ABSTRACT
The antibody–drug conjugate (ADC) field has undergone a renaissance, with substantial recent developmental investment and subsequent drug approvals over the past 6 y. In November 2022, ElahereTM became the latest ADC to be approved by the US Food and Drug Administration (FDA). To date, over 260 ADCs have been tested in the clinic against various oncology indications. Here, we review the clinical landscape of ADCs that are currently FDA approved (11), agents currently in clinical trials but not yet approved (164), and candidates discontinued following clinical testing (92). These clinically tested ADCs are further analyzed by their targeting tumor antigen(s), linker, payload choices, and highest clinical stage achieved, highlighting limitations associated with the discontinued drug candidates. Lastly, we discuss biologic engineering modifications preclinically demonstrated to improve the therapeutic index that if incorporated may increase the proportion of molecules that successfully transition to regulatory approval.
KEYWORDS: Antibody–drug conjugate, ADC, payload, linker, drug-to-antibody ratio, site-directed conjugation, clinical trials
ADCs as a new class of targeted therapeutics
A new class of precision medicines, antibody–drug conjugates (ADCs), was ushered into oncology clinical practice in 2000 with the US Food and Drug Administration (FDA)’s approval of MylotargTM for the treatment of acute myeloid leukemia (AML). ADC molecules marry the precision of antibody-mediated tumor antigen targeting with potent cytotoxic agents, thereby creating a targeted delivery vehicle for malignant tumors. In this manner, ADCs provide a means to reduce off-tumor toxicities by limiting payload exposure in normal tissues. While most ADC clinical candidates utilize cytotoxic chemotherapeutic payloads, recent ADC candidates have also incorporated targeted small molecules1 and immunomodulatory agents.2 In the 23 years since MylotargTM’s first registration, only 12 of 267 clinically tested ADCs have made it to regulatory approval; 10 occurring in the last 6 years [Figure 1]. Insights into biologic engineering and utilization of less potent linker-payloads (e.g., EnhertuTM) have re-energized the field and ushered a new wave of drug approvals.
Figure 1.
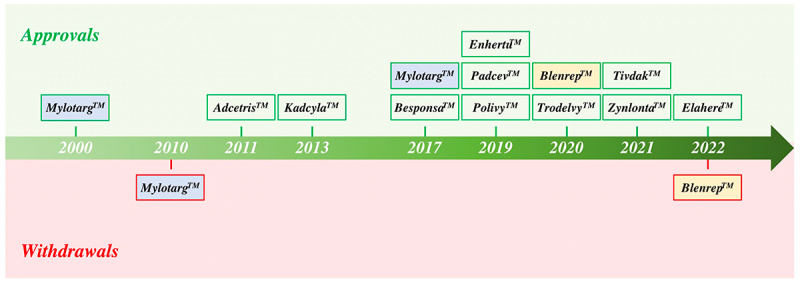
Timeline of FDA Approvals. To date, 12 ADCs have been granted FDA approval (green boxes). Two candidates, MylotargTM and BlenrepTM, had their approvals withdrawn (red boxes) due to failure to meet requisite endpoints in post-approval trials. MylotargTM was subsequently re-approved at a lower dose in combination with chemotherapy. Eleven ADC therapeutics are currently FDA approved.
Factors affecting activity of ADCs
ADCs offer several advantages over standard chemotherapies, notably: 1) precision delivery of cytotoxic payloads to cells expressing the selected target antigen, 2) enablement of more potent cytotoxic payload utilization than can be administered systemically, and 3) potential minimization of on target/off tumor toxicity. The promise of ADCs, when successfully designed, is the ability to broaden the therapeutic index over that of systemically administered chemotherapy. By directly delivering the cytotoxic payloads to the tumor tissue, the minimum effective dose (MED) is lowered with corresponding reduction in on target/off tumor adverse events.
Effective analysis of the clinically tested ADC molecules necessitates a fundamental understanding of the factors that modulate their biological activity. The basic cellular processes underlying ADC cytotoxic payload delivery have three key parts. First, the antibody binds to the target antigen on the surface of an antigen-positive cell. Second, the antigen-ADC complex is internalized into the target cell by receptor-mediated endocytosis. Third, the antigen-ADC complex is digested by lysosomal enzymes, releasing the cytotoxic payload that triggers cell death. As illustrated in Figure 2 and discussed below, the effectiveness of these basic cellular processes underlying ADC clinical activity are further modulated by various factors, notably the target antigen, functional attributes of the created antibody, conjugation chemistries, linker attributes, and payload potency and effectiveness for a chosen tumor indication.
Figure 2.
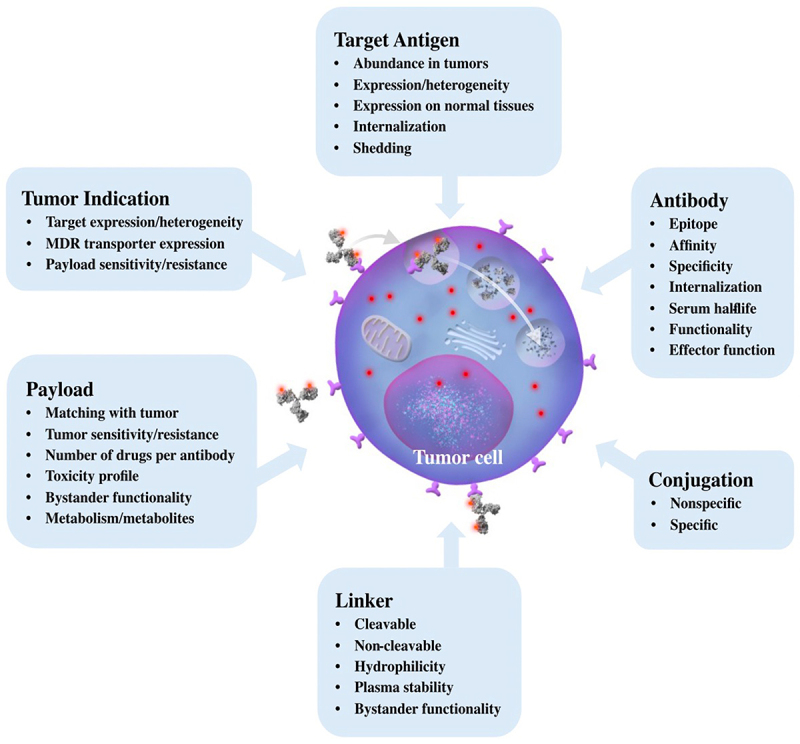
Factors Governing ADC Activity. Grey arrows indicate the path of an ADC into a cell. The antibody binds to the target antigen on the surface of the cell, the antigen-ADC complex is internalized by endocytosis, and the antigen-ADC complex is either recycled back to the cell surface, or transitions to the lysosomal compartment. Lysosomal processing releases the cytotoxic payload (red dots) ultimately triggering cell death. Factors governing this process include the target antigen, the antibody, the conjugation methodology to attach the payload to the biologic, the linker, the payload, and the selected tumor indication.
Target antigen
For an ADC to be effectively internalized within a given cell, a requisite target antigen density needs to exist to trigger efficient receptor-mediated endocytosis. A target antigen density of approximately 10,000 copies/cell or greater has been proposed as a minimum threshold for efficient biologic-mediated ADC internalization.3 Cells with target antigens expressed at lower molecular densities exhibit inefficient ADC internalization with a subsequent reduction in payload delivery. Inefficient ADC internalization can also result in ADC recycling outside of the cell prior to payload processing and release, further reducing the ADC’s cytotoxic effect.4 In addition to requisite tumor antigen densities to trigger efficient internalization, the ideal targets chosen for ADC drug development would demonstrate significantly elevated tumor antigen expression over that of normal tissues to minimize the potential for on target/off tumor toxicities. A favorable example of a target that is significantly overexpressed in tumor tissues relative to normal tissues is the HER2/neu antigen that is expressed at lower levels on a subset of normal cells, but expressed at hundreds of thousands to over a million copies on HER2+ cancer cells.5 Indeed, ADCs targeting the HER2 antigen have demonstrated robust internalization into HER2-targeted tumor cells with efficient payload delivery6,7 that has translated to clinical benefit and ultimate drug approval.8,9 In contrast, ADCs targeting tumor antigens with heterogeneous/low target antigen expression, such as the prolactin receptor with antigen densities of thousands to tens of thousands of molecules/cell,10 failed to demonstrate clinical responses at the biologic doses tested and were subsequently terminated from future clinical development.11
Antibody
Target epitope choice of a given biologic can greatly alter the effectiveness of the created ADC. Notably, biologics targeting epitopes that promote rapid receptor-mediated internalization show greater activity than biologics targeting non-internalizing epitopes.12 In addition to epitope choice, biologic affinity can also alter the effectiveness of ADC biologics. Indeed, biologics with lower affinities may demonstrate insufficient binding and/or internalization at lower target antigen densities13 and biologics with too high cellular affinities may result in reduced receptor occupancy and/or internalization.14 Biologic affinity tuning may also help mitigate on target/off tumor toxicities for antigens expressed in normal tissues of concern. Creating biologics with lower cellular affinities could help mitigate toxicity toward target positive normal cells while retaining potency against tumor cells where the given antigen is overexpressed. A preclinical example of this concept is the low affinity EGFR ADC RN765C that demonstrated robust killing of EGFR-positive cell lines/tumor models where EGFR is overexpressed with reduced toxicity against EGFR-positive normal human keratinocytes.13
Conjugation
Most ADCs use nonspecific lysine or cysteine residue-directed biologic conjugation. Both conjugation approaches have been found to generate heterogenous ADC products.15,16 In contrast, site-specific conjugation to native or engineered amino acid residues has been shown to generate more homogenous ADC drug products with improved pharmacokinetic (PK) properties and safety profiles.17,18
Linker
Linkers can be cleavable or non-cleavable. Cleavable linkers are designed to release the payload inside the targeted cell by protonolysis, thiol reduction, proteolysis, or carbohydrate hydrolysis. In addition to cytosolic payload release, cleavable linkers have also been shown to be cleaved extracellularly due to the presence of cleaving agents in the blood and/or tumor microenvironment (TME). These linkers can be associated with both increased adverse events (due to systemic payload release)19 and increased efficacy due to noted “bystander effects” (wherein released payload can diffuse across the plasma membrane of a higher tumor antigen expressing cell to adjacent tumor cells with lower antigen expression).20 An ADC can also be created with a non-cleavable linker that only releases payload after proteolysis by lysosomal enzymes. These released payload-adducts are modified such that they do not diffuse across plasma membranes, which limits both their systemic adverse effects but also mitigates the efficacy benefit to neighboring tumor cells due to diminished bystander diffusion.21 An excellent example of this concept is the approved clinical ADC, KadcylaTM, that employs a non-cleavable linker, limiting its systemic toxicity as well as efficacy to bystander cells expressing lower target antigen densities. EnhertuTM, in contrast, uses a cleavable linker, and demonstrates bystander killing and greater clinical activity in tumors with lower HER2 target expression.9 In a head-to-head clinical trial, EnhertuTM demonstrated superior clinical activity (mPFS 28.8 months, EnhertuTM versus 6.8 months, KadcylaTM) with comparable incidence of Grade 3 or higher treatment-emergent adverse events (56%, EnhertuTM versus 52%, KadcylaTM) and serious treatment-emergent adverse events (25%, EnhertuTM versus 22%, KadcylaTM).22 In addition to linker choice, choice of payload and presence of tumor drug efflux pumps could have also contributed to these clinical results. Linkers can also vary by their degree of hydrophilicity. Indeed, more hydrophilic linkers have been shown to increase the solubility and favorable PK properties of the ADCs, especially those that use more hydrophobic drug payloads.23
Payload
The traditional chemotherapeutic ADC payloads fall into three general classes: 1) microtubule inhibitors, 2) DNA-damaging agents, and most recently 3) topoisomerase I inhibitors. The potencies of these payload classes dictate the ADC efficacy and toxicity. Early ADC candidates utilizing low potency payloads of systemically administered chemotherapies (e.g., doxorubicin, IC50 ~ 10–7 M) were ultimately abandoned due to insufficient clinical activity at administered drug exposures.24,25 As a result, the ADC field pivoted to the use of increasingly more potent cytotoxic payloads, such as the DNA damaging agents calicheamicin (IC50 ~ 10−10 M) and pyrrolobenzodiazepines (PBDs) (IC50 ~ 10−12 M) and microtubule inhibitors such as monomethyl auristatin E, MMAE (IC50 ~ 10−10 M) for follow-on drug development.26 Utilization of very potent payloads, however, limited the biologic doses that could be administered, often resulting in suboptimal payload delivery to tumors with lower target antigen densities.27–31 In addition to payload choice, payload ADC effectiveness is also influenced by the 1) number of payload molecules per ADC (drug–antibody ratio, DAR), 2) presence of multi-drug resistance (MDR) efflux pumps in tumors that can expel select payloads, 3) potential bystander functionality of the payload once released, and 4) payload clearance. Bystander functionality is determined by whether the free payload, once released, can diffuse across cellular membranes to trigger a cytotoxic effect. The net charge on the released payload has been found to influence this functionality. For example, released neutral lipophilic MMAE payloads can diffuse across cell membranes to produce a bystander effect, whereas charged MMAF (monomethyl auristatin F) molecules cannot.32
Payload hydrophobicity has been found to modulate the clearance of the payload. More hydrophobic payloads tend to exhibit more rapid clearance, altering the on-target efficacy and off-target toxicity of a given ADC.23 In vivo payload metabolism can also modulate ADC safety and efficacy. For example, the SN-38 payload becomes inactivated in the liver with the opening of the lactone ring, dampening its cytotoxic functionality.33 Finally, clinical success of the ADC depends upon appropriate matching of the payload class to the desired indication as described below.
Indication
The clinical effectiveness of an ADC also depends on the nature of the tumor being targeted. In general, tumors with heterogeneous and/or low target antigen levels are difficult targets for ADCs. Engineering ADCs with bystander activity may in part overcome this challenge as was demonstrated with EnhertuTM’s recent approval in HER2 low breast cancer.9 Tumors with robust expression of multidrug efflux pumps, which expel payloads from tumors, also present challenges for certain classes of ADC payloads. Indeed, ADC resistance in these high efflux tumors can be circumvented with different payload utilization.34,35
In summary, matching the appropriate tumor antigen to selected ADC linker-payloads for a given cancer indication is critical for development of successful ADC therapeutics.
Analysis of oncology ADCs that have entered clinical trials
Here, we review ADCs registered for at least one human clinical trial for an oncology indication by January 1, 2023, that were included in the Beacon Targeted Therapies Clinical Trials and Pipeline Database (beacon-intelligence.com). We included ADCs that possessed the following two elements: 1) a targeting moiety comprising an antibody, antibody-fusion, or antibody fragment and 2) a payload. The utilized payload is one from either a conventional chemotherapeutic class or a targeted small molecule and/or immune-modulator. Radioisotope ADCs were excluded from this analysis.
In the 26 years since the first ADC clinical trial in 1997, 266 additional ADCs have been tested in over 1200 clinical trials. During this period, 54 ADC programs have been formally discontinued and 38 ADCs have been removed from company pipelines. ADCs covered in this review are classified as 1) Approved (by FDA), 2) Active (not approved by FDA but currently in ≥1 clinical trial), and 3) Discontinued (no longer listed in the company’s clinical pipeline, irrespective of an announcement of discontinuation) [Figure 3]. It should be noted that all Approved ADCs are also currently active in several clinical trials though they are not included in the ‘Active’ category for the purpose of this review (to eliminate double-counting). Additionally, all of the FDA Approved ADCs are approved in other countries in addition to the United States.
Figure 3.
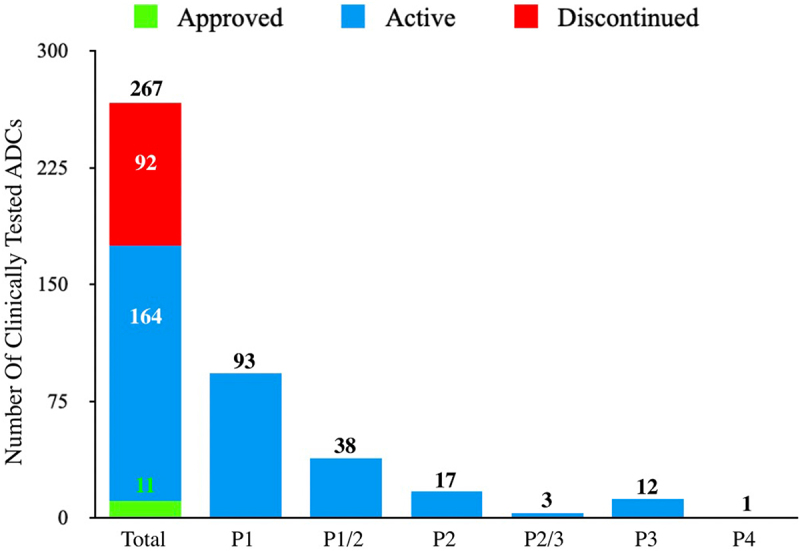
Clinically Tested ADCs. This bar graph captures the 267 ADC that have undergone clinical testing of which: 11 are FDA Approved (green sector), 164 are in Active clinical testing (blue sectors), and 92 have been Discontinued (red sector). Additionally, for the Active ADCs, they have been broken down to highlight their highest development stage (Phase 1-Phase 4, P1-P4). The one candidate in this class listed in Phase 4 (P4), disitamab vedotin, has been approved in China and is not yet approved by the FDA.
Summary of tumor antigens targeted by clinically tested ADCs
The tumor antigen targets and the most advanced stage of clinical testing are illustrated in Figure 4. To date, a total of 106 tumor antigens have been targeted by ADC drug candidates. The 11 approved ADCs target 10 unique cancer antigens: 5 ADCs target hematologic cancer antigens and 6 target solid tumors [Figure 5, Table 1]. Select antigens are the targets of multiple ADCs, including HER2 (41 candidates), Trop-2 (14), CLDN18.2 (11), and EGFR (11). Fewer than 2% of the clinical ADC candidates target more than 1 epitope of selected cancer antigen(s): four bispecific and one biparatopic ADCs are included in this review.
Figure 4.
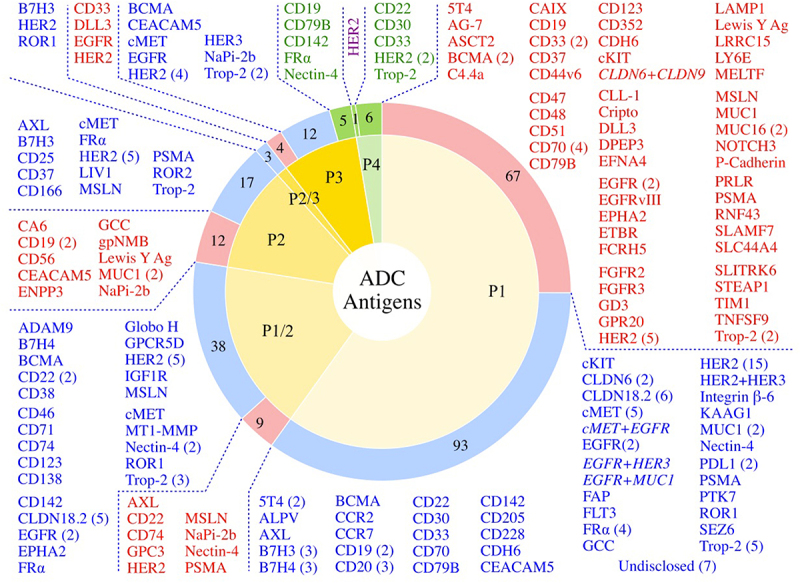
Antigen Targets of the Clinically Tested ADCs. Of the 267 clinically tested ADCs, 260 have known antigens (7 are undisclosed). Numbers of ADCs targeting a given tumor antigen in various stages of clinical testing (Phase 1-Phase 4, P1-P4) are shown in the categories of FDA Approved ADCs (green sectors, green text), Active ADCs (blue sectors, blue text), and Discontinued ADCs (red sectors, red text). Dual antigen targeting ADCs are shown in italics. The Phase 4 HER2 candidate shown in purple text is disitamab vedotin, that has been approved in China and is not yet approved by the FDA.
Figure 5.
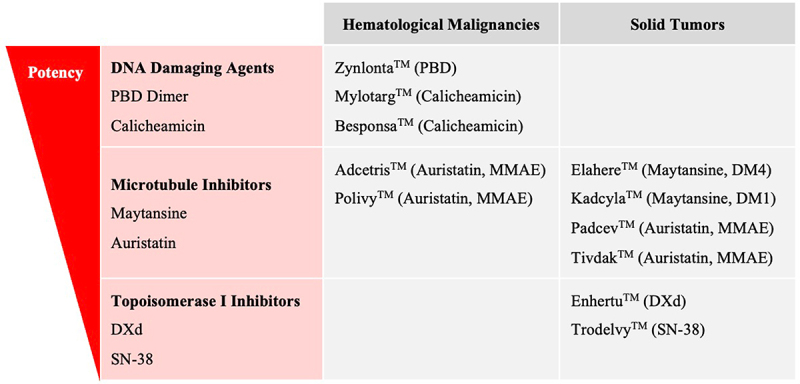
Approved ADCs Classified by Payload Class and Malignancy Setting. Approved ADC drug name and payload are provided. ADCs are listed from top to bottom based upon the potency of the payload utilized with PBD payloads being the most potent and SN-38 payloads the least potent.
Table 1.
Attributes of FDA Approved ADCs and Approval Indications.
| Hematological Malignancies |
Solid Tumors |
||
|---|---|---|---|
| ADC | Indication(s) | ADC | Indication(s) |
|
AdcetrisTM brentuximab vedotin Target: CD30 Conjugation: Nonspecific cysteine Linker: Cleavable, Val-Cit Payload: MMAE DAR ~4 |
Hodgkin’s Lymphoma after ASCT failure, > 2 L when ASCT is not an option, >1 L sALCL (Accelerated approval based on ORR) |
KadcylaTM trastuzumab emtansine Target: HER2 Conjugation: Nonspecific lysine Linker: Non-cleavable, SMCC Payload: DM1 DAR ~3.5 |
HER2+ mBC after treatment with trastuzumab and a taxane, separately or together, HER2+ Early BC as adjuvant treatment in patients with residual disease after neoadjuvant taxane and trastuzumab-based treatment |
|
BesponsaTM inotuzumab ozogamicin Target: CD22 Conjugation: Nonspecific lysine Linker: Cleavable, AcBut acyl hydrazone-disulfide Payload: Calicheamicin DAR ~2-3 |
r/r B cell precursor ALL |
EnhertuTM trastuzumab deruxtecan Target: HER2 Conjugation: Specific cysteine Linker: Cleavable, GGFG Payload: DXd DAR ~8 |
>1 L u/r/m HER2+ BC, HER2-low BC,>1 L HER2+ NSCLC, HER2+ GC/GEJC after trastuzumab-based therapy(Accelerated approval based on ORR, DOR and PFS) |
|
MylotargTM gemtuzumab ozogamicin Target: CD33 Conjugation: Nonspecific lysine Linker: Cleavable, AcBut acyl hydrazone-disulfide Payload: Calicheamicin DAR ~2-3 |
CD33+ AML in adults, CD33 + r/r AML in patients above 2 y in age |
PadcevTM enfortumab vedotin Target: Nectin-4 Conjugation: Nonspecific cysteine Linker: Cleavable, Val-Cit Payload: MMAE DAR ~4 |
Locally advanced or metastatic Urothelial Cancer (la/m UC) after treatment with αPD-1/PD-L1 and a platin-containing chemotherapy(Accelerated approval based on OS, PFS, ORR) |
|
PolivyTM polatuzumab vedotin Target: CD79b Conjugation: Nonspecific cysteine Linker: Cleavable, Val-Cit Payload: MMAE DAR ~3.5 |
>2 L for DLBCL in combination with bendamustine and a rituximab product (Accelerated approval based on CRR) |
TivdakTM tisotumab vedotin Target: Tissue Factor Conjugation: Nonspecific cysteine Linker: Cleavable, Val-Cit Payload: MMAE DAR ~4 |
r/m Cervical Cancer with disease progression on/after chemotherapy(Accelerated approval based on ORR and DOR) |
|
ZynlontaTM loncastuximab tesirine Target: CD19 Conjugation: Nonspecific cysteine Linker: Cleavable, Val-Ala Payload: PBD DAR ~2.3 |
>1 L r/r BCL (including DLBCL) (Accelerated approval based on ORR) |
TrodelvyTM sacituzumab govitecan Target: Trop-2 Conjugation: Specific cysteine Linker: Cleavable, CL2A Payload: SN38 DAR ~7.6 |
>3 L mTNBCla/m, UC after platin-based and αPD-1/PD-L1 therapy(Accelerated approval based on ORR and DOR) |
|
ElahereTM mirvetuximab soravtansine Target: Folate Receptor Alpha Conjugation: Nonspecific lysine Linker: Cleavable, Sulfo-SPDB Payload: DM4 DAR ~3.4 |
2–4 L FRα+ platin-resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer(Accelerated approval based on ORR and DOR) | ||
Abbreviations: 1 L, first line; 2 L, second line; 3 L, third line; AcBut, 4-(4-acetylphenoxy) butanoic acid; Ala, alanine; ALL, acute lymphoblastic leukemia; ASCT, allogeneic stem cell transplant; BC, breast cancer; BCL, B cell lymphoma; Cit, citrulline; CRR, complete response rate; DAR, drug–antibody ratio; DLBCL, diffuse large B cell lymphoma; DM1, mertansine; DM4, ravtansine; DOR, duration of response; DXd, deruxtecan; FRα, folate receptor alpha; GC, gastric cancer; GEJC, gastroesophageal junction cancer; GGFG, glycine-glycine-phenylalanine-glycine; la/m, locally advanced or metastatic; mBC, metastatic breast cancer; MMAE, monomethyl auristatin E; mTNBC, metastatic triple-negative breast cancer; mUC, urothelial cancer; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PBD, pyrrolobenzodiazepine; PFS, progression free survival; r/m, relapsed/metastatic; r/r, relapsed/refractory; sALCL, systemic anaplastic large cell lymphoma; SMCC, succinimidyl-4-(N-maleimidomethyl cyclohexane)-1-carboxylate; u/r/m, unresectable/recurrent/metastatic; Val, valine.
Summary of linkers utilized by clinically tested ADCs
Linkers fall into two major classes: cleavable and non-cleavable [Figure 6]. Of the clinical ADCs, 54% use cleavable linkers, which represent the most utilized linker class. Ten of 11 clinically approved ADCs use protease-cleavable linkers. Of the clinically tested ADCs, 16% use non-cleavable linkers, including the clinically active ADC BlenrepTM. Only one approved ADC, KadcylaTM, uses a non-cleavable linker. Linker class was not disclosed for 31% of the clinically tested ADCs.
Figure 6.
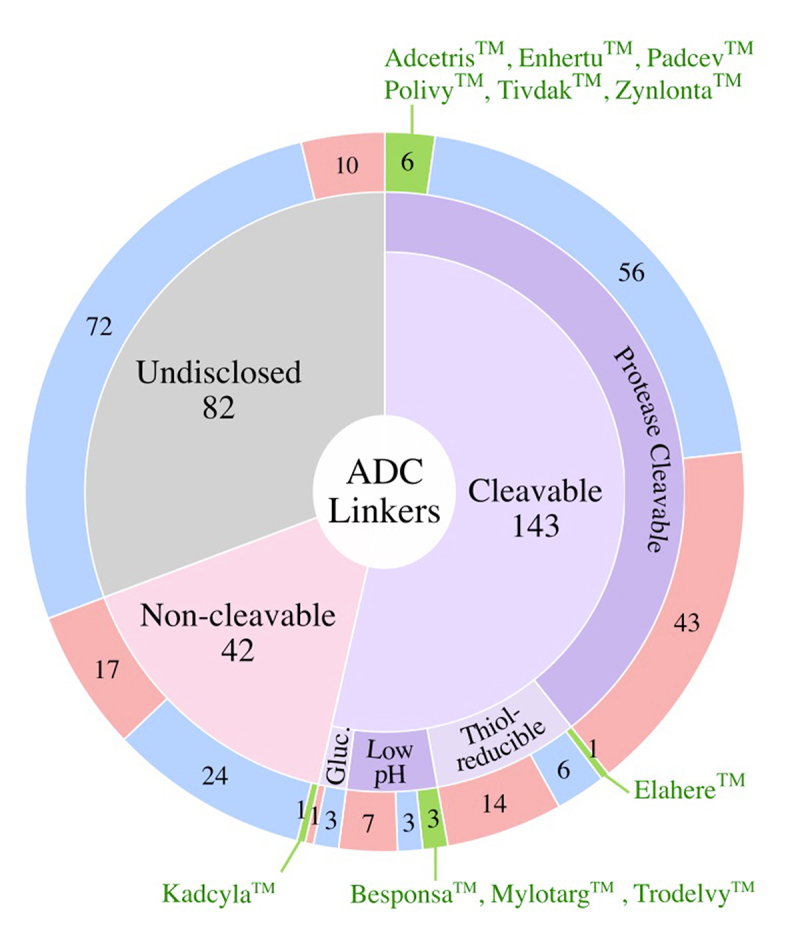
Linkers Used in Clinically Tested ADCs. Numbers of ADCs utilizing different linker classes are shown in the outer ring for the FDA-approved ADCs (green), active ADCs (blue), and discontinued ADCs (red). FDA approved ADCs are shown alongside their respective linkers. Gluc., α-Glucuronide.
Summary of payloads utilized by clinically tested ADCs
Payloads fall into four major classes: 1) microtubule inhibitors, 2) DNA-damaging agents, 3) topoisomerase I inhibitors, and 4) targeted small molecules (SM) [Figure 7]. Microtubule disrupting agents represent the largest payload class (57%) that have undergone clinical testing. Seven of the 11 approved ADCs use microtubule inhibitor payloads. DNA damaging agents comprise the next largest payload class (17%) of ADCs. In this subgroup, 26 of 45 molecules use highly potent PBD payloads, only one of which was granted FDA approval. Two additional approved ADCs employ the DNA damaging class by utilizing the calicheamicin payload. Topoisomerase I inhibitors are included in 7% of clinically tested ADCs. Of the 11 approved ADCs, two use topoisomerase I inhibitor payloads. In addition to these traditional chemotherapeutic payload classes, roughly 5% of ADCs incorporate targeted small molecules such as Bcl-xL inhibitors, as well as immunomodulatory agents such as TLR and STING agonists. No candidate in this non-chemotherapeutic payload class has yet been granted FDA approval. Payloads for 15% of the clinically tested ADCs are not disclosed.
Figure 7.
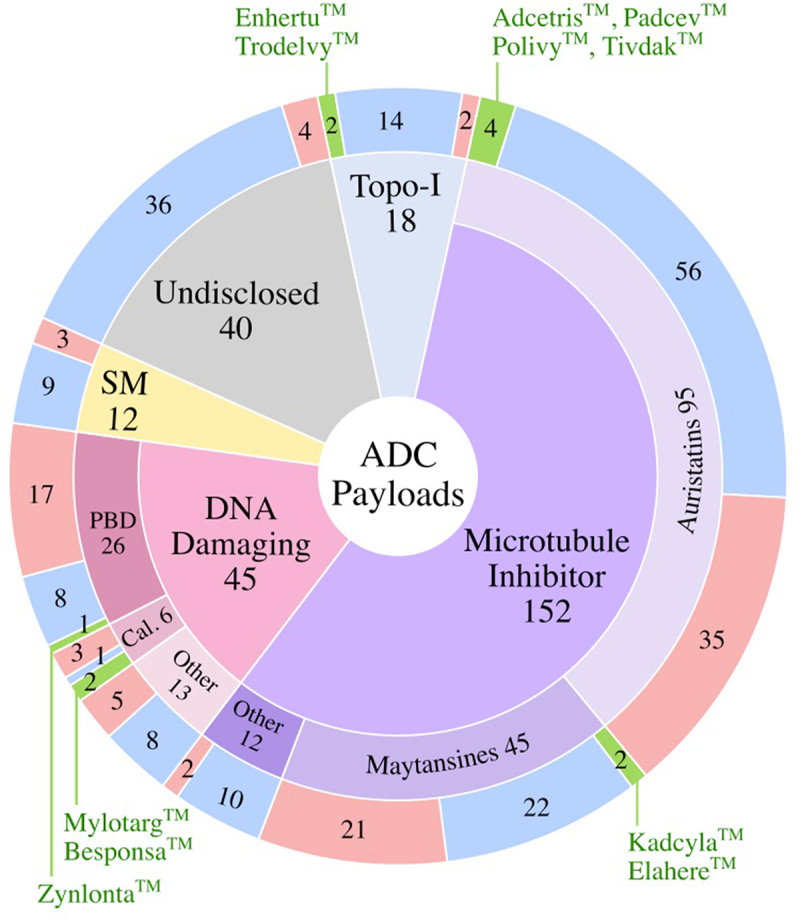
Payloads Used in Clinically Tested ADCs. Numbers of ADCs corresponding to the type of payload are shown are shown in the outer ring for the FDA-approved ADCs (green), active ADCs (blue), and discontinued ADCs (red) sectors. Topo-I, Topoisomerase I Inhibitor; SM, targeted small molecules; PBD, pyrrolobenzodiazepine; Cal., calicheamicin.
Summary of conjugation methods utilized by clinically tested ADCs
Of the 267 clinical ADCs, 111 candidates utilized nonspecific amino acid conjugation, 72 candidates utilized site-specific conjugation, and 84 candidates did not disclose the conjugation method for ADC creation. Of the ADC candidates that utilized site-specific ADC conjugation, 2 Approved (EnhertuTM and TrodelvyTM), 50 Active, and 26 Discontinued ADCs underwent clinical testing. With the exception of the DAR = 8 ADCs (e.g., EnhertuTM and TrodelvyTM) that utilize all natural disulfide bonds for conjugation, the remaining ADCs utilized site-specific conjugation methods that either retain the four inter-chain disulfide bonds or replace these with chemical covalent bonds (e.g., disulfide rebridging).36
Approved ADCs
The FDA has approved 12 ADCs to date [Figure 1, Figure 5, Figure 8, and Table 1], 6 each for hematologic and solid tumor malignancies, respectively [Figure 5, Table 1]. Accelerated conditional approvals were granted to 9 of the 12 approved ADCs. Approvals were withdrawn for 2 (MylotargTM and BlenrepTM) of the 12 ADCs [Figure 1]. MylotargTM was withdrawn in 2010 due to safety versus clinical benefit concerns but was re-approved in 2017 at a lower dose in combination with chemotherapy.37 BlenrepTM was withdrawn in 2022 when the confirmatory trial did not meet the requisite post-approval efficacy endpoints.38
Figure 8.
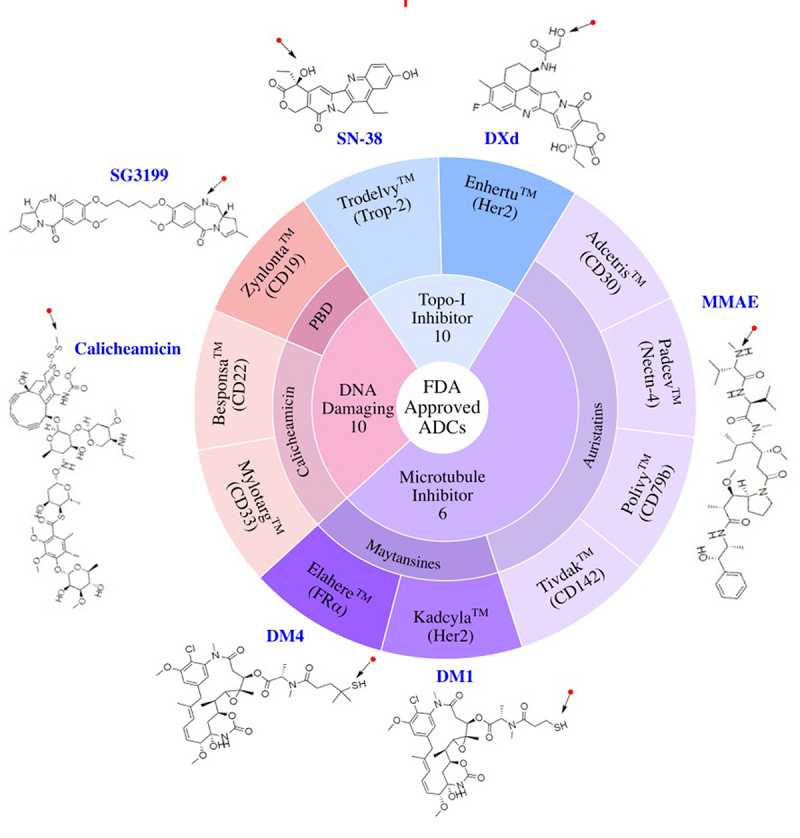
FDA Approved ADCs Classified by Payload Class. ADC drug name, target antigen, and names and chemical structures of payloads are shown. Arrows mark the point of attachment of payload to the antibody. Topo-I, Topoisomerase I Inhibitor; PBD, pyrrolobenzodiazepine.
Of the 11 currently FDA approved ADCs, 6 utilize microtubule inhibitor payloads. Three approved ADCs use DNA damaging payloads, while 2 carry payloads that inhibit topoisomerase I [Figure 5, Figure 8]. These payloads span a range of potency from the highly potent DNA damaging agent PBD (IC50 ~ pM) to the lower potency topoisomerase I inhibitor SN-38 (IC50 ~ nM).39 Although the sample size is small, approved ADCs used higher potency payloads when targeting hematological malignancies and lower potency payloads were used in ADCs targeting solid tumors. Higher drug exposures required for efficacy in the solid tumor setting may limit utilization of higher potency payloads with reported increased systemic toxicity at the preferred biologic dose.
Active ADCs
Of the 164 Active ADCs, ~7% are in Phase 3 clinical testing. These active late-stage ADCs target the following tumor antigens: BCMA (belantamab mafodotin), CEACAM5 (tusamitamab ravtansine), c-Met (telisotuzumab vedotin), HER2 (trastuzumab duocarmazine and trastuzumab rezetecan), HER3 (patritumab deruxtecan), NaPi-2b (upifitamab rilsodotin), and Trop-2 (datopotamab deruxtecan and SKB264).
Microtubule inhibitor payloads are utilized by most ADCs in the active ADC group (~54%), followed by DNA damaging (10%), and topoisomerase I inhibitor (~9%) payloads. Payloads of ~22% of Active ADCs are undisclosed [Figure 9]. Among microtubule inhibitor ADCs, auristatins are most abundant, followed by maytansines. In the DNA damaging payload class, PBDs comprise ~50% of the clinically active ADCs.
Figure 9.
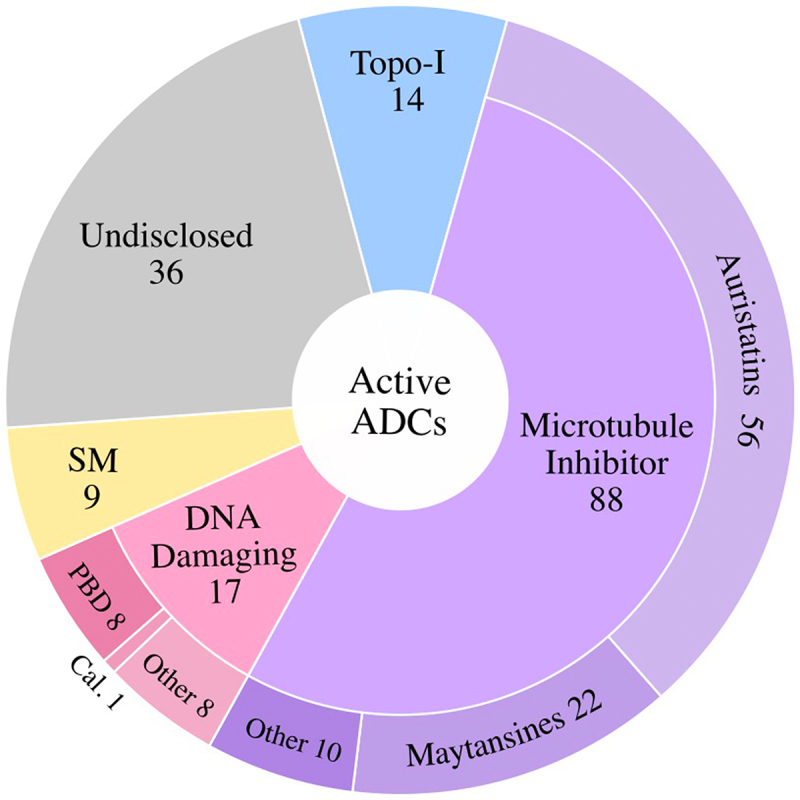
Active ADCs Classified by Payload Class. Of the active ADCs in clinical testing, the majority utilize microtubule inhibitor payloads, followed by DNA Damaging Agents, Topoisomerase I Inhibitors (Topo-I), and targeted small molecules (SM). ~22% of active ADCs have not disclosed the payload utilized (Undisclosed). PBD, pyrrolobenzodiazepine; Cal., calicheamicin.
Of the cancer antigens targeted by the clinically active ADCs, ~16% target hematologic tumor antigens, ~80% target solid tumor antigens, and ~4% are directed against a cancer antigen that is expressed in both hematologic and solid tumor malignancies. The most frequently targeted tumor antigens in the Active ADC category include HER2 (32 candidates), Trop-2 (11), CLDN18.2 (11), and EGFR (8).
Discontinued ADCs
Discontinuation of ADCs can be ascribed to one or more of the following three reasons: 1) insufficient therapeutic benefit due to intolerable toxicity, 2) therapeutic benefit not superior to current standard of care due to insufficient efficacy, and/or 3) business/commercial considerations. Details of all the discontinued ADCs are shown in Table 2.
Table 2.
Discontinued ADCs by Payload Class and Malignancy Setting.
| No. | Target Molecule Conjugation Amino Acid Linker Payload DAR Company Years in Pipeline |
Phase Regimen Trial Identifier Status |
Indication(s) | Trial Design Results |
Ref |
|---|---|---|---|---|---|
| |
Microtubule Inhibitors – Auristatins – MMAE: Solid Tumors (16) |
||||
| 1 |
Tyrosine-protein kinase receptor UFO (AXL)enapotamab vedotin; AXL-107-MMAE; HuMax- AXL-ADCNonspecific Cys conj.Cleavable, Val-Cit linker MMAE payloadDAR~4Genmab; Seagen Inc.2016–2020 |
Phase 1/2 Monotherapy NCT02988817 Completed, minimal efficacy at tolerated doses |
OC, Cervical Cancer, NSCLC, Thyroid Cancer, Melanoma, Sarcoma |
n = 306, dose escal. 1Q3W (0.3–2.4 mg/kg) and 3Q4W (0.6–1.2 mg/kg); dose exp. at 2.2 mg/kg 1Q3W and 1.0 mg/kg 3Q4W.Safety: Dose escal. DLTs noted in 13.3% pts. Dose exp., 49% pts. had TESAEs, 90% pts. had treatment emergent infusion AEs. 61% pts. had Gr ≥3 TRAEs.Efficacy: Dose exp., 8% ORR.Minimal efficacy at tolerated doses. |
25 |
| 2 |
Carbonic anhydrase 9 (CA9) BAY79-4620 Nonspecific Cys conj. Cleavable, Val-Cit linker MMAE payload DAR~ Undisclosed Bayer; MorphoSys 2009–2011 |
Phase 1 Monotherapy NCT01028755 Completed |
Solid Tumors |
n = 12, no published results |
25 |
| Phase 1 Monotherapy NCT01065623 Terminated, safety |
Solid Tumors |
n = 2, no published results |
25 |
||
| 3 |
Epidermal growth factor receptor (EGFR)losatuxizumab vedotin; ABBV-221Nonspecific Cys conj.Cleavable, Val-Cit linkerMMAE payloadDAR~ 3AbbVie2015–2018 |
Phase 1 Monotherapy NCT02365662 Terminated, safety |
HNSCC, NSCLC, TNBC, CRC, GBM |
n = 45, doses 0.3–6.0 mg/kg Q3W.Safety: high incidence of infusion-related AEs (49%; Gr ≥3 in 9%). Several mitigation strategies were explored.Efficacy: 1 PR (2%).Trial halted due to high frequency of infusion-related AEs. |
40 |
| 4 |
Endothelin receptor type B (EDNRB)DEDN6526A; RG7636Nonspecific Cys conj.Cleavable, Val-Cit linkerMMAE payloadDAR~ UndisclosedRoche-Genentech; Seagen2012–2014 |
Phase 1MonotherapyNCT01522664Completed, minimal clinical efficacy at tolerated doses |
Melanoma |
n = 53, dose escal. 0.3–2.8 mg/kg Q3W, RP2D 2.4 mg/kg Q3W.Safety: DLTs included infusion-related reactions, increased ALT/AST, and liver injury. At RP2D, Gr 3+ AEs observed in 38% pts. including neutropenia (25%), ALT increase (7%), infusion reactions and PN (3% each).Efficacy, RP2D: 12.5% PR.Minimal clinical efficacy at tolerated doses. |
41 |
| 5 |
Guanylyl cyclase C (GUCY2C)indusatumab vedotin; 5F9-vcMMAE; MLN0264; TAK-264Nonspecific Cys conj.Cleavable, Val-Cit linkerMMAE payloadDAR ~3.7Takeda; Millennium Pharmaceuticals, Inc.;Seagen Inc.2012–2018Descriptions of Ph. 1 trials (NCT02391038, NCT01577758) are not included. |
Phase 2 Monotherapy NCT02202785 Terminated, minimal activity at tolerated doses |
PC |
n = 43, 1.8 mg/kg Q3W.Safety: 35% pts. experienced ≥3 Gr AEs; 12% had SAEs. Nausea (33%), fatigue (28%), and neutropenia (23%) noted.Efficacy: 3% ORR, 1 PR. Minimal clinical activity noted at tolerated doses. |
42 |
| Phase 2 Monotherapy NCT02202759 Terminated, minimal efficacy at tolerated doses |
Stomach Cancers, GEJC | n = 38, 1.8 mg/kg Q3W.Safety: 37% pts. experienced ≥3 Gr AEs that included anemia, diarrhea, and neutropenia.Efficacy: 6% ORR.Limited efficacy at tolerated doses. | 43 | ||
|
6. |
Glycoprotein NMB (GPNMB)glembatumumab vedotin; CDX-011; CR011vcMMAENonspecific Cys conj.Cleavable, Val-Cit linkerMMAE payloadDAR~2.7Celldex Therapeutics; Seagen Inc.2008–2018Descriptions of the six Ph2 trials are presented. Descriptions of Ph.1/2 (NCT03326258, NCT02713828, NCT00704158, NCT00412828), Ph. 1 (NCT03473691, ACTRN12617001621303), and Expanded Access (NCT03067935) trials are not included. |
Phase 2 Monotherapy NCT02487979 Completed, minimal efficacy noted at doses tested |
OS |
n = 22, Q3W, dose not specified.Safety: SAEs in 36.4% pts. including rash (13%), anemia (9%), hypokalemia (9%), and febrile neutropenia (4.5%).Efficacy: 4.5% ORR. |
25 |
| Phase 2 Monotherapy NCT02363283 Completed, minimal efficacy noted at doses administered |
Uveal Melanoma |
n = 35, 1.9 mg/kg Q3W.Safety: The most common Gr 3/4 AE was neutropenia (48%), others occurring in 3–6% of pts. included elevated ALT/AST and leukopenia. One Gr 5 encephalopathy was noted.Efficacy: 6% PR, mDOR 8.6 months, mPFS 3.1 months, mOS 11.9 months. Minimal efficacy noted at doses administered. |
44 |
||
| Phase 2 Combination with varlilumab, nivolumab, pembrolizumab, or CDX-301 (Flt3L) NCT02302339 Terminated, minimal efficacy at doses tested |
Melanoma |
n = 132; of these 62 pts. given 1.9 mg/kg (reduced to 1.3/1.0 mg/kg in case of DLT).Safety: Gr ≥3 occurred in 37% pts., the most common of these were neutropenia (19%), rash (8%), and neuropathy (7%). A fatal pneumonia was deemed possibly drug related.Efficacy: 11% ORR, mDOR 6.0 months, mPFS 4.4 months, mOS 9.0 months. |
45 |
||
| Phase 2 (METRIC) Combination with capecitabine NCT01997333 Completed, study did not meet primary efficacy PFS endpoint |
gpNMB+ TNBC |
n = 327, pivotal trial, randomly assigned to 2 arms: ADC (n = 218) vs capecitabine (n = 109).Safety: Gr ≥3 AEs for ADC included neutropenia (28%), rash (12%), leukopenia (9%). SAEs included septic shock that resulted in death (3 pts.).Efficacy: ADC vs capecitabine-mPFS 2.9 vs 2.8 months (HR 1.13), mOS 8.9 vs 8.7 months (HR 1.06),ORR 16% vs 15%.Study did not meet primary endpoint of improved PFS. |
46 |
||
| Phase 2 (EMERGE) Monotherapy vs investigator’s choice (IC) chemotherapy NCT01156753 Completed, ADC arm did not provide improved efficacy over IC comparator arm |
BC |
n = 124, selected for gpNMB+ in ≥ 5% of epithelial/stromal cells by IHC; randomly assigned 2:1 to ADC (n = 83, 1.9 mg/kg Q3W) or investigator’s choice (IC, n = 41).Safety: ADC vs IC:DLTs 8% vs 5%; 40% of ADC pts. had Gr 3/4 AEs including neutropenia (22%), fatigue (7%), PN (3%).Efficacy: ADC vs IC:ORR 12% vs 12%,PR 6% vs 7%,mOS 7.5 vs 7.4 months.ADC arm did not provide improved efficacy over comparator. |
47 |
||
| Phase 2 Monotherapy, biomarker analysis NCT02487979 Completed, limited efficacy with DLTs noted in 6/22 pts. with one possible fatal TRAE |
OS |
n = 22 adolescents/YAs, 1.9 mg/kg, Q3W.Safety: DLTs in 6 pts. Gr ≥3 AEs included rash (9.8%) and hypokalemia (6.6%), one possible fatal TRAE, end organ failure.Efficacy: 4.5% PR.Limited efficacy with DLTs noted in 6/22 pts. with one possible fatal TRAE. |
48 |
||
|
7. |
Leucine-rich repeat- containing protein 15 (LRRC15) samrotamab vedotin; ABBV-085 Nonspecific Cys conj. Cleavable Val-Cit linker MMAE payload DAR~2 AbbVie; Seagen Inc. 2015–2019 |
Phase 1 Monotherapy NCT02565758 Completed, Tolerable safety with evidence of anti-tumor activity |
Solid Tumors |
n = 85, 0.3–6.0 mg/kg Q2W, dose exp. (n = 45) at 3.6 mg/kg Q2W.Safety, 3.6 mg/kg: Gr ≥3 AEs occurred in 31% of pts. including fatigue (7%), anemia (4%), and neuropathy (4%).Efficacy, 3.6 mg/kg: 20% ORR for osteosarcoma and undifferentiated pleomorphic sarcoma.Tolerable safety with evidence of anti-tumor activity. |
49 |
|
8. |
Lymphocyte antigen 6E (LY6E) DLYE5953A; RG7841 Nonspecific Cys conj. Cleavable Val-Cit linker MMAE payload DAR~ Undisclosed Roche-Genentech; Seagen 2014–2017 |
Phase 1 Monotherapy NCT02092792 Completed, preliminary efficacy noted at doses tested |
Solid Tumors including BC and NSCLC |
n = 68, 0.2–2.4 mg/kg Q3W; no DLTs, MTD not reached. RP2D 2.4 m/kg Q3W.Safety, RP2D: Gr ≥3 AEs occurred in 25% of pts., including neutropenia (10%) and hypertension (5%).Efficacy, RP2D: 11.5% PR in BC, 22% PR in NSCLC. Preliminary efficacy noted at doses tested. |
50 |
|
9. |
Mesothelin (MSLN) DMOT4039A; αMSLN-MMAE; RG7600 Nonspecific Cys conj. Cleavable Val-Cit linker MMAE payload DAR~3.5 Roche-Genentech; Seagen Inc. 2011–2014 Description of Ph. 1 radio-imaging trial (NCT01832116) is not included. |
Phase 1 Monotherapy NCT01469793 Completed, limited efficacy at MTD |
OC, PC |
n = 71 (40 PC, 31 OC), Q3W (n = 54) 0.2–2.8 mg/kg or QW (n = 17) 0.8–1.2 mg/kg. Q3W-MTD and RP2D = 2.4 mg/kg. QW- MTD 1.2 mg/kg and RP2D = 1 mg/kg.Safety, RP2D: Gr ≥3 AEs occurred in 38% of pts., including pyrexia, gastroparesis, hypotension, sinus tachycardia, and infection.Efficacy, RP2D: PC 8% PR, mPFS 1.7 months; OC 30% PR, mPFS 4.9 months.Tolerated but with limited efficacy at MTD. |
51 |
|
10. |
Mucin-16 (MUC16) sofituzumab vedotin; DMUC5754A; RG7458 Nonspecific Cys conj. Cleavable Val-Cit linker MMAE payload DAR~3.5 Roche-Genentech; Seagen Inc. 2011–2016 |
Phase 1 Monotherapy NCT01335958 Completed, limited efficacy noted at doses tested |
OC, PC |
n = 77 (66 PSOC, 11 PC), two dosing regimens: Q3W (n = 54) 0.3–3.2 mg/kg and Q1W (n = 23) 0.8–1.6 mg/kg. RP2D of 2.4 mg/kg Q3W or 1.4 mg/kg QW.Safety, RP2D Q3W: Gr ≥3 AEs included neutropenia (10%), fatigue (10%), and peripheral neuropathy (15%). SAEs included small intestine obstruction, hypocalcemia, and neutropenia. 4 patient deaths were due to AEs: respiratory failure (2), sepsis (1), and acute renal failure (1).Efficacy, RP2D: ~17% ORRLimited efficacy noted at doses tested. |
52 |
| 11. |
Mucin-16 (MUC16) DMUC4064A; D-4064a; RG7882 Specific Cys (THIOMABTM) conj. Cleavable Val-Cit linker MMAE payload DAR~2.0 Roche-Genentech; Seagen Inc. 2014–2018 |
Phase 1 Monotherapy NCT02146313 Completed, evidence of antitumor efficacy at tolerated doses |
OC, PC | n = 65, 1.0–5.6 mg/kg. MTD not reached. RP2D of 5.2 mg/kg, Q3W.Safety, RP2D Q3W: Gr ≥3 AEs in 62% pts. including fatigue (15%), keratitis (12%), and blurred vision (4%).Efficacy, RP2D: ~42% ORR, mPFS 5.3 months.Evidence of antitumor efficacy at tolerated doses. | 53 |
|
12. |
Sodium-dependent phosphate transport protein 2B, NaPi-2b (SLC34A2)lifastuzumab vedotin; DNIB0600A; NaPi2b ADC; RG7599Nonspecific Cys conj.Cleavable Val-Cit linkerMMAE payloadDAR~3.5Roche-Genentech; Seagen Inc.2011–2016Descriptions of Ph. 1b trial (NCT01995188) and Ph. 1a trial (NCT01363947) are not included. |
Phase 2 Monotherapy vs PLD (PEGylated liposomal doxorubicin)NCT01991210Terminated, primary efficacy PFS endpoint not met |
OC |
n = 95, randomized 1:1 to ADC (n = 47) vs PLD (n = 48).Safety: ADC Gr ≥3 AEs occurred in 46% pts. including 30% SAEs. Additional AEs included abdominal pain (46%), constipation (24%), diarrhea (35%), neutropenia (28%), and stomatitis (7%).Efficacy: ADC vs PLD – mPFS 5.3 vs 3.1 months, mDOR 5.5 vs 3.9 months,34% ORR (2% CR, 32% PR) vs 15% ORR (2.1% CR and 12.5% PR).Tolerated with objective responses but primary efficacy PFS endpoint not met. |
54 |
|
13. |
Choline transporter-like protein 4 (SLC44A4)ASG-5ME; AGS-5; AGS-5MENonspecific Cys conj.Cleavable Val-Cit linkerMMAE payloadDAR~ UndisclosedAgensys, Inc.; Astellas Pharma Inc.; Seagen Inc.2010–2013 |
Phase 1 Monotherapy NCT01228760 Completed, minimal efficacy at MTD |
PC, GC, CRPC |
n = 35; PC, n = 20, 0.3–1.5 mg/kg, 3XQW-one week off cycle. MTD 1.2 mg/kg; GC, n = 15, dose exp. at MTD.Safety: Gr ≥3 AEs in 68.6% PC pts. included neutropenia (20%), anemia (8.6%), and pleural effusion (5.7%). Gr ≥3 AEs in 87.6% GC pts. included keratitis (20%), dyspnea and ascites (13% each) and sepsis (6.7%). 1 probable drug-related death, sepsis, in GC patient.Efficacy: 1 PR for PC; 1 PR for GC; DCR of 33% for PC and 47% for GC.n = 46 CRPC, dose esc. (n = 26) 0.3–3.0 mg/kg Q3W, dose exp. (n = 20) at 2.4/2.7 mg/kg Q3W, MTD 2.7 mg/kg Q3W.Safety: Gr ≥3 AEs in 55% dose exp. pts. including 2 deaths attributable to drug (1 multiorgan failure, 1 sepsis).Efficacy: 2 PR.Limited efficacy at MTD. |
55,56 |
|
14. |
SLIT and NTRK-like protein 6 (SLITRK6) sirtratumab vedotin; ASG-15ME; AGS-15E Nonspecific Cys conj. Cleavable Val-Cit linker MMAE payload DAR~4 Seagen Inc.; Astellas Pharma Inc. 2013–2018 |
Phase 1 Monotherapy NCT01963052 Completed |
Urothelial Cancer |
n = 93, no published results |
25 |
| 15. | Metalloreductase STEAP-1 (STEAP1)vandortuzumab vedotin; DSTP3086S; RG7450Nonspecific Cys conj.Cleavable Val-Cit linkerMMAE payloadDAR~2.0Roche-Genentech; Seagen Inc.2011–2016 | Phase 1 Monotherapy NCT01283373 Completed, minimal efficacy at doses tested |
Prostate Cancer | n = 84 (Q3W, n = 77; QW n = 7), 0.3–2.8 mg/kg Q3W, RP2D 2.4 mg/kg Q3W.Safety: Gr ≥3 AEs occurred in 24% of pts., the most common of which were peripheral neuropathy (5%) and ALT increase (5%).Efficacy: >50% PSA reduction in 14% pts.; 4% PR.Minimal efficacy at doses tested. | 57 |
|
16. |
Hepatitis A virus cellular receptor 1, TIM-1 (HAVCR1)CDX-014Nonspecific Cys conj.Cleavable Val-Cit linkerMMAE payloadDAR~4.5Celldex Therapeutics2016–2018 |
Phase 1 Monotherapy NCT02837991 Terminated, limited efficacy at tolerated doses |
RCC, Kidney Cancers, OC |
n = 16, 0.15 − 2.0 mg/kg Q3W or 1.2 mg/kg Q2W; RP2D 1.8 mg/kg Q3W.Safety: 1 patient death due to multiorgan failure at 2 mg/kg. Other Gr ≥3 included hyperglycemia (19%) and Gr 4 urosepsis in 1 patient.Efficacy: 6% PR, mPFS 2.7 months, OS 12.6 months. Limited efficacy at tolerated doses. |
58 |
| |
Microtubule Inhibitors – Auristatins – MMAE: Hematological Malignancies (6) |
||||
|
17. |
B-cell receptor CD22 (CD22) pinatuzumab vedotin; DCDT2980; RG7593 Nonspecific Cys conj. Cleavable Val-Cit linker MMAE payload DAR~3.5 Roche-Genentech 2010–2015 Description of Ph. 1 trial (combination with rituximab; NCT01209130) is not included. |
Phase 1/2 (ROMULUS) Combination with obinutuzumab or rituximab compared against polatuzumab vedotin (CD79b) combination arm NCT01691898 Completed, program halted to advance polatuzumab vedotin based on superior clinical activity |
DLBCL, FL |
n = 231; comparison of rituximab + pinatuzumab vedotin (R-Pina) vs rituximab + polatuzumab vedotin (R-Pola); Arms DLBCL (n = 81; 42 given R-Pina, 39 given R-Pola) and FL (n = 41; 21 given R-Pina, 20 given R-Pola).Safety, R-Pina vs R-Pola: Gr ≥3 AEs in DLBCL 79% vs 77%, in FL 62% vs 50%.Efficacy, R-Pina vs R-Pola: DLBCL- ORR 60% vs 54%; CR 26% vs 21%.FL- ORR 62% vs 70%;CR 5% vs 45%.Pola was selected for advancement based on superior clinical activity. |
59 |
|
18. |
Leukocyte antigen CD37 (CD37) AGS67E Nonspecific Cys conj. Cleavable Val-Cit linker MMAE payload DAR~2.3 Astellas Pharma Inc.; Seagen Inc. 2014–2018 |
Phase 1 Monotherapy NCT02175433 Completed |
Lymphoid Malignancy |
n = 71, no published results. |
25 |
| Phase 1 Monotherapy NCT02610062 Terminated, business decision |
AML |
n = 23, no published results. |
25 |
||
| 19. |
CD48 antigen (CD48) SGN-CD48A Specific Cys conj. Cleavable β-glucuronidase (BG) linker MMAE payload DAR~8 Seagen Inc. 2018–2019 |
Phase 1 Monotherapy NCT03379584 Terminated, overall benefit/risk profile |
MM | n = 14, no published results. | 25 |
|
20. |
B-cell antigen receptor complex-associated protein beta chain (CD79B)iladatuzumab vedotin; DCDS0780A; RG7986Specific engineered Cys (THIOMABTM) conj.Cleavable Val-Cit linkerMMAE payloadDAR~2Roche-Genentech; Seagen Inc.2015–2018 |
Phase 1Monotherapy and Combination with rituximab or obinutuzumabNCT02453087 Completed, did not demonstrate superior efficacy vs PolivyTM with enhanced ocular toxicity |
NHL |
n = 60, Monotherapy dose esc. (n = 51) 0.3–4.8 mg/kg Q3W; Combination (n = 9) 3.6 or 4.8 mg/kg with rituximab, 375 mg/m2.Safety: Gr ≥3 AEs in (5%) pts. included neutropenia (23%), hypercalcemia (5%), thrombocytopenia (5%), and decreased white blood cell count (5%). 53% of monotherapy and 55% of combination group had ocular toxicity.Efficacy: ADC monotherapy- 47% ORR, 18% PR, 28% CR; ADC + rituximab- 59% ORR, mPFS for all pts. 4.4 months, DLBCL mPFS 3.9 months.Did not demonstrate superior efficacy vs PolivyTM + rituximab with enhanced ocular toxicity. |
60 |
|
21. |
Fc receptor-like protein 5 (FCRL5) DFRF4539A; RG7598 Nonspecific Cys conj. Cleavable Val-Cit linker MMAE payload DAR~ Undisclosed Roche-Genentech; Seagen Inc. 2011–2014 |
Phase 1 Monotherapy NCT01432353 Completed, minimal efficacy noted at doses tested |
MM |
n = 39, 0.3–2.4 mg/kg Q3W or 0.8–1.1 mg/kg QW; RP2D 2.4 mg/kg Q3W.Safety, at RP2D: Gr ≥ 3 AEs in 47% pts. including neutropenia (17.6%), thrombocytopenia, acute renal failure, hyponatremia, and nervous system disorders (11.8% each). SAEs in 21% pts. PN in 21% pts.Efficacy, at RP2D: 5% PR, 3% MR.Completed, minimal efficacy noted at doses tested. |
61 |
|
22. |
SLAM family member 7 (SLAMF7)azintuxizumab vedotin; ABBV-838Nonspecific Cys conj.Cleavable Val-Cit linkerMMAE payloadDAR~ UndisclosedAbbVie2015–2017Description of withdrawn Ph.1 trial (NCT02951117) is not included. |
Phase 1Combination with pomalidomide, dexamethasoneNCT02462525Terminated, limited efficacy at tolerated doses |
MM |
n = 75, dose escal. (n = 32) 0.6–6.0 mg/kg Q3W or (n = 8) 1.5 mg/kg Q1W or (n = 6) 3.0 mg/kg Q2W. Dose exp. (n = 29) at 5.0 mg/kg Q3W.Safety: 73.3% Gr >3 AEs including neutropenia (20.0%), anemia (18.7%), and leukopenia (13.3%). SAEs in 36.0% pts. PN in 18.7% of pts.Efficacy: 10.7% ORR (2.7% VGPR, 8.0% PR), mDOR 4 months.Limited efficacy at tolerated doses. |
62 |
| |
Microtubule Inhibitors – Auristatins – MMAF: Solid Tumors (5) |
||||
|
23. |
Trophoblast glycoprotein, 5T4 (TPBG) PF-06263507 Nonspecific Cys conj. Non-cleavable mc linker MMAE payload DAR~ 4 Pfizer 2013–2015 |
Phase 1 Monotherapy NCT01891669 Terminated, severe dose-limiting ocular toxicity without corresponding tumor reduction |
Neoplasms, NSCLC, BC, OC |
n = 26, 0.5–6.5 mg/kg Q3W, MTD and RP2D 4.34 mg/kg Q3W.Safety: At the RP2D, 16.7% of the pts. had treatment related Gr 3/4 events including ocular toxicity, infection, hypophosphatemia, and embolism. 38.5% of pts. experienced Gr 1/2 ocular toxicity.Efficacy: No ORR.No ORR with severe ocular toxicity. |
63 |
|
24. |
Epidermal growth factor receptor variant III (EGFRvIII)depatuxizumab mafodotin; ABT-414; Depatux-MNonspecific Cys conj.Non-cleavable mc linkerMMAF payloadDAR~ 4AbbVie; Seagen Inc.2013–2018Results of Ph. 2 trials (NCT02343406, NCT02590263), Ph. 1/2 trial (NCT02590263), Ph. 1 trials (NCT01800695, NCT01741727), and one Expanded Access trial (NCT03123952) are not included. |
Phase 3b (UNITE) Combination with temozolomide (TMZ) + radiation (RT) vs TMZ + RT assessing differing prophylactic ophthalmologic treatmentsNCT03419403Terminated, ADC was discontinued due to lack of survival benefit |
GBM |
n = 40Independent Data Monitoring Committee responsible for interim analysis review recommended study termination due to lack of survival benefit. |
25 |
| Phase 2/3Combination with temozolomide (TMZ) + radiation (RT) vs TMZ + RTNCT02573324Completed, no difference in OS between ADC and comparator arm |
GB, GS |
n = 639, double blind randomization 1:1 into 2 Arms, ADC (n = 323; 1.25 mg/kg Q2W) + TMZ + RT vs placebo (n = 316) TMZ + RT; trial amended to Ph. 3 with OS as primary endpoint based on early results of Ph. 2 INTELLANCE-2 trial.Safety: Gr >3 AEs in 80% of ADC group vs 58% placebo. Ocular AEs- 94% vs 36%; thrombocytopenia- 61% vs 36%; gamma-glutamyltransferase increase-10.8% vs 1%.Efficacy: OS 18.9 vs 18.7 months (HR 1.02); mPFS EGFRvIIIm group 8.3 vs 5.9 months; mPFS for pts. without EGFRvIIIm 6.9 vs 7.9 months.ADC provided no OS benefit over placebo arm. |
64 |
||
|
25. |
Ectonucleotide pyrophosphatase/ phosphodiesterase family member 3 (ENPP3)AGS16F; AGS-16C3F; AGS-16M8FNonspecific Cys conj.Non-cleavable mc linkerMMAF payloadDAR~ 4Astellas Pharma Inc.; Seagen Inc.2010–2019Descriptions of Ph. 1 trials (NCT01114230, NCT01672775) are not included. |
Phase 2Monotherapy vs axitinibNCT0263912Completed, did not meet primary PFS efficacy endpoint |
RC |
n = 133, randomized 1:1 ADC at 1.8 mg/kg Q3W vs axitinib.Safety: ADC common AEs included fatigue (53%), nausea (47%), and ocular (44%). ADC Gr >3 AEs included fatigue, dry eye, and thrombocytopenia (3–5%).Efficacy: ADC vs. axitinib – mPFS 2.9 vs. 5.7 months (p = .015), mOS 13.1 vs. 15. 4 months (p = .747).Did not meet primary efficacy endpoint of improved PFS. |
65 |
| 26. |
Ephrin type-A receptor 2 (EPHA2) MEDI-547; MI-CP177 Nonspecific Cys conj. Non-cleavable mc linker MMAF payload DAR~ 4 AstraZeneca; Seagen Inc. 2012–2019 |
Phase 1MonotherapyNCT00796055Terminated, intolerable toxicity with no clinical responses | Solid Tumors | n = 6, 0.08 mg/kg Q3W.Safety: 4/6 (66.7%) pts. experienced SAEs including conjunctival hemorrhage, liver disorder, and hemorrhage deemed to be treatment related.Efficacy: All pts. discontinued treatment due to progressive disease (n = 4) or plan to pursue alternative treatment (n = 2). No clinical responses were observed.Study terminated due to intolerable toxicity with no efficacy benefit. | 66 |
|
27. |
CD70 antigen (CD70) vorsetuzumab mafodotin; SGN-75 Nonspecific Cys conj. Non-cleavable mc linker MMAF payload DAR~ 4 Seagen Inc. 2009–2013 Description of Ph. 1 trial (NCT01677390) is not included. |
Phase 1 Monotherapy NCT01015911 Completed, minimal efficacy at tolerated doses |
RCC, NHL |
n = 58 (39 RCC, 19 NHL), 0.3–4.5 mg/kg Q3W or 0.3/0.6 mg/kg Q1W.RCC: MTD 3 mg/kg Q3WNHL: dose esc. terminated due to thrombocytopenic purpura in 2 pts.Safety: Q3W AEs included dry eye (32%), nausea (30%), ocular AEs (57%), and thrombocytopenia (26%). The most common Gr ≥3 AE was thrombocytopenia (19%).Efficacy: Q3W, 1 CR (NHL, MCL), 2 PR (RCC).Minimal activity at tolerated doses. |
67 |
| |
Microtubule Inhibitors – Auristatins – MMAF: Hematological Malignancies (1) |
||||
|
28. |
B-lymphocyte antigen CD19 (CD19) denintuzumab mafodotin; SGN-CD19A Nonspecific Cys conj. Non-cleavable mc linker MMAF payload DAR~ Undisclosed Seagen Inc. 2012–2019 Descriptions of Ph. 1 trials (NCT01786135, NCT01786096) are not included; neither has published results. |
Phase 2 Combination with rituximab (R) cyclophosphamide (C), doxorubicin (H), vincristine (O), prednisone (P)NCT02855359Terminated, portfolio prioritization |
DLBCL, FL, Transformed Lymphoma |
n = 24; ADC (3 mg/kg Q6W) + RCHP, n = 11 and ADC (3 mg/kg Q6W) + RCHOP, n = 13.Safety: ADC + RCHP-100% pts. Gr >3 TEAE, 45.5% pts. with SAEs, AE with outcome of death-18.25%; ADC + RCHOP-92.3% pts. TEAE, 23.1% pts. SAEs, AE with outcome of death-7.7%.Efficacy: Efficacy outcomes of mPFS, OS, and ORR not assessed due to lack of study progression to these endpoints. |
25 |
| Phase 2 Combination with rituximab, ifosfamide, carboplatin, etoposide (RICE) vs RICENCT02592876Terminated, portfolio prioritization |
BCL, DLBCL, FL |
n = 81, ADC (3 mg/kg Q3W) + RICE, n = 40 vs RICE, n = 41.Safety: ADC + RICE vs RICE; SAEs 47.5% vs 32.5%, all-cause mortality, 22.5% vs 15%.Efficacy: ADC + RICE vs RICE, 62.5% vs 45% CRR, 80% vs 75% ORR.Insufficient efficacy benefit over comparator arm with enhanced toxicity. |
25 |
||
| |
Microtubule Inhibitors – Other Auristatins: Solid Tumors (7) |
||||
|
29. |
Tumor antigen AG-7 AbGn-107; Ab1-18Hr1 Nonspecific Cys conj. Cleavable Val-Cit linker MMAD payload DAR~ 2.5 AltruBio Inc. 2017–2021 |
Phase 1 Monotherapy NCT02908451 Terminated due to COVID |
GC, CRC, PC, Biliary Cancer |
n = 39, no published results. |
25 |
| 30. |
Urokinase plasminogen activator surface receptor, C4.4a (PLAUR)lupartumab amadotin; BAY1129980Nonspecific Cys conj.Non-cleavable mc-hydrazide linkerAuristatin W analog payloadDAR~ 4Bayer; Seagen Inc.2014–2019 |
Phase 1 Monotherapy NCT02134197 Terminated, reason not disclosed |
Neoplasms | n = 69, no published results. | 25 |
|
31. |
Fibroblast growth factor receptor 2 (FGFR2)aprutumab ixadotin; BAY1187982Nonspecific Lys conj.Non-cleavable Caproyl linkerAuristatin W analog payloadDAR~ 4Bayer2015–2017 |
Phase 1 Monotherapy NCT02368951 Terminated, intolerable toxicity with no clinical responses |
FGFR2+ Solid Tumors |
n = 20; 0.1–1.3 mg/kg Q3W, MTD 0.2 mg/kg Q3W.Safety: Gr ≥3 TEAEs included anemia, aspartate aminotransferase increase, proteinuria, and thrombocytopenia.Efficacy: No clinical responses were observed.Poorly tolerated with MTD reached below the therapeutic threshold estimated for efficacy. |
68 |
|
32. |
Receptor tyrosine-protein kinase erbB-2, HER2 (ERBB2)XMT-1522; TAK-522Nonspecific Cys conj.Cleavable Fleximer Polymer linkerAuristatin F-HPA payloadDAR~ 12Mersana Therapeutics; Takeda2016–2019 |
Phase 1 Monotherapy NCT02952729 Completed, minimal clinical activity at the doses tested |
HER2+ BC, GC, NSCLC |
n = 120 (estimated), prelim results with 19 pts., dose escal. 2.0–21.3 mg/m2, no DLTs or SAEs, MTD and RP2D not reached.Safety: TRAEs included elevated liver enzymes, fatigue, nausea, and vomiting (Gr 1 or 2).Efficacy: At doses of 16 and 21.3 mg/m2 (6 pts.), 1 PR was observed.Tolerable safety profile but minimal clinical activity at doses tested. |
69 |
|
33. |
Sodium-dependent phosphate transport protein 2B, NaPi-2b (SLC34A2)XMT-1592Specific conj. (amino acid not disclosed)Cleavable undisclosed linkerAuristatin F-HPA payloadDAR~ 6Mersana Therapeutics; Synaffix2020–2022 |
Phase 1/2 Monotherapy NCT04396340 Active, not recruiting |
OC, NSCLC |
n = 120, no published results |
25 |
|
34. |
Neurogenic locus notch homolog protein 3 (NOTCH3)PF-06650808Nonspecific Cys conj.Cleavable Val-Cit linkerAuristatin (Aur 101) payloadDAR~ UndisclosedPfizer2014–2016 |
Phase 1 Monotherapy NCT02129205 Terminated, portfolio prioritization |
Solid Tumors including BC |
n = 40, 0.2–6.4 mg/kg Q3W, MTD 2.4 mg/kg Q3W.Safety: At MTD, 27.3% pts. had DLTs including Gr 3 rash, diarrhea, and thromboembolic event. 54.5% Gr 3 TRAEs included neutropenia, lymphopenia, and AST increase.Efficacy: At MTD, 14.3% pts. had objective responses.Minimal efficacy noted at MTD. |
25,70 |
|
35. |
Tumor-associated calcium signal transducer 2, Trop-2 (TACD2)PF-06664178; RN927CSpecific Glu conj.Cleavable Val-Cit linkerAuristatin (Aur 101) payloadDAR~ 2Pfizer2014–2016 |
Phase 1 Monotherapy NCT02122146 Terminated, unacceptable toxicity |
Solid Tumors |
n = 31, 0.15–4.8 mg/kg Q3W.Safety: ≥1 DLTs in 22.5% pts. at doses >3.6 mg/kg. Significant DLTs in skin and mucosa in the dose ranges tested. Gr 3/4 TRAEs noted in 45.5% pts. The most common Gr 4 TRAE was neutropenia and most common Gr 3 TRAE was rash.Efficacy: 0% ORR.Program discontinued due to unacceptable toxicity. |
71 |
| |
Microtubule Inhibitors – Maytansine DM1: Solid Tumors (9) |
||||
|
36. |
Mucin-1, sialylated carbohydrate tumor antigen CA242 of Mucin-1 (MUC1)cantuzumab mertansine; huC242-DM1; SB- 408075Nonspecific Lys conj.Cleavable SPP linkerDM1 payloadDAR~ 3.5GlaxoSmithKline; ImmunoGen, Inc.1999–2014Description of Ph. 1 trial (HWID128964) is not included. |
Phase 1 Monotherapy HWID128999 Completed, tolerated with minimal activity at doses tested |
CA242+ Solid Tumors |
n = 37; 22–295 mg/m2 Q3W, recommended dose 235 mg/m2 Q3W.Safety: Reversable elevations of hepatic transaminases were the principal adverse events. Nausea, vomiting, and diarrhea were common but rarely severe at elevated doses.Efficacy: 2 minor regressions noted.Tolerated but with little clinical activity. |
72 |
| Phase 1 Monotherapy HWID128907 Completed, no objective responses noted up to and including MTD |
CA242+ Solid Tumors |
n = 39, 40–138 mg/m2 Q1W, MTD: 115 mg/m2.Safety: Gr ≥3 AEs at MTD included elevated lipase and alkaline phosphatase (4.3% each).Efficacy: No objective clinical responses (PR or CR) were noted.No objective responses noted up to and including MTD. |
73 |
||
|
37. |
CD44 antigen, variant 6 (CD44v6)bivatuzumab mertansine; B1W1-1Nonspecific Lys conj.Cleavable SPP linkerDM1 payloadDAR~ UndisclosedBoehringer Ingelheim; ImmunoGen, Inc.2002–2005Descriptions of Ph. 1 trials (NCT02254031, NCT02254018, NCT02254005) are not included. |
Phase 1 Monotherapy NCT02254044 Terminated, intolerable, dose limiting skin toxicity |
HNSCC |
n = 7, dose esc. 20–140 mg/m2 Q1W.Safety: Principal AEs- rashes, blisters, desquamation. 3 pts. developed desquamation 5–6 d after 1st or 2nd dose; one pt. (140 mg/m2) died of toxic epidermal necrolysis.Efficacy: No objective clinical responses were observed at the doses tested.Program halted due to severe skin toxicity. |
74,75 |
|
38. |
Neural cell adhesion molecule 1, CD56 (NCAM1)lorvotuzumab mertansine; BB-10901; huN901- DM1; IMGN901Nonspecific Lys conj.Cleavable SPP linkerDM1 payloadDAR~ 3.5ImmunoGen, Inc.2002–2017Descriptions of Ph. 2 trial (NCT02420873), Ph. 1/2 trials (NCT01237678, NCT00065429), and Ph. 1 trials (NCT00991562, NCT00346385, NCT00346255) are not included. |
Phase 2 Combination with carboplatin and etoposide (CE) vs CENCT01237678Terminated, intolerable toxicity without efficacy benefit |
SCLC |
n = 91; ADC arm, n = 44, 90 mg/m2, D1/D8 of 21-d cycle + CE vs CE, n = 47.Safety: ADC arm-88% of pts. experienced Gr ≥4 TRAEs including anemia (19%), peripheral sensory neuropathy (18%), neutropenia (17%), and thrombocytopenia (11%). TRAEs resulting in death occurred in 25% of pts. due to lethal infections.Efficacy: ADC arm vs CE-mPFS 6.2 vs 6.7 months (HR = 0.93); mOS estimates 10.1 vs 11 months.Intolerable toxicity without efficacy benefit. |
76,77 |
|
39. |
CD70 antigen (CD70) AMG 172 Nonspecific Lys conj. Non-cleavable MCC linker DM1 payload DAR~ Undisclosed Amgen; ImmunoGen, Inc. 2012–2016 |
Phase 1 Monotherapy NCT01497821 Completed, poor tolerability with limited clinical activity |
RCC |
n = 172; dose esc. 0.15–2.4 mg/kg BIW, MTD 1.6 mg/kg.Safety: TRAEs included thrombocytopenia (59%), anemia (32.4%), hypophosphatemia (29.7%), and AST increase (27%). Drug related DLTs included thrombocytopenia and hepatocellular injury.At 1.6 mg/kg dose, 3/10 pts. had DLTs: Gr 3 liver injury, in 2 pts., Gr 3/4 thrombocytopenia in 2 pts., Gr 3 AST elevations in 2 pts.Efficacy: 5.4% PR.Poor tolerability with limited clinical activity. |
78 |
|
40. |
Mast/stem cell growth factor receptor Kit, c-Kit (KIT)LOP628Nonspecific Lys conj.Non-cleavable SMCC linkerDM1 payloadDAR~ UndisclosedImmunoGen, Inc.; Novartis Pharmaceuticals2014–2016 |
Phase 1 Monotherapy NCT02221505 Terminated, intolerable toxicity |
c-KIT+ Solid Tumors |
n = 3 GISTs; 0.3 mg/kg (without premedication) 1 patient and 0.15 mg/kg (with premedication) 2 pts.Safety: Hypersensitivity reactions (HSR) noted in all pts. within minutes of infusion of 1st, 2nd, or 3rd dose; pts. were rescued with appropriate treatment; pre-medication controlled HSR, but reactions recurred in subsequent doses and ceased when dosing was discontinued. High serum tryptase noted in all pts.Study was terminated for safety. |
79 |
|
41. |
Epidermal growth factor receptor (EGFR)laprituximab emtansine; IMGN289; J2898ANonspecific Lys conj.Non-cleavable SMCC linkerDM1 payloadDAR~ UndisclosedImmunoGen, Inc.2013–2015 |
Phase 1 Monotherapy NCT01963715 Terminated, reason not disclosed |
EGFR+ Solid Tumors |
n = 20, no results published |
25 |
|
42. |
Epidermal growth factor receptor variant III (EGFRvIII)AMG 595Nonspecific Lys conj.Non-cleavable SMCC linkerDM1 payloadDAR~ 3.5Amgen; ImmunoGen, Inc.2012–2016 |
Phase 1 Monotherapy NCT01475006 Completed, poor tolerability and minimal clinical activity at MTD |
GBM, AA |
n = 32 GBM, 0.5–3.0 mg/kg Q3W, MTD 2.0 mg/kg.Safety: DLT, Gr 4 thrombocytopenia in 31.25% pts., Gr ≥3 TRAEs noted in 53% pts. including thrombocytopenia (44%) and neutropenia, ALT/AST increase, and purpura (3% each).Efficacy: 6% PR.Minimal clinical activity at MTD. |
80 |
| 43. |
Cadherin-3, P-Cadherin (CDH3) PCA062 Nonspecific Lys conj. Non-cleavable SMCC linker DM1 payload DAR~ 3.8 ImmunoGen, Inc.; Novartis Pharmaceuticals 2015–2022 |
Phase 1 Monotherapy NCT02375958 Completed, poor tolerability with minimal clinical activity |
TNBC, HNC, Esophageal Cancer | n = 47, 0.4–5.0 mg/kg Q2W, MTD 3.6 mg/kg.Safety: Frequent AEs included elevated AST, anemia, pyrexia, and thrombocytopenia (34% each). 66.0% pts. had SAEs with 55.3% Gr≥3. The most frequently occurring SAE was pyrexia (6.4%). 17% of pts. observed at least one DLT event including thrombocytopenia, AST increase, and anemia.Efficacy: 1 PR (2%).Insufficient efficacy noted at MTD. | 81 |
|
44. |
Prostate-specific membrane antigen, PSMA (FOLH1)MLN2704Nonspecific Lys conj.Cleavable SPP linkerDM1 payloadDAR~ 3–4Millennium Pharmaceuticals, Inc.2002–2006Description of one Ph.1 trial (NCT00052000) is not included here. |
Phase 1/2MonotherapyNCT00070837Completed, dose-limiting neurotoxicity with an absence of objective tumor responses |
Prostate Cancer |
n = 62, various dose esc. Cohorts: 60–165 mg/m2 Q1W (n = 12), 120–330 mg/m2 Q2W (n = 15), 330/426 mg/m2 Q3W (n = 18), 330 mg/m2, D1D15Q6W (n = 17).Safety: PN (71%) – 10% of which were Gr 3/4; nausea (61%), fatigue (60%).Efficacy: Only 8% of pts. experienced ≥ 50% decline in PSA. No objective tumor responses were noted.Neurotoxicity was dose-limiting with no objective tumor responses, attributable in part to labile linker. |
82 |
| |
Microtubule Inhibitors – Maytansines – DM1: Hematological Malignancies (1) |
||||
|
45. |
Tumor necrosis factor receptor superfamily member 17, BCMA (TNFRSF17)AMG 224Nonspecific Lys conj.Non-cleavable MCC linkerDM1 payloadDAR~ UndisclosedAmgen2015–2019 |
Phase 1 Monotherapy NCT02561962 Completed, evidence of antitumor activity noted at MTD |
MM |
n = 40; dose escal. 30–250 mg Q3W, n = 29; dose exp. 3 mg/kg Q3W with 2 cohorts: A (prior exposure to anti-CD38 Ab) and B (no prior exposure to anti-CD38 Ab). MTD 190 mg Q3W.Safety: Dose exp., Gr ≥ 3 TEAEs were thrombocytopenia (55%), neutropenia (27%), and anemia (18%). Treatment-emergent ocular AEs (all Gr 1 or 2) occurred in 36%) pts. Drug-related SAEs occurred in 36% pts.Efficacy: 23% ORR (5% sCRs, 5% VG PRs, 13% PRs, mDOR 14.7 months.Evidence of antitumor activity noted at MTD. |
83 |
| |
Microtubule Inhibitors – Maytansines – DM4: Solid Tumors (7) |
||||
|
46. |
Mucin-1 associated sialoglycotope CA6 (MUC1)SAR566658; ACT14884Nonspecific Lys conj.Cleavable SPBD linkerDM4 payloadDAR~ UndisclosedImmunoGen, Inc.; Sanofi2010–2018Description of Ph.1 trial (NCT01156870) is not included. |
Phase 2 Monotherapy NCT02984683 Terminated, limited clinical benefit with higher-than- expected rate of ophthalmological toxicity |
TNBC |
n = 23, 90 mg/m2 (n = 11) and 120 mg/m2 (n = 12) dosed on day 1 and 8 of a 21-d cycle.Safety: 100% of subjects experienced TRAEs; 90 mg/m2- 9.1% of pts. experienced SAE, 27.3% had TEAEs leading to discontinuation; 120 mg/m2-50% experienced SAE; 25% had TEAEs leading to discontinuation. Corneal toxicities were noted in 3/11 (90 mg/m2) and 5/12 (120 mg/m2) pts.Efficacy: 0% ORR.Limited clinical benefit with higher-than-expected incidence of ophthalmological toxicity. |
25 |
|
47. |
Mucin-1 associated sialoglycotope CA242 (MUC1)cantuzumab ravtansine; IMGN242; HuC242-DM4Nonspecific Lys conj.Cleavable SPBD linkerDM4 payloadDAR~ UndisclosedImmunoGen, Inc.2005–2009Description of Ph.1 trial (NCT00352131) is not included. |
Phase 2 Monotherapy NCT00620607 Withdrawn, lack of accrual |
Stomach Neoplasms, GC, GEJC |
n = 0, study not conducted. |
25 |
|
48. |
Integrin alpha-V, CD51 (ITGAV) IMGN388 Nonspecific Lys conj. Cleavable SPBD linker DM4 payload DAR~ Undisclosed ImmunoGen, Inc. 2008–2011 |
Phase 1 Monotherapy NCT00721669 Completed |
Solid Tumors |
n = 60, no published results. |
25 |
|
49. |
Cadherin-6 (CDH6) HKT288; CDH6-ADC Nonspecific Lys conj. Cleavable Sulfo-SPBD linker DM4 payload DAR~ Undisclosed Novartis Pharmaceuticals 2016–2018 |
Phase 1 Monotherapy NCT02947152 Terminated, unanticipated neurotoxicity with unknown mechanism |
RCC, EOC |
n = 9 (5 RCC, 4 EOC), starting dose 0.3 mg/kg Q3W.Safety: Common AEs included pyrexia (44.4%), constipation (44.4%), fatigue and vomiting (both 33.3%); Gr 2 neurological AEs in 3 pts. (30%). Unanticipated neurotoxicity with unknown mechanism.Efficacy: No objectives responses observed in doses tested.Limited clinical benefit with unanticipated neurotoxicity with unknown mechanism. |
84 |
|
50. |
Teratocarcinoma-derived growth factor 1, Cripto (TDGF1)BIIB015Nonspecific Lys conj.Cleavable SPBD linkerDM4 payloadDAR~ UndisclosedBiogen Idec; ImmunoGen, Inc.2008–2011 |
Phase 1 Monotherapy NCT00674947 Completed |
Solid Tumors |
n = 55, no published results. |
25 |
|
51. |
Fibroblast growth factor receptor 3 (FGFR3)LY3076226Nonspecific Lys conj.Cleavable Sulfo-SPBD linkerDM4 payloadDAR~ UndisclosedEli Lilly and Company; ImmunoGen, Inc.2015–2019 |
Phase 1 Monotherapy NCT02529553 Completed, 0% ORR |
Solid Tumors |
n = 25; dose escal. n = 22, 0.2–5.0 mg/kg Q3W; dose exp. n = 3, 5.0 mg/kg Q3W.Safety: Most TEAEs were Gr 1/2 and included PN (16%), thrombocytopenia (16%), and diarrhea (32%). Gr 3 AEs in 8% pts. included Gr 3 pulmonary embolism (4%) and Gr 3 thrombocytopenia.Efficacy: 0% ORR.Acceptable safety with no reported clinical activity. |
85 |
|
52. |
Lysosome-associate membrane glycoprotein 1 (LAMP-1)SAR428926Nonspecific Lys conj.Cleavable SPBD linkerDM4 payloadDAR~ UndisclosedImmunoGen, Inc.; Sanofi2015–2018 |
Phase 1 Monotherapy NCT02575781 Completed |
Solid Tumors |
n = 34, no published results. |
25 |
| |
Microtubule Inhibitors – Maytansines – DM4: Hematological Malignancies (2) |
||||
|
53. |
B-lymphocyte antigen CD19 (CD19) coltuximab ravtansine; SAR3419 Nonspecific Lys conj. Cleavable SPBD linker DM4 payload DAR~ 3.5 ImmunoGen, Inc. 2007–2018 Descriptions of Ph.1 trials (NCT00796731, NCT00549185) are not included. |
Phase 2 Monotherapy NCT01472887 Completed, tolerable with evidence of antitumor activity |
DLBCL |
n = 61, 55 mg/m2 4xQ1W/4xQ2W; 20 pts. eliminated from efficacy analysis (some misclassified).Safety: Gr ≥3 AEs in 38% pts. including hepatotoxicity (3%) and abdominal pain. Ocular AEs observed in 25% of pts.Efficacy: 43.9% ORR, 14.6% CR, mDOR 4.7 months, mPFS 4.4 months, OS 9.2 months. |
86 |
| Phase 2 Combination with rituximab NCT01470456 Completed, primary objective of ORR was not met |
DLBCL |
n = 52, 55 mg/m2 + rituximab 4XQ1W/8XQ2W, dose reduced to 40 mg/m2 (in case of Gr ≥3 AE.Safety: Gr ≥3 TEAEs in 52% of pts., mostly hematologic AEs including lymphopenia, neutropenia, thrombocytopenia, and anemia. 2 SAEs noted: Gr 1 sinus tachycardia and Gr 4 bronchospasm.Efficacy: 31.1% ORR, 8.9% CR, 22.2% PR, mDOR 8.6 months, mPFS 3.9 months, mOS 9.0 months.Insufficient efficacy: primary objective of ORR, ≥40%, was not met. |
87 |
||
| Phase 2 Monotherapy NCT01440179 Terminated, modest activity compared to competitors |
ALL |
n = 36, dose esc. (n = 19) 55–90 mg/m2; dose exp. (n = 17) at 70 mg/m2.Safety: Gr ≥3 TEAEs in 44% of pts. (55 mg/m2), 63% (70 mg/m2), and 88% of pts. (90 mg/m2) on study. SAEs were reported in 22%, 74%, and 88% of pts. – correlating with dose group. The most common SAEs were bacteremia, pneumonia, and febrile neutropenia.Efficacy (dose exp.): 25.47% ORR (1 CR, 2 CRi, 1 PR), mDOR 1.94 months. Tolerated with low clinical responses vs competitors. |
88 |
||
|
54. |
Myeloid cell surface antigen CD33 (CD33)AVE9633; IMGN-633Nonspecific Lys conj.Cleavable SPBD linkerDM4 payloadDAR~ 3.5ImmunoGen, Inc.; Sanofi2007–2009 |
Phase 1 Monotherapy NCT00543972 Terminated, minimal clinical activity at MTD |
AML |
n = 54, 3 dosing regimens: Q3W, D1D8 or D1D4D7 of 28-d cycle, MTD 150 mg/m2 for D1D4D7 and 130 mg/m2 for D1D8 group.Safety: Main toxicity was allergic reaction during infusion. DLTs of keratitis, liver toxicity for D1D4D7 group. 15% of D1D8 pts. had Gr 3/4 AEs including bronchospasm, keratitis, and liver toxicity. 20% of D1D4D7 pts. had Gr 3/4 events including bronchospasm, erythema, and liver toxicity.Reduction in CD33- blasts noted in D1D8 group.Efficacy: D1D8 − 10% ORR (5% CRi, 5%PR) and D1D4D7 − 0% ORR.Minimal clinical activity at MTD. |
89 |
| |
Microtubule Inhibitors – Other Maytansines: Solid Tumors (2) |
||||
|
55. |
Receptor tyrosine-protein kinase erbB-2, HER2 (ERBB2)BAT8001Nonspecific Cys conj.Non-cleavable 3AA linkerMaytansine payloadDAR~ 3.5Bio-Thera Solutions Ltd.2017–2021Ph. 1/2 trials (ChiCTR1900022300, NCT04151329) are not included; neither had published results. |
Phase 3 Monotherapy vs lapatinib + capecitabine NCT04185649 Unknown, last update 12/4/2019 |
HER2+ BC |
Expected/estimated n = 410, results not published. |
25 |
| Phase 1 Monotherapy NCT04182911 Active, not recruiting |
HER2+ BC or GC |
n = 29, 1.2–6.0 mg/kg Q3W; MTD 3.6 mg/kg.Safety: DLTs of Gr 4 thrombocytopenia and Gr 3 transaminase elevation noted. Gr ≥3 occurred in 48.3% pts., including thrombocytopenia (41.4%), increased AST (13.8%), increased γ‐glutamyl transferase (6.9%), and increased alanine aminotransferase (6.9%) of pts.Efficacy: 41.4% ORR, mPFS 4.3 months.Tolerated with evidence of antitumor efficacy. |
90 |
||
|
56. |
Tumor-associated calcium signal transducer 2, Trop-2(TACD2)BAT8003Specific Engineered Cys conj.Non-cleavable 3AA linkerMaytansine payloadDAR~ 2Bio-Thera Solutions Ltd.2017–2019 |
Phase 1 Monotherapy NCT03884517 Unknown status; last update March 21, 2019 |
Solid Tumors |
Expected/estimated n = 50, no published results. |
25 |
| |
Other Microtubule Inhibitor: Solid Tumors (2) |
||||
|
57. |
Glypican-3 (GPC3) BMS-986183 Nonspecific Lys conj. Cleavable Val-Cit linker Tubulysin payload DAR~ 3–3.5 Bristol-Myers Squibb 2016–2018 |
Phase 1/2 Combination with nivolumab (Ph. 2) NCT02828124 Terminated; portfolio prioritization |
HCC |
n = 25, Combination dose escal. 3–36 mg (n = 10) with nivolumab (Nivo).Safety, Nivo combo dose escal: 40% pts. had SAEs.Efficacy, Nivo combo dose escal.: 0% pts. had objective responses.0% objective responses noted at doses tested. |
25 |
|
58. |
Receptor tyrosine-protein kinase erbB-2, HER2 (ERBB2)MEDI4276Specific Engineered Cys conj.Cleavable mc-lysine linkerTubulysin analogue payloadDAR~ 4AstraZeneca2016–2018(Biparatopic antibody) |
Phase 1 Monotherapy NCT02576548 Completed, limited efficacy at MTD |
HER2+ BC, GC, Stomach Cancers |
n = 47, 0.05–0.9 mg/kg Q3W; MTD 0.75 mg/kg Q3W, t½ 0.5–2 d.Safety: Gr ≥3 TRAE occurred in 36.2% pts. The most common Gr ≥3 TRAE included increased AST (21.3%), increased ALT (14.9%), and increased blood bilirubin (6.4%). 10% of pts. had TRAE leading to discontinuation. At the MTD (0.75 mg/kg), MEDI4276 had poor tolerability, as evidenced by the fact that 75.0% of pts. experienced ≥1 serious and/or Gr ≥3 event.Efficacy: BC- 9.4% ORR, 3% CR, 6% PR, mPFS 1.3–15.4 months, mOS 19.1 months.GC -no ORR, mPFS 1.8 months.Limited efficacy at MTD. |
91 |
| |
DNA Damaging – PBDs: Solid Tumors (11) |
||||
|
59. |
Claudin-6 (CLDN6), Claudin-9 (CLDN9) SC-004 Specific Engineered Cys conj. Cleavable Val-Cit linker PBD Dimer (SG1882) payload DAR~ 2 AbbVie; Stemcentrx 2017–2020 |
Phase 1/1b Monotherapy or Combination with ABBV-181 (αPD1) NCT03138408 Terminated, low tolerability with limited clinical activity |
Epithelial Ovarian Cancer, Endometrial Cancer |
n = 24 (11 OC, 13 EC), 0.005–0.3 mg/kg Q3W, MTD 0.2 mg/kg Q3W.Safety: Gr ≥3 TRAEs in 33% pts. including pericardial effusion, pleural effusion, renal failure, and respiratory failure (8% each).Efficacy: 5% PR.Low tolerability with limited clinical activity. |
30 |
|
60. |
Delta-like protein 3 (DLL3) rovalpituzumab tesirine; Rova-T; SC16LD6.5 Nonspecific Cys conj. Cleavable Val-Ala linker PBD Dimer (SG1882) payload DAR~ 2 AbbVie; Stemcentrx; Spirogen 2013–2019 Descriptions of Ph.3 (NCT03334487, study withdrawn), Ph. 2 (NCT03543358, NCT02674568), Ph. 1/2 (NCT03026166, NCT02709889), Ph. 1 (NCT01901653, NCT03086239, NCT03000257, NCT02874664, NCT02819999), and one Expanded Access trial (NCT03503890) are not included here. |
Phase 3 (TAHOE) Monotherapy vs topotecan NCT03061812 Completed, Rova-T failed to demonstrate clinical benefit vs topotecan |
SCLC |
n = 444 (600 were needed for sufficient power), antigen-high (by IHC).ADC dosed at 2 × 0.3 mg/kg Q6W.Safety: ADC vs topotecan-Gr ≥3 AEs in 64% pts. ADC arm vs 88% pts.in Topotecan arm. Serious TRAEs in ADC arm (56%) included malignant neoplasm progression (10%), pneumonia (7%), pleural effusion (6%), and dyspnea (6%).Efficacy: ADC vs topotecan-ORR 15% vs 21%, mDOR 3.5 vs 4.9 months, mOS 6.3 vs 8.6 months (HR 1.46).ADC failed to demonstrate improved clinical benefit vs topotecan. |
29 |
| Phase 3 (MERU) Monotherapy vs placebo maintenance after 1 L platin-based therapyNCT03033511Terminated, recommendation of IDMC (toxicity) |
SCLC |
n = 748; 372 in ADC arm and 376 in placebo arm: ADC dosed at 0.3 mg/kg Q6W, omitting every 3rd cycle.Safety: Gr ≥3 TEAEs in 59% pts. in ADC arm vs 30% pts. in placebo arm. The most common Gr ≥3 TEAE in ADC arm was thrombocytopenia (9%). TRAEs lead to death in 10% of ADC and placebo arms. ADC discontinuation due to TRAEs in 20% of pts.Efficacy: ADC vs placebo- mOS 8.5 vs 9.8 months; HR = 1.07 favoring placebo arm.ADC showed lack of survival benefit (did not meet primary endpoint). |
28 |
||
|
61. |
Delta-like protein 3 (DLL3) SC-002 Specific Engineered Cys conj. Cleavable Val-Ala linker PBD Dimer (SG1882) payload DAR~ 2 AbbVie; Stemcentrx 2016–2019 |
Phase 1 Monotherapy NCT02500914 Terminated, systemic toxicity with insufficient efficacy |
SCLC |
n = 35, 0.025–0.4 mg/kg Q3W, MTD 0.4 mg/kg Q9WSafety: 66% of pts. experienced ≥ 1 SAE and in at least 37% of the pts. these were considered drug related including one case of lethal pneumonia.Efficacy: 14% PR.Systemic toxicity was postulated to limit the efficacy as was seen with Rova-T. |
92 |
|
62. |
Dipeptidase 3 (DPEP3) tamrintamab pamozirine; SC-003 Specific Engineered Cys conj. Cleavable Val-Ala linker PBD Dimer (SG1882) payload DAR~ 2 AbbVie; Stemcentrx 2015–2018 |
Phase 1a/b Combination with ABBV-181 (αPD1) NCT02539719 Terminated, lack of requisite safety and efficacy |
OC |
n = 74 (n = 29 for dose esc; n = 45 dose exp.; n = 3 ABBV-181 combination), MTD 0.3 mg/kg Q3W.Safety: At MTD, 66% of pts. experienced ≥ 1 SAE; 7% experienced Gr 4/5 AEs. 1 death due to kidney injury was deemed treatment related. Common TRAEs included pleural effusion (35%) and peripheral edema (34%).Efficacy: 4% ORR, responses were not durable.Lack of requisite safety and efficacy. |
27 |
|
63. |
Receptor tyrosine-protein kinase erbB-2, HER2 (ERBB2)ADCT-502Specific Engineered Cys conj.Cleavable Val-Ala linkerPBD Dimer (SG1882) payloadDAR~ 1.7ADC Therapeutics S.A.2017–2018 |
Phase 1 Monotherapy NCT03125200 Terminated, safety |
HER2+ BC, NSCLC, GEC, Bladder Cancer |
n = 21, 0.030–0.240 mg/kg Q3W, MTD 0.240 mg/kg.Safety: At doses ≥0.060. mg/kg, 33% pts. had SAEs; 7% experienced Gr 4 /5 events. At doses ≥0.060 mg/kg, 36.8% pts. had treatment emergent SAE including small intestinal obstruction (14%) and peripheral edema, sepsis, pneumonia, pleural effusion (4.8% each).Efficacy: 4.8% PR.Lack of requisite safety and efficacy. |
25 |
|
64. |
Receptor tyrosine-protein kinase erbB-2, HER2 (ERBB2)DHES0815A; RG6148Specific Engineered Cys (THIOMABTM) conj.Cleavable disulfide linkerPBD-MA payloadDAR~ 2Roche-Genentech2018–2019 |
Phase 1 Monotherapy NCT03451162 Completed, insufficient efficacy at tolerated doses |
BC |
n = 14, dose escal. 0.6–6.0 mg/kg Q3W.Safety: 29% pts. discontinued treatment due to AEs. Skin events were reported in 50% of pts. (all doses) and related included pruritus (36%), rash (36%), and skin hyperpigmentation (21%). Ocular toxicities were reported in 57% of pts. with 3 pts. having Gr 3 ocular events. Lung toxicities were reported in 36% of pts., including pneumonitis (14%). Due to these AEs, ADC dose was decreased to 2.4 mg/kg Q3W for all enrolled pts. and accrual was stopped.Efficacy: 7% CR.Insufficient efficacy at tolerated doses. |
93,94 |
|
65. |
Melanotrasferrin (MELTF) SC-005 Undisclosed conj. method Undisclosed linker PBD payload DAR~ Undisclosed AbbVie; Stemcentrx 2018–2019 |
Phase 1 Monotherapy NCT03316794 Terminated, sponsor strategic alignment |
BC |
n = 2, no published results. |
25 |
|
66. |
Prolactin receptor (PRLR) rolinsatamab talirine; ABBV-176 Specific Engineered Cys conj. Cleavable Val-Ala linker PBD Dimer (SFD-1882) payload DAR~ 2 AbbVie; Seagen Inc. 2017–2019 |
Phase 1 Monotherapy NCT03145909 Terminated, safety |
PRLR+ Solid Tumors |
n = 19, 0.0027–0.10935 mg/kg Q3W.Safety: Possible cumulative toxicity in the form of effusion and edema.DLTs of thrombocytopenia, neutropenia, and pancytopenia were noted. Effusions and edema were common, and timing of onset suggested possible cumulative ABBV-176 toxicity.Efficacy: No ORR.Significant toxicity with absence of objective tumor responses. |
11 |
|
67. |
Prostate-specific membrane antigen, PSMA (FOLH1)ADCT-401; MEDI3726Specific Engineered Cys conj.Cleavable Val-Ala linkerPBD Dimer (SFD-1882) payloadDAR~ 1.8ADC Therapeutics S.A.; AstraZeneca2017–2019 |
Phase 1 Monotherapy NCT02991911 Completed, lack of clinical benefit at tolerated doses |
mCRPC |
n = 33; 0.015–0.3 mg/kg Q3W, MTD not identified; max. administered dose 0.3 mg/kg.Safety: TRAEs in ~91% pts., primarily skin toxicities and effusions. Gr ≥3 TRAEs in ~46% pts.; 33.3% pts. discontinued due to TRAEs. Gr 3/4 TRAEs included increased gamma-glutamyltransferase (21.2%), thrombocytopenia, capillary leak syndrome (each 9.1%), and increased ALT (6%).Efficacy: 0% ORR, 3% uPR, mPFS 3.6 months, mOS 8.9 months.Lack of efficacy benefit at tolerated doses. |
31 |
|
68. |
E3 ubiquitin-protein ligase (RNF43) SC-006 Specific Engineered Cys conj. Cleavable Val-Ala linker PBD (SC-DR003) payload DAR~ 2 Stemcentrx; AbbVie 2017–2019 |
Phase 1 Combination with ABBV-181 (αPD1) NCT03035279 Terminated, strategic considerations |
CRC |
n = 29, no results published. |
25 |
|
69. |
Tumor necrosis factor ligand superfamily member 9 (TNFSF9)SC-007Undisclosed conj. methodUndisclosed linkerPBD payloadDAR~ UndisclosedAbbVie; Stemcentrx2017–2018 |
Phase 1 Monotherapy NCT03253185 Terminated, benefit/risk imbalance |
CRC, GC |
n = 7, no results published. |
25,95 |
| |
DNA Damaging – PBDs: Hematological Malignancies and Solid Tumors (1) |
||||
|
70. |
CD70 antigen (CD70) SGN-CD70A Specific Engineered Cys conj. Cleavable Val-Ala linker PBD Dimer (SGD-1882) payload DAR~ 2 Seagen Inc. 2014–2016 |
Phase 1 Monotherapy NCT02216890 Completed, drug-related thrombocytopenia severity and prevalence cited as reasons for program discontinuation |
RCC, MCL, DLBCL, FL |
n = 20 NHL; 0.008–0.20 mg/kg Q3W amended to Q6W due to thrombocytopenia, MTD 0.030 mg/kg Q6W.Safety: TEAEs ≥ Gr 3 occurred in 90% pts., including thrombocytopenia (65%), anemia (50%), and fatigue (50%). 55% of pts. experienced drug related SAEs.Efficacy: 20% ORR, 5% CR, 15% PR.Poorly tolerated with insufficient efficacy. |
67,96 |
| |
DNA Damaging – PBDs: Hematological Malignancies (7) |
||||
|
71. |
B-lymphocyte antigen CD19 (CD19) SGN-CD19B Specific Engineered Cys conj. Cleavable Val-Cit linker PBD Dimer (SG1882) payload DAR~ 2 Seagen Inc. 2015–2018 |
Phase 1 Monotherapy NCT02702141 Terminated, reason not disclosed |
NHL, DLBCL, FL |
n = 44, no published results. |
25 |
|
72. |
Neutral amino acid transporter B(0), ASCT2 (SLC1A5)MEDI7247Specific Engineered Cys conj.Cleavable Val-Ala linkerPBD payloadDAR~ 2AstraZeneca2017–2020 |
Phase 1 Monotherapy NCT03106428 Completed |
AML, MM, DLBCL |
n = 67; no published results. |
25 |
|
73. |
Tumor necrosis factor receptor superfamily member 17, BCMA (TNFRSF17)MEDI2228Specific Engineered Cys conj.Cleavable Val-Ala linkerPBD Dimer (SG3199) payloadDAR~ 2AstraZeneca2018–2021 |
Phase 1 Monotherapy NCT03489525 Completed |
MM |
n = 107, no published results. |
25 |
|
74. |
Myeloid cell surface antigen CD33 (CD33)vadastuximab talirine; SGN-CD33ASpecific Engineered Cys conj.Cleavable Val-Ala linkerPBD Dimer (SGD-1882) payloadDAR~ 1.9Seagen Inc.2013–2018Ph. 1/2 (NCT02706899, NCT02614560) and Ph. 1 trial (NCT02326584) are not included; neither had published results. |
Phase 3 Combination with azacitidine or decitabine vs placebo NCT02785900 Terminated, safety; higher rates of deaths, including fatal infections |
AML |
n = 240; Trial was halted following a review of unblinded data and consultation with the Independent Data Monitoring Committee. Pts. assigned to the vadastuximab talirine arm had higher rates of death than those in the control arm, including fatal infections. |
25 |
| Phase 1 Combination with HMA (hypomethylating agents) azacitidine (AZA) or decitabine (DEC) NCT01902329 Completed, combination with HMA had evidence of antitumor activity at tolerated doses |
AML, APML |
n = 195, Monotherapy dose escal. (n = 131) 0.005–0.06 mg/kg; expansion dose 0.04 mg/kg selected. Combination (n = 53) AZA for 7 d, n = 23 or DEC for 5 d, n = 27 with ADC dosed (0.01 mg/kg) on last day of AZA/DEC treatment; 28 d treatment cycle.Safety-Monotherapy: Most TEAEs consistent with myelosuppression. 100% of 0.04 mg/kg pts. experienced Gr ≥3 TEAEs that included febrile neutropenia (72%), anemia (42%), and thrombocytopenia (25%).Efficacy–Monotherapy: 0.04 mg/kg monotherapy: CRc (composite remission rate) 28%.Safety-Combination: Gr ≥3 TEAEs reported in 98% of pts. Gr ≥3 TEAEs that included thrombocytopenia (57%), febrile neutropenia (49%), and anemia (45%).Efficacy-Combination: 70% CRc, mOS 11.3 months.Combination with HMA had evidence of antitumor activity at tolerated doses. |
97,98 |
||
|
75. |
Interleukin-3 receptor subunit alpha, CD123 (IL3RA)SGN-CD123ASpecific Engineered Cys conj.Cleavable Val-Ala linkerPBD Dimer (SGD-1882) payloadDAR~ 2Seagen Inc.2016–2018 |
Phase 1 Monotherapy NCT02848248 Terminated, reason not disclosed |
AML |
n = 17, no published results. |
25 |
|
76. |
SLAM family member 6, CD352 (SLAMF6)SGN-CD352ASpecific Engineered Cys conj.Cleavable Val-Ala linkerPBD Dimer (SGD-1882) payloadDAR~ 2Seagen Inc.2016–2018 |
Phase 1 Monotherapy NCT02954796 Terminated, sponsor portfolio prioritization |
MM |
n = 27, no published results. |
25 |
|
77. |
C-type lectin domain family 12 member A, CLL-1 (CLEC12A)DCLL9718S; RG6109Specific Engineered Cys (THIOMABTM) conj.Undisclosed cleavable linkerPBD payloadDAR~ 2 Roche-Genentech2017–2019 |
Phase 1 Combination with azacitidine NCT03298516 Completed, limited tolerability and lack of clinical activity |
AML |
n = 18, dose esc. 0.01–0.16 mg/kg Q3W, MTD not identified.Safety: AEs of Gr ≥3 in 67% pts., including febrile neutropenia (33%) and pneumonia (28%).Efficacy: 0% ORR (CR/PR).Limited tolerability and lack of clinical activity. |
99 |
| |
DNA Damaging – Calicheamicin: Solid Tumors (3) |
||||
|
78. |
Ephrin-A4 (EFNA4) PF-06647263 Nonspecific Lys conj. Cleavable AcBut acyl hydrazone-disulfide linker Calicheamicin payload DAR~ 4.6 Pfizer 2014–2019 |
Phase 1 Monotherapy NCT02078752 Terminated, limited efficacy response at tolerated doses |
TNBC, Solid Tumors |
n = 60 (48 in dose esc., 12 in dose exp.); dose esc. 0.015–0.134 mg/kg Q3W or 0.01–0.02 mg/kg QW; dose exp. at RP2D of 0.015 mg/kg QW.Safety: TRAEs in >82% pts., AEs of Gr 3/4 in 53.3% including thrombocytopenia (20%). Notable TRAEs are mucosal inflammation (28%), stomatitis (28%), and rash (24%).Efficacy: 10.4% ORR in dose esc; in dose exp. group, 8.3% ORR; no CR.Limited anti-tumor activity at tolerated doses. |
100 |
|
79. |
Lewis Y antigenCMD-193Nonspecific Lys conj.Cleavable AcBut acyl hydrazone-disulfide linkerCalicheamicin payloadDAR~ UndisclosedWyeth; Pfizer2004–2014 |
Phase 1 Monotherapy (radiolabeled) NCT00293215 Terminated, abnormal distribution with rapid hepatic uptake and low tumor uptake with rapid clearance from blood |
Solid Tumors |
n = 9, objective was to determine the biodistribution and PK of48 In-CMD-193; CMD-193 demonstrated rapid blood clearance and increased hepatic uptake compared with prior studies of the parental antibody hu3S193.Safety: Myelosuppression and prolonged liver uptake which affected liver function were the most significant adverse events. Gr ≥3 AEs included thrombocytopenia (20%) and abnormal liver function (20%).Efficacy: No objective responses were observed.Abnormal drug distribution and low tumor uptake with lack of objective responses. |
101 |
|
80. |
Mucin-1 (MUC-1)CMB-401; hCTMOl-calicheamicinNonspecific Lys conj.Cleavable AcBut acyl hydrazone-disulfide linkerCalicheamicin payloadDAR~ 2–3Pfizer, Celltech Therapeutics1997–2016Description of Ph. 1 trial (PMID 10942064) is not included. |
Phase 1 Monotherapy NCT00257881 Terminated, reason not disclosed |
Solid Tumors |
n = 46 (Japan), no published results. |
25 |
| Phase 2 Monotherapy Registry Trial Identifier PMID 12669249 Completed, lack of efficacy noted at tolerated doses |
PSEOC |
n = 21, pre-dosed with Ab hCTMO1, ADC given at 16 mg/m2 Q4W.Safety: 66% of pts. experienced at least 1 Gr ≥3 AE. TRAEs included epistaxis, anemia, AST elevation, and thrombocytopenia. Gr 4 anemia and peritonitis occurred in 1 pt. each.Efficacy: 0% ORR.No anti-tumor activity at tolerated doses.Hypothesized that amide linker may have contributed to the failure to induce PR. |
102,103 |
||
| |
Other DNA Damaging: Solid Tumors (2) |
||||
|
81. |
Lewis Y antigen SGN-15; BMS-182248; br96-doxPh Nonspecific Cys conj. Cleavable hydrazone linker Doxorubicin payload DAR~ Undisclosed Seagen Inc. 2000–2005 Descriptions of Ph. 2 trials (NCT00031187, NCT00028483, NCT00051584, NCT00086333) are not included; none have published results. |
Phase 2 Combination with docetaxel vs docetaxel NCT00051571 Completed, insufficient activity benefit vs comparator arm |
NSCLC |
n = 62, randomized 2:1 into Arm A (ADC 200 mg/m2 Q1W + docetaxel, n = 40) and Arm B (docetaxel alone, n = 19); intrapatient dose escal. up to 350 mg/m2 Q1W.Safety: Gr ≥3 AEs in Arm A: increased lipase (25%), nausea/vomiting (18%), asthenia/fatigue (13%); Arm B: respiratory distress (16%), pneumonia (11%), asthenia/fatigue (11%).Efficacy: Arm A vs Arm B, OR 46% vs 37%,mPFS 31.4 vs 25.3 weeks, 1-y survival 29% vs 24%.Insufficient efficacy benefit versus comparator arm. |
24 |
|
82. |
Mesothelin (MSLN) BMS-986148; MDX-1204 Nonspecific Lys conj. Cleavable Val-Cit linker Duocarmycin payload DAR~ 1.4 Bristol-Myers Squibb; Medarex 2009–2018 Description of Ph. 1 trial (NCT02884726) is not included here. |
Phase 1/2 Monotherapy and Combination with nivolumab (Nivo) NCT02341625 Terminated, limited clinical activity |
MESO, NSCLC, OC, PC, GC |
n = 126, Arm A: ADC alone (n = 96; dose esc. 0.1–1.6 mg/kg Q3W/QW) and Arm B: ADC (n = 30, 0.4/0.6/1.2 mg/kg Q3W) + Nivo.Safety: Arm A: 50% pts. treated at 1.6 mg/kg had DLTs, 40% transaminase elevations, 10% Gr 3 pleuritic pain. Combination Arm B: 33% Gr 3/4 AE of ALT/AST increase and pleuritic pain.Efficacy: 2% ORR for ADC monotherapy; 6% ORR in combination group.Limited clinical activity observed alone or in combination with Nivo. |
104 |
| |
Other DNA Damaging: Hematological Malignancies and Solid Tumors (1) |
||||
|
83. |
CD70 antigen (CD70) MDX-1203; BMS936561; aCD70_MED-A Nonspecific Lys conj. Cleavable Val-Cit linkerMED-A/DNAMGBA toxin (Duocarmycin) payloadDAR~ 1.25Bristol-Myers Squibb; Medarex2009–2018 |
Phase 1 Monotherapy NCT00944905 Completed, DLTs of Gr 3 hypersensitivity (13%); no efficacy observed at doses tested |
RCC, NHL |
n = 26, 0.5 − 15 mg/kg Q3W; MTD not defined, RP2D of 8 mg/kg Q3W.Safety: Gr 3 hypersensitivity DLTs (13%) at highest dose; delayed toxicities of facial edema and pleural/pericardial effusion occurred in 38% of pts. at highest dose.Efficacy: 0% OR (PR/CR). No efficacy at doses tested. |
105 |
| |
Other DNA Damaging: Hematological Malignancies (2) |
||||
|
84. |
Myeloid cell surface antigen CD33 (CD33)IMGN779Nonspecific Lys conj.Cleavable Sulfo-SPDB linkerDGN462 (DNA alkylator) payloadDAR~ 3ImmunoGen, Inc.; Jazz Pharmaceuticals2016–2019 |
Phase 1 Monotherapy NCT02674763 Completed |
AML |
n = 62; initial results from 17 pts., dose escal. from 0.02–0.26 mg/kg on days 1 and 15 of a 28-d cycle.Safety: SAEs included Gr 3 febrile neutropenia (29%) and pneumonia (24%). Rash, respiratory failure, and constipation were reported in ≥ 24% of pts. No correlation of frequency and severity of AEs to dose was observed.Efficacy was not reported. |
106 |
| 85. |
HLA class II histocompatibility antigen gamma chain (CD74)milatuzumab doxorubicin; CD74-DOX; hLL1-DOX; IMMU-110Specific Cys conj.Cleavable Hydrazone linkerDoxorubicin payloadDAR~ 8Immunomedics, Inc.2010–2019 |
Phase 1/2 Monotherapy NCT01101594 Terminated, lack of efficacy |
MM |
n = 17, no published results. |
25 |
| Phase 1/2 Monotherapy NCT01585688 Terminated, lack of efficacy |
NHL, CLL |
n = 13, no published results. |
25 |
||
| |
Topoisomerase Inhibitors: Solid Tumors (2) |
||||
|
86. |
Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5)labetuzumab govitecan; hMN14-SN38; IMMU-130Specific Cys conj.Cleavable CL2A linkerSN-38 payloadDAR~ 7.6Immunomedics, Inc.2011–2021Descriptions of Ph. 1/2 trial (NCT01605318) and Ph. 1 trial (NCT01270698) are not included; neither has published results. |
Phase 2 Monotherapy NCT01915472 Withdrawn; no participants enrolled |
CRC |
n = 0, no participants enrolled. |
25 |
| Phase 1/2 Monotherapy NCT01270698 Completed, minimal efficacy noted at doses tested |
CRC |
n = 86 pts., dose exp. 4–10 mg/kg QW or BIW on weeks 1 and 2 of 3-week cycle.Safety: Gr ≥ 3 AEs included neutropenia (16%), leukopenia (11%), and anemia (9%). Toxicity was similar among the 4 cohorts although Gr ≥ 3 AEs were more frequent in the 10 mg/kg QW group (36%).Efficacy: 1 PR (1.16%) from 6 mg/kg BIW treatment group.Minimal efficacy noted at doses tested. |
107 |
||
|
87. |
G-protein coupled receptor 20 (GPR20) DS-6157; DS-6157a; GPR20 ADC Specific Cys conj. Cleavable GGFG linker DXd/DX8951 payload DAR~ 8 Daiichi Sankyo, Inc.; Sarah Cannon Research Institute 2020–2021 |
Phase 1 Monotherapy NCT04276415 Completed, insufficient efficacy noted at tolerated doses |
GIST |
n = 34, dose esc 1.6–6.4 mg/kg QW3; MTD was 6.4 mg/kg QW3.Safety: Gr ≥3 TRAEs occurred in 41% of pts. including decreased platelets (21%) and anemia (18%). Serious TEAEs (SAEs) occurred in 27% of pts. Related Gr 4 SAEs in 2 pts. included abnormal hepatic function, neutropenia, thrombocytopenia, and leukopenia. There was 1 treatment related death (hepatic failure).Efficacy: One patient had a confirmed PR (~3%), mPFS across all dose levels was 3.6 months.Insufficient efficacy noted at tolerated doses. |
108 |
| |
Immunomodulatory Payloads: Solid Tumors (3) |
||||
|
88. |
Receptor tyrosine-protein kinase erbB-2, HER2 (ERBB2)NJH395Specific Cys conj.Noncleavable linkerUndisclosed TLR7 agonist payloadDAR~ 4Novartis2018–2020 |
Phase 1 Monotherapy NCT03696771 Completed, insufficient efficacy noted at tolerated doses |
Non-breast HER2+ Malignancies |
n = 18, dose esc 0.1–1.6 mg/kg.Safety: The most common AE was cytokine release syndrome (55.6% Gr ≥2), pyrexia (44.4%), and AST (33.3%) and ALT (27.8%) increase. The most common Gr ≥3 AEs included lymphopenia (27.8%) and AST increase (11.1%). Five Gr 3 DLTs were reported, 2 AST increase, 1 ALT increase, 1 aseptic meningitis, and 1 meningism.Efficacy: 0% ORR.Insufficient efficacy noted at tolerated doses. |
109 |
|
89. |
Receptor tyrosine-protein kinase erbB-2, HER2 (ERBB2)SBT6050Undisclosed Cys conj. methodUndisclosed cleavable linkerUndisclosed TLR8 agonist payloadDAR~ 4Silverback Therapeutics2020–2022 |
Phase 1/2 NCT05091528 Terminated, sponsor portfolio prioritization |
HER2+ BC, GC, CRC, NSCLC |
n = 2, study not conducted. |
25 |
| Phase 1/1bMonotherapy and Combination with pembrolizumab/cemiplimabNCT04460456Active, not recruiting |
HER2+ Solid Tumors |
n = 58Interim analysis on first 18 pts.; dose escal. 0.15–1.2 mg/kg Q2W.Safety: The most frequent TRAEs (25%) were chills, diarrhea, fatigue, hypotension, injection site reaction, nausea, pyrexia, and vomiting. Gr 3 DLTs were observed at 1.2 mg/kg Q2W. Was concluded that a dose of 0.6 mg/kg Q2W had a tolerable safety profile with evidence of target saturation.Efficacy: 7% PR.Insufficient efficacy noted at tolerated doses. |
110 |
||
|
90. |
Nectin-4 (NECTIN4) SBT6290 Undisclosed Cys conj. method Undisclosed linker Undisclosed TLR8 agonist payload DAR~ Undisclosed ARS Pharmaceuticals; Silverback Therapeutics2022–2022 |
Phase 1/2 Monotherapy and Combination with pembrolizumab NCT05234606 Withdrawn, sponsor strategic realignment |
UC, TNBC, NSCLC, HNSCC, HR+ HER2- BC |
n = 0, study not conducted. |
25 |
| |
Undisclosed Payloads: Solid Tumors (2) |
||||
|
91. |
Leukocyte surface antigen CD47 (CD47) SGN-CD47M Undisclosed conj. method Undisclosed linker Undisclosed payload DAR~ Undisclosed Seagen Inc. 2019–2021 |
Phase 1 Monotherapy NCT03957096 Terminated, sponsor portfolio prioritization |
STS, CRC, HNSCC, NSCLC, BC, OC, Exocrine PC, GC, Melanoma |
n = 16, no published results. |
25 |
| 92. |
Alpha-N-acetylneuraminide alpha-2,8- sialtransferase, GD3 (ST8SIA1)PF-06688992; GD3-ADCUndisclosed conj. methodUndisclosed linkerUndisclosed payloadDAR~ UndisclosedPfizer2017–2019 |
Phase 1 Monotherapy NCT03159117 Completed |
Melanoma | n = 7, no published results. | 25 |
Abbreviations: Ab, antibody; ADC, antibody–drug conjugate; AE, adverse event; Ala, alanine; ALT, alanine transaminase; AML, acute myeloid leukemia; APML, acute promyelocytic leukemia; AST, aspartate transferase; AZA, azacytidine; BIW, twice weekly; Cit, citrulline; CLL, chronic lymphocytic leukemia; conj., conjugation; CRPC, castrate resistant prostate cancer; CR, complete response; CRc, composite complete remission; CRC, colorectal cancer; CRi, complete remission with incomplete blood count recovery; Cys, cysteine; D1D15, day 1 and day 15; DAR, drug–antibody ratio; DCR, disease control rate; DLBCL, diffuse large B cell lymphoma; DLT, dose-limiting toxicity; DM1, mertansine; DM4, ravtansine; EOC, epithelial ovarian cancer; escal., escalation; exp., expansion; FL, follicular lymphoma; Gr, grade; GBM, glioblastoma multiforme; GC, gastric cancer; GEJC, gastroesophageal junction cancer; GIST, gastrointestinal stromal tumors; HNSCC, head and neck squamous cell carcinoma; HR, hazard ratio; HR+, hormone receptor positive; HSR, hypersensitivity reactions; Lys, lysine; mc, maleimidocaproyl; MCL, mantle cell lymphoma; mDOR, medium duration of response; mg/m2, milligrams per meter squared; mg/kg, milligrams per kilogram; MM, multiple myeloma; MMAE, monomethyl auristatin E; MR, mixed response; mOS, medium overall survival; mPFS, medium progression free survival; MTD, maximum tolerated dose; NHL, non-Hodgkin lymphoma; Nivo, nivolumab; NSCLC, non-small cell lung cancer; OC, ovarian cancer; ORR, overall response rate; PBD, pyrrolobenzodiazepine; PC, prostate cancer; PK, pharmacokinetic; PLD, pegylated liposomal doxorubicin; PN, peripheral neuropathy; PR, partial response; PSA, prostate-specific antigen; PSOC, platin-sensitive ovarian cancer; pts., patients; QW, weekly; Q2W, every two weeks; Q3W, every three weeks; Q4W, every four weeks; Q6W, every six weeks; Q9W, every nine weeks; RCC, renal cell carcinoma; RP2D, recommended phase 2 dose; RT, radiation; SAE, serious adverse event; sCR, stringent complete response; SMCC, Succinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate; SPP, N-succinimidyl 4-(2-pyridyldithio) pentanoate; SPBD, N-succinimidyl 4-(2-pyridyldithio) butanoate; TEAE, treatment emergent adverse event; TESAE, treatment emergent serious adverse event; TMZ, temozolomide; TNBC, triple negative breast cancer; TRAE, treatment related adverse event; uPR, unconfirmed partial response; Val, valine; VGPR, very good partial response; YA, young adults.
Potential factors contributing to insufficient therapeutic benefit due to intolerable toxicity include 1) on target/off tumor toxicity, 2) utilization of very high potency payloads for antigens requiring higher biologic exposures, 3) labile linkers leading to off-tumor release of payload, 4) off-target toxicity, possibly due to pinocytosis of the ADC, and 5) metabolic conversion of the payload to a more toxic metabolite. Approximately 29% of the clinically tested ADCs cited intolerable toxicity as a reason for program termination. Examples of ADCs with intolerable toxicity that could in part be due to on target/off tumor toxicity include bivatuzumab mertansine (CD44v6, expressed in skin keratinocytes) – fatal desquamation,74 MEDI-547 (EphA2) – bleeding and coagulation adverse effects (adverse events not typically associated with the MMAE payload),66 and PF-06664178 – rash adverse events (Trop-2, expressed on the surface of normal epithelial including skin).71 For the latter example of PF-06664178, an additional potential contributing factor to the severity of skin toxicity noted is the potent auristatin payload pairing with this Trop-2-targeting ADC. Indeed, the severity of the skin toxicity of PF-06664178 is markedly different from the approved Trop-2-targeting ADC, TrodelvyTM, which uses a lower potency topoisomerase I inhibitor payload.111 Additionally, skin toxicity has also been noted for another auristatin ADC, PadcevTM, targeting Nectin-4 (also expressed in the skin).112
Microtubule inhibitor payload ADCs account for 63% of discontinued candidates, followed by DNA damaging (~27%) payloads. Topoisomerase I inhibitors, targeted small molecules, and undisclosed payloads combined comprise 10% of discontinued ADCs [Figure 10]. Utilization of high potency payloads for antigens requiring higher biologic exposures was a likely contributing factor to the intolerable toxicity of several discontinued ADC candidates. The payload choice of biparatopic tetravalent HER2-directed ADC MEDI4276 could have contributed to the intolerable toxicity at doses >0.3 mg/kg.91 Indeed, the chosen tubulysin analogue payload (IC50 ~ low pM) is in the potency range of PBD payloads.113 None of the clinically approved ADCs for solid tumors (including 2 ADCs targeting the HER2 antigen) use payloads in this potency range – the most active of which is an ADC employing the less potent payload (EnhertuTM).22 Safety was noted as the reason for termination HER2 for the PBD-conjugated ADCs ADCT-50225 and DHES0815A.93,94
Figure 10.
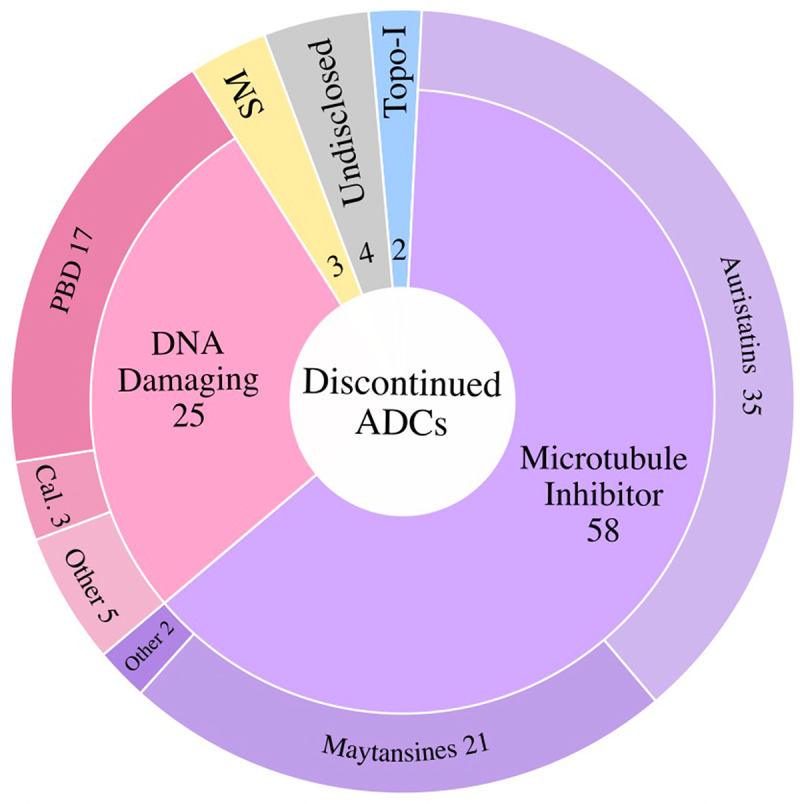
Discontinued ADCs Classified by Payload Class. The major payload classes utilized in the discontinued ADCs are the microtubule inhibitors and DNA Damaging Agents. Topoisomerase I Inhibitors (Topo-1), targeted small molecules (SM), and undisclosed candidates combined make up ~9% of the discontinued ADCs. PBD, pyrrolobenzodiazepine; Cal., calicheamicin.
ADCs targeting six tumor antigens of the approved ADCs (CD19, CD22, CD33, CD79b, HER2, and Trop-2) have also been discontinued, some due to intolerable toxicity. TrodelvyTM, the approved Trop-2 ADC using the lower potency topoisomerase I payload SN-38 (IC50 ~ nM), requires high biologic exposures to achieve the desired efficacy benefit (10 mg/kg on days 1 and 8 of a 21-day treatment cycle). Two ADCs targeting Trop-2 have been discontinued, most likely due to too potent payload selection pairing with a tumor antigen target requiring higher biologic exposures. PF-06664178, which uses a highly potent auristatin analog payload (IC50~ low pM),114 generated dose-limiting toxicities without any partial and/or complete responses in patients treated with doses up to 4.8 mg/kg every 3 weeks (doses ≥3.6 mg/kg deemed intolerable due to dose-limiting toxicities of rash, mucositis, and neutropenia).71 No clinical trial data have been published surrounding the highly potent maytansine payload ADC, BAT8003, although dose-limiting toxicities are suspected.
CD79b is targeted by the approved ADC PolivyTM. A follow-on site-specific CD79b-targeting ADC, iladatuzumab vedotin, was tested in combination with rituximab. Iladatuzumab vedotin was ultimately discontinued because no improvement in the therapeutic index (vs PolivyTM) was noted due to ocular toxicity at higher doses.60
Three ADCs targeting CD33, the target of MylotargTM, were also discontinued. AVE9633 (DM4 payload) showed no clinical activity below toxic doses;89 IMGN779 (indolino-benzodiazepine dimer payload) where efficacy was not reported;106 and vadastuximab talirine (PBD payload) that was discontinued following combination studies with hypomethylating agents citing safety concerns that included fatal infections.97 One CD33 targeting ADC with a tubulysin payload, DXC007, is currently in Phase 1 (Registration number CTR20221074), although safety and efficacy data have yet to be released.
Infusion-related adverse events were cited for the discontinuation of LOP628 (c-KIT)79 and losatuxizumab vedotin (EGFR).40 Additionally, poor tolerability and lack of objective responses of DCLL9718S (CLL-1) at doses tested did not justify its further development.99 In some discontinued ADCs, the clinical toxicity profile did not match preclinical observations, such as the CDH6 targeting ADC, HKT288, that showed neurological toxicity in patients not observed in preclinical models.84 Similarly, aprutumab ixadotin (FGFR2) had a clinical MTD below the therapeutic threshold estimated preclinically.68 These latter two examples highlight the need for better predictive models to guide ADC clinical development.
In addition to intolerable toxicity, insufficient efficacy is also a cause of ADC discontinuation. Factors contributing to insufficient efficacy include 1) low tumor target antigen densities and/or poor internalization properties of discontinued ADCs, 2) insufficient payload potency, 3) heterogenous DAR ADC products resulting in sub-optimal doses of payload, 4) off-tumor payload release and/or incomplete drug release in tumors, 5) rapid clearance of ADC due to poor PK properties, 6) failure to demonstrate efficacy superiority over standard of care, and 7) multidrug resistance mediated through elevated drug efflux transporters in tumors.
Of the discontinued ADC candidates where data is available, insufficient efficacy was a likely contributing factor in ~47% of the cases. Candidates that were reported to demonstrate insufficient efficacy to warrant further clinical testing include, but are not limited to, tamrintamab pamozirine (DPEP3),27 PF-06647263 (EFNA4),100 and PCA062 (P-Cadherin).81 It is possible that some of these ADC targets had heterogenous tumor expression and/or insufficient tumor antigen densities to induce efficient ADC internalization.
Utilization of payloads with insufficient potency, contributing to insufficient efficacy, was a possible contributing factor leading to discontinuation of the HER2-targeting immunomodulatory ADCs NJH395 and SBT6050. No objective responses were observed in 18 patients treated with NJH395 (TLR7 agonist payload).109 Likewise, only one of 14 patients achieved a partial response with SBT6050 (TLR8 agonist payload).110 For these TLR agonist ADCs, it is also possible that the lack of clinical activity is tied to suboptimal activation of an antitumor immune response. The clinical HER2 maytansinoid ADC BAT800190 was discontinued, possibly to advance a less potent topoisomerase I inhibitor payload ADC (BAT8010). This discontinuation/advancement decision is in line with the clinical experience of the two approved HER2 ADCs, KadcylaTM and EnhertuTM, where the ADC employing the lower potency payload (EnhertuTM) demonstrates greater clinical activity.115
ADCs with heterogenous DAR mixtures resulting in sub-optimal doses of payload was the likely cause of the lower efficacy observed with the nonspecific cysteine conjugate MUC16 ADC, sofituzumab vedotin,52 when compared to the specific cysteine (THIOMABTM) conjugate ADC, DMUC4064A.53 CMB-401 (MUC1) is an example of an ADC discontinued due to insufficient efficacy that may in part be due to poor linker choice leading to off-tumor payload release.102 It was suggested that the failure of this calicheamicin ADC to elicit a single partial remission was due to the utilization of the labile amid linker.102 MEDI4267 is an example of an ADC discontinued due to poor PK properties (and intolerable toxicity). It was noted that this HER2-targeted tubulysin ADC, at MTD, had a very short half-life and high clearance relative to the HER2-targeted ADC, KadcylaTM, at its MTD.91
Seven ADCs were discontinued due to failure to demonstrate superiority over standard chemotherapy comparator arms: rovalpituzumab tesirine (DLL3),28,29 depatuxizumab mafodotin (EGFRvIII),64,116,117 AMG 595 (EGFRvIII),80 AGS16F (ENPP3),65 glembatumumab vedotin (gpNMB),46 and lifastuzumab vedotin (NaPi-2b).54 Supplementing standard chemotherapy with lorvotuzumab mertansine (CD56) increased incidence of adverse events without enhancing efficacy.76,118
Clinical information regarding the remaining 22 of the 92 discontinued ADCs remains unpublished (AbGn-107, AGS67E, BAT8003, BIIB015, cantuzumab ravtansine, IMGN388, milatuzumab doxorubicin, laprituximab emtansine, lupartumab amadotin, MEDI2228, MEDI7247, PF-06688992, SAR428926, SBT6290, SC-005, SC-006, SGN-CD19B, SGN-CD48A, SGN-CD123A, SGN-CD352A, sirtratumab vedotin, and XMT-1592). Of these 22, companies cited portfolio prioritization/strategic considerations and lack of accrual for 48% and 2% of discontinuations, respectively, but no reason for discontinuation was given for the remaining 50%.
Implications for future ADC drug design
Development of the next generation of ADCs with a potential to improve their therapeutic index can be broken down into the three main components of the ADC (antibody, linker, payload) and the conjugation technology used to link the antibody to the payload. Also, consideration of the need to match the appropriate payload to a given tumor indication is required, while being mindful of the cancer antigen densities of the tumor targeting biologic.
Improvements to the biologic
Improvements in antibody design include binder selection and engineering to 1) select epitope(s)/affinities that promote maximal internalization, 2) optimize/lower affinity of binders for targets with higher expression on normal tissues of concern, and 3) fine tune the net charge of the ADC to mitigate target-independent toxicity.
Biologics targeting epitopes that promote rapid receptor-mediated internalization show greater activity than biologics targeting non-internalization antigen epitopes.12 Additionally, biparatopic and bispecific ADC biologics have been reported to improve ADC internalization, increasing the ADC effectiveness in tumors with lower target antigen densities.119,120 Biparatopic and bispecific ADCs currently in testing include REGN5093-M114 (c-MET, c-MET), zanidatamab zovodotin (HER2, HER2), IMGN151 (FRα FRα), BL-B01D1 (EGFR, HER3), M1231 (EGFR, MUC1), and ORM-5029 (HER2, HER3).
In addition to selecting internalizing epitopes and/or biparatope/bispecific antigen targeting, biologic affinity optimization of ADC biologics would need to be tailored for the antigen(s) of choice. Indeed, biologics with lower affinities may demonstrate insufficient binding and/or internalization at lower target antigen densities13 and biologics with too high cellular affinities may result in reduced receptor occupancy and/or internalization.14 Biologic affinity tuning may also help mitigate on target/off-tumor toxicities for antigens expressed in normal tissues of concern. Affinity de-tuning has been shown to lower target-dependent toxicity in normal tissues while maintaining activity on tumor cells with higher target antigen expression.13,121
Finally, optimizing the net charge of an ADC has been demonstrated to mitigate target-independent toxicity. An example of this is the reduction in ocular toxicity via introduction of a single Lys to Asp mutation into the biologic of the ADC, AGS-16C3F.122 These results suggest that creating a net negative surface charge on the ADC could dampen target-independent toxicity.
Improvements to the linker
Linkers are not mere inert bridges between an antibody and a payload; they influence the stability and PK of a given ADC. Poor performance of some early ADCs, like CMB-401, has been attributed to labile linkers.102 Improvements in ADC linkers have been shown to decrease systemic payload release and improve PK properties. Along these lines, improvements in linker development could include 1) payload masking linkers, 2) hydrophilic linkers, 3) branched linkers to increase the drug load, 4) tandem cleavage linkers, and 5) dual cleavage-specific linkers.
Modifying the linker to mask the hydrophobic payload can increase the therapeutic index.123 In general, reducing hydrophobicity of an ADC improves PK and therapeutic activity,23 at least in part due to reduced micropinocytosis-induced off-target toxicity.124 Indeed, incorporating hydrophilic macrocycles in the ADC to mask the hydrophobic payloads improved the in vivo activity of AdcetrisTM-like ADCs.125
Modifying the linker to increase drug load is another strategy to increase the effectiveness of ADCs that incorporate low potency payloads. One challenge in creating traditional cytotoxic ADCs with higher DAR loads is the increased hydrophobicity of the ADC molecule due to increased numbers of hydrophobic payloads that both increase the probability of aggregation126 and hasten clearance of the ADC from the organism.16,23 Creating polymer linkers, such as FleximerTM linkers127 or PEG chain additions, either between the antibody and the linker or branching from a location within a traditional linker,23 can increase the drug load on the ADC molecule without the associated liabilities of biologic degradation and/or clearance. Using such methods, the DAR can be increased without increasing the overall ADC hydrophobicity. Additionally, polypeptides composed of a pseudo-repeating pattern of hydrophilic neutral or negatively charged amino acids (Ala, Gly, Pro, Ser, Thr, Glu; XTENTM-peptide based platform) can yield ADCs with DARs as high as 18 without compromising PK.128 Increasing linker hydrophilicity can alter the toxicity profile of the ADC by modulating the bystander effect through reduced expulsion of the payload metabolites by the MDR1 pump.129 However, this approach may not work for all ADCs.130
Lastly, modifying cleavable linkers to minimize systemic release while still maintaining tumor bystander effect could improve the therapeutic index of follow-on ADC molecules. Engineering linkers requiring successive cleavage by enzymes only found inside lysosomes could achieve this property. Such an example was described for a glucuronidase-cleavable linker that when cleaved uncovered a cathepsin cleavage site that enabled payload release – ensuring that both cleavage steps only occurred inside of lysosomes.131 Such tandem cleavage linkers were found to improve both the stability as well as tolerability of an ADC in a rat toxicity model.131
Improvements to the payload
Modifications to the payloads that could improve the therapeutic benefit of follow-on ADCs include the creation of 1) prodrug-based payloads to mitigate off-tumor toxicity, 2) creation of hydrophilic cytotoxic payloads, and 3) the creation of bifunctional payloads to increase tumor efficacy. Prodrug payloads exploit the acidic, hypoxic, hyper-sialylated, and protease-rich TME to trigger active payload release in tumors.132 Prodrugs can involve masking toxic, hydrophobic payloads such as PBDs by “capping”. The prodrug cap is designed to be cleaved by the TME enzymes, such as beta-glucuronidases, to minimize off-tumor payload release.133 The identification of additional endosomal trafficking modulators and lysosomal pathway regulators for payload release could aid in design of the next generation of prodrug payloads.134
The creation of hydrophilic cytotoxic payloads is another potential advancement to develop ADCs with elevated DARs that retain biologic integrity with good PK attributes. An example of this is the hydrophilic payload auristatin β-D-glucuronide MMAU.135 This glycoside-payload had the added benefit of being relatively inert in its unconjugated, free form. Lysosomal enzymatic processing to a deglycosylated state activates the payload’s cytotoxic and bystander activity.
The potency of an ADC can also be enhanced with the creation of dual payloads to increase tumor efficacy. Conjugation to two or more different payloads to a given biologic has been shown to have greater antitumor activity over that of a mixture of ADCs carrying the individual payloads. Preclinical studies exploring dual payload ADCs include the two different microtubule inhibitor payloads, MMAE and MMAF,136 as well as a microtubule inhibitor payload coupled with a DNA damaging agent such as MMAE and PBD,137 or MMAF and PNU-159682.138 All of these dual payload ADCs have been shown to increase the antitumor activity over that of a mixture of mono payload ADCs. Additionally, tolerability of these dual payload ADCs in healthy mice was found to be similar to the mono payload ADCs as measured by body weight loss and liver clinical chemistries.139
Improvements in payload conjugation
Site-specific attachment of payload yields ADC preparations with controlled and defined DAR. The first method to produce such ADCs via cysteine amino acid engineering gave homogeneous preparations demonstrating superior preclinical PK properties and safety profiles compared to randomly conjugated ADCs.18 These findings triggered enthusiasm in the field and led to the development of additional methods for site-specific conjugation. To date, site-selective conjugation methods fall into eight categories: cysteine engineering, non-natural amino acid engineering, conjugation to native cysteines, peptide tags, glycan modification, enzymatic modification, disulfide rebridging, and conjugation to native lysines.140 No method used to date for site-specific conjugation has been shown to have a direct effect on FcRn recycling that can alter ADC PK, efficacy, and safety.
Linker-payload conjugation via non-natural amino acid methods is currently being explored. However, it has been noted that the position of non-natural amino acid conjugation for linker-payload attachment caused a marked effect on tumor killing, although the stability and PK were equivalent.141
Examples of site-specific conjugation using peptide tag technology are the SMARTagTM and Glutamine Tag. SMARTagTM achieves site-specific conjugation with the use of an aldehyde tag attaching the linker-payload to formylglycine.142 Glutamine Tag technology utilizes transglutaminase to attach the linker-payload.143 Both technologies were shown to improve PK and efficacy.142,143
GlycoConnectTM is an example of a site-specific glycan modification conjugation method. Here, site-specific conjugation is achieved with attachment of the linker-payload following glycan remodeling of the antibody at the Asparagine-297 site.144 However, since Asparagine-297 glycans are important for antibody Fcγ-receptor effector functions, this method needs to be balanced against the loss of Fc effector function that could otherwise provide an efficacy benefit to the developed ADC.145
A notable advance in site-specific technology is the AJICAPTM method that utilizes native lysines for site-specific linker-payload attachment. This method does not require antibody engineering or enzymatic reactions. ADCs so produced were shown to have an improved therapeutic index in preclinical models.17
Clinically, the site-specific ADC DMUC4064A (MUC16) could be administered at higher biologic doses with higher overall response rates53 than the nonspecific, cysteine-conjugated counterpart sofituzumab vedotin (MUC16).52 While promising, site-specific payload conjugation has not always resulted in therapeutic improvement. For example, the site-specific conjugated ADCs iladatuzumab vedotin (CD79b) and SC-002 (DLL3) did not demonstrate an improvement in clinical responses/therapeutic index over that of the nonspecific cysteine-conjugated ADCs PolivyTM60 and rovalpituzumab tesirine.29,92
Concluding remarks
Of the 267 ADCs tested for oncology indications, 11 have gained FDA approval; 92 have been discontinued. Analyses of the limitations associated with the discontinued drug candidates can help inform the design and selection of the next series of molecules. Importantly, new biologic engineering modifications have been shown preclinically to improve the therapeutic index. Taking an integrated, multifactorial approach of careful target selection with simultaneous optimization of the antibody, linker, and payload – matched to the indications of interest – will hopefully usher in the next wave of new ADC approvals.
Acknowledgments
The authors wish to thank Beacon Intelligence for providing access to the Beacon Targeted Therapies Clinical Trials and Pipeline Database. The authors would also like to thank Allison Bruce for technical assistance with figure generation.
Funding Statement
No funding was associated with the work of this article.
Abbreviations
- ADC
Antibody-Drug Conjugate
- ALA
Alanine
- AML
Acute Myeloid Leukemia
- Asp
Aspartic acid
- DAR
Drug-to-Antibody Ratio
- FcRn
neonatal Fc receptor
- FDA
Food and Drug Administration
- Glu
Glutamic acid
- Gly
Glycine
- IC50
half-maximal inhibitory concentration
- Lys
lysine
- M
Molar
- MDR
Multi-Drug Resistance
- MED
minimum effective dose
- mg/kg
milligrams per kilogram
- MMAE
Monomethyl auristatin E
- MMAF
Monomethyl auristatin F
- mPFS
Medium Progression Free Survival
- MTD
Maximum Tolerated Dose
- nM
Nanomolar
- PBD
Pyrrolobenzodiazepine
- PK
Pharmacokinetic
- pM
Picomolar
- Pro
Proline
- Ser
Serine
- SM
Targeted Small Molecules
- STING
Stimulator of Interferon Genes
- Thr
Threonine
- TLR
Toll-Like Receptor
- TME
Tumor Microenvironment
- US
United States
Disclosure statement
All authors are employees of Aarvik Therapeutics, Inc. and may have stock and/or stock options or interests in Aarvik Therapeutics, Inc.
References
- 1.Tolcher AW, Carneiro BA, Dowlati A, Razak AR, Chae YK, Villella JA, Coppola S, Englert S, Phillips AC, Souers AJ, et al. A first-in-human study of mirzotamab clezutoclax as monotherapy and in combination with taxane therapy in relapsed/refractory solid tumors: dose escalation results. J Clin Oncol. 2021;39:3015–43. doi: 10.1200/JCO.2021.39.15_suppl.3015. [DOI] [Google Scholar]
- 2.Sharma M, Carvajal RD, Hanna GJ, Li BT, Moore KN, Pegram MD, Rasco DW, Spira AI, Alonso M, Fang L, et al. Preliminary results from a phase 1/2 study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. J Clin Oncol. 2021;39(15_suppl):2549–2549. doi: 10.1200/JCO.2021.39.15_suppl.2549. [DOI] [Google Scholar]
- 3.Sharma S, Li Z, Bussing D, Shah DK.. Evaluation of quantitative relationship between target expression and antibody-drug conjugate exposure inside cancer cells. Drug Metab Dispos. 2020;48:368–77. doi: 10.1124/dmd.119.089276. PMID: 32086295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammood M, Craig AW, Leyton JV. Impact of endocytosis mechanisms for the receptors targeted by the currently approved Antibody-Drug Conjugates (ADCs)-A necessity for future ADC research and development. Pharmaceuticals (Basel). 2021;14 doi: 10.3390/ph14070674. PMID: 34358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li JY, Perry SR, Muniz-Medina V, Wang X, Wetzel LK, Rebelatto MC, Hinrichs MJ, Bezabeh BZ, Fleming RL, Dimasi N, et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell. 2016;29:117–29. doi: 10.1016/j.ccell.2015.12.008. PMID: 26766593. [DOI] [PubMed] [Google Scholar]
- 6.Liang K, Mei S, Gao X, Peng S, Zhan J. Dynamics of endocytosis and degradation of antibody-drug conjugate T-DM1 in HER2 positive cancer cells. Drug Des Devel Ther. 2021;15:5135–50. PMID: 34992350. doi: 10.2147/DDDT.S344052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, et al. DS-8201a, A Novel HER2-targeting ADC with a Novel DNA topoisomerase i inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–108. doi: 10.1158/1078-0432.CCR-15-2822. PMID: 27026201. [DOI] [PubMed] [Google Scholar]
- 8.Von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A, Redondo A, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–28. doi: 10.1056/NEJMoa1814017. PMID: 30516102. [DOI] [PubMed] [Google Scholar]
- 9.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–21. doi: 10.1056/NEJMoa1914510. PMID: 31825192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson MG, Zhang Q, Rodriguez LE, Hecquet CM, Donawho CK, Ansell PJ, Reilly EB. ABBV-176, a PRLR antibody drug conjugate with a potent DNA-damaging PBD cytotoxin and enhanced activity with PARP inhibition. BMC Cancer. 2021;21(1):681.doi: 10.1186/s12885-021-08403-5. PMID: 34107902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemech C, Woodward N, Chan N, Mortimer J, Naumovski L, Nuthalapati S, Tong B, Jiang F, Ansell P, Ratajczak CK, et al. A first-in-human, phase 1, dose-escalation study of ABBV-176, an antibody-drug conjugate targeting the prolactin receptor, in patients with advanced solid tumors. Invest New Drugs. 2020;38(6):1815–25. doi: 10.1007/s10637-020-00960-z. PMID: 32524319. [DOI] [PubMed] [Google Scholar]
- 12.Du X, Beers R, Fitzgerald DJ, Pastan I. Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68(15):6300–05.doi: 10.1158/0008-5472.CAN-08-0461. PMID: 18676854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong OK, Tran -T-T, Ho W-H, Casas MG, Au M, Bateman M, Lindquist KC, Rajpal A, Shelton DL, Strop P, et al. RN765C, a low affinity EGFR antibody drug conjugate with potent anti-tumor activity in preclinical solid tumor models. Oncotarget. 2018;9(71):33446–58. doi: 10.18632/oncotarget.26002. PMID: 30323890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Clark S, Wong T, Chen Y, Chen Y, Dennis MS, Luis E, Zhong F, Bheddah S, Koeppen H, et al. Armed antibodies targeting the mucin repeats of the ovarian cancer antigen, MUC16, are highly efficacious in animal tumor models. Cancer Res. 2007;67(10):4924–32. doi: 10.1158/0008-5472.CAN-06-4512. PMID: 17510422. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Amphlett G, Blattler WA, Lambert JM, Zhang W. Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry. Protein Sci. 2005;14(9):2436–46. doi: 10.1110/ps.051478705.PMID: 16081651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–70. doi: 10.1158/1078-0432.CCR-04-0789. PMID: 15501986. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda Y, Seki T, Yamada K, Ooba Y, Takahashi K, Fujii T, Kawaguchi S, Narita T, Nakayama A, Kitahara Y, et al. Chemical site-specific conjugation platform to improve the pharmacokinetics and therapeutic index of Antibody–Drug conjugates. Mol Pharm. 2021;18(11):4058–66. doi: 10.1021/acs.molpharmaceut.1c00473. PMID: 34579528. [DOI] [PubMed] [Google Scholar]
- 18.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–32. doi: 10.1038/nbt.1480. PMID: 18641636. [DOI] [PubMed] [Google Scholar]
- 19.Wynn CP, Patel R, Hillegass WB, Tang ,S-C. Increased systemic toxicities from antibody-drug conjugates (ADCs) with cleavable versus non-cleavable linkers: a meta-analysis of commercially available ADCs. Journal of Clinical Oncology. 2022;40(16_suppl):3032–3032. doi: 10.1200/JCO.2022.40.16_suppl.3032.35820082 [DOI] [Google Scholar]
- 20.Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, Leece BA, Chittenden T, Blattler WA, Goldmacher VS. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66(6):3214–21. doi: 10.1158/0008-5472.CAN-05-3973. PMID: 16540673. [DOI] [PubMed] [Google Scholar]
- 21.Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS −8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46. doi: 10.1111/cas.12966. PMID: 27166974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurvitz SA, Hegg R, Chung W-P, Im S-A, Jacot W, Ganju V, Chiu JWY, Xu B, Hamilton E, Madhusudan S, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–17. doi: 10.1016/S0140-6736(22)02420-5. PMID: 36495879. [DOI] [PubMed] [Google Scholar]
- 23.Lyon RP, Bovee TD, Doronina SO, Burke PJ, Hunter JH, Neff-LaFord HD, Jonas M, Anderson ME, Setter JR, Senter PD. Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nat Biotechnol. 2015;33(7):733–35. doi: 10.1038/nbt.3212. PMID: 26076429. [DOI] [PubMed] [Google Scholar]
- 24.Ross HJ, Hart LL, Swanson PM, Rarick MU, Figlin RA, Jacobs AD, McCune DE, Rosenberg AH, Baron AD, Grove LE, et al. A randomized, multicenter study to determine the safety and efficacy of the immunoconjugate SGN-15 plus docetaxel for the treatment of non-small cell lung carcinoma. Lung Cancer. 2006;54(1):69–77. doi: 10.1016/j.lungcan.2006.05.020. PMID: 16934909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.https://clinicaltrials.gov. [accessed 2023 Jan 1]
- 26.Conilh L, Sadilkova L, Viricel W, Dumontet C. Payload diversification: a key step in the development of antibody-drug conjugates. J Hematol Oncol. 2023;16:3. doi: 10.1186/s13045-022-01397-y. PMID: 36650546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton E, O’Malley DM, O’Cearbhaill R, Cristea M, Fleming GF, Tariq B, Fong A, French D, Rossi M, Brickman D, et al. Tamrintamab pamozirine (SC-003) in patients with platinum-resistant/refractory ovarian cancer: findings of a phase 1 study. Gynecol Oncol. 2020;158:640–45. doi: 10.1016/j.ygyno.2020.05.038. PMID: 32513564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson ML, Zvirbule Z, Laktionov K, Helland A, Cho BC, Gutierrez V, Colinet B, Lena H, Wolf M, Gottfried M, et al. Rovalpituzumab tesirine as a maintenance therapy after first-line platinum-based chemotherapy in patients with extensive-stage-SCLC: results from the phase 3 MERU study. J Thorac Oncol. 2021;16:1570–81. doi: 10.1016/j.jtho.2021.03.012. PMID: 33823285. [DOI] [PubMed] [Google Scholar]
- 29.Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, Majem M, Nackaerts K, Syrigos K, Hansen K, et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J Thorac Oncol. 2021;16:1547–58. doi: 10.1016/j.jtho.2021.02.009. PMID: 33607312. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton E, Fleming GF, Thaker PH, Subbiah S, Chen C, Fong A, Brickman D, Moore K. First-in-human study of SC-004, an antibody-drug conjugate targeting CLDN6/9, in patients with epithelial ovarian cancers. Cancer Res. 2020:80. doi: 10.1158/1538-7445.AM2020-CT124. [DOI] [Google Scholar]
- 31.De Bono JS, Fleming MT, Wang JS, Cathomas R, Miralles MS, Bothos J, Hinrichs MJ, Zhang Q, He P, Williams M, et al. Phase I study of MEDI3726: a prostate-specific membrane antigen-targeted antibody-drug conjugate, in patients with mCRPC after failure of abiraterone or enzalutamide. Clin Cancer Res. 2021;27:3602–09. doi: 10.1158/1078-0432.CCR-20-4528. PMID: 33795255. [DOI] [PubMed] [Google Scholar]
- 32.Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, Oflazoglu E, Toki BE, Sanderson RJ, Zabinski RF, et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem. 2006;17:114–24. doi: 10.1021/bc0502917. PMID: 16417259. [DOI] [PubMed] [Google Scholar]
- 33.Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res. 2001;7:2182–94. https://www.ncbi.nlm.nih.gov/pubmed/11489791. PMID: 11489791. [PubMed] [Google Scholar]
- 34.Yu SF, Zheng B, Go M, Lau J, Spencer S, Raab H, Soriano R, Jhunjhunwala S, Cohen R, Caruso M, et al. A Novel anti-CD22 anthracycline-based Antibody-Drug Conjugate (ADC) that overcomes resistance to auristatin-based ADCs. Clin Cancer Res. 2015;21:3298–306. doi: 10.1158/1078-0432.CCR-14-2035. PMID: 25840969. [DOI] [PubMed] [Google Scholar]
- 35.Takegawa N, Nonagase Y, Yonesaka K, Sakai K, Maenishi O, Ogitani Y, Tamura T, Nishio K, Nakagawa K, Tsurutani J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer. 2017;141:1682–89. doi: 10.1002/ijc.30870. PMID: 28677116. [DOI] [PubMed] [Google Scholar]
- 36.Hull EA, Livanos M, Miranda E, Smith ME, Chester KA, Baker JR. Homogeneous bispecifics by disulfide bridging. Bioconjug Chem. 2014;25:1395–401. doi: 10.1021/bc5002467. PMID: 25033024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jen EY, Ko CW, Lee JE, Del Valle PL, Aydanian A, Jewell C, Norsworthy KJ, Przepiorka D, Nie L, Liu J, et al. FDA approval: gemtuzumab ozogamicin for the treatment of adults with newly diagnosed CD33-positive acute myeloid leukemia. Clin Cancer Res. 2018;24:3242–46. doi: 10.1158/1078-0432.CCR-17-3179. PMID: 29476018. [DOI] [PubMed] [Google Scholar]
- 38.https://www.gsk.com/en-gb/media/press-releases/gsk-provides-update-on-blenrep-us-marketing-authorisation/.
- 39.Goldenberg DM, Sharkey RM. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: a case study of anti-TROP-2 sacituzumab govitecan. MAbs. 2019;11:987–95. doi: 10.1080/19420862.2019.1632115. PMID: 31208270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleary JM, Calvo E, Moreno V, Juric D, Shapiro GI, Vanderwal CA, Hu B, Gifford M, Barch D, Roberts-Rapp L, et al. A phase 1 study evaluating safety and pharmacokinetics of losatuxizumab vedotin (ABBV-221), an anti-EGFR antibody-drug conjugate carrying monomethyl auristatin E, in patients with solid tumors likely to overexpress EGFR. Invest New Drugs. 2020;38:1483–94. doi: 10.1007/s10637-020-00908-3. PMID: 32189093. [DOI] [PubMed] [Google Scholar]
- 41.Sandhu S, McNeil CM, LoRusso P, Patel MR, Kabbarah O, Li C, Sanabria S, Flanagan WM, Yeh R-F, Brunstein F, et al. Phase I study of the anti-endothelin B receptor antibody-drug conjugate DEDN6526A in patients with metastatic or unresectable cutaneous, mucosal, or uveal melanoma. Invest New Drugs. 2020;38(3):844–54. doi: 10.1007/s10637-019-00832-1. PMID: 31385109. [DOI] [PubMed] [Google Scholar]
- 42.Almhanna K, Wright D, Mercade TM, Van Laethem J-L, Gracian AC, Guillen-Ponce C, Faris J, Lopez CM, Hubner RA, Bendell J, et al. A phase II study of antibody-drug conjugate, TAK-264 (MLN0264) in previously treated patients with advanced or metastatic pancreatic adenocarcinoma expressing guanylyl cyclase C. Invest New Drugs. 2017;35(5):634–41. doi: 10.1007/s10637-017-0473-9. PMID: 28527133. [DOI] [PubMed] [Google Scholar]
- 43.Almhanna K, Miron ML, Wright D, Gracian AC, Hubner RA, Van Laethem J-L, Lopez CM, Alsina M, Munoz FL, Bendell J, et al. Phase II study of the antibody-drug conjugate TAK-264 (MLN0264) in patients with metastatic or recurrent adenocarcinoma of the stomach or gastroesophageal junction expressing guanylyl cyclase C. Invest New Drugs. 2017;35(2):235–41. doi: 10.1007/s10637-017-0439-y. PMID: 28188407. [DOI] [PubMed] [Google Scholar]
- 44.Hasanov M, Rioth MJ, Kendra K, Hernandez-Aya L, Joseph RW, Williamson S, Chandra S, Shirai K, Turner CD, Lewis K, et al. A phase II study of glembatumumab vedotin for metastatic uveal melanoma. Cancers (Basel). 2020;12(8):2270. doi: 10.3390/cancers12082270. PMID: 32823698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ott PA, Pavlick AC, Johnson DB, Hart LL, Infante JR, Luke JJ, Lutzky J, Rothschild NE, Spitler LE, Cowey CL, et al. A phase 2 study of glembatumumab vedotin, an antibody-drug conjugate targeting glycoprotein NMB, in patients with advanced melanoma. Cancer. 2019;125(7):1113–23. doi: 10.1002/cncr.31892. PMID: 30690710. [DOI] [PubMed] [Google Scholar]
- 46.Vahdat LT, Schmid P, Forero-Torres A, Blackwell K, Telli ML, Melisko M, Mobus V, Cortes J, Montero AJ, Ma C, et al. Glembatumumab vedotin for patients with metastatic, gpNMB overexpressing, triple-negative breast cancer (“METRIC”): a randomized multicenter study. NPJ Breast Cancer. 2021;7:57. doi: 10.1038/s41523-021-00244-6. PMID: 34016993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yardley DA, Weaver R, Melisko ME, Saleh MN, Arena FP, Forero A, Cigler T, Stopeck A, Citrin D, Oliff I, et al. EMERGE: a randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein NMB–expressing breast cancer. J Clin Oncol. 2015;33(14):1609–19. doi: 10.1200/JCO.2014.56.2959. PMID: 25847941. [DOI] [PubMed] [Google Scholar]
- 48.Kopp LM, Malempati S, Krailo M, Gao Y, Buxton A, Weigel BJ, Hawthorne T, Crowley E, Moscow JA, Reid JM, et al. Phase II trial of the glycoprotein non-metastatic B-targeted antibody–drug conjugate, glembatumumab vedotin (CDX-011), in recurrent osteosarcoma AOST1521: a report from the Children’s oncology group. Eur J Cancer. 2019;121:177–83. doi: 10.1016/j.ejca.2019.08.015. PMID: 31586757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demetri GD, Luke JJ, Hollebecque A, Powderly JD 2nd, Spira AI, Subbiah V, Naumovski L, Chen C, Fang H, Lai DW, et al. First-in-human phase I Study of ABBV-085, an Antibody–Drug conjugate targeting LRRC15, in sarcomas and other advanced solid tumors. Clin Cancer Res. 2021;27(13):3556–66. doi: 10.1158/1078-0432.CCR-20-4513. PMID: 33820780. [DOI] [PubMed] [Google Scholar]
- 50.Tolaney SM, Do KT, Eder JP, LoRusso PM, Weekes CD, Chandarlapaty S, Chang C-W, Chen S-C, Nazzal D, Schuth E, et al. A phase I study of DLYE5953A, an anti-LY6E antibody covalently linked to monomethyl auristatin E, in patients with refractory solid tumors. Clin Cancer Res. 2020;26(21):5588–97. doi: 10.1158/1078-0432.CCR-20-1067. PMID: 32694157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weekes CD, Lamberts LE, Borad MJ, Voortman J, McWilliams RR, Diamond JR, De Vries EG, Verheul HM, Lieu CH, Kim GP, et al. Phase I study of DMOT4039A, an Antibody–Drug conjugate targeting mesothelin, in patients with unresectable pancreatic or platinum-resistant ovarian cancer. Mol Cancer Ther. 2016;15(3):439–47. doi: 10.1158/1535-7163.MCT-15-0693. PMID: 26823490. [DOI] [PubMed] [Google Scholar]
- 52.Liu JF, Moore KN, Birrer MJ, Berlin S, Matulonis UA, Infante JR, Wolpin B, Poon KA, Firestein R, Xu J, et al. Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann Oncol. 2016;27:2124–30. doi: 10.1093/annonc/mdw401. PMID: 27793850. [DOI] [PubMed] [Google Scholar]
- 53.Liu J, Burris H, Wang JS, Barroilhet L, Gutierrez M, Wang Y, Vaze A, Commerford R, Royer-Joo S, Choeurng V, et al. An open-label phase I dose-escalation study of the safety and pharmacokinetics of DMUC4064A in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2021;163:473–80. doi: 10.1016/j.ygyno.2021.09.023. PMID: 34627611. [DOI] [PubMed] [Google Scholar]
- 54.Banerjee S, Oza AM, Birrer MJ, Hamilton EP, Hasan J, Leary A, Moore KN, Mackowiak-Matejczyk B, Pikiel J, Ray-Coquard I, et al. Anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer in a randomized, open-label, phase II study. Ann Oncol. 2018;29:917–23. doi: 10.1093/annonc/mdy023. PMID: 29401246. [DOI] [PubMed] [Google Scholar]
- 55.Coveler AL, Ko AH, Catenacci DV, Von Hoff D, Becerra C, Whiting NC, Yang J, Wolpin B. A phase 1 clinical trial of ASG-5ME, a novel drug-antibody conjugate targeting SLC44A4, in patients with advanced pancreatic and gastric cancers. Invest New Drugs. 2016;34(3):319–28.doi: 10.1007/s10637-016-0343-x. PMID: 26994014. [DOI] [PubMed] [Google Scholar]
- 56.McHugh D, Eisenberger M, Heath EI, Bruce J, Danila DC, Rathkopf DE, Feldman J, Slovin SF, Anand B, Chu R, et al. A phase I study of the antibody drug conjugate ASG-5ME, an SLC44A4-targeting antibody carrying auristatin E, in metastatic castration-resistant prostate cancer. Invest New Drugs. 2019;37(5):1052–60. doi: 10.1007/s10637-019-00731-5. PMID: 30725389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Danila DC, Szmulewitz RZ, Vaishampayan U, Higano CS, Baron AD, Gilbert HN, Brunstein F, Milojic-Blair M, Wang B, Kabbarah O, et al. Phase I study of DSTP3086S, an antibody-drug conjugate targeting six-transmembrane epithelial antigen of prostate 1, in metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37(36):3518–27. doi: 10.1200/JCO.19.00646. PMID: 31689155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGregor BA, Gordon M, Flippot R, Agarwal N, George S, Quinn DI, Rogalski M, Hawthorne T, Keler T, Choueiri TK. Safety and efficacy of CDX-014, an antibody-drug conjugate directed against T cell immunoglobulin mucin-1 in advanced renal cell carcinoma. Invest New Drugs. 2020;38(6):1807–14.doi: 10.1007/s10637-020-00945-y. PMID: 32472319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morschhauser F, Flinn IW, Advani R, Sehn LH, Diefenbach C, Kolibaba K, Press OW, Salles G, Tilly H, Chen AI, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol. 2019;6(5):e254–e265. doi: 10.1016/S2352-3026(19)30026-2. PMID: 30935953. [DOI] [PubMed] [Google Scholar]
- 60.Herrera AF, Patel MR, Burke JM, Advani R, Cheson BD, Sharman JP, Penuel E, Polson AG, Liao CD, Li C, et al. Anti-CD79B antibody-drug conjugate DCDS0780A in patients with B-cell non-Hodgkin lymphoma: phase 1 dose-escalation study. Clin Cancer Res. 2022;28:1294–301. doi: 10.1158/1078-0432.CCR-21-3261. PMID: 34980599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart AK, Krishnan AY, Singhal S, Boccia RV, Patel MR, Niesvizky R, Chanan-Khan AA, Ailawadhi S, Brumm J, Mundt KE, et al. Phase I study of the anti-FcRH5 antibody-drug conjugate DFRF4539A in relapsed or refractory multiple myeloma. Blood Cancer J. 2019;9(2):17. doi: 10.1038/s41408-019-0178-8. PMID: 30718503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vij R, Nath R, Afar DEH, Mateos M-V, Berdeja JG, Raab MS, Guenther A, Martinez-Lopez J, Jakubowiak AJ, Leleu X, et al. First-in-human phase I study of ABBV-838, an Antibody–Drug conjugate targeting SLAMF7/CS1 in patients with relapsed and refractory multiple myeloma. Clin Cancer Res. 2020;26(10):2308–17. doi: 10.1158/1078-0432.CCR-19-1431. PMID: 31969330. [DOI] [PubMed] [Google Scholar]
- 63.Shapiro GI, Vaishampayan UN, LoRusso P, Barton J, Hua S, Reich SD, Shazer R, Taylor CT, Xuan D, Borghaei H. First-in-human trial of an anti-5T4 antibody-monomethylauristatin conjugate, PF-06263507, in patients with advanced solid tumors. Invest New Drugs. 2017;35(3):315–23.doi: 10.1007/s10637-016-0419-7. PMID: 28070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lassman AB, Pugh SL, Wang TJC, Aldape K, Gan HK, Preusser M, Vogelbaum MA, Sulman EP, Won M, Zhang P, et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: a phase III randomized clinical trial. Neuro Oncol. 2023;25:339–50. doi: 10.1093/neuonc/noac173. PMID: 35849035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kollmannsberger C, Choueiri TK, Heng DYC, George S, Jie F, Croitoru R, Poondru S, Thompson JA. A randomized phase II study of AGS-16C3F versus axitinib in previously treated patients with metastatic renal cell carcinoma. Oncologist. 2021;26:182–e361. doi: 10.1002/onco.13628. PMID: 33289953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Annunziata CM, Kohn EC, LoRusso P, Houston ND, Coleman RL, Buzoianu M, Robbie G, Lechleider R. Phase 1, open-label study of MEDI-547 in patients with relapsed or refractory solid tumors. Invest New Drugs. 2013;31:77–84. PMID: 22370972. doi: 10.1007/s10637-012-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tannir NM, Forero-Torres A, Ramchandren R, Pal SK, Ansell SM, Infante JR, de Vos S, Hamlin PA, Kim SK, Whiting NC, et al. Phase I dose-escalation study of SGN-75 in patients with CD70-positive relapsed/refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma. Invest New Drugs. 2014;32(6):1246–57. doi: 10.1007/s10637-014-0151-0. PMID: 25142258. [DOI] [PubMed] [Google Scholar]
- 68.Kim SB, Meric-Bernstam F, Kalyan A, Babich A, Liu R, Tanigawa T, Sommer A, Osada M, Reetz F, Laurent D, et al. First-in-human phase I study of aprutumab ixadotin, a fibroblast growth factor receptor 2 antibody-drug conjugate (BAY 1187982) in patients with advanced cancer. Target Oncol. 2019;14:591–601. doi: 10.1007/s11523-019-00670-4. PMID: 31502117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamilton EP, Barve MA, Bardia A, Beeram M, Bendell JC, Mosher R, Hailman E, Bergstrom DA, Burris HA, Soliman HH. Phase 1 dose escalation of XMT-1522, a novel HER2-targeting antibody-drug conjugate (ADC), in patients (pts) with HER2-expressing breast, lung and gastric tumors. J Clin Oncol. 2018;36(15_suppl):2546. doi: 10.1200/JCO.2018.36.15_suppl.2546. [DOI] [Google Scholar]
- 70.Rosen LS, Wesolowski R, Baffa R, Liao K-H, Hua SY, Gibson BL, Pirie-Shepherd S, Tolcher AW. A phase I, dose-escalation study of PF-06650808, an anti-Notch3 antibody–drug conjugate, in patients with breast cancer and other advanced solid tumors. Invest New Drugs. 2020;38(1):120–30.doi: 10.1007/s10637-019-00754-y. PMID: 30887250. [DOI] [PubMed] [Google Scholar]
- 71.King GT, Eaton KD, Beagle BR, Zopf CJ, Wong GY, Krupka HI, Hua SY, Messersmith WA, El-Khoueiry AB. A phase 1, dose-escalation study of PF-06664178, an anti-Trop-2/Aur0101 antibody-drug conjugate in patients with advanced or metastatic solid tumors. Invest New Drugs. 2018;36:836–47. doi: 10.1007/s10637-018-0560-6. PMID: 29333575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tolcher AW, Ochoa L, Hammond LA, Patnaik A, Edwards T, Takimoto C, Smith L, de Bono J, Schwartz G, Mays T, et al. Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. J Clin Oncol. 2003;21(2):211–22. doi: 10.1200/JCO.2003.05.137. PMID: 12525512. [DOI] [PubMed] [Google Scholar]
- 73.Helft PR, Schilsky RL, Hoke FJ, Williams D, Kindler HL, Sprague E, DeWitte M, Martino HK, Erickson J, Pandite L, et al. A phase I study of cantuzumab mertansine administered as a single intravenous infusion once weekly in patients with advanced solid tumors. Clin Cancer Res. 2004;10(13):4363–68. doi: 10.1158/1078-0432.CCR-04-0088. PMID: 15240523. [DOI] [PubMed] [Google Scholar]
- 74.Riechelmann H, Sauter A, Golze W, Hanft G, Schroen C, Hoermann K, Erhardt T, Gronau S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:823–29. doi: 10.1016/j.oraloncology.2007.10.009. PMID: 18203652. [DOI] [PubMed] [Google Scholar]
- 75.Tijink BM, Buter J, de Bree R, Giaccone G, Lang MS, Staab A, Leemans CR, van Dongen GA. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res. 2006;12(20):6064–72.doi: 10.1158/1078-0432.CCR-06-0910. PMID: 17062682. [DOI] [PubMed] [Google Scholar]
- 76.Socinski MA, Kaye FJ, Spigel DR, Kudrik FJ, Ponce S, Ellis PM, Majem M, Lorigan P, Gandhi L, Gutierrez ME, et al. Phase 1/2 study of the CD56-targeting antibody-drug conjugate lorvotuzumab mertansine (IMGN901) in combination with carboplatin/etoposide in small-cell lung cancer patients with extensive-stage disease. Clin Lung Cancer. 2017;18:68–76 e62. doi: 10.1016/j.cllc.2016.09.002. PMID: 28341109. [DOI] [PubMed] [Google Scholar]
- 77.Geller JI, Pressey JG, Smith MA, Kudgus RA, Cajaiba M, Reid JM, Hall D, Barkauskas DA, Voss SD, Cho SY, et al. ADVL1522: a phase 2 study of lorvotuzumab mertansine (IMGN901) in children with relapsed or refractory Wilms tumor, rhabdomyosarcoma, neuroblastoma, pleuropulmonary blastoma, malignant peripheral nerve sheath tumor, or synovial sarcoma-A Children’s Oncology Group study. Cancer. 2020;126(24):5303–10. doi: 10.1002/cncr.33195. PMID: 32914879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Massard C, Soria J-C, Krauss J, Gordon M, Lockhart AC, Rasmussen E, Upreti VV, Patel S, Ngarmchamnanrith G, Henary H. First-in-human study to assess safety, tolerability, pharmacokinetics, and pharmacodynamics of the anti-CD27L antibody-drug conjugate AMG 172 in patients with relapsed/refractory renal cell carcinoma. Cancer Chemother Pharmacol. 2019;83(6):1057–63.doi: 10.1007/s00280-019-03796-4. PMID: 30915497. [DOI] [PubMed] [Google Scholar]
- 79.L’Italien L, Orozco O, Abrams T, Cantagallo L, Connor A, Desai J, Ebersbach H, Gelderblom H, Hoffmaster K, Lees E, et al. Mechanistic insights of an immunological adverse event induced by an anti-KIT antibody drug conjugate and mitigation strategies. Clin Cancer Res. 2018;24:3465–74. doi: 10.1158/1078-0432.CCR-17-3786. PMID: 29615457. [DOI] [PubMed] [Google Scholar]
- 80.Rosenthal M, Curry R, Reardon DA, Rasmussen E, Upreti VV, Damore MA, Henary HA, Hill JS, Cloughesy T. Safety, tolerability, and pharmacokinetics of anti-EGFRvIII antibody-drug conjugate AMG 595 in patients with recurrent malignant glioma expressing EGFRvIII. Cancer Chemother Pharmacol. 2019;84:327–36. doi: 10.1007/s00280-019-03879-2. PMID: 31154523. [DOI] [PubMed] [Google Scholar]
- 81.Duca M, Lim DW, Subbiah V, Takahashi S, Sarantopoulos J, Varga A, D’Alessio JA, Abrams T, Sheng Q, Tan EY, et al. A first-in-human, phase I, multicenter, open-label, dose-escalation study of PCA062: an antibody-drug conjugate targeting P-cadherin, in patients with solid tumors. Mol Cancer Ther. 2022;21:625–34. doi: 10.1158/1535-7163.MCT-21-0652. PMID: 35131875. [DOI] [PubMed] [Google Scholar]
- 82.Milowsky MI, Galsky MD, Morris MJ, Crona DJ, George DJ, Dreicer R, Tse K, Petruck J, Webb IJ, Bander NH, et al. Phase 1/2 multiple ascending dose trial of the prostate-specific membrane antigen-targeted antibody drug conjugate MLN2704 in metastatic castration-resistant prostate cancer. Urol Oncol. 2016;34(12):530 e515–530 e521. doi: 10.1016/j.urolonc.2016.07.005. PMID: 27765518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee HC, Raje NS, Landgren O, Upreti VV VV, Wang J, Avilion AA, Hu X, Rasmussen E, Ngarmchamnanrith G, Fujii H, et al. Phase 1 study of the anti-BCMA antibody-drug conjugate AMG 224 in patients with relapsed/refractory multiple myeloma. Leukemia. 2021;35(1):255–58. doi: 10.1038/s41375-020-0834-9. PMID: 32317775. [DOI] [PubMed] [Google Scholar]
- 84.Schoffski P, Concin N, Suarez C, Subbiah V, Ando Y, Ruan S, Wagner JP, Mansfield K, Zhu X, Origuchi S, et al. A phase 1 study of a CDH6-targeting antibody-Drug conjugate in patients with advanced solid tumors with evaluation of inflammatory and neurological adverse events. Oncol Res Treat. 2021;44:547–56. doi: 10.1159/000518549. PMID: 34515215. [DOI] [PubMed] [Google Scholar]
- 85.Kollmannsberger C, Britten CD, Olszanski AJ, Walker JA, Zang W, Willard MD, Radtke DB, Farrington DL, Bell-McGuinn KM, Patnaik A. A phase 1 study of LY3076226, a fibroblast growth factor receptor 3 (FGFR3) antibody–drug conjugate, in patients with advanced or metastatic cancer. Invest New Drugs. 2021;39(6):1613–23.doi: 10.1007/s10637-021-01146-x. PMID: 34264412. [DOI] [PubMed] [Google Scholar]
- 86.Trneny M, Verhoef G, Dyer MJ, Ben Yehuda D, Patti C, Canales M, Lopez A, Awan FT, Montgomery PG, Janikova A, et al. A phase II multicenter study of the anti-CD19 antibody drug conjugate coltuximab ravtansine (SAR3419) in patients with relapsed or refractory diffuse large B-cell lymphoma previously treated with rituximab-based immunotherapy. Haematologica. 2018;103(8):1351–58. doi: 10.3324/haematol.2017.168401. PMID: 29748443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coiffier B, Thieblemont C, de Guibert S, Dupuis J, Ribrag V, Bouabdallah R, Morschhauser F, Navarro R, Le Gouill S, Haioun C, et al. A phase II, single-arm, multicentre study of coltuximab ravtansine (SAR3419) and rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. Br J Haematol. 2016;173(5):722–30. doi: 10.1111/bjh.13992. PMID: 27010483. [DOI] [PubMed] [Google Scholar]
- 88.Kantarjian HM, Lioure B, Kim SK, Atallah E, Leguay T, Kelly K, Marolleau J-P, Escoffre-Barbe M, Thomas XG, Cortes J, et al. A phase II study of coltuximab ravtansine (SAR3419) monotherapy in patients with relapsed or refractory acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2016;16(3):139–45. doi: 10.1016/j.clml.2015.12.004. PMID: 26775883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lapusan S, Vidriales MB, Thomas X, de Botton S, Vekhoff A, Tang R, Dumontet C, Morariu-Zamfir R, Lambert JM, Ozoux ML, et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Invest New Drugs. 2012;30:1121–31. doi: 10.1007/s10637-011-9670-0. PMID: 21519855. [DOI] [PubMed] [Google Scholar]
- 90.Hong R, Xia W, Wang L, Lee K, Lu Q, Jiang K, Li S, Yu J, Wei J, Tang W, et al. Safety, tolerability, and pharmacokinetics of BAT8001 in patients with HER2-positive breast cancer: an open- label, dose-escalation, phase I study. Cancer Commun (Lond). 2021;41:171–82. doi: 10.1002/cac2.12135. PMID: 33528890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pegram MD, Hamilton EP, Tan AR, Storniolo AM, Balic K, Rosenbaum AI, Liang M, He P, Marshall S, Scheuber A, et al. First-in-human, phase 1 dose-escalation study of biparatopic anti-HER2 antibody-drug conjugate MEDI4276 in patients with HER2-positive advanced breast or gastric cancer. Mol Cancer Ther. 2021;20:1442–53. doi: 10.1158/1535-7163.MCT-20-0014. PMID: 34045233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morgensztern D, Johnson M, Rudin CM, Rossi M, Lazarov M, Brickman D, Fong A. SC-002 in patients with relapsed or refractory small cell lung cancer and large cell neuroendocrine carcinoma: phase 1 study. Lung Cancer. 2020;145:126–31. doi: 10.1016/j.lungcan.2020.04.017. PMID: 32438272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lewis G, Li G, Guo J, Yu SF, Fields C, Lee G, Zhang D, Dragovich P, Pillow T, Wei B, et al. Development of DHES0815A; a novel HER2-directed antibody-drug conjugate comprised of a reduced potency mono-alkylating agent linked to a domain I binding HER2 antibody. Research Square. 2022;Preprint. doi: 10.21203/rs.3.rs-2322502/v1. [DOI] [Google Scholar]
- 94.Krop I, Hamilton E, Jung KH, Modi S, Kalinsky KM, Phillips G, Shi R, Monemi S, Mamounas M, Saad O, et al. A phase I dose-escalation study of DHES0815A, a HER2-targeting antibody-drug conjugate with a DNA monoalkylator payload, in patients with HER2-positive breast cancer. Cancer Res. 2022;82. doi: 10.1158/1538-7445.SABCS21-P2-13-25. [DOI] [Google Scholar]
- 95.Portillo S. AbbVie halts SC-007 development program in gastric cancer.ADC Review; 2018. [Google Scholar]
- 96.Phillips T, Barr PM, Park SI, Kolibaba K, Caimi PF, Chhabra S, Kingsley EC, Boyd T, Chen R, Carret A-S, et al. A phase 1 trial of SGN-CD70A in patients with CD70-positive diffuse large B cell lymphoma and mantle cell lymphoma. Invest New Drugs. 2019;37(2):297–306. doi: 10.1007/s10637-018-0655-0. PMID: 30132271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fathi AT, Erba HP, Lancet JE, Stein EM, Ravandi F, Faderl S, Walter RB, Advani AS, DeAngelo DJ, Kovacsovics TJ, et al. A phase 1 trial of vadastuximab talirine combined with hypomethylating agents in patients with CD33-positive AML. Blood. 2018;132:1125–33. doi: 10.1182/blood-2018-03-841171. PMID: 30045838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stein EM, Walter RB, Erba HP, Fathi AT, Advani AS, Lancet JE, Ravandi F, Kovacsovics T, DeAngelo DJ, Bixby D, et al. A phase 1 trial of vadastuximab talirine as monotherapy in patients with CD33-positive acute myeloid leukemia. Blood. 2018;131(4):387–96. doi: 10.1182/blood-2017-06-789800. PMID: 29196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Daver N, Salhotra A, Brandwein JM, Podoltsev NA, Pollyea DA, Jurcic JG, Assouline S, Yee K, Li M, Pourmohamad T, et al. A Phase I dose-escalation study of DCLL9718S, an antibody-drug conjugate targeting C-type lectin-like molecule-1 (CLL-1) in patients with acute myeloid leukemia. Am J Hematol. 2021;96:E175–E179. doi: 10.1002/ajh.26136. PMID: 33617672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garrido-Laguna I, Krop I, Burris HA 3rd, Hamilton E, Braiteh F, Weise AM, Abu-Khalaf M, Werner TL, Pirie-Shepherd S, Zopf CJ, et al. First-in-human, phase I study of PF-06647263, an anti-EFNA4 calicheamicin antibody-drug conjugate, in patients with advanced solid tumors. Int J Cancer. 2019;145:1798–808. doi: 10.1002/ijc.32154. PMID: 30680712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herbertson RA, Tebbutt NC, Lee F-T, MacFarlane DJ, Chappell B, Micallef N, Lee S-T, Saunder T, Hopkins W, Smyth FE, et al. Phase I biodistribution and pharmacokinetic study of Lewis Y–Targeting immunoconjugate CMD-193 in patients with advanced epithelial cancers. Clin Cancer Res. 2009;15(21):6709–15. doi: 10.1158/1078-0432.CCR-09-0536. PMID: 19825951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chan SY, Gordon AN, Coleman RE, Hall JB, Berger MS, Sherman ML, Eten CB, Finkler NJ. A phase 2 study of the cytotoxic immunoconjugate CMB-401 (hCTM01-calicheamicin) in patients with platinum-sensitive recurrent epithelial ovarian carcinoma. Cancer Immunol Immunother. 2003;52:243–48. doi: 10.1007/s00262-002-0343-x. PMID: 12669249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gillespie AM, Broadhead TJ, Chan SY, Owen J, Farnsworth AP, Sopwith M, Coleman RE. Phase I open study of the effects of ascending doses of the cytotoxic immunoconjugate CMB-401 (hCTMO1-calicheamicin) in patients with epithelial ovarian cancer. Ann Oncol. 2000;11(6):735–41.doi: 10.1023/a:1008349300781. PMID: 10942064. [DOI] [PubMed] [Google Scholar]
- 104.Rottey S, Clarke J, Aung K, Machiels J-P, Markman B, Heinhuis KM, Millward M, Lolkema M, Patel SP, de Souza P, et al. Phase I/IIa trial of BMS-986148, an Anti-mesothelin Antibody–drug conjugate, alone or in combination with nivolumab in patients with advanced solid tumors. Clin Cancer Res. 2022;28(1):95–105. doi: 10.1158/1078-0432.CCR-21-1181. PMID: 34615718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Owonikoko TK, Hussain A, Stadler WM, Smith DC, Kluger H, Molina AM, Gulati P, Shah A, Ahlers CM, Cardarelli PM, et al. First-in-human multicenter phase I study of BMS-936561 (MDX-1203), an antibody-drug conjugate targeting CD70. Cancer Chemother Pharmacol. 2016;77(1):155–62. doi: 10.1007/s00280-015-2909-2. PMID: 26576779. [DOI] [PubMed] [Google Scholar]
- 106.Cortes J, DeAngelo D, Wang E, Arana-Yi C, Zweidler-McKay P, Munteanu M, Andreu-Vieyra C, Erba H, Blum W, Traer E. Initial results from a first-in-human study of IMGN779, a CD33-targeting antibody-drug conjugate (ADC) with Novel DNA alkylating activity, in Patients with Relapsed or Refractory AML. EHA Conference; 2017; Madrid, Spain. [Google Scholar]
- 107.Dotan E, Cohen SJ, Starodub AN, Lieu CH, Messersmith WA, Simpson PS, Guarino MJ, Marshall JL, Goldberg RM, Hecht JR, et al. Phase I/II trial of Labetuzumab govitecan (Anti-CEACAM5/SN-38 antibody-drug conjugate) in patients with refractory or relapsing metastatic colorectal cancer. J Clin Oncol. 2017;35(29):3338–46. doi: 10.1200/JCO.2017.73.9011. PMID: 28817371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.George S, Heinrich MC, Somaiah N, Tine BA, McLeod R, Laadem A, Cheng B, Nishioka S, Kundu MG, Qian X, et al. A phase 1, multicenter, open-label, first-in-human study of DS-6157a in patients (pts) with advanced gastrointestinal stromal tumor (GIST). J Clin Oncol. 2022;40(16_suppl):11512–11512. doi: 10.1200/JCO.2022.40.16_suppl.11512. [DOI] [Google Scholar]
- 109.Janku F, Han SW, Doi T, Amatu A, Ajani JA, Kuboki Y, Cortez A, Cellitti SE, Mahling PC, Subramanian K, et al. Preclinical characterization and phase I study of an anti-HER2-TLR7 immune-stimulator antibody conjugate in patients with HER2+ malignancies. Cancer Immunol Res. 2022;10:1441–61. doi: 10.1158/2326-6066.CIR-21-0722. PMID: 36129967. [DOI] [PubMed] [Google Scholar]
- 110.Klempner SJ, Beeram M, Sabanathan D, Chan A, Hamilton E, Loi S, Oh D, Emens LA, Patnaik A, Kim JE, et al. Interim results of a Phase 1/1b study of SBT6050 monotherapy and pembrolizumab combination in patients with advanced HER2-expressing or amplified solid tumors. ESMO Conference. 2021; Paris, France* (Virtual meeting due to COVID) [Google Scholar]
- 111.https://www.askgileadmedical.com/docs/trodelvy/trodelvy-incidence-and-management-of-rash.
- 112.Lacouture ME, Patel AB, Rosenberg JE, O’Donnell PH. Management of dermatologic events associated with the Nectin-4-directed antibody-drug conjugate enfortumab vedotin. Oncologist. 2022;27:e223–e232. doi: 10.1093/oncolo/oyac001. PMID: 35274723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Faria M, Peay M, Lam B, Ma E, Yuan M, Waldron M, Mylott WR Jr, Liang M, Rosenbaum AI. Multiplex LC-MS/MS assays for clinical bioanalysis of MEDI4276, an antibody-drug conjugate of tubulysin analogue attached via cleavable linker to a biparatopic humanized antibody against HER-2. Antibodies (Basel). 2019;8 doi: 10.3390/antib8010011. PMID: 31544817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maderna A, Doroski M, Subramanyam C, Porte A, Leverett CA, Vetelino BC, Chen Z, Risley H, Parris K, Pandit J, et al. Discovery of cytotoxic dolastatin 10 analogues with N-terminal modifications. J Med Chem. 2014;57:10527–43. doi: 10.1021/jm501649k. PMID: 25431858. [DOI] [PubMed] [Google Scholar]
- 115.Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, Kim MH, Tseng LM, Petry V, Chung CF, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–54. doi: 10.1056/NEJMoa2115022. PMID: 35320644. [DOI] [PubMed] [Google Scholar]
- 116.Narita Y, Muragaki Y, Kagawa N, Asai K, Nagane M, Matsuda M, Ueki K, Kuroda J, Date I, Kobayashi H, et al. Safety and efficacy of depatuxizumab mafodotin in Japanese patients with malignant glioma: a nonrandomized, phase 1/2 trial. Cancer Sci. 2021;112:5020–33. doi: 10.1111/cas.15153. PMID: 34609773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clement PMJ, Dirven L, Eoli M, Sepulveda-Sanchez JM, Walenkamp AME, Frenel JS, Franceschi E, Weller M, Chinot O, De Vos F, et al. Impact of depatuxizumab mafodotin on health-related quality of life and neurological functioning in the phase II EORTC 1410/INTELLANCE 2 trial for EGFR-amplified recurrent glioblastoma. Eur J Cancer. 2021;147:1–12. doi: 10.1016/j.ejca.2021.01.010. PMID: 33601293. [DOI] [PubMed] [Google Scholar]
- 118.Berdeja JG, Hernandez-Ilizaliturri F, Chanan-Khan A, Patel M, Kelly KR, Running KL, Murphy M, Guild R, Carrigan C, Ladd S, et al. Phase I study of Lorvotuzumab Mertansine (LM, IMGN901) in combination with Lenalidomide (Len) and Dexamethasone (Dex) in patients with CD56-positive relapsed or relapsed/refractory Multiple Myeloma (MM). Blood. 2012;120:728. doi: 10.1182/blood.V120.21.728.728.22563087 [DOI] [Google Scholar]
- 119.Hamblett KJ, Barnscher SD, Davies RH, Hammond PW, Hernandez A, Wickman GR, Fung VK, Ding T, Garnett G, Galey AS, et al. ZW49, a HER2 targeted biparatopic antibody drug conjugate for the treatment of HER2 expressing cancers. Cancer Res. 2019;79:6–17-13. doi: 10.1158/1538-7445.SABCS18-P6-17-13. [DOI] [Google Scholar]
- 120.Knuehl C, Toleikis L, Dotterweich J, Ma J, Kumar S, Ross E, Wilm C, Schmitt M, Grote HJ, Amendt C. M1231 is a bispecific anti-MUC1xEGFR antibody-drug conjugate designed to treat solid tumors with MUC1 and EGFR co-expression. Cancer Res. 2022;82:5284. doi: 10.1158/1538-7445.AM2022-5284. [DOI] [Google Scholar]
- 121.Mazor Y, Sachsenmeier KF, Yang C, Hansen A, Filderman J, Mulgrew K, Wu H, Dall’Acqua WF. Enhanced tumor-targeting selectivity by modulating bispecific antibody binding affinity and format valence. Sci Rep. 2017;7:40098. doi: 10.1038/srep40098. PMID: 28067257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao H, Atkinson J, Gulesserian S, Zeng Z, Nater J, Ou J, Yang P, Morrison K, Coleman J, Malik F, et al. Modulation of macropinocytosis-mediated internalization decreases ocular toxicity of antibody-drug conjugates. Cancer Res. 2018;78:2115–26. doi: 10.1158/0008-5472.CAN-17-3202. PMID: 29382707. [DOI] [PubMed] [Google Scholar]
- 123.Viricel W, Fournet G, Beaumel S, Perrial E, Papot S, Dumontet C, Joseph B. Monodisperse polysarcosine-based highly-loaded antibody-drug conjugates. Chem Sci. 2019;10:4048–53. doi: 10.1039/c9sc00285e. PMID: 31015945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burke PJ, Hamilton JZ, Jeffrey SC, Hunter JH, Doronina SO, Okeley NM, Miyamoto JB, Anderson ME, Stone IJ, Ulrich ML, et al. Optimization of a PEGylated glucuronide-monomethylauristatin E linker for antibody-drug conjugates. Mol Cancer Ther. 2017;16:116–23. doi: 10.1158/1535-7163.MCT-16-0343. PMID: 28062707. [DOI] [PubMed] [Google Scholar]
- 125.Evans N, Grygorash R, Williams P, Kyle A, Kantner T, Pathak R, Sheng X, Simoes F, Makwana H, Resende R, et al. Incorporation of hydrophilic macrocycles into drug-linker reagents produces antibody-drug conjugates with enhanced in vivo performance. Front Pharmacol. 2022;13:764540. doi: 10.3389/fphar.2022.764540. PMID: 35784686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gandhi AV, Randolph TW, Carpenter JF. Conjugation of emtansine onto trastuzumab promotes aggregation of the antibody-drug conjugate by reducing repulsive electrostatic interactions and increasing hydrophobic interactions. J Pharm Sci. 2019;108:1973–83. doi: 10.1016/j.xphs.2019.01.029. PMID: 30735687. [DOI] [PubMed] [Google Scholar]
- 127.Yurkovetskiy AV, Bodyak ND, Yin M, Thomas JD, Clardy SM, Conlon PR, Stevenson CA, Uttard A, Qin L, Gumerov DR, et al. Dolaflexin: a novel antibody-drug conjugate platform featuring high drug loading and a controlled bystander effect. Mol Cancer Ther. 2021;20:885–95. doi: 10.1158/1535-7163.MCT-20-0166. PMID: 33722857. [DOI] [PubMed] [Google Scholar]
- 128.Zacharias N, Podust VN, Kajihara KK, Leipold D, Del Rosario G, Thayer D, Dong E, Paluch M, Fischer D, Zheng K, et al. A homogeneous high-DAR antibody-drug conjugate platform combining THIOMAB antibodies and XTEN polypeptides. Chem Sci. 2022;13:3147–60. doi: 10.1039/d1sc05243h. PMID: 35414872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kovtun YV, Audette CA, Mayo MF, Jones GE, Doherty H, Maloney EK, Erickson HK, Sun X, Wilhelm S, Ab O, et al. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010;70:2528–37. doi: 10.1158/0008-5472.CAN-09-3546. PMID: 20197459. [DOI] [PubMed] [Google Scholar]
- 130.Bryden F, Martin C, Letast S, Lles E, Vieitez-Villemin I, Rousseau A, Colas C, Brachet-Botineau M, Allard-Vannier E, Larbouret C, et al. Impact of cathepsin B-sensitive triggers and hydrophilic linkers on in vitro efficacy of novel site-specific antibody-drug conjugates. Org Biomol Chem. 2018;16:1882–89. doi: 10.1039/c7ob02780j. PMID: 29473076. [DOI] [PubMed] [Google Scholar]
- 131.Chuprakov S, Ogunkoya AO, Barfield RM, Bauzon M, Hickle C, Kim YC, Yeo D, Zhang F, Rabuka D, Drake PM. Tandem-cleavage linkers improve the in vivo stability and tolerability of antibody-drug conjugates. Bioconjug Chem. 2021;32:746–54. doi: 10.1021/acs.bioconjchem.1c00029. PMID: 33689309. [DOI] [PubMed] [Google Scholar]
- 132.Weidle UH, Tiefenthaler G, Georges G. Proteases as activators for cytotoxic prodrugs in antitumor therapy. Cancer Genomics Proteomics. 2014;11:67–79. https://www.ncbi.nlm.nih.gov/pubmed/24709544. PMID: 24709544. [PubMed] [Google Scholar]
- 133.Gregson SJ, Barrett AM, Patel NV, Kang GD, Schiavone D, Sult E, Barry CS, Vijayakrishnan B, Adams LR, Masterson LA, et al. Synthesis and evaluation of pyrrolobenzodiazepine dimer antibody-drug conjugates with dual beta-glucuronide and dipeptide triggers. Eur J Med Chem. 2019;179:591–607. doi: 10.1016/j.ejmech.2019.06.044. PMID: 31279293. [DOI] [PubMed] [Google Scholar]
- 134.Tsui CK, Barfield RM, Fischer CR, Morgens DW, Li A, Smith BAH, Gray MA, Bertozzi CR, Rabuka D, Bassik MC. CRISPR-Cas9 screens identify regulators of antibody-drug conjugate toxicity. Nat Chem Biol. 2019;15:949–58. doi: 10.1038/s41589-019-0342-2. PMID: 31451760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Satomaa T, Pynnonen H, Vilkman A, Kotiranta T, Pitkanen V, Heiskanen A, Herpers B, Price LS, Helin J, Saarinen J. Hydrophilic auristatin glycoside payload enables improved antibody-drug conjugate efficacy and biocompatibility. Antibodies (Basel). 2018;7 doi: 10.3390/antib7020015. PMID: 31544867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Levengood MR, Zhang X, Hunter JH, Emmerton KK, Miyamoto JB, Lewis TS, Senter PD. Orthogonal cysteine protection enables homogeneous multi-drug antibody-drug conjugates. Angew Chem Int Ed Engl. 2017;56:733–37. doi: 10.1002/anie.201608292. PMID: 27966822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kumar A, Kinneer K, Masterson L, Ezeadi E, Howard P, Wu H, Gao C, Dimasi N. Synthesis of a heterotrifunctional linker for the site-specific preparation of antibody-drug conjugates with two distinct warheads. Bioorg Med Chem Lett. 2018;28:3617–21. doi: 10.1016/j.bmcl.2018.10.043. PMID: 30389292. [DOI] [PubMed] [Google Scholar]
- 138.Nilchan N, Li X, Pedzisa L, Nanna AR, Roush WR, Rader C. Dual-mechanistic antibody-drug conjugate via site-specific selenocysteine/cysteine conjugation. Antib Ther. 2019;2:71–78. doi: 10.1093/abt/tbz009. PMID: 31930187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yamazaki CM, Yamaguchi A, Anami Y, Xiong W, Otani Y, Lee J, Ueno NT, Zhang N, An Z, Tsuchikama K. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat Commun. 2021;12:3528. doi: 10.1038/s41467-021-23793-7. PMID: 34112795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Walsh SJ, Bargh JD, Dannheim FM, Hanby AR, Seki H, Counsell AJ, Ou X, Fowler E, Ashman N, Takada Y, et al. Site-selective modification strategies in antibody-drug conjugates. Chem Soc Rev. 2021;50:1305–53. doi: 10.1039/d0cs00310g. PMID: 33290462. [DOI] [PubMed] [Google Scholar]
- 141.Yin G, Stephenson HT, Yang J, Li X, Armstrong SM, Heibeck TH, Tran C, Masikat MR, Zhou S, Stafford RL, et al. RF1 attenuation enables efficient non-natural amino acid incorporation for production of homogeneous antibody drug conjugates. Sci Rep. 2017;7:3026. doi: 10.1038/s41598-017-03192-z. PMID: 28596531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu J, Barfield RM, Rabuka D. Site-specific bioconjugation using SMARTag((R)) technology: a practical and effective chemoenzymatic approach to generate antibody-drug conjugates. Methods Mol Biol. 2019;2033:131–47. doi: 10.1007/978-1-4939-9654-4_10. PMID: 31332752. [DOI] [PubMed] [Google Scholar]
- 143.Strop P, Liu S-H, Dorywalska M, Delaria K, Dushin RG, Tran -T-T, Ho W-H, Farias S, Casas MG, Abdiche Y, et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol. 2013;20(2):161–67. doi: 10.1016/j.chembiol.2013.01.010. PMID: 23438745. [DOI] [PubMed] [Google Scholar]
- 144.Van Geel R, Wijdeven MA, Heesbeen R, Verkade JM, Wasiel AA, Van Berkel SS, van Delft FL. Chemoenzymatic conjugation of toxic payloads to the globally conserved N-glycan of native mAbs provides homogeneous and highly efficacious antibody–Drug conjugates. Bioconjug Chem. 2015;26(11):2233–42.doi: 10.1021/acs.bioconjchem.5b00224. PMID: 26061183. [DOI] [PubMed] [Google Scholar]
- 145.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al. Unique carbohydrate–carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108(31):12669–74. doi: 10.1073/pnas.1108455108. PMID: 21768335. [DOI] [PMC free article] [PubMed] [Google Scholar]


