Mycetoma is a chronic granulomatous infection of the subcutaneous tissue caused by true fungi or higher bacteria; hence, it is classified as eumycetoma or actinomycetoma, respectively (5). Mycetoma is endemic in (sub)tropical areas, and the Sudan seems to be the mycetoma homeland, with hundreds of new patients attending specialized clinics each year. Mycetoma pathogens such as the fungus Madurella mycetomatis can be found in certain types of soil or as colonizers of plants and show widespread environmental distribution. Infection may be initiated once fungal material is inoculated into the subcutaneous tissues through minor trauma (7). M. mycetomatis is the main cause of eumycetoma, being responsible for approximately 70% of all mycetoma cases in Sudan (2, 6). Mycetoma usually presents as a slowly progressing subcutaneous nodule which increases in size. Multiple secondary nodules may evolve as well. The nodules may suppurate, and characteristic (black) grains as well as purulent material may be discharged through multiple sinus tracts (Fig. 1 and reference 2). Mycetoma is usually painless (4), but in some cases, patients seek medical advice because of persisting pain. The pain may be produced by bone invasion or it may be due to secondary bacterial infection (SBI) (3). The latter is diagnosed upon bacterial cultures positive for potentially pathogenic species other than the microorganisms causing the mycetoma and obtained from deep within the sinus tracts. Little is known about the incidence of SBI in mycetoma, the organisms involved, and its potential contribution to morbidity.
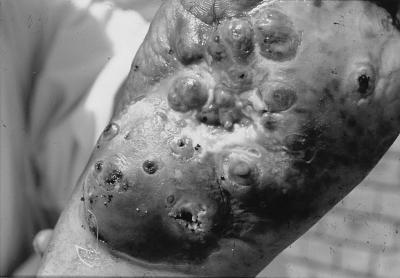
FIG. 1.
Mycetoma lesions on the sole of a foot. The picture clearly shows the severity of the lesions, which rendered the patient invalid. Closed as well as open sinuses are visible. Note the shedding of purulent material and the characteristic black grains consisting of fungus from the open sinuses.
We, therefore, prospectively studied SBIs in mycetoma patients in the Sudan. Specimens were collected from 98 consecutive patients attending the Mycetoma Research Centre, Soba University Hospital, University of Khartoum, Sudan. The mean age of the patients was 23 years (range, 15 to 70 years), and 77 males and 21 females were included, of whom 89 had eumycetoma and 9 had actinomycetoma. Feet and legs were the limbs most frequently involved (97%). All patients were receiving treatment, which in our clinic consists of ketoconazole for eumycetoma and a combination of streptomycin and dapsone for actinomycetoma, both prior to eventual surgery. Most of the patients (78 [80%]) were in the active stage of the disease.
For isolation of bacteria involved in SBI, sterile cotton swabs were inserted deeply into the sinus tracts. In patients with closed, inactive fistulae, clinical material was obtained by percutaneous fluid aspiration from the lesion. In some cases, sinuses were opened with a sterile hypodermic needle prior to deep insertion of a cotton swab. All swabs were inoculated directly onto 5% blood agar, MacConkey agar, and mannitol salt agar plates, which were incubated aerobically at 37°C for 24 h. It appeared that 62 patients (63%) had SBI caused by one or more of the following potentially pathogenic microorganisms: 39 (56%) isolates were Staphylococcus aureus, 24 isolates (34%) were Streptococcus pyogenes, and 7 isolates (10%) were Proteus mirabilis. All S. aureus strains were sensitive to amoxicillin-clavulanic acid combination (Augmentin) and resistant to penicillin, whereas all S. pyogenes isolates were sensitive to both antibiotics. Some of the P. mirabilis strains were multidrug resistant, which included some aminoglycosides and broad-spectrum cephalosporins. Overall, SBI was identified in 59 of 89 of all patients suffering from eumycetoma versus 3 of 9 of the patients with actinomycetoma (P = 0.06). It was also established that patients with closed inactive sinuses are less likely than patients with open sinuses to have SBI in their lesions (P < 0.05).
Our findings indicate that most of the patients with mycetoma, especially those with eumycetoma, are susceptible to SBI in their lesions. These results contradict the previous impression that the sinuses’ discharge is usually sterile due to antibiotics produced by the fungi (1). Upon testing the M. mycetomatis strains for antibiotic production, no such inhibitory compounds to which standard clinically relevant bacterial species were susceptible were detected (data not shown). S. aureus appeared to be the most-common pathogen in this type of infection. Although the number of patients with actinomycetoma included in the present study is small, SBI might be less common in this group because these patients receive streptomycin as part of antimicrobial therapy.
In conclusion, a high prevalence of secondary bacterial infection has been found in lesions of patients suffering from various types of mycetoma. SBI appears to be a serious problem in mycetoma. It may cause pain and increased disability. Moreover, some superinfected patients develop concomitant bacteremia or septicemia which incidentally results in death (7). The clinical significance of our present findings with respect to patient management needs to be assessed in future case control studies.
REFERENCES
- 1.Fahal A H, El Hassan A M. Mycetoma. Br J Surg. 1992;79:1138–1141. doi: 10.1002/bjs.1800791107. [DOI] [PubMed] [Google Scholar]
- 2.Fahal A H, Suliman S H. Clinical presentation of mycetoma. Sudan Med J. 1994;32:46–66. [Google Scholar]
- 3.Fahal A H, Sharfi A R, El Hassan A M, Mahgoub E S. Internal fistula formation: an unusual complication of mycetoma. Trans R Soc Trop Med Hyg. 1996;90:550–552. doi: 10.1016/s0035-9203(96)90318-1. [DOI] [PubMed] [Google Scholar]
- 4.Fahal A H, El Toum E A, Gumaa S A, Mahgoub E S, El Hassan A M. A preliminary study on the ultrastructure of Actinomadura pelletieri and its host tissue reaction. J Med Vet Mycol. 1994;32:343–348. [PubMed] [Google Scholar]
- 5.Fahal A H, El Toum E A, Gumaa S A, Mahgoub E S, El Hassan A M. Host tissue reaction to Madurella mycetomatis: new classification. J Med Vet Mycol. 1995;33:15–17. [PubMed] [Google Scholar]
- 6.Gumaa S A. The aetiology and epidemiology of mycetoma. Sudan Med J. 1994;32:14–22. [Google Scholar]
- 7.Magana M. Mycetoma. Int J Dermatol. 1984;23:221–236. doi: 10.1111/j.1365-4362.1984.tb01238.x. [DOI] [PubMed] [Google Scholar]