Abstract
Variations in the dietary Ca concentration may affect inositol phosphate (InsP) degradation, and thereby, P digestibility in pigs. This study assessed the effects of dietary Ca concentration and exogenous phytase on InsP degradation, nutrient digestion and retention, blood metabolites, and microbiota composition in growing pigs with ileal cannulation. In a completely randomized row–column design with four periods, eight ileal-cannulated barrows (initial body weight 27 kg) were fed four corn–soybean- and rapeseed meal-based diets containing 5.5 or 8.5 g Ca/kg dry matter (DM), with or without 1,500 FTU of an exogenous hybrid-6-phytase/kg diet. No mineral P was added and the P concentration in the feed was 4.8 g P/kg DM. Prececal InsP6 disappearance in pigs fed diets containing exogenous phytase was lower (P = 0.022) with additional Ca than without. Concentrations of InsP2-4 isomers and myo-inositol in the distal ileal digesta and prececal P digestibility were greater (P < 0.001) with exogenous phytase than without exogenous phytase. In feces, InsP6 disappearance was lower (P < 0.002) and concentration of InsP5 and InsP4 isomers was higher (P ≤ 0.031) with additional Ca compared to without additional Ca. The prececal amino acid digestibility, energy digestibility, and hindgut disappearance of energy did not differ. The Shannon diversity index of the microbiota in the distal ileal digesta and feces was similar among the diets but was lower in the distal ileal digesta than in the feces (P < 0.001). Permutation analysis of variance revealed no dietary differences between the bacterial groups within the ileal digesta and fecal samples (P > 0.05). In conclusion, additional Ca reduced the effect of exogenous phytase on prececal InsP6 degradation. Endogenous InsP degradation was impaired by additional Ca only in the hindgut but the abundance of bacterial genera in feces was not affected.
Keywords: calcium, exogenous phytase, inositol phosphate, microbiota, phosphorus, pig
Increasing the dietary calcium concentration reduces the effects of exogenous phytase on prececal phytate degradation and endogenous phytate degradation in the hindgut of growing pigs.
Introduction
Phytate is the salt of phytic acid (myo-inositol 1,2,3,4,5,6-hexakis [dihydrogen phosphate]s; InsP6) and is the main storage form of phosphorus (P) in plant feed ingredients (Eeckhout and Paepe, 1994). The release of InsP6–P depends on exogenous and plant intrinsic phytases in the feed because intestinal endogenous phytase activity is very low in pigs (Hu et al., 1996). However, InsP6 disappearance from the digestive tract may be reduced by the addition of Ca for different reasons. First, with more Ca in the diet, more Ca is available in the gastrointestinal tract for complexing with InsP6, whereby InsP6 hydrolysis progresses less readily (Wise and Gilburt, 1981). Second, dietary Ca concentration generally varies with limestone addition, which is associated with a high acid binding capacity as it takes 15 ± 2 mEq of 0.1 M HCl to lower 1 g of limestone to pH 3 (Lawlor et al., 2005). This is crucial, as Ca–InsP6 complexes are considered insoluble at an intestinal pH greater than 5 (Selle et al., 2009), thus, chelated InsP6 is no longer available for hydrolysis by phytase. Lastly, surplus Ca2+ ions may compete directly with the active site of the phytase (Qian et al., 1996).
The concentrations of Ca and P in the feed may also affect the microbial composition and bacterial activity in the gastrointestinal tract of pigs (Metzler-Zebeli et al., 2011, 2013). However, few studies have examined the interactions between dietary Ca concentration and exogenous phytase in the gastrointestinal tract of pigs. Hu et al. (2023) observed an effect of Ca on InsP6 disappearance, irrespective of the presence of exogenous phytase, whereas Sandberg et al. (1993) observed no effect of dietary Ca on InsP6 disappearance by the end of the ileum, but rather, in the feces when diets without exogenous phytase were fed.
Therefore, the objectives of this study were to investigate the effects of dietary Ca concentration and exogenous phytase on 1) InsP degradation, nutrient digestibility and retention, blood metabolites, and 2) microbial composition in the ileal digesta and feces of pigs, as well as the concentration of volatile fatty acids (VFA) in the feces. We hypothesized that the effects of exogenous phytase on InsP degradation, nutrient digestibility, and microbial groups in the digesta and feces are dependent on the Ca concentration of the feed.
Materials and Methods
The experiment was approved by the Regierungspräsidium Stuttgart, Germany, in agreement with the German Animal Welfare Regulations (approval No. 35-9185.81/0494). The care of the animals throughout the trial was in accordance with Directive 2010/63/EU (European Parliament and the Council of the European Union, 2010).
Experimental diets
The experimental diets followed a 2 × 2 factorial arrangement with two dietary Ca concentrations achieved by limestone addition (6 and 14 g/kg diet) and two inclusion levels of exogenous 6-phytase (0 and 1,500 FTU/kg diet, added on top of the mix; Table 1). A hybrid-6-phytase (Natuphos E; Rychen et al., 2017) was used. The inclusion of 6 g limestone/kg diet targeted a Ca:digestible P ratio of 2:1 in the diet (assuming a P digestibility of 60% when exogenous phytase is present; Gesellschaft für Ernährungsphysiologie (GfE, 2008), whereas the inclusion of 14 g limestone/kg diet was chosen to target a Ca:digestible P ratio of 3:1. The inclusion of 8 g/kg diet of diatomaceous earth balanced the mass differences between the dietary Ca concentrations. All the diets were based on corn, soybean meal, and rapeseed meal. Titanium dioxide was used as an indigestible marker. Mineral P was not added to the diets.
Table 1.
Ingredient composition of the experimental diets
| Dietary Ca, g/kg DM | 5.5 | 8.5 | |||||
|---|---|---|---|---|---|---|---|
| Exogenous phytase, FTU/kg of diet | 0 | 1,500 | 0 | 1,500 | |||
| Ingredients, g/kg as-fed | |||||||
| Corn1 | 591 | ||||||
| Soybean meal1 | 250 | ||||||
| Rapeseed meal1 | 100 | ||||||
| Soybean oil | 20 | ||||||
| P-free mineral and vitamin premix2 | 20 | ||||||
| Titanium dioxide | 5 | ||||||
| Limestone | 6 | 14 | |||||
| Diamol3 | 8 | — | |||||
| Exogenous phytase, FTU/kg of diet | 0 | 1,500 | 0 | 1,500 | |||
1Analyzed concentration of nutrients is described in Supplementary Table S1.
2BASU Mineralfutter GmbH, Bad Sulza, Germany; provided the following per kilogram of diet: Ca, 0.88 g; Na, 1.0 g; Mg, 200 mg; Fe, 80 mg (iron sulfate); Mn, 50 mg (manganese oxide and sulfate); Zn, 60 mg (zinc oxide and sulfate); Cu, 10 mg (copper sulfate); I, 1.34 mg (calcium iodate); Se, 0.26 mg (sodium selenite); vitamin A, 7,000 IU; vitamin D, 1,000 IU; vitamin E; 80 mg; vitamin K, 1.0 mg; vitamin B1, 1.0 mg; vitamin B2, 3.1 mg; vitamin B6, 2.5 mg; vitamin B12, 20µg; niacin, 12.5 mg; pantothenic acid, 8.0 mg; folic acid, 0.4 mg; biotin, 0.08 mg; choline chlorides, 160 mg.
3Provided by BASU Mineralfutter GmbH, Bad Sulza, Germany.
Animals, experimental design, sample collection, and preparation
Eight barrows (initial BW 27.3 ± 1.1 kg; final BW 59.0 ± 2.4 kg; German Landrace × Piétrain) were obtained from the Agricultural Research Station, Unterer Lindenhof (Eningen unter Achalm, Germany) of the University of Hohenheim. They were kept individually in stainless steel metabolism units (1.5 × 0.8 × 1.0 m). Room temperature was controlled at 22.0 ± 0.5 °C. The pigs were surgically fitted with a simple T-cannula in the distal ileum (Li et al., 1993). Pigs were fed three times the estimated energy requirement for maintenance (440 kJ ME/kg0.75 BW; GfE, 2008) per day, provided in two equal meals at 0715 and 1915 hours. The feed was mixed with water (1:1; w:w) immediately before feeding and ingested within 15 min. Feed residues in the trough were rare and were collected and frozen for subsequent analysis.
The experiment was arranged in a completely randomized row–column design with eight pigs, four diets, and four periods to achieve eight replicates per diet. The experimental periods of 12 d each consisted of a 5-d dietary adaption, followed by a 4-d feces and urine collection, 2 d of ileal digesta collection, and 1 d of blood collection. Feces were collected from the floor by hand immediately after defecation. Urine was collected by placing a clean bucket under the pig as soon as the pig started urinating. Two persons were present in the barn during day time to ensure the start of urination was noticed. A subsample of each defecation was taken with a sterile spatula for microbiota analysis and was immediately frozen at −18 °C. Ileal digesta were collected between 0715 and 1915 hours by attaching plastic bags to the open barrel of the cannula with rubber bands, as described by Rosenfelder-Kuon et al. (2020). A subsample of each filled plastic bag was taken by pouring a few milliliters of ileal digesta into a sterile container for microbiota analysis. Samples were frozen immediately at −18 °C. On the last day of each period, a blood sample was obtained from each pig via venipuncture 4 h after the morning meal, as described by Klein et al. (2021).
The ileal digesta, feces, and urine subsamples were pooled for each pig and period. The ileal digesta and feces were lyophilized (Delta 1-24 LSC, Martin Christ Gefriertrocknungsanlagen, Osterode am Harz, Germany). Ingredients, diets, lyophilized feces, and ileal digesta were ground through a 0.5-mm sieve (Ultra-Zentrifugalmühle ZM 200, Retsch, Haan, Germany) and pulverized using a vibrating cup mill (Pulverisette 9, Fritsch, Idar-Oberstein, Germany) or a mixer mill (MM 400, Retsch, Haan, Germany).
Chemical analyses
Dry matter, crude nutrients, and gross energy (GE) were determined in the feed ingredients, diets, feces, and ileal digesta using standard assays (VDLUFA, 2007). Concentrations of total P, Ca, and Ti in diets, feces, and digesta, and total P and Ca in urine were analyzed using the modified sulfuric and nitric acid wet digestion method (Boguhn et al., 2009), followed by measurement using an inductively coupled plasma optical emission spectrometer (Vista Pro, Varian Inc., Australia). The concentrations of K in the diet, feces, and urine were analyzed accordingly, but measured at a wavelength of 766 nm. Inorganic P (Pi) and Ca in blood serum were analyzed as described by Sommerfeld et al. (2018a). Activity of alkaline phosphatase (ALP) was determined by measuring the formation of p-nitrophenol from p-nitrophenyl phosphate in the presence of 2-amino-2-methyl-1-propanol. Blood urea nitrogen (BUN) was measured using a Beckman Olympus AU480 instrument (Beckman Coulter, Krefeld, Germany), based on an adaptation of the enzymatic method described by Talke and Schubert (1965). Blood analyses were conducted by IDEXX BioAnalytics (Kornwestheim, Germany).
The concentrations of InsP6 and InsP3-5 isomers in the feed ingredients, diets, feces, and digesta were determined according to the method of Zeller et al. (2015), with modifications as described by Sommerfeld et al. (2018b). For the extraction of InsP1-2 isomers, a buffer containing 50 mM Tris, 50 mM glycine, and 0.2 M sodium fluoride at a pH of 9 was used. All InsP were measured using high-pressure ion chromatography (ICS-3000 system, Dionex, Idstein, Germany). Because the Ins(1,2,6)P3, Ins(1,4,5)P3, and Ins(2,4,5)P3 isomers co-elute in this measurement, they were summarized as InsP3x. The results did not differentiate between the d- and l-forms of the measured InsP. Myo-inositol was determined using a gas chromatograph/mass spectrometer (7890B/5977A, Agilent Technologies, Waldbronn, Germany) as described by Sommerfeld et al. (2018b). The phytase activity of the feed was analyzed according to ISO EN 30024 (2009). Diets and ileal digesta were analyzed for amino acids (AA; Rodehutscord et al., 2004) using an L-8900 Amino Acid Analyzer (VWR, Hitachi, Tokyo, Japan), following sample oxidation and acid hydrolysis. The content of N-acetylneuraminic acid (Neu5Ac), a marker of mucin synthesis (Mesina et al., 2019), was determined using high performance anion exchange chromatography with pulsed amperometric detection. Mucin was extracted from ileal digesta samples with 0.15 M NaCl solution and cleaned up by repeated overnight precipitation with ethanol at −18 °C, following a protocol adapted from Lien et al. (1997). After clean-up, mucin was hydrolyzed to obtain Neu5Ac, according to Hurum and Rohrer (2011), by incubation for 3 h at 80 °C in 2 M acetic acid. The supernatant was filtered through a 0.2-µm polyamide syringe filter and diluted 1:25 for measurements. Neu5Ac measurements were conducted on a Dionex ICS 5000 + system (Thermo Fisher Scientific, Waltham, MA, USA), using a CarboPac MA1 column and an isocratic eluent of 250 mM sodium acetate in 100 mM sodium hydroxide. Quantification was performed using an external five-point-calibration of Neu5Ac standards at concentrations between 0.1 and 10 mg/L. The VFA concentrations in the feces were determined using ultrapure water-diluted samples by using vacuum distillation and gas chromatography (Hewlett-Packard 6890; Agilent Technologies), as described by Wischer et al. (2013).
DNA extraction, illumina amplicon sequencing, and data analysis
The DNA was extracted from 0.25 g of each ileal digesta and fecal sample using FastDNA SPIN Kit for Soil (MP Biomedical, Solon, OH, USA) with some adjustments (Burbach et al., 2016). The DNA was quantified with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific) and stored at −20 °C. Amplification of the V1 to V2 region of the 16S rRNA gene was performed according to the method described by Kaewtapee et al. (2017). Amplicons were verified by agarose gel electrophoresis, purified, and normalized using the SequalPrep Normalization Kit (Invitrogen, Carlsbad, CA, USA). Samples were sequenced using 250 bp paired-end sequencing chemistry on an Illumina NovaSeq 6000 (Illumina Solutions, Berlin, Germany).
Calculations
Prececal (pc) InsP6 disappearance and digestibilities of nutrients and GE were calculated as follows:
| (1) |
where y(X) is the disappearance or digestibility of X (%) and X is the concentration of InsP6, DM, nitrogen (N), AA, Ca, P (g/kg), or GE (MJ/kg). The corresponding values for the total digestive tract were calculated using the fecal concentrations.
Hindgut disappearance of InsP6, P, Ca, and N (g/kg) was calculated as follows:
| (2) |
where y(X) is the hindgut disappearance of X in g/kg or MJ/kg of feed, and XTT and Xpc are the total tract or pc disappearance of N, Ca, P, InsP6 (g/kg) and GE (MJ/kg) of feed.
The amount of excreted urine (kg/d) was calculated as follows:
| (3) |
where KTT is the total tract digested amount of K in g/d; KA is the assumed accretion of K by the pig in g/d, according to GfE (2008) (1.9 g/kg BW gain) and KU is the analyzed K concentration in the urine in g/kg. It was assumed that the difference between KTT and KA must represent the amount of K excreted via the urine.
The urinary excretion of P, Ca, myo-inositol, and N was calculated as the product of the measured concentration in the urine and the amount of urine calculated by Equation 3. The retained quantities of Ca and P were calculated as the difference between the intake and excretion of the respective elements in the feces and urine.
Statistical analysis
Data were analyzed using a 2 × 2 factorial analysis of variance using the MIXED procedure in SAS (version 9.3; SAS Institute Inc., Cary, NC, USA). The model was
| (4) |
where yijkl is the response variable, µ is the overall mean, a is the fixed effect of dietary Ca concentration (i = 5.5 or 8.5 g/kg DM), b is the fixed effect of exogenous phytase inclusion (j = 0 or 1,500 FTU/kg), (ab)ij is the fixed interaction effect between the ith dietary Ca concentration and the jth exogenous phytase inclusion, c is the random effect of the animal (k = 1 to 8), τ is the random effect of the period (l = 1 to 4), and eijkl is the residual error.
The InsP6 disappearance and nutrient digestibility data were logit-transformed, and concentrations in the digesta, feces, urine, and blood were log- or square-root-transformed to meet the assumptions of variance homogeneity and normality. If the F-test results were significant, a multiple t-test was used for pairwise treatment comparisons. All results are presented via a letter-based display as the least-squares means of the untransformed data. Statistical significance was set at P ≤ 0.05.
Bioinformatics assembly and alignment of raw sequencing reads were performed using the MOTHUR pipeline (Kozich et al., 2013). UCHIME was used to identify possible chimeras (Edgar et al., 2011). The amplicon sequence variants (ASV) were identified using the SILVA database (Release 138.1). For unclassified ASV, the nearest representative was manually detected using seqmatch from the Ribosomal Database Project (Cole et al., 2014). Only ASV with an average relative abundance > 0.0001% and >250 bp were used for further analyses. One fecal sample was excluded from further analysis because of a lower number of reads. The Shannon diversity index was calculated to evaluate bacterial diversity within the samples using the vegan package in R (version 4.0.0; 2020-04-24).
After the sequencing reads were standardized using total reads, a sample dissimilarity matrix was constructed using the Bray–Curtis dissimilarity coefficient. This was subjected to a permutation analysis of variance (PERMANOVA) using the Adonis function to compare the microbial communities associated with diets within sample types (digesta and feces) as well as between digesta and fecal samples.
To visualize the ordination and clustering of the samples, nonmetric multidimensional scaling (NMDS) was plotted using the R package, ggplot2. The Wilcoxon test followed by the Benjamini–Hochberg procedure was conducted to analyze differences in abundance at the phylum and genus levels between both sample types. Spearman’s correlation coefficients between fecal genera with an average relative abundance > 1% and items from hindgut disappearance, total tract digestibility, and VFA concentrations were calculated after the determination of the z-scores for standardization. Visualization was performed using the R package, svglite. Statistical significance for all results was set at P ≤ 0.05. Sequences were submitted to the European Nucleotide Archive under the accession number, PRJEB61474.
Results
The Ca concentration ranged from 5.4 g/kg DM in the diets without additional Ca to 8.4 and 8.6 g/kg DM in diets with additional Ca (Table 2). The analyzed total P concentration was 4.8 g/kg DM in all diets, whereof 3 g was bound in InsP5-6 provided by corn (45%), followed by soybean (30%), and rapeseed meal (25%; Supplementary Table S1). The results confirmed that intrinsic plant phytase activity in the diets without phytase was not detectable (<50 FTU/kg diet), and phytase activity in diets with exogenous phytase ranged from 1,470 to 1,620 FTU/kg. Analyzed AA concentrations of the diets are shown in Supplementary Table S2 and exceeded the formulated values of indispensable AA between 0.6 g/kg DM (Met) and 3.6 g/kg DM (Leu).
Table 2.
Analyzed chemical composition of the experimental diets (g/kg DM unless otherwise stated)
| Dietary Ca, g/kg DM | 5.5 | 8.5 | ||
|---|---|---|---|---|
| Exogenous phytase, FTU/kg of diet | 0 | 1,500 | 0 | 1,500 |
| DM, g/kg | 896 | 895 | 900 | 897 |
| GE, MJ/kg DM | 19.2 | 19.1 | 19.0 | 19.0 |
| CP (nitrogen × 6.25) | 229 | 222 | 220 | 222 |
| Ether extract | 34 | 33 | 34 | 34 |
| aNDFom1 | 128 | 130 | 127 | 125 |
| ADFom2 | 58 | 60 | 61 | 59 |
| ADL | 20 | 17 | 18 | 15 |
| Crude fiber | 36 | 37 | 37 | 37 |
| Crude ash | 73 | 75 | 69 | 72 |
| Calcium | 5.4 | 5.4 | 8.6 | 8.4 |
| Total phosphorus (P) | 4.8 | 4.8 | 4.8 | 4.8 |
| InsP6–P | 2.9 | 2.9 | 2.8 | 2.7 |
| InsP6, µmol/g DM3 | 15.5 | 15.5 | 14.9 | 14.8 |
| Ins(1,2,4,5,6)P5, µmol/g DM | 1.1 | 1.1 | 1.0 | 1.0 |
| Ins(1,2,3,4,5)P5, µmol/g DM | 0.6 | 0.5 | 0.6 | 0.6 |
| Myo-inositol, µmol/g DM | 1.1 | 1.1 | 1.1 | 1.1 |
| Phytase activity, FTU/kg | <50 | 1,470 | <50 | 1,620 |
1aNDFom, neutral detergent fiber assayed with heat-stable amylase and expressed exclusive of residual ash.
2ADFom, acid detergent fiber expressed exclusive of residual ash.
3Inositol phosphate (InsP) isomers not mentioned in the table were not detectable or below limit of quantification.
The pc InsP6 disappearance was increased with supplemental phytase, but this increase was lower with additional Ca than without additional Ca (22.7% to 84.8% vs. 21.9% to 89.6%), implying a interaction (P < 0.022; Table 3). Total tract InsP6 disappearance was lower (P = 0.002) with additional Ca than without additional Ca and higher (P = 0.023) with supplemented phytase than without it. The hindgut disappearance of InsP6 was lower with additional Ca in diets without added phytase, but not in diets with added phytase (P = 0.045). The pc and total tract digestibility of P and Ca were greater (P < 0.001) with phytase than without phytase. Urinary P excretion was greater with phytase supplementation only in the diet without additional Ca (P < 0.001). Urinary Ca excretion was greater (P < 0.001) with additional Ca than without additional Ca, but lower (P < 0.001) with phytase than without phytase. The Ca retention was greater with additional Ca (P = 0.004) and phytase supplementation (P < 0.001) than without. The calculated disappearance of P and Ca from the hindgut did not differ. The digestibility and hindgut disappearance of GE also did not differ among the diets (Table 4). Total tract N digestibility was greater (P ≤ 0.019) with phytase than without phytase; however, N excretion in urine and N retention by pigs were similar. The pc digestibility of AA did not differ among the diets (Supplementary Table S3). Fecal VFA concentrations did not differ among the diets, except for isobutyric acid and isovaleric acid, which were greater with additional Ca (P ≤ 0.037; Supplementary Table S4).
Table 3.
Prececal, total tract, and hindgut InsP6 disappearance, prececal and total tract digestibility, hindgut disappearance (HD), and retention of P and Ca by growing pigs1
| Dietary Ca, g/kg DM | 5.5 | 8.5 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Exogenous phytase, FTU/kg of diet |
0 | 1,500 | 0 | 1,500 | Ca | Phytase | Ca × phytase | |
| InsP6 | ||||||||
| Prececal disappearance, % | 21.9c | 89.6a | 22.7c | 84.8b | 1.42 | 0.068 | <0.001 | 0.022 |
| Total tract disappearance, % | 95.9 | 98.5 | 87.0 | 96.0 | 3.34 | 0.002 | 0.023 | 0.988 |
| HD, µmol/g DM | 11.5a | 1.4c | 9.6b | 1.7c | 0.59 | 0.368 | <0.001 | 0.045 |
| P | ||||||||
| Prececal digestibility, % | 22.9c | 63.5a | 25.9b | 60.1a | 1.37 | 0.840 | <0.001 | 0.009 |
| Total tract digestibility, % | 28.2 | 61.2 | 26.3 | 58.6 | 1.51 | 0.086 | <0.001 | 0.906 |
| HD, g/kg DM | 0.3 | -0.1 | 0.0 | -0.1 | 0.07 | 0.164 | 0.101 | 0.621 |
| Intake, g/d | 6.5 | 6.5 | 6.5 | 6.4 | 0.83 | — | — | — |
| In feces, g/d | 4.6 | 2.5 | 4.8 | 2.7 | 0.47 | 0.054 | <0.001 | 0.792 |
| In urine, mg/d | 17b | 355a | 16b | 18b | 54 | <0.001 | <0.001 | <0.001 |
| Retention, g/d | 1.8 | 3.6 | 1.6 | 3.8 | 0.37 | 0.703 | <0.001 | 0.134 |
| Ca | ||||||||
| Prececal digestibility, % | 48.9c | 72.0a | 49.6c | 64.2b | 2.13 | 0.017 | <0.001 | 0.007 |
| Total tract digestibility, % | 47.3c | 71.2a | 46.9c | 63.9b | 2.62 | 0.024 | <0.001 | 0.038 |
| HD, g/kg DM | -0.1 | 0.0 | -0.2 | 0.0 | 0.10 | 0.662 | 0.166 | 0.645 |
| Intake, g/d | 7.3 | 7.3 | 11.6 | 11.4 | 1.21 | <0.001 | 0.548 | 0.593 |
| In feces, g/d | 3.9 | 2.1 | 6.3 | 4.1 | 0.66 | <0.001 | <0.001 | 0.055 |
| In urine, g/d | 1.6b | 0.3c | 3.0a | 1.7b | 0.21 | <0.001 | <0.001 | 0.002 |
| Retention, g/d | 1.8 | 4.9 | 2.3 | 5.6 | 0.53 | 0.004 | <0.001 | 0.650 |
| Urinary myo-inositol excretion, mg/d | 23 | 38 | 23 | 60 | 15 | 0.004 | <0.001 | 0.440 |
1Least squares means based on eight observations per diet.
a,b,cWithin a row, individual treatment means without a common superscript differ significantly (P < 0.05).
Table 4.
Prececal and total tract digestibility, calculated hindgut disappearance (HD) of GE and N, and N retention by growing pigs1
| Dietary Ca, g/kg DM | 5.5 | 8.5 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Exogenous phytase, FTU/kg of diet |
0 | 1,500 | 0 | 1,500 | Ca | Phytase | Ca × phytase | |
| GE | ||||||||
| Prececal digestibility, % | 68.7 | 69.1 | 69.5 | 69.7 | 0.62 | 0.236 | 0.618 | 0.953 |
| Total tract digestibility, % | 84.6 | 84.9 | 85.1 | 85.4 | 0.38 | 0.079 | 0.236 | 0.999 |
| HD, MJ/kg DM | 3.0 | 3.0 | 3.0 | 3.0 | 0.12 | 0.569 | 0.903 | 0.790 |
| N | ||||||||
| Prececal digestibility, % | 76.0 | 75.9 | 75.1 | 76.2 | 0.68 | 0.617 | 0.448 | 0.377 |
| Total tract digestibility, % | 84.4 | 85.8 | 84.6 | 86.2 | 0.98 | 0.602 | 0.019 | 0.807 |
| HD, g/kg DM | 3.1 | 3.5 | 3.3 | 3.6 | 0.40 | 0.427 | 0.105 | 0.614 |
| Intake, g/d | 49.4 | 47.8 | 47.7 | 48.1 | 6.17 | — | — | — |
| In feces, g/d | 7.6 | 6.7 | 7.3 | 6.5 | 0.67 | 0.372 | 0.009 | 0.784 |
| In urine, g/d | 21.2 | 19.9 | 20.7 | 19.5 | 4.89 | 0.907 | 0.743 | 0.653 |
| Retention, g/d | 20.7 | 21.2 | 19.7 | 22.0 | 1.66 | 0.744 | 0.226 | 0.425 |
1Least squares means based on eight observations per diet.
In the ileal digesta, the concentrations of InsP6, Ins(1,2,3,4,5)P5, and Ins(1,2,4,5,6)P5 were lower (P < 0.001) with phytase, whereas the concentrations of the InsP4 isomers, InsP3x, and myo-inositol were higher (P < 0.001) than those without phytase (Table 5). Ins(1,2)P2 was only detected in the ileal digesta of pigs fed diets containing phytase, and its concentration was lower (P = 0.013) with the addition of Ca. In the feces, the concentrations of InsP6, Ins(1,2,4,5,6)P5, and Ins(1,2,5,6)P4 were higher (P < 0.031) with additional Ca than without. Fecal concentrations of InsP6, Ins(1,2,4,5,6)P5, and myo-inositol were lower (P ≤ 0.015) with phytase than without. The Neu5Ac concentration in the ileal digesta was higher by a trend in pigs fed diets with additional Ca (P = 0.064) than without additional Ca. Urinary myo-inositol excretion was higher (P < 0.001) with phytase than without phytase and higher (P = 0.004) with additional Ca (Table 3).
Table 5.
Concentrations of inositol phosphates (InsP) and myo-inositol in ileal digesta and feces of pigs1
| Dietary Ca, g/kg DM | 5.5 | 8.5 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Exogenous phytase, FTU/kg of diet | 0 | 1,500 | 0 | 1,500 | Calcium | Phytase | Calcium × phytase | |
| Ileal digesta2, µmol/g DM | ||||||||
| InsP6 | 33.3a | 4.5c | 33.1a | 6.6b | 0.59 | 0.025 | <0.001 | 0.023 |
| Ins(1,2,4,5,6)P5 | 2.4 | 0.3 | 2.5 | 0.5 | 0.05 | 0.027 | <0.001 | 0.078 |
| Ins(1,2,3,4,5)P5 | 1.4 | 0.6 | 1.4 | 0.8 | 0.09 | 0.305 | <0.001 | 0.516 |
| Ins(1,2,3,4,6)P5 | 0.7 | n.d. | 0.7 | n.d. | 0.03 | 0.968 | — | — |
| Ins(1,2,5,6)P4 | 0.5 | 1.9 | 0.4 | 2.3 | 0.23 | 0.646 | <0.001 | 0.349 |
| Ins(1,2,3,4)P4 | 0.3 | 2.3 | 0.3 | 2.9 | 0.31 | 0.389 | <0.001 | 0.468 |
| InsP3x3 | 0.5 | 8.1 | 0.4 | 8.1 | 0.59 | 0.880 | <0.001 | 0.856 |
| Ins(1,2)P2 | n.d. | 4.9 | n.d. | 2.9 | 0.63 | 0.013 | — | — |
| Myo-inositol | 1.5 | 7.0 | 1.5 | 7.0 | 0.76 | 0.748 | <0.001 | 0.562 |
| Neu5Ac4, g/kg DM | 0.35 | 0.30 | 0.36 | 0.37 | 0.04 | 0.064 | 0.300 | 0.154 |
| Feces2, µmol/g DM | ||||||||
| InsP6 | 3.7 | 1.4 | 11.6 | 3.8 | 2.89 | 0.004 | 0.015 | 0.487 |
| Ins(1,2,4,5,6)P5 | 0.8 | n.d. | 1.3 | 0.3 | 0.21 | 0.013 | <0.001 | — |
| Ins(1,2,3,4,5)P5 | n.d. | n.d. | 0.4 | 0.2 | 0.15 | — | 0.361 | — |
| Ins(1,2,5,6)P4 | 0.2 | n.d. | 0.3 | n.d. | 0.05 | 0.031 | — | — |
| Myo-inositol | 0.8 | 0.4 | 0.7 | 0.4 | 0.08 | 0.537 | 0.014 | 0.442 |
1Least squares means based on eight observations per diet.
2Inositol phosphate isomers not mentioned here were not detectable (n.d.) or below limit of quantification.
3A clear discrimination was not possible because of co-elution, at least one of the following isomers Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
4 N-acetylneuraminic acid.
a,b,cWithin a row, individual treatment means without a common superscript differ significantly (P < 0.05).
The serum Pi concentration was higher with added phytase than without, and lower with additional Ca in the diet without exogenous phytase, causing an interaction (P = 0.037; Table 6). The serum Ca concentration increased to a greater extent with additional Ca than with exogenous phytase (P < 0.001). The plasma myo-inositol concentration was increased by phytase supplementation (P < 0.001), whereas BUN concentration was reduced by phytase supplementation (P = 0.002). The serum ALP activity in the blood of one pig was consistently higher than the mean of the other pigs by a factor of 2.2. When the data of this pig were disregarded, the serum ALP activity decreased (P = 0.016) with the addition of phytase.
Table 6.
Concentrations of inorganic P (Pi), calcium (Ca), alkaline phosphatases (ALP), urea nitrogen (BUN), and myo-inositol in the blood of pigs1
| Dietary Ca, g/kg DM | 5.5 | 8.5 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Exogenous phytase, FTU/kg of diet | 0 | 1,500 | 0 | 1,500 | Calcium | Phytase | Calcium × phytase | |
| Pi, mmol/L | 2.6b | 3.4a | 2.1c | 3.2a | 0.14 | <0.001 | <0.001 | 0.037 |
| Ca, mmol/L | 3.1b | 3.0c | 3.5a | 3.1b | 0.04 | <0.001 | <0.001 | <0.001 |
| ALP, U/L | 253 | 232 | 237 | 224 | 34.67 | 0.950 | 0.051 | 0.767 |
| ALP2, U/L | 209 | 192 | 219 | 200 | 11.0 | 0.181 | 0.016 | 0.917 |
| Myo-inositol, µg/mL | 9.4 | 17.2 | 8.6 | 17.4 | 0.96 | 0.407 | <0.001 | 0.499 |
| BUN, mg/dL | 21.5 | 17.9 | 19.6 | 18.3 | 2.07 | 0.269 | 0.002 | 0.065 |
1Least squares means based on eight observations per diet.
2Least squares means without one pig that had a 2.2-fold higher ALP activity than the mean of the other pigs.
a,b,cWithin a row, individual treatment means without a common superscript differ significantly (P < 0.05).
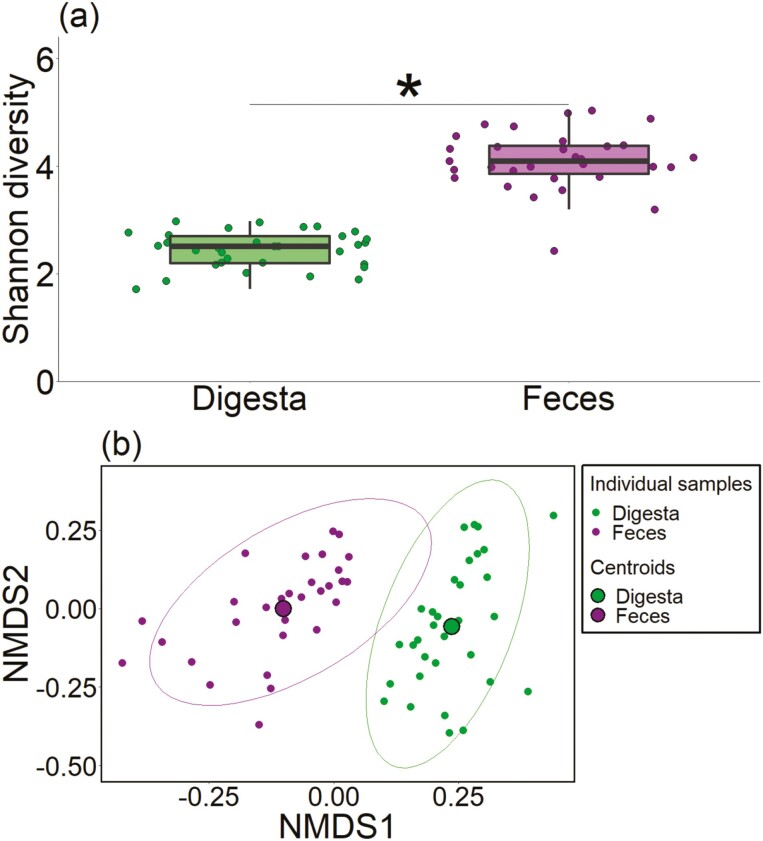
Amplicon sequencing of fecal and ileal digesta samples showed an average of 44,156 ± 3,213 reads per sample, and 2,648 ASV were identified from the reads. The Shannon diversity index in ileal digesta and fecal samples did not differ among the diets. However, the Shannon diversity index was lower in the ileal digesta than in the fecal samples (P < 0.001; Figure 1a). The PERMANOVA revealed no differences in the bacterial community of the ileal digesta among the diets (Supplementary Table S5) but showed a trend for the interaction of additional Ca × phytase in fecal samples (P = 0.09; Supplementary Table S6). Differences were found between the ileal digesta and fecal bacterial communities (P < 0.001; Supplementary Table S7), as indicated by the two distinct clusters in the NMDS plot and the distance between the two centroids (Figure 1b).
Figure 1.
Comparison of the microbial community in the ileal digesta and fecal samples of pigs. (a) Shannon diversity index (*significant difference, P < 0.05), and (b) NMDS plot with centroids per group of samples.s
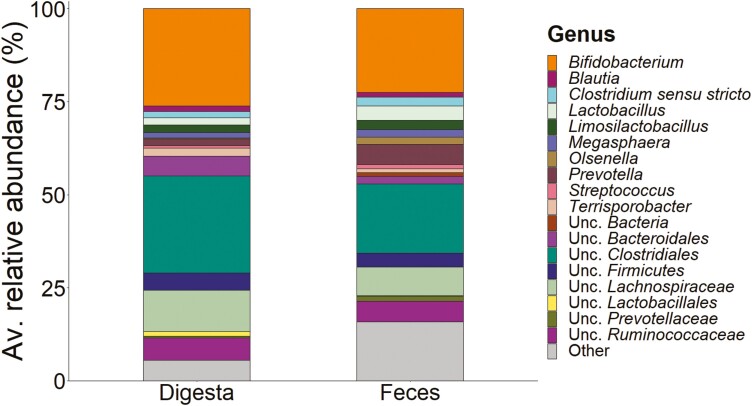
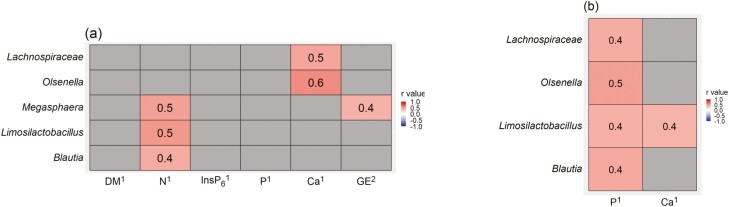
The most abundant phyla in both sample types were Firmicutes (ileal digesta, 64%; feces, 60.2%), followed by Actinobacteria (ileal digesta, 26.8%; feces, 26.2%), and Bacteroidetes (ileal digesta, 8.5%; feces, 11.0%). At the genus level, the most abundant group was Bifidobacterium, with no marked difference between ileal digesta and feces (Figure 2). Terrisporobacter was more abundant in ileal digesta than feces (P < 0.05; Supplementary Table S8), whereas Lactobacillus and Limosilactobacillus were less abundant in ileal digesta than feces (P ≤ 0.016). The relative abundance of Megasphaera (P = 0.012), Limosilactobacillus (P = 0.002), and Blautia (P = 0.021) were positively correlated with N hindgut disappearance (Figure 3a). Megasphaera abundance was positively correlated with GE hindgut disappearance (P = 0.025). The relative abundance of unclassified Lachnospiraceae (P = 0.011) and Olsenella (P < 0.001) were positively correlated with the hindgut disappearance of Ca. Positive correlations with the total tract digestible P were detected for the relative abundance of Olsenella (P = 0.005), Limosilactobacillus (P = 0.016), Blautia (P = 0.098), and unclassified Lachnospiraceae (P = 0.014; Figure 3b). Most correlations found between fecal bacterial abundance and fecal VFA concentration were negative, except for Prevotella and Bifidobacterium (Supplementary Figure S1).
Figure 2.
Comparison of the microbial community in the ileal digesta and fecal samples at genus level (‘Other’ includes genera with a relative abundance of <1%).
Figure 3.
Significant correlations (P < 0.05) between bacteria in the feces (relative abundance > 1%), (a) hindgut disappearance, and (b) total tract digestibility (P < 0.05). 1g/kg DM, 2MJ/kg DM.
Discussion
Inositol phosphate degradation
In the present study, when the feed did not contain added phytase, pc InsP6 disappearance was not affected by the additional Ca. Variations in dietary Ca concentration from 3 to 11 g Ca/kg DM also did not affect pc InsP6 disappearance in pigs fed diets without exogenous phytase (Sandberg et al., 1993). Hu et al. (2023) detected a decrease in InsP6 disappearance when a basal diet without limestone (2 g Ca/kg DM) was added with limestone to a Ca concentration of 6 g Ca/kg DM, but not when further limestone was added to a Ca concentration of 10 g Ca/kg DM. In all these studies, pc InsP6 disappearance was low (≤35%) at the lowest Ca concentration in the feed. This might explain why a further reduction in pc InsP6 disappearance was hardly observed with the addition of Ca. The generally low pc InsP6 disappearance in pigs fed diets without phytase has often been reported in the literature (Rodehutscord et al., 2022) and is most likely related to low endogenous phytase activity in the small intestine (Hu et al., 1996; Yi and Kornegay, 1996). Phytase added to a diet with a low Ca concentration increased pc InsP6 disappearance to 90% in the present study, which confirmed the level found in phytase-supplemented diets in previous studies (Mesina et al., 2019; Lu et al., 2020; Rosenfelder-Kuon et al., 2020). A reduction in InsP6 disappearance by 5 percentage points in diets with phytase was found with additional Ca in the present study. Hu et al. (2023) also observed the reducing effect of additional Ca. The pH optima of the used phytase in both studies suggest a notable InsP6 degradation in the stomach (Greiner, 2021; Menezes-Blackburn et al., 2022). Insoluble Ca–InsP6 complexes precipitate at pH 5.4 (Kaufman and Kleinberg, 1971). Thus, most of the InsP6 might be hydrolyzed by the exogenous phytases in the stomach before insoluble complexes are formed at a decisive pH in the small intestine. Nevertheless, the addition of Ca by limestone increased the pH in the stomach from 3.8 to 4.1 (Hu et al., 2023). A shift in stomach pH away from the phytase optimum cannot be ruled out in the present study and may explain the reduction in InsP6 disappearance with additional Ca in the phytase-containing feed.
Consistent with previous studies, phytase addition in the present study increased the concentration of partially dephosphorylated InsP in the distal ileum digesta (Rosenfelder et al., 2020; Hu et al., 2023). The higher concentration of Ins(1,2,3,4,5)P5 compared to other InsP5 isomers in the presence of phytase confirmed its classification by Rychen et al. (2017) as a 6-phytase. The Ins(1,2,3,4,6)P5 isomer was detected only in the ileal digesta of pigs fed diets without phytase. Thus, mucosal phytase activity in pigs, although low overall, may include 5-phytase, as it has been previously suggested for laying hens (Sommerfeld et al., 2020a, 2020b) and gnotobiotic broiler chickens (Sommerfeld et al., 2019). Hybrid phytase used in the present study increased the concentrations of Ins(1,2,3,4)P4 and Ins(1,2,5,6)P4 in the distal ileum digesta, which is consistent with the results of other studies that used this phytase (Krieg et al., 2020). In studies that used an Escherichia coli 6-phytase, only Ins(1,2,5,6)P4 increased in the ileal digesta of pigs (Rosenfelder-Kuon et al., 2020). In contrast, the InsP3x concentration increased upon phytase addition to a greater extent in the present study than in the study by Rosenfelder-Kuon et al. (2020). These differences in the distal ileum digesta indicate that the two 6-phytases differ in their degradation pathways. The concentration of Ins(1,2)P2 in the ileal digesta was lower with additional Ca in the phytase-supplemented diet. This is likely a consequence of the greater InsP6 concentration in the ileal digesta of pigs fed the respective diets. If the initial dephosphorylation step is impaired, less Ins(1,2)P2 is produced at the end of the ileum. This was consistent with the higher concentrations (although not significant) of all intermediate dephosphorylation products.
The myo-inositol concentration was markedly increased by adding exogenous phytase in ileum digesta and blood plasma of pigs. A similar effect has been reported in previous studies on pigs and poultry (Mesina et al., 2019; Lu et al., 2020; Klein et al., 2021; Novotny et al., 2023). Urinary myo-inositol excretion was also increased by phytase. Rats fed myo-inositol-deficient diets exhibited reduced urinary myo-inositol concentrations (Burton et al., 1976). The present study suggests that the increased release of myo-inositol caused by exogenous phytase might increase the overall myo-inositol pool in the animal, providing a plausible explanation for the observed increase in urinary and plasma myo-inositol levels. Myo-inositol excretion in urine was higher with additional Ca carbonate. However, the calculated myo-inositol excretion of one individual fed additional Ca carbonate with phytase was four times higher than the LSMean calculated for this diet (213 vs. 60.1 mg/d).
Results of fecal InsP6 disappearance indicated that microbial InsP6 breakdown in the large intestine was impaired by the additional Ca in the feed. This is consistent with the results of Sandberg et al. (1993) and might be attributed to Ca–InsP6 complexes formed along the gastrointestinal tract, which would remain insoluble in the large intestine because the physiological pH is above 6.0 (Merchant et al., 2011). The fecal concentrations of Ins(1,2,4,5,6)P5 and Ins(1,2,5,6)P4 were similarly affected but to a lesser extent than that of InsP6. The diminishing effect of Ca with each further hydrolysis step of InsP concurs with in vitro results of Kaufman and Kleinberg (1971). They observed a decreased capacity of lower InsP molecules to form insoluble Ca–InsP complexes under physiological pH conditions. The effects of Ca on fecal InsP concentrations were not related to variations in the relative abundance of any of the genera detected in this dataset. This might imply that the variable release of InsP6–P among the diets was not decisive for the abundance of bacteria in the large intestine.
Mineral digestibility and mineral balance
In diets with phytase, Ca addition caused a reduction in pc digestible P by 0.2 g/kg DM (dietary P concentration multiplied by pc P digestibility). Although this effect was not significant, it reflects the reduction in pc InsP6–P disappearance, which was 0.3 g/kg DM (dietary InsP6–P concentration multiplied by InsP6 disappearance). Most of the difference of 0.1 g/kg DM may reflect P contained in partially degraded InsP and therefore remained indigestible to the pig (Rodehutscord and Bikker, 2022). Similarly, additional Ca did not affect the absorption of P released from InsP6, as observed by Hu et al. (2023).
Except for the diet without additional Ca and with phytase, the urinary excretion of P was approximately 17 mg P/d. This value was close to the estimated urinary P loss of 0.35 mg/kg BW/d (or 17.5 mg P/d for a pig with 50 kg BW), which was considered inevitable for growing pigs by Rodehutscord et al. (1998). This indicates that the absorbed P was completely utilized for P retention by the pigs in the present study, and P supply was the limiting factor for retention. However, in the diet without additional Ca and with phytase, urinary P excretion increased by a factor of 20. In addition, serum Pi concentration increased with this diet, indicating that the amount of Ca absorbed was insufficient to retain the P that became digestible upon phytase addition, although the Ca digestibility increased to 72% and Ca excretion in urine decreased to 0.3 g/d.
The Ca intake in diets with additional Ca increased by 4.2 g/d compared to diets without additional Ca, of which 2.2 and 1.4 g/d were excreted in feces and urine, respectively, leaving 0.5 to 0.7 g/d of Ca additionally retained compared to diets without additional Ca. This confirmed that absorption, endogenous secretion, and urinary excretion are involved in Ca homeostasis (Schröder and Breves, 2006). When phytase was added to the diet with additional Ca, a further increase in Ca retention of 0.7 g/d was observed compared to the phytase diet without additional Ca. Likewise, this reflects the effect of increased P digestibility and retention causing an increased metabolic demand of Ca for bone formation. Except for the treatment without phytase and without additional Ca, the ratio of Ca retention and P retention was in a narrow range of 1.36 to 1.47. Differences in serum Ca concentrations coincided with differences in urinary Ca excretion, which is consistent with the results of Schlegel and Gutzwiller (2017). The serum ALP activity was decreased with the addition of phytase, which is consistent with the study of Kiarie et al (2022), who observed a decrease in plasma ALP activity in nursery pigs with the addition of exogenous phytase to a low-P corn-based diet. The calculated hindgut disappearance of P and Ca did not differ, and the values were close to zero, indicating no net absorption of these minerals in the hindgut. Although the release of P from InsP6 in the hindgut was remarkable in diets without phytase, the released P was not absorbed, which is consistent with the results of previous studies (Schlemmer et al., 2001; Rutherford et al., 2014; Mesina et al., 2019; Rosenfelder-Kuon et al., 2020; Klein et al., 2021).
Nitrogen balance, amino acid digestibility, and sialic acid
Pigs fed diets without phytase exhibited greater fecal N excretion. This suggests that when more P enters the hindgut, bacterial cell replication and metabolism increase, leading to increased N incorporation into microbial proteins, which are subsequently excreted (Metzler and Mosenthin, 2008). In addition to greater fecal N excretion, pigs fed diets without phytase showed higher BUN concentrations. Phosphate is involved in protein synthesis and insufficient digestible P intake may impair such processes, leading to higher AA catabolism and subsequent urea concentrations at the time of blood sampling. However, the quantitative urinary N excretion of pigs did not differ, indicating that any effect on the metabolic level was not generally relevant to N retention of the pigs. Additional Ca and phytase did not affect pc AA digestibility, indicating that AA was equally available for protein synthesis in all treatments. Mesina et al. (2019) rejected the hypothesis that dietary InsP6 could increase the endogenous secretion of proteins such as mucin, thereby increasing endogenous AA losses. Consistent with this, the Neu5Ac concentration in the distal ileum digesta, an indicator of mucin, was not significantly affected. The only indication of a trend was that mucin secretion may have been increased by the addition of Ca to the diet in the present study. In a meta-analysis of 34 publications, the pc AA digestibility was increased by an average of 0.9 (Met) to 1.5 percentage units (Thr; Zouaoui et al., 2018). However, phytase effects on AA digestibility were inconsistent among studies and overall may be smaller in pigs than in broiler chickens (Rodehutscord et al., 2022).
Microbial composition
In the present study, the microbial community composition in the feces and distal ileum digesta was not affected by the treatment. Bacterial communities may stabilize in response to dietary changes (Metzler-Zebeli et al., 2013). However, Tilocca et al. (2017) found that restructuring a bacterial community after a change in diet takes several weeks. Wood and Clark (1988) reported that excess P could be stored in bacterial cells in the form of polyphosphates and be used as an energy source. Therefore, it cannot be ruled out that the adaptation period in the present study was not long enough for the microbiota to adapt, which contributed to the absence of any measured treatment effects on the microbiota composition.
Consistent with other studies (Looft et al., 2014; Zhao et al., 2015; Gierse et al., 2020), the composition of the microbial community in the distal ileum digesta was different from that in the feces. This can be attributed to the various functions and environments that exist in intestinal sections. While most digestion and absorption occur in the small intestine, in the large intestine, it is mainly the formation of VFA by fermentation. The abundance of Lactobacillus was lower in the distal ileum digesta than in the feces, whereas that of Bifidobacterium was higher. Yang et al. (2020) identified Bifidobacterium as the genus with the highest abundance in the ileum and colon. Metzler-Zebeli et al. (2019) found a relationship between the abundance of lactic acid-producing groups in feces and the supply of resistant starch. However, starch entry into the large intestine was not likely to differ in the present study when pc GE digestibility was used as an indicator.
Megasphaera correlated positively with fecal GE digestibility. Megaspahera elsdenii belongs to the lactate-utilizing bacterial group and can metabolize lactate as an intermediate product of carbohydrate fermentation in the hindgut (Tsukahara et al., 2002; Hashizume et al., 2003). Corn was the ingredient with the greatest proportion in all diets, and the hindgut microbiota uses resistant starch as a substrate for fermentation (Englyst et al., 1992). The genus Megasphaeara may utilize lactate derived from fermentation as a substrate.
Prevotella correlated positively with isobutyric, butyric, isovaleric, and valeric acid concentrations in feces. Prevotella produces acetate, which other bacteria can use to produce butyric acid (Kovatcheva-Datchary et al., 2015; Bernad-Roche et al., 2021). The positive correlation between Bifidobacterium and isovaleric acid concentration in feces might be due to the glycolytic abilities attributed to this genus, which generates peptidase to facilitate the utilization of N sources (Schell et al., 2002).
Conclusion
Negative effects of dietary Ca concentration on pc InsP6 disappearance depend on exogenous phytase. These results imply that dietary Ca concentration must be precisely adjusted in feed formulations to avoid metabolic P loss or decreased pc InsP6–P release in low-P, corn-based diets with exogenous phytase. The variation in dietary Ca and the presence of exogenous phytase did not directly alter the microbial composition of the ileum and feces in the study period.
Supplementary Material
Acknowledgments
N. Klein and N. Sarpong received a doctoral scholarship from the H. Wilhelm Schaumann Stiftung, Hamburg, which is gratefully acknowledged. The study was funded by BASF SE, Ludwigshafen, Germany. We thank D. Berghaus for help in conducting animal trials. We appreciate the excellent service provided by the animal clinic of the University of Hohenheim in surgery and care of the animals, and the chemical analyses conducted by the laboratory team of the Animal Nutrition Department. We also thank the High Performance and Cloud Computing Group at the Zentrum für Datenverarbeitung of the University of Tübingen for providing the bwHPC.
Glossary
Abbreviations
- AA
amino acid
- ALP
alkaline phosphatases
- ASV
amplicon sequence variant
- BUN
blood urea nitrogen
- FTU
phytase unit
- GE
gross energy
- InsP
inositol phosphate
- InsP6
myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate)
- N
nitrogen
- Neu5Ac
N-acetylneuraminic acid
- NMDS
non-metric multidimensional scaling
- pc
prececal
- PERMANOVA
permutation analysis of variance
- Pi
inorganic phosphorus
- VFA
volatile fatty acid
Contributor Information
Nicolas Klein, Institute of Animal Science, University of Hohenheim, 70599 Stuttgart, Germany.
Naomi Sarpong, Institute of Animal Science, University of Hohenheim, 70599 Stuttgart, Germany.
Tanja Melzer, Core Facility Hohenheim, University of Hohenheim, 70599 Stuttgart, Germany.
Dieter Feuerstein, BASF SE, 67063 Ludwigshafen, Germany.
Charlotte M E Heyer, Institute of Animal Science, University of Hohenheim, 70599 Stuttgart, Germany.
Amélia Camarinha-Silva, Institute of Animal Science, University of Hohenheim, 70599 Stuttgart, Germany.
Markus Rodehutscord, Institute of Animal Science, University of Hohenheim, 70599 Stuttgart, Germany.
Conflict of interest statement
Dieter Feuerstein is an employee of BASF SE. All authors declare no real or perceived conflicts of interest.
Literature Cited
- Bernad-Roche, M., Bellés A., Grasa L., Casanova-Higes A., and Mainar-Jaime R. C.. . 2021. Effects of dietary supplementation with protected sodium butyrate on gut microbiota in growing-finishing pigs. Animals. 11:2137. doi: 10.3390/ani11072137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguhn, J., Baumgärtel T., Dieckmann A., and Rodehutscord M.. . 2009. Determination of titanium dioxide supplements in different matrices using two methods involving photometer and inductively coupled plasma optical emission spectrometer measurements. Arch. Anim. Nutr. 63:337–342. doi: 10.1080/17450390903052623. [DOI] [PubMed] [Google Scholar]
- Burbach, K., Seifert J., Pieper D. H., and Camarinha-Silva A.. . 2016. Evaluation of DNA extraction kits and phylogenetic diversity of the porcine gastrointestinal tract based on Illumina sequencing of two hypervariable regions. Microbiologyopen. 5:70–82. doi: 10.1002/mbo3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, L. E., Ray R. E., Bradford J. R., Orr J. P., Nickerson J. A., and Wells W. W.. . 1976. Myo-inositol metabolism in the neonatal and developing rat fed a myo-inositol-free diet. J. Nutr. 106:1610–1616. doi: 10.1093/jn/106.11.1610. [DOI] [PubMed] [Google Scholar]
- Cole, J. R., Wang Q., Fish J. A., Chai B., McGarrell D. M., Sun Y., Brown C. T., Porras-Alfaro A., Kuske C. R., and Tiedje J. M.. . 2014. Ribosomal database project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, R. C., Haas B. J., Clemente J. C., Quince C., and Knight R.. . 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout, W., and de Paepe M.. . 1994. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed Sci. Technol. 47:19–29. doi: 10.1016/0377-8401(94)90156-2. [DOI] [Google Scholar]
- Englyst, H. N., Kingman S. M., and Cummings J. H.. . 1992. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 46:S33–S50. [PubMed] [Google Scholar]
- European Parliament and the Council of the European Union. 2010. Directive 2010/63/EU of the European parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. OJEU. 53:33–79. doi: 10.3000/17252555.L_2010.276.eng. [DOI] [Google Scholar]
- GfE. 2008. Recommendations for the supply of energy and nutrients to pigs. Frankfurt am Main, Germany: DLG Verlag. [Google Scholar]
- Gierse, L. C., Meene A., Schultz D., Schwaiger T., Karte C., Schröder C., Wang H., Wünsche C., Methling K., Kreikemeyer B., . et al. 2020. A multi-omics protocol for swine feces to elucidate longitudinal dynamics in microbiome structure and function. Microorganisms. 8:1887. doi: 10.3390/microorganisms8121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner, R. 2021. Limitations of an in vitro model of the poultry digestive tract on the evaluation of the catalytic performance of phytases. J. Sci. Food Agric. 101:2519–2524. doi: 10.1002/jsfa.10878. [DOI] [PubMed] [Google Scholar]
- Hashizume, K., Tsukahara T., Yamada K., Koyama H., and Ushida K.. . 2003. Megasphaera elsdenii JCM1772T normalizes hyperlactate production in the large intestine of fructooligosaccharide-fed rats by stimulating butyrate production. J. Nutr. 133:3187–3190. doi: 10.1093/jn/133.10.3187. [DOI] [PubMed] [Google Scholar]
- Hu, Y., Hendriks W., van Baal J., Resink J.-W., Rodehutscord M., van Krimpen M. M., and Bikker P.. . 2023. The impact of dietary calcium content on phosphorus absorption and retention in growing pigs is enhanced by dietary microbial phytase supplementation. Br. J. Nutr. 129:955–966. doi: 10.1017/S0007114522001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, H. L., Wise A., and Henderson C.. . 1996. Hydrolysis of phytate and inositol tri-, tetra-, and penta-phosphates by intestinal mucosa of the pig. Nutr. Res. 16:781–787. doi: 10.1016/0271-5317(96)00070-x. [DOI] [Google Scholar]
- Hurum, D. C., and Rohrer J. S.. . 2011. Five-minute glycoprotein sialic acid determination by high-performance anion exchange chromatography with pulsed amperometric detection. Anal. Biochem. 419:67–69. doi: 10.1016/j.ab.2011.08.002. [DOI] [PubMed] [Google Scholar]
- ISO. 2009. Animal feeding stuffs—determination of phytase activity [accessed 15 February 2023]. https://www.iso.org/standard/45787.html.
- Kaewtapee, C., Eklund M., Wiltafsky M., Piepho H. -P., Mosenthin R., and Rosenfelder P.. . 2017. Influence of wet heating and autoclaving on chemical composition and standardized ileal crude protein and amino acid digestibility in full-fat soybeans for pigs. J. Anim. Sci. 95:779–788. doi: 10.2527/jas.2016.0932. [DOI] [PubMed] [Google Scholar]
- Kaufman, H. W., and Kleinberg I.. . 1971. Effect of pH on calcium binding by phytic acid and its inositol phosphoric acid derivatives and on the solubility of their calcium salts. Arch. Oral Biol. 16:445–460. doi: 10.1016/0003-9969(71)90168-3. [DOI] [PubMed] [Google Scholar]
- Kiarie, E. G., Song X., Lee J., and Zhu C.. . 2022. Efficacy of enhanced Escherichia coli phytase on growth performance, bone quality, nutrient digestibility, and metabolism in nursery pigs fed corn-soybean meal diet low in calcium and digestible phosphorous. Transl. Anim. Sci. 6:1–10. doi: 10.1093/tas/txac020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, N., Papp M., Rosenfelder-Kuon P., Schroedter A., Avenhaus U., and Rodehutscord M.. . 2021. Phosphorus digestibility and phytate degradation in pigs fed wheat-based diets with different intrinsic phytase activity and added microbial phytase. Arch. Anim. Nutr. 75:450–464. doi: 10.1080/1745039X.2021.1988814. [DOI] [PubMed] [Google Scholar]
- Kovatcheva-Datchary, P., Nilsson A., Akrami R., Lee Y. S., de Vadder F., Arora T., Hallen A., Martens E., Björck I., and Bäckhed F.. . 2015. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. 22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Kozich, J. J., Westcott S. L., Baxter N. T., Highlander S. K., and Schloss P. D.. . 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg, J., Siegert W., Berghaus D., Bock J., Feuerstein D., and Rodehutscord M.. . 2020. Phytase supplementation effects on amino acid digestibility depend on the protein source in the diet but are not related to InsP6 degradation in broiler chickens. Poult. Sci. 99:3251–3265. doi: 10.1016/j.psj.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor, P. G., Lynch P. B., Caffrey P. J., O’Reilly J. J., and O’Connell M. K.. . 2005. Measurements of the acid-binding capacity of ingredients used in pig diets. Ir. Vet. J. 58:447–452. doi: 10.1186/2046-0481-58-8-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., Sauer W. C., and Fan M. Z.. . 1993. The effect of dietary crude protein level on ileal and fecal amino acid digestibility in early-weaned pigs. J. Anim. Physiol. Anim. Nutr. 70:117–128. doi: 10.1111/j.1439-0396.1993.tb00314.x. [DOI] [Google Scholar]
- Lien, K. A., Sauer W. C., and Fenton M.. . 1997. Mucin output in ileal digesta of pigs fed a protein-free diet. Eur. J. Nutr. 36:182–190. doi: 10.1007/BF01611398. [DOI] [PubMed] [Google Scholar]
- Looft, T., Allen H. K., Cantarel B. L., Levine U. Y., Bayles D. O., Alt D. P., Henrissat B., and Stanton T. B.. . 2014. Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. 8:1566–1576. doi: 10.1038/ismej.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H., Shin S., Kuehn I., Bedford M., Rodehutscord M., Adeola O., and Ajuwon K. M.. . 2020. Effect of phytase on nutrient digestibility and expression of intestinal tight junction and nutrient transporter genes in pigs. J. Anim. Sci. 98:skaa206. doi: 10.1093/jas/skaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Blackburn, D., Greiner R., and Konietzny U.. . 2022. Phytases: biochemistry, enzymology and characteristics relevant to animal feed use. In: Bedford, M. R., Partridge G., Hruby M. , Walk C. L.,, editors. Enzymes in farm animal nutrition. GB: CABI Publishers. p. 103–123. doi: 10.1079/9781789241563.0007 [DOI] [Google Scholar]
- Merchant, H. A., McConnell E. L., Liu F., Ramaswamy C., Kulkarni R. P., Basit A. W., and Murdan S.. . 2011. Assessment of gastrointestinal pH, fluid and lymphoid tissue in the guinea pig, rabbit and pig, and implications for their use in drug development. Eur. J. Pharm. Sci. 42:3–10. doi: 10.1016/j.ejps.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Mesina, V. G. R., Lagos L. V., Sulabo R. C., Walk C. L., and Stein H. H.. . 2019. Effects of microbial phytase on mucin synthesis, gastric protein hydrolysis, and degradation of phytate along the gastrointestinal tract of growing pigs. J. Anim. Sci. 97:756–767. doi: 10.1093/jas/sky439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler, B. U., and Mosenthin R.. . 2008. A review of interactions between dietary fiber and the gastrointestinal microbiota and their consequences on intestinal phosphorus metabolism in growing pigs. Asian-Aust. J. Anim. Sci. 21:603–615. doi: 10.5713/ajas.2008.r.03. [DOI] [Google Scholar]
- Metzler-Zebeli, B. U., Canibe N., Montagne L., Freire J., Bosi P., Prates J. A. M., Tanghe S., and Trevisi P.. . 2019. Resistant starch reduces large intestinal pH and promotes fecal lactobacilli and bifidobacteria in pigs. Animal. 13:64–73. doi: 10.1017/S1751731118001003. [DOI] [PubMed] [Google Scholar]
- Metzler-Zebeli, B. U., Mann E., Schmitz-Esser S., Wagner M., Ritzmann M., and Zebeli Q.. . 2013. Changing dietary calcium-phosphorus level and cereal source selectively alters abundance of bacteria and metabolites in the upper gastrointestinal tracts of weaned pigs. Appl. Environ. Microbiol. 79:7264–7272. doi: 10.1128/AEM.02691-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Zebeli, B. U., Zijlstra R. T., Mosenthin R., and Gänzle M. G.. . 2011. Dietary calcium phosphate content and oat β-glucan influence gastrointestinal microbiota, butyrate-producing bacteria and butyrate fermentation in weaned pigs. FEMS Microbiol. Ecol. 75:402–413. doi: 10.1111/j.1574-6941.2010.01017.x. [DOI] [PubMed] [Google Scholar]
- Novotny, M., Sommerfeld V., Krieg J., Kühn I., Huber K., and Rodehutscord M.. . 2023. Mucosal phosphatase activity, phytate degradation, and mineral digestibility in 6-week-old turkeys and broilers at different dietary levels of phosphorus and phytase and comparison with 3-week-old animals. Poult. Sci. 102:102476. doi: 10.1016/j.psj.2023.102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, H., Kornegay E. T., and Conner D. E.. . 1996. Adverse effects of wide calcium:phosphorus ratios on supplemental phytase efficacy for weanling pigs fed two dietary phosphorus levels. J. Anim. Sci. 74:1288–1297. doi: 10.2527/1996.7461288x. [DOI] [PubMed] [Google Scholar]
- Rodehutscord, M., and Bikker P.. . 2022. Phytate degradation, P and Ca absorption and digestibility in pigs. Anim. Sci. Proc. 13:133–136. doi: 10.1016/j.anscip.2022.03.167. [DOI] [Google Scholar]
- Rodehutscord, M., Haverkamp R., and Pfeffer E.. . 1998. Inevitable losses of phosphorus in pigs, estimated from balance data using diets deficient in phosphorus. Arch. Anim. Nutr. 51:27–38. doi: 10.1080/17450399809381903. [DOI] [PubMed] [Google Scholar]
- Rodehutscord, M., Kapocius M., Timmler R., and Dieckmann A.. . 2004. Linear regression approach to study amino acid digestibility in broiler chickens. Br. Poult. Sci. 45:85–92. doi: 10.1080/00071660410001668905. [DOI] [PubMed] [Google Scholar]
- Rodehutscord, M., Sommerfeld V., Kühn I., and Bedford M.. . 2022. Phytases: potentials and limits of phytate destruction in the digestive tract of pigs and poultry. In: Bedford, M., Partridge G., Hruby M., and Walk C., editors. Enzymes in farm animal nutrition. 3rd ed.Wallingford, UK: CAB International. doi: 10.1079/9781789241563.0008. [DOI] [Google Scholar]
- Rosenfelder-Kuon, P., Klein N., Zegowitz B., Schollenberger M., Kühn I., Thuringer L., Seifert J., and Rodehutscord M.. . 2020. Phytate degradation cascade in pigs as affected by phytase supplementation and rapeseed cake inclusion in corn-soybean meal-based diets. J. Anim. Sci. 98:1–12. doi: 10.1093/jas/skaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford, S. M., Chung T. K., and Moughan P. J.. . 2014. Effect of microbial phytase on phytate P degradation and apparent digestibility of total P and Ca throughout the gastrointestinal tract of the growing pig. J. Anim. Sci. 92:189–197. doi: 10.2527/jas.2013-6923. [DOI] [PubMed] [Google Scholar]
- Rychen, G., Aquilina G., Azimonti G., Bampidis V., Bastos M. L., Bories G., Chesson A., Flachowsky G., Gropp J., Kolar B., . et al. 2017. Safety and efficacy of Natuphos® E (6-phytase) as a feed additive for avian and porcine species. EFSA J. 15:e05024. doi: 10.2903/j.efsa.2017.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg, A. S., Larsen T., and Sandström B.. . 1993. High dietary calcium level decreases colonic phytate degradation in pigs fed a rapeseed diet. J. Nutr. 123:559–566. doi: 10.1093/jn/123.3.559. [DOI] [PubMed] [Google Scholar]
- Schell, M. A., Karmirantzou M., Snel B., Vilanova D., Berger B., Pessi G., Zwahlen M.-C., Desiere F., Bork P., Delley M., . et al. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. U.S.A. 99:14422–14427. doi: 10.1073/pnas.212527599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel, P., and Gutzwiller A.. . 2017. Effect of dietary calcium level and source on mineral utilisation by piglets fed diets containing exogenous phytase. J. Anim. Physiol. Anim. Nutr. 101:e165–e174. doi: 10.1111/jpn.12582. [DOI] [PubMed] [Google Scholar]
- Schlemmer, U., Jany K. D., Berk A., Schulz E., and Rechkemmer G.. . 2001. Degradation of phytate in the gut of pigs – pathway of gastro-intestinal inositol phosphate hydrolysis and enzymes involved. Arch. Anim. Nutr. 55:255–280. doi: 10.1080/17450390109386197. [DOI] [PubMed] [Google Scholar]
- Schröder, B., and Breves G.. . 2006. Mechanisms and regulation of calcium absorption from the gastrointestinal tract in pigs and ruminants: comparative aspects with special emphasis on hypocalcemia in dairy cows. Anim. Health Res. Rev. 7:31–41. doi: 10.1017/S1466252307001144. [DOI] [PubMed] [Google Scholar]
- Selle, P. H., Cowieson A. J., and Ravindran V.. . 2009. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest. Sci. 124:126–141. doi: 10.1016/j.livsci.2009.01.006. [DOI] [Google Scholar]
- Sommerfeld, V., Huber K., Bennewitz J., Camarinha-Silva A., Hasselmann M., Ponsuksili S., Seifert J., Stefanski V., Wimmers K., and Rodehutscord M.. . 2020a. Phytate degradation, myo-inositol release, and utilization of phosphorus and calcium by two strains of laying hens in five production periods. Poult. Sci. 99:6797–6808. doi: 10.1016/j.psj.2020.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld, V., Künzel S., Schollenberger M., Kühn I., and Rodehutscord M.. . 2018a. Influence of phytase or myo-inositol supplements on performance and phytate degradation products in the crop, ileum, and blood of broiler chickens. Poult. Sci. 97:920–929. doi: 10.3382/ps/pex390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld, V., Omotoso A. O., Oster M., Reyer H., Camarinha-Silva A., Hasselmann M., Huber K., Ponsuksili S., Seifert J., Stefanski V., . et al. 2020b. Phytate degradation, transcellular mineral transporters, and mineral utilization by two strains of laying hens as affected by dietary phosphorus and calcium. Animals. 10:1736. doi: 10.3390/ani10101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld, V., Schollenberger M., Kühn I., and Rodehutscord M.. . 2018b. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 97:1177–1188. doi: 10.3382/ps/pex404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld, V., van Kessel A. G., Classen H. L., Schollenberger M., Kühn I., and Rodehutscord M.. . 2019. Phytate degradation in gnotobiotic broiler chickens and effects of dietary supplements of phosphorus, calcium, and phytase. Poult. Sci. 98:5562–5570. doi: 10.3382/ps/pez309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talke, H., and Schubert G. E.. . 1965. Enzymatische Harnstoffbestimmung in Blut und Serum im optischen Test nach Warburg. Klein. Wochenschr. 43:174–175. doi: 10.1007/BF01484513. [DOI] [PubMed] [Google Scholar]
- Tilocca, B., Burbach K., Heyer C. M. E., Hoelzle L. E., Mosenthin R., Stefanski V., Camarinha-Silva A., and Seifert J.. . 2017. Dietary changes in nutritional studies shape the structural and functional composition of the pigs fecal microbiome: from days to weeks. Microbiome. 5:144. doi: 10.1186/s40168-017-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara, T., Koyama H., Okada M., and Ushida K.. . 2002. Stimulation of butyrate production by gluconic acid in batch culture of pig cecal digesta and identification of butyrate-producing bacteria. J. Nutr. 132:2229–2234. doi: 10.1093/jn/132.8.2229. [DOI] [PubMed] [Google Scholar]
- VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten. 2007. Handbuch der landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA–Methodenbuch). Vol. 3: Die Chemische Untersuchung von Futtermitteln. Darmstadt, Germany: VDLUFA-Verlag. [Google Scholar]
- Wischer, G., Boguhn J., Steingaß H., Schollenberger M., Hartung K., and Rodehutscord M.. . 2013. Effect of monensin on in vitro fermentation of silages and microbial protein synthesis. Arch. Anim. Nutr. 67:219–234. doi: 10.1080/1745039X.2013.793050. [DOI] [PubMed] [Google Scholar]
- Wise, A., and Gilburt D. J.. . 1981. Binding of cadmium and lead to the calcium-phytate complex in vitro. Toxicol. Lett. 9:45–50. doi: 10.1016/0378-4274(81)90173-9. [DOI] [PubMed] [Google Scholar]
- Wood, H. G., and Clark J. E.. . 1988. Biological aspects of inorganic polyphosphates. Annu. Rev. Biochem. 57:235–260. doi: 10.1146/annurev.bi.57.070188.001315. [DOI] [PubMed] [Google Scholar]
- Yang, G., Shi C., Zhang S., Liu Y., Li Z., Gao F., Cui Y., Yan Y., and Li M.. . 2020. Characterization of the bacterial microbiota composition and evolution at different intestinal tract in wild pigs (Sus scrofa ussuricus). PeerJ. 8:e9124. doi: 10.7717/peerj.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, Z., and Kornegay E. T.. . 1996. Sites of phytase activity in the gastrointestinal tract of young pigs. Anim. Feed Sci. Technol. 61:361–368. doi: 10.1016/0377-8401(96)00959-5. [DOI] [Google Scholar]
- Zeller, E., Schollenberger M., Witzig M., Shastak Y., Kühn I., Hoelzle L. E., and Rodehutscord M.. . 2015. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 94:1018–1029. doi: 10.3382/ps/pev087. [DOI] [PubMed] [Google Scholar]
- Zhao, W., Wang Y., Liu S., Huang J., Zhai Z., He C., Ding J., Wang J., Wang H., Fan W., . et al. 2015. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS One. 10:e0117441. doi: 10.1371/journal.pone.0117441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouaoui, M., Létourneau-Montminy M. P., and Guay F.. . 2018. Effect of phytase on amino acid digestibility in pig: a meta-analysis. Anim. Feed Sci. Technol. 238:18–28. doi: 10.1016/j.anifeedsci.2018.01.019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.