species (ROS) [274]. Gan et al. proposed that a ferroptosis inducer, IR780-SPhF, could enable TNBC imaging and treatment by targeting mitochondria and that IR780-SPhF had a stronger anticancer effect than cyclophosphamide, suggesting that IR780-SPhF could hold promise for the treatment of TNBC patients [275]. Glutathione peroxidase 4 (GPX4), an antioxidant enzyme, acts as an inhibitor of ferroptosis. Ding et al. found that DMOCPTL is capable of promoting the ferroptosis of TNBC cells by inducing GPX4 ubiquitination [276]. Prevention of GPX4-stimulated ferroptosis and increased sensitivity of TNBC cells in response to gefitinib [276]. The small-molecule compound erastin sensitizes TNBC cells to ferroptosis, but its application has been hampered by nephrotoxicity [277]. Yang et al. developed an exosome (erastin@FA-exo) that tagged folic acid (FA) and contained erastin. Erastin@FA-exo inhibits GPX4 expression and promotes ferroptosis in TNBC cells, and also inhibits TNBC cell proliferation more strongly than regular erastin [278]. This provides a novel therapeutic approach and direction for TNBC therapy. Tumor-associated macrophages (TAMs) are a critical element in the tumor microenvironment and are involved in tumor initiation and progression [279, 280]. IL-6 generated from TNBC cells stimulates TGF-β1 secretion by TAMs, which in turn allows hepatic leukemia factor (HLF) to trigger γ-glutamyl transferase 1 (GGT1) to promote ferroptosis resistance in TNBC cells, eventually causing the progression of TNBC [281]. In addition, iron-saturated Lf facilitated ferroptosis in TNBC cells via the production of ROS and enhanced the sensitivity of TNBC to radiotherapy [282]. Moreover, Zhang et al. indicated that MTHFD2 knockdown could induce ferroptosis in TNBC and inhibit TNBC progression, and may be a promising therapeutic target [283]. In the presence of ACSL3, mammary adipocytes protected TNBC cells from ferroptosis via oleic acid, which may offer new insights and targets for tumor therapy [284].
Interestingly, Zhimin Shao’s group revealed that there is high metabolic heterogeneity within TNBC, with the LAR-type being the ferroptosis-sensitive subtype, and AR-driven GPX4 being a critical molecule for mediating ferroptosis in the LAR subtype of TNBC. Moreover, GPX4 inhibitors not only suppressed the proliferation of LAR subtype cells, but also remodeled the tumor microenvironment [285]. Therefore, the combination of GPX4 inhibitors with ICIs may involve a prospective therapeutic approach for LAR subtype TNBC.
New models for triple-negative breast cancer research
In recent years, the maturation of organoid technology has opened up a novel tool for tumor modeling. Organoids are in vitro cultured 3D tumor tissues that can more accurately reflect information associated with primary tumors and provide a more accurate tumor model for precision medicine [286–288]. Guillen et al. discovered that patient-derived xenografts (PDXs)-derived organoid (PDxO) could be utilized to screen promising therapeutic drugs such as birinapant, which exhibited powerful antitumor activity in the TNBC-organoid, and which has been validated in PDXs [289, 290]. Chemotherapy resistance is also a major barrier in the current treatment of TNBC patients, and inhibition of lysyl oxidase (LOX) in TNBC-organoid has been identified to enhance drug penetration, restrict FAK/Src signaling pathway, and overcome chemoresistance in TNBC [291]. In addition, liquid biopsy is crucial for precise cancer diagnosis and therapy [292, 293]. Salvador et al. predicted response to neoadjuvant chemotherapy at the time of TNBC diagnosis using immunosuppression-related biomarkers in blood samples and tumor biopsies [294]. The aforementioned emerging technologies are guiding the diagnosis and treating TNBC, and we expect to obtain reassuring strategies for TNBC treatment through these technologies in the future.
Prospects
TNBC is characterized by a highly aggressive, largely heterogeneous, and highly malignant nature. At the same time, TNBC patients are susceptible to drug resistance and have a poor prognosis. Currently, there is a lack of valid targeted strategies for TNBC patients, and chemotherapy remains the main treatment method. With histological research and in-depth analysis of TNBC molecular typing, targeted therapy, and immunotherapy, as well as individualized therapy guided by TNBC molecular typing, light is being shed on the precision treatment for patients with TNBC [295].
The progression of TNBC and its malignant biological behaviors involve the aberrant activation of multiple signaling pathways. Exploring these relevant signaling pathways could help us to better understand the pathogenesis of TNBC, develop molecules with more diagnostic value or molecular markers with precision prognostic value, and provide a theoretical basis for molecularly targeted tumor therapy. Various antitumor drugs that target abnormally activated signaling pathways have been developed and have achieved excellent results in the pre-clinical setting. It is expected that more and more targeted drugs will be used in clinical settings in the future, bringing hope to TNBC patients.
"Fudan typing" plays a pivotal role in promoting precise therapy for TNBC and guiding researchers towards a more profound comprehension of TNBC heterogeneity. This advancement enables tailoring treatment plans based on individual TNBC patient characteristics, facilitating precise clinical trials aimed at enhancing the prognosis of TNBC patients. Nonetheless, the existing therapeutic approaches for TNBC remain constrained. Enhancing the effectiveness of treatments for TNBC patients stands as a pressing concern and formidable challenge. For instance, there's a pressing need to comprehensively analyze viable clinical targets for TNBC patients, explore superior treatment strategies, and surmount instances of drug resistance.
Acknowledgements
Not applicable.
Abbreviations
- TNBC
Triple-negative breast cancer
- BC
Breast cancer
- HER2
Human epidermal growth factor receptor-2
- EGFR
Epidermal growth factor receptor
- TROP-2
Trophoblast cell-surface antigen 2
- FDA
Food and drug administration
- BL1
Basal-like 1
- BL2
Basal-like 2
- MSL
Mesenchymal stem-like
- IM
Immunomodulatory
- LAR
Luminal androgen receptor
- IHC
Immunohistochemistry
- PARP
Poly (ADP-ribose) polymerase
- OS
Overall survival
- iDFS
Invasive disease-free survival
- DDFS
Distant disease-free survival
- ORR
Objective response rate
- pCR
Pathologic complete response
- CBR
Clinical benefit rate
- mTNBC
Metastatic TNBC
- mAb
Monoclonal antibody
- DLL4
Delta-like ligand 4
- CAR-T
Chimeric antigen receptor-T
- NK
Natural killer
- JAK
Janus kinase
- TGF
Transforming growth factor
- ADC
Antibody–drug conjugate
- SG
Sacituzumab govitecan
- PFS
Progression-free survival
- HDAC
Histone deacetylase
- ICIs
Immune checkpoint inhibitors
- PD-1
Programmed death receptor-1
- PD-L1
Programmed death ligand-1
- RCD
Regulated cell death
- ROS
Reactive oxygen species
- GPX4
Glutathione peroxidase 4
- FA
Folic acid
- TAMs
Tumor-associated macrophages
- HLF
Hepatic leukemia factor
- GGT1
γ-Glutamyl transferase 1
- PDXs
Patient-derived xenografts
- LOX
Lysyl oxidase
Author Contributions
SZ drafted the manuscript and prepared the figures. YW, BS, YY, and MY collected the related references and participated in the discussion. KW and QM designed this review and revised the manuscript. All authors contributed to this manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No.82073370, 82272794).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qi Mei, Email: borismq@163.com.
Kongming Wu, Email: wukm_lab@163.com.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Yi M, Li T, Niu M, et al. Epidemiological trends of women's cancers from 1990 to 2019 at the global, regional, and national levels: a population-based study. Biomark Res. 2021;9:55. doi: 10.1186/s40364-021-00310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borri F, Granaglia A. Pathology of triple negative breast cancer. Semin Cancer Biol. 2021;72:136–145. doi: 10.1016/j.semcancer.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Poteat TC, Adams MA, Malone J, et al. Delays in breast cancer care by race and sexual orientation: results from a national survey with diverse women in the United States. Cancer. 2021;127:3514–3522. doi: 10.1002/cncr.33629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 7.Morris GJ, Naidu S, Topham AK, et al. Differences in breast carcinoma characteristics in newly diagnosed african-american and caucasian patients: a single-institution compilation compared with the national cancer institute's surveillance, epidemiology, and end results database. Cancer. 2007;110:876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 8.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 9.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 10.Duffy MJ, McGowan PM, Crown J. Targeted therapy for triple-negative breast cancer: Where are we? Int J Cancer. 2012;131:2471–2477. doi: 10.1002/ijc.27632. [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Qian W, Sun X, Jiang S. Small-molecule inhibitors, immune checkpoint inhibitors, and more: FDA-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J Hematol Oncol. 2022;15:143. doi: 10.1186/s13045-022-01362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9:176–198. doi: 10.1158/2159-8290.CD-18-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ. 2023;381:e071674. doi: 10.1136/bmj-2022-071674. [DOI] [PubMed] [Google Scholar]
- 14.Kumar H, Gupta NV, Jain R, et al. A review of biological targets and therapeutic approaches in the management of triple-negative breast cancer. J Adv Res; 2023. [DOI] [PubMed]
- 15.Yi M, Zhang D, Song B, et al. Increased expression of ECT2 predicts the poor prognosis of breast cancer patients. Exp Hematol Oncol. 2022;11:107. doi: 10.1186/s40164-022-00361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spínola-Lasso E, Montero JC, Jiménez-Monzón R, et al. Chemical-proteomics identify Peroxiredoxin-1 as an actionable target in triple-negative breast cancer. Int J Biol Sci. 2023;19:1731–1747. doi: 10.7150/ijbs.78554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong B, Yi M, Luo S, Li A, Wu K. RDGN-based predictive model for the prognosis of breast cancer. Exp Hematol Oncol. 2020;9:13. doi: 10.1186/s40164-020-00169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L, Kong D, Liu J, et al. Breast cancer heterogeneity and its implication in personalized precision therapy. Exp Hematol Oncol. 2023;12:3. doi: 10.1186/s40164-022-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang YZ, Ma D, Suo C, et al. Genomic and transcriptomic landscape of triple-negative breast cancers: subtypes and treatment strategies. Cancer Cell. 2019;35(428–40):e5. doi: 10.1016/j.ccell.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Xiao Y, Ma D, Yang YS, et al. Comprehensive metabolomics expands precision medicine for triple-negative breast cancer. Cell Res. 2022;32:477–490. doi: 10.1038/s41422-022-00614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Zhang H, Merkher Y, et al. Recent advances in therapeutic strategies for triple-negative breast cancer. J Hematol Oncol. 2022;15:121. doi: 10.1186/s13045-022-01341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Jiang Z. Chinese society of clinical oncology breast cancer (CSCO BC) guidelines in 2022: Stratification and classification. Cancer Biol Med. 2022;19:769–773. doi: 10.20892/j.issn.2095-3941.2022.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner NC, Reis-Filho JS. Tackling the diversity of triple-negative breast cancer. Clin Cancer Res. 2013;19:6380–6388. doi: 10.1158/1078-0432.CCR-13-0915. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu YR, Jiang YZ, Xu XE, et al. Comprehensive transcriptome analysis identifies novel molecular subtypes and subtype-specific RNAs of triple-negative breast cancer. Breast Cancer Res. 2016;18:33. doi: 10.1186/s13058-016-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao S, Ma D, Xiao Y, et al. Molecular subtyping of triple-negative breast cancers by immunohistochemistry: molecular basis and clinical relevance. Oncologist. 2020;25:e1481–e1491. doi: 10.1634/theoncologist.2019-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang YZ, Liu Y, Xiao Y, et al. Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: the future trial. Cell Res. 2021;31:178–186. doi: 10.1038/s41422-020-0375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li T, Wang X, Qin S, et al. Targeting PARP for the optimal immunotherapy efficiency in gynecologic malignancies. Biomed Pharmacother. 2023;162:114712. doi: 10.1016/j.biopha.2023.114712. [DOI] [PubMed] [Google Scholar]
- 29.Yi M, Dong B, Qin S, et al. Advances and perspectives of PARP inhibitors. Exp Hematol Oncol. 2019;8:29. doi: 10.1186/s40164-019-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo L, Keyomarsi K. PARP inhibitors as single agents and in combination therapy: The most promising treatment strategies in clinical trials for BRCA-mutant ovarian and triple-negative breast cancers. Expert Opin Investig Drugs. 2022;31:607–631. doi: 10.1080/13543784.2022.2067527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eikesdal HP, Yndestad S, Elzawahry A, et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann Oncol. 2021;32:240–249. doi: 10.1016/j.annonc.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 33.Robson ME, Tung N, Conte P, et al. Olympiad final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant Olaparib for patients with brca1- or brca2-mutated breast cancer. N Engl J Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litton JK, Scoggins ME, Hess KR, et al. Neoadjuvant Talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J Clin Oncol. 2020;38:388–394. doi: 10.1200/JCO.19.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beniey M, Hubert A, Haque T, et al. Sequential targeting of PARP with carboplatin inhibits primary tumour growth and distant metastasis in triple-negative breast cancer. Br J Cancer. 2023;128:1964–1975. doi: 10.1038/s41416-023-02226-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bianchini G, De Angelis C, Licata L, Gianni L. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19:91–113. doi: 10.1038/s41571-021-00565-2. [DOI] [PubMed] [Google Scholar]
- 39.Gerratana L, Basile D, Buono G, et al. Androgen receptor in triple negative breast cancer: a potential target for the targetless subtype. Cancer Treat Rev. 2018;68:102–110. doi: 10.1016/j.ctrv.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Khadela A, Chavda VP, Soni S, et al. Anti-androgenic therapies targeting the luminal androgen receptor of a typical triple-negative breast cancer. Cancers (Basel) 2022;15:233. doi: 10.3390/cancers15010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y, Yang F, Huang D, Guan X. Androgen blockade based clinical trials landscape in triple negative breast cancer. Biochim Biophys Acta Rev Cancer. 2018;1870:283–290. doi: 10.1016/j.bbcan.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Gucalp A, Tolaney S, Isakoff SJ, et al. Phase ii trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19:5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36:884–890. doi: 10.1200/JCO.2016.71.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnefoi H, Grellety T, Tredan O, et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12–1) Ann Oncol. 2016;27:812–818. doi: 10.1093/annonc/mdw067. [DOI] [PubMed] [Google Scholar]
- 45.Min A, Jang H, Kim S, et al. Androgen receptor inhibitor enhances the antitumor effect of PARP inhibitor in breast cancer cells by modulating DNA damage response. Mol Cancer Ther. 2018;17:2507–2518. doi: 10.1158/1535-7163.MCT-18-0234. [DOI] [PubMed] [Google Scholar]
- 46.Luo J, Jin J, Yang F, et al. The correlation between PARP1 and BRCA1 in AR positive triple-negative breast cancer. Int J Biol Sci. 2016;12:1500–1510. doi: 10.7150/ijbs.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehmann BD, Abramson VG, Sanders ME, et al. TBCRC 032 Ib/II multicenter study: Molecular insights to AR antagonist and PI3K inhibitor efficacy in patients with AR (+) metastatic triple-negative breast cancer. Clin Cancer Res. 2020;26:2111–2123. doi: 10.1158/1078-0432.CCR-19-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choupani E, Madjd Z, Saraygord-Afshari N, Kiani J, Hosseini A. Combination of androgen receptor inhibitor enzalutamide with the CDK4/6 inhibitor ribociclib in triple negative breast cancer cells. PLoS ONE. 2022;17:e0279522. doi: 10.1371/journal.pone.0279522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bury M, Le Calvé B, Ferbeyre G, Blank V, Lessard F. New insights into CDK regulators: novel opportunities for cancer therapy. Trends Cell Biol. 2021;31:331–344. doi: 10.1016/j.tcb.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Beykou M, Arias-Garcia M, Roumeliotis TI, et al. Proteomic characterization of triple negative breast cancer cells following CDK4/6 inhibition. Sci Data. 2022;9:395. doi: 10.1038/s41597-022-01512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asghar US, Barr AR, Cutts R, et al. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin Cancer Res. 2017;23:5561–5572. doi: 10.1158/1078-0432.CCR-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdelmalak M, Singh R, Anwer M, et al. The renaissance of CDK inhibitors in breast cancer therapy: An update on clinical trials and therapy resistance. Cancers (Basel) 2022;14:5388. doi: 10.3390/cancers14215388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cretella D, Fumarola C, Bonelli M, et al. Pre-treatment with the CDK4/6 inhibitor palbociclib improves the efficacy of paclitaxel in TNBC cells. Sci Rep. 2019;9:13014. doi: 10.1038/s41598-019-49484-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu X, Chen L, Huang B, et al. Efficacy and mechanism of the combination of PARP and CDK4/6 inhibitors in the treatment of triple-negative breast cancer. J Exp Clin Cancer Res. 2021;40:122. doi: 10.1186/s13046-021-01930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li S, Wang L, Wang Y, et al. The synthetic lethality of targeting cell cycle checkpoints and parps in cancer treatment. J Hematol Oncol. 2022;15:147. doi: 10.1186/s13045-022-01360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teo ZL, Versaci S, Dushyanthen S, et al. Combined CDK4/6 and PI3Kα inhibition is synergistic and immunogenic in triple-negative breast cancer. Cancer Res. 2017;77:6340–6352. doi: 10.1158/0008-5472.CAN-17-2210. [DOI] [PubMed] [Google Scholar]
- 57.Cretella D, Ravelli A, Fumarola C, et al. The anti-tumor efficacy of CDK4/6 inhibition is enhanced by the combination with PI3K/AKT/mTOR inhibitors through impairment of glucose metabolism in TNBC cells. J Exp Clin Cancer Res. 2018;37:72. doi: 10.1186/s13046-018-0741-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyu L, Zhang S, Deng Y, et al. Regulatory mechanisms, functions, and clinical significance of CircRNAs in triple-negative breast cancer. J Hematol Oncol. 2021;14:41. doi: 10.1186/s13045-021-01052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Im SA, Mukai H, Park IH, et al. Palbociclib plus letrozole as first-line therapy in postmenopausal Asian women with metastatic breast cancer: Results from the phase iii, randomized paloma-2 study. J Glob Oncol. 2019;5:1–19. doi: 10.1200/JGO.18.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 61.Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 62.Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 63.Mayer EL, Dueck AC, Martin M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22:212–222. doi: 10.1016/S1470-2045(20)30642-2. [DOI] [PubMed] [Google Scholar]
- 64.Mayer EL, Fesl C, Hlauschek D, et al. Treatment exposure and discontinuation in the palbociclib collaborative adjuvant study of PALbociclib with adjuvant endocrine therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative early breast cancer (PALLAS/AFT-05/ABCSG-42/BIG-14-03) J Clin Oncol. 2022;40:449–458. doi: 10.1200/JCO.21.01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gnant M, Dueck AC, Frantal S, et al. Adjuvant palbociclib for early breast cancer: the PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03) J Clin Oncol. 2022;40:282–293. doi: 10.1200/JCO.21.02554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loibl S, Marmé F, Martin M, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-the Penelope-B trial. J Clin Oncol. 2021;39:1518–1530. doi: 10.1200/JCO.20.03639. [DOI] [PubMed] [Google Scholar]
- 67.Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 68.Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571–1581. doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 69.Sledge GW, Jr, Toi M, Neven P, et al. Monarch 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 70.Dey N, De P, Leyland-Jones B. PI3K-AKT-mTOR inhibitors in breast cancers: From tumor cell signaling to clinical trials. Pharmacol Ther. 2017;175:91–106. doi: 10.1016/j.pharmthera.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 71.Pascual J, Turner NC. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann Oncol. 2019;30:1051–1060. doi: 10.1093/annonc/mdz133. [DOI] [PubMed] [Google Scholar]
- 72.Chan JJ, Tan TJY, Dent RA. Novel therapeutic avenues in triple-negative breast cancer: PI3K/AKT inhibition, androgen receptor blockade, and beyond. Ther Adv Med Oncol. 2019;11:1758835919880429. doi: 10.1177/1758835919880429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-AKT-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin Cancer Biol. 2022;80:1–17. doi: 10.1016/j.semcancer.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 74.Isakoff SJ, Engelman JA, Irie HY, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 75.Li G, Robinson GW, Lesche R, et al. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 2002;129:4159–4170. doi: 10.1242/dev.129.17.4159. [DOI] [PubMed] [Google Scholar]
- 76.Campbell IG, Russell SE, Choong DY, et al. Mutation of the PI3KCA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 77.Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: Role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35:515–524. doi: 10.1007/s10555-016-9637-x. [DOI] [PubMed] [Google Scholar]
- 78.Alves CL, Ditzel HJ. Drugging the PI3K/AKT/mTOR pathway in er+ breast cancer. Int J Mol Sci. 2023;24:4522. doi: 10.3390/ijms24054522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalimutho M, Parsons K, Mittal D, et al. Targeted therapies for triple-negative breast cancer: Combating a stubborn disease. Trends Pharmacol Sci. 2015;36:822–846. doi: 10.1016/j.tips.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 80.Khan MA, Jain VK, Rizwanullah M, Ahmad J, Jain K. PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: a review on drug discovery and future challenges. Drug Discov Today. 2019;24:2181–2191. doi: 10.1016/j.drudis.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Nicholson KM, Quinn DM, Kellett GL, Warr JR. LY294002, an inhibitor of phosphatidylinositol-3-kinase, causes preferential induction of apoptosis in human multidrug resistant cells. Cancer Lett. 2003;190:31–36. doi: 10.1016/S0304-3835(02)00615-8. [DOI] [PubMed] [Google Scholar]
- 82.Singh AR, Joshi S, George E, Durden DL. Anti-tumor effect of a novel PI3-kinase inhibitor, SF1126, in (12) V-Ha-Ras transgenic mouse glioma model. Cancer Cell Int. 2014;14:105. doi: 10.1186/s12935-014-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Joshi S, Singh AR, Durden DL. Pan-PI-3 kinase inhibitor SF1126 shows antitumor and antiangiogenic activity in renal cell carcinoma. Cancer Chemother Pharmacol. 2015;75:595–608. doi: 10.1007/s00280-014-2639-x. [DOI] [PubMed] [Google Scholar]
- 84.Mahadevan D, Chiorean EG, Harris WB, et al. Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and b-cell malignancies. Eur J Cancer. 2012;48:3319–3327. doi: 10.1016/j.ejca.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng M, Wang J, Chen Y, Zhang L, Liu D. Combination of SF1126 and gefitinib induces apoptosis of triple-negative breast cancer cells through the PI3K/AKT/mTOR pathway. Anticancer Drugs. 2015;26:422–427. doi: 10.1097/CAD.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 86.Singh AR, Joshi S, Burgoyne AM, et al. Single agent and synergistic activity of the "first-in-class" dual PI3K/BRD4 inhibitor SF1126 with sorafenib in hepatocellular carcinoma. Mol Cancer Ther. 2016;15:2553–2562. doi: 10.1158/1535-7163.MCT-15-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan Y, Long H, Zhou Z, Fu Y, Jiang B. PI3K-AKT-targeting breast cancer treatments: Natural products and synthetic compounds. Biomolecules. 2023;13:93. doi: 10.3390/biom13010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banerji U, Dean EJ, Pérez-Fidalgo JA, et al. A phase I open-label study to identify a dosing regimen of the pan-AKT inhibitor AZD5363 for evaluation in solid tumors and in PIK3CA-mutated breast and gynecologic cancers. Clin Cancer Res. 2018;24:2050–2059. doi: 10.1158/1078-0432.CCR-17-2260. [DOI] [PubMed] [Google Scholar]
- 89.de Bono JS, De Giorgi U, Rodrigues DN, et al. Randomized phase II study evaluating AKT blockade with Ipatasertib, in combination with Abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res. 2019;25:928–936. doi: 10.1158/1078-0432.CCR-18-0981. [DOI] [PubMed] [Google Scholar]
- 90.Kalinsky K, Hong F, McCourt CK, et al. Effect of Capivasertib in patients with an AKT1 E17k-mutated tumor: NCI-match subprotocol EAY131-Y nonrandomized trial. JAMA Oncol. 2021;7:271–278. doi: 10.1001/jamaoncol.2020.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmid P, Abraham J, Chan S, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: The PAKT trial. J Clin Oncol. 2020;38:423–433. doi: 10.1200/JCO.19.00368. [DOI] [PubMed] [Google Scholar]
- 92.Kim SB, Dent R, Im SA, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (lotus): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18:1360–1372. doi: 10.1016/S1470-2045(17)30450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oliveira M, Saura C, Nuciforo P, et al. Fairlane, a double-blind placebo-controlled randomized phase II trial of neoadjuvant Ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann Oncol. 2019;30:1289–1297. doi: 10.1093/annonc/mdz177. [DOI] [PubMed] [Google Scholar]
- 94.Wu X, Xu Y, Liang Q, et al. Recent advances in dual PI3K/mTOR inhibitors for tumour treatment. Front Pharmacol. 2022;13:875372. doi: 10.3389/fphar.2022.875372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Omeljaniuk WJ, Krętowski R, Ratajczak-Wrona W, Jabłońska E, Cechowska-Pasko M. Novel dual PI3K/mTOR inhibitor, Apitolisib (GDC-0980), inhibits growth and induces apoptosis in human glioblastoma cells. Int J Mol Sci. 2021;22:11511. doi: 10.3390/ijms222111511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao B, Li H, Xia Y, et al. Immune checkpoint of H7–H3 in cancer: From immunology to clinical immunotherapy. J Hematol Oncol. 2022;15:153. doi: 10.1186/s13045-022-01364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Getu AA, Tigabu A, Zhou M, et al. New frontiers in immune checkpoint B7–H3 (CD276) research and drug development. Mol Cancer. 2023;22:43. doi: 10.1186/s12943-023-01751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cerma K, Piacentini F, Moscetti L, et al. Targeting PI3K/AKT/mTOR pathway in breast cancer: From biology to clinical challenges. Biomedicines. 2023;11:109. doi: 10.3390/biomedicines11010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sobande F, Dušek L, Matějková A, et al. EGFR in triple negative breast carcinoma: significance of protein expression and high gene copy number. Cesk Patol. 2015;51:80–86. [PubMed] [Google Scholar]
- 100.Talukdar S, Emdad L, Das SK, Fisher PB. EGFR: an essential receptor tyrosine kinase-regulator of cancer stem cells. Adv Cancer Res. 2020;147:161–188. doi: 10.1016/bs.acr.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 101.Sabbah DA, Hajjo R, Sweidan K. Review on epidermal growth factor receptor (EGFR) structure, signaling pathways, interactions, and recent updates of EGFR inhibitors. Curr Top Med Chem. 2020;20:815–834. doi: 10.2174/1568026620666200303123102. [DOI] [PubMed] [Google Scholar]
- 102.Eccles SA. The epidermal growth factor receptor/Erb-B/HER family in normal and malignant breast biology. Int J Dev Biol. 2011;55:685–696. doi: 10.1387/ijdb.113396se. [DOI] [PubMed] [Google Scholar]
- 103.Corkery B, Crown J, Clynes M, O'Donovan N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol. 2009;20:862–867. doi: 10.1093/annonc/mdn710. [DOI] [PubMed] [Google Scholar]
- 104.Baselga J, Gómez P, Greil R, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31:2586–2592. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: Randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30:2615–2623. doi: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Q, He L, Li S, et al. Homer3 facilitates growth factor-mediated β-catenin tyrosine phosphorylation and activation to promote metastasis in triple negative breast cancer. J Hematol Oncol. 2021;14:6. doi: 10.1186/s13045-020-01021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang AH, Hoefer RA, Guye ML, Bear HD. Persistent EGFR/K-RAS/SIAH pathway activation drives chemo-resistance and early tumor relapse in triple-negative breast cancer. Cancer Drug Resist. 2022;5:691–702. doi: 10.20517/cdr.2022.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.El Guerrab A, Bamdad M, Bignon YJ, Penault-Llorca F, Aubel C. Co-targeting EGFR and mTOR with gefitinib and everolimus in triple-negative breast cancer cells. Sci Rep. 2020;10:6367. doi: 10.1038/s41598-020-63310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17:318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 110.Pérez Piñero C, Giulianelli S, Lamb CA, Lanari C. New insights in the interaction of FGF/FGFR and steroid receptor signaling in breast cancer. Endocrinology. 2022;163:bqab265. doi: 10.1210/endocr/bqab265. [DOI] [PubMed] [Google Scholar]
- 111.Cheng CL, Thike AA, Tan SY, et al. Expression of FGFR1 is an independent prognostic factor in triple-negative breast cancer. Breast Cancer Res Treat. 2015;151:99–111. doi: 10.1007/s10549-015-3371-x. [DOI] [PubMed] [Google Scholar]
- 112.Lee HJ, Seo AN, Park SY, et al. Low prognostic implication of fibroblast growth factor family activation in triple-negative breast cancer subsets. Ann Surg Oncol. 2014;21:1561–1568. doi: 10.1245/s10434-013-3456-x. [DOI] [PubMed] [Google Scholar]
- 113.Francavilla C, O'Brien CS. Fibroblast growth factor receptor signalling dysregulation and targeting in breast cancer. Open Biol. 2022;12:210373. doi: 10.1098/rsob.210373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Turner N, Lambros MB, Horlings HM, et al. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene. 2010;29:2013–2023. doi: 10.1038/onc.2009.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.André F, Bachelot T, Campone M, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res. 2013;19:3693–3702. doi: 10.1158/1078-0432.CCR-13-0190. [DOI] [PubMed] [Google Scholar]
- 116.Pearson A, Smyth E, Babina IS, et al. High-level clonal FGFR amplification and response to fgfr inhibition in a translational clinical trial. Cancer Discov. 2016;6:838–851. doi: 10.1158/2159-8290.CD-15-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.André F, Cortés J. Rationale for targeting fibroblast growth factor receptor signaling in breast cancer. Breast Cancer Res Treat. 2015;150:1–8. doi: 10.1007/s10549-015-3301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117–124. doi: 10.1016/j.semcancer.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 119.Shiau JP, Wu CC, Chang SJ, et al. Fak regulates VEGFR2 expression and promotes angiogenesis in triple-negative breast cancer. Biomedicines. 2021;9:1789. doi: 10.3390/biomedicines9121789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shashni B, Nishikawa Y, Nagasaki Y. Management of tumor growth and angiogenesis in triple-negative breast cancer by using redox nanoparticles. Biomaterials. 2021;269:120645. doi: 10.1016/j.biomaterials.2020.120645. [DOI] [PubMed] [Google Scholar]
- 121.Robert NJ, Diéras V, Glaspy J, et al. Ribbon-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 122.Brufsky AM, Hurvitz S, Perez E, et al. Ribbon-2: A randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011;29:4286–4293. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- 123.von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (Geparsixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 124.Gerber B, von Minckwitz G, Eidtmann H, et al. Surgical outcome after neoadjuvant chemotherapy and bevacizumab: results from the Geparquinto study (GBG 44) Ann Surg Oncol. 2014;21:2517–2524. doi: 10.1245/s10434-014-3606-9. [DOI] [PubMed] [Google Scholar]
- 125.Loibl S, Weber KE, Timms KM, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from Geparsixto. Ann Oncol. 2018;29:2341–2347. doi: 10.1093/annonc/mdy460. [DOI] [PubMed] [Google Scholar]
- 126.Bell R, Brown J, Parmar M, et al. Final efficacy and updated safety results of the randomized phase III BEATRICE trial evaluating adjuvant bevacizumab-containing therapy in triple-negative early breast cancer. Ann Oncol. 2017;28:754–760. doi: 10.1093/annonc/mdw665. [DOI] [PubMed] [Google Scholar]
- 127.Gao Z, Shi M, Wang Y, Chen J, Ou Y. Apatinib enhanced anti-tumor activity of cisplatin on triple-negative breast cancer through inhibition of VEGFR-2. Pathol Res Pract. 2019;215:152422. doi: 10.1016/j.prp.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 128.Yang C, Zhang J, Zhang Y, et al. Low-dose Apatinib combined with neoadjuvant chemotherapy in the treatment of early-stage triple-negative breast cancer (lancet): a single-center, single-arm, phase trial. Ther Adv Med Oncol. 2022;14:17588359221118053. doi: 10.1177/17588359221118053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li DD, Tao ZH, Wang BY, et al. Apatinib plus vinorelbine versus vinorelbine for metastatic triple-negative breast cancer who failed first/second-line treatment: the nan trial. NPJ Breast Cancer. 2022;8:110. doi: 10.1038/s41523-022-00462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu J, Liu Q, Li Y, et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial. J Immunother Cancer. 2020;8:e000696. doi: 10.1136/jitc-2020-000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou B, Lin W, Long Y, et al. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7:95. doi: 10.1038/s41392-022-00934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yuan X, Wu H, Xu H, et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett. 2015;369:20–27. doi: 10.1016/j.canlet.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 133.Weijzen S, Rizzo P, Braid M, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- 134.Yuan X, Zhang M, Wu H, et al. Expression of notch1 correlates with breast cancer progression and prognosis. PLoS ONE. 2015;10:e0131689. doi: 10.1371/journal.pone.0131689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yuan X, Wu H, Xu H, et al. Meta-analysis reveals the correlation of notch signaling with non-small cell lung cancer progression and prognosis. Sci Rep. 2015;5:10338. doi: 10.1038/srep10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang H, Yang Y, Li X, Yuan X, Chu Q. Targeting the notch signaling pathway and the notch ligand, dll3, in small cell lung cancer. Biomed Pharmacother. 2023;159:114248. doi: 10.1016/j.biopha.2023.114248. [DOI] [PubMed] [Google Scholar]
- 137.O'Neill CF, Urs S, Cinelli C, et al. Notch2 signaling induces apoptosis and inhibits human mda-mb-231 xenograft growth. Am J Pathol. 2007;171:1023–1036. doi: 10.2353/ajpath.2007.061029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Giuli MV, Giuliani E, Screpanti I, Bellavia D, Checquolo S. Notch signaling activation as a hallmark for triple-negative breast cancer subtype. J Oncol. 2019;2019:8707053. doi: 10.1155/2019/8707053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Espinoza I, Miele L. Notch inhibitors for cancer treatment. Pharmacol Ther. 2013;139:95–110. doi: 10.1016/j.pharmthera.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sharma A, Paranjape AN, Rangarajan A, Dighe RR. A monoclonal antibody against human notch1 ligand-binding domain depletes subpopulation of putative breast cancer stem-like cells. Mol Cancer Ther. 2012;11:77–86. doi: 10.1158/1535-7163.MCT-11-0508. [DOI] [PubMed] [Google Scholar]
- 141.Kume T. Ligand-dependent notch signaling in vascular formation. Adv Exp Med Biol. 2012;727:210–222. doi: 10.1007/978-1-4614-0899-4_16. [DOI] [PubMed] [Google Scholar]
- 142.Krishna BM, Jana S, Singhal J, et al. Notch signaling in breast cancer: From pathway analysis to therapy. Cancer Lett. 2019;461:123–131. doi: 10.1016/j.canlet.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qin JJ, Yan L, Zhang J, Zhang WD. STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review. J Exp Clin Cancer Res. 2019;38:195. doi: 10.1186/s13046-019-1206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ma JH, Qin L, Li X. Role of stat3 signaling pathway in breast cancer. Cell Commun Signal. 2020;18:33. doi: 10.1186/s12964-020-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jackson JG, Lozano G. TNBC invasion: downstream of STAT3. Oncotarget. 2017;8:20517–20518. doi: 10.18632/oncotarget.15259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wei W, Tweardy DJ, Zhang M, et al. STAT3 signaling is activated preferentially in tumor-initiating cells in claudin-low models of human breast cancer. Stem Cells. 2014;32:2571–2582. doi: 10.1002/stem.1752. [DOI] [PubMed] [Google Scholar]
- 147.Lu L, Dong J, Wang L, et al. Activation of STAT3 and Bcl-2 and reduction of reactive oxygen species (ROS) promote radioresistance in breast cancer and overcome of radioresistance with niclosamide. Oncogene. 2018;37:5292–5304. doi: 10.1038/s41388-018-0340-y. [DOI] [PubMed] [Google Scholar]
- 148.Zhong Y, Deng L, Shi S, et al. The novel STAT3 inhibitor WZ-2-033 causes regression of human triple-negative breast cancer and gastric cancer xenografts. Acta Pharmacol Sin. 2022;43:1013–1023. doi: 10.1038/s41401-021-00718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu X, Zhang L, He X, et al. TGF-β plays a vital role in triple-negative breast cancer (TNBC) drug-resistance through regulating stemness, emt and apoptosis. Biochem Biophys Res Commun. 2018;502:160–165. doi: 10.1016/j.bbrc.2018.05.139. [DOI] [PubMed] [Google Scholar]
- 150.Ding MJ, Su KE, Cui GZ, et al. Association between transforming growth factor-β1 expression and the clinical features of triple negative breast cancer. Oncol Lett. 2016;11:4040–4044. doi: 10.3892/ol.2016.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pinilla K, Drewett LM, Lucey R, Abraham JE. Precision breast cancer medicine: Early stage triple negative breast cancer-a review of molecular characterisation, therapeutic targets and future trends. Front Oncol. 2022;12:866889. doi: 10.3389/fonc.2022.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bhola NE, Balko JM, Dugger TC, et al. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123:1348–1358. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yi M, Li T, Niu M, et al. TGF-β: a novel predictor and target for anti-pd-1/pd-l1 therapy. Front Immunol. 2022;13:1061394. doi: 10.3389/fimmu.2022.1061394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bai X, Yi M, Jiao Y, Chu Q, Wu K. Blocking TGF-β signaling to enhance the efficacy of immune checkpoint inhibitor. Onco Targets Ther. 2019;12:9527–9538. doi: 10.2147/OTT.S224013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang X, Zhang G, Liang T. Cancer environmental immunotherapy: Starving tumor cell to death by targeting TGFβ on immune cell. J Immunother Cancer. 2021;9:e002823. doi: 10.1136/jitc-2021-002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lan Y, Yeung TL, Huang H, et al. Colocalized targeting of TGF-β and pd-l1 by bintrafusp alfa elicits distinct antitumor responses. J Immunother Cancer. 2022;10:e004122. doi: 10.1136/jitc-2021-004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yi M, Wu Y, Niu M, et al. Anti-TGF-β/PD-L1 bispecific antibody promotes t cell infiltration and exhibits enhanced antitumor activity in triple-negative breast cancer. J Immunother Cancer. 2022;10:e005543. doi: 10.1136/jitc-2022-005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yi M, Zhang J, Li A, et al. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1. J Hematol Oncol. 2021;14:27. doi: 10.1186/s13045-021-01045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yi M, Niu M, Zhang J, et al. Combine and conquer: Manganese synergizing anti-TGF-β/PD-L1 bispecific antibody YM101 to overcome immunotherapy resistance in non-inflamed cancers. J Hematol Oncol. 2021;14:146. doi: 10.1186/s13045-021-01155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yi M, Niu M, Wu Y, et al. Combination of oral sting agonist MSAA-2 and anti-TGF-β/PD-L1 bispecific antibody YM101: a novel immune cocktail therapy for non-inflamed tumors. J Hematol Oncol. 2022;15:142. doi: 10.1186/s13045-022-01363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17:630–641. doi: 10.1038/nrg.2016.93. [DOI] [PubMed] [Google Scholar]
- 162.Muhammad A, Forcados GE, Katsayal BS, et al. Potential epigenetic modifications implicated in triple- to quadruple-negative breast cancer transition: a review. Epigenomics. 2022;14:711–726. doi: 10.2217/epi-2022-0033. [DOI] [PubMed] [Google Scholar]
- 163.Yang X, Phillips DL, Ferguson AT, et al. Synergistic activation of functional estrogen receptor (er)-alpha by DNA methyltransferase and histone deacetylase inhibition in human er-alpha-negative breast cancer cells. Cancer Res. 2001;61:7025–7029. [PubMed] [Google Scholar]
- 164.Tan B, Zhou K, Liu W, et al. RNA N(6) -methyladenosine reader YTHDC1 is essential for TGF-beta-mediated metastasis of triple negative breast cancer. Theranostics. 2022;12:5727–5743. doi: 10.7150/thno.71872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiang XC, Tu FH, Wei LY, et al. Discovery of a novel g-quadruplex and histone deacetylase (HDAC) dual-targeting agent for the treatment of triple-negative breast cancer. J Med Chem. 2022;65:12346–12366. doi: 10.1021/acs.jmedchem.2c01058. [DOI] [PubMed] [Google Scholar]
- 166.Chiu HW, Yeh YL, Wang YC, et al. Combination of the novel histone deacetylase inhibitor YCW1 and radiation induces autophagic cell death through the downregulation of BNIP3 in triple-negative breast cancer cells in vitro and in an orthotopic mouse model. Mol Cancer. 2016;15:46. doi: 10.1186/s12943-016-0531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tan WW, Allred JB, Moreno-Aspitia A, et al. Phase I study of Panobinostat (IBH589) and letrozole in postmenopausal metastatic breast cancer patients. Clin Breast Cancer. 2016;16:82–86. doi: 10.1016/j.clbc.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ramaiah MJ, Tangutur AD, Manyam RR. Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy. Life Sci. 2021;277:119504. doi: 10.1016/j.lfs.2021.119504. [DOI] [PubMed] [Google Scholar]
- 169.Fedele P, Orlando L, Cinieri S. Targeting triple negative breast cancer with histone deacetylase inhibitors. Expert Opin Investig Drugs. 2017;26:1199–1206. doi: 10.1080/13543784.2017.1386172. [DOI] [PubMed] [Google Scholar]
- 170.Tucker N. Junshi biosciences announces toripalimab in combination with chemotherapy for treatment of advanced triple-negative breast cancer met primary endpoint in phase 3 clinical study. https://bit.ly/3Stq86o. Accessed 22 Feb 2023.
- 171.Toripalimab in combination with nab-paclitaxel for patients with metastatic or recurrent triple-negative breast cancer (TNBC) with or without systemic treatment (Torchlight). https://clinicaltrials.gov/ct2/show/NCT04085276?term=TORCHLIGHT&draw=2&rank=1. Accessed 22 Feb 2023.
- 172.Fan L, Linxiaoxi M, Wu S, et al. Future-super: A randomized, subtyping-based umbrella phase ii trial for first-line treatment of metastatic triple-negative breast cancer. Cell Res. 2023;41:3011. [Google Scholar]
- 173.Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw. 2020;18:479–489. doi: 10.6004/jnccn.2020.7554. [DOI] [PubMed] [Google Scholar]
- 174.Zhu Y, Zhu X, Tang C, Guan X, Zhang W. Progress and challenges of immunotherapy in triple-negative breast cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188593. doi: 10.1016/j.bbcan.2021.188593. [DOI] [PubMed] [Google Scholar]
- 175.Xu L, Zou C, Zhang S, et al. Reshaping the systemic tumor immune environment (STIE) and tumor immune microenvironment (TIME) to enhance immunotherapy efficacy in solid tumors. J Hematol Oncol. 2022;15:87. doi: 10.1186/s13045-022-01307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 179.Girard N, Bar J, Garrido P, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: Findings from the pacific-r study. J Thorac Oncol. 2023;18:181–193. doi: 10.1016/j.jtho.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 180.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Escudier B, Motzer RJ, Sharma P, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur Urol. 2017;72:368–376. doi: 10.1016/j.eururo.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 182.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Agostinetto E, Losurdo A, Nader-Marta G, et al. Progress and pitfalls in the use of immunotherapy for patients with triple negative breast cancer. Expert Opin Investig Drugs. 2022;31:567–591. doi: 10.1080/13543784.2022.2049232. [DOI] [PubMed] [Google Scholar]
- 184.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dai M, Liu M, Yang H, Küçük C, You H. New insights into epigenetic regulation of resistance to PD-1/PD-L1 blockade cancer immunotherapy: Mechanisms and therapeutic opportunities. Exp Hematol Oncol. 2022;11:101. doi: 10.1186/s40164-022-00356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu Z, Yu X, Xu L, Li Y, Zeng C. Current insight into the regulation of PD-L1 in cancer. Exp Hematol Oncol. 2022;11:44. doi: 10.1186/s40164-022-00297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ali HR, Glont SE, Blows FM, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26:1488–1493. doi: 10.1093/annonc/mdv192. [DOI] [PubMed] [Google Scholar]
- 188.Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Darvin P, Toor SM, Sasidharan NV, Elkord E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp Mol Med. 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tarantino P, Antonarelli G, Ascione L, Curigliano G. Investigational immunomodulatory drugs for enhancement of triple negative breast cancer (TNBC) immunotherapy: early phase development. Expert Opin Investig Drugs. 2022;31:499–513. doi: 10.1080/13543784.2021.1972968. [DOI] [PubMed] [Google Scholar]
- 191.Wang Y, Zhang H, Liu C, et al. Immune checkpoint modulators in cancer immunotherapy: Recent advances and emerging concepts. J Hematol Oncol. 2022;15:111. doi: 10.1186/s13045-022-01325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:397–404. doi: 10.1093/annonc/mdy517. [DOI] [PubMed] [Google Scholar]
- 194.Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:405–411. doi: 10.1093/annonc/mdy518. [DOI] [PubMed] [Google Scholar]
- 195.Winer EP, Lipatov O, Im SA, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (Keynote-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:499–511. doi: 10.1016/S1470-2045(20)30754-3. [DOI] [PubMed] [Google Scholar]
- 196.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 197.Huang M, O'Shaughnessy J, Haiderali A, et al. Q-twist analysis of pembrolizumab combined with chemotherapy as first-line treatment of metastatic triple-negative breast cancer that expresses PD-L1. Eur J Cancer. 2022;177:45–52. doi: 10.1016/j.ejca.2022.09.029. [DOI] [PubMed] [Google Scholar]
- 198.Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase Ib open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31:569–581. doi: 10.1016/j.annonc.2020.01.072. [DOI] [PubMed] [Google Scholar]
- 199.Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6:676–684. doi: 10.1001/jamaoncol.2019.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schmid P, Cortes J, Dent R, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556–567. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 201.Santa-Maria CA, O'Donnell M, Nunes R, Wright JL, Stearns V. Integrating immunotherapy in early-stage triple-negative breast cancer: Practical evidence-based considerations. J Natl Compr Canc Netw. 2022;20:738–744. doi: 10.6004/jnccn.2022.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fasching PA, Hein A, Kolberg HC, et al. Pembrolizumab in combination with nab-paclitaxel for the treatment of patients with early-stage triple-negative breast cancer—a single-arm phase II trial (Neoimmunoboost, AGO-B-041) Eur J Cancer. 2023;184:1–9. doi: 10.1016/j.ejca.2023.01.001. [DOI] [PubMed] [Google Scholar]
- 203.Shah M, Osgood CL, Amatya AK, et al. FDA approval summary: Pembrolizumab for neoadjuvant and adjuvant treatment of patients with high-risk early-stage triple-negative breast cancer. Clin Cancer Res. 2022;28:5249–5253. doi: 10.1158/1078-0432.CCR-22-1110. [DOI] [PubMed] [Google Scholar]
- 204.Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (Impassion031): A randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 205.Narayan P, Wahby S, Gao JJ, et al. FDA approval summary: Atezolizumab plus paclitaxel protein-bound for the treatment of patients with advanced or metastatic TNBC whose tumors express PD-L1. Clin Cancer Res. 2020;26:2284–2289. doi: 10.1158/1078-0432.CCR-19-3545. [DOI] [PubMed] [Google Scholar]
- 206.Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (Impassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 207.Emens LA, Molinero L, Loi S, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the Impassion130 study. J Natl Cancer Inst. 2021;113:1005–1016. doi: 10.1093/jnci/djab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Emens LA, Adams S, Barrios CH, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: impassion 130 final overall survival analysis. Ann Oncol. 2021;32:983–993. doi: 10.1016/j.annonc.2021.05.355. [DOI] [PubMed] [Google Scholar]
- 209.Miles D, Gligorov J, André F, et al. Primary results from Impassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32:994–1004. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 210.Loibl S, Untch M, Burchardi N, et al. A randomised phase ii study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30:1279–1288. doi: 10.1093/annonc/mdz158. [DOI] [PubMed] [Google Scholar]
- 211.Loibl S, Schneeweiss A, Huober J, et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol. 2022;33:1149–1158. doi: 10.1016/j.annonc.2022.07.1940. [DOI] [PubMed] [Google Scholar]
- 212.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. 'Off-the-shelf' allogeneic CAR T cells: Development and challenges. Nat Rev Drug Discov. 2020;19:185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 213.Labanieh L, Majzner RG, Klysz D, et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell. 2022;185:1745-63. e22. doi: 10.1016/j.cell.2022.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Liu H, Pan C, Song W, et al. Novel strategies for immuno-oncology breakthroughs with cell therapy. Biomark Res. 2021;9:62. doi: 10.1186/s40364-021-00316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xie Y, Hu Y, Zhou N, et al. CAR T-cell therapy for triple-negative breast cancer: Where we are. Cancer Lett. 2020;491:121–131. doi: 10.1016/j.canlet.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 216.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor t cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yan T, Zhu L, Chen J. Current advances and challenges in CAR T-cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment. Exp Hematol Oncol. 2023;12:14. doi: 10.1186/s40164-023-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yin L, Chen GL, Xiang Z, et al. Current progress in chimeric antigen receptor-modified T cells for the treatment of metastatic breast cancer. Biomed Pharmacother. 2023;162:114648. doi: 10.1016/j.biopha.2023.114648. [DOI] [PubMed] [Google Scholar]
- 219.Harrasser M, Gohil SH, Lau H, et al. Inducible localized delivery of an anti-PD-1 SCFV enhances anti-tumor activity of ror1 car-t cells in TNBC. Breast Cancer Res. 2022;24:39. doi: 10.1186/s13058-022-01531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hu Z. Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer. Sci Rep. 2020;10:2815. doi: 10.1038/s41598-020-59736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang L, Meng Y, Feng X, Han Z. Car-NK cells for cancer immunotherapy: From bench to bedside. Biomark Res. 2022;10:12. doi: 10.1186/s40364-022-00364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xia L, Zheng ZZ, Liu JY, et al. EGFR-targeted car-t cells are potent and specific in suppressing triple-negative breast cancer both in vitro and in vivo. Clin Transl Immunology. 2020;9:e01135. doi: 10.1002/cti2.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol Cancer. 2019;18:128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huo W, Yang X, Wang B, et al. Biomineralized hydrogel dc vaccine for cancer immunotherapy: a boosting strategy via improving immunogenicity and reversing immune-inhibitory microenvironment. Biomaterials. 2022;288:121722. doi: 10.1016/j.biomaterials.2022.121722. [DOI] [PubMed] [Google Scholar]
- 225.Huang L, Rong Y, Tang X, et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol Cancer. 2022;21:45. doi: 10.1186/s12943-022-01515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bai X, Zhou Y, Yokota Y, et al. Adaptive antitumor immune response stimulated by bio-nanoparticle based vaccine and checkpoint blockade. J Exp Clin Cancer Res. 2022;41:132. doi: 10.1186/s13046-022-02307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Razazan A, Behravan J. Single peptides and combination modalities for triple negative breast cancer. J Cell Physiol. 2020;235:4089–4108. doi: 10.1002/jcp.29300. [DOI] [PubMed] [Google Scholar]
- 228.Overholser J, Ambegaokar KH, Eze SM, et al. Anti-tumor effects of peptide therapeutic and peptide vaccine antibody co-targeting HER-1 and HER-2 in esophageal cancer (EC) and her-1 and igf-1r in triple-negative breast cancer (TNBC) Vaccines (Basel) 2015;3:519–543. doi: 10.3390/vaccines3030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Petrina M, Martin J, Basta S. Granulocyte macrophage colony-stimulating factor has come of age: from a vaccine adjuvant to antiviral immunotherapy. Cytokine Growth Factor Rev. 2021;59:101–110. doi: 10.1016/j.cytogfr.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu J, Fu M, Wang M, et al. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J Hematol Oncol. 2022;15:28. doi: 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jin S, Wang Q, Wu H, Pang D, Xu S. Oncolytic viruses for triple negative breast cancer and beyond. Biomark Res. 2021;9:71. doi: 10.1186/s40364-021-00318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21:141–162. doi: 10.1038/s41573-021-00339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nie Y, Yun X, Zhang Y, Wang X. Targeting metabolic reprogramming in chronic lymphocytic leukemia. Exp Hematol Oncol. 2022;11:39. doi: 10.1186/s40164-022-00292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li S, Zeng H, Fan J, et al. Glutamine metabolism in breast cancer and possible therapeutic targets. Biochem Pharmacol. 2023;210:115464. doi: 10.1016/j.bcp.2023.115464. [DOI] [PubMed] [Google Scholar]
- 235.Wu HL, Gong Y, Ji P, et al. Targeting nucleotide metabolism: A promising approach to enhance cancer immunotherapy. J Hematol Oncol. 2022;15:45. doi: 10.1186/s13045-022-01263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yi M, Zheng X, Niu M, et al. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol Cancer. 2022;21:28. doi: 10.1186/s12943-021-01489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhu S, Zhang T, Zheng L, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 2021;14:156. doi: 10.1186/s13045-021-01164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wu SY, Xu Y, Chen L, et al. Combined angiogenesis and PD-1 inhibition for immunomodulatory TNBC: concept exploration and biomarker analysis in the future-c-plus trial. Mol Cancer. 2022;21:84. doi: 10.1186/s12943-022-01536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhu L, Liu J, Chen J, Zhou Q. The developing landscape of combinatorial therapies of immune checkpoint blockade with DNA damage repair inhibitors for the treatment of breast and ovarian cancers. J Hematol Oncol. 2021;14:206. doi: 10.1186/s13045-021-01218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang Z, Sun K, Xiao Y, et al. Niraparib activates interferon signaling and potentiates anti-pd-1 antibody efficacy in tumor models. Sci Rep. 2019;9:1853. doi: 10.1038/s41598-019-38534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019;5:1132–1140. doi: 10.1001/jamaoncol.2019.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pusztai L, Yau C, Wolf DM, et al. Durvalumab with olaparib and paclitaxel for high-risk her2-negative stage ii/iii breast cancer: Results from the adaptively randomized i-spy2 trial. Cancer Cell. 2021;39:989-98. e5. doi: 10.1016/j.ccell.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chu Y, Zhou X, Wang X. Antibody-drug conjugates for the treatment of lymphoma: clinical advances and latest progress. J Hematol Oncol. 2021;14:88. doi: 10.1186/s13045-021-01097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Rose S. "Very compelling" results for ADC in TNBC trial. Cancer Discov. 2022;12:280–281. doi: 10.1158/2159-8290.CD-NB2021-0407. [DOI] [PubMed] [Google Scholar]
- 245.Wu M, Huang W, Yang N, Liu Y. Learn from antibody-drug conjugates: consideration in the future construction of peptide-drug conjugates for cancer therapy. Exp Hematol Oncol. 2022;11:93. doi: 10.1186/s40164-022-00347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jeon Y, Jo U, Hong J, Gong G, Lee HJ. Trophoblast cell-surface antigen 2 (Trop2) expression in triple-negative breast cancer. BMC Cancer. 2022;22:1014. doi: 10.1186/s12885-022-10076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cortesi M, Zanoni M, Maltoni R, et al. Trop2 (trophoblast cell-surface antigen 2): a drug target for breast cancer. Expert Opin Ther Targets. 2022;26:593–602. doi: 10.1080/14728222.2022.2113513. [DOI] [PubMed] [Google Scholar]
- 248.Bailly C. Irinotecan: 25 years of cancer treatment. Pharmacol Res. 2019;148:104398. doi: 10.1016/j.phrs.2019.104398. [DOI] [PubMed] [Google Scholar]
- 249.Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II immu-132-01 basket trial. Ann Oncol. 2021;32:746–756. doi: 10.1016/j.annonc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 250.Bardia A, Tolaney SM, Punie K, et al. Biomarker analyses in the phase III ascent study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32:1148–1156. doi: 10.1016/j.annonc.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 251.Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 252.Qiu S, Zhang J, Wang Z, et al. Targeting Trop-2 in cancer: Recent research progress and clinical application. Biochim Biophys Acta Rev Cancer. 2023;1878:188902. doi: 10.1016/j.bbcan.2023.188902. [DOI] [PubMed] [Google Scholar]
- 253.Adams E, Wildiers H, Neven P, Punie K. Sacituzumab govitecan and trastuzumab deruxtecan: Two new antibody-drug conjugates in the breast cancer treatment landscape. ESMO Open. 2021;6:100204. doi: 10.1016/j.esmoop.2021.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rugo HS, Bardia A, Tolaney SM, et al. Tropics-02: A phase III study investigating sacituzumab govitecan in the treatment of HR+/HER2- metastatic breast cancer. Future Oncol. 2020;16:705–715. doi: 10.2217/fon-2020-0163. [DOI] [PubMed] [Google Scholar]
- 255.Rugo HS, Bardia A, Marmé F, et al. Sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2022;40:3365–3376. doi: 10.1200/JCO.22.01002. [DOI] [PubMed] [Google Scholar]
- 256.Furlanetto J, Marmé F, Loibl S. Sacituzumab govitecan: Past, present and future of a new antibody-drug conjugate and future horizon. Future Oncol. 2022;18:3199–3215. doi: 10.2217/fon-2022-0407. [DOI] [PubMed] [Google Scholar]
- 257.Demeule M, Charfi C, Currie JC, et al. The TH1902 docetaxel peptide-drug conjugate inhibits xenografts growth of human SORT1-positive ovarian and triple-negative breast cancer stem-like cells. Pharmaceutics. 2022;14:1910. doi: 10.3390/pharmaceutics14091910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Demeule M, Charfi C, Currie JC, et al. TH1902, a new docetaxel-peptide conjugate for the treatment of sortilin-positive triple-negative breast cancer. Cancer Sci. 2021;112:4317–4334. doi: 10.1111/cas.15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Charfi C, Demeule M, Currie JC, et al. New peptide-drug conjugates for precise targeting of sort1-mediated vasculogenic mimicry in the tumor microenvironment of TNBC-derived mda-mb-231 breast and ovarian es-2 clear cell carcinoma cells. Front Oncol. 2021;11:760787. doi: 10.3389/fonc.2021.760787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Okajima D, Yasuda S, Maejima T, et al. Datopotamab deruxtecan, a novel Trop2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther. 2021;20:2329–2340. doi: 10.1158/1535-7163.MCT-21-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Shastry M, Jacob S, Rugo HS, Hamilton E. Antibody-drug conjugates targeting Trop-2: clinical development in metastatic breast cancer. Breast. 2022;66:169–177. doi: 10.1016/j.breast.2022.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 263.Cortés J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 264.Hurvitz SA, Hegg R, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401:105–117. doi: 10.1016/S0140-6736(22)02420-5. [DOI] [PubMed] [Google Scholar]
- 265.Poh A. T-dxd: New standard for HER2-low breast cancer. Cancer Discov. 2022;12:1828. doi: 10.1158/2159-8290.CD-NB2022-0043. [DOI] [PubMed] [Google Scholar]
- 266.Sidaway P. T-DXd active in HER2-low disease. Nat Rev Clin Oncol. 2022;19:493. doi: 10.1038/s41571-022-00663-9. [DOI] [PubMed] [Google Scholar]
- 267.Modi S, Park H, Murthy RK, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: Results from a phase Ib study. J Clin Oncol. 2020;38:1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.M-Rabet M, Cabaud O, Josselin E, et al. Nectin-4: a new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann Oncol. 2017;28:769–776. doi: 10.1093/annonc/mdw678. [DOI] [PubMed] [Google Scholar]
- 270.Guo P, Huang J, Zhu B, et al. A rationally designed ICAM1 antibody drug conjugate eradicates late-stage and refractory triple-negative breast tumors in vivo. Sci Adv. 2023;9:eabq7866. doi: 10.1126/sciadv.abq7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 272.Liao M, Qin R, Huang W, et al. Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies. J Hematol Oncol. 2022;15:44. doi: 10.1186/s13045-022-01260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Qin R, You FM, Zhao Q, et al. Naturally derived indole alkaloids targeting regulated cell death (RCD) for cancer therapy: from molecular mechanisms to potential therapeutic targets. J Hematol Oncol. 2022;15:133. doi: 10.1186/s13045-022-01350-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jiang L, Gao XM, Cao J. The achilles heel of TNBCs: ferroptosis heterogeneity. Cell Metab. 2023;35:1–2. doi: 10.1016/j.cmet.2022.11.014. [DOI] [PubMed] [Google Scholar]
- 275.Gan H., Huang X., Luo X., et al. A mitochondria-targeted ferroptosis inducer activated by glutathione-responsive imaging and depletion for triple negative breast cancer theranostics. Adv Healthc Mater. 2023: e2300220. [DOI] [PubMed]
- 276.Song X, Wang X, Liu Z, Yu Z. Role of GPX4-mediated ferroptosis in the sensitivity of triple negative breast cancer cells to Gefitinib. Front Oncol. 2020;10:597434. doi: 10.3389/fonc.2020.597434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yang C, Zhang J, Liao M, et al. Folate-mediated one-carbon metabolism: a targeting strategy in cancer therapy. Drug Discov Today. 2021;26:817–825. doi: 10.1016/j.drudis.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 279.Zhu S, Yi M, Wu Y, Dong B, Wu K. Roles of tumor-associated macrophages in tumor progression: implications on therapeutic strategies. Exp Hematol Oncol. 2021;10:60. doi: 10.1186/s40164-021-00252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chen X, Yang M, Yin J, et al. Tumor-associated macrophages promote epithelial-mesenchymal transition and the cancer stem cell properties in triple-negative breast cancer through CCL2/AKT/β-catenin signaling. Cell Commun Signal. 2022;20:92. doi: 10.1186/s12964-022-00888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Li H, Yang P, Wang J, et al. HLF regulates ferroptosis, development and chemoresistance of triple-negative breast cancer by activating tumor cell-macrophage crosstalk. J Hematol Oncol. 2022;15:2. doi: 10.1186/s13045-021-01223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhang Z, Lu M, Chen C, et al. Holo-lactoferrin: the link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics. 2021;11:3167–3182. doi: 10.7150/thno.52028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhang H, Zhu S, Zhou H, et al. Identification of MTHFD2 as a prognostic biomarker and ferroptosis regulator in triple-negative breast cancer. Front Oncol. 2023;13:1098357. doi: 10.3389/fonc.2023.1098357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Xie Y, Wang B, Zhao Y, et al. Mammary adipocytes protect triple-negative breast cancer cells from ferroptosis. J Hematol Oncol. 2022;15:72. doi: 10.1186/s13045-022-01297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Yang F, Xiao Y, Ding JH, et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023;35:84–100.e8. doi: 10.1016/j.cmet.2022.09.021. [DOI] [PubMed] [Google Scholar]
- 286.Xu H, Jiao D, Liu A, Wu K. Tumor organoids: applications in cancer modeling and potentials in precision medicine. J Hematol Oncol. 2022;15:58. doi: 10.1186/s13045-022-01278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Fang Z, Li P, Du F, Shang L, Li L. The role of organoids in cancer research. Exp Hematol Oncol. 2023;12:69. doi: 10.1186/s40164-023-00433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Xu H, Jiao Y, Qin S, et al. Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Exp Hematol Oncol. 2018;7:30. doi: 10.1186/s40164-018-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Guillen KP, Fujita M, Butterfield AJ, et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat Cancer. 2022;3:232–250. doi: 10.1038/s43018-022-00337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Foo MA, You M, Chan SL, et al. Clinical translation of patient-derived tumor organoids- bottlenecks and strategies. Biomark Res. 2022;10:10. doi: 10.1186/s40364-022-00356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Saatci O, Kaymak A, Raza U, et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat Commun. 2020;11:2416. doi: 10.1038/s41467-020-16199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Freitas AJA, Causin RL, Varuzza MB, et al. Liquid biopsy as a tool for the diagnosis, treatment, and monitoring of breast cancer. Int J Mol Sci. 2022;23:9952. doi: 10.3390/ijms23179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Manoochehri M, Borhani N, Gerhäuser C, et al. DNA methylation biomarkers for noninvasive detection of triple-negative breast cancer using liquid biopsy. Int J Cancer. 2023;152:1025–1035. doi: 10.1002/ijc.34337. [DOI] [PubMed] [Google Scholar]
- 294.Salvador-Coloma C, Santaballa A, Sanmartín E, et al. Immunosuppressive profiles in liquid biopsy at diagnosis predict response to neoadjuvant chemotherapy in triple-negative breast cancer. Eur J Cancer. 2020;139:119–134. doi: 10.1016/j.ejca.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 295.Swaminathan H, Saravanamurali K, Yadav SA. Extensive review on breast cancer its etiology, progression, prognostic markers, and treatment. Med Oncol. 2023;40:238. doi: 10.1007/s12032-023-02111-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.