Abstract
Shiga toxin-producing Escherichia coli O111:H2 strains from an outbreak of hemolytic-uremic syndrome showed aggregative adhesion to HEp-2 cells and harbored large plasmids which hybridized with the enteroaggregative E. coli probe PCVD432. These strains present a novel combination of virulence factors and might be as pathogenic to humans as the classic enterohemorrhagic E. coli.
Enterohemorrhagic Escherichia coli (EHEC) is a well-known cause of severe disease, such as hemorrhagic colitis and hemolytic-uremic syndrome (HUS) (10). The bacterium produces Shiga toxins (Stx; also known as verocytotoxins) (10), harbors large plasmids which code for production of enterohemolysin (EHEC-Hly) (24) and catalase-peroxidase (KatP) (4), and possesses the intimin-coding gene eaeA (27). The gene is part of a chromosomal gene cluster termed LEE, for locus of enterocyte effacement (16), which determines the production of attaching and effacing lesions on the intestinal mucosa and localized adhesion to HEp-2 cells (8, 12). Besides O157, by far the most important in human disease (10), EHEC strains belong to a restricted number of serogroups (10), among which O111 has been associated with both sporadic cases (9, 10) and outbreaks (3, 5, 20) of HUS.
During a study aimed at characterizing Stx-producing E. coli (STEC) O111 strains from different countries (data not shown), we found that eight strains isolated in France during an outbreak of HUS (3) showed aggregative adhesion (AA), instead of the typical localized adhesion, to HEp-2 cells (18) and possessed the genetic markers of enteroaggregative E. coli (EAggEC). EAggEC, defined by its aggregating pattern of adherence to Hep-2 cells (18), has been associated with protracted diarrhea in children in developing countries (6) and with cases of childhood diarrhea in Europe (9, 11, 25). AA is associated with the presence of large plasmids carrying genes coding for bundle-forming fimbriae (17) and the production of EAggEC heat-stable enterotoxin 1 (EAST1) (23). Fragments from these plasmids have been used as DNA probes (2) or PCR targets (25) for identifying EAggEC. Since AA and Stx production have never been found to be associated in E. coli isolates, we describe here the molecular characteristics of these unusual strains.
The E. coli O111:H2 strains, designated RD1 to RD8, gave negative results in the PCR analyses for the eaeA gene (26) and the EHEC plasmid markers ehec-hly (24) and katP (4). In addition, they did not produce hemolysin and did not hybridize with the eaeA (12) and EHEC (15) probes, even under low-stringency conditions. When tested in the HEp-2 cell assay (9), all these strains showed the aggregative pattern of adhesion typical of EAggEC (Fig. 1). Accordingly, they agglutinated rat erythrocytes in the presence of 0.5% mannose (17) and gave positive PCR amplification with the primer pairs which amplify a 630-bp region of the EAggEC probe (25) and the astA determinant of EAST1 (23). Moreover, they hybridized with the EAggEC (2) and astA (23) probes. The Vero cell assay (9) and the PCR analyses with Stx1- and Stx2-specific primers (21) confirmed that all the strains produced Stx2 alone. Taq cycle sequencing of the toxin B-subunit gene (21) showed 100% homology with the nucleotide sequence of the stx2 B gene from the O157:H7 strain EDL933. Table 1 summarizes the characteristics of the E. coli O111:H2 isolates in comparison with reference EAggEC and EHEC strains used as controls in all the experiments. As previously described by Savarino and coworkers (22), the O157:H7 strain EDL933 hybridized with a probe produced by PCR amplification of the astA gene present in strain 17-2 (23). However, it was negative in the astA PCR, thus suggesting the existence of a degree of variability in the astA nucleotide sequences present in the different groups of E. coli.
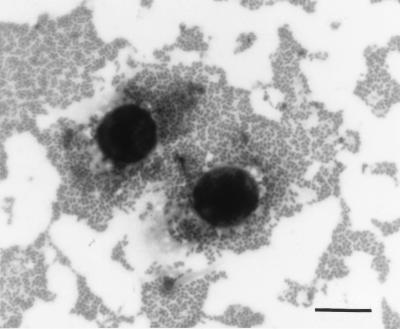
FIG. 1.
Pattern of AA to HEp-2 cells of a representative STEC O111:H2 strain. Bar = 15 μm.
TABLE 1.
Virulence properties of STEC O111:H2 strains and reference EHEC and EAggEC strains
| Strain | Stx production | HEp-2 cell adhesion | Positive hybridization with correlated probe
|
Positive PCR as defined in text
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EAggEC probe | EAST1 gene | eaeA gene | EHEC probe | EAST1 gene | eaeA gene | EHEC-Hly gene | KatP gene | |||
| STEC O111:H2 | + | Aggregative | + | + | − | − | + | − | − | − |
| EHEC O157 EDL933 | + | Localized | − | + | + | + | − | + | + | + |
| EAggEC 17-2 | − | Aggregative | + | + | − | − | + | − | − | − |
All the E. coli O111:H2 strains harbored two plasmids of approximately 100 and 7 kb, respectively. Southern analysis showed that the large plasmids hybridized with the EAggEC probe but not with an stx2 probe, which reacted only with the total cellular DNAs. These results indicated that the EAggEC gene cluster was located on the large plasmid, as in the EAggEC strain 17-2, and that the stx2 gene was present on the chromosome, as in the EHEC strain EDL933.
Stx genes are usually phage encoded in both O157 and non-O157 EHEC (19), and a phage λ regulatory gene, designated p, is usually located near both the stx1 and stx2 genes (7). The E. coli O111:H2 isolates were negative in a PCR assay performed with a primer pair complementary to p (7) but hybridized with a probe produced by PCR amplification of the p gene present in strain EDL933. An attempt to induce phages was performed by UV light treatment. Lysates of the E. coli O111:H2 strains obtained according to the protocol described by O’Brien et al. (19) did not contain infectious phages, while strain EDL933, included as a control in all the experiments, consistently yielded lysates containing 104 PFU/ml. The absence of inducible phages, however, does not exclude the possibility that the stx2 determinant is associated with a defective phage, and further work is needed to clarify this issue.
The E. coli O111:H2 strains described here present a combination of virulence factors found in both EHEC and EAggEC, a finding that, to the best of our knowledge, has never been described before in E. coli strains. These isolates can be classified as EHEC because they have been isolated from HUS patients and produce Stx. However, they do not possess the eaeA gene and show an aggregative pattern of adhesion to HEp-2 cells instead of the localized adhesion usually exhibited by EHEC (8, 9, 27). Stx production alone does not appear to confer human pathogenicity on STEC. In fact, most EHEC strains associated with disease in humans and cattle possess the eaeA determinant and EHEC plasmids detectable with the CVD419 probe (8, 9, 13, 15, 20, 27). Conversely, the eaeA gene and the EHEC plasmids are significantly less common among STEC strains isolated from healthy cattle (1, 13). Based on this evidence, when strains from animals or food are screened, the presence of eaeA is often considered to be a better predictor of the pathogenicity of STEC in humans than the Stx genes themselves. The STEC O111:H2 strains described here lack both the eaeA gene and the EHEC plasmid markers usually considered in diagnostic studies and should not strictly be considered EHEC. However, they have been associated with a severe outbreak of HUS (3), a typical EHEC-associated disease. In vitro these strains showed all the properties of EAggEC, and it has been reported that EAggEC cells are able to attach to human intestinal mucosa explants (14). It is therefore conceivable that the AA ability has allowed these E. coli O111:H2 strains to colonize the intestinal mucosa of children as efficiently as the typical eaeA-positive EHEC strains, and hence to cause disease.
In conclusion, E. coli strains possessing the novel combination of virulence factors described here, i.e., Stx production and enteroaggregative adhesion ability, might be as pathogenic to humans as the classic EHEC strains. Therefore, STEC from animal reservoirs or food should also be examined for EAggEC properties, in addition to EHEC plasmid markers and the characteristics associated with the attaching and effacing property, before excluding the possibility of their pathogenicity.
Acknowledgments
This work was partially supported by grant 95.01662.CT04 from Consiglio Nazionale delle Ricerche and by grant BMH4-CT96-0970 from the Commission of the European Communities.
REFERENCES
- 1.Barrett T J, Kaper J B, Jerse A E, Wachsmuth I K. Virulence factors in Shiga-like toxin-producing Escherichia coli isolated from humans and animals. J Infect Dis. 1992;165:979–980. doi: 10.1093/infdis/165.5.979. [DOI] [PubMed] [Google Scholar]
- 2.Baudry B, Savarino S J, Vial P, Kaper J B, Levine M M. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrhoeal pathogen. J Infect Dis. 1990;161:1249–1251. doi: 10.1093/infdis/161.6.1249. [DOI] [PubMed] [Google Scholar]
- 3.Boudaillez B, Berquin P, Mariani-Kurkdjian P, Bef D, Cuvelier B, Capek I, Tribout B, Bingen E, Piussan C. Possible person-to-person transmission of Escherichia coli O111-associated hemolytic uremic syndrome. Pediatr Nephrol. 1996;11:36–39. doi: 10.1007/s004670050229. [DOI] [PubMed] [Google Scholar]
- 4.Brunder W, Schmidt H, Karch H. Katp, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology. 1996;142:3305–3315. doi: 10.1099/13500872-142-11-3305. [DOI] [PubMed] [Google Scholar]
- 5.Caprioli A, Luzzi I, Rosmini F, Resti C, Edefonti A, Perfumo F, Farina C, Goglio A, Gianviti A, Rizzoni G. Communitywide outbreak of hemolytic-uremic syndrome associated with non-O157 verocytotoxin-producing Escherichia coli. J Infect Dis. 1994;169:208–211. doi: 10.1093/infdis/169.1.208. [DOI] [PubMed] [Google Scholar]
- 6.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 7.Datz M, Janetzki-Mittmann C, Franke S, Gunzer F, Schmidt H, Karch H. Analysis of the enterohemorrhagic Escherichia coli O157 DNA region containing lambdoid phage gene p and Shiga-like toxin structural genes. Appl Environ Microbiol. 1996;62:791–797. doi: 10.1128/aem.62.3.791-797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giammanco A, Maggio M, Giammanco G, Morelli R, Minelli F, Scheutz F, Caprioli A. Characteristics of Escherichia coli strains belonging to enteropathogenic E. coli serogroups isolated in Italy from children with diarrhea. J Clin Microbiol. 1996;34:689–694. doi: 10.1128/jcm.34.3.689-694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 11.Huppertz H I, Rutkowski S, Aleksic S, Karch H. Acute and chronic diarrhoea and abdominal colic associated with enteroaggregative Escherichia coli in young children living in western Europe. Lancet. 1997;349:1660–1662. doi: 10.1016/S0140-6736(96)12485-5. [DOI] [PubMed] [Google Scholar]
- 12.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson R P, Clarke R C, Wilson J B, Read S C, Rhan K, Renwick S A, Shadu K A, Alves D, Karmali M A, Lior H, McEwen S A, Spika J S, Gyles C. Growing concern and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J Food Prot. 1996;59:1112–1122. doi: 10.4315/0362-028X-59.10.1112. [DOI] [PubMed] [Google Scholar]
- 14.Knutton S, Shaw R K, Bhan M K, Smith H R, McConnell M M, Cheasty T, Williams P H, Baldwin T J. Ability of enteroaggregative Escherichia coli strains to adhere in vitro to human intestinal mucosa. Infect Immun. 1992;60:2083–2091. doi: 10.1128/iai.60.5.2083-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine M M, Xu J, Kaper J B, Lior H, Prado V, Nataro J, Karch H, Wachsmuth I K. A DNA probe to identify enterohaemorrhagic Escherichia coli O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987;156:175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- 16.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 17.Nataro J P, Deng Y, Meneval D R, German A L, Martin W C, Levine M M. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect Immun. 1992;60:2297–2304. doi: 10.1128/iai.60.6.2297-2304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nataro J P, Kaper J B, Robins-Browne R, Prado V, Vial P A, Levine M M. Patterns of adherence of diarrheagenic Escherichia coli to Hep-2 cells. Pediatr Infect Dis J. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien A D, Newland J W, Miller S F, Holmes R K, Smith H W, Formal S B. Shiga-like toxin converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 20.Paton A W, Ratcliff R M, Doyle R M, Seymour-Murray J, Davos D, Lanser J A, Paton J C. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like-toxin-producing Escherichia coli. J Clin Microbiol. 1996;34:1622–1627. doi: 10.1128/jcm.34.7.1622-1627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russmann H, Kothe E, Schmidt H, Franke S, Harmsen D, Caprioli A, Karch H. Genotyping of Shiga-like toxin genes in non-O157 Escherichia coli strains associated with hemolytic uremic syndrome. J Med Microbiol. 1995;42:404–410. doi: 10.1099/00222615-42-6-404. [DOI] [PubMed] [Google Scholar]
- 22.Savarino S J, McVeigh A, Watson J, Cravioto A, Molina J, Echevarria P, Bhan M K, Levine M M, Fasano A. Enteroaggregative Escherichia coli heat-stable enterotoxin is not restricted to enteroaggregative E. coli. J Infect Dis. 1996;173:1019–1022. doi: 10.1093/infdis/173.4.1019. [DOI] [PubMed] [Google Scholar]
- 23.Savarino S J, Fasano A, Watson J, Martin B M, Levine M M, Guandalini S, Guerry P. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc Natl Acad Sci USA. 1993;90:3093–3097. doi: 10.1073/pnas.90.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL933. Infect Immun. 1995;63:1055–1061. doi: 10.1128/iai.63.3.1055-1061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt H, Knop C, Franke S, Aleksic S, Heesemann J, Karch H. Development of PCR for screening of enteroaggregative Escherichia coli. J Clin Microbiol. 1995;33:7011–7015. doi: 10.1128/jcm.33.3.701-705.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt H, Plaschke B, Franke S, Russmann H, Schwarzkopf A, Heesemann J, Karch H. Differentiation in virulence patterns of Escherichia coli possessing the eae genes. Med Microbiol Immunol. 1994;183:23–31. doi: 10.1007/BF00193628. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Kaper J B. Cloning and characterization of the eae gene of enterohemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992;6:411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]