Abstract
Background:
There has been a recent focus among anesthesiologists on reducing the use of perioperative opioids in favor of multimodal analgesic regimens. Gabapentin has played an integral role in this evolution of practice. This comprehensive review assesses the current clinical evidence on the efficacy of perioperative gabapentin regarding postoperative pain and opioid requirements among the pediatric surgery population.
Data Sources:
Pubmed, CINAHL, Embase, Scopus, and Web of Science Review
Methods:
This scoping review of the above databases includes all studies examining the use of gabapentin perioperatively in pediatric patients and its association with postoperative pain intensity and postoperative opioid consumption through July 2021. The inclusion criteria encompassed all studies evaluating gabapentin in the perioperative pediatric population through randomized controlled trials (RCTs) and retrospective studies. Relevant metadata from each study were abstracted and descriptive statistics were used to summarize the results.
Results:
Fifteen papers met the inclusion criteria for this review, including eleven RCTs and four retrospective studies. Sample sizes ranged from 20–144 patients. Administered doses varied widely, mainly between 5–20 mg/kg. The studies included primarily orthopedic (10) and neck surgery cases (3). Seven papers had gabapentin provided preoperatively only, two postoperative only, and six both pre- and postoperatively. Of the studies assessing postoperative pain, 6/11 studies saw a decrease in postoperative pain in at least one period for the gabapentin group. Of the studies considering opioid requirements, 6/10 reported a reduction, 1/10 an increase, and 3/10 no difference in opioid requirements for the gabapentin groups. Yet, most of these pain and opioid requirement findings were only significant at 1–2 time points in the study follow-up periods, and the actual decreases had minimal clinical significance.
Conclusions:
The current data on perioperative gabapentin in pediatric patients is insufficient to support the routine use of gabapentin in pediatric patients. Additional high-quality RCTs with more standardized protocols for gabapentin administration and outcome measures are necessary to provide more definitive conclusions.
Keywords: Gabapentin, anesthesia, pediatric, pediatric anesthesia, perioperative pain, multimodal analgesia, opioids
Introduction
Recently, there has been an increased emphasis on rethinking perioperative pain management using multimodal analgesic approaches in pediatric patients. This extends beyond the use of traditional analgesics used in children such acetaminophen, morphine, and ibuprofen to other opioids, non-steroidal anti-inflammatory drugs (NSAIDs), and non-opioid adjuncts.1–3 These approaches are often used as an integral component of the enhanced recovery program (ERP) care pathways that improve a patient’s pain optimization, recovery time, surgical outcome, and care experience.4,5 ERP protocols favors the planned and coordinated optimization of non-opioid analgesics, such as acetaminophen and NSAIDs, along with other non-opioid adjuncts, such as ketamine, lidocaine, and gabapentin, alongside opioids into the pain relief regimen.6 Gabapentin is traditionally used as an oral anticonvulsant and non-opioid analgesic in the treatment of epilepsy and chronic neuropathic pain.7 However, there has been a recently growing body of evidence supporting the clinical benefit of gabapentin in surgical patients, particularly as it relates to reducing opioid use in the perioperative period and potentially even preventing the incidence of chronic postsurgical pain in adult patients.8,9
While opioids provide excellent short-term analgesia, they come with short-term dose-related side effects such as postoperative nausea and vomiting (PONV), urinary retention, intestinal obstruction, pruritus, and respiratory depression.10,11 The ill effects of opioid therapy, alongside the potential for overdose and long-term dependence, especially in older children and adolescents, make reducing perioperative opioid therapy preferable whenever possible.12,13
Extensive reviews have been performed to assess the efficacy of gabapentin in reducing acute, subacute, and chronic postoperative pain in adults. Some smaller systematic reviews have identified gabapentin as an effective adjunct in postoperative pain control,9,14–16 including a decrease in postoperative opioid consumption.14,15 Larger scale reviews have since been conducted, one of which identified 132 randomized control trials (RCTs) with 9,498 adult patients and concluded that the quality of evidence supporting the regular use of gabapentin perioperatively was low. This was due to imprecision, inconsistency, and risk of bias.17 More recently, a systematic review with meta-analysis was conducted using 281 RCTs with 24,682 participants, yielding similar results to Fabritius et al.18 Currently, there is insufficient evidence to support the routine administration of gabapentin perioperatively among adults.
The current data for efficacy of perioperative gabapentin on postoperative pain and opioid use in pediatrics is even more sparse. A recent systematic review of seven studies assessing the efficacy and safety of gabapentinoids for pain, including postoperative, neuropathic, and fibromyalgia pain, in children identified insufficient evidence for its use in pediatric patients.1 Work such as this has made recommendations for perioperative gabapentin in children unclear. Although findings in adult patients are more comprehensive, these cannot translate to children, given the differences in body composition, drug absorption, distribution, clearance, dosage, and interval.19
Even though gabapentin is often thought of as a safer medication as compared to opioids, it is certainly not benign. When used, gabapentin is often administered at 15–20 mg/kg orally one to two hours before induction for children undergoing major surgery that may also have a neuropathic pain component.20 No more than 600 mg is given in a single dose preoperatively to avoid an increased risk for delayed emergence from anesthesia and prolonged sedation20. However, there is currently limited evidence for the optimal dose and timing, leading to wide variability in reported dosing. Further, gabapentin is typically discontinued postoperatively to avoid the increased risk of respiratory depression when used in conjunction with postoperative opioids.21
The objective of this scoping review is to identify and characterize the current state of knowledge regarding the effect of perioperative gabapentin use on postoperative pain intensity (based on pain scores) and postoperative opioid consumption. This is pertinent knowledge as gabapentin continues to be incorporated into perioperative multimodal non-opioid analgesic approaches. This article will then further discuss potential reasons for the sparsity of studies and the current lack of clinical evidence on this topic and how the field may proceed to help guide evidence-based perioperative pain management practices in pediatrics.
Methods
Literature Search
Following PRISMA Scoping Review (PRISMA-ScR) guidelines, searches were conducted in 5 databases: PubMed (MEDLINE), CINAHL, Embase, Scopus, and Web of Science, from their inception to July 29, 2021. The following search strategy was used in PubMed:
#1 Topic = (Gabapentin* or Neurontin).
#2 Topic = (pain* or neuralgi* or neuropath* or analgesi* or anesthesi* or anaesthesi* or preemptive* or pre-emptive* or preventive* or prophylax*).
#3 Topic = (surg* or operati* or periop* or peri-op* or postop* or post-op* or preop* or pre-op* or intraop* or intra-op* or procedur* or preincision*).
#4 Topic = (child* or adolescent* or “young adult*” or infant* or youth* or toddler* or newborn* or neonat* or pediatric*).
#5 = #4 AND #3 AND #2 AND #1.
Similar search strategies were conducted for CINAHL, Embase, Scopus, and Web of Science.
Inclusion Criteria
This review encompasses randomized controlled trials (RCTs) and retrospective studies (cohort or case-control) that assessed the analgesic effect of perioperative gabapentin (all doses and frequencies) in the pediatric population – children and adolescents <18 years of age through July 2021. Only full-text English language articles were included. Figure 1 details exclusion reasons.
Figure 1.
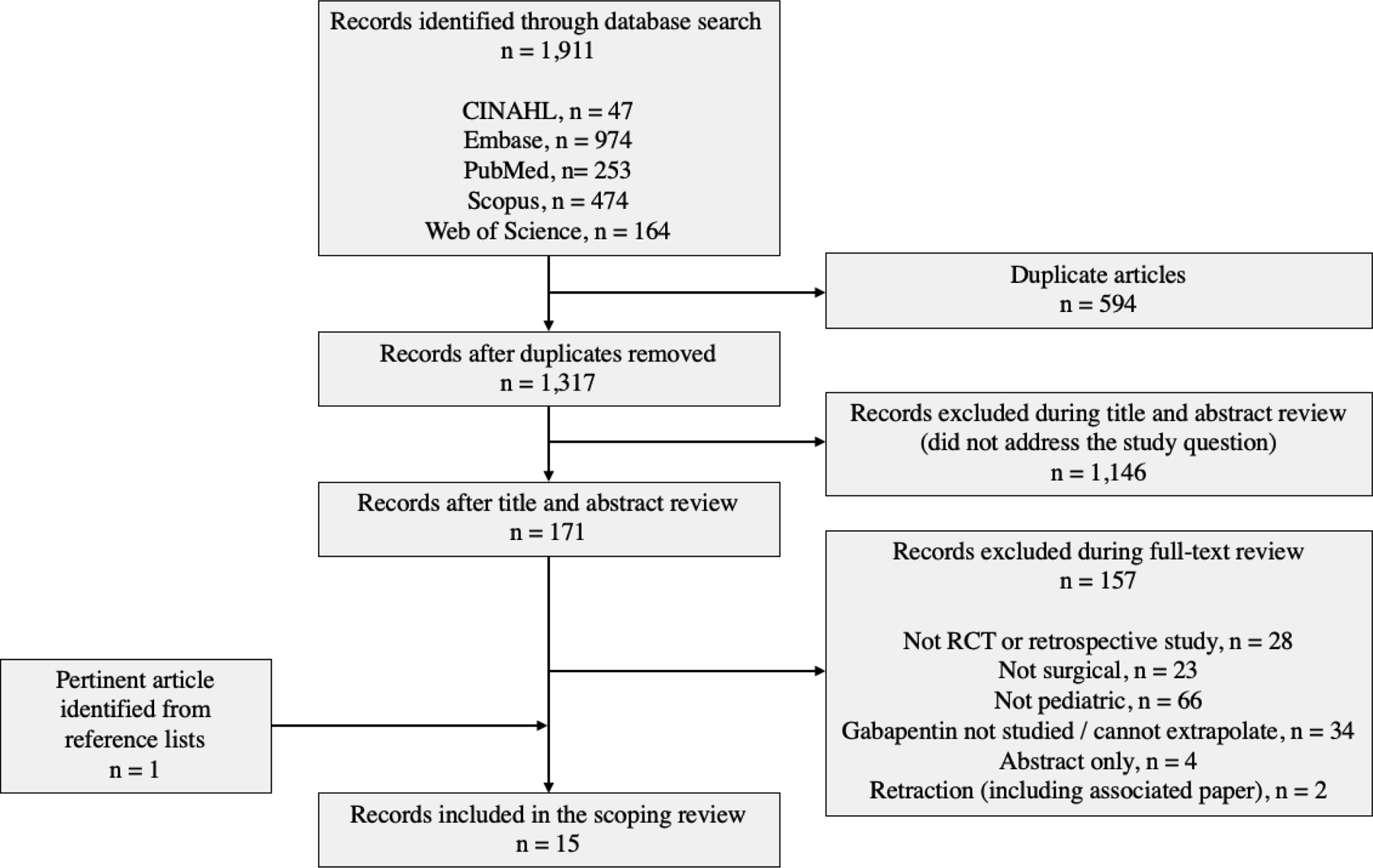
Flow diagram summarizing search process and results.
Data abstraction
Citations from initial database searches were compiled into a reference management program (EndNote), and an independent review process was performed. After removing duplicates, each abstract was assessed, and further full-text reviews were conducted to identify articles that met the inclusion criteria. Using a qualitative descriptive approach, the authors extracted and categorized data from each article into tables that captured the study design, intervention description, sample characteristics, outcome measures, and results relating to postoperative pain and opioid consumption (Tables 1–4). Text eligibility and review were initially assessed by one author (OC). Full-text review was then confirmed via independent review by authors (OC and JBC).
Table 1.
Study Design and Baseline Subject Characteristics
| Reference | Study Design | Type of Surgery | Intervention (No. of Participants) | Comparator (No. of Participants) | Comparator (No. of Participants) | Age Range (years) | Sex (M/F) |
|---|---|---|---|---|---|---|---|
| Anderson28 | RCT | Orthopedic (PSF for Idiopathic Scoliosis) | Gabapentin (24) | Placebo (26) | --- | 10–19 | 12 M / 38 F |
| Badawy39 | RCT | Ophthalmic (Strabismus Repair) | Gabapentin (35) | Placebo (35) | --- | 2–6 | 49 M / 16 F |
| Baxter27 | Retrospective Cohort | Abdominal (Single and 3-port Laparoscopic Appendectomy) | Gabapentin (29) | No Gabapentin (58) | --- | Not stated | 72 M / 15 F |
| Choudhry21 | Retrospective Cohort (3 groups M-PCA, M-PCA + Gabapentin, M-PCA + Gabapentin +Transdermal Clonidine) | Orthopedic (PSF for Idiopathic Scoliosis) | Gabapentin + M-PCA (45) | M-PCA only (42) | Gabapentin + M-PCA + Transdermal Clonidine (40) | 11–20 | 26 M / 101 F |
| Gettis32 | RCT | Orthopedic (ACL reconstruction) | Gabapentin (26) | Placebo (26) | --- | 12–18 | 25 M / 27 F |
| Haddadi20 | RCT | Head and Neck (Adenotonsillectomy) | Gabapentin (30) | Acetaminophen (30) | --- | 7–15 | Not stated |
| Mayell33 | RCT | Orthopedic (PSF for Idiopathic Scoliosis Surgery) | Gabapentin (18) | Placebo (17) | --- | 10–17 | 6 M, 29 F |
| Mohamed19 | RCT | Head and Neck (Adenotonsillectomy) | Gabapentin (72) | Placebo (72) | --- | 4–8 | 82 M, 62 F |
| Pinto Filho31 | RCT | Orthopedic (Unilateral limb surgery: minor, medium & major) | Gabapentin (40) | Control (44) | --- | 3 months - 16 years | 53 M, 31 F |
| Rusy26 | RCT | Orthopedic (PSF for Idiopathic Scoliosis Surgery) | Gabapentin (29) | Placebo (30) | --- | 9–18 | 14 M, 45 F |
| Salman40 | RCT | Head and Neck (Tonsillectomy or Adenoidectomy) | Gabapentin (23) | Saline (23) | --- | 3–12 | 19 M / 27 F |
| Thomas25 | Retrospective Cohort | Orthopedic (PSF for Idiopathic Scoliosis Surgery) | Gabapentin (50) | No Gabapentin (51) | --- | 10–18 | 16 M / 85 F |
| Tomaszek24 | RCT | Thoracic (Ravitch Procedure) | Gabapentin (20) | Placebo (20) | --- | 9–17 | 34 M, 6 F |
| Trzcinski23 | Retrospective Cohort | Orthopedic (PSF for Idiopathic Scoliosis Surgery) | Gabapentin (24) | No Gabapentin (105) | --- | 10–21 | 33 M / 96 F |
| Wang22 | RCT | Orthopedic (AKA and BKA) | Gabapentin (23) | Placebo (22) | --- | 10–17 | 23 M, 22 F |
RCT, randomized controlled trial; M-PCA, morphine patient-controlled analgesia; PSF, posterior spinal fusion; ACL, anterior cruciate ligament; AKA, above the knee amputation; BKA, below the knee amputation
Table 4.
Efficacy of Intervention vs. Comparator with Regards to Postoperative Analgesia Administration
| Reference | Method of Measuring Opioid Consumption | Time Period(s) | Intervention Group Opioid Consumption | Comparator Group Opioid Consumption | Significance |
|---|---|---|---|---|---|
| Anderson28 | Total Opioids Administered (in mg/kg ME) – mean (SD) | a. Operative Day b. Postoperative Period c. Total Perioperative Period |
a. 0.20 (0.13) b. 3.38 (1.79) c. 3.58 (1.82) |
a. 0.28 (0.21) b. 5.05 (3.16) c. 5.33 (3.20) |
Significantly less MMEs for the postoperative (p=0.03) and total perioperative (p=0.02) periods only |
| Badawy39 | Percentage of Patients Requiring Postoperative Meperidine | Postoperative period | 30.3% | 52.9% | Significantly less (p=0.03) |
| Baxter27 | Total Opioids Administered (in mg/kg ME) – mean for: 1. Simple appendicitis 2. Perforated appendicitis 3. Overall |
Postoperative period | 1. 0.010 2. 0.057 3. 0.034 |
1. 0.055 2. 0.153 3. 0.106 |
Significantly less opioids for simple (p=0.01), complicated (p=0.03), and overall appendicitis cases (p<0.01) |
| Choudhry21 | Daily Opioids Administered (in mg/kg/hr) ME – mean | POD 0 and POD 1 | Gabapentin alone: POD 0: 0.041 POD 1: 0.028 Gabapentin + clonidine: POD 0: 0.045 POD 1: 0.023 |
POD 0: 0.048 POD 1: 0.042 |
Significantly less opioids on POD 1 for gabapentin (p<0.001) and clonidine + gabapentin (p<0.001) compared to control groups |
| Gettis32 | Daily Opioids Administered (in mg/kg ME) – median (IQR) | POD 1 through POD 5 | POD 1: 0.14 (0.09, 0.21) POD 2: 0.10 (0.04, 0.29) POD 3: 0.08 (0.00, 0.20) POD 4: 0.04 (0.00, 0.14) POD 5: 0.04 (0.00, 0.13) |
POD 1: 0.18 (0.11, 0.26) POD 2: 0.16 (0.00, 0.31) POD 3: 0.07 (0.00, 0.24) POD 4: 0.00 (0.00, 0.10) POD 5: 0.00 (0.00, 0.09) |
Not significant at any time |
| Mayell33 | Cumulative Opioids Administered (in mg/kg ME) – mean (SD) | 1, 4, 8, 24, 48, and 72 hours postop | 1 hour: 0.087 (0.6) 4 hours: 0.24 (0.12) 8 hours: 0.44 (0.17) 24 hours: 1.29 (0.44) 48 & 72 hours: Quantitative data not given |
1 hour: 0.121 (0.06) 4 hours: 0.35 (0.16) 8 hours: 0.56 (0.27) 24 hours: 1.46 (0.68) 48 & 72 hours: quantitative data not given |
Not significant at any time |
| Mohamed19 | Percentage of Patients Requiring Postoperative Analgesia with Intravenous Ketorolac | Postoperative period | 19.4% | 48.6% | Significantly less (p=0.0004) |
| Pinto Filho31 | 1. Time to first morphine administration – mean (SD) 2. Percentage of patients requiring postop morphine 3. Daily consumption of morphine (in mg/kg/day) – mean |
Perioperative period | 1. 7.3 (4.6) hours 2. 30% 3. 0.09 |
1. 7.8 (3.6) hours 2. 44% 3. 0.08 |
Not significant for any measures |
| Rusy26 | Daily Opioids Administered (in mg/kg/hr ME) – mean (SD) | POD 0 through POD 5 | POD 0: 0.044 (0.017) POD 1: 0.046 (0.016) POD 2: 0.036 (0.016) POD 3–5: Not stated |
POD 0: 0.064 (0.031) POD 1: 0.055 (0.017) POD 2: 0.047 (0.019) POD 3–5: Not stated |
Significantly less on POD 0 (p=0.003) and POD 2 (p=0.018), close to significantly less on POD 1 (p=0.051), no difference on POD 3–5 |
| Salman40 | Number of Postoperative Analgesia Administrations with Acetaminophen – mean | 24 hours after surgery | 1.68 | 3.29 | Significantly less (p<0.01) |
| Thomas25 | Daily Opioids Administered (in mg ME) – mean (SD) | POD 1 through 3 | POD 1: 20 (17) POD 2: 17 (10) POD 3: 13 (9) |
POD 1: 13 (13) POD 2: 14 (10) POD 3: 10 (7) |
Significantly higher on POD 1 (p=0.0357) only |
| Trzcinski23 | Daily Opioids Administered (in mg/kg ME) – mean | POD 1 through POD 4 | POD 1: 0.38 POD 2: 0.68 POD 3: 0.72 POD 4: 0.66 |
POD 1: 0.75 POD 2: 1.s00 POD 3: 0.82 POD 4: 0.55 |
Significantly lower on POD 1 (p<0.001) and POD 2 (p=0.019) only |
POD, postoperative day; ME, morphine equivalents; SD, Standard Deviation; IQR, Interquartile Range
Results
Literature Review
From the five databases, 1,911 publications were gathered, of which 594 were duplicates. Of the 1,317 unique articles screened, 1,146 were omitted because they were not relevant or specific to the topic of interest. Among the remaining 171 articles assessed for eligibility, 28 were excluded because they were not RCTs or retrospective studies (i.e., they were reviews, case reports without controls, or commentary/response articles), 23 were excluded because they were not surgical, and 66 were excluded because they were not pediatric studies. Another 34 were excluded because either gabapentin was not studied, or it was part of a multimodal intervention in which results for gabapentin were not able to be separated. Four were excluded for lacking full-text availability (abstract only), and two were excluded due to the retraction of one and its association with another by the same author. The latter was omitted for the potential concern of validity. The remaining fourteen articles met the inclusion criteria and were utilized in this review. An additional publication was identified from the reference list of an article found from the above search strategy and met the inclusion criteria resulting in a total of fifteen included articles.22 (Figure 1)
Study Characteristics
The tables below summarize baseline characteristics (Table 1), intervention characteristics (Table 2), and outcome measures between the two groups – intervention and its comparator with regards to postoperative pain and postoperative analgesia administered. (Tables 3–4)
Table 2.
Intervention Characteristics
| Reference | Pre-operative Gabapentin | Dosage | Frequency of Administration | Duration of Administration |
|---|---|---|---|---|
| Anderson28 | Yes | 15 mg/kg pre-op / 10 mg/kg post-op | Once pre-op / TID post-op | Not stated pre-op / 5 days post-op |
| Badawy39 | Yes | 5 mg/kg pre-op | Once | 1 hour pre-op |
| Baxter27 | No | 4.4–30.4 mg/kg/day post-op (median 10.1) | TID post-op | Not stated post-op |
| Choudhry21 | Yes | Gabapentin10 mg/kg pre-op (maximum of 600mg) and 200 mg TID (if >50 kg) or 100 mg TID (if <50 kg) total post-op Clonidine (0.1mg) transdermal patch 0.05 mg/d folded in half and covered with patch |
Gabapentin once pre-op / TID post-op | Gabapentin 1 hour pre-op / until discharge post-op Clonidine patch kept in place for 7 days post-op |
| Gettis32 | Yes | 15 mg/kg (maximum of 600 mg) | Once | 30–60 minutes pre-op |
| Haddadi20 | Yes | Gabapentin 10 mg/kg Acetaminophen 40 mg/kg suppository (after induction) |
Once | Gabapentin 2 hours pre-op Acetaminophen – After induction |
| Mayell33 | Yes | 600 mg | Once | 1 hour pre-op |
| Mohamed19 | Yes | 20 mg/kg | Once | 2 hours pre-op |
| Pinto Filho31 | Yes | 10 mg/kg (maximum of 600 mg) | Once | 1–2 hours pre-op |
| Rusy26 | Yes | 15 mg/kg pre-op / 5 mg/ kg post-op | Once pre-op / TID post-op | 25–30 minutes pre-op / 5 days post-op |
| Salman40 | Yes | 15 mg/kg | Once | 30 minutes pre-op |
| Thomas25 | Yes | If >40kg, 300mg capsule pre- and post-op / If 20–40kg, 200 mg capsule pre- and post-op or 5 mg/kg pre- and post-op (if they could not swallow) |
Once pre-op / TID post-op | Not stated pre-op / until discharge post-op |
| Tomaszek24 | Yes | 15 mg/kg pre-op / 7.5 mg/kg post-op | Once pre-op / BID post-op | 1 hour pre-op / 3 days post-op |
| Trzcinski23 | Yes | 10 mg/kg pre-op / 5 mg/kg post-op | Once pre-op / TID post-op | Morning of surgery pre-op / 4 days post-op |
| Wang22 | No | 300 mg total | Once on Day 1, BID on Day 2, TID for Day 3 onward | 30 days |
TID, 3 times daily, BID, 2 times daily.
Table 3.
Efficacy of Intervention vs. Comparator with Regards to Postoperative Pain
| Reference | Pain Score | Time Period(s) | Intervention Group Pain Score | Comparator Group Pain Score | Significance |
|---|---|---|---|---|---|
| Anderson28 | VAS | Preoperative day, operative day, and POD 1 through POD 5 | No numerical value: lower VAS score visualized on figure | No numerical value: higher VAS score visualized on figure | Significantly lower pain score (p=0.02) on operative day only |
| Baxter27 | Time to VAS ≤ 3 – mean | Postoperative Period | 12.21 hours | 17.01 hours | Not significant (p=0.23) |
| Gettis32 | VAS – mean (SD) | POD 1 | 4.76 (2.09) | 5.01 (1.76) | Not significant (p=0.658) |
| Haddadi20 | VAS – mean (SD) | 0, 2, 4, 6, 12, and 24 hours postop | 0 hours: 3.20 (1.94) 2 hours: 1.67 (0.92) 4 hours: 1.83 (1.15) 6 hours: 1.33 (0.84) 12 hours: 1.20 (1.21) 24 hours: 0.93 (1.26) |
0 hours: 2.73 (1.57) 2 hours: 1.23 (0.86) 4 hours: 1.53 (1.07) 6 hours: 1.53 (1.07) 12 hours: 0.93 (0.69) 24 hours: 0.60 (0.62) |
Not significant at any time |
| Mayell33 | NRS | 1, 4, 8, 24, 48, and 72 hours postop | No numerical value: similar pain score visualized on figures | No numerical value: similar pain score visualized on figures | Not significant at any time |
| Pinto Filho31 | CRIES, CHIPPS, and Wong-Baker Faces scale depending on developmental level | 1, 4, 8, 12, 18, and 24 hours postop | No numerical value; higher frequency of “without pain” category | No numerical value; lower frequency of “without pain” category | Significantly lower pain only 4th and 8th hour post-op (p<0.05) |
| Rusy26 | NRS – mean (SD) | POD 0 through POD 5 (AM and PM) | POD 0: 2.5 (2.8) POD 1 AM: 3.2 (2.6) POD 1 PM-5 PM: Not stated |
POD 0: 6.0 (2.4) POD 1 AM: 5.0 (2.2) POD 1 PM-5 PM: Not stated |
Significantly lower pain score on POD 0 (p<0.001) and POD 1 AM (p<0.05) only |
| Thomas25 | NRS – mean (SD) | POD 1 through 3 | POD 1: 5 (2) POD 2: 5 (2) POD 3: 5 (3) |
POD 1: 6 (2) POD 2: 7 (2) POD 3: 6 (3) |
Significantly lower on POD 2 (p=0.0025) only |
| Tomaszek24 | NRS at rest, during deep breathing, and during cough – median (IQR) | POD 0 through POD 3 | At rest: POD 0: 0.5 (0.3–0.8) POD 1: 0.4 (0.0–0.7) POD 2: 0.0 (0.0–0.7) POD 3: 0.0 (0.0–0.6) During deep breathing: POD 0: 0.4 (0.3–0.9) POD 1: 0.3 (0.1–0.6) POD 2: 0.1 (0.0–0.3) POD 3: 0.0 (0.0–0.2) During cough: POD 0: 0.6 (0.3–0.7) POD 1: 0.5 (0.2–0.5) POD 2: 0.3 (0.0–0.5) POD 3: 0.0 (0.0–0.3) |
At rest: POD 0: 0.5 (0.3–1.0) POD 1: 0.1 (0.0–0.8) POD 2: 0.5 (0.0–1.0) POD 3: 0.5 (0.0–1.1) During deep breathing: POD 0: 0.3 (0.1–0.6) POD 1: 0.2 (0.0–0.4) POD 2: 0.0 (0.0–0.2) POD 3: 0.0 (0.0–0.3) During cough: POD 0: 0.4 (0.2–0.8) POD 1: 0.3 (0.0–0.7) POD 2: 0.1 (0.0–0.3) POD 3: 0.0 (0.0–0.6) |
Not significant at any time |
| Trzcinski23 | NRS or Wong-Baker FACES scale depending on developmental level – mean | POD 1 through POD 4 | POD 1: 3.51 POD 2: 3.57 POD 3: 3.35 POD 4: 3.12 |
POD 1: 4.00 POD 2: 4.12 POD 3: 3.83 POD 4: 3.43 |
Significantly lower on POD 1 (p=0.012), POD 2 (p=0.002), and POD 3 (0.037) only |
| Wang22 | VAS | Day of hospitalization, day of randomization, operative day, and POD 1 through 14 | No numerical value: lower VAS score visualized on figure from POD 4 though POD 9 | No numerical value: higher VAS score visualized on figure from POD 4 though POD 9 | Significantly lower pain score (p<0.05) from POD 4–9 only |
VAS, visual analog scale (0–10); POD, postoperative day; NRS, numerical rating score (0–10); ME, morphine equivalents; SD, Standard Deviation; IQR, Interquartile Range
Of the fifteen included articles, eleven were RCTs, and four were retrospective analyses. The studies included orthopedic (10), abdominal (1), thoracic (1), ophthalmic (1), and head and neck surgery cases (3). All RCT articles used a placebo in the control group, apart from one that used acetaminophen.23 One of the included retrospective cohort studies used gabapentin and morphine together as the intervention, comparing them to morphine only. This study also had an additional arm looking at the additive effects of a clonidine patch on gabapentin and morphine.24 Most publications had group sizes between 20–100 participants. Mohamed et al. had the largest sample size with 144 patients .22 Some articles enrolled participants of childhood age, adolescent age, or a range encompassing both. (Table 1)
The dosage of gabapentin mostly varied between 5–20 mg/kg among the selected publications. The frequency of administration ranged from one single dose to two to three times daily over multiple days. The timing of dosing varied widely, with seven papers providing preoperative dosing alone, two having postoperative dosing alone, and six having both pre- and postoperative dosing. Of the eight studies, including postoperative administration, the time course ranged from 3 to 30 days after surgery.24–31 (Table 2)
Perioperative Pain and Opioid Requirements
Eleven of the studies assessed postoperative pain but varied in the utilized pain scale between the visual analog scale (VAS), numerical rating score (NRS), Wong-Baker FACES, CRIES, and CHIPPS. Of those assessing postoperative pain, 6/11 (55%) saw a decrease in pain in the gabapentin groups. (Table 3) Some articles evaluated postoperative opioid consumption as another outcome, if not the primary measure. Of those studies considering opioid requirements, 6/10 (60%) reported a reduction while, 1/10 (10%) an increase, and 3/10 (30%) no difference in administered opioids for the gabapentin group. Yet, most of the pain and requirement findings were only significant in 1–2 time points in the follow-up period, and the actual decreases had minimal clinical significance. (Table 4).
Discussion
The potential for gabapentin as a component of multimodal analgesic regimens in reducing postoperative pain and opioid prescriptions is a current area of interest within the literature. Among the fifteen publications assessed in this review, the evidence regarding the efficacy of perioperative gabapentin in reducing postoperative pain and opioid use were lacking. Some articles demonstrated a statistically significant decrease in postoperative pain intensity score and/or opioid consumption in the gabapentin compared to the control group.24–26,29–32 Others identified no significant difference between the two groups in either of those outcomes.23,27,33,34 Varying evidence from these limited studies helps to explain why current recommendations and guidelines for perioperative gabapentin usage, dosage, and timing in pediatric cases are limited.
Pain Assessment Tools
One consideration for the inconclusive evidence from these collective studies is the variability and reliability of pain assessment tools/scales.3 Scales used in the selected publications primarily included the VAS and NRS. The CRIES, Wong-Baker FACES, and CHIPPS scales were also used for patients too young to utilize the VAS and NRS (typically <5 years old).26,32,35,36 The included studies did include appropriate scales based on patient age but even so, each of these scores carries their own criteria and limitations that make them hard to directly compare. For instance, the most commonly used pain assessment tool is the VAS which consists of a 100 mm horizontal or vertical line, with one end labeled as no pain and the other labeled as unbearable/worst pain.37 Patients are asked to mark where they would rate their pain along the line. Studies utilizing the VAS often differ on the definition of mild, moderate, and severe pain thresholds and which marks are clinically meaningful.37
Also, pain scales, in general, are inherently subjective and partly depend on factors other than pain, such as mood, anxiety, and environment.38,39 This fact alongside the inconsistencies between and within scales limits the utility of these scales in assessing how gabapentin impacts perioperative pain outcomes.
Heterogeneity of Studies
A large factor in the lack of substantial supporting evidence for the perioperative use of gabapentin in pediatrics is the heterogeneity of outcome measures in the studies included in the review. The studies varied from looking at pain to opioid use, side effects, and numerous other measures. Additionally, the measurement intervals greatly varied for studies looking at the same outcomes. This was also seen in the large-scale systematic review with a meta-analysis of 281 RCTs consisting of 24,682 adult patients for the evaluation of perioperative gabapentinoid usage.18 In a pediatric systematic review of gabapentinoid use for postoperative, neuropathic, and fibromyalgia pain, there was no meta-analysis conducted due to the heterogeneity of study outcomes in the five RCTs included for review.1 Some measured pain intensity scores, total postoperative morphine consumption, postoperative nonsteroidal anti-inflammatory IV analgesics, or a combination of these making them impossible to directly compare.
Our review included articles beyond the time that the paper by Egunsola et al. (2019) was published while also specifically focusing on gabapentin use in the perioperative setting in children and including retrospective studies. Even the articles that used postoperative pain intensity scores as their primary outcome varied in the pain scale(s) used within their study. Additionally, pain intensity scores were measured at multiple postoperative time points that varied between studies. Results showed significantly lower pain scores in the gabapentin group in only a select few time points, if at all, for studies that used pain scores as an outcome. (Table 3)
Studies also varied in the frequency and duration of gabapentin administration and the length of the intervention period assessed. In summary, seven studies administered gabapentin only preoperatively, six administered gabapentin both pre- and postoperatively, and two only administered gabapentin postoperatively (Table 2). Additionally, for the control group, one article used a non-opioid analgesic (acetaminophen) instead of a placebo like the rest of the included RCTs.23 Another article investigated gabapentin and morphine together as the intervention group, comparing it to morphine only.24 These differences also contribute to the vast heterogeneity between included studies.
Another consideration is the administration of postoperative opioids, assessed in several ways – any opioid use, the amount used, by postoperative day, and/or in total.24,26,28–34,40,41 For some studies for which the age range was between 9–19, it was self-directed administration with a patient-controlled analgesia (PCA) pump.24,28,29,31,34 For other studies with a wider age range (3 months-18 years), opioid administration was primarily based on the presence of moderate-to-severe pain based on relevant pain scales.30,32,33,40 Children must be able to comprehend the concept of delayed pain control following the push of a button; it has been shown that children as young as five are capable of safely utilizing PCA.42 The varying methods of opioid administration may be contributing to the heterogeneity of studies.
Because of this heterogeneity of studies, conclusions on the optimal dosing and timing of perioperative gabapentin cannot be made. Larger and more comprehensive studies are needed to determine the efficacy, safety, and long-term benefits, if any, of perioperative gabapentin in children and replicate results before shifting clinical guidance/management of perioperative pain management in the pediatric population.
Randomized Controlled Trials
When comparing the number of RCTs included in the systematic review on the use of perioperative gabapentin in adults by Fabritius et al. with the number included in this review (132 vs. 11), it is evident that investigation of perioperative gabapentin usage in pediatrics lags far behind. Does the inadequate evidence of the safety and analgesic efficacy of perioperative gabapentin in adults impact the decision to conduct RCTs in children and adolescents? Certainly, this scoping review has highlighted the sparsity of these RCTs in children. Current clinical guidelines may be skewed by one of the few research studies on this topic, which was subsequently retracted and excluded from this review.43–45 More RCT studies in pediatrics are needed to help guide evidence-based perioperative pain management practices, though there may be difficulties with patient recruitment, especially given the rightful special protections for children as research subjects. Looking at the sample sizes of groups in the included RCTs, most had less than 100 participants, except for Mohamed and Al-Sersy (2014), where both groups had 72 participants each (Table 1). Perhaps future studies can focus on enrolling larger sample sizes/participants to help offset the variability of pain scales/scores as an outcome measure as patients often experience and express pain differently.
Retrospective Cohort Studies
This scoping review was broadened by including retrospective studies in the literature search. These studies do not yield the same level of evidence as RCTs because of their non-randomized retrospective design. This allows for the introduction of potential selection bias and the inclusion of non-standard treatment doses and placebo controls. During the full-text review process, several retrospective studies were omitted because their intervention was gabapentin and pregabalin combined under the class gabapentinoids, so the results from gabapentin alone could not be elucidated.
Among the four included retrospective studies, there were varying gabapentin doses and frequencies. Additionally, one article used gabapentin and morphine together as the intervention compared to morphine alone, while all other studies had gabapentin alone as the intervention.24 Because gabapentin is part of a multimodal analgesic regimen in the perioperative setting, there are often other non-opioid adjuncts used concomitantly. This presents a potentially confounding variable(s) in these retrospective studies because of the lack of standardization of non-opioid analgesics that complement gabapentin, such as acetaminophen, ketorolac, and others. However, these studies tended to have larger sample sizes and thus provide helpful insights for future studies.
Limitations
One of the main limitations of this review is the paucity of studies on this topic in this population. Additionally, this article includes retrospective studies which do not deliver the same level of evidence as RCTs and may also face selection bias due to their research design. Lastly, non-English language publications were not included in this review.
Directions for Future Research
There is currently no universal pain scale for children and likely will not be one since it must be age-appropriate for comprehension.3 Moving forward, it may be beneficial to use multiple validated and age-appropriate pain scales with standardized cutoffs in studies on perioperative gabapentin in children within a given study to better account for variation in participant pain assessment. Other suggestions include identifying standardized non-opioid analgesic adjuncts (dosing and frequency) within a multimodal regimen for participants in each study (at a particular institution) and enrolling a larger sample size in each to increase the power of the studies.
Conclusion
Overall, this scoping review found sparse evidence supporting the perioperative use of gabapentin for pain in children and adolescents. Existing studies on the topic vary widely in the types of surgical cases analyzed, methodologies for delivering perioperative gabapentin, and how pain and postoperative analgesia were measured and delivered. These current findings do not support the routine use of gabapentin in perioperative pain protocols in pediatric patients. Additional high-quality RCTs are necessary to elucidate the efficacy and safety of perioperative gabapentin administration in children. These studies should seek to determine standardized pain scales for pediatric patients and employ more standardized methodologies for perioperative gabapentin administration and postoperative pain and analgesia measurement.
Funding:
This research was supported by an NCI R25 Grant to the Memorial Sloan Kettering Cancer Center. This review was also supported, in part, by the National Institutes of Health/National Cancer Institute (Bethesda, Maryland) Cancer Support Grant P30 CA008748.
Footnotes
Conflict of Interest Disclosures: The authors have no potential conflicts of interest to disclose.
Data statement: Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Egunsola O, Wylie CE, Chitty KM, Buckley NA. Systematic Review of the Efficacy and Safety of Gabapentin and Pregabalin for Pain in Children and Adolescents. Anesth Analg 2019;128(4):811–819. [DOI] [PubMed] [Google Scholar]
- 2.Shah SA, Guidry R, Kumar A, White T, King A, Heffernan MJ. Current Trends in Pediatric Spine Deformity Surgery: Multimodal Pain Management and Rapid Recovery. Global Spine J 2020;10(3):346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schug SA, Palmer GM, Scott DA, Alcock M, R. H, Mott JF, eds. Acute Pain Management: Scientific Evidence Fifth ed: Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine; 2020. [Google Scholar]
- 4.Rafeeqi T, Pearson EG. Enhanced recovery after surgery in children. Transl Gastroenterol Hepatol 2021;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salaün JP, Ecoffey C, Orliaguet G. Enhanced recovery in children: how could we go further? World J Pediatr Surg 2021;4(2):e000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gai N, Naser B, Hanley J, Peliowski A, Hayes J, Aoyama K. A practical guide to acute pain management in children. Journal of Anesthesia 2020;34(3):421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathieson S, Lin CC, Underwood M, Eldabe S. Pregabalin and gabapentin for pain. Bmj 2020;369:m1315. [DOI] [PubMed] [Google Scholar]
- 8.Hah J, Mackey SC, Schmidt P, et al. Effect of Perioperative Gabapentin on Postoperative Pain Resolution and Opioid Cessation in a Mixed Surgical Cohort: A Randomized Clinical Trial. JAMA Surg 2018;153(4):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg 2012;115(2):428–442. [DOI] [PubMed] [Google Scholar]
- 10.Dunkman WJ, Manning MW. Enhanced Recovery After Surgery and Multimodal Strategies for Analgesia. Surg Clin North Am 2018;98(6):1171–1184. [DOI] [PubMed] [Google Scholar]
- 11.Gelman D, Gelmanas A, Urbanaitė D, et al. Role of Multimodal Analgesia in the Evolving Enhanced Recovery after Surgery Pathways. Medicina (Kaunas) 2018;54(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward A, De Souza E, Miller D, et al. Incidence of and Factors Associated With Prolonged and Persistent Postoperative Opioid Use in Children 0–18 Years of Age. Anesth Analg 2020;131(4):1237–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua K-P, Brummett CM, Conti RM, Bohnert AS. Opioid prescribing to US children and young adults in 2019. Pediatrics 2021;148(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurley RW, Cohen SP, Williams KA, Rowlingson AJ, Wu CL. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med 2006;31(3):237–247. [DOI] [PubMed] [Google Scholar]
- 15.Clivatti J, Sakata RK, Issy AM. Review of the use of gabapentin in the control of postoperative pain. Rev Bras Anestesiol 2009;59(1):87–98. [DOI] [PubMed] [Google Scholar]
- 16.Penprase B, Brunetto E, Dahmani E, Forthoffer JJ, Kapoor S. The efficacy of preemptive analgesia for postoperative pain control: a systematic review of the literature. Aorn j 2015;101(1):94–105.e108. [DOI] [PubMed] [Google Scholar]
- 17.Fabritius ML, Geisler A, Petersen PL, et al. Gabapentin for post-operative pain management - a systematic review with meta-analyses and trial sequential analyses. Acta Anaesthesiol Scand 2016;60(9):1188–1208. [DOI] [PubMed] [Google Scholar]
- 18.Verret M, Lauzier F, Zarychanski R, et al. Perioperative Use of Gabapentinoids for the Management of Postoperative Acute Pain: A Systematic Review and Meta-analysis. Anesthesiology 2020;133(2):265–279. [DOI] [PubMed] [Google Scholar]
- 19.Lim SY, Pettit RS. Pharmacokinetic considerations in pediatric pharmacotherapy. Am J Health Syst Pharm 2019;76(19):1472–1480. [DOI] [PubMed] [Google Scholar]
- 20.UpToDate. Schechter W. Pharmacologic management of acute perioperative pain in infants and children https://www.uptodate.com/contents/pharmacologic-management-of-acute-perioperative-pain-in-infants-and-children#H599075171. Published 2021. Accessed June 30, 2021.
- 21.Cavalcante AN, Sprung J, Schroeder DR, Weingarten TN. Multimodal Analgesic Therapy With Gabapentin and Its Association With Postoperative Respiratory Depression. Anesth Analg 2017;125(1):141–146. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed MH, Al-Sersy H. Preoperative gabapentin decreases the incidence of postoperative vomiting and analgesic requirements after pediatric adenotonsillectomy. The Egyptian Journal of Otolaryngology 2014;30(3):225–228. [Google Scholar]
- 23.Haddadi S, Marzban S, Parvizi A, et al. Effects of Gabapentin Suspension and Rectal Acetaminophen on Postoperative Pain of Adenotonsillectomy in Children. Iran J Otorhinolaryngol 2020;32(111):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhry DK, Brenn BR, Sacks K, Shah S. Evaluation of Gabapentin and Clonidine Use in Children Following Spinal Fusion Surgery for Idiopathic Scoliosis: A Retrospective Review. J Pediatr Orthop 2019;39(9):e687–e693. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Yi Y, Tang D, et al. Gabapentin as an Adjuvant Therapy for Prevention of Acute Phantom-Limb Pain in Pediatric Patients Undergoing Amputation for Malignant Bone Tumors: A Prospective Double-Blind Randomized Controlled Trial. J Pain Symptom Manage 2018;55(3):721–727. [DOI] [PubMed] [Google Scholar]
- 26.Trzcinski S, Rosenberg RE, Montes DV, et al. Use of Gabapentin in Posterior Spinal Fusion is Associated With Decreased Postoperative Pain and Opioid Use in Children and Adolescents. Clinical Spine Surgery 2019;32(5):210–214. [DOI] [PubMed] [Google Scholar]
- 27.Tomaszek L, Fenikowski D, Maciejewski P, Komotajtys H, Gawron D. Perioperative Gabapentin in Pediatric Thoracic Surgery Patients-Randomized, Placebo-Controlled, Phase 4 Trial. Pain Med 2020;21(8):1562–1571. [DOI] [PubMed] [Google Scholar]
- 28.Thomas JJ, Levek C, Quick HD, Brinton JT, Garg S, Cohen MN. Utility of gabapentin in meeting physical therapy goals following posterior spinal fusion in adolescent patients with idiopathic scoliosis. Paediatr Anaesth 2018;28(6):558–563. [DOI] [PubMed] [Google Scholar]
- 29.Rusy LM, Hainsworth KR, Nelson TJ, et al. Gabapentin use in pediatric spinal fusion patients: a randomized, double-blind, controlled trial. Anesth Analg 2010;110(5):1393–1398. [DOI] [PubMed] [Google Scholar]
- 30.Baxter KJ, Hafling J, Sterner J, et al. Effectiveness of gabapentin as a postoperative analgesic in children undergoing appendectomy. Pediatr Surg Int 2018;34(7):769–774. [DOI] [PubMed] [Google Scholar]
- 31.Anderson DE, Duletzke NT, Pedigo EB, Halsey MF. Multimodal pain control in adolescent posterior spinal fusion patients: a double-blind, randomized controlled trial to validate the effect of gabapentin on postoperative pain control, opioid use, and patient satisfaction. Spine Deform 2020;8(2):177–185. [DOI] [PubMed] [Google Scholar]
- 32.Pinto Filho WA, Silveira LHJ, Vale ML, Fernandes CR, Alves Gomes J. The Effect of Gabapentin on Postoperative Pain of Orthopedic Surgery of Lower Limb by Sciatic and Femoral Blockage in Children: A Clinical Trial. Anesth Pain Med 2019;9(4):e91207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gettis M, Nusz D, Roberts J. Gabapentin Premedication for Pediatric Anterior Cruciate Ligament Reconstruction: A Feasibility Study. Pediatric Nursing 2020;46(6):282–290. [Google Scholar]
- 34.Mayell A, Srinivasan I, Campbell F, Peliowski A. Analgesic effects of gabapentin after scoliosis surgery in children: a randomized controlled trial. Paediatr Anaesth 2014;24(12):1239–1244. [DOI] [PubMed] [Google Scholar]
- 35.Birnie KA, Hundert AS, Lalloo C, Nguyen C, Stinson JN. Recommendations for selection of self-report pain intensity measures in children and adolescents: a systematic review and quality assessment of measurement properties. Pain 2019;160(1):5–18. [DOI] [PubMed] [Google Scholar]
- 36.Giordano V, Edobor J, Deindl P, et al. Pain and Sedation Scales for Neonatal and Pediatric Patients in a Preverbal Stage of Development: A Systematic Review. JAMA Pediatrics 2019;173(12):1186–1197. [DOI] [PubMed] [Google Scholar]
- 37.Reed MD, Van Nostran W. Assessing pain intensity with the visual analog scale: a plea for uniformity. J Clin Pharmacol 2014;54(3):241–244. [DOI] [PubMed] [Google Scholar]
- 38.Williams G, Howard RF, Liossi C. Persistent postsurgical pain in children and young people: prediction, prevention, and management. Pain Rep 2017;2(5):e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagemakers SH, van der Velden JM, Gerlich AS, Hindriks-Keegstra AW, van Dijk JFM, Verhoeff JJC. A Systematic Review of Devices and Techniques that Objectively Measure Patients’ Pain. Pain Physician 2019;22(1):1–13. [PubMed] [Google Scholar]
- 40.Badawy AA, Kasem SA, Rashwan D, et al. The role of Gabapentin oral solution in decreasing desflurane associated emergence agitation and delirium in children after stabismus surgery, a prospective randomized double-blind study. BMC Anesthesiol 2018;18(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salman AE, Camkiran A, Oguz S, Donmez A. Gabapentin premedication for postoperative analgesia and emergence agitation after sevoflurane anesthesia in pediatric patients. Agri 2013;25(4):163–168. [DOI] [PubMed] [Google Scholar]
- 42.Lehr VT, BeVier P. Patient-controlled analgesia for the pediatric patient. Orthop Nurs 2003;22(4):298–304; quiz 305–296. [DOI] [PubMed] [Google Scholar]
- 43.Retraction: Evaluation of gabapentin and dexamethasone alone or in combination for pain control after adenotonsillectomy in children. Saudi J Anaesth 2018;12(4):662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amin SM, Amr YM. Comparison between preemptive gabapentin and paracetamol for pain control after adenotonsillectomy in children. Anesth Essays Res 2011;5(2):167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amin SM. Evaluation of gabapentin and dexamethasone alone or in combination for pain control after adenotonsillectomy in children. Saudi J Anaesth 2014;8(3):317–322. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


