Abstract
This study aimed to evaluate the effects of ketamine, on behavioral parameters, oxidative stress, and inflammation in the brain of male and female rats submitted to the animal model of maternal deprivation (MD). Wistar rats were deprived of maternal care in the first 10 days of life (three hours daily). As adults, male and female rats were divided: control + saline deprived + saline and deprived + ketamine (15 mg/kg). The behavior was evaluated through the open field and forced swimming tests. Then brain was removed for analysis of oxidative damage, the activity of superoxide dismutase (SOD), catalase (CAT), and myeloperoxidase (MPO) activity, and levels of interleukin-6 (IL-6). MD induced depressive behavior in males and ketamine reversed these changes. MD induced an increase in lipid peroxidation in males and females; ketamine reversed these effects in males. Protein carbonylation was increased in males and females, with ketamine decreasing such effects. The concentration of nitrite/nitrate increased in males and females, whereas ketamine decreased this in the PFC of males. SOD and CAT activities were decreased in male and female deprived groups and deprived groups treated with ketamine. MPO activity and IL-6 levels increased in males subjected to MD and ketamine reversed this effect. The results suggest that stressful events in early life can induce behavioral, neuroimmune changes, and oxidative stress, however, such effects depend on sex and brain area. Ketamine presents anti-inflammatory and antioxidant properties and could be considered an alternative for individuals who are resistant to classical treatments.
Keywords: Oxidative stress, interleukin-6, Ketamine, Inflammation, Maternal deprivation, Major depressive disorder
1. Introduction
Major depressive disorder (MDD) is complex, impacts multiple systems in the brain and periphery, and alters emotional and cognitive processes that are integral to healthy daily functioning and quality of life (Albert and Newhouse, 2019). Sex-specific factors have also been identified in the clinical presentation, prevalence, and resiliency of MDD (Sramek et al., 2016). According to Kessler (2005), the incidence and prevalence of MDD in women are two to three times higher than in men. In men, new-onset rates and 12-month prevalence of MDD remain fairly constant from puberty to old age, but increase in women at puberty and remain higher than in men until menopause (Kessler, 2003). Besides, there is an emerging body of literature that shows women and men differ not only in the rate of MDD but also in the presentation of mood symptoms (Millett et al., 2019). Women experience more somatic depression, marked by fatigue, sleep disturbance, pain, and anxiety (Silverstein et al., 2013), whereas men may present with symptoms such as aggression, impulsivity, and substance abuse (Martin et al., 2013).
Depressive episodes may also leave a scar, such as a cognitive bias for negative information, that results in maladaptive behavior and dependent stressful events (De Raedt and Koster, 2010). Stress is defined as a sudden inconsistent physical, physiological, and social-environmental change experienced by an organism (Shah et al., 2005) and there is strong accumulated evidence that episodic stressors play a causal role in many instances of MDD (Hammen, 2005). Exposure to stress during early life can have short- or long-term effects on brain development, and these effects may include learning deficits and/or psychiatric disorders such as generalized anxiety and depression (Lupien et al., 2009).
There is evidence that MDD is associated with increased peripheral markers of oxidative stress. Oxidative stress occurs when a biological system is overwhelmed by reactive oxygen species (ROS), that includes superoxide anion, hydroxyl radical, and hydrogen peroxide, due to its inability to counteract these free radicals, leading to modifications of lipids, proteins, and DNA (Liu et al., 2015; Kattoor et al., 2017). These free radicals can trigger lipid peroxidation reactions, as well oxidative stress, and DNA mutations (Kujoth et al., 2006). As the imbalance continues, oxidative stress can induce apoptosis, a programmed cell death (Samhan-Arias et al., 2004).
Sex differences in oxidative stress have been observed in numerous basic and clinical studies, wherein males exhibit higher oxidative stress than females (Dantas et al., 2004; Wong et al., 2015). Interestingly, sex differences have been observed in the enzyme NADPH oxidase (NOX) (Dantas et al., 2004; Wong et al., 2015), which is a major oxidative stress generator in cells (Drummond et al., 2011). Many human and animal studies have implicated inflammation as a mediator of oxidative stress in the pathophysiology of MDD (Millett et al., 2019). Interestingly, females and males have known differences in immune activation (Fish, 2008), and thus the inciting, moderating, or mediating influence of inflammation in MDD may also differ between the sexes, and could potentially underlie the gender-based differences in MDD symptomatology and prevalence. Sex differences in markers of MDD were reported by Domenici et al. (2010), who found 11 plasma analytes with significant interactions between sex and diagnosis, including growth hormone and proteins involved in the immune response.
During the past decade, one of the most striking discoveries in the treatment of MDD was the finding that infusion of a single subanesthetic dose of the N-methyl-d-aspartate (NMDA) receptor antagonist ketamine induces rapid and sustained antidepressant effects in treatment-resistant MDD patients and rodents subjected to various antidepressant-predictive animal models (Réus et al., 2011, 2019). However, intricate behavioral and neurobiological mechanisms underlying ketamine’s antidepressant actions appear to be brain region- and dose-dependent and have not yet been fully elucidated. Preclinical studies have shown that the antidepressant effects of ketamine could also be related to sex difference response in animal models (Carrier and Kabbaj, 2013; Franceschelli et al., 2015). Previous studies from our group have been shown the effects of maternal deprivation as well as ketamine treatment in the oxidative stress and inflammatory parameters. Indeed, early life stress can induce behavioral changes throughout development, besides inducing oxidative stress, neuroinflammation, and epigenetic changes (Réus et al., 2013, 2015a, 2015b, 2015c; 2017). However, these studies it was performed only in male Wistar rats. Studies exploring the effects of maternal deprivation and ketamine acute treatment have been little explored in the context of sex. Thus, to understand the molecular mechanisms underlying sex differences in the antidepressant-like response to ketamine, the objective of this study was to evaluate the effects of a single dose of ketamine on stress oxidative parameters and IL-6 levels in the hippocampus and PFC of male and female Wistar rats submitted to early life stress.
2. Experimental procedures
2.1. Animals
For this study, male and female Wistar rats (3 months of age, weighing 250–280 g) were obtained from the breeding colony of Universidade do Extremo Sul Catarinense (UNESC, Cricúma, SC, Brazil) and were housed for 1 week in the presence of males for mating purposes. At the end of 7 days, the pregnant rats were housed individually with ad libitum access to food and water. The pregnant rats remained individually housed for the birth of the pups and their sexual identification. All mothers and pups were kept on a 12-h light/dark cycle (06:00 a.m. to 06:00 p.m.), at a temperature of 23 ± 1 °C. One day after birth, the maternal deprivation protocol was applied to a percentage of the male and female pups from days 1–10 after birth; the other males and females were used as controls. All of the experimental procedures involving animals were performed following the NIH Guidelines for the Care and Usage of Laboratory Animals, within the Brazilian Society for Neuroscience and Behaviour recommendations for animal care, and with the approval of the local Ethics Committee under protocol number 132–2019.
2.2. Experimental groups and maternal deprivation (MD) protocol
The deprivation protocol consisted of removing the mother from the residence box and taking her to another room. The pups were maintained in their home cage (grouped in the nest in the presence of maternal odor). The pups were deprived of the mother for 3 h per day during the postnatal day (PND) 1–10. We prefer this MD protocol because it does not require the manipulation of the pups (Réus et al., 2017). At the end of each daily deprivation session, the mothers were returned to their home cages; this procedure was carried out during the light part of the cycle, between 9:00 a.m. and 12:00 p.m. The control rats remained in their resident boxes together with their mothers throughout the experiment.
2.3. Experimental groups
On PND 60, male and female Wistar ratswere divided into three experimental groups (n = 12): 1) control (non-deprived) + sal; 2) deprived + sal; 3) deprived + ketamine. Ketamine was administered intraperitoneally at a dose of 15 mg/kg sixty minutes before behavioral tests. All treatments were administered in a volume of 1 mL/kg (Réus et al., 2015a).
2.4. Behavioral tests
2.4.1. Open field test (OFT)
Male and female Wistar rats were subjected to the OFT to evaluate possible effects on spontaneous locomotor activity. The test was performed in an apparatus that consisted of a brown plywood 45 × 9 × 60 cm arena surrounded by 50 cm high wooden walls and containing a frontal glass wall. The floor of the open field was divided into nine rectangles (15 × 9 × 20 cm each) by black lines. The animals were gently placed in the left rear quadrant and left to explore the arena, and then, the number of horizontal (crossings) and vertical (rearings) movements performed by each rat during the 5 min observation period was counted by an expert observer (Réus et al., 2011).
2.4.2. Forced swimming test (FST)
After the OFT, the same animals were subjected to the FST. The FST was conducted according to previous reports (Porsolt et al., 1977; Réus et al., 2011). The test involves two individual exposures to a cylindrical tank filled with water, in which rats cannot touch the bottom of the tank or escape. The tank is made of transparent Plexiglas, 80 cm tall, 30 cm in diameter, and filled with water (22–23 °C) to a depth of 40 cm. On PND 60, the rats were individually placed in the cylinder containing water for 15 min (pre-test session). On PND 61, one hour after treatments, the rats were again subjected to the FST for a 5 min session (test session), and the immobility, swimming, and climbing times of rats were recorded in seconds.
2.5. Brain samples
After the behavioral tests were complete, the animals were killed by decapitation. The skulls were removed, then the whole brain was removed and placed on a filter paper and Petri dish on ice and then and the PFC and hippocampus were quickly isolated by hand dissection using a magnifying glass, a spatula, and a thin brush by a qualified researcher. Also, the dissection was based on the histological distinctions described by Paxinos and Watson (1986). After the removal of the structures, they were placed in microcentrifuge tubes and stored in a freezer at −70 °C before biochemical analysis (n = 5).
2.6. IL- 6 levels
The level of interleukin (IL)-6 was tested using an ELISA test kit (RAB0311 - Sigma Aldrich). The operations were carried out in strict accordance with the kit instructions. The test kit was maintained at room temperature for 20 min, and the detergents were prepared. A total of ten standard wells (including 2 blank control wells, no samples, and enzyme labeling reagents) were set on the enzyme-labeled plate, and the standard curve was plotted after standard dilution. The samples were diluted and then placed into the sample wells of the enzyme-labeled plate. The plates were shaken gently after the addition of samples and then sealed for incubation for 30 min at 37 °C. The liquid in the wells was removed, detergents were added and removed after 30 s. The process was repeated 5 times, and then the samples were dried. The enzyme labeling reagents (50 μL) were added and incubated for 30 min at 37 °C. Then the liquid in the wells was removed, the detergents were added and removed after 30 s. The process was repeated five times and then the sample was dried. Next, chromogenic agents, A was added to each well. After gentle mixing, the samples were incubated at 37 °C for 15 min, and 50 μL stop buffer was added. The OD value per well was measured respectively at an excitation wavelength of 450 nm using a microplate reader (Spectramax ID3) within 10 min with the blank well serving as the control. The concentration standard curve was plotted, and the sample concentration was recorded according to the standard curve. Besides, standards and samples run were in duplicate.
2.7. Oxidative stress parameters
2.7.1. Thiobarbituric acid reactive species
The formation of thiobarbituric acid reactive substances (TBARS) during an acid-heating reaction was measured as an index of oxidative stress as previously described (Esterbauer, 1990). Briefly, the samples were mixed with 1 mL of 10 % trichloroacetic acid and 1 mL of 0.67 % thiobarbituric acid (Sigma-Aldrich, Saint Louis, MO, USA) and then heated in a boiling water bath for 15 min. Malondialdehyde (MDA) equivalents were determined at an absorbance of 535 nm using 1,1,3, 3-tetramethoxypropane (Sigma-Aldrich, Saint Louis, MO, USA) as an external standard.
2.7.2. Protein carbonyl
The oxidative damage to proteins was assessed by the determination of carbonyl groups based on the reaction with dinitrophenylhydrazine (Sigma-Aldrich, Saint Louis, MO, USA) as previously described (Levine et al., 1990). Briefly, proteins were precipitated by the addition of 20 % trichloroacetic acid and redissolved in dinitrophenylhydrazine, with absorbance being read at 370 nm. The results were expressed as protein carbonyls per mg of protein.
2.7.3. Myeloperoxidase (MPO) activity
Neutrophil infiltrate in tissues was measured by MPO activity (De Young et al., 1989). Brain tissues were homogenized (50 mg/mL) in 0.5 % hexadecyltrimethylammonium bromide and centrifuged at 15,000 x g for 40 min. The suspension was then sonicated three times for 30 s. An aliquot of the supernatant was mixed with a solution of 1.6 mM tetra-methylbenzidine and 1 mM H2O2. The activity was measured spectrophotometrically as the change in absorbance at 650 nm at 37 °C. Data were expressed as mU per mg of protein.
2.7.4. Measurement of nitrite/nitrate concentration
Total nitrite concentrations were measured using the Griess reaction, by adding 100 μL of Griess reagent 0.1 % (w/v) naphthyl ethyl and amide dihydrochloride in H2O and 1% (w/v) sulphanilamide in 5% (v/v) concentrated H3PO4, vol. [1:1] to the 100 μL sample. Absorbance was recorded in a spectrophotometer at 550 nm (Green et al., 1982). Data were expressed as nmol of nitrite/nitrate concentration per mg of protein.
2.7.5. Oxidative damage to proteins by assessing the integrity of sulfhydryl groups
The number of total thiol groups in the PCF and the hippocampus was determined using 5,5′-Dithiobis (2-nitrobenzoic acid) (DTNB) (Aksenov and Markesbery, 2001). Briefly, 30 u L of a sample was mixed with 1 mL of PBS / 1 mM EDTA (pH, 7.5). The reaction was started by adding 30 u L of 10 mM DTNB solution in PBS. Control samples, which do not include DTNB or protein, were evaluated simultaneously. After 30 min of incubation at room temperature, the absorbance of 412 nm was measured and quantities of TNB formed (equivalent to the amount of SH groups formed) determined. The results are expressed as levels of sulfhydryl proteins per milligram of protein.
2.7.6. Analysis of superoxide dismutase (SOD) activity
The method used for the assay of SOD activity was based on the capacity of pyrogallol to autoxidize, a process highly dependent on peroxide ion, a substrate for SOD (Aebi, 1984). The inhibition of autoxidation of this compound occurs when SOD is present; the enzymatic activity can then be indirectly assayed spectrophotometrically at 420 nm using a double-beam spectrophotometer with temperature control. A calibration curve was generated using purified SOD as the standard to calculate the specific activity of SOD in the samples. A total of 50 % inhibition of pyrogallol autoxidation is defined as 1 unit of SOD, and the specific activity is represented as units per mg of protein.
2.7.7. Analysis of catalase (CAT) activity
The activity of CAT was assayed using a double-beam spectrophotometer with temperature control. This method is based on the disappearance of H2O2 at 240 nm in a reaction medium containing 20 mM H2O2, 0.1 % Triton X-100, 10 Mm potassium phosphate buffer, and 0.1–0.3 mg protein/mL at a pH of 7.0 (Bannister and Calabrese, 1987). One unit of CAT is defined as 1 mol of hydrogen peroxide consumed per minute, and the specific activity is reported as units per mg protein.
2.8. Protein determination
All biochemical measures were normalized to the protein content using bovine albumin as the standard (Lowry et al., 1951).
2.9. Statistical analysis
The data are presented as mean ± standard error of the mean (S.E.M). Differences among experimental groups in the assessment of behavior, oxidative stress parameters, and IL-6 levels were determined by one-way ANOVA, followed by Tukey post-hoc test when ANOVA was significant. Differences between sex and group interaction were determined by two-way ANOVA. P values < 0.05 were considered to be statistically significant.
3. Results
3.1. Effects of MD and ketamine treatment on the behavior of males and females Wistar rats
Fig. 1A shows the effects of MD and ketamine treatment on the forced swimming test in male and female Wistar rats. In males, the deprived group that received saline had an increase in immobility time when compared to non-deprived rats; and still, the deprived group treated with ketamine was able to reverse this increase (F = 2–20 11.323; p = 0.001; Fig. 1A). Also, the deprived males treated with saline decreased the climbing time when compared to the non-deprived group (F = 2–20 4.693; p = 0.021; Fig. 1A) and the deprived males treated with ketamine increased the swimming time when compared to deprived rats treated with saline (F = 2–20 6.334; p = 0.007; Fig. 1A). Females did not show statistical differences, in either groups, for immobility (F = 2–20 2.790; p = 0.073; Fig. 1A), climbing (F = 2–20 2.790; p = 0.073) and swimming times (F = 2–20 0.968; p = 0.389; Fig. 1A) when compared to non-deprived group. Also, two-way ANOVA revealed differences for sex and groups interaction in the immobility (F = 2–60 6.423; p = 0.002), in the climbing (F = 2–60 4.079; p = 0.021), and in the swimming times (F = 2–60 6.459; p = 0.002). In the immobility and climbing times it was showed differences for sex in the maternal deprivation, and in the swimming for ketamine treatment.
Fig. 1.
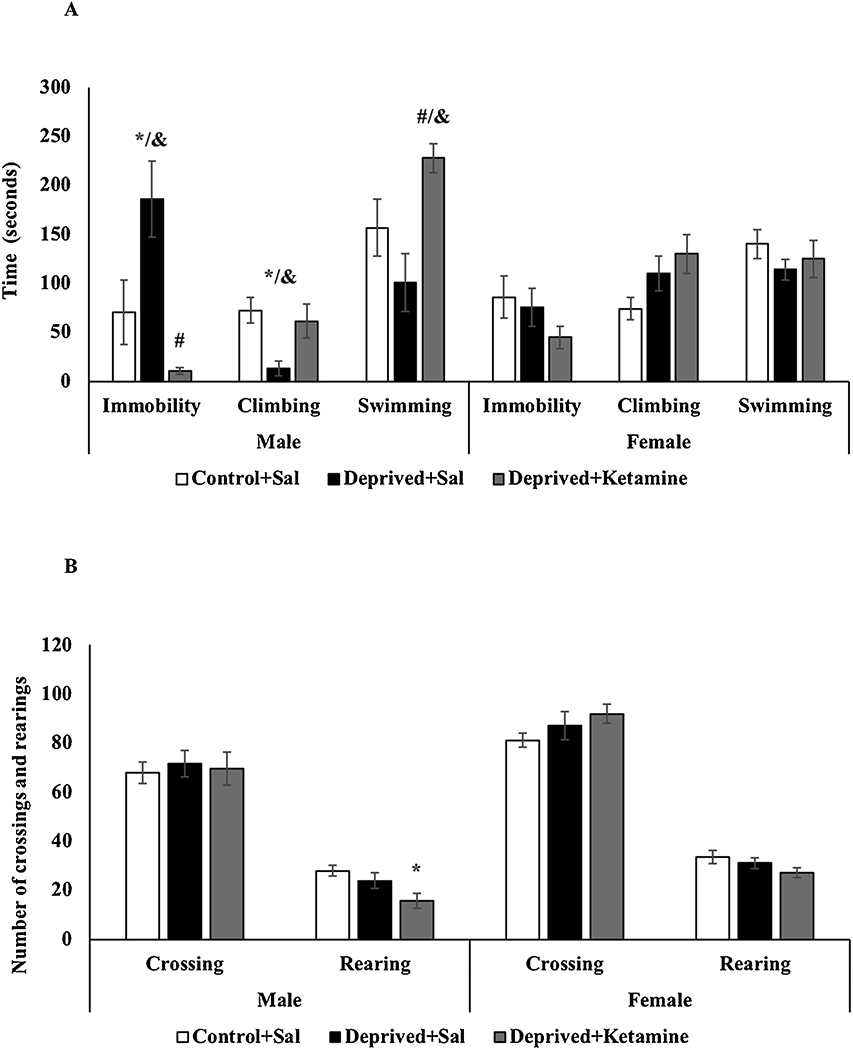
A: Effects of MD and ketamine treatment on immobility, swimming, and climbing time in male and female Wistar rats. Values are expressed as mean ± E.P.M. *p < 0.05 vs. Control + Sal; #p < 0.05 vs. Deprived + Sal, according to oneway ANOVA followed by Tukey’s post-hoc test; &p<0.05 vs. sex and groups interaction according to two-way ANOVA. B: Effects of MD and ketamine treatment on the number of crossings and rearings in male and female Wistar rats. Values are expressed as mean ± E.P.M. *p < 0.05 vs. Control + Sal, according to one-way ANOVA followed by Tukey’s post-hoc test.
Fig. 1B shows the effects of MD and ketamine treatment on the locomotor activity of male and female Wistar rats. In males, there was a decrease in the number of rearings in the deprived group treated with ketamine when compared to non-deprived animals (F = 2–20 4.817; p = 0.020; Fig. 1B). There was no statistical difference in the number of crossings in deprived males treated with saline and ketamine when compared to the control animals (F = 2–20 0.084; p = 0.920; Fig. 1B). Regarding females, there was no statistical difference, in any of the groups, for the numbers of crossings (F = 2–20 1.237; p = 0.301; Fig. 1B) and rearings (F = 2–20 1.535; p = 0.228; Fig. 1B) when compared to non-deprived group. Two-way ANOVA did not revealed differences for sex and groups interaction in the numbers of crossings (F = 2–60 0.283; p = 0.754) and rearings (F = 2–60 0.582; p = 0.561).
3.2. Effects of MD and ketamine treatment on oxidative stress parameters in PFC and hippocampus of male and female Wistar rats
Fig. 2 shows the effects of MD and ketamine treatment on oxidative stress parameters in the PFC and hippocampus of male and female Wistar rats. Deprived male rats treated with saline had an increase in TBARS levels in PFC (F = 2–12 22.356; p < 0.0001; Fig. 2A) and hippocampus F = 2–14 61.982; p < 0.0001; Fig. 2A) when compared to non-deprived males, however, in the deprived group treated with ketamine there was a decrease in TBARS levels compared to the deprived group treated with saline in the two brain structures. In females, the deprived group treated with saline increase the TBARS levels in the hippocampus when compared to the non-deprived group (F = 2–15 7.089; p = 0.007; Fig. 2A). There was no statistical difference for TBARS levels in the PFC of any female groups (F = 2–15 0.418; p = 0.666; Fig. 2A). Two-way ANOVA revealed differences for sex and groups interaction on TBARS levels in the PFC (F = 2–27 14.952; p < 0.0001) and hippocampus (F = 2–29 16.755; p < 0.0001).
Fig. 2.
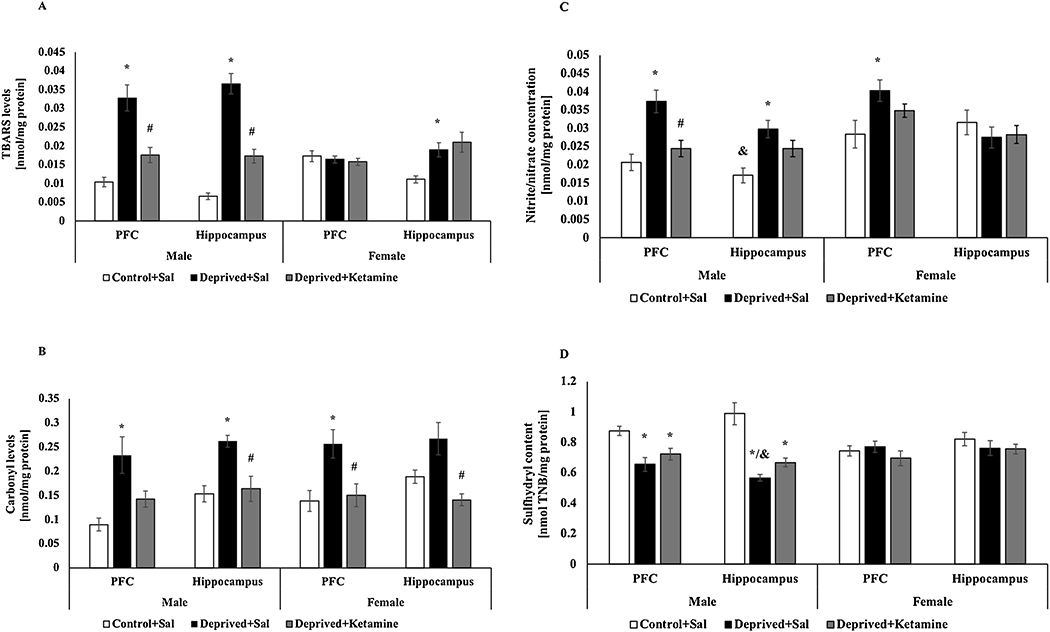
A: Effects of MD and ketamine treatment on TBARS levels in PFC and hippocampus of male and female Wistar rats. Values are expressed as mean ± E.P.M. *p < 0.05 vs. Control + Sal, #p < 0.05 vs. Deprived + Sal, according to one-way ANOVA followed by Tukey’s post-hoc test; &p<0.05 vs. sex and groups interaction according to two-way ANOVA. B: Effects of MD and ketamine treatment on protein carbonyl levels in the PFC and hippocampus of male and female Wistar rats. Values are expressed as mean ± E.P.M. *p < 0.05 vs. Control + Sal; #p < 0.05 vs. Deprived + Sal, according to one-way ANOVA followed by Tukey’s post-hoc test. C: Effects of MD and ketamine treatment on nitrite/nitrate concentration in PFC and hippocampus of male and female Wistar rats. Values are expressed as mean ± E.P.M. * p < 0.05 vs. Control + Sal; #p < 0.05 vs. Deprived + Sal, according to one-way ANOVA followed by Tukey’s post-hoc test; &p<0.05 vs. sex and groups interaction according to two-way ANOVA. D: Effects of MD and ketamine treatment on the sulfhydryl content in PFC and hippocampus of male and female Wistar rats. Values are expressed as mean ± E.P.M. * p < 0.05 vs. Control + Sal, according to one-way ANOVA followed by Tukey’s post-hoc test; &p<0.05 vs. sex and groups interaction according to two-way ANOVA.
Regarding the protein carbonyl levels observed in Fig. 2B, there was an increase in PFC of deprived male rats treated with saline compared to the control group (F = 2–12 8.275; p = 0.006; Fig. 2B). In the hippocampus of male rats, there was also an increase in protein carbonyl levels in deprived rats treated with saline compared to the control group, however, the deprived animals treated with ketamine reversed this increase (F = 2–14 11.132; p = 0.001; Fig. 2B). In females’ rats, there was an increase in protein carbonyl levels, in PFC, in deprived rats treated with saline compared to non-deprived rats and in PFC (F = 2–15 6.623; p = 0.009; Fig. 2B) and hippocampus (F = 2–15 8.432; p = 0.004; Fig. 2B) the deprived female rats treated with ketamine reduced these levels. Two-way ANOVA did not revealed differences for sex and groups interaction on carbonyl protein levels in the PFC (F = 2–27 0.367; p = 0.695) and hippocampus (F = 2–29 0.934 p = 0.404).
The nitrite/nitrate concentration is shown in Fig. 2C. In the PFC of the male rats, an increase in nitrite/nitrate concentration was observed in deprived rats treated saline compared to the non-deprived group and the deprived group treated with ketamine were able to reserve this increase (F = 2–13 11.956; p = 0.001; Fig. 2C). In the hippocampus of male rats, there was an increase in nitrite/nitrate concentration in deprived rats treated with saline compared to the control group (F = 2–13 8.329; p = 0.05; Fig. 2C). In the PFC of female rats, it was observed an increase in nitrite/nitrate concentration in deprived rats treated with saline compared to the non-deprived group (F = 2–15 3.979; p = 0.041; Fig. 2C). There was no statistical difference in the hippocampus of females’ rats for any groups (F = 2–15 0.552; p = 0.587; Fig. 2C). Two-way ANOVA did not revealed differences for sex and groups interaction on nitrite/nitrate concentration in the PFC (F = 2–28 0.471; p = 0.628); however, in the hippocampus differences it was found for sex between control groups (F = 2–28 7.868 p = 0.001).
The sulfhydryl content was showed in the Fig. 2D. A reduction was found in both the PFC (F = 2–12 8.199; p = 0.006; Fig. 2D) and the hippocampus (F = 2–14 21.731; p < 0.0001; Fig. 2D) of deprived male rats treated with saline compared to control group and this effect was not reversed by treatment with ketamine. In the PFC (F = 2–15 0.740; p = 0.494) or hippocampus (F = 2–15 0.740; p = 0.495; Fig. 2D) of females, no statistical differences were shown in this parameter. Two-way ANOVA revealed differences for sex and groups interaction on sulfhydryl content in the PFC (F = 2–27 4.681; p = 0.017) and hippocampus (F = 2–29 8.543; p = 0.01).
3.3. Effects of MD and ketamine treatment on the activity of the antioxidant enzymes SOD and CAT in PFC and hippocampus of male and female Wistar rats
Fig. 3 demonstrates the effects of MD and ketamine treatment on the activity of the antioxidant enzymes SOD and CAT in the PCF and hippocampus of male and female Wistar rats. As seen in Fig. 3A, in the hippocampus (F = 2–14 47.833; p < 0.0001; Fig. 3A) and in the PFC (F = 2–12 12.967; p = 0.001; Fig. 3A) of male rats, SOD activity was decreased in the deprived groups treated with saline and ketamine when compared with the control animals. In females, there was a decrease in SOD activity in the PFC of the deprived group treated with ketamine compared to the control (F = 2–15 4.689; p = 0.026; Fig. 3A) and a decrease in the hippocampus of the deprived groups treated with saline and ketamine compared to control animals (F = 2–14 19.651; p < 0.0001; Fig. 3A). Two-way ANOVA did not revealed differences for sex and groups interaction on SOD activity in the PFC (F = 2–27 0.910; p = 0.413); however, in the hippocampus differences it was found for sex between control groups (F = 2–29 5.165 p = 0.012). The activity of the antioxidant enzyme CAT is illustrated in Fig. 3B. In male rats, CAT activity was decreased in PFC (F = 2–13 16.820; p < 0.0001; Fig. 3B) and in the hippocampus (F = 2–14 7.176; p = 0.007; Fig. 3B) of deprived animals treated with saline and ketamine when compared to the control group. In female rats, a decrease on CAT activity was also observed in PFC (F = 2–15 20.465; p < 0.0001; Fig. 3B) and hippocampus (F = 2–15 45.243; p < 0.0001; Fig. 3B) of deprived groups treated with saline and ketamine compared to the control group. Two-way ANOVA did not revealed differences for sex and groups interaction on CAT activity in the PFC (F = 2–28 0.817; p = 0.203) and hippocampus (F = 2–29 0.910 p = 0.413).
Fig. 3.
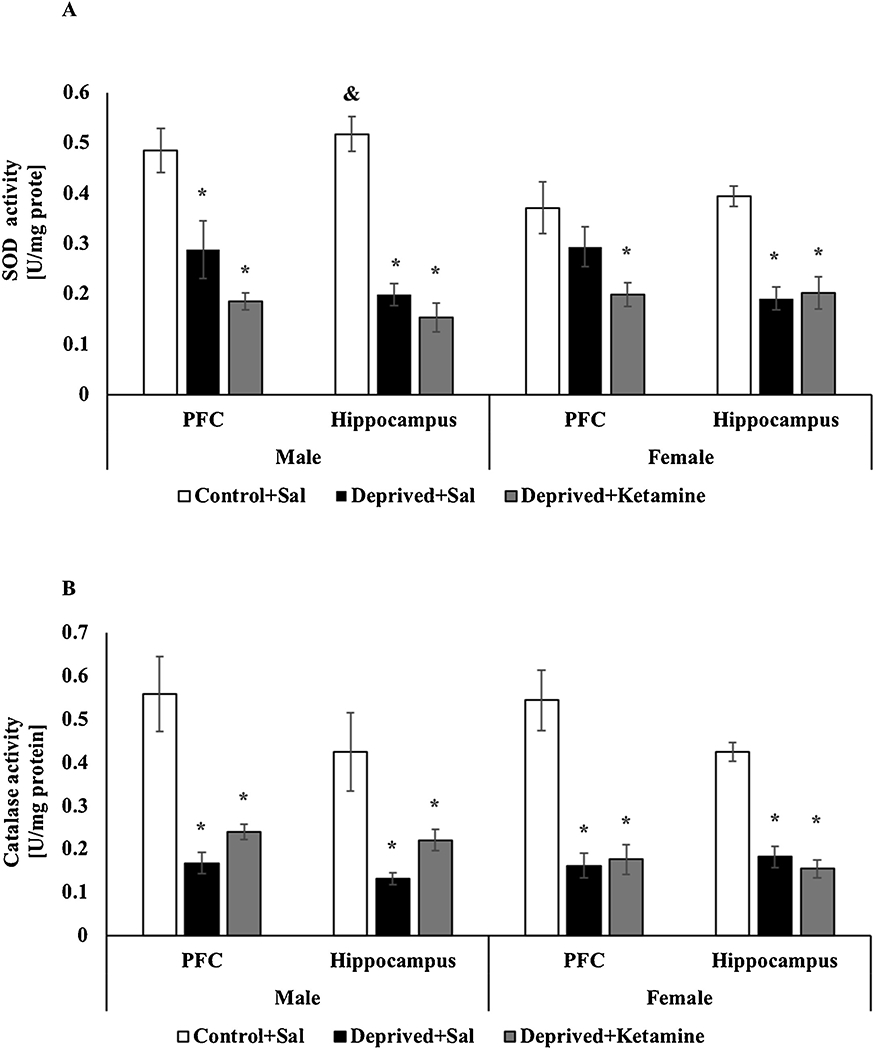
A: Effects of MD and ketamine treatment on SOD activity in PFC and hippocampus of male and female Wistar rats. Values are expressed as mean ± E.P.M. * p < 0.05 vs. Control + Sal, according to one-way ANOVA followed by Tukey’s post-hoc test; &p<0.05 vs. sex and groups interaction according to two-way ANOVA. B: Effects of MD and ketamine treatment on CAT activity in PFC and hippocampus of male and female Wistar rats. Values are expressed as mean ± E.P.M. * p < 0.05 vs. Control + Sal, according to one-way ANOVA followed by Tukey’s post-hoc test.
3.4. Effects of MD and ketamine treatment on inflammatory parameters in PFC and hippocampus of male and female Wistar rats
Fig. 4A demonstrates that in the hippocampus of deprived male rats treated with saline there was an increase in MPO activity when compared to the control group, but in deprived animals treated with ketamine, there was a decrease (F = 2–13 165.004; p < 0.0001; Fig. 4A). No statistical difference was observed in the PFC of male rats in any groups (F = 2–13 2931; p = 0.089; Fig. 4A). In females, there was a decrease in MPO activity in the PFC of the deprived group treated with ketamine compared to the control group and the deprived group treated with saline (F = 2–13 12.654; p = 0.001; Fig. 4A). There was no statistical difference in the hippocampus of females rats for any groups (F = 2–15 2.542; p = 0.112; Fig. 4A). Two-way ANOVA revealed differences for sex and groups interaction on MPO activity in the PFC (F = 2–28 40.160; p < 0.0001) and hippocampus (F = 2–28 40.160; p < 0.0001). Effects it was observed for sex in the maternal deprivation and ketamine treatment in the hippocampus and the ketamine treatment in the PFC.
Fig. 4.
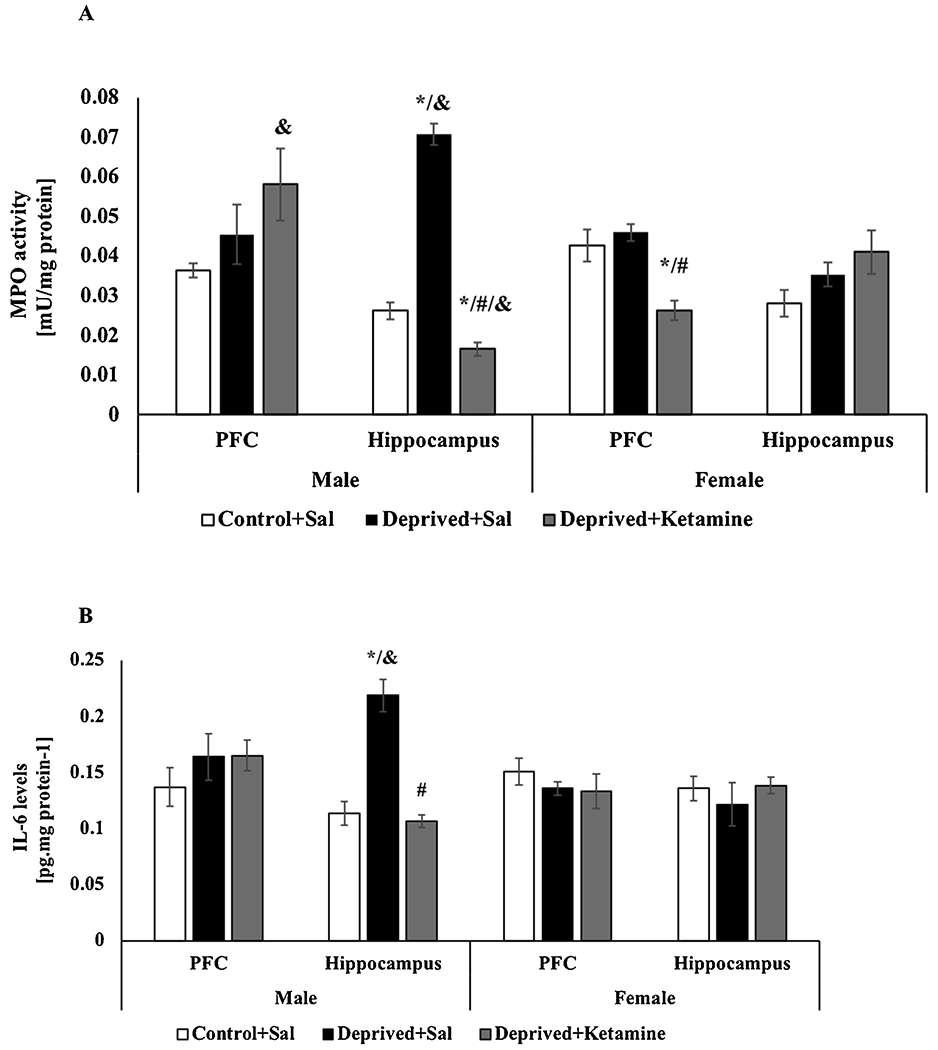
A: Effects of MD and ketamine treatment on MPO activity in PFC and hippocampus of male and female Wistar rats. Values are expressed as mean ± E.P.M. * p < 0.05 vs. Control + Sal; #p < 0.05 vs. Deprived + Sal, according to one-way ANOVA followed by Tukey’s post-hoc test; &p<0.05 vs. sex and groups interaction according to two-way ANOVA. B: Effects of MD and ketamine treatment on IL-6 levels in PFC and hippocampus of male and female Wistar rats. Values are expressed as mean ± E.P.M. * p < 0.05 vs. Control + Sal; #p < 0.05 vs. Deprived + Sal, according to one-way ANOVA followed by Tukey’s post-hoc test; &p<0.05 vs. sex and groups interaction according to two-way ANOVA.
Fig. 4B demonstrates the effects of MD and ketamine treatment on IL-6 levels in the PCF and hippocampus of male and female Wistar rats. In male rats, an increase in IL-6 levels was observed in the hippocampus of deprived rats treated with saline compared to the control group and the deprived rats treated with ketamine had a reversion of these effects (F = 2–12 34.425; p < 0.0001; Fig. 4B). In the PFC of male rats there was no statistical difference for any (F = 2–11 0.793; p < 0.0001; Fig. 4B). In the PFC (F = 2–12 0.633; p = 0.548; Fig. 4B) and hippocampus (F = 2–12 0.456; p < 0.644; Fig. 4B) of female rats, there was also no statistical difference for any groups. Two-way ANOVA did not revealed differences for sex and groups interaction on IL-6 levels in the PFC (F = 2–23 1.700; p = 0.204); however, in the hippocampus differences it was found for sex in the maternal deprivation (F = 2–24 16.252 p < 0.0001).
4. Discussion
Some studies have observed differences between females and males in responses to treatment with antidepressants (Khan et al., 2005), in the ability to remember and mood changes (Loewhental et al., 1995), and in the modulation of depressive symptoms. Still, the influence of social and psychological aspects seems important not only in the genesis of depressive states but also in the way of presenting symptoms and the role that they can play in social and family relationships (Justo et al., 2007). In some contexts, women seem to have more somatic symptoms than men. Regarding the possible differences in the depressive manifestations between men and women, in a biological context, the hormonal functioning and its consequences call attention (Justo et al., 2006). Concerning the pharmacological treatment of MDD, there is controversy about the difference in the effectiveness of antidepressants between genders (Gorman, 2006).
Still, the behavioral and neurobiological mechanisms underlying the antidepressant action of ketamine appear to be related to the brain region and the dose used. Preclinical evidence indicates that ketamine has rapid effects on synaptogenesis in PFC and hippocampus, two brain regions that have been strongly implicated in the pathophysiology of MDD (Li et al., 2010). Studies show that these two brain regions have been implicated in sex-related neurobehavioral responses to stress and antidepressant treatments (Anderson et al., 2011). Specifically, it is believed that the changes induced by ketamine in the hippocampus and PFC trigger molecular cascades related to neuroplasticity that, in turn, regulate the synthesis of synaptic proteins involved in synaptogenesis and presynaptic release machinery (Li et al., 2010). Evidence indicates that females are more sensitive to NMDA receptor antagonists, such as dizocilpine (MK-801), phencyclidine, and ketamine than males (Honack and Loscher, 1993). In fact, Honack and Loscher (1993) showed that female rats are more responsive to the motor properties of MK-801 and lower doses of the drug. Further, a previous study has shown that female rats tend to sleep more than males after the administration of anesthetic doses of ketamine (Douglas and Dagirmanjian, 1975). Jevtovic-Todorovic et al. (2001) observed that the neurotoxic effects of ketamine (40—80 mg/kg) on the retrosplenial cortex are more severe in female rats. Besides, a clinical study has shown that women who received ketamine in anesthetic doses had more psychotropic effects, such as hallucinations than men (Jevtovic-Todorovic et al., 2001). Although women can develop MDD at approximately twice the rate of men (Grigoriadis and Robinson, 2007), research on the relevant neurobiological antidepressant effects of ketamine has concentrated if almost exclusively in males. Studies have shown that maternal deprivation exposure increased plasma levels of adrenocorticotropic hormone and corticosterone in adult offspring, suggesting hyperactivation of the HPA axis and insensitivity to negative feedback of the stress hormone, cortisol (Gesing et al., 2001). In addition, the cortisol response to stress has already shown differences between the sexes by decreasing during the high estradiol phases of the menstrual cycle in females (Albert and Newhouse, 2019). The present study showed that male rats submitted to maternal deprivation increased the immobility time and decreased the climbing time in the forced swim test, demonstrating a depressive-like behavior and ketamine was able to reverse these changes, showing an antidepressant effect. Corroborating the present study, other findings also demonstrated the antidepressant effect of ketamine in male rats submitted to maternal deprivation and tested in the forced swimming test (Réus et al., 2013, 2015a; Maciel et al., 2018). Unlike males, the present study showed that maternal deprivation was not able to induce depressive-like behavior in females. In fact, sex differences have already been described (Drossopoulou et al., 2004; Dalla et al., 2005), indicating that male rats exhibit longer immobility times than in females (Bielajew et al., 2003), although previous studies have reported contradictory results (Drossopoulou et al., 2004; Dalla et al., 2005). Carrier and Kabbaj (2013) and Franceschelli et al. (2015) demonstrated that female rats were more sensitive to the rapid antidepressant effects of ketamine in the forced swimming test. This variation in results may be related to behavioral changes associated with the estrous cycle of females (Frye and Wall, 2002). In the open field test, the present study showed that there was a decrease in the number of rearings in deprived male rats treated with ketamine, with no significant change in the number of crossings in males and crossings and rearings in females. Previous studies have already shown that ketamine did not affect the open field test (Garcia et al., 2008a, b). However, another study showed that C57BL/6 mice treated with ketamine had an increase in locomotor activity compared to Balb/c mice (Akillioglu et al., 2012). The results of the present study suggest that the decrease in the number of rearings in the open field test may be related to the increase in immobility time in the forced swimming test, however, more studies are needed to explain why ketamine had this effect in the deprived animals. Constant exposure to different stress protocols induces the formation of an oxidative stress condition, which is characterized by the imbalance between the increase in the production of highly reactive species and the decrease in tissue antioxidant capacity. Sexual differences in oxidative stress parameters have been observed in preclinical and clinical studies, in which males had higher oxidative stress than females (Dantas et al., 2004; Wong et al., 2015). Gender differences were observed in the enzyme NADPH oxidase (Dantas et al., 2004; Wong et al., 2015), which is an important generator of stress oxidative in cells (Drummond et al., 2011).
Studies indicate the presence of high markers of oxidative stress and inflammation in rats submitted to the maternal deprivation protocol (Réus et al., 2017). The present study showed that in deprived male rats there was an increase in TBARS levels in the PFC and the hippocampus, however, the treatment with ketamine was able to reverse this increase. In deprived females, there was an increase in TBARS levels in the hippocampus. Regarding the levels of protein carbonylation, in both deprived males and females, there was an increase in protein carbonylation levels, and treatment with ketamine reduced these levels. Corroborating the present work, a previous study showed that male rats submitted to the maternal deprivation protocol had an increase in the levels of lipid peroxidation and protein carbonylation and that the administration of a single dose of ketamine-S reversed this damage when evaluated 14 days after the application (Réus et al., 2015b). Maternal deprivation promotes hyperfunction of the hypothalamic-pituitary-adrenal axis and exacerbated corticosterone release and numerous studies correlate the increase in glucocorticoids with the increase in oxidative stress (Schiavone et al., 2015). Findings in the literature have already shown that increased levels of nitrous oxide and nitrite are associated with the pathophysiology of MDD (Maes et al., 2011). The present study showed an increase in nitrite/nitrate concentration in the PFC and hippocampus of deprived male rats and the treatment with ketamine reversed this increase only in the PFC. In the deprived females, there was an increase in nitrite/nitrate concentration in the PFC, however, the treatment with ketamine was not able to reverse this increase. Maciel et al. (2018) showed that treatment with ketamine was able to reverse the increase in nitrite/nitrate concentration in male rats submitted to the maternal deprivation and chronic mild stress protocols only in some brain regions. This is the first study that shows the effects of maternal deprivation and ketamine treatment on nitrite/nitrate concentration in female rats. These results suggest that the neuroprotective effect of ketamine on the nitrite/nitrate concentration may be related to sex and different brain regions. The present study also demonstrated an increase in MPO activity in the hippocampus of male rats submitted to maternal deprivation and treatment with ketamine was able to reverse this increase; and in deprived females, treatment with ketamine decreased the activity of MPO in PFC concerning the control and deprived groups. In fact, the increase in MPO activity has been correlated with lipid peroxidation and depressive symptoms in humans (Vaccarino et al., 2008). In addition to ROS, MPO induces the production of pro-inflammatory cytokines involved in neurodegenerative diseases (Lefkowitz and Lefkowitz, 2008) and loss of neurogenesis in the hippocampus (Ekdahl et al., 2003). According to the present findings, Maciel et al. (2018), showed that acute treatment with ketamine was able to decrease the activity of MPO in some brain structures related to MDD. Thus, the present study suggests that ketamine is capable of having a neuroprotective effect on MPO activity. Several pathologies such as cardiovascular diseases, atherosclerosis, cancer, and neurodegenerative diseases are associated with oxidation or modification of sulfhydryl groups to suggest their role in the pathophysiology of these conditions. In fact, the present study showed that only male rats subjected to maternal deprivation decreased the sulfhydryl content in the PFC and the hippocampus, suggesting that maternal deprivation led to higher damage to proteins in these brain regions and that this may also be related to sex. In addition, ketamine did not affect the sulfhydryl content of male or female rats submitted to the maternal deprivation protocol. This is the first study that shows the effect of ketamine on the sulfhydryl content in brain regions of male and female rats submitted to maternal deprivation, suggesting that the antidepressant effect of ketamine may be related to other brain pathways related to oxidative stress, such as peroxidation lipid and protein carbonylation.
The present study showed that maternal deprivation was able to decrease the SOD and CAT activities in the PFC and hippocampus of male and female rats. In fact, other authors have already observed that in animals submitted to the maternal deprivation protocol, SOD and CAT activities are reduced in brain regions related to mood regulation, such as PFC, hippocampus, and nucleus accumbens (Réus et al., 2015c; Maciel et al., 2018). Besides, the present study also showed that deprived male and female rats treated with ketamine had no changes in SOD and CAT activities. Gazal et al. (2014) demonstrated that treatment with ketamine was able to decrease the activity of the antioxidant enzymes SOD and CAT in animals submitted to an animal model of mania. Furthermore, Hou et al. (2013) showed that the chronic administration of ketamine in animals submitted to the animal model of schizophrenia was able to increase nitric oxide in the PFC, hippocampus, and serum, and decreased the activity of SOD in the hippocampus. However, depending on the time that ketamine is administered, it may have an antioxidant effect. As shown by Réus et al. (2015c), SOD activity was increased in control animals that received ketamine for 14 days in PFC and nucleus accumbens and were decreased in deprived animals that received saline and ketamine.
Studies have already shown that women and men have differences in immune activation (Fish, 2008) and that these differences in inactivation may be related to the symptoms and prevalence of MDD. In fact, sexual differences in inflammatory markers related to MDD were reported by Domenici et al. (2010), who found significant interactions between sex and diagnosis, including growth hormones and proteins involved in the immune response. IL-6 is a potent inflammatory cytokine, with a redundant and pleiotropic activity that mediates a series of physiological functions, including lymphocyte differentiation, cell proliferation, and survival, in addition to potentiating apoptotic signals (Kamimura et al., 2003). Pre-clinical and clinical studies have shown that IL-6 may have an important role in the pathophysiology of MDD as well as in the effects of its treatment (Nukina et al., 2001; Jankord et al., 2010). Increased levels of IL-6 can lead to hypothalamic-pituitary-adrenal axis dysfunction, changes in synaptic neurotransmission, and reduced neurotrophic factors. The present study showed that deprived male rats had an increased level of IL-6 in the hippocampus and treatment with ketamine was able to reverse this effect. Nukina et al. (2001) demonstrated that plasma concentrations of IL-6 increased after an hour of stress, and shortly thereafter, gradually decreased. Still, another study showed that there was an increase in IL-6 mRNA expression with an increase in circulating of IL-6 levels in the hypothalamus of rats subjected to the stress protocol (Jankord et al., 2010). Regarding the treatment with ketamine, Yang et al. (2013) showed that administration of this drug significantly decreased the immobility time of the rats in the forced swimming test and decreased the expression of IL-6 in the PFC and hippocampus. The findings of the present study suggest that maternal deprivation was able to induce an inflammatory state in some brain regions related to MDD and the antidepressant effects induced by ketamine may be associated with decreased levels of IL-6 in the brain.
5. Conclusions
The results obtained with this researchmade it possible to observe the differences in the action of ketamine in brain areas involved with MDD and also the activity of biomarkers associated with depressive behaviors in male and female animals. Such results include ketamine as a promising way of treating MDD, considering the reduction and reversal of neuroinflammatory biomarkers. The experimental model of maternal deprivation was also fundamental to obtain the results. However, it is necessary to observe the variables highlighted in the discussion presented, considering the results obtained in male and female rats as well as the variables observed in different regions of the brain involved with MDD. In this context, even though the results are effective with inflammatory and oxidative stress biomarkers, further studies are suggested to evaluate the behavior of females and males in other periods of life, as well as the possible inhibition of the estrous cycle, for investigation of the discrepancies observed about maternal deprivation and the effects of ketamine in other stages of life, especially in females. Finally, it is considered that the findings of this study will contribute to future research in the search for new and increasingly effective treatment alternatives for MDD.
Acknowledgments
Translational Psychiatry Program (USA) is funded by a grant from the National Institute of Health/National Institute of Mental Health (1R21MH117636-01A1, to JQ). Center of Excellence on Mood Disorders (USA) is funded by the Pat Rutherford Jr. Chair in Psychiatry, John S. Dunn Foundation and Anne and Don Fizer Foundation Endowment for Depression Research. Translational Psychiatry Laboratory (Brazil) is funded by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (GZR and JQ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (JQ and GZR), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) (GZR and JQ), and Instituto Cérebro e Mente (JQ and GZR). JQ is a 1A and GZR is a 2 CNPq Researches Fellow.
Abbreviations:
- MD
maternal deprivation
- SOD
superoxide dismutase
- CAT
catalase
- MPO
myeloperoxidase
- IL-6
interleukin-6
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Aebi H, 1984. Catalase in vitro. Met. Enzym 105, 121–126. [DOI] [PubMed] [Google Scholar]
- Akillioglu K, Melik EB, Melik E, Boga A, 2012. Effect of ketamine on exploratory behaviour in BALB/C and C57BL/6 mice. Pharmacol. Biochem. Behav 100, 513–517. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Markesbery WR, 2001. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci. Lett 302, 141–145. [DOI] [PubMed] [Google Scholar]
- Albert KM, Newhouse PA, 2019. Estrogen, stress, and depression: cognitive and biological interactions. Annu. Rev. Clin. Psychol 15, 399–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IM, Juhasz G, Thomas E, Downey D, McKie S, Deakin JF, Elliott R, 2011. The effect of acute citalopram on face emotion processing in remitted depression: a pharmacoMRI study. Eur. Neuropsychopharmacol 21, 140–148. [DOI] [PubMed] [Google Scholar]
- Bannister JV, Calabrese L, 1987. Assays for superoxide dismutase. Met. Biochem. Anal 32, 279–312. [DOI] [PubMed] [Google Scholar]
- Bielajew C, Konkle A, Kentiner A, Baker S, Stewart A, Hutchins A, et al. , 2003. Strain and gender specific effects in the forced swin test: effects of prévios stress exposure. Estress. 6, 269–280. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M, 2013. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacol. 70, 27–34. [DOI] [PubMed] [Google Scholar]
- Dalla C, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z, 2005. Chronic mild stress impact: are females more vulnerable? Neuroci. 135, 703–714. [DOI] [PubMed] [Google Scholar]
- Dantas AP, do Franco MC, Silva-Antonialli MM, Tostes RC, Fortes ZB, Nigro D, Carvalho MH, 2004. Gender differences in superoxide generation in microvessels of hypertensive rats: role of NAD(P)H-oxidase. Cardiovasc. Res 61, 22–29. [DOI] [PubMed] [Google Scholar]
- De Raedt R, Koster EHW, 2010. Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cogn. Affect. Behav. Neurosci 10, 50–70. [DOI] [PubMed] [Google Scholar]
- De Young LM, Kheifets JB, Ballaron SJ, Young JM, 1989. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 126, 335–341. [DOI] [PubMed] [Google Scholar]
- Domenici E, Willé DR, Tozzi F, Prokopenko I, Miller S, McKeown A, et al. , 2010. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One 5, e9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas BG, Dagirmanjian R, 1975. The effects of magnesium deficiency of ketamine sleeping times in the rat. Br. J. Anaesth 47, 336–340. [DOI] [PubMed] [Google Scholar]
- Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, Papadopoulou-Daifoti Z, 2004. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neurosci. 12, 849–857. [DOI] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, Sobey CG, 2011. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov 10, 453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O, 2003. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. U.S.A 100, 13632–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, 1990. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 21, 186–207. [DOI] [PubMed] [Google Scholar]
- Fish EN, 2008. The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol 8, 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM, 2015. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neurosci. 49–60. [DOI] [PubMed] [Google Scholar]
- Frye C, Walf A, 2002. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm. Behav 41, 306–315. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J, 2008a. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 140–144. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Andreazza AC, Stertz L, et al. , 2008b. Chronic administration of ketamine elicits entidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels basic. Clin. Phasmacol. Toxicol 103, 502–506. [DOI] [PubMed] [Google Scholar]
- Gazal M, Valente MR, Acosta BA, Kaufmann FN, Braganhol E, Lencina CL, Stefanello FM, Ghisleni G, Kaster MP, 2014. Neuroprotective and antioxidant effects of curcumin in a ketamine-induced model of mania in rats. Eur. J. Pharmacol 724, 132–139. [DOI] [PubMed] [Google Scholar]
- Gesing A, Bilang-Bleuel A, Droste SK, Linthorst AC, Holsboer F, Reul JM, 2001. Psychological stress increases hippocampal mineralocorticoid receptor levels: involvement of corticotropin-releasing hormone. J. Neurosci 21, 4822–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, 2006. Gender differences in depression and response to psychotropic medication. J. Gend. Med 3, 93–109. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR, 1982. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal. Biochem 126, 131–138. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, Robinson GE, 2007. Gender issues in depression. Ann. Clin. Psychiatry 19, 247–255. [DOI] [PubMed] [Google Scholar]
- Hammen C, 2005. Stress and depression. Annu. Rev. Clin. Psychol 1, 293–319. [DOI] [PubMed] [Google Scholar]
- Honack W, Loscher, 1993. Sex differences in NMDA receptor mediated responses in rats. Brain Res. 620, 167–170. [DOI] [PubMed] [Google Scholar]
- Hou Y, Zhang H, Xie G, Cao X, Zhao Y, Liu Y, Mao Z, Yang J, Wu C, 2013. Neuronal injury, but not microglia activation, is associated with ketamine-induced experimental schizophrenic model in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 45, 107–116. [DOI] [PubMed] [Google Scholar]
- Jankord R, Zhang R, Flak JN, Solomon MB, Albertz J, Herman JP, 2010. Stress activation of il-6 neurons in the hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol 299, R343–R351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Wozniak DF, Benshoff ND, Olney JW, 2001. A comparative evaluation of the neurotoxic properties of ketamine and nitrous oxide. Brain Res. 895, 264–267. [DOI] [PubMed] [Google Scholar]
- Justo LP, Calil HM, 2006. Depression – does it affect equally men and women? Rev. Psiq. Clín 33, 74–79. [Google Scholar]
- Justo LP, Borsonelo EC, Guerra ABG, Calil HM, 2007. Anxious and depressive symptoms in Latin American women: questions about societal factors. J. Affect. Disord 102, 259–264. [DOI] [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T, 2003. IL-6 signal transduction and its physiological roles: the signal orchestration model. Ver. Physiol. Biochem. Pharmacol 149, 1–38. [DOI] [PubMed] [Google Scholar]
- Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL, 2017. Oxidative stress in atherosclerosis. Curr. Atheroscler. Repo 19, 42. [DOI] [PubMed] [Google Scholar]
- Kessler RC, 2003. Epidemiology of women and depression. J. Affect. Disord 74, 5–13. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and ageof-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA, 2005. Sex differences in antidepressant response in recent antidepressant clinical trials. J. Clin. Psychopharmacol 25, 318–324. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Leeuwenburgh C, Prolla TA, 2006. Mitochondrial DNA mutations and apoptosis in mammalian aging. Cancer Res. 66, 7386–7389. [DOI] [PubMed] [Google Scholar]
- Lefkowitz DL, Lefkowitz SS, 2008. Microglia and myeloperoxidase: a deadly partnership in neurodegenerative disease. Free Radic. Biol. Med 45, 726–731. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, 1990. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 186, 464–478. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS, 2010. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhong S, Liao X, Chen J, He T, Lai S, Jia Y, 2015. A meta-analysis of oxidative stress markers in depression. PLoS One 10, e0138904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewhental K, Goldblatt V, Gordon T, Lubitsch G, Bicknell H, Fellowes D, et al. , 1995. Gender and depression in anglo-jewry. Psychol. Med 25, 1051–1063. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ, 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem 193, 265–275. [PubMed] [Google Scholar]
- Lupien SJ, Mcewen BS, Gunnar MR, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Rev. Neurosci 10, 434–445. [DOI] [PubMed] [Google Scholar]
- Maciel AL, Abelaira HM, de Moura AB, de Souza TG, Rosa T, Matos D, Tuon T, Garbossa L, Strassi AP, Fileti ME, Goldim MP, Mathias K, Petronilho F, Quevedo J, Réus GZ, 2018. Acute treatment with ketamine and chronic treatment with minocycline exert antidepressant-like effects and antioxidant properties in rats subjected different stressful events. Brain Res. Bull 137, 204–216. [DOI] [PubMed] [Google Scholar]
- Maes M, Galecki P, Chang YS, Berk M, 2011. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 676–692. [DOI] [PubMed] [Google Scholar]
- Martin LA, Neighbors HW, Griffith DM, 2013. The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. JAMA Psychiatry 70, 1100–1106. [DOI] [PubMed] [Google Scholar]
- Millett CE, Phillips BE, Saunders EFH, 2019. The sex-specific effects of LPS on depressive-like behavior and oxidative stress in the Hippocampus of the mouse. Neuroscience. 399, 77–88. [DOI] [PubMed] [Google Scholar]
- Nukina H, Sudo N, Aiba Y, Oyama N, Koga Y, Kubo C, 2001. Restraint stress elevates the plasma interleukin-6 levels in germ-free mice. J. Neuroimmunol 115, 46–52. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1986. The Rat Brain: Stereotaxic Coordinates, second ed. Academic Press, Australia. [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M, 1977. Animal model of depression. Nature 266, 730–732. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Stringari RB, Ribeiro KF, Cipriano AL, Panizzutti BS, Stertz L, Lersch C, Kapczinski F, Quevedo J, 2011. Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochem. Res 36, 460–466. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Abelaira HM, dos Santos MA, Carlessi AS, Tomaz DB, Neotti MV, Liranço JL, Gubert C, Barth M, Kapczinski F, Quevedo J, 2013. Ketamine and imipramine in the nucleus accumbens regulate histone deacetylation induced by maternal deprivation and are critical for associated behaviors. Behav. Brain Res 256, 451–456. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Nacif MP, Abelaira HM, Tomaz DB, dos Santos MA, Carlessi AS, da Luz JR, Gonçalves RC, Vuolo F, Dal-Pizzol F, Carvalho AF, Quevedo J, 2015a. Ketamine ameliorates depressive-like behaviors and immune alterations in adult rats following maternal deprivation. Neurosci. Lett 584, 83–87. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Carlessi AS, Titus SE, Abelaira HM, Ignácio ZM, da Luz JR, Matias BI, Bruchchen L, Florentino D, Vieira A, Petronilho F, Quevedo J, 2015b. A single dose of S-ketamine induces long-term antidepressant effects and decreases oxidative stress in adulthood rats following maternal deprivation. Dev. Neurobiol 75, 1268–1281. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Nacif MP, Abelaira HM, Tomaz DB, dos Santos MA, Carlessi AS, Matias BI, da Luz JR, Steckert AV, Jeremias GC, Scaini G, Morais MO, Streck EL, Quevedo J, 2015c. Ketamine treatment partly reverses alterations in brain derived- neurotrophic factor, oxidative stress and energy metabolism parameters induced by an animal model of depression. Curr. Neurovasc. Res 12, 73–84. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Fernandes GC, De Moura AB, Silva RH, Darabas C, De Souza TG, Abelaira HM, Carneiro C, Wendhausen D, Michels M, Pescador B, Dal-Pizzol F, Macêdo D, Quevedo J, 2017. Early life experience contributes to the developmental programming of depressive-like behaviour, neuroinflammation and oxidative stress. J. Psychiatr. Res 95, 196–207. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Silva RH, de Moura AB, Presa JF, Abelaira HM, Abatti M, Vieira A, Pescador B, Michels M, Ignácio ZM, Dal-Pizzol F, Quevedo J, 2019. Early maternal deprivation induces microglial activation, alters glial fibrillary acidic protein immunoreactivity and indoleamine 2,3-Dioxygenase during the development of offspring rats. Mol. Neurobiol 56, 1096–1108. [DOI] [PubMed] [Google Scholar]
- Samhan-Arias AK, Martin-Romero FJ, Gutierrez-Merino C, 2004. Kaempferol blocks oxidative stress in cerebellar granule cells and reveals a key role for reactive oxygen species production at the plasma membrane in the commitment to apoptosis. Free Radic. Biol. Med 37, 48–61. [DOI] [PubMed] [Google Scholar]
- Schiavone S, Colaianna M, Curtis L, 2015. Impact of early life stress on the pathogenesis of mental disorders: relation to brain oxidative stress. Curr. Pharm. Des 21, 404–412. [DOI] [PubMed] [Google Scholar]
- Shah ZA, Gilani RA, Sharma P, Vohora SB, 2005. Attenuation of stress-elicited brain catecholamines, serotonin and plasma corticosterone levels by calcined gold preparations used in Indian system of medicine. Basic Clin. Pharmacol. Toxicol 96, 469–474. [DOI] [PubMed] [Google Scholar]
- Silverstein B, Edwards T, Gamma A, Ajdacic-Gross V, Rossler W, et al. , 2013. The role played by depression associated with somatic symptomatology in accounting for the gender difference in the prevalence of depression. Soc. Psychiatr. Epidemiol 48, 257–263. [DOI] [PubMed] [Google Scholar]
- Sramek JJ, Murphy MF, Cutler NR, 2016. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci 18, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Brennan ML, Miller AH, Bremner JD, Ritchie JC, Lindau F, Veledar E, Su S, Murrah NV, Jones L, Jawed F, Dai J, Goldberg J, Hazen SL, 2008. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol. Psychiatry 64, 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PS, Randall MD, Roberts RE, 2015. Sex differences in the role of NADPH oxidases in endothelium-dependent vasorelaxation in porcine isolated coronary arteries. Vascul. Pharmacol 72, 83–92. [DOI] [PubMed] [Google Scholar]
- Yang C, Hong T, Shen J, Ding J, Dai XW, Zhou ZQ, Yang JJ, 2013. Ketamine exerts antidepressant effects and reduces il-1beta and il-6 levels in rat prefrontal cortex and hippocampus. Exp. Med 5, 1093–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]


