Abstract
Significant decreases in fertility have been observed over the past 50 years, with female conception rates dropping by 44% and male sperm counts decreasing by over 50%. This dramatic decrease in fertility can be attributed in part to our increasing exposure to endocrine disrupting chemicals (EDCs). Diethylstilbestrol (DES) is an estrogenic EDC that was prescribed to millions of pregnant women between 1940 and 1970 and resulted in detrimental reproductive effects in the offspring that were exposed in utero. Women who were exposed to DES in utero experienced higher rates of infertility, pregnancy complications, and reproductive cancers. Alarmingly, there is evidence to suggest that these effects may persist in the grandchildren and great grandchildren of exposed women. To define the transgenerational reproductive impacts in females following exposure to DES, gestating mice were exposed to DES and the effects monitored in the female descendants across 3 generations. There was a trend for reduced pregnancy rate and fertility index seen across the generations and moreover, the anogenital distance (AGD) was significantly reduced up until the third, unexposed generation. The onset of puberty was also significantly affected, with the timing of vaginal opening occurring significantly earlier in DES descendants. These results indicate a transgenerational effect of DES on multiple reproductive parameters including fertility, timing of puberty, and AGD. These data have significant implications for more than 50 million DES descendants worldwide as well as raising concerns for the ongoing health impacts caused by exposures to other estrogenic EDCs which are pervasive in our environment.
Keywords: fertility, DES, transgenerational, EDC
Infertility is an increasing issue in society today, with 15% of reproductive aged couples being affected by infertility globally (Boivin et al., 2007). Male sperm counts have dropped by 50% in the past 50 years, and female conception rates have also dropped by 44% in the past 50 years (Hamilton and Ventura, 2006; Levine et al., 2017). This decrease in fertility can, in part, be attributed to our continued exposure to endocrine disrupting chemicals (EDC). EDCs are among the most pervasive toxins found in our environment and are defined as exogenous agents that can interfere or block the synthesis, secretion, transport, action, or elimination of natural hormones in the body (Kabir et al., 2015; Skinner, 2014; Skinner et al., 2010). We are continuously exposed to EDCs in everyday life as they can be naturally occurring chemicals present in food or synthetic chemicals added to a variety of products including plastics, pesticides, and pharmaceutical drugs (Diamanti-Kandarakis et al., 2009). Xenoestrogens are one of the most harmful classes of EDCs present in our environment as they are able to bind to estrogen receptors (ER) and interfere and overstimulate normal estrogen signaling pathways. Exposure to estrogenic EDCs during development is particularly harmful as they can activate ERs and alter the fetal hormonal environment, predisposing individuals to reproductive tract tumors, infertility, and genital abnormalities later in life (Nordkap et al., 2012; Prins, 2008; Sharp and Cole, 1991).
Diethylstilbestrol (DES) is a potent xenoestrogen that was prescribed to over 10 million pregnant women worldwide between 1940 and 1970 with the hopes to prevent miscarriage and premature labor (Al Jishi and Sergi, 2017; Veurink et al., 2005). However, it was later found that daughters who had been exposed to DES in utero presented with an increased incidence of clear cell vaginal adenocarcinoma, a very rare genital tract tumor (Herbst et al., 1971). It was due to this association that the use of DES was banned in 1971 (Palmer et al., 2000; Prins, 2008). Since then, it has been shown that women who were exposed to DES in utero have a 33% rate of infertility compared with 14% in unexposed females (Hoover et al., 2011). Moreover, these women experience numerous pregnancy complications. They are nearly 3 times more likely to have a preterm delivery, 5 times more likely to have an ectopic pregnancy, twice as likely to have spontaneous abortions and stillbirth occurs in 8.9% of DES daughter pregnancies compared with 2.6% in unexposed women (Hoover et al., 2011; Kaufman et al., 2000). However, there is now evidence to suggest that these effects can persist into subsequent generations, known as transgenerational effects (Titus, 2021; Titus et al., 2019; Titus-Ernstoff et al., 2006).
When a gestating female (F0) is exposed to an environmental factor (eg, DES) both the F0 and the developing F1 fetus are directly exposed. The F1 fetus will develop in an altered hormonal environment, which may lead to an increased disease susceptibility later in life. Furthermore, within the developing F1 fetus are the germ cells that will produce the F2 generation. Therefore, while the F2 develops in a normal hormonal environment, exposure as a germ cell also has the potential to cause an increased rate of disease. These exposures (F0, F1, and F2) are referred to as multigenerational, where each generation has been directly exposed to the EDC, albeit at vastly different developmental stages (F0 exposed as an adult, F1 exposed as fetus, and F2 exposed as germ cells). However, what is of utmost concern is the potential for EDCs to cause impacts beyond the F2 generation, without any direct exposure to the causative agent. Such impacts are referred to as transgenerational effects and indicate a permanent change to the genome.
Multigenerational impacts of DES have been reported in a large cohort study of 796 DES exposed and 469 unexposed women. The DES granddaughters (F2 generation) were shown to have an increased risk of menstrual irregularity, amenorrhea, preterm birth, and possibly ectopic pregnancy, although they did not find any differences in rates of infertility (Titus et al., 2019). However, due to the young age of this cohort, follow-up studies are needed to further assess the impacts DES has on fertility and pregnancy complications. Animal studies have also highlighted the multigenerational effects of DES, with increased incidence of reproductive tract abnormalities, including uterine tumors, persisting into the second generation after prenatal DES exposure (Mahawong et al., 2014; Newbold et al., 1998).
There are limited human studies that have investigated the transgenerational effects of EDCs, however, studies in mice have shown the ability of EDCs to have effects that persist into the F3 generation. Prenatal exposure to BPA significantly impacts the timing of puberty and reduces fertility in female mice in a transgenerational manner (Ziv-Gal et al., 2015). The plasticizer DEHP also has transgenerational effects with exposure leading to early onset of puberty, altered estrous cyclicity, and decreased anogenital distance (AGD) in the F3 generation (Rattan et al., 2018). Moreover, rats treated with a mixture of plasticizers (BPA, DEHP, and DBP) resulted in pubertal abnormalities and polycystic ovaries in F3 females (Manikkam et al., 2013). Whilst there have been many studies that have investigated the effects of DES on reproductive development, very few have studied its long-term transgenerational effects. Although DES is no longer in use, there are over 50 million descendants today who may be impacted by its long-term effects (Prins, 2008; Titus, 2021). Therefore, understanding the transgenerational effects of DES would be of value to these descendants, and the adverse reproductive outcomes we observe from DES exposure can provide a basis for understating how other potent estrogenic EDCs can impact female fertility and reproductive development.
This study defines the transgenerational effects of DES on female fertility and reproductive health. DES was administered prenatally at 3 doses, 2000 ng/l, 50 µg/kg, and 100 µg/kg. The lowest dose, 2000 ng/l, was administered through the drinking water to represent environmental exposures to estrogenic EDCs (Aris et al., 2014). The higher doses were administered via subcutaneous injection and are representative of the spectrum of doses that was clinically delivered to pregnant women between 1940 and 1970 (Al Jishi and Sergi, 2017). We analyzed the transgenerational effect of DES on fertility and birth outcomes, timing of puberty, body weight, and AGD in the F1–F3 generation of female mice.
Materials and methods
Animals and DES treatments
C57BL/6 mice were raised in the animal facility in BioSciences 4 at the University of Melbourne under a 13 h light/11 h dark cycle at 22–23°C and fed a standard diet (GR2 rat and mouse cubes; Ridley AgriProducts) and tap water ad libitum. The University of Melbourne Ethics Committee, ethics number 1714163, approved all procedures.
Eight-week-old female mice were mated to male mice and the presence of a vaginal plug was considered day 0 of gestation (10–15 damns per treatment group). These dams were considered the F0 generation. Dams were treated every second day of gestation from days 9 to 17 inclusive to ensure exposure occurred throughout the window of fetal sexual differentiation and development (Figure 1) (Arango and Donahoe, 2008). Dams were assigned to 1 of 4 treatment groups (vehicle, 2000 ng/l, 50 µg/kg, and 100 µg/kg). DES (obtained from Abcam) was dissolved in 100% ethanol and then diluted to 40 µg/ml in 1% ethanol in sterile phosphate buffered saline to make up the stock solution. The 50- and 100-µg/kg doses of DES were administered via subcutaneous injection using a 26-guage needle into the flank. This regimen was chosen to represent one of the many ways women received DES and the doses were chosen to encapsulate the range of DES exposure pregnant women received between 1940 and 1970 (Al Jishi and Sergi, 2017). The 2000-ng/l dose of DES was administered via the drinking water and was refreshed every second day throughout the treatment period. This dose and administration is representative of an environmental exposure to estrogenic EDCs (Aris et al., 2014). Assuming a mouse’s average intake of 5 ml per day and weight of 20 g, this dose equates to 1 µg/kg every second day and will be referred to as 1 µg/kg from here on (Barbour and Trace, 1936). Control female mice (vehicle) were treated with 1% ethanol in phosphate buffered saline through subcutaneous injection or via the drinking water, as no differences in control groups were observed, these cohorts were combined and will be referred to as the vehicle treatment.
Figure 1.
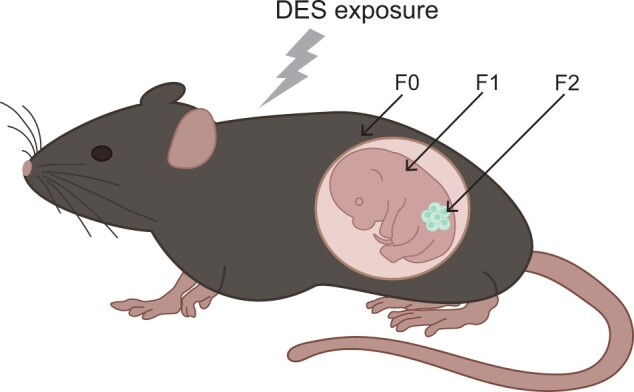
Schematic representation of DES exposure. The F0 female is exposed to DES every second day from days 9 to 17 of gestation. The F1 has a direct fetal exposure to DES and develops in an altered hormonal environment. The F2 is exposed as germ cells within the F1 fetus, and the F3 generation has no direct exposure to DES and is the first transgenerational generation.
At 8 weeks of age the F1 female mice (exposed as fetus) were mated and housed with unexposed males for 2 weeks to generate the F2 generation. The F2 and F3 females were used to generate the F3 and F4 generation in the same manner.
Fertility tests
At 8 weeks of age, female mice were paired with untreated males for 2 weeks. Vaginal plugs were checked daily to determine timed pregnancies and females were weighed daily to monitor pregnancy gain or loss. Female fertility was determined according to standard methods using the following equations (Tyl et al., 2008):
Litter size, pup death, and sex ratio were recorded. Where possible, dead pups were sexed via PCR, and pup deaths were recorded as a percentage of dead pups per litter.
Super ovulation
Vehicle, 1 and 100 µg/kg DES females were super-ovulated and oocytes collected at each generation. At 6 weeks of age 5 IU (0.1 ml) of pregnant mare’s serum gonadotrophin was administered through intraperitoneal injection using a 25-gauge needle. Forty-eight hours later, 5 IU (0.1 ml) of human chorionic gonadotrophin (hCG) was administered in the same manner. Twenty-four hours after hCG administration females were sacrificed, and oviducts dissected out and placed in M2 media. The ampulla of the oviducts was pierced with an 18-gauge needle to release the cumulus masses. The cumulus masses were transferred to 2 ml M2 media with 75 µl hyaluronidase (10 mg/ml). Once the cumulus cells were removed the oocytes were transferred to M2 media with 10% fetal bovine serum and counted.
Onset of puberty, body weight, and AGD
Timing of puberty for female pups was determined by checking for presence of vaginal opening daily from postnatal-day 21.
Females were sacrificed at 5 months of age and body weight and AGD measured. AGD was standardized by dividing the AGD by the cubed root of body weight (Gallavan et al., 1999). Exact animal numbers used for each analysis can be found in Supplementary Table 1.
Statistical analysis
Where data had equal variance and normal distribution, a 1-way analysis of variance followed by a Tukey’s test was performed. Where data did not have either equal variance or a normal distribution, a Kruskal-Wallis rank sum test followed by a Wilcoxon test was carried out. A p value of <.05 was considered significant.
Results
Effects of DES exposure on fertility indices
Although not statistically significant, a reduction in fertility outcomes was seen across multiple generations and doses after exposure to DES. Mating index, pregnancy rate, fertility index, and gestational index were all 100% for vehicle treated animals (Figure 2, n = 10–16 dams/treatment group). Pregnancy rate and fertility index were reduced in the 100-µg/kg DES F1 and F2 generation (Figs. 2B and 2C, pregnancy rate = 75% and 91%, fertility index = 80% and 91%, respectively), whereas 50 µg/kg DES led to a reduction in both pregnancy rate and fertility index up until the F3 generation (Figs. 2B and 2C, pregnancy rate = 92%, 85%, and 83%, fertility index = 92%, 85%, and 91%, respectively). Gestational index was reduced in 100 µg/kg DES exposure in the F1 and F2 generations (Figure 2D, gestational index = 83% and 90%, respectively), and exposure to 1 µg/kg DES caused a reduction in the F3 generation (Figure 2D, gestational index = 90%). Mating index was reduced in the F1 100 µg/kg and F3 50 µg/kg DES exposure (Figure 2A, mating index = 94% and 92%, respectively).
Figure 2.
The effects of prenatal DES exposure on fertility related indices in the F1, F2, and F3 generation. The mating index (A), pregnancy rate (B), fertility index (C), and gestational index (D) are represented.
Effect of DES on oocyte numbers
Females from vehicle, 1 and 100 µg/kg DES groups were super-ovulated at 6 weeks of age and oocytes collected. F1 100 µg/kg exposed females ovulated significantly fewer oocytes compared with control animals (Figure 3, n = 10–14 females/treatment group, p = .0054), whereas no differences in oocyte numbers were observed in the 100-µg/kg F2 and F3 generation compared with the control. No significant difference in oocyte numbers were observed after exposure to 1 µg/kg DES in any generation.
Figure 3.

The effect of prenatal DES exposure on oocyte number. **p < .01.
Effect of DES on litter size, pup death, and sex ratio
Prenatal exposure to 50 and 100 µg/kg of DES significantly decreased litter size in the F1 generation (Figure 4A, n = 9–16 dams/treatment group; p = .0014 and p = .0004, respectively). However, this was recovered by the F2 generation. Litter size was not reduced after exposure to 1 µg/kg DES across any generations. DES exposure did not increase pup death percentage across any generation or dose (Figure 4B). DES exposure did not impact sex ratio across any generation (data not shown).
Figure 4.

The effects of prenatal DES exposure on litter size (A) and pup death percentage (B). ***p < .001.
Effect of DES on timing of vaginal opening
Vaginal opening is an indication of the start of puberty in female mice. Timing of vaginal opening occurred significantly earlier in all F1 DES exposure groups (Figure 5, n = 11–44 females/treatment group, p = .015, p = .001, and p = .029, 1 µg/kg, 50 µg/kg, 100 µg/kg, respectively). In the F2 generation, only exposure to 50 µg/kg DES resulted in vaginal opening occurring significantly earlier (p = .0002). No significant differences were seen in the F3 generation, however in the F4 generation, 50 µg/kg resulted in significantly earlier vaginal opening (p = .015) and 1 µg/kg group trended towards significance (p = .056).
Figure 5.

Prenatal DES exposure leads to earlier timing of vaginal opening across multiple generations and doses. *p < .05; **p < .01; ***p < .001.
Effect of DES on body weight and AGD
Prenatal exposure to DES did not have any effect on body weight (Figure 6A, n = 7–25 dams/treatment group). DES exposure significantly reduced the standardized AGD in the F1 generation at 100 µg/kg DES (Figure 6B, n = 7–25 dams/treatment group, p = .005). In the F2 generation, the AGD was significantly reduced at 1 and 100 µg/kg DES (p = .046 and .0005, respectively), whilst in the F3 generation the AGD was significantly reduced at all 3 doses of DES (p = .0001, <.0001, and <.0001 for 1 µg/kg, 50 µg/kg, and 100 µg/kg, respectively).
Figure 6.
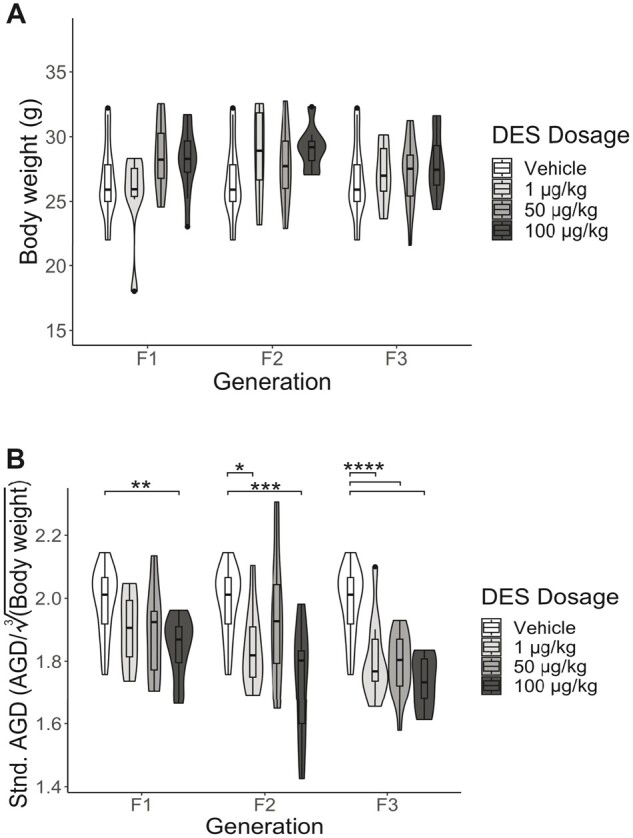
DES exposure did not impact body weight (A) but did significantly reduce the AGD across multiple generations and doses (B). *p < .05; **p < .01; ***p < .001; ****p < .0001.
Discussion
DES is an estrogenic EDC and pharmaceutical drug that was prescribed to millions of pregnant women world-wide, resulting in detrimental reproductive effects in the offspring that were exposed in utero. There is growing evidence to suggest that exposure to EDCs can have long lasting transgenerational effects, therefore, the purpose of this study was to determine if prenatal DES exposure can impact female fertility and reproductive development beyond the F1 generation into the F2 and more importantly, the unexposed F3, and at which doses DES is able to cause these effects. The human F3 DES population is only now just reaching reproductive age, therefore, animal studies are essential in determining the long-lasting effects of DES so that these descendants can be aware of their reproductive risks. In this study we saw that prenatal DES exposure results in long-lasting, transgenerational impacts on fertility index, pregnancy rate, AGD, and timing of puberty, suggesting that DES descendants may still be at risk of reproductive effects due to their ancestral exposure.
There are multiple aspects to consider when analyzing the effects of DES exposure on female fertility in this study, including the generation, reproductive parameter, and dosage. Our study found that prenatal DES exposure impacts female fertility in the F1, F2, and F3 generation. However, DES is not impacting all aspects of fertility, DES did not impact the ability of these females to mate, however it did impact the pregnancy rate of these females, and this persisted into the F3 generation. The ability to maintain pregnancy to term was only impacted in the F1 and F2 generations, showing that DES does not act in a linear dose response. Although exposure to 100 µg/kg DES had the most impact in the F1 generation, fertility was recovered by the F3 at this dose. Exposure to 50 µg/kg DES resulted in transgenerational effects, with reduced fertility persisting in the F3 generation. The lowest dose of DES, 1 µg/kg, did not have any impact on female fertility. It should be noted that our fertility tests were only completed for 1 time point, and different effects may have been observed if this was completed over a longer period. However, these results still indicate that a clinically relevant dose of DES causes a heritable, transgenerational effect on infertility. Although the changes to fertility that were observed in this study were subtle, this has biological relevance for human fertility, as subtle changes to fertility may severely impact females already at risk of subfertility or infertility.
Our results are in contrast to a previous study that found fertility was only impacted in the F1 generation after DES exposure (Newbold et al., 1998). However, this study used different doses of DES (2.5 µg/kg, 5 µg/kg, and 10 µg/kg), and measured fertility across a 20-week continuous breeding scheme (Newbold et al., 1998). Our data are consistent with studies of BPA which has been shown to have transgenerational effects, with prenatal exposure reducing fertility index and pregnancy rate up until the F3 generation (Ziv-Gal et al., 2015). Moreover, in humans, women experiencing infertility have higher levels of serum BPA compared with fertile women (Caserta et al., 2013). Although the direct cause of reduced fertility in our study is not known, previous studies have shown that exposure to a mixture of plastics that include BPA, reduces the ovarian reserve in the F1 and F3 generation (Manikkam et al., 2013). Human cohort studies have also found an association between high levels of BPA in urine samples and decreased antral follicle counts (Souter et al., 2013). Therefore, DES may be reducing fertility through altering normal ovarian function, either through depleting the ovarian reserve or altering folliculogenesis. Another mechanism by which DES may be impacting fertility is through altering the uterine environment. DES exposure has adverse impacts on the uterus in humans and mice, including increased uterine leiomyomas, hyperplasia, and uterine adenocarcinoma, indicating that DES may be impacting fertility through altering the uterus and creating an unsuitable environment for implantation and growth (Al Jishi and Sergi, 2017; Newbold et al., 1998, 2002; Senekjian et al., 1988; Suen et al., 2018). Overall, DES exposure is able to alter normal hormonal signaling and regulation which may then impact any aspect of the reproductive tract, such as the ovaries or uterus, leading to reduced fertility that persists for multiple generations.
Litter size was significantly reduced after exposure to 50 and 100 µg/kg of DES in the F1 but not in the F2 and F3 generation. Reasons that may explain reduced litter size include less conceptuses, increased number of reabsorptions, or early postnatal mortality. One mechanism we explored in this study was the impact of DES on ovulation. F1 100 µg/kg DES exposed females ovulated significantly fewer oocytes compared with control animals, whereas no differences in oocyte numbers were observed in the F2 and F3 generations. Therefore, DES may be having a direct effect on ovulation which can then lead to reduced litter size. However, as this reduced ovulation and litter size is only observed in the F1 generation, it indicates a direct fetal exposure is needed to cause this effect. Similarly, a cohort study in women undergoing infertility treatment found an association between increased levels of BPA in urine and decreased total follicle numbers, further highlighting the effect EDCs can have on oocyte numbers and ovulation (Mok-Lin et al., 2010).
Prenatal DES exposure did not impact the body weight of these females, although, the medium body weight of all groups was slightly raised compared with the control across all generations. Exposure to EDCs, including DES, increases the rate of obesity, as DES is able to alter hormones involved in weight regulation (Hao et al., 2012; Manikkam et al., 2013). Although we did not observe any significant changes to body weight in this study, many aspects of reproductive development were impacted, suggesting that reproductive development is more sensitive to hormonal changes than metabolism.
The AGD is highly hormonal dependent and is thus used as a marker of the hormonal environment in which the embryo developed. Therefore, the reduced AGD that we observed in the F1 generation was expected and indicates that DES was able to effectively cross the placenta and impact reproductive tract development at a dose of 100 µg/kg. Our results agree with previous studies that have also shown a reduced AGD in the F1 generation after developmental exposure to DES, whereas pre- and neo-natal exposure to BPA also leads to a reduced AGD in rats (Christiansen et al., 2014; Stewart et al., 2018). Alarmingly, our results indicate an effect on AGD beyond the F1. AGD was reduced in the F2 1 µg/kg DES and 100 µg/kg DES groups, however, AGD was also reduced in the F3 generation at all doses, highlighting the transgenerational effect of DES on AGD development. The F2 and F3 generation developed in a normal hormonal environment, with the F2 being exposed as germ cells, and the F3 having no direct exposure to DES. Therefore, DES may be causing permanent and heritable changes to hormonal signaling pathways or gene regulatory networks which can then lead to this sustained reduction of the AGD. It is known that the sonic hedgehog (Shh) pathway is involved in AGD development, and any alteration or mutation in this pathway results in a reduced AGD (He et al., 2016). Moreover, Efnb2 has been shown to be involved in cloacal septation in early embryonic development and disruption to its gene expression also results in a reduced AGD in female mice (Mattiske et al., 2020). Therefore, the legacy of DES may be impacting key developmental genes, like Shh and Efnb2, leading to a reduced AGD in the F2 and F3 generation.
Our data indicate that DES exposure accelerates the onset of puberty in the F1 generation at all doses. Estrogen plays an important role in activating and regulating female puberty, therefore exposure to exogenous estrogens can disrupt normal pubertal timing (DiVall and Radovick, 2009; Mayer et al., 2010). The accelerated puberty observed in the F1 generation is due to a direct fetal exposure to DES, however, we also observed accelerated puberty in the F2 and F4 50 µg/kg DES generations. This continued disruption suggests DES may be having a permanent effect on genes involved in the regulation of puberty. Puberty is delayed in female mice that have been exposed to a mixture of EDCs, and key hypothalamic genes that control puberty and ovulation are altered in the F2 and F3 generation (López-Rodríguez et al., 2021). Therefore, similar to what we observed with the sustained reduction of the AGD, DES may cause permanent changes to hormonally regulated genes which then impacts many aspects of reproductive development like puberty and AGD. It is surprising that we saw puberty accelerated in the F4 generation but not the F3 because both the F3 and F4 have had no direct exposure to DES. However, this may be explained through tertiary epimutations. A tertiary epimutation is when an exposure to a toxicant, like an EDC, can lead to a random accumulation of mutations in subsequent generations, such that by the F4 generation, there are multiple epimutations resulting in disease onset, or in our case, altered timing of puberty (McCarrey et al., 2016). The occurrence of pubertal abnormalities in humans has been greatly increasing over time, with the average age of the onset of puberty steadily decreasing (DiVall and Radovick, 2009). Specifically in the DES daughters and granddaughters, the average age of menarche is not affected; however, they do attain menstrual regularity at a slightly older age, and they are more likely to experience menstrual irregularity (Titus-Ernstoff et al., 2006). Therefore, this may be an effect that persists for multiple generations.
The main mechanism by which EDCs are able to induce transgenerational effects is through altering the epigenome, specifically, altering DNA methylation (Bromer et al., 2009; Dolinoy et al., 2007; Sato et al., 2009). Neonatal exposure to DES causes demethylation of the CpG site −464 in the lactoferrin promoter in the mature mouse uterus (Li et al., 1997). Whilst prenatal DES exposure significantly increases methylation in the Hoxa10 promoter as well as increases DNA methyltransferase expression (Bromer et al., 2009). Therefore, in this study, DES may be altering methylation patterns of key developmental and hormonal genes in a permanent and heritable manner, thus resulting in transgenerational effects on fertility, puberty, and AGD.
Our results have shown that DES does not act in a linear dose response, with the most effects being observed after exposure to 50 µg/kg DES. Although the higher dose of DES had the most impact on fertility in the F1 generation, this was recovered by the F3 generation, whereas exposure to 50 µg/kg DES led to transgenerational effects on fertility, and furthermore, puberty was significantly impacted for multiple generations after exposure to 50 µg/kg and not 100 µg/kg DES. However, there have been many examples of EDCs acting in a nonlinear dose response (Vandenberg, 2014; Vandenberg et al., 2012). Reasons that may explain this nonlinear dose response include EDCs binding multiple different receptors at a high dose which then elicit opposite effects, or saturation of the metabolic system at a high dose which can result in the parent compound causing effects and not the metabolite, or a high dose resulting in desensitization of receptors (Lagarde et al., 2015).
Our results showed that prenatal exposure to DES caused multi- and transgenerational effects on female fertility and reproductive development. Specifically, DES reduced fertility index, pregnancy rate, timing of puberty, and AGD across all 3 generations. Although DES is no longer in use, these results have implications for the millions of DES descendants, as they may be at higher risk of experiencing reproductive abnormalities due to their ancestral exposure. Moreover, this study also has broader implications for society in general as it adds to the growing evidence that EDC exposure negatively impacts human fertility and reproductive health. Therefore, it is essential that we continue to monitor the effects of EDCs and regulate policy around our exposure to these chemicals, as well as implementing more stringent rules on new chemical development and educating individuals on the risk of EDC exposure especially during pregnancy.
Supplementary Material
Acknowledgments
We would like to thank Laura Cook for her assistance with the data analysis and presentation.
Contributor Information
Rachael E Rogers, School of BioSciences, The University of Melbourne, Parkville, Victoria 3010, Australia.
Shuyi Chai, School of BioSciences, The University of Melbourne, Parkville, Victoria 3010, Australia.
Andrew J Pask, School of BioSciences, The University of Melbourne, Parkville, Victoria 3010, Australia.
Deidre M Mattiske, School of BioSciences, The University of Melbourne, Parkville, Victoria 3010, Australia.
Supplementary data
Supplementary data are available at Toxicological Sciences online.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was funded by a John McKenzie fellowship awarded to Deidre Mattiske from the University of Melbourne.
References
- Al Jishi T., Sergi C. (2017). Current perspective of diethylstilbestrol (DES) exposure in mothers and offspring. Reprod. Toxicol. 71, 71–77. [DOI] [PubMed] [Google Scholar]
- Arango N. A., Donahoe P. K. (2008). Sex differentiation in mouse and man and subsequent development of the female reproductive organs. In StemBook (The Stem Cell Research Community, Ed.). Harvard Stem Cell Institute, Cambridge, MA. [PubMed] [Google Scholar]
- Aris A. Z., Shamsuddin A. S., Praveena S. M. (2014). Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: a review. Environ. Int. 69, 104–119. [DOI] [PubMed] [Google Scholar]
- Barbour H. G., Trace J. (1936). Standard metabolism in the white mouse. Am. J. Physiol. 118, 77–86. [Google Scholar]
- Boivin J., Bunting L., Collins J. A., Nygren K. G. (2007). International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum. Reprod. 22, 1506–1512. [DOI] [PubMed] [Google Scholar]
- Bromer J. G., Wu J., Zhou Y., Taylor H. S. (2009). Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology 150, 3376–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta D., Bordi G., Ciardo F., Marci R., La Rocca C., Tait S., Bergamasco B., Stecca L., Mantovani A., Guerranti C., et al. (2013). The influence of endocrine disruptors in a selected population of infertile women. Gynecol. Endocrinol. 29, 444–447. [DOI] [PubMed] [Google Scholar]
- Christiansen S., Axelstad M., Boberg J., Vinggaard A. M., Pedersen G. A., Hass U. (2014). Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 147, 477–487. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J. P., Giudice L. C., Hauser R., Prins G. S., Soto A. M., Zoeller R. T., Gore A. C. (2009). Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr. Rev. 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiVall S. A., Radovick S. (2009). Endocrinology of female puberty. Curr. Opin. Endocrinol. Diabetes. Obes. 16, 1–4. [DOI] [PubMed] [Google Scholar]
- Dolinoy D. C., Huang D., Jirtle R. L. (2007). Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. U.S.A. 104, 13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallavan R. H., Holson J. F., Stump D. G., Knapp J. F., Reynolds V. L. (1999). Interpreting the toxicologic significance of alterations in anogenital distance: potential for confounding effects of progeny body weights. Reprod. Toxicol. 13, 383–390. [DOI] [PubMed] [Google Scholar]
- Hamilton B. E., Ventura S. J. (2006). Fertility and abortion rates in the United States, 1960-2002. Int. J. Androl. 29, 34–45. [DOI] [PubMed] [Google Scholar]
- Hao C.-J., Cheng X.-J., Xia H.-F., Ma X. (2012). The endocrine disruptor diethylstilbestrol induces adipocyte differentiation and promotes obesity in mice. Toxicol. Appl. Pharmacol. 263, 102–110. [DOI] [PubMed] [Google Scholar]
- He F., Akbari P., Mo R., Zhang J. J., Hui C.-C., Kim P. C., Farhat W. A. (2016). Adult Gli2+/−;Gli3Δ699/+ male and female mice display a spectrum of genital malformation. PLoS One 11, e0165958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst A. L., Ulfelder H., Poskanzer D. C. (1971). Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 284, 878–881. [DOI] [PubMed] [Google Scholar]
- Hoover R. N., Hyer M., Pfeiffer R. M., Adam E., Bond B., Cheville A. L., Colton T., Hartge P., Hatch E. E., Herbst A. L., et al. (2011). Adverse health outcomes in women exposed in utero to diethylstilbestrol. N. Engl. J. Med. 365, 1304–1314. [DOI] [PubMed] [Google Scholar]
- Kabir E. R., Rahman M. S., Rahman I. (2015). A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 40, 241–258. [DOI] [PubMed] [Google Scholar]
- Kaufman R. H., Adam E., Hatch E. E., Noller K., Herbst A. L., Palmer J. R., Hoover R. N. (2000). Continued follow-up of pregnancy outcomes in diethylstilbestrol-exposed offspring. Obstet. Gynecol. 96, 483–489. [DOI] [PubMed] [Google Scholar]
- Lagarde F., Beausoleil C., Belcher S. M., Belzunces L. P., Emond C., Guerbet M., Rousselle C. (2015). Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ. Health 14, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H., Jorgensen N., Martino-Andrade A., Mendiola J., Weksler-Derri D., Mindlis I., Pinotti R., Swan S. H. (2017). Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum. Reprod. Update 23, 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Washburn K. A., Moore R., Uno T., Teng C., Newbold R. R., McLachlan J. A., Negishi M. (1997). Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 57, 4356–4359. [PubMed] [Google Scholar]
- López-Rodríguez D., Aylwin C. F., Delli V., Sevrin E., Campanile M., Martin M., Franssen D., Gérard A., Blacher S., Tirelli E., et al. (2021). Multi- and transgenerational outcomes of an exposure to a mixture of endocrine-disrupting chemicals (EDCs) on puberty and maternal behavior in the female rat. Environ. Health Perspect. 129, 87003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahawong P., Sinclair A., Li Y., Schlomer B., Rodriguez E., Ferretti M. M., Liu B., Baskin L. S., Cunha G. R. (2014). Prenatal diethylstilbestrol induces malformation of the external genitalia of male and female mice and persistent second-generation developmental abnormalities of the external genitalia in two mouse strains. Differentiation 88, 51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M., Tracey R., Guerrero-Bosagna C., Skinner M. K. (2013). Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One 8, e55387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiske D., Behringer R. R., Overbeek P. A., Pask A. J. (2020). A novel long non-coding RNA, Leat1, causes reduced anogenital distance and fertility in female mice. Differentiation 112, 1–6. [DOI] [PubMed] [Google Scholar]
- Mayer C., Acosta-Martinez M., Dubois S. L., Wolfe A., Radovick S., Boehm U., Levine J. E. (2010). Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc. Natl. Acad. Sci. U.S.A. 107, 22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey J. R., Lehle J. D., Raju S. S., Wang Y., Nilsson E. E., Skinner M. K. (2016). Tertiary epimutations—a novel aspect of epigenetic transgenerational inheritance promoting genome instability. PLoS One 11, e0168038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok-Lin E., Ehrlich S., Williams P. L., Petrozza J., Wright D. L., Calafat A. M., Ye X., Hauser R. (2010). Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int. J. Androl. 33, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. R., Hanson R. B., Jefferson W. N., Bullock B. C., Haseman J., McLachlan J. A. (1998). Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis 19, 1655–1663. [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Moore A. B., Dixon D. (2002). Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES). Toxicol. Pathol. 30, 611–616. [DOI] [PubMed] [Google Scholar]
- Nordkap L., Joensen U. N., Blomberg Jensen M., Jorgensen N. (2012). Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol. Cell. Endocrinol. 355, 221–230. [DOI] [PubMed] [Google Scholar]
- Palmer J. R., Anderson D., Helmrich S. P., Herbst A. L. (2000). Risk factors for diethylstilbestrol-associated clear cell adenocarcinoma. Obstet. Gynecol. 95, 814–820. [PubMed] [Google Scholar]
- Prins G. S. (2008). Estrogen imprinting: when your epigenetic memories come back to haunt you. Endocrinology 149, 5919–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S., Brehm E., Gao L., Flaws J. A. (2018). Di(2-Ethylhexyl) phthalate exposure during prenatal development causes adverse transgenerational effects on female fertility in mice. Toxicol. Sci. 163, 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Fukata H., Kogo Y., Ohgane J., Shiota K., Mori C. (2009). Neonatal exposure to diethylstilbestrol alters expression of DNA methyltransferases and methylation of genomic DNA in the mouse uterus. Endocr. J. 56, 131–139. [DOI] [PubMed] [Google Scholar]
- Senekjian E. K., Potkul R. K., Frey K., Herbst A. L. (1988). Infertility among daughters either exposed or not exposed to diethylstilbestrol. Am. J. Obstet. Gynecol. 158, 493–498. [DOI] [PubMed] [Google Scholar]
- Sharp G. B., Cole P. (1991). Identification of risk factors for diethylstilbestrol-associated clear cell adenocarcinoma of the vagina: similarities to endometrial cancer. Am. J. Epidemiol. 134, 1316–1324. [DOI] [PubMed] [Google Scholar]
- Skinner M. K. (2014). Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol. Cell. Endocrinol. 398, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. K., Manikkam M., Guerrero-Bosagna C. (2010). Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 21, 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souter I., Smith K. W., Dimitriadis I., Ehrlich S., Williams P. L., Calafat A. M., Hauser R. (2013). The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod. Toxicol. 42, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. K., Mattiske D. M., Pask A. J. (2018). In utero exposure to both high- and low-dose diethylstilbestrol disrupts mouse genital tubercle development. Biol. Reprod. 99, 1184–1193. [DOI] [PubMed] [Google Scholar]
- Suen A. A., Jefferson W. N., Williams C. J., Wood C. E. (2018). Differentiation patterns of uterine carcinomas and precursor lesions induced by neonatal estrogen exposure in mice. Toxicol. Pathol. 46, 574–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus L. (2021). Evidence of intergenerational transmission of diethylstilbestrol health effects: hindsight and insight. Biol. Reprod. 105, 681–686. [DOI] [PubMed] [Google Scholar]
- Titus L., Hatch E. E., Drake K. M., Parker S. E., Hyer M., Palmer J. R., Strohsnitter W. C., Adam E., Herbst A. L., Huo D., et al. (2019). Reproductive and hormone-related outcomes in women whose mothers were exposed in utero to diethylstilbestrol (DES): a report from the US national cancer institute DES third generation study. Reprod. Toxicol. 84, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus-Ernstoff L., Troisi R., Hatch E. E., Wise L. A., Palmer J., Hyer M., Kaufman R., Adam E., Strohsnitter W., Noller K., et al. (2006). Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES). Int. J. Epidemiol. 35, 862–868. [DOI] [PubMed] [Google Scholar]
- Tyl R. W., Myers C. B., Marr M. C., Sloan C. S., Castillo N. P., Veselica M. M., Seely J. C., Dimond S. S., Van Miller J. P., Shiotsuka R. N., et al. (2008). Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 104, 362–384. [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N. (2014). Non-Monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol A as a case study. Dose Response 12, 259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L. N., Colborn T., Hayes T. B., Heindel J. J., Jacobs D. R. Jr, Lee D.-H., Shioda T., Soto A. M., vom Saal F. S., Welshons W. V., et al. (2012). Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veurink M., Koster M., Berg L. T. (2005). The history of DES, lessons to be learned. Pharm. World Sci. 27, 139–143. [DOI] [PubMed] [Google Scholar]
- Ziv-Gal A., Wang W., Zhou C., Flaws J. A. (2015). The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol. Appl. Pharmacol. 284, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



