Abstract
Core needle biopsy (CNB) of breast lesions is routine for diagnosis and treatment planning. Despite refinement of diagnostic criteria, the diagnosis of breast lesions on CNB can be challenging. At many centers, including ours, confirmation of diagnoses rendered in other laboratories is required before treatment planning. We identified CNBs first diagnosed elsewhere that were reviewed in our department over the course of one year because the patients sought care at our center and in which a change in diagnosis had been recorded. The outside and in-house CNB diagnoses were then classified based on Breast WHO 5th ed. diagnostic categories. The impact of the change in diagnosis was estimated based on the subsequent surgical management. Findings in follow-up surgical excisions (EXC) were used for validation. In 2018, 4950 outside cases with CNB were reviewed at our Center. 403 CNBs diagnoses were discrepant. Of these, 147 had a change in the WHO diagnostic category: 80 (54%) CNBs had a more severe diagnosis and 44 (30%) a less severe diagnosis. In 23 (16%) CNBs the change of diagnostic category had no impact on management. Intraductal proliferations (n=54), microinvasive carcinoma (n=18) and papillary lesions (n=35) were the most disputed diagnoses. The in-house CNB diagnosis was confirmed in most cases with available EXC. Following CNB reclassification, 22/147 (15%) lesions were not excised. A change affecting the surgical management at our center occurred in 2.5% of all CNBs. Our results support routine review of outside breast CNB as a clinically significant practice before definitive treatment.
Keywords: breast, consultation, core biopsy, second opinion, quality assurance
Introduction
Accurate pathological diagnosis of breast lesions is essential for optimal patient care. Continuous refinement and standardization of diagnostic criteria has been fundamental to improving diagnostic reproducibility.1–3 Nonetheless, the diagnosis of breast lesions remains challenging and interobserver diagnostic agreement is not perfect.4–7 As treatment options for breast carcinomas have increased in complexity, patients and practitioners frequently pursue second opinions before initiating definitive treatment.8 High-risk breast lesions may invite even greater disagreement than malignant lesions, and second opinions play a critical role in avoiding overdiagnosis and guiding surveillance and risk reduction strategies.9 Accordingly, confirmation of pertinent prior pathologic diagnoses is required at most tertiary care centers, including ours, before initiating definitive treatment.
The CNB diagnosis of breast lesions is especially important, as it usually sets the treatment course. In 2004, a study comparing local and central CNB diagnoses found diagnostic agreement in 96% of cases, with 99% agreement for benign lesions and 97% for invasive cancers.10 However, in the past two decades the surgical management of breast cancer and high risk lesions has undergone substantial changes. Currently no EXC is performed routinely after CNB diagnosis of classic lobular neoplasia or papilloma without atypia with radiologic-pathologic concordance.11 In contrast, patients with stage II-III triple-negative or HER2-positive carcinomas, or tumors larger than 5 cm usually received neoadjuvant chemotherapy before definitive surgery. Because of these changes, diagnostic confirmation by “second look” of the tumor in the treatment-naïve EXC specimen, or in the follow-up surgical EXC of a high-risk lesion is no longer routine. In this context, review of the original CNB material and confirmation of the CNB diagnosis rendered elsewhere becomes even more critical.
This study aimed to assess the frequency and severity of changes in the diagnosis of breast CNBs documented at our institution over the course of one year. In cases with available follow-up surgical excision (EXC), the diagnosis was used for validation of the CNB interpretation.
Materials and Methods
Identification of cases
The study was approved by the Institutional Review Board. A search of the anatomic pathology laboratory information system identified all breast pathology cases diagnosed elsewhere that were reviewed in the department between January 1st and December 31st, 2018 because patients sought care at our center. These “departmental consults” (DCs) do not include “personal consults” submitted directly to our breast pathologists for an expert opinion on diagnosis. On any given day breast DC cases are reviewed and diagnosed by the staff breast pathologist on service. If the breast pathologist does not fully agree with the outside diagnosis, the DC case is reviewed at the intradepartmental breast pathology consensus conference and/ or by at least one senior staff breast pathologist to adjudicate the diagnosis. At the time of sign out the staff pathologist records agreement or disagreement with outside diagnosis in the quality-assurance module. In 2018, the breast pathology team consisted of 13 breast pathologists with 2 to 25 years of practice.
An electronic search of the 2018 breast DC cases identified all cases recorded as diagnostic disagreements. One of the study pathologists (C.C.) verified the specimen types of all cases with a diagnostic disagreement. Only cases with a disagreement pertaining to the CNB diagnosis were included in the study.
Classification and grouping of the histological diagnoses
The histological diagnoses were grouped according to the World Health Organization Classification of Tumours of the Breast (5th Edition, 2019), with minor modifications.12 The diagnostic categories are listed in Table 1. Diagnostic discrepancies were identified by comparing the diagnosis rendered by the original pathologist (“outside diagnosis”) with the diagnosis rendered by the staff breast pathologist who reviewed the case at our center (“in-house diagnosis”). Discrepancies involving non-mammary malignancies (e.g. hematolymphoid malignancy, mesenchymal tumors, skin adnexal tumors, extramammary metastasis) were classified separately.
Table 1.
Diagnostic categories (modified from WHO 5th ed. classification).
| 1 | Benign breast | Normal (non-atypical) breast parenchyma | Normal breast Inflammatory change Radiation change |
|---|---|---|---|
| Benign (non-atypical) epithelial proliferations | Fibrocystic changes Columnar cell change Sclerosing adenosis Mucocele-like lesion without atypia | ||
| 2 | Radial scar without atypia | ||
| Radial scar with atypia / complex sclerosing lesion | |||
| 3 | Flat epithelial atypia (FEA) (columnar cell change with atypia) | ||
| 4 | Classic lobular carcinoma in situ (cLCIS) Atypical lobular hyperplasia (ALH) | ||
| 5 | Pleomorphic / florid LCIS | ||
| 6 | Atypical ductal hyperplasia (ADH) | ||
| 7 | Ductal carcinoma in situ (DCIS) | ||
| 8 | Microinvasive carcinoma (mIC) | ||
| 9 | Invasive breast carcinoma (IBC) (any type) | ||
| 10 | Papilloma | Without atypia | |
| With ADH | |||
| With DCIS | |||
| 11 | Papillary carcinoma | Without invasion | Encapsulated papillary carcinoma (EPC) w/o invasion Solid papillary carcinoma (SPC) in situ |
| With microinvasion | EPC with mIC SPC with mIC |
||
| Invasive | Invasive papillary carcinoma Invasive SPC IBC near EPC or SPC |
||
| 12 | Fibroadenoma | ||
| 13 | Fibroepithelial lesion (not specified as fibroadenoma) Phyllodes tumor |
||
Identification of changes in diagnosis with impact on surgical management
The surgical management of breast lesions diagnosed at CNB is standardized based on clinical presentation, imaging findings, and CNB diagnosis, according to guidelines endorsed by the National Comprehensive Cancer Network (NCCN) (https://www.nccn.org/guidelines)13 and American Society of Breast Surgeons (https://www.breastsurgeons.org/resources/statements).11 At our center, surgical management decisions also take into account information obtained by retrospective analysis of our own data.14–16 For example, the CNB diagnosis of ADH prompts EXC of the lesion, whereas the CNB diagnosis of DCIS or DCIS suspicious but not diagnostic of microinvasion usually prompts EXC together with removal of separate margin specimens. A sentinel lymph node biopsy is usually performed only if the CNB diagnosis documents definite (micro)invasion. As another example, a radiology-pathology concordant CNB diagnosis of papilloma without atypia rarely mandates EXC, whereas in most cases EXC is still routinely performed for a CNB diagnosis of radial scar.
Building on this knowledge and with input from our breast surgeons, changes in the diagnosis with substantial impact on the patient’s surgical management were identified.
Correlation with the findings in the follow-up surgical excision
Whenever available, information of the findings in the follow-up surgical excision specimen was correlated with the in-house CNB diagnoses.
Results
Identification of core needle biopsies with diagnostic disagreement impacting surgical management
A total of 6635 breast DC cases were identified within the study period, including an estimated 4950 cases with breast CNB(s). Of the 6635 cases, 599 had a disagreement reported in the pathology data system. Upon re-review, 5 cases were excluded because no diagnostic disagreement was identified. Hence a total of 594/6635 (9%) cases were confirmed as discordant.
The 594 cases recorded as having a diagnostic disagreement included 403 (68%) CNBs, 144 (24%) surgical specimens (75 lumpectomies, 48 mastectomies, and 21 margin excisions), 29 (5%) lymph nodes, 13 (2%) cytology specimens (including fine needle aspirations and pleural fluids), and 5 (0.8%) biopsies of metastases to extramammary sites.
Of the 403 CNBs with diagnostic disagreements recorded in the pathology data system, 256 CNBs had a disagreement pertaining to parameters such as tumor grade, size, lymphovascular invasion, presence or absence of DCIS together with an invasive carcinoma, or other incidental findings with no substantial impact on the immediate management of the patient. These CNBs were excluded from further analysis. In the remaining 147 (36%) CNBs the diagnostic disagreement entailed a change in the WHO 5th ed. diagnostic classification category. These biopsies constitute our study cohort.
The process of case selection is illustrated in Figure 1.
Figure 1.
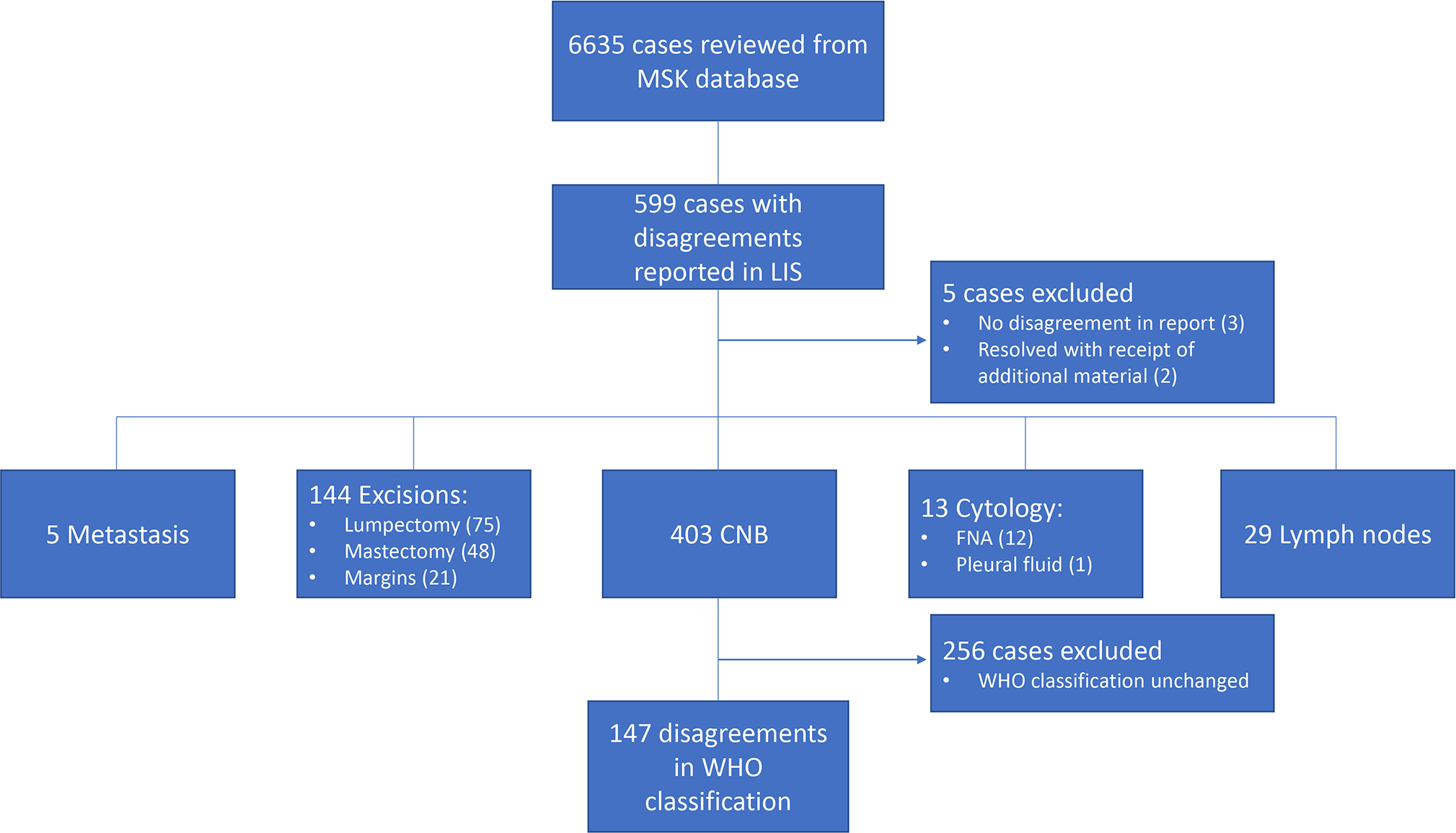
Case selection.
CNBs Study Cohort
The 147 CNBs with differences in the diagnostic category were from 146 patients, including one patient with bilateral CNBs. All patients were females. The mean patient age at diagnosis was 56 years (range 24–92 years). The outside and in-house diagnoses of these cases are summarized in Table 2.
Table 2.
Changes in CNB diagnoses based on WHO diagnostic categories between outside (y-axis) and MSK (x-axis).
| In-House/MSK Diagnosis | Total | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DUCTAL | LOBULAR | mIC | IBC | PAPILLOMA | PAPILLARY CARCINOMA | Fibroadenoma | Fibroepithelial tumors | |||||||||||||
| Benign breast | Radial scar/ complex sclerosing lesion | FEA | ADH | DCIS | cLCIS/ ALH | Pleomorphic/ florid LCIS | without atypia | with ADH | with DCIS | without invasion | with mIC | invasive | ||||||||
| Outside Diagnosis | DUCTAL | Benign breast | 12 | 2 | 1 | 1 | 1 | 17 | ||||||||||||
| Radial scar/ complex sclerosing lesion | 1 | 1 | 1 | 1 | 4 | |||||||||||||||
| FEA | 6 | 3 | 1 | 10 | ||||||||||||||||
| ADH | 11 | 1 | 17 | 3 | 2 | 2 | 36 | |||||||||||||
| DCIS | 10 | 3 | 2 | 17 | 4 | 1 | 1 | 38 | ||||||||||||
| LOBULAR | cLCIS/ALH | 3 | 1 | 4 | ||||||||||||||||
| Pleomorphic/ florid LCIS | 0 | |||||||||||||||||||
| mIC | 0 | |||||||||||||||||||
| IBC | 1 | 1 | 2 | 1 | 5 | |||||||||||||||
| PAPILLOMA | without atypia | 5 | 2 | 4 | 2 | 3 | 16 | |||||||||||||
| with ADH | 1 | 1 | 2 | |||||||||||||||||
| with DCIS | 2 | 2 | ||||||||||||||||||
| PAPILLARY CARCINOMA | without invasion | 2 | 1 | 3 | ||||||||||||||||
| with mIC | 1 | 1 | ||||||||||||||||||
| invasive | 1 | 1 | ||||||||||||||||||
| Fibroadenoma | 2 | 2 | 4 | |||||||||||||||||
| Fibroepithelial tumors | 1 | 1 | 2 | |||||||||||||||||
| Total | 25 | 2 | 1 | 33 | 21 | 9 | 2 | 18 | 11 | 3 | 6 | 0 | 6 | 2 | 0 | 4 | 2 | 145 * | ||
ADH = atypical ductal hyperplasia, ALH = atypical lobular hyperplasia, cLCIS = classic lobular carcinoma in situ, DCIS = ductal carcinoma in situ, FEA = flat epithelial atypia, IBC = invasive breast carcinoma, mIC = microinvasive carcinoma.
Does not include one case of skin adnexal tumor and one case of spindle cell sarcoma.
Overall, a more severe diagnosis was rendered in 80 (54%) CNBs [i.e. benign to atypical or in situ carcinoma, in situ carcinoma to (micro)invasive carcinoma] and a less severe diagnosis in 44 (30%). According to our current patient practices, reclassification of these 124 CNBs had an immediate impact on the patients’ surgical management. These 124 CNBs constituted 2.5% of approximately 4950 cases with CNBs reviewed over the course of one year.
In the remaining 23 (16%) CNBs the change in diagnosis involved reclassification to a different diagnostic group with equivalent surgical management, at least at our center, compatibly with radiologic-pathologic concordance (e.g. benign papilloma to normal breast, or DCIS to solid papillary carcinoma in situ).
A summary of all CNBs with diagnostic disagreement (with or without surgical management impact) is presented below, with cases grouped according to the final diagnosis.
Normal breast / benign epithelial proliferations
A total of 25/147 (17%) cases were reclassified as normal breast/benign epithelial proliferations without atypia. The original diagnoses included 6 (24%) FEA, 11 (44%) ADH, 5 (20%) papillomas without atypia, 2 (8%) fibroadenomas, and 1 (4%) fibroepithelial lesion (reclassified as fibroadenomatoid changes).
Radial scar/complex sclerosing lesion
A total of 2/147 (1%) cases were reclassified as radial scar/complex sclerosing lesion. The original diagnosis was papilloma without atypia in both cases.
FEA
One of 147 (0.7%) case was reclassified as FEA. The original diagnosis was ADH.
Classic LCIS/ALH
A total of 9/147 (6%) cases were reclassified as classic LCIS/ ALH. The original diagnoses included 2 (22%) normal breast/benign epithelial proliferations without atypia, 1 (11%) radial scar, 3 (33%) ADH (Supplemental Figure 1), and 3 (33%) DCIS.
Pleomorphic/florid LCIS
A total of 2/147 (1%) cases, both with original diagnoses of DCIS, were reclassified as florid LCIS (Supplemental Figure 2). Loss of E-cadherin expression in the tumor cells supported a lobular phenotype in both cases. In one case the E-cadherin stain was performed in-house using submitted unstained slides. In the other case the E-Cadherin stain had been received together with the rest of the slides, but the interpretation differed.
ADH
A total of 33/147 (22%) cases were reclassified as ADH. The original diagnoses included 12 (36%) normal breast/benign epithelial proliferations without atypia (Supplemental Figure 3), 1 (3%) radial scar, 3 (9%) FEA, 3 (9%) classic LCIS/ALH, 10 (30%) DCIS, and 4 (12%) papillomas without atypia.
DCIS
A total of 21/147 (14%) cases were reclassified as DCIS (without microinvasion). The original diagnoses included 1 (5%) radial scar/complex sclerosing lesion (Figure 2A–B), 17 (81%) ADH, 1 (5%) IBC (reclassified as DCIS involving a sclerosing lesion), and 2 (10%) papillomas without atypia (Figure 2E–F).
Figure 2.
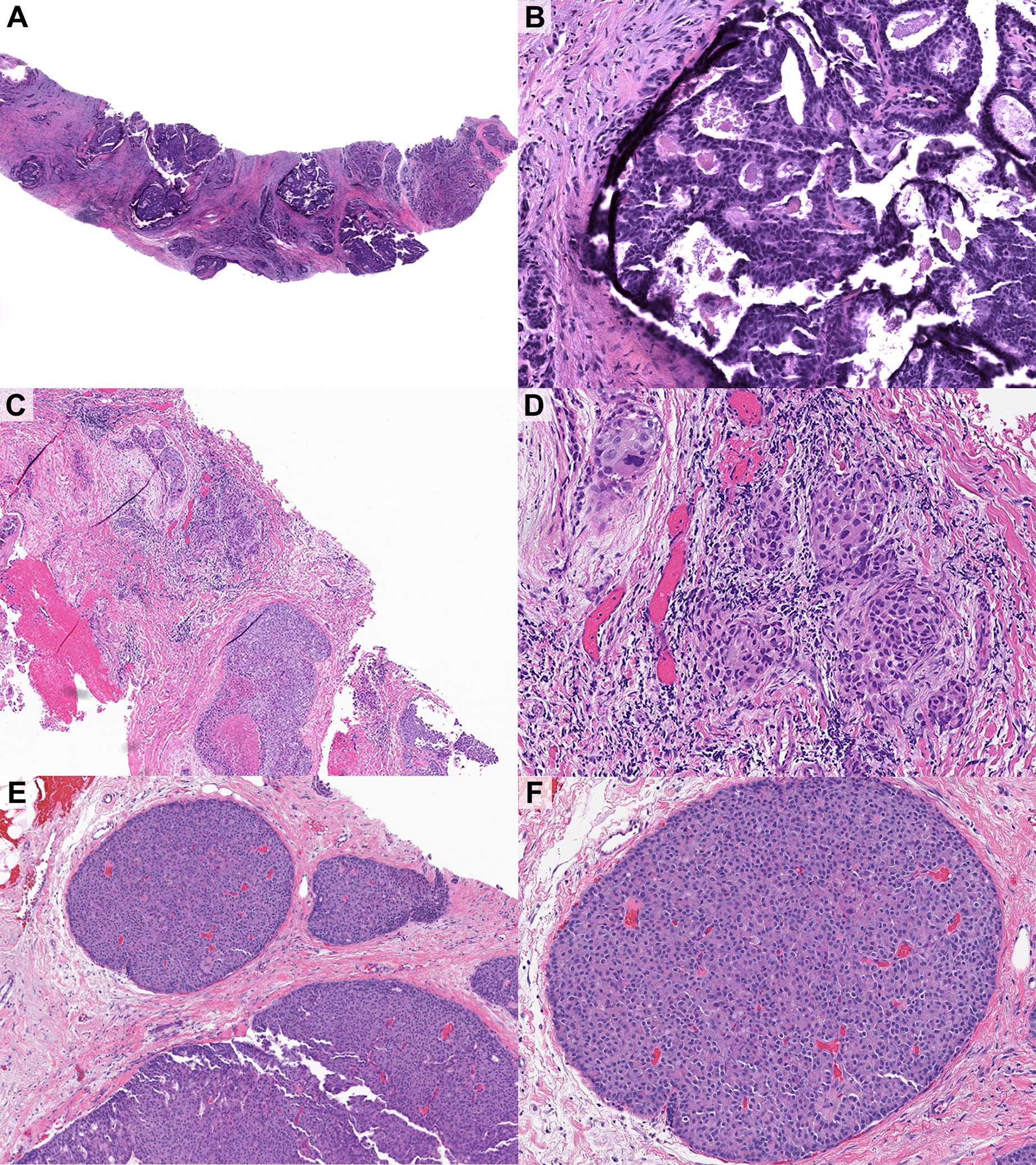
Examples of disagreements in core needle biopsy diagnoses. A-B) DCIS arising in the background of a complex sclerosing lesion, diagnosed outside as complex sclerosing lesion. Excision showed approximately 1 cm of DCIS. C-D) Microinvasive carcinoma arising in the background of solid high-grade DCIS, diagnosed outside as DCIS. Excision showed multiple foci of microinvasive carcinoma. The patient also underwent sentinel lymph node biopsy. E) DCIS, solid papillary type, diagnosed outside as benign intraductal papilloma. Excision showed residual DCIS.
Microinvasive carcinoma
A total of 18/147 (12%) cases were reclassified as microinvasive carcinoma, including 1 microinvasive lobular carcinoma. The original diagnoses included 17 (94%) DCIS (Figure 2C–D) and 1 (6%) classic LCIS.
An additional case with original diagnosis of DCIS was reclassified as DCIS suspicious for microinvasion, with no implications for the immediate surgical management.
Invasive carcinoma
A total of 11/147 (7%) cases were reclassified as IBC of no special type (NST). The largest span of invasive carcinoma was 9 mm. The outside diagnoses included 1 (9%) normal breast/benign epithelial proliferation without atypia (note: in this case the invasive carcinoma was obscured by a dense inflammatory infiltrate), 2 (18%) ADH, 4 (36%) DCIS (including one case diagnosed outside as suspicious for but not diagnostic of invasion), 2 (18%) SPC in situ suspicious for invasion, and 1 (9%) SPC with microinvasive carcinoma. An additional case with original diagnosis of SPC with invasion was reclassified as invasive carcinoma NST, a change in tumor subtype without any clinical impact.
One case with original diagnosis of “rare atypical ductal epithelium suspicious for malignancy” (ADH) was reclassified as invasive ductal carcinoma. The patient had prior history of contralateral invasive ductal carcinoma with apocrine morphology and had developed local recurrence and pleural metastasis. The carcinoma in the index CNB bore close morphologic resemblance to the contralateral primary invasive carcinoma.
Papillary lesions
In 35/147 (24%) cases the reclassification involved the diagnosis of papillary lesions. A total of 18/147 (12%) cases were reclassified as papillary lesions, including 9 cases originally diagnosed as papillary lesions of a different type (e.g. papilloma without atypia reclassified as papillary carcinoma). In 17 additional cases the original diagnosis of papillary lesion was not confirmed (Supplemental Table 1).
Fibroepithelial neoplasms
A total of 6/147 (4%) cases were reclassified as fibroepithelial neoplasms. Four of the six cases were reclassified as fibroadenomas and none was excised; the original diagnoses included 2 (50%) ADH, 1 (25%) FEA, and 1 (25%) fibroepithelial lesion. Two additional cases with original diagnosis of fibroadenoma were reclassified as fibroepithelial lesions with increased stromal cellularity.
Two special cases
In two cases the final diagnosis could not be categorized into one of the breast-specific entities based on the WHO Classification of Breast Tumours 5th ed.
A tumor diagnosed at the outside institution as “consistent with poorly differentiated carcinoma” was reclassified as radiation-induced spindle cell sarcoma (Figure 3). Per report, the patient had bilateral IBC 14 years earlier, treated with breast conserving surgery, and adjuvant radiotherapy and chemotherapy. An in-breast recurrence had been treated with mastectomy and additional radiotherapy. Subsequently, the patient developed an ipsilateral chest wall mass. CNB yielded a high-grade spindle cell malignancy. In-house workup of the outside CNB material revealed focal weak immunoreactivity for GATA3, ERG, and CD31, with no staining for keratins (CAM5.2, 34βE12, CK7 and CK18), and SOX10. Next-generation sequencing using a multi-gene solid tumor panel17 revealed an INSR mutation and DSCAM-TP53 fusion. Altogether the findings were most compatible with a radiation-induced sarcoma. No further follow-up information was available.
Figure 3.
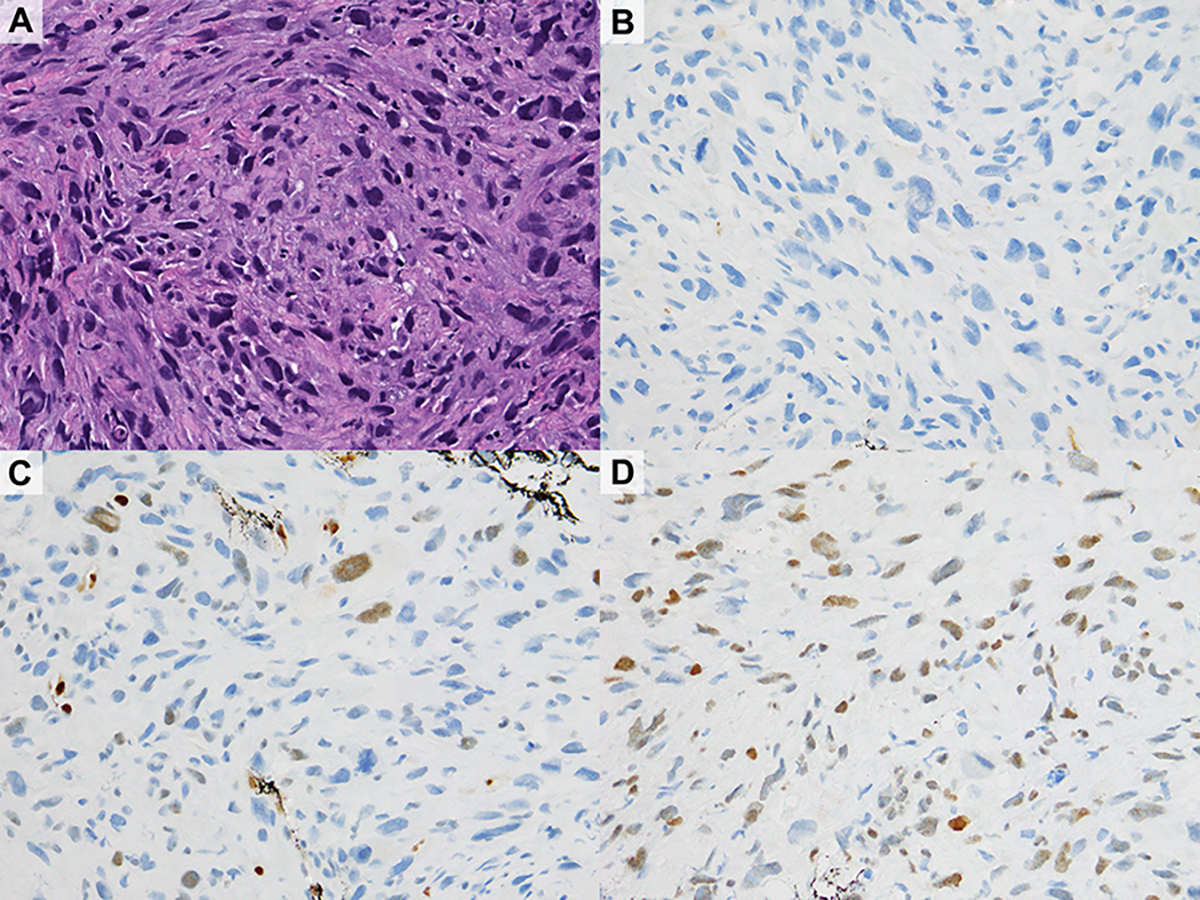
Radiation-induced spindle cell sarcoma, diagnosed outside as “consistent with poorly differentiated carcinoma” (A). Immunohistochemical staining for multiple keratins (CAM5.2 shown in B) and SOX10 were negative; there was weak expression of ERG (C), GATA3 (D), and CD31.
In the other case, the patient was a 30-year-old woman with a palpable sub/periareolar mass. Ultrasound examination showed a 1.3 cm complex cystic lesion, that grew to 2.0 cm in size at 6-month follow-up. The outside CNB diagnosis was DCIS. Review at out center revealed an intracystic papillary lesion composed of layers of low-grade cuboidal cells surmounted by cells with abundant eosinophilic or vacuolated cytoplasm (Figure 4A). A diagnosis of atypical papillary lesion was rendered. EXC yielded a 2.5 cm tumor composed of small cuboidal cells and luminal “umbrella” cells, with periductal inflammation and evidence of cyst rupture (Figure 4B–C). Scattered cells with cytoplasmic mucin were also seen. Immunohistochemistry showed scattered positivity for ER and diffuse positivity for 34βE12 (Figure 4D) and GATA3; p63 was positive in the cuboidal but not the luminal cells. Although the differential diagnosis in this case included a low grade mucoepidermoid carcinoma, given the tumor very superficial location right under the nipple a final diagnosis of nodular cystic hidradenoma was rendered in consultation with the dermatopathology team. The patient had no clinical or imaging evidence of disease at 4 years follow-up.
Figure 4.
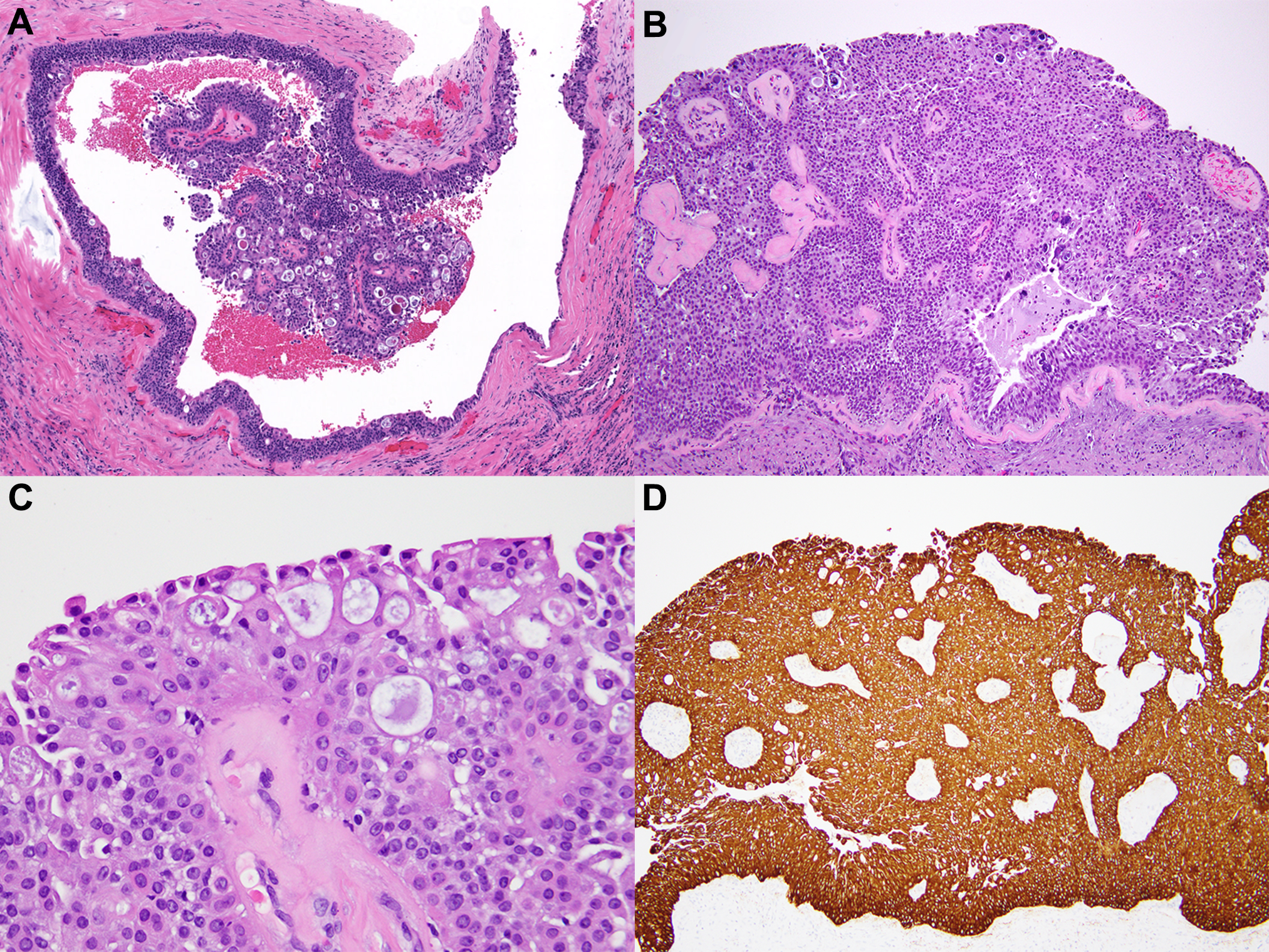
Nodular cystic hidradenoma. Core biopsy (A), diagnosed outside as DCIS, was called atypical papillary lesion. Final diagnosis was rendered on the excision specimen (B) with consultation from a dermatopathologist. High power view shows a mixture of cell types (C). The cells are diffusely positive for high molecular weight cytokeratin (34βE12 shown in D).
Correlation with the findings in surgical excision specimens of CNB
Information on the histologic findings in the follow-up EXC specimen was available for a subset of cases. Establishing definitive correlation between the in-house CNB diagnosis and EXC diagnosis was not always possible because some patients had (an)other synchronous ipsilateral lesion(s) that required surgery, and the site of the index CNB was not always specified. Below is a summary of the findings in the surgical excision specimens grouped according to the CNB diagnosis rendered at our center.
In-house CNB diagnosis of ADH.
Information of the findings in the EXC was available for 30/33 CNBs with in-house diagnosis of ADH. ADH was present in 7/30 (23%) EXC, DCIS in 8/30 (27%), and invasive carcinoma in 2/30 (7%). ALH/classic LCIS was present in 4/30 (13%) EXC, and 8/30 (27%) EXC had no residual atypia or carcinoma. In one additional case, EXC was performed after neoadjuvant chemotherapy for a synchronous invasive carcinoma, precluding definitive correlation. If the latter case is excluded, the findings at EXC confirmed the in-house CNB diagnosis of ADH or a more severe lesion in 17/29 (57%) cases.
In-house CNB diagnosis of DCIS.
Information on the findings in the EXC was available for 17/21 cases with inhouse CNB diagnosis of DCIS. DCIS was present in 9/17 (53%) EXC, SPC in situ in 2/17 (12%), and invasive carcinoma in 2/17 (12%). ADH and ALH were present in 2/17 EXC (12%). In two additional cases, EXC was performed after neoadjuvant chemotherapy for synchronous invasive carcinoma, precluding definitive correlation. If the latter two cases are excluded, the findings at EXC confirmed the in-house CNB diagnosis of DCIS or a more severe lesion in 13/19 (68%) cases.
In-house CNB diagnosis of microinvasive carcinoma.
Information on the findings in the EXC was available for 16/18 cases with inhouse CNB diagnosis of microinvasive carcinoma. Microinvasive carcinoma was present in 8/16 (50%) EXC, and invasive carcinoma in 4/6 (25%). DCIS with no residual microinvasion was identified in 2/6 (12.5%) cases. One EXC (7%) had no residual atypia or carcinoma. In one additional case, EXC was performed after neoadjuvant chemotherapy for a synchronous invasive carcinoma, precluding definitive correlation. If the latter case is excluded, the findings at EXC confirmed the in-house CNB diagnosis of microinvasive carcinoma or a more severe lesion in 12/18 (67%) cases.
In-house CNB diagnosis of invasive carcinoma.
Information on the findings in the EXC was available for 7/11 cases with inhouse CNB diagnosis of invasive carcinoma. Invasive carcinoma was confirmed in 6/7 (86%) EXCs. The seventh EXC was benign, but the invasive carcinoma in the corresponding CNB had measured only 1.5 mm microscopically. In this group of cases, EXC confirmed the in-house CNB diagnosis of invasive carcinoma in 86% of cases.
In-house CNB diagnosis of papillary lesions.
Information on the findings in the EXC was available for 10/14 cases with final CNB of papillary lesions, excluding papilloma without atypia. EXC of 4 lesions with CNB diagnosis of papillary DCIS/solid papillary carcinoma in situ yielded the same diagnosis in 2/10 (20%) cases, and invasive carcinoma in the other two (20%). EXC of six cases with CNB diagnosis of atypical papilloma/ papilloma with ADH, yielded 3 (30%) papillary DCIS, one (10%) papilloma with ADH, and one (10%) residual papilloma without atypia. The fourth tumor diagnosed as atypical papilloma on CNB (10%) yielded a tall cell carcinoma with reversed polarity at EXC. In this group of cases, EXC confirmed the in-house CNB diagnosis of atypical papilloma, papillary carcinoma or a more severe lesion in 9/10 (90%) of cases.
In-house CNB diagnosis of high-risk lesions no longer excised routinely.
In 22 cases the final CNB diagnosis was a benign or high-risk lesion for which EXC is not performed routinely at our institution in cases with radiology-pathology concordance. The inhouse CNB diagnoses included 4 normal breast, 7 benign epithelial proliferation without atypia, 7 ALH/classic LCIS, 1 FEA, 2 fibroadenomas, and 1 papilloma without atypia. The original CNB diagnoses were 17 ADH, 1 papilloma with ADH, 3 DCIS, and 1 radial scar. Based on the in-house CNB diagnoses and according to our management protocols, 22 of 147 (15%) discrepant CNBs in our study (approximately 0.4% of all outside CNBs reviewed at our center over the course of one year), were spared surgery.
Discussion
Core needle biopsy is a minimally invasive procedure used routinely for the diagnosis of benign and malignant breast lesions. In the past two decades, the pivotal role of CNB diagnosis in treatment planning has progressively increased, in parallel with changes in the management of patients with benign and malignant breast lesions. While in the past EXC was performed routinely, currently, de-escalation of surgery is accepted for patients with radiology-pathology concordant CNB diagnosis of high-risk lesions such as classic LCIS and papilloma without atypia given low rates of upgrade at EXC.11,15,16,18,19 In contrast, neoadjuvant chemotherapy is usually administered before definitive surgical treatment to patients with CNB diagnosis of high-grade triple negative or HER2-positive carcinoma.13 In this context, the diagnostic accuracy and reproducibility of CNB diagnoses has become even more critical, as the opportunity to verify the characteristics of many benign or malignant lesions upon review of the follow-up EXC specimen is no longer available.
Most studies of breast pathology second opinions have focused on review of surgical excision specimens rather than CNB. We examined our experience with outside CNBs reviewed at our center over the course of one year and assessed the impact on surgical management of changes in the CNB diagnoses.
Especially high levels of variability – even among highly experienced pathologists who subspecialize in breast – concern the classification of intraductal epithelial proliferations. A salient example is the differential diagnosis of ADH versus low-grade DCIS with limited extent. A consensus diagnosis is reached in only 11–48% of ADH, with other interpretations including benign, FEA, DCIS, and invasive carcinoma.20–22 Kappa statistics for diagnosing ADH and DCIS were 0.34–0.35 and 0.78 respectively.20,23
We encountered 27 cases in which ADH was reclassified as DCIS or vice versa. While these lesions exist on a continuous morphologic and biologic spectrum and harbor similar molecular aberrations, the standard management of a patient with CNB diagnosis of ADH involves follow-up EXC and endocrine chemoprevention if there is no upgrade to carcinoma; but a CNB diagnosis of DCIS mandates wide EXC (or EXC with separately submitted margin specimens) followed by adjuvant radiotherapy and hormone therapy in most cases.24 Prospective clinical trials with observation of low to intermediate grade DCIS without (micro)invasion are currently ongoing.25
The diagnosis of microinvasive carcinoma may also be subject to interobserver disagreement on CNB. In our study, 12% (17/147) of discrepant CNB diagnoses entailed an upgrade from DCIS to microinvasive carcinoma. Based on our published experience,26 at our center patients with CNB diagnosis of microinvasive carcinoma currently undergo sentinel lymph node biopsy, which is not performed routinely in patients with CNB diagnosis of DCIS or DCIS “suspicious for microinvasion”. The use of immunohistochemical stains for myoepithelial cells can greatly improve concordance rates for microinvasion, from 9 to 25 out of 50 cases in one study, though consensus was still not achieved in half of the cases.27
The diagnosis of papillary lesions is also challenging. In a study with central pathology review of 116 CNBs with original diagnosis of intraductal papilloma without atypia, 31 (27%) cases were reclassified, including 21 cases reclassified as benign mimics of papilloma and 10 cases found to have ductal atypia within or near the papilloma.18 In our study, disagreements involving papillary lesions constituted 24% (35/147) of CNBs with changes in the diagnostic category. Reclassification to a more severe diagnosis occurred in 15/35 (43%) cases, while only one (3%) papillary lesion was reclassified to a less severe diagnosis, consistent with the observation that most papillary lesions tend to be under-called in general practice.18,28 Reclassification of the papillary lesions led to 10 EXC that would not have been performed based on the original diagnosis, while one patient was spared EXC.
We also encountered two unusual cases. A subareolar papillary and cystic neoplasm was ultimately classified as eccrine acrospiroma – also known as hidradenoma – a rare skin tumor of sweat gland origin which can mimic breast carcinoma.29 The original CNB diagnosis in this case was DCIS, and ours was atypical papillary lesion. Acrospiroma is an indolent tumor of clear, polygonal, mucinous, or squamoid cells and dark cuboidal poroid cells. It is typically negative or shows heterogeneous staining for ER and PR. The treatment of acrospiroma is wide local EXC.30 A high index of suspicion for this entity should be maintained for a superficial tumor with unusual morphology. The other unusual case was a radiation-induced sarcoma, originally diagnosed as recurrent poorly differentiated carcinoma. It is important to distinguish radiation-induced sarcoma from local recurrence of breast carcinoma because the former tumors behave aggressively and frequently recur even after resection with clear margins. Chemotherapy is usually warranted for inoperable or advanced disease.31
To our knowledge, ours is the first study assessing diagnostic disagreements in breast CNBs with surgical management implications. Our study confirms that routine in-house review of breast pathology from patients seeking care from outside institutions is a useful and clinically important practice. Establishing guidelines for the reporting and communication of diagnostic disagreements is critical to ensure consistent and optimal care.
Supplementary Material
Acknowledgements:
We thank Bruce Crilly (MSKCC Digital Pathology Team) for assistance with the photographs.
Funding:
The authors were supported in part by a Cancer Center Support Grant of the National Institute of Health/National Cancer Institute (grant number P30CA008748).
Footnotes
Conflicts of interest: The authors have no relevant conflicts of interest to disclose. Unrelated: M.G.H. is a consultant of PaigeAI and advisor of PathPresenter. M.M. has received honoraria from Roche and Exact Sciences.
Ethics approval: The study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSKCC).
Data availability:
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Schnitt SJ, Connolly JL, Tavassoli FA, et al. Interobserver reproducibility in the diagnosis of ductal proliferative breast lesions using standardized criteria. 1992;16:1133–1143. [DOI] [PubMed] [Google Scholar]
- 2.O’Malley FP, Mohsin SK, Badve S, et al. Interobserver reproducibility in the diagnosis of flat epithelial atypia of the breast. Mod Pathol. 2006;19:172–179. [DOI] [PubMed] [Google Scholar]
- 3.Perkins C, Balma D, Garcia R and Group tMotC. Why current breast pathology practices must be evaluated. A susan g. Komen for the cure white paper: June 2006. 2007;13:443–447. [DOI] [PubMed] [Google Scholar]
- 4.Romanoff AM, Cohen A, Schmidt H, et al. Breast pathology review: Does it make a difference? Ann Surg Oncol. 2014;21:3504–3508. [DOI] [PubMed] [Google Scholar]
- 5.Rosai J Borderline epithelial lesions of the breast. 1991;15:209–221. [DOI] [PubMed] [Google Scholar]
- 6.Rakha EA, El-Sayed ME, Reed J, Lee AHS, Evans AJ and Ellis IO. Screen-detected breast lesions with malignant needle core biopsy diagnoses and no malignancy identified in subsequent surgical excision specimens (potential false-positive diagnosis). Eur J Cancer. 2009;45:1162–1167. [DOI] [PubMed] [Google Scholar]
- 7.Elmore JG, Longton GM, Carney PA, et al. Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA. 2015;313:1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heeg E, Civil YA, Hillen MA, et al. Impact of second opinions in breast cancer diagnostics and treatment: A retrospective analysis. Ann Surg Oncol. 2019;26:4355–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swapp RE, Aubry MC, Salomao DR and Cheville JC. Outside case review of surgical pathology for referred patients: The impact on patient care. Arch Pathol Lab Med. 2013;137:233–240. [DOI] [PubMed] [Google Scholar]
- 10.Collins LC, Connolly JL, Page DL, et al. Diagnostic agreement in the evaluation of image-guided breast core needle biopsies: Results from a randomized clinical trial. Am J Surg Pathol. 2004;28:126–131. [DOI] [PubMed] [Google Scholar]
- 11.The American Society of Breast Surgeons. Consensus guideline on concordance assessment of image-guided breast biopsies and management of borderline or high-risk lesions. 2018. Available at: https://www.breastsurgeons.org/docs/statements/Consensus-Guideline-on-Concordance-Assessment-of-Image-Guided-Breast-Biopsies.pdf?v2. Accessed June 18, 2022.
- 12.Allison KH, Brogi E, Ellis IO, et al. WHO classification of tumours editorial board. Breast tumours. In: Series WHO classification of tumours editorial board. Breast tumours. 5th ed. Lyon, France: IARC; 2019. [Google Scholar]
- 13.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): Breast cancer. 2022. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed June 18, 2022.
- 14.Grabenstetter A, Brennan S, Salagean ED, Morrow M and Brogi E. Flat epithelial atypia in breast core needle biopsies with radiologic-pathologic concordance: Is excision necessary? 2020;44:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pareja F, Corben AD, Brennan SB, et al. Breast intraductal papillomas without atypia in radiologic-pathologic concordant core-needle biopsies: Rate of upgrade to carcinoma at excision. Cancer. 2016;122:2819–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray MP, Luedtke C, Liberman L, Nehhozina T, Akram M and Brogi E. Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: Outcomes of prospective excision. Cancer. 2013;119:1073–1079. [DOI] [PubMed] [Google Scholar]
- 17.Cheng DT, Mitchell TN, Zehir A, et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (msk-impact): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakhlis F, Baker GM, Pilewskie M, et al. The incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patients with intraductal papilloma without atypia on core biopsy: Results from a prospective multi-institutional registry (tbcrc 034). Ann Surg Oncol. 2021;28:2573–2578. [DOI] [PubMed] [Google Scholar]
- 19.Rageth CJ, O’Flynn EAM, Pinker K, et al. Second international consensus conference on lesions of uncertain malignant potential in the breast (b3 lesions). Breast Cancer Res Treat. 2019;174:279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain RK, Mehta R, Dimitrov R, et al. Atypical ductal hyperplasia: Interobserver and intraobserver variability. Modern Pathology. 2011;24:917–923. [DOI] [PubMed] [Google Scholar]
- 21.Allison KH, Rendi MH, Peacock S, Morgan T, Elmore JG and Weaver DL. Histological features associated with diagnostic agreement in atypical ductal hyperplasia of the breast: Illustrative cases from the b-path study. Histopathology. 2016;69:1028–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tozbikian G, Brogi E, Vallejo CE, et al. Atypical ductal hyperplasia bordering on ductal carcinoma in situ. Int J Surg Pathol. 2017;25:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elston CW, Sloane JP, Amendoeira I, et al. Causes of inconsistency in diagnosing and classifying intraductal proliferations of the breast. European Journal of Cancer. 2000;36:1769–1772. [DOI] [PubMed] [Google Scholar]
- 24.Allison KH, Reisch LM, Carney PA, et al. Understanding diagnostic variability in breast pathology: Lessons learned from an expert consensus review panel. Histopathology. 2014;65:240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanbayashi C, Thompson AM, Hwang E-SS, et al. The international collaboration of active surveillance trials for low-risk dcis (loris, lord, comet, loretta). Journal of Clinical Oncology. 2019;37:15_suppl, TPS603–TPS603. [Google Scholar]
- 26.Flanagan MR, Stempel M, Brogi E, Morrow M and Cody HS, 3rd. Is sentinel lymph node biopsy required for a core biopsy diagnosis of ductal carcinoma in situ with microinvasion? Ann Surg Oncol. 2019;26:2738–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cserni G, Wells CA, Kaya H, et al. Consistency in recognizing microinvasion in breast carcinomas is improved by immunohistochemistry for myoepithelial markers. Virchows Arch. 2016;468:473–481. [DOI] [PubMed] [Google Scholar]
- 28.Rakha EA, Ahmed MA and Ellis IO. Papillary carcinoma of the breast: Diagnostic agreement and management implications. Histopathology. 2016;69:862–870. [DOI] [PubMed] [Google Scholar]
- 29.Lee HH, Park SH, Choi HY and Park HK. Eccrine spiradenoma arising in the breast misdiagnosed as an epidermal inclusion cyst. Korean J Radiol. 2011;12:256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cyrlak D, Barr RJ and Wile AG. Malignant eccrine acrospiroma of the breast. Int J Dermatol. 1995;34:271–273. [DOI] [PubMed] [Google Scholar]
- 31.Dogan A, Kern P, Schultheis B, Hausler G, Rezniczek GA and Tempfer CB. Radiogenic angiosarcoma of the breast: Case report and systematic review of the literature. BMC Cancer. 2018;18:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


