Abstract
Background/Aims:
To investigate the factors associated with choroidal microvasculature dropout (MvD) enlargement detected by optical coherence tomography angiography (OCT-A) in glaucomatous eyes.
Methods:
Ninety-one eyes of 68 primary open angle glaucoma (POAG) patients were enrolled. Only eyes with a minimum of four good quality OCT-A and OCT scans of the optic nerve head acquired at least and with a minimum of 2 years follow-up were included. Area and angular circumference of MvD were analyzed on en-face images. Univariable and multivariable mixed effects models were constructed to identify the factors contributing to MvD area and angular circumference change over time.
Results:
Peripapillary MvD was detected in 53 (58.2%) eyes at baseline and in an additional 17 (18.6%) eyes during follow-up, whereas MvD was not detected in 21 (23.0 %) eyes during the entire follow-up period. In multivariable analysis, worse baseline VF MD (ß=0.27, 95%CI: 0.10, 0.44, P=0.002), greater intraocular pressure (IOP) fluctuations (ß=0.86, 95% CI: 0.24, 1.48, P=0.007), higher peak IOP (ß=0.17, 95% CI: −0.01, 0.35, P=0.067) and greater number of IOP lowering medications (ß =1.36, 95% CI: 0.67, 2.05, P<0.001) were associated with faster MvD area enlargement. Worse baseline VF MD and greater IOP fluctuation were also associated with significantly faster MvD circumferential enlargement in multivariable models.
Conclusion:
Greater IOP fluctuation, higher peak IOP, worse baseline VF MD and greater number of glaucoma medications were significantly associated with MvD enlargement in glaucomatous eyes. The identification of factors associated with MvD enlargement may improve our understanding of the role of choroidal vasculature in glaucoma.
Keywords: Choroidal microvasculature dropout, Optical coherence tomography angiography, glaucoma, glaucoma progression
INTRODUCTION
Glaucoma is a progressive optic neuropathy characterized by optic nerve head (ONH) damage and visual field (VF) damage.[1] Even though the pathogenesis is still unclear, the retinal and choroidal microvasculature, have been suggested as having an important role in the pathophysiology of glaucoma.[2 3] The advent of optical coherence tomography angiography (OCT-A) has allowed objective and reproducible evaluation and measurement of this microvasculature.[4] In particular, localized parapapillary choroidal perfusion impairment detected with OCT-A, known as choroidal microvasculature dropout (MvD), has been observed in glaucomatous eyes.[5] It is unclear whether such impairment is a primary cause of glaucomatous optic nerve damage or a secondary result of the damage. Regardless, the presence of MvD suggests that perfusion is compromised in the deep ONH tissues, since choroidal circulation in the parapapillary area is supplied by the short posterior ciliary arteries that also supply the laminar and prelaminar regions of the ONH.[4]
MvD has been detected more frequently in glaucoma eyes with central visual field (VF) defects, more advanced stage[4 6] and faster progression of the disease.[7–9] Several systemic risk factors, such as lower ocular perfusion pressure, lower diastolic blood pressure, cold extremities and migraine, have been more commonly seen in OAG eyes with MvD compared to eyes without MvD.[4 10–12] An enlargement of MvD area and angular circumference has been observed in 21.8% to 32.4% of patients with glaucoma over a mean of two-year follow-up. This increase in MvD size between baseline and last visit was significantly associated with faster rates of RNFL thinning and VF loss throughout the follow-up period.[13 14] However, whether other factors are associated with changes in the area of impaired perfusion over time have not yet been determined. The current study aims to evaluate whether risk factors such as mean IOP during follow-up, peak IOP and IOP fluctuation are associated with rates of change of MvD area and angular circumference over time in a cohort of open-angle glaucoma patients.
MATERIALS AND METHODS
This longitudinal study included POAG patients enrolled in Diagnostic Innovations in Glaucoma Study (DIGS)[15 16] who underwent OCT-A (Angiovue; Optovue Inc., Fremont, CA) and Spectral-domain OCT (Spectralis, Heidelberg Engineering, Germany) imaging. Participants were assessed longitudinally according to a standard protocol consisting of follow-up visits with annual clinical examinations, imaging and functional testing every 6 months. All participants from the DIGS study who met the inclusion criteria described below were included. Informed consent was obtained from all study participants. This study received the institutional review board approval of The University of California, San Diego (NCT00221897) and the methodology adhered to tenets of the Declaration of Helsinki.
This study included eyes with a minimum of four qualified OCT-A and OCT scans of ONH, acquired at least six months apart, and a minimum of 2 years follow-up. Tests (i.e. OCT-A and IOP) were censored after glaucoma surgery, including trabeculectomy, tube shunt surgery or bleb revision) during the follow-up. Eyes were classified as glaucomatous at the OCT-A baseline visit if they had repeatable (at least 2 consecutive) abnormal VF test results and evidence of glaucomatous optic neuropathy – defined as excavation, presence of focal thinning, notching of neuroretinal rim, or localized or diffuse atrophy of the retinal nerve fiber layer on the basis of masked grading of optic disc photographs by 2 graders. An abnormal VF test was defined as a pattern standard deviation (PSD) value at the 5% level or a Glaucoma Hemifield Test result outside normal limits. Inclusion criteria also included (1) older than 18 years of age, (2) open angles on gonioscopy, (3) best-corrected visual acuity of 20/40 or better at study entry. Exclusion criteria were (1) history of trauma or intraocular surgery at OCT-A baseline (except for uncomplicated cataract surgery), (2) coexisting retinal disease, (3) uveitis, (4) non-glaucomatous optic neuropathy, and (5) axial length of 26 mm or more. Participants with the diagnosis of systemic diseases such as Parkinson’s disease, Alzheimer’s disease, dementia, or a history of stroke were excluded.
Choroidal microvasculature dropout detection
Scans (4.5 × 4.5 mm2 - 304 B-scans x 304 A-scans per B-scan) centered on the ONH were acquired with the AngioVue OCT-A system (software version 2018.1.1.63). The retinal layers of each scan were segmented automatically by the AngioVue software and the en-face choroidal vessel density map was acquired. Only good-quality images were included. OCT-A and OCT image quality review was completed according to the Imaging Data Evaluation and Analysis Reading Center standard protocol on scans processed with standard AngioVue software. Poor-quality images were excluded; these were defined as images with 1) low scan quality with quality index (QI) of less than 4; 2) poor clarity; 3) residual motion artifacts visible as irregular vessel pattern or disc boundary on the en-face angiogram; 4) image cropping or local weak signal resulting from vitreous opacity; or 5) segmentation errors that could not be corrected.
MvD was defined as a complete loss of the choriocapillaris without any visible microvasculature network within the βPPA, as in previous studies.[13 17]
Dropout was required to be present in at least 4 consecutive horizontal B-scans >200 μm in diameter in at least one b-scan and in contact with the OCT disc boundary.[4] The optic disc boundary was automatically detected by the Optovue software. For errors in disc demarcation, one trained observer (E.M.) masked to the clinical information of the participants corrected the disc boundary manually by searching for the position of Bruch’s membrane opening (BMO), as previously described.[18] Two observers (E.M. and N.E.N.), who were masked to the clinical characteristics of the participants independently determined the presence or absence of MvD for each patient. Figure 1 shows a representative case of choroidal microvasculature dropout.
Figure 1.
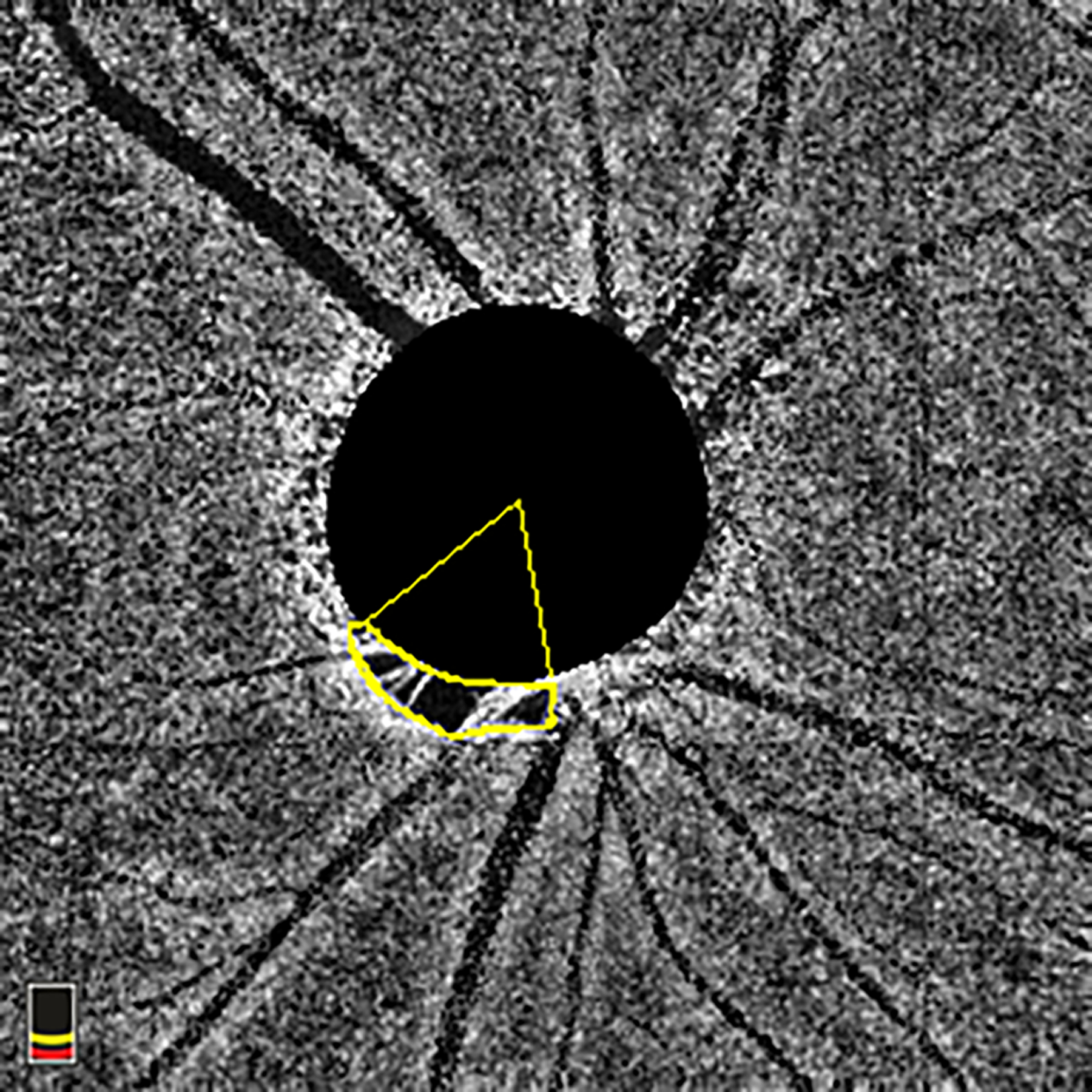
En-face choroidal vessel density image showing Choroidal Microvasculature Dropout (MvD) area and angular circumference. MvD area was manually outlined using ImageJ software. The two points at which the extreme borders of MvD area met the optic nerve head (ONH) border were identified and defined as angular circumferential margins. The angular circumference was Then determined by drawing two lines connecting the ONH center to the angular circumference margins of the MvD. MvD = microvasculature dropout; ONH = optic nerve head.
Assessment of MvD area, circumferential angle, and location
Optic disc and parapapillary atrophy (PPA) margins were detected by simultaneously viewing the stereoscopic optic disc photographs and the scanning laser ophthalmoscopic (SLO) images that were obtained along with the OCT-A images. MvD area was manually demarcated on en-face choroidal vessel density maps using the line tool provided by ImageJ software (Version 1.53; available at http://imagej.nih.gov/ij/download.html; National Institute of Health, Bethesda, Maryland, USA).
MvD angular circumference was measured as previously described. In brief, the two points at which the extreme borders of MvD area met the ONH border were identified and defined as angular circumferential margins. The angular circumference was then determined by drawing two lines connecting the ONH center to the angular circumference margins of the MvD. The optic disc center was automatically provided by the AngioVue software, as in previous studies.[13]
Both area and angular circumference of the MvD were assessed by two trained graders (E.M. and N.E.N.), who were masked to the clinical data of the patients, including cpRNFL data.
Disagreement in the MvD detection was resolved by a glaucoma specialist, designated as the adjudicator (S.M.). MvD area that included large retinal vessels was included as part of the total MvD area if the MvD extended beyond the vessels. In cases where the retinal vessels were located at the border of the MvD, the area covered by the vessels was excluded from the measured MvD area. En-face images with reflectance or shadowing of large vessels were excluded from the quantitative analysis. In eyes with more than one MvD, the area and the angular extent of each MvD were calculated separately and then added together to determine the total area and the total angular extension of MvD for each eye. To define the location of each MvD, a line was drawn to equally bisect the angular circumferential margins of the MvD from the ONH center, as previously reported[13]. MvD area and angle were manually outlined by 2 graders and their measurements were averaged. Figure 2 shows a representative case of MvD enlargement and presents the method used to measure MvD area and angular circumference.
Figure 2.
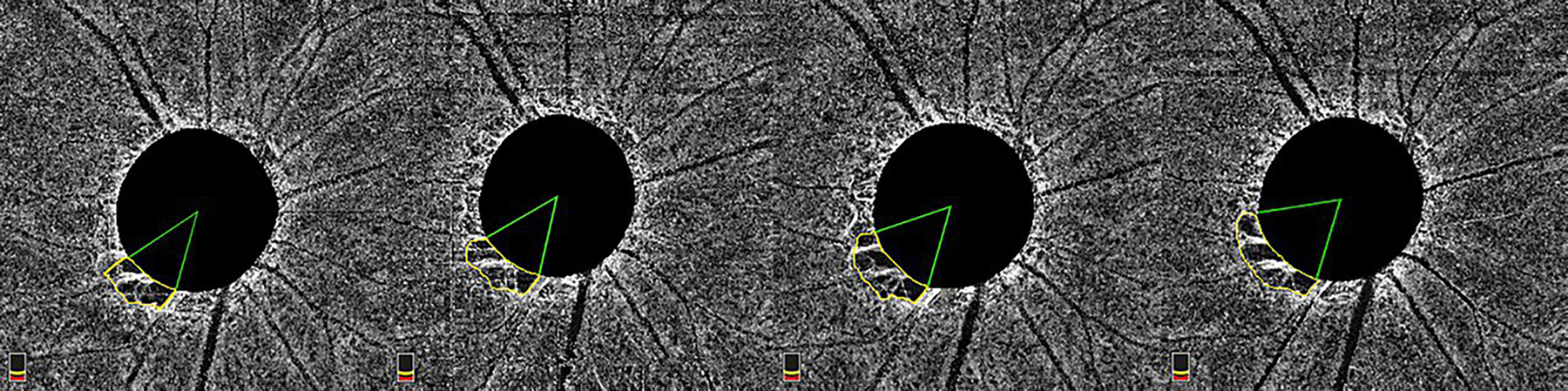
En-face image showing MvD enlargement over 4 years of follow-up. MvD area was manually measured (yellow outline) in each image of the follow-up. The two points at which the extreme borders of MvD area met the ONH border were identified and defined as angular circumferential margins. The angular circumference was then determined by drawing two lines connecting the ONH center to the angular circumference margins of the MvD.
To take into consideration the ocular magnification in OCT-A, Littmann’s formula was used to correct measurements of MvD area obtained using ImageJ software.[4] Details of the formula are provided in previous studies.[19] The Avanti SD OCT has a default axial length of 23.95 mm and a default anterior corneal curvature radius of 7.77 mm.
Statistical analysis
Patient and eye characteristics data were presented as mean (95% confidence interval (CI)) for continuous variables and count (%) for categorical variables. In addition to baseline variables, summaries of IOP parameters (mean, peak and IOP fluctuation) also are presented. The mean IOP was calculated by averaging all IOP measurements during follow-up. If patients underwent cataract surgery during the follow-up period, all post-operative IOP measurements made within 6 months after surgery were excluded. IOP fluctuation was defined as the standard deviation of IOP measurements during follow-up. Peak IOP was the highest single measurement during the entire follow-up. Categorical variables were compared using the chi-square test. We investigated the effect of different baseline parameters as well as IOP parameters and medications on MvD area and angular circumference change over time. Univariable and multivariable linear mixed effects models were constructed to estimate the effect of factors on MvD area and angular circumference change over time. This standard technique has been described in detail by Laird and Ware.[20] Briefly, mixed models take into account the natural correlation of longitudinal data over time, as well as the fact that each patient may contribute two eyes in the analysis. Differences in rates of change between eyes and participants are taken into account by introducing random slopes and random intercept. Univariable models were first used to evaluate the effect of demographic and clinical characteristics and IOP parameters on rates of MvD area and angular circumference change over time. Finally, we built multivariable models for each of the IOP parameters that include additional adjustment for age and any variable in which the P value was <0.1 in univariable analysis. Statistical analyses were performed using Stata version 16.0 (StataCorp, College Station, TX). P values of less than 0.05 were considered statistically significant for all analyses.
RESULTS
A total of 101 POAG eyes were initially enrolled in the study. Of these, 10 eyes of 7 patients were excluded due to the poor quality of their OCT-A images, resulting in inclusion of 91 eyes of 68 POAG patients included in the study analysis. Eleven (12.1%) eyes that underwent glaucoma or complicated cataract surgery were censored during the follow-up. The demographic characteristics are provided in Table 1. Mean age (95%CI) was 69.7 (67.0, 72.4) years and mean baseline VF MD (95% CI) was −4.7 (−5.8, −3.6) dB. Mean number of OCT and OCT-A visits (95%CI) were 6.2 (5.8, 6.6) and 5.7 (5.4, 6.0) over a mean (95% CI) follow-up of 3.9 (3.8, 4.1) years.
Table 1.
Demographics and Clinical characteristics of the participants
| Variables* | MvD at baseline | No MvD at baseline | Total | p-value |
|---|---|---|---|---|
| N, patients (eyes) | 46 (53) | 22 (38) | 68 (91) | |
| Baseline age (years) | 69.9 (66.7, 73.0) | 69.3 (64.0, 74.6) | 69.7 (67.0, 72.4) | |
| Gender (Female/ Male) | 23/23 | 11/11 | 34/34 | 1.000 |
| Race (African American/ Non-African American), n (%) | 9/37 | 10/12 | 19 (28%)/49 (72%) | 0.026 |
| Self-reported hypertension, n (%) | 31 (67.4%) | 14 (63.6%) | 45 (66.2%) | 0.759 |
| Self-reported diabetes, n (%) | 8 (17.4%) | 2 (9.1%) | 10 (14.7%) | 0.366 |
| Axial length (mm) | 24.3 (24.0, 24.6) | 23.9 (23.6, 24.3) | 24.1 (23.9, 24.4) | 0.085 |
| CCT (μm) | 532.2 (519.6, 544.9) | 533.1 (517.8, 548.3) | 532.7 (523.3, 542.1) | 0.941 |
| Baseline IOP (mmHg) | 13.5 (12.2, 14.8) | 15.2 (14.0, 16.4) | 14.2 (13.3, 15.1) | 0.055 |
| Mean IOP (during follow-up (mmHg) | 14.0 (13.0, 15.0) | 15.1 (13.8, 16.4) | 14.5 (13.7, 15.3) | 0.190 |
| Peak IOP during follow-up (mmHg) | 17.2 (15.9, 18.4) | 18.5 (16.6, 20.5) | 17.7 (16.6, 18.8) | 0.191 |
| IOP fluctuation during follow-up (mmHg) | 2.5 (2.2, 2.9) | 2.6 (2.0, 3.2) | 2.6 (2.3, 2.9) | 0.787 |
| IOP follow-up visits (n) | 6.0 (5.4, 6.5) | 5.2 (4.6, 5.9) | 5.7 (5.3, 6.1) | 0.072 |
| Disease Severity by baseline 24–2 VF MD | 0.001 | |||
| Early glaucoma (MD≥−6 dB), Eye No. (%) | 32 (60.4%) | 35 (92.1%) | 67 (73.6%) | |
| Moderate and advanced glaucoma (MD<−6 dB), Eye n. (%) | 21 (39.6%) | 3 (7.9%) | 24 (26.4%) | |
| Baseline VF MD (dB) | −6.2 (−7.9, −4.4) | −2.6 (−3.4, −1.8) | −4.7 (−5.8, −3.6) | 0.001 |
| Baseline RNFL thickness (μm) | 73.2 (68.5, 77.9) | 85.1 (80.8, 89.4) | 78.1 (74.7, 81.6) | <0.001 |
| Corrected MvD area at baseline (mm2) | 0.15 (0.09, 0.22) | NA | ||
| Corrected MvD area at the last visit (mm2) | 0.32 (0.24, 0.40) | NA | ||
| MvD angle at baseline (degree) | 41.3 (29.3, 53.4) | NA | ||
| MvD angle at the last visit (degree) | 85.4 (69.4, 101.4) | NA | ||
| OCTA follow-up visits (n) | 5.8 (5.5, 6.2) | 3.9 (3.7, 4.1) | 5.7 (5.5, 6.0) | 0.485 |
| Follow-up (years) | 4.0 (3.8, 4.1) | 3.9 (3.7, 4.1) | 3.9 (3.8, 4.1) | 0.598 |
| Number of glaucoma medications during the follow-up (n) | 2.1 (1.7, 2.4) | 2.0 (1.5, 2.5) | 2.0 (1.8, 2.3) | 0.852 |
| Glaucoma medications use during the follow-up, Eye No. (%) | ||||
| Prostaglandin | 44 (83.0%) | 30 (79.0%) | 74 (81.3%) | 0.623 |
| β-blocker | 22 (41.5%) | 16 (42.1%) | 38 (41.8%) | 0.955 |
| CAI | 26 (49.1%) | 17 (44.7%) | 43 (47.3%) | 0.684 |
| Brimonidine | 16 (30.2%) | 13 (34.2%) | 29 (31.9%) | 0.685 |
| Glaucoma surgeries, n (%) | 16 (32.7%) | 7 (19.4%) | 23 (25.3%) | 0.176 |
| Cataract surgeries, n (%) | 20 (37.7%) | 21 (55.3%) | 41 (45.1%) | 0.097 |
| Scan Quality Score | 7.2 (7.0, 7.4) | 7.1 (7.0, 7.3) | 7.2 (7.0, 7.3) | 0.708 |
CCT = central corneal thickness; IOP = intraocular pressure; MD = mean deviation; MvD = microvascular dropout; OCTA = optical coherence tomography angiography; VF = visual field.
Data is presented as mean (95% CI) unless otherwise indicated. Statistically significant P values are shown in bold.
Parapapillary MvD was detected in 53 (58.2%) eyes at baseline, while 17 (18.7%) eyes without MvD at baseline developed MvD during follow-up. MvD was not detected in 21 (23.0%) eyes during the follow-up period. In eyes with MvD, the mean MvD area (95%CI) was 0.15 (0.09, 0.22) mm2 at baseline and 0.32 mm2 (0.24, 0.41) at final follow-up (P <0.001). In eyes with MvD, the mean MvD angular circumference (95%CI) was 41.3° (29.3, 53.4) at baseline, while it was 85.4° (69.4.4, 101.4) at the final follow-up (P < 0.001). The mean rates of change of MvD area and angular circumference (95%CI) were 0.05 (0.04, 0.06) mm2/year and 13.22 (10.67, 15.76) degree/year, respectively. Interobserver reproducibility for the MvD measurement was reported in detail in our previous study.[9]
Table 2 summarizes the factors contributing to the MvD area change over time. In univariable analysis, baseline VF MD (ß coefficient (95% CI): 0.27 (0.10, 0.44) mm2/year per 1 dB higher; P=0.002), mean IOP fluctuations during follow-up (coefficient (95% CI): 0.86 (0.24, 1.48) mm2/year per 1 mmHg higher; P=0.007) and the number of medications (coefficient (95% CI) 1.36 (−0.67, 2.05) degree /year per 1 added medication; P<0.001) were significantly associated with the rate of MvD area change over time. We present two multivariable models adjusting for age and baseline VF MD that contain different IOP parameters: (1) peak IOP and (2) IOP fluctuation. The effect of peak IOP was statistically significant in Model 1 with coefficient (95% CI) 0.24 (0.06, 0.41) mm2/year per 1 mmHg higher; P=0.008. The effect of IOP fluctuation also remained statistically significant in Model 2, with coefficient (95% CI) 0.96 (0.37, 1.56) mm2/year per 1 mmHg higher; P=0.001.
Table 2.
Factors Contributing to the Rate of Corrected MvD Area change Over Time: Univariable and Multivariable Mixed Effects Model Analyses
| Variables | Univariable Model | Multivariable Model 1 (Includes peak IOP) | Multivariable Model 2 (Includes IOP fluctuation) | Multivariable Model 3 (Includes number of glaucoma medications) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β, 95 % CI | P value | β, 95 % CI | P value | β, 95 % CI | P value | β, 95 % CI | P value | |
| Baseline Age, per 10 years older | −0.09 (−0.91, 0.73) | 0.827 | 0.39 (−0.40, 1.18) | 0.331 | 0.36 (−0.40, 1.13) | 0.358 | 0.16 (−0.60, 0.92) | 0.679 |
| Gender: Female | −1.53 (−3.39, 0.32) | 0.106 | ||||||
| Race: African American |
−0.22 (−2.34, 1.90) | 0.839 | ||||||
| Self-reported hypertension | −0.96 (−2.91, 0.99) | 0.333 | ||||||
| Self-reported diabetes | −0.75 (−3.42, 1.91) | 0.580 | ||||||
| Axial length, per 1mm longer | 0.38 (−0.53, 1.28) | 0.416 | ||||||
| CCT, per 100 μm thinner | −0.77 (−2.95,1.40) | 0.486 | ||||||
| Baseline VF MD, per 1 dB worse | 0.27 (0.10, 0.44) | 0.002 | 0.30 (0.13, 0.47) | 0.001 | 0.28 (0.11, 0.45) | 0.001 | 0.19 (0.02, 0.36) | 0.029 |
| Baseline RNFL thickness, μm per 1 μm thicker | −0.08 (−0.13, −0.02) | 0.006 | ||||||
| Baseline IOP, per 1 mmHg higher | −0.01 (−0.23, 0.21) | 0.945 | ||||||
| Mean IOP during follow-up, per 1 mmHg higher | 0.10 (−0.15, 0.36) | 0.414 | ||||||
| Peak IOP during follow-up, per 1 mmHg higher | 0.17 (−0.01, 0.35) | 0.067 | 0.22 (0.04, 0.40) | 0.012 | ||||
| IOP fluctuation, per 1 mmHg higher | 0.86 (0.24, 1.48) | 0.007 | 0.93 (0.33, 1.52) | 0.002 | ||||
| IOP follow-up visits (n), per 1 mmHg | 0.44 (−0.03, 0.91) | 0.066 | 0.24 (−0.21, 0.69) | 0.296 | 0.21 (−0.23, 0.65) | 0.349 | ||
| No. of glaucoma medications during follow-up (n) | 1.4 (0.7, 2.0) | <0.001 | 1.12 (0.41, 1.82) | 0.002 | ||||
| Prostaglandin, yes | 2.18 (−0.19, 4.54) | 0.071 | ||||||
| β-blocker, yes | 2.40 (0.56, 4.24) | 0.011 | ||||||
| CAI, yes | 2.37 (0.55, 4.19) | 0.011 | ||||||
| Brimonidine, yes | 3.05 (1.14, 4.96) | 0.002 | ||||||
| Scan Quality Score, per 1 higher | −0.05 (−1.53, 1.43) | 0.949 | ||||||
| Follow-up period, per 1 year longer | 0.76 (−0.67, 2.19) | 0.300 | ||||||
| No. of follow-up visits, per 1 visit more | 0.55 (−0.13, 1.23) | 0.111 | ||||||
CCT = central corneal thickness; IOP = intraocular pressure; MD = mean deviation; MvD = microvascular dropout; VF = visual field. Values are shown in β coefficient (95% confidence interval).
The value of MvD area was multiplied by one hundred to enhance the readability of the Table. Statistically significant P values are shown in bold.
The mean (95% CI) number of IOP visits was 0.44 (−0.03, 0.91), P= 0.066 in univariable Model of MvD area change, but it was not significant in multivariable Model 2 (P=0.349). IOP parameters and the number of medications were correlated, therefore the number of medications was included separately (Model 3).
Table 3 summarizes the factors associated with MvD development in eyes that did not have MvD detected at baseline but developed MvD during follow-up compared to eyes that did not develop MvD. In eyes that developed MvD during follow-up, mean (95% CI) IOP fluctuation (3.5 [2.3, 4.7] vs. 1.9 [1.5, 2,3] mmHg, P=0.012) and the number of glaucoma medications were significantly greater compared to eyes that did not show develop detectable MvD during follow-up. (2.5 [1.8, 3.3] vs. 1.6 [1.0, 2.2], P=0.044). Although both the mean follow-up and the number of follow-up visits were greater in the group of patients that developed MvD compared to the group that did not show MvD, no significant differences were found in the number of cataract or glaucoma surgeries at baseline between the two groups.
Table 3.
Comparison of Demographics and Clinical characteristics between eyes that developed MvD and eyes that did not develop MvD during the follow-up
| Variables | No MvD (21 eyes of 18 patients) |
MvD during follow-up (17 eyes of 16 patients) |
P value |
|---|---|---|---|
| Baseline Age (yrs) | 70.2 (64.8, 75.6) | 71.7 (66.2, 77.1) | 0.701 |
| Gender (Female/ Male) | 10/8 | 8/8 | 0.746 |
| Race (African American/ Non-African American) | 7/11 | 6/10 | 0.934 |
| Self-reported hypertension, n (%) | 11 (61.1%) | 9 (56.3%) | 0.774 |
| Self-reported diabetes, n (%) | 1 (5.6%) | 4 (25.0%) | 0.110 |
| Axial length (mm) | 23.9 (23.4, 24.4) | 23.9 (23.3, 24.5) | 0.972 |
| CCT (μm) | 522.4 (498.9, 545.9) | 545.6 (526.3, 565.0) | 0.108 |
| Baseline VF MD (dB) | −2.1 (−3.2, −1.1) | −3.1 (−4.4, −1.8) | 0.207 |
| Baseline RNFL Thickness (um) | 88.6 (82.6, 94.7) | 80.7 (74.6, 86.7) | 0.064 |
| Baseline IOP (mmHg) | 15.0 (13.4, 16.7) | 15.5 (13.5, 17.4) | 0.742 |
| Mean IOP during follow-up (mmHg) | 14.9 (13.6, 16.3) | 15.4 (12.7, 18.1) | 0.773 |
| IOP fluctuation (mmHg) | 1.9 (1.5, 2.3) | 3.5 (2.3, 4.7) | 0.012 |
| Peak IOP during follow-up (mmHg) | 17.4 (15.9, 18.9) | 20 (15.7, 24.3) | 0.260 |
| No. of glaucoma medications during the follow-up (n) | 1.6 (1, 2.2) | 2.5 (1.8, 3.3) | 0.044 |
| No. of eyes using glaucoma medication during the follow-up, n (%) | 7.2 (6.9, 7.5) | 7.1 (6.8, 7.4) | 0.506 |
| Glaucoma surgeries at baseline, n (%) | 2 (10.0%) | 5 (29.4%) | 0.133 |
| Cataract surgeries at baseline, n (%) | 11 (52.4%) | 10 (58.8%) | 0.691 |
| Scan Quality Score | 7.2 (6.9, 7.5) | 7.1 (6.8, 7.4) | 0.506 |
| Follow-up period (years) | 3.7 (3.4, 4.0) | 4.2 (3.8, 4.5) | 0.021 |
| No. of follow-up visits | 5.0 (4.4, 5.5) | 6.4 (5.6, 7.3) | 0.002 |
CCT = central corneal thickness; IOP = intraocular pressure; MD = mean deviation; MvD = microvascular dropout; OCTA = optical coherence tomography angiography; VF = visual field.
Data is presented as mean (95% CI) unless otherwise indicated. Statistically significant P values are shown in bold.
Supplemental Table 1 summarizes the factors contributing on the MvD Angle change over time. In univariable analysis, IOP fluctuations during follow-up (coefficient (95% CI) 1.57 (0.06, 3.09) degree /year per 1 mmHg higher; P=0.041) and the number of medications (coefficient (95% CI) 2.73 (1.05, 4.41) degree /year per 1 higher; P=0.001) were significantly associated with the rate of MvD angle change over time. As in Table 2, we compared three multivariable models including peak IOP, IOP fluctuation or the number of medications. The effect of IOP fluctuation remained statistically significant in the multivariable model (Model 2), with coefficient (95% CI) 1.77 (0.28, 3.26) degree /year per 1 mmHg higher; P=0.02, whereas the effect of peak IOP was not significant in Model 2, with coefficient (95% CI) 0.39 (−0.05, 0.83) degree /year per 1 mmHg higher; P=0.081. Figure 3 shows one case of non-progressive MvD and three representative cases of MvD enlargement: One with worse baseline VF MD, one with high IOP fluctuations and one with high IOP peak.
Figure 3.
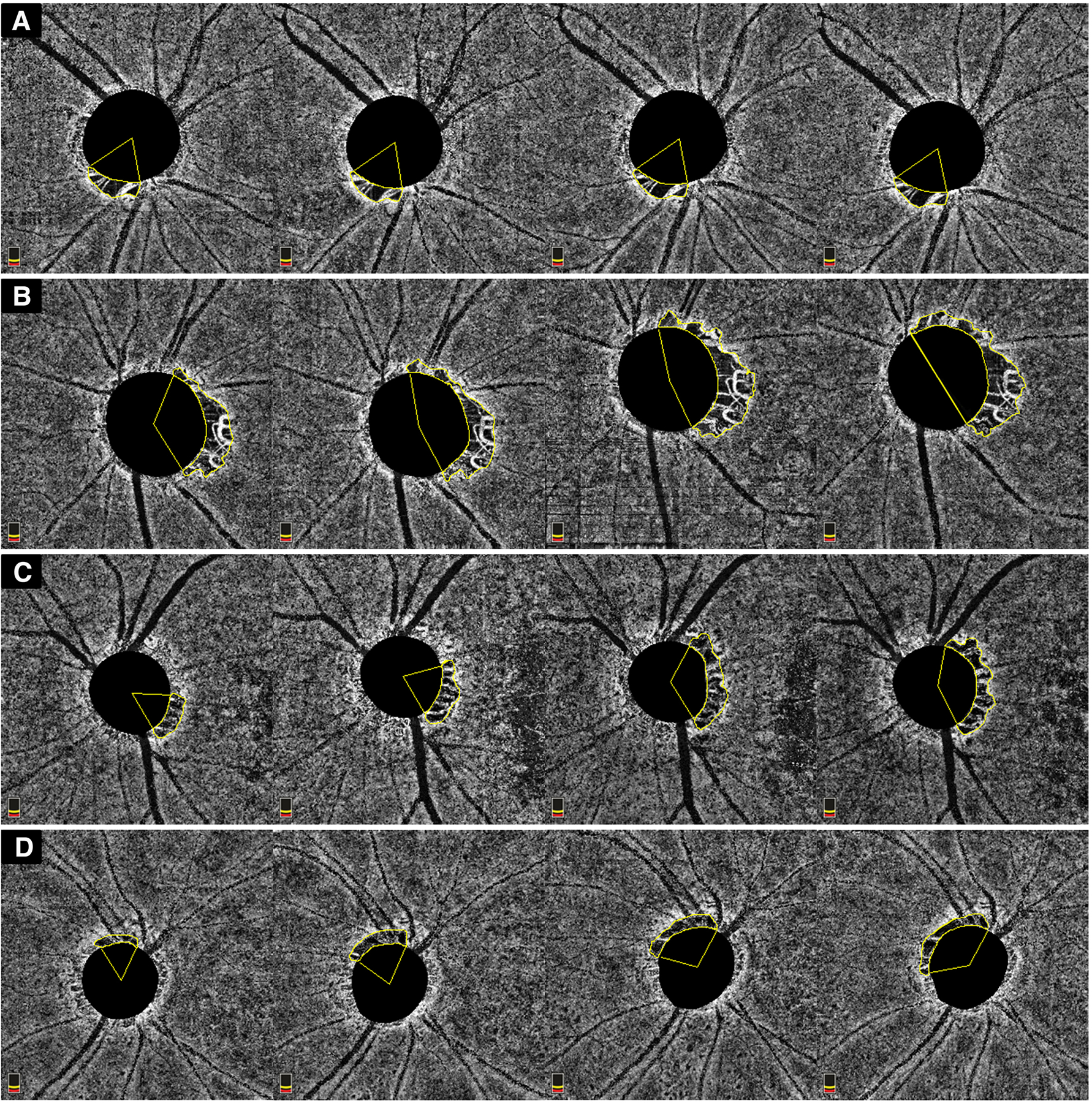
Representative case of MvD showing no enlargement over time (A); MvD enlargement in an OAG eye with worse VF MD at baseline (MD −11.4 dB); MvD enlargement in an OAG eye with high IOP fluctuations IOP (IOP SD: 4.7) during follow-up; MvD enlargement in an OAG eye with high IOP peak (IOP 28 mmHg) during follow-up.
DISCUSSION
With four years of follow-up in the current study, dropout of choroidal microvasculature within the ß-PPA was detected in more than three quarters of glaucoma eyes at baseline or during follow-up. Worse baseline VF MD, higher IOP fluctuations, higher peak IOP during follow-up and higher number of IOP-lowering medications were significantly associated with faster MvD area and angular circumference enlargement in POAG eyes. Eyes that developed MVD after the baseline visit showed greater IOP fluctuations compared to eyes that did not develop MVD over the follow-up period. These findings suggest that greater fluctuations in IOP and worse glaucoma severity at baseline are the main factors associated with the development and rate of enlargement of choroidal perfusion defects in glaucomatous eyes.
More than half of POAG eyes (n= 53) showed evidence of MvD at baseline, while 17 eyes with no MvD at baseline developed it over the follow-up. In contrast, 21 eyes (23.0%) did not show any MvD over the given follow-up period. Whether these eyes subsequently will develop MvD or not remains to be determined. Only a few previous studies[13 14] evaluated the longitudinal changes of MvD over time. As they only included eyes with MvD at baseline in these studies, data were not available regarding the development of MvD during the follow-up period. What causes MvD is not well-understood. It may reflect a disruption of the microvasculature contained in the laminar and prelaminar tissues of the ONH tissues, as the parapapillary choroid and deep ONH structures share short posterior ciliary artery as a common source of perfusion.[21 22] Since vascular factors may have a significant role in the development and progression of OAG,[2 3] the development and rate of enlargement of MvD over time may constitute a potential risk factor for the progression of glaucoma. Due to the complex vascular anatomy of parapapillary choroid, either an enlargement of area and angular circumference seems to be present and might show significant associations.
Alternatively, MvD changes may be secondary to worsening of glaucoma. Indeed, factors associated with MvD enlargement in the present study (i.e., IOP fluctuation, peak IOP, baseline VF MD) are also risk factors of the disease progression (i.e., RNFL thinning and VF progression).
Given that MvD and disease progression are closely related, it is possible that these factors are not directly associated with the MvD enlargement, but are an epiphenomenon of glaucoma progression. The temporal relationship between the vascular damage and the structural damage is controversial. Although the current study does not answer this question, it does enhance our understanding of the mechanisms of the vascular damage.
In the present study, worse baseline VF mean deviation (MD) was found to be significantly associated with faster rates of MvD area and angular circumference changes over a mean of 4-year follow-up. In a previous cross-sectional study, the presence of choroidal perfusion defects detected by OCT-A was significantly associated with glaucoma severity[4], suggesting an association between MvD and advanced disease. On the other hand, baseline MD was found to be a significant predictor of VF worsening in several cohorts of glaucoma patients.[23–25] In the JAMDIG study[25], baseline VF damage was a prognostic factor of glaucoma progression, specifically in eyes with low mean IOP. In the current study, faster enlargement of MvD may be associated with faster glaucoma progression in eyes with more advanced disease, even when IOP is seemingly well-controlled.
The relationship between the localized choroidal perfusion defects and IOP parameters is still unclear. Although several studies did not find any associations between MvD and IOP[4 10], a few studies found that peak IOP was more frequently present in glaucoma patients with MvD and mean IOP < 21 mm Hg.[12] In the present study, no significant associations were found between mean IOP and MvD changes during the follow-up period. However, larger peak IOP was significantly associated with faster MvD area changes during the follow-up. Furthermore, higher long-term fluctuations of IOP were significantly associated with faster enlargement of MvD as well as its development. Indeed, the group of eyes that developed MvD after the first OCT-A visit showed greater IOP fluctuation compared with those that did not develop any MvD. These findings suggests that variations in IOP, rather than mean IOP, may have a role in the progression and development of MvD in these glaucoma patients.
The role of long-term IOP fluctuation on VF deterioration has been evaluated in several studies.[25–28] In a cohort from the Advanced Glaucoma Interventional study,[26] long-term fluctuations were associated with VF progression in patients with low mean IOP (10.8 mmHg), but not in patients with high mean IOP (20.6 mmHg). A study by Asrani et al.[27] noted that in glaucoma patients with normal IOP range during the day, IOP fluctuation, measured with home tonometry, was a significant risk factor for glaucoma progression. In the present study, most of the patients were undergoing intensive glaucoma treatment, with a mean IOP of 14.1 mmHg during follow-up. Since MvD changes were associated with progressive glaucoma damage, the association between long-term IOP fluctuations and faster rates of MvD changes may suggest that IOP fluctuations have a role in patients with seemingly well-controlled IOP and VF progression. Although the mechanism remains unclear, long-term IOP fluctuations and peak IOP may disrupt homeostatic mechanisms, causing loading and unloading stresses and, as opposed to conditions of static stress, the tissue is unable to compensate. As a result, further damage occurs.
When eyes were categorized based on the number of IOP lowering medications, the use of beta-blockers, carbonic anhydrases inhibitors (CAI) and alpha-agonists were significantly associated with the rates of change of MvD area and angular circumference during follow-up. The role of medications on vessel changes has been discussed in many studies.[29–33] Topical beta-blockers, especially timolol, have been found to have a vasoconstricting effect on superficial retinal and choroidal layers. Although the present study found a significant relationship between the use of beta-blockers and faster rates of MvD changes, beta-blockers were usually prescribed as second-line therapy in combination with other medications. Thus, the severity of the disease, history of glaucoma or cataract surgery and number of IOP measurements may be confounders in these patients. Further studies should be performed to clarify the role of different IOP-lowering medications on MvD.
Previous studies revealed a significant association between MvD and axial length, suggesting that the elongation of the eye in myopic eyes may result in increased mechanical stress on the ONH and peripapillary area leading to vascular insufficiency or dropout in the choroidal layers. Other studies have suggested that MvD is more related to the pathophysiological changes of glaucomatous damage than to myopic changes. For example, Na et al. found that MvD was detected in more than three quarters of myopic POAG eyes whereas none of the myopic non-POAG eyes showed any dropout.[34] The exclusion of eyes with axial length>27 mm in the current study might account for us not finding any association between MvD enlargement and axial length.
Several limitations affect this study. First, area and angular circumference were measured using the en-face choroidal vessel density image, which may be affected by large overlying vessels or disc hemorrhages that project on it, making it difficult to detect or define MvD boundaries. To reduce subjectivity and potential false negatives of MvD measurements, we defined MvD as a complete loss of the choroidal microvasculature with a size of at least 200 microns in diameter within the βPPA.[4] Although the reproducibility of MvD measurement was excellent in this study, enlargement of MvD assessed manually might be affected by test–retest variability. In addition, OCT beam penetration due to shadowing or projection artifact overlaid on the choroid can contribute to the inter-visit variability, and this can hinder the accurate measurement of the MvD change. The detection of MvD may also be affected by the βPPA, which was not measured in the present study. Second, the automatic demarcation of the BMO was not accurate in some eyes. Therefore, a trained observer manually corrected potential disc margin errors before the quantitative analysis of MvD size. Also, there was a difference in the number of OCT-A visits between the group with MvD at baseline and the group without MvD at baseline. Although this difference was not significant, it may lead to a falsely low rate of MvD detection in eyes without MvD at baseline. Third, the choroid is not segmented specifically by OCT-A and one cannot assume that the en-face choroidal vessel density map represents only the choroidal layer. Fourth, IOP measurements are mere snapshots of true IOP over time and our understanding of real IOP behavior is limited. The application of sensors that continual measurements of IOP would provide substantially more information and would strengthen the assessment of IOP variability.[35 36] Fifth, the sample size of eyes the developed MvD after the baseline visit is small, so results may not be generalizable to other populations. Last, duration of IOP-lowering medication use was not considered in this study. However, only medications used during the given follow-up were included.
In conclusion, higher levels of IOP fluctuations, peak IOP, worse VF MD at baseline and number of IOP lowering medications during follow-up were the main factors related to faster MvD enlargement in glaucomatous eyes. IOP fluctuations were significantly greater in eyes that developed MvD compared to eyes that did not develop MvD during follow-up. Further studies are needed to clarify the relationship between MvD and IOP metrics and other factors, as well as the temporal relationship between the vascular damage and the structural damage.
Supplementary Material
KEY MESSAGES:
What is already known on this topic:
Choroidal Microvascularure Dropout (MvD) tends to enlarge over time in eyes with glaucoma. However, factors that are associated with its enlargement still needs to be investigated.
What this study adds:
The present study demonstrated that greater IOP fluctuation, higher peak IOP, worse baseline VF MD are factors associated with the rate of MvD enlargement in glaucomatous eyes.
How this study might affect research, practice or policy
The identification of factors associated with MvD enlargement may help physicians and researchers understand the role of choroidal vasculature or blood flow in glaucoma.
SYNOPSIS:
In this observational cohort study, greater IOP fluctuation, higher peak IOP, worse baseline VF MD and greater number of glaucoma medications were significantly associated with choroidal microvasculature dropout enlargement in glaucomatous eyes.
Acknowledgment/financial support:
National Institutes of Health/National Eye Institute Grants R01EY029058, R01EY011008, R01EY026574, R01EY019869 and R01EY027510; Core Grant P30EY022589; by the donors of the National Glaucoma Research Program (no grant number), a program of the BrightFocus Foundation grant (G2017122), an unrestricted grant from Research to Prevent Blindness (New York, NY); UC Tobacco Related Disease Research Program (T31IP1511); and grants (grant number not applicable) for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck, and Santen. The sponsor or funding organizations had no role in the design or conduct of this research.
Footnotes
STATEMENTS
Financial Disclosures:
Eleonora Micheletti: none; Sasan Moghimi: none; Takashi Nishida: none; Nevin W. El-Nimri: none; Golnoush Mahmoudinezhad: none; Alireza Kamalipour: none; Vahid Mohammadzadeh: none; Linda M. Zangwill: National Eye Institute (F), Carl Zeiss Meditec Inc. (F), Heidelberg Engineering GmbH (F), OptoVue Inc. (F, R), Topcon Medical Systems Inc. (F, R) Merck (C); Robert N. Weinreb: Allergan (C), Eyenovia (C), Topcon (C), Heidelberg Engineering (F), Carl Zeiss Meditec (F), Konan (F), OptoVue (F), Topcon (F), Centervue (F).
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004;363(9422):1711–20. [DOI] [PubMed] [Google Scholar]
- 2.Weinreb RN. Ocular blood flow in glaucoma. Can J Ophthalmol 2008;43(3):281–3. [DOI] [PubMed] [Google Scholar]
- 3.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002;21(4):359–93. [DOI] [PubMed] [Google Scholar]
- 4.Suh MH, Zangwill LM, Manalastas PI, et al. Deep Retinal Layer Microvasculature Dropout Detected by the Optical Coherence Tomography Angiography in Glaucoma. Ophthalmology 2016;123(12):2509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh MH, Na JH, Zangwill LM, Weinreb RN. Deep-layer Microvasculature Dropout in Preperimetric Glaucoma Patients. J Glaucoma 2020;29(6):423–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon J, Shin JW, Lee J, Kook MS. Choroidal Microvasculature Dropout Is Associated With Parafoveal Visual Field Defects in Glaucoma. Am J Ophthalmol 2018;188:141–54. [DOI] [PubMed] [Google Scholar]
- 7.Park HL, Kim JW, Park CK. Choroidal Microvasculature Dropout Is Associated with Progressive Retinal Nerve Fiber Layer Thinning in Glaucoma with Disc Hemorrhage. Ophthalmology 2018;125(7):1003–13. [DOI] [PubMed] [Google Scholar]
- 8.Kwon JM, Weinreb RN, Zangwill LM, Suh MH. Parapapillary Deep-Layer Microvasculature Dropout and Visual Field Progression in Glaucoma. Am J Ophthalmol 2019;200:65–75. [DOI] [PubMed] [Google Scholar]
- 9.Micheletti E, Moghimi S, El-Nimri N, et al. Relationship of macular ganglion cell complex thickness to choroidal microvasculature drop-out in primary open-angle glaucoma. Br J Ophthalmol 2022 [DOI] [PubMed]
- 10.Lee EJ, Kim TW, Kim JA, Kim JA. Central Visual Field Damage and Parapapillary Choroidal Microvasculature Dropout in Primary Open-Angle Glaucoma. Ophthalmology 2018;125(4):588–96. [DOI] [PubMed] [Google Scholar]
- 11.Lee EJ, Kim S, Hwang S, Han JC, Kee C. Microvascular Compromise Develops Following Nerve Fiber Layer Damage in Normal-Tension Glaucoma Without Choroidal Vasculature Involvement. J Glaucoma 2017;26(3):216–22. [DOI] [PubMed] [Google Scholar]
- 12.Shin JW, Jo YH, Song MK, Won HJ, Kook MS. Nocturnal blood pressure dip and parapapillary choroidal microvasculature dropout in normal-tension glaucoma. Sci Rep 2021;11(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JY, Shin JW, Song MK, Hong JW, Kook MS. An Increased Choroidal Microvasculature Dropout Size is Associated With Progressive Visual Field Loss in Open-Angle Glaucoma. Am J Ophthalmol 2021;223:205–19. [DOI] [PubMed] [Google Scholar]
- 14.Kim JA, Lee EJ, Kim TW. Evaluation of Parapapillary Choroidal Microvasculature Dropout and Progressive Retinal Nerve Fiber Layer Thinning in Patients With Glaucoma. JAMA Ophthalmol 2019;137(7):810–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009;127(9):1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol 2010;128(5):541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin JW, Kwon J, Lee J, Kook MS. Choroidal Microvasculature Dropout is Not Associated With Myopia, But is Associated With Glaucoma. J Glaucoma 2018;27(2):189–96. [DOI] [PubMed] [Google Scholar]
- 18.Zang P, Gao SS, Hwang TS, et al. Automated boundary detection of the optic disc and layer segmentation of the peripapillary retina in volumetric structural and angiographic optical coherence tomography. Biomed Opt Express 2017;8(3):1306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aykut V, Oner V, Tas M, Iscan Y, Agachan A. Influence of axial length on peripapillary retinal nerve fiber layer thickness in children: a study by RTVue spectral-domain optical coherence tomography. Curr Eye Res 2013;38(12):1241–7. [DOI] [PubMed] [Google Scholar]
- 20.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38(4):963–74. [PubMed] [Google Scholar]
- 21.Anderson DR, Braverman S. Reevaluation of the optic disk vasculature. Am J Ophthalmol 1976;82(2):165–74. [DOI] [PubMed] [Google Scholar]
- 22.Onda E, Cioffi GA, Bacon DR, Van Buskirk EM. Microvasculature of the human optic nerve. Am J Ophthalmol 1995;120(1):92–102. [DOI] [PubMed] [Google Scholar]
- 23.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007;114(11):1965–72. [DOI] [PubMed] [Google Scholar]
- 24.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108(11):1943–53. [DOI] [PubMed] [Google Scholar]
- 25.Lee JM, Caprioli J, Nouri-Mahdavi K, et al. Baseline prognostic factors predict rapid visual field deterioration in glaucoma. Invest Ophthalmol Vis Sci 2014;55(4):2228–36. [DOI] [PubMed] [Google Scholar]
- 26.Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 2008;115(7):1123–29 e3. [DOI] [PubMed] [Google Scholar]
- 27.Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000;9(2):134–42. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros FA, Weinreb RN, Zangwill LM, et al. Long-term intraocular pressure fluctuations and risk of conversion from ocular hypertension to glaucoma. Ophthalmology 2008;115(6):934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoste AM. In vitro studies of the effects of beta-adrenergic drugs on retinal and posterior ciliary microarteries. Surv Ophthalmol 1999;43 Suppl 1:S183–90. [DOI] [PubMed] [Google Scholar]
- 30.Grunwald JE. Effect of two weeks of timolol maleate treatment on the normal retinal circulation. Invest Ophthalmol Vis Sci 1991;32(1):39–45. [PubMed] [Google Scholar]
- 31.Leung M, Grunwald JE. Short-term effects of topical levobunolol on the human retinal circulation. Eye (Lond) 1997;11 ( Pt 3):371–6. [DOI] [PubMed] [Google Scholar]
- 32.Durmus Ece BS, Saricaoglu MS. Examination of retinal vascular density changes via optical coherence tomography angiography in patients with glaucoma. Int Ophthalmol 2021;41(2):687–98. [DOI] [PubMed] [Google Scholar]
- 33.Lin YH, Su WW, Huang SM, Chuang LH, Chen LC. Optical Coherence Tomography Angiography Vessel Density Changes in Normal-tension Glaucoma Treated With Carteolol, Brimonidine, or Dorzolamide. J Glaucoma 2021;30(8):690–96. [DOI] [PubMed] [Google Scholar]
- 34.Na HM, Lee EJ, Lee SH, Kim TW. Evaluation of Peripapillary Choroidal Microvasculature to Detect Glaucomatous Damage in Eyes With High Myopia. J Glaucoma 2020;29(1):39–45. [DOI] [PubMed] [Google Scholar]
- 35.Mansouri K, Gillmann K, Rao HL, Weinreb RN, Group A-S. Weekly and seasonal changes of intraocular pressure measured with an implanted intraocular telemetry sensor. Br J Ophthalmol 2021;105(3):387–91. [DOI] [PubMed] [Google Scholar]
- 36.Mansouri K, Rao HL, Weinreb RN, Group A-S. Short-Term and Long-Term Variability of Intraocular Pressure Measured with an Intraocular Telemetry Sensor in Patients with Glaucoma. Ophthalmology 2021;128(2):227–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.


