Lifelong pharmacological immunosuppression is required to achieve graft acceptance, but this approach is imperfect with numerous side-effects and an inability to fully mitigate graft rejection. The induction of long-term allograft-specific immunological tolerance remains therefore the gold standard for clinical transplantation, the requirement for the currently available nonspecific immunosuppression. Many approaches have been identified to yield transplantation tolerance in pre-clinical models but few of them have successfully achieved operational tolerance in the clinic, for example those involving hematopoietic stem cell transplantation (1). Notably, while successful tolerance induction is rare for most types of clinical organ transplants, pregnancy represents a reliably induced physiological state of tolerance to semi-allogeneic fetuses that is mediated by multiple mechanisms effective both locally within the uterine environment and systemically, including the induction of fetus-specific regulatory T cells in addition to T cell anergy or dysfunction(2).
Paradoxically, pregnancy has long been known to cause humoral sensitization; indeed, serum from multiparous women was instrumental to the initial discovery of HLA antigens(3). Recent studies by our own group have reconciled these divergent immunological effects in a murine model of semi-allogeneic pregnancy, demonstrating that fetus-specific T cells were indeed tolerized by pregnancy, whereas fetus-specific B cells became sensitized(4). The effect of humoral sensitization was dominant in the context of a subsequent offspring-matched heart transplantation and was sufficient to override the pro-tolerogenic effects of co-stimulation blockade with anti-CD154. Using B cell-deficient mice to circumvent the effects of humoral sensitization, we have been able to show that pregnancy-induced fetus-specific T tolerance was persistent systemically and sufficient to allow for the spontaneous acceptance of fetus-matched heart grafts. In contrast, skin-sensitization resulted in an accelerated rejection of donor-matched heart grafts in B cell-deficient recipients.
In a recent publication in the Journal of Experimental Medicine, Emma Lewis and co-workers confirmed that sensitization by semi-allogeneic pregnancy results in enhanced offspring-matched skin graft survival in B cell-deficient mice, while adoptive transfer of serum from wild-type post-partum mice into these recipients accelerated skin graft rejection(5). The authors then utilized registry data from the Organ Procurement and Transplantation Network (OPTN; n = 382,780 kidney transplant recipients) to compare kidney transplant allograft survival between parous female recipients of offspring kidneys and non-parous recipients of a second haplotype-matched kidney that was identical to their first kidney donor at the HLA-A, -B, and -DR loci (“non-offspring” recipients). This analysis revealed a significantly increased risk of all-cause allograft failure among non-offspring recipients compared with offspring recipients (n=3001, hazard ratio, 3.04; 95% confidence interval, 1.18–7.86). These results complement findings in mice that sensitization with pregnancy promotes hyporesponsive states in T cells that can result in superior graft outcomes compared to sensitization by allografts.
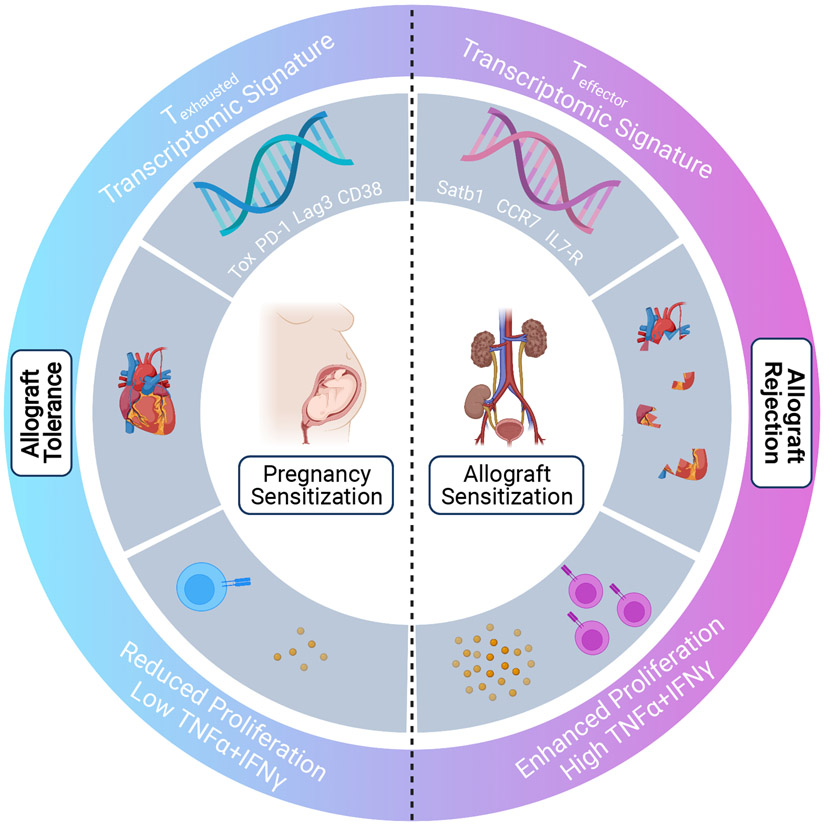
Emma Lewis and co-workers then executed a fascinating in-depth molecular exploration into the fate of maternal fetus-specific CD8+ T cells after pregnancy (Fig 1). To this end, the authors designed a clonally restricted pregnancy model in which wild-type C57BL/6 (WTB6) females harboring congenic OT-I cells were either mated with or received a skin graft from Act-mOVA.B6 mice. Act-mOVA.B6 mice ubiquitously express membrane-bound chicken ovalbumin (OVA), while OT-I cells are monoclonal T cell receptor (TCR)-transgenic CD8+ T cells specific for OVA257-264 peptide presented by major histocompatibility complex (MHC) Class I, Kb. The authors first confirmed that pregnancy indeed induces hypofunction in OT-1 cells, defined by a reduced capacity for secondary expansion, impaired cytolytic function, diminished cytokine production and decreased expression of transcription factors TCF-1 and FoxO1, which program effector T cell differentiation. Lewis et al. then utilized RNA-sequencing of OT-1 cells for a genome-wide transcriptional signature acquired during pregnancy. By pairing these data with cutting-edge bioinformatics analysis to integrate multiple previously published transcriptomic datasets, the authors demonstrated that pregnancy programmed OT-1 cells into a state of exhaustion that resembled T cell responses during chronic infection and cancer. The most striking features of this exhausted signature were the phenotypic and transcriptional upregulation of co-inhibitory markers including PD-1, LAG-3, CD38, and most notably TOX, a master regulator of CD8+ T cell exhaustion(6). Another interesting aspect of exhaustion found to be conserved during pregnancy is translational repression characterized by downregulation of 30 ribosomal genes, along with their respective upstream transcriptional regulators. This finding was identified and visualized via network analysis.
Figure 1.
Emma Lewis et al. (5) demonstrate the ability of pregnancy to induce a hypofunctional state in fetus-specific CD8+ T cells. IFNγ, interferon-g; IL7R, interleukin-7 receptor, TNFα, tumor necrosis factor-α; Tox, PD-1, Lag3, CD38, co-inhibitory markers associated with T cell dysfunction and exhaustion; Satb1, CCR7, IL7-R, markers associated with typical effector T cell response
By synthesizing results from previous studies of T cell exhaustion during cancer and chronic infection, the authors hypothesized that continuous signaling by the transcription factor, Nuclear Factor of Activated T-cells (NFAT) signaling is a major driver of the exhausted signature in post-partum OT-1 cells(7-9). They first compared the transcriptome of pregnancy with a previously published transcriptional dataset with a constitutively active form of NFAT that cannot partner with the transcription factor, AP-1(10). This so-called “partnerless NFAT” dataset produced a strong correlation with the transcriptional signature of pregnancy when analyzed via Gene Set Enrichment Analysis (GSEA). The authors then demonstrated that treatment with an NFAT inhibitor, FK506, during pregnancy resulted in decreased expression of PD-1 and TOX in fetus-specific CD8+ T cells, along with a modest restoration of cytokine production compared to pregnancy without FK506 treatment. However, cytokine production in the presence of FK506 remained inferior to that of CD8+ T cells primed with skin graft. Moreover, multiple markers known to be modified during pregnancy remained unchanged even under FK506 treatment. For example, the exhaustion-associated transcription factor, Aiolos persisted in both untreated and FK506-treated pregnancy. Meanwhile, FK506 treatment did not increase levels of SATB1 expression, a marker associated with T cell activation. Taken together, the authors concluded that pregnancy drives the exhaustion of CD8+ T cells via both NFAT-dependent and -independent mechanisms.
That semi-allogeneic pregnancy primes fetus-specific CD8+ T cells to enter a state of exhaustion raises new research questions including a delineation of the molecular mechanisms driving the hypofunction in CD4+ T conventional cells reported by Suah and others, and the ways memory B cells and fetus-specific antibodies affect the programming or maintenance of hypofunction in CD8+ T cells in the setting of offspring-matched organ transplantation. Furthermore, the interplay between T cell hypofunction and humoral sensitization has profound implications for transplantation in the parous female and underscores the importance of humoral desensitization to reveal the unexpected pro-tolerogenic effects of pregnancy for off-spring matched allografts. Finally, the use of maintenance calcineurin inhibitors to combat both transplant rejection and autoimmunity might prevent the programming of T cell hypofunction in patients, and this will be important to dissect. It is foreseeable that the remarkable capacity of pregnancy to induce systemic and persistent fetus-specific T cell hypofunction will continue to yield novel mechanistic insights. Understanding them may provide translational opportunities to get us closer to the gold-standard of transplantation tolerance.
References
- 1.Messner F, Etra JW, Dodd-o JM, Brandacher G. 2019. Chimerism, Transplant Tolerance and Beyond. Transplantation 103:1556–1567. 10.1097/TP.0000000000002711 [DOI] [PubMed] [Google Scholar]
- 2.PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, Mor G, Schulz L, Soares M, Spencer T, Strominger J, Way SS, Yoshinaga K. 2015. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. Apr;16(4):328–34. 10.1038/ni.3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Rood J, Eernisse J & Van Leeuwen A 1958. Leucocyte Antibodies in Sera from Pregnant Women. Nature 181:1735–1736. 10.1038/1811735a0 [DOI] [PubMed] [Google Scholar]
- 4.Suah AN, Tran DV, Khiew SH, Andrade MS, Pollard JM, Jain D, Young JS, Yin D, Chalasani G, Alegre M-L, and Chong AS. 2021. Pregnancy-induced humoral sensitization overrides T cell tolerance to fetus-matched allografts in mice. J. Clin. Invest 131:e140715. 10.1172/JCI140715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis EL, Xu R, Beltra JC, Nglow SF, Cohen J, Telange R, Crane A, Sawinski D, Wherry EJ, and Porrett PM. 2021. NFAT-dependent and -independent exhaustion circuits progam CD8 T cell hypofunction in pregnancy. J. Exp. Med 219:e20201599. 10.1084/jem.20201599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, et al. 2019. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 571:211–218. 10.1038/s41586-019-1325-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, Trivedi P, Menocal L, Appleby H, Camara S, et al. 2019. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 571: 270–274. 10.1038/s41586-019-1324-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y, et al. 2019. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 571:265–269. 10.1038/s41586-019-1326-9 [DOI] [PubMed] [Google Scholar]
- 9.Seo H, Chen J, Gonzalez-Avalos E, Samaniego-Castruita D, Das A, Wang YH´, López-Moyado IF, Georges RO, Zhang W, Onodera A, et al. 2019. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl. Acad. Sci. USA 116:12410–12415. 10.1073/pnas.1905675116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez GJ, Pereira RM, Aij ¨ o T, Kim EY, Marangoni F, Pipkin ME, Togher S¨, Heissmeyer V, Zhang YC, Crotty S, et al. 2015. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity. 42:265–278. 10.1016/j.immuni.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]