Abstract
Background
Uveitis is a term used to describe a group of intraocular inflammatory diseases. Uveitis is the fifth most common cause of vision loss in high‐income countries, with the highest incidence of disease in the working‐age population. Corticosteroids are the mainstay of treatment for all subtypes of non‐infectious uveitis. They can be administered orally, topically with drops, by periocular (around the eye) or intravitreal (inside the eye) injection, or by surgical implantation.
Objectives
To determine the efficacy and safety of steroid implants in people with chronic non‐infectious posterior uveitis, intermediate uveitis, and panuveitis.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register), MEDLINE Ovid, Embase, PubMed, LILACS, and three trials registries to November 2021.
Selection criteria
We included randomized controlled trials comparing either fluocinolone acetonide (FA) or dexamethasone (DEX) intravitreal implants with standard‐of‐care therapy or sham procedures, with at least six months of follow‐up after treatment. We included studies that enrolled participants of all ages, who had chronic non‐infectious posterior uveitis, intermediate uveitis, or panuveitis with vision that was better than hand‐motion.
Data collection and analysis
We applied standard Cochrane methodology.
Main results
We included data from four trials (683 participants, 907 eyes) that compared corticosteroid implants with either sham or standard‐of‐care therapy.
Study characteristics and risk of bias Of the two trials that compared corticosteroid implants with sham procedure, one examined a 0.18 mg FA implant, and the other, a 0.7 mg DEX implant. The other two trials compared a 0.59 mg FA implant with standard‐of‐care therapy, which included systemic corticosteroids and immunosuppressive medications, if needed. Considering improvement in visual acuity, we assessed the four trials to be at either low risk, or with some concerns of risk of bias across all domains.
Findings Using sham procedure as control, combined results at the six‐month primary time point suggested that corticosteroid implants may decrease the risk of uveitis recurrence by 60% (relative risk [RR] 0.40, 95% confidence interval [CI] 0.30 to 0.54; 2 trials, 282 participants; low‐certainty evidence); and lead to a greater improvement in best‐corrected visual acuity (BCVA; mean difference [MD] 0.15 logMAR, 95% CI 0.06 to 0.24; 1 trial, 153 participants; low‐certainty evidence). Evidence based on a single‐study report (146 participants) suggested that steroid implants may have no effects on visual functioning quality of life, measured on the National Eye Institute 25‐Item Visual Function Questionnaire (MD 2.85, 95%CI ‐3.64 to 9.34; 1 trial, 146 participants; moderate‐certainty evidence).
Using standard‐of care therapy as control, combined estimates at the 24‐month primary time point suggested that corticosteroid implants were likely to decrease the risk of recurrence of uveitis by 54% (RR 0.46, 95% CI 0.35 to 0.60; 2 trials, 619 eyes). Combined estimates at 24 months also suggested that steroid implants may have little to no effects on improving BCVA (MD 0.05 logMAR, 95% CI ‐0.02 to 0.12; 2 trials, 619 eyes; low‐certainty evidence). Evidence based on a single‐study report (232 participants) suggested that steroid implants may have minimal clinical effects on visual functioning (MD 4.64, 95% CI 0.13 to 9.15; 1 trial, 232 participants; moderate‐certainty evidence); physical functioning (SF‐36 physical subscale MD 2.95, 95% CI 0.55 to 5.35; 1 trial, 232 participants; moderate‐certainty evidence); or mental health (SF‐36 mental subscale MD 3.65, 95% CI 0.52 to 6.78; 1 trial, 232 participants; moderate‐certainty evidence); but not on EuroQoL (MD 6.17, 95% CI 1.87 to 10.47; 1 trial, 232 participants; moderate‐certainty evidence); or EuroQoL‐5D scale (MD 0.02, 95% CI ‐0.04 to 0.08; 1 trial, 232 participants; moderate‐certainty evidence).
Adverse effects Compared with sham procedures, corticosteroid implants may slightly increase the risk of cataract formation (RR 2.69, 95% CI 1.17 to 6.18; 1 trial, 90 eyes; low‐certainty evidence), but not the risk of cataract progression (RR 2.00, 95% CI 0.65 to 6.12; 1 trial, 117 eyes; low‐certainty evidence); or the need for surgery (RR 2.98, 95% CI 0.82 to 10.81; 1 trial, 180 eyes; low‐certainty evidence), during up to 12 months of follow‐up. These implants may increase the risk of elevated intraocular pressure ([IOP] RR 2.81, 95% CI 1.42 to 5.56; 2 trials, 282 participants; moderate‐certainty evidence); and the need for IOP‐lowering eyedrops (RR 1.85, 95% CI 1.05 to 3.25; 2 trials, 282 participants; moderate‐certainty evidence); but not the need for IOP‐lowering surgery (RR 0.72, 95% CI 0.13 to 4.17; 2 trials, 282 participants; moderate‐certainty evidence).
Evidence comparing the 0.59 mg FA implant with standard‐of‐care suggested that the implant may increase the risk of cataract progression (RR 2.71, 95% CI 2.06 to 3.56; 2 trials, 210 eyes; low‐certainty evidence); and the need for surgery (RR 2.98, 95% CI 2.33 to 3.79; 2 trials, 371 eyes; low‐certainty evidence); along with the risk of elevated IOP (RR 3.64, 95% CI 2.71 to 4.87; 2 trials, 605 eyes; moderate‐certainty evidence); and the need for medical (RR 3.04, 95% CI 2.36 to 3.91; 2 trials, 544 eyes; moderate‐certainty evidence); or surgical interventions (RR 5.43, 95% CI 3.12 to 9.45; 2 trials, 599 eyes; moderate‐certainty evidence).
In either comparison, these implants did not increase the risk for endophthalmitis, retinal tear, or retinal detachment (moderate‐certainty evidence).
Authors' conclusions
Our confidence is limited that local corticosteroid implants are superior to sham therapy or standard‐of‐care therapy in reducing the risk of uveitis recurrence. We demonstrated different effectiveness on BCVA relative to comparators in people with non‐infectious uveitis. Nevertheless, the evidence suggests that these implants may increase the risk of cataract progression and IOP elevation, which will require interventions over time.
To better understand the efficacy and safety profiles of corticosteroid implants, we need future trials that examine implants of different doses, used for different durations. The trials should measure core standard outcomes that are universally defined, and measured at comparable follow‐up time points.
Keywords: Humans; Adrenal Cortex Hormones; Adrenal Cortex Hormones/adverse effects; Cataract; Panuveitis; Quality of Life; Uveitis, Intermediate
Plain language summary
Steroid implants for chronic uveitis not caused by infection
What is chronic non‐infectious uveitis?
Uveitis is a group of eye diseases caused by inflammation (redness, swelling, pain, etc.) inside the eye, which can lead to vision loss. Uveitis can result from infections, or non‐infectious causes. Non‐infectious uveitis can result from a disease somewhere else in the body. The uvea (middle layer of the eye) has many blood vessels. If the immune system is fighting a problem in one area, the cells and chemicals it makes can travel through the bloodstream and enter the eye, leading to inflammation. Acute uveitis lasts less than three months; chronic uveitis lasts longer than three months.
How it is treated?
Chronic non‐infectious uveitis is generally treated with steroids, applied near or inside the eye, or other medicines, taken either by mouth or injection, to control the inflammation. However, these medicines, including steroids, suppress the immune system and result in unwanted side effects.
What did we want to find out?
We assessed whether a steroid‐containing implant (a small capsule that slowly releases steroids inside the eye) can reduce the return of uveitis, improve vision, or improve quality of life. We also evaluated whether these implants increased any unwanted side effects.
What we did
We searched for trials that randomly assigned children and adults with chronic non‐infectious uveitis to receive either steroid‐containing implants or another treatment; the other treatment could be a pretend (sham) procedure or other standard way of delivering care. We summarized the study findings, and assessed how confident we were in the findings.
What we found
We found two studies (282 participants) that compared surgically‐placed implants that released fluocinolone acetonide into the eye with a sham procedure. The type and amount of medicine released was different in both studies. The steroid‐containing implants appeared to reduce the risk of uveitis coming back, and lead to better vision and quality of life.
We found two studies (683 participants) that compared surgically‐placed implants that released fluocinolone acetonide into the eye with standard treatment. Both studies used the same implant. The results did not show that the steroid‐containing implants reduced the risk of uveitis coming back, or improved much vision, but the participants appeared to have a better quality of life.
Steroid‐containing implants seemed to increase the risk for developing cataracts (clouding of the lens of the eye), and for increasing the pressure in the eye. All four studies included participants from multiple countries.
What are the limitations of the evidence?
We only included four studies. They did not enroll large numbers of participants, and had some flaws in their study design. Therefore, we have moderate to limited confidence in our findings.
How up to date is this evidence?
The evidence is current to November 2021.
Summary of findings
Summary of findings 1. Steroid implant versus sham procedure.
| Steroid implant versus sham procedure for chronic non‐infectious uveitis | ||||||
|
Patient or population: people with chronic non‐infectious uveitis Settings: eye clinics in North America, Europe, Middle East, and South Asia Intervention: fluocinolone acetonide 0.18 mg or dexamethasone 0.7 mg implant Comparison: sham procedure | ||||||
| Outcomes | Illustrative comparative risks (95% CI) |
Relative Effect (95% CI) |
No. of participants (studies) |
Certainty of evidence (GRADE) |
Comments | |
| Assumed risk* with sham procedure |
Corresponding risk** with steroid implant |
|||||
| Proportion of participants with recurrence of uveitis |
Primary time point: 6 months 57 events per 100 participants |
23 events (17 to 31) per 100 persons |
RR 0.40 (0.30 to 0.54) |
282 (2) | Lowa,b | Lower is better |
|
Secondary time points: ≥ 12 months 12 months: 98 events per 100 participants 36 months: 98 events per 100 participants |
12 months: 38 events (29 to 50) per 100 persons 36 months: 66 events (56 to 77) per 100 persons |
RR 0.39 (0.30 to 0.51) RR 0.67 (0.57 to 0.79) |
129 (1) | |||
| Mean difference in BCVA (logMAR) |
Primary time point: 6 months The mean improvement in BCVA in the sham group was 0.07 (SD 0.28) |
The mean improvement in BCVA in the steroid implant group was 0.15 higher (0.06 to 0.24) | 153 (1) | Lowa,b | Results are presented as improvement in logMAR, with positive differences indicating more improvement. | |
|
Secondary time points: ≥ 12 months The mean improvement in BCVA in the sham group at 12 months: 0.07 (SD 0.26) 36 months: 0.05 (SD 0.28) |
The mean improvement in BCVA in the steroid implant group was: 12 months: 0.05 higher (0.05 lower to 0.15 higher) 36 months: 0.13 higher (0.03 to 0.23) |
129 (1) | ||||
| Mean difference in quality of life scores (NEI‐VFQ25) |
Primary time point: 6 months The mean quality of life scores in the sham group was 73.38 (SD 21.19) |
The mean quality of life scores in the steroid implant group was 2.85 higher (3.64 lower to 9.34 higher) | 146 (1) | Moderateb | MCID was 4 to 6 points (Suner 2009). | |
| Proportion of participants with cataract formation/progression, or surgery |
Cataract formation 13 events per 100 eyes |
34 events (15 to 79) per 100 eyes |
RR 2.69 (1.17 to 6.18) |
90 eyes (1) | Lowb,c | Lower is better. Up to 6 to 12 months post‐treatment. |
|
Cataract progression 7 events per 100 eyes |
15 events (5 to 45) per 100 eyes |
RR 2.00 (0.65 to 6.12) |
117 eyes (1) | |||
|
Cataract surgery 4 events per 100 eyes |
12 events (3 to 43) per 100 eyes |
RR 2.98 (0.82 to 10.81) |
180 eyes (1) | |||
| Proportion of participants with elevated IOP or receiving intervention |
Elevated IOP 8 events per 100 participants |
22 events (11 to 44) per 100 participants |
RR 2.81 (1.42 to 5.56) |
282 (2) | Moderateb | Lower is better. Up to 6 to 12 months post‐treatment. |
|
Requiring medication 9 events per 100 participants |
17 events (9 to 29) per 100 participants |
RR 1.85 (1.05 to 3.25) |
||||
|
Requiring surgery 20 events per 1000 participants |
14 events (3 to 83) per 1000 participants |
RR 0.72 (0.13 to 4.17) |
||||
| Proportion of participants with endophthalmitis | 17 events per 1000 participants | 8 events (2 to 39) per 1000 participants |
RR 0.47 (0.10 to 2.30) |
280 (2) | Moderateb | Lower is better. Up to 6 to 12 months post‐treatment. |
| Proportion of participants with retinal tear or detachment | 17 events per 1000 participants | 19 events (4 to 98) per 1000 participants |
RR 1.11 (0.21 to 5.75) |
280 (2) | Moderateb | Lower is better. Up to 6 to 12 months post‐treatment. |
| *The basis for the assumed risk is the mean baseline risk from the studies in the meta‐analysis; the total number of events in the control group divided by the total number of participants in the control groups, scaled to 100 or 1000. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **The corresponding risk was the absolute risk (number of events divided by number of participants in the intervention group). The 95% CI was calculated using a binomial distribution. BCVA: best‐corrected visual acuity; CI: confidence interval; DEX: dexamethasone; FA: fluocinolone acetonide; IOP: intraocular pressure; MCID: minimal clinically important difference; MD: mean difference; No: number; NEI‐VFQ25: National Eye Institute 25‐Item Visual Function Questionnaire; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty. We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low‐certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded for risk of bias (‐1) bDowngraded for imprecision (‐1) cDowngraded for indirectness (‐1)
Summary of findings 2. Steroid implant versus standard‐of‐care therapy.
| Steroid implant versus systemic therapy for chronic non‐infectious uveitis | ||||||
|
Patient or population: people with chronic non‐infectious uveitis Settings: eye clinics in North America, Europe, Middle East, and Australia Intervention: fluocinolone acetonide 0.59 mg implant Comparison: standard‐of‐care therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of eyes (Studies) | Certainty of the evidence (GRADE) | Comments | |
|
Assumed risk* with standard‐of‐care therapy |
Corresponding risk** with steroid implant |
|||||
| Proportion of eyes with recurrence of uveitis |
Primary time point: 24 months 38 per 100 eyes |
17 events (13 to 23) per 100 eyes | RR 0.46 (95% CI: 0.35 to 0.60) | 619 (2) | Lowa,b | Lower is better. Combined results were similar at 24 months when using data that excluded inferred recurrence from Pavesio 2010 (RR 0.37, 95% CI 0.27 to 0.51) |
|
Secondary time point: 6 months 43 events per 100 eyes |
19 events (15 to 25) per 100 eyes |
RR 0.45 (95% CI: 0.35 to 0.59) |
||||
| Mean difference in BCVA (logMAR) |
Primary time point: 24 months The mean improvement in BCVA in the standard‐of‐care group was 0.04 (SD 0.51) |
The mean improvement in BCVA in the steroid implant group was 0.05 higher (0.02 lower to 0.12 higher) | ‐ | 619 (2) | Lowa,c | Results represent improvement in BCVA, with positive differences indicating more improvement. Single‐study estimates reported at 6 months by Pavesio 2010 (140 eyes) were similar (MD 0.02, 95% CI ‐0.08 to 0.12). |
|
Secondary time point: 12 months The mean improvement in BCVA in the standard‐of‐care group was 0.06 (SD 0.53) |
The mean improvement in BCVA in the steroid implant group was 0.01 higher (0.06 lower to 0.08 higher) | ‐ | ||||
| Mean difference in quality of life scores*** |
NEI‐VFQ25 composite score The mean difference in the standard‐of‐care group was 6.8 (SD 16.87) |
The mean difference in the steroid implant group was 4.64 higher (0.13 to 9.15) | ‐ | 232 (1) | Moderatec | MCID was 4 to 6 points (Suner 2009). |
|
SF‐36 physical The mean difference in the standard‐of‐care group was ‐1.8 (SD 9.61) |
The mean difference in the steroid implant group was 2.95 higher (0.55 to 5.35) | ‐ | MCID was 3 to 5 points (Hays 2001). | |||
|
SF‐36 mental The mean difference in the standard‐of‐care group was ‐1.1 (SD 12.28) |
The mean difference in the steroid implant group was 3.65 higher (0.52 to 6.78) | ‐ | MCID was 3 to 5 points (Hays 2001). | |||
|
EuroQoL (VAS) The mean difference in the standard‐of‐care group was ‐0.88 (SD 19.01) |
The mean difference in the steroid implant group was 6.17 higher (1.87 to 10.47) | ‐ | MCID was 7 points (Pickard 2007). | |||
|
EuroQoL‐5D The mean difference in the standard‐of‐care group was 0 (SD 0.21) |
The mean difference in the steroid implant group was 0.02 higher (0.04 lower to 0.08 higher) | ‐ | MCID was 0.06 to 0.07 points (Pickard 2007). | |||
| Proportion of eyes with cataract formation or progression, or surgery |
Cataract progression 33 events per 100 eyes |
89 events (68 to 117) per 100 eyes |
RR 2.71 (2.06 to 3.56) |
210 (2) | Lowb,c | Lower is better |
|
Cataract surgery 27 events per 100 eyes |
80 events (63 to 102) per 100 eyes |
RR 2.98 (2.33 to 3.79) |
371 (2) | |||
| Proportion of eyes with elevated IOP or receiving intervention |
Elevated IOP 14 events per 100 eyes |
51 events (38 to 68) per 100 eyes |
RR 3.64 (2.71 to 4.87) |
605 (2) | Moderatec | Lower is better |
|
Requiring medications 20 events per 100 eyes |
61 events (47 to 78) per 100 eyes |
RR 3.04 (2.36 to 3.91) |
544 (2) | |||
|
Requiring surgery 5 events per 100 eyes |
27 events (16 to 47) per 100 eyes |
RR 5.43 (3.12 to 9.45) |
599 (2) | |||
| Proportion of eyes with endophthalmitis**** | 3 events (0.3 to 22) per 1000 eyes | 20 events per 1000 eyes |
RR 7.30 (0.91 to 58.72) |
607 (2) | Moderatec | Lower is better |
| Proportion of eyes with retinal tear or detachment | 10 events per 1000 eyes | 21 events (5 to 84) per 1000 eyes |
RR 2.07 (0.51 to 8.40) |
606 (2) | Moderatec | Lower is better |
| *The basis for the assumed risk is the mean baseline risk from the studies in the meta‐analysis; the total number of events in the control group divided by the total number of participants in the control groups, scaled to 100 or 1000. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **The corresponding risk was the absolute risk (number of events divided by number of participants in the intervention group). The 95% CI was calculated using a binomial distribution. ***A favorable direction of changes differs by questionnaire. ****The corresponding risk is the total number of events in the intervention group divided by the total number of eyes in the intervention groups, scaled to 1000. The assumed risk (and its 95% CI) is based on the assumed risk in the intervention group and the relative effect of the intervention (and its 95% CI). BCVA: best‐corrected visual acuity; CI: confidence interval; FA, fluocinolone acetonide; IOP: intraocular pressure; MCID: minimal clinically important difference; MD: mean difference; No: number; NEI‐VFQ25: the National Eye Institute 25‐Item Visual Function Questionnaire; RR: risk ratio; SD: standard deviation; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High‐certainty. We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low‐certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded for risk of bias (‐1) bDowngraded for indirectness (‐1) cDowngraded for imprecision (‐1)
Background
Description of the condition
Uveitis is a term used to describe a heterogeneous group of intraocular inflammatory diseases of the anterior, intermediate, and posterior uveal tract (iris, ciliary body, choroid). Uveitis is the fifth most common cause of vision loss in high‐income countries, accounting for 5% to 20% of legal blindness (Durrani 2004; Nussenblatt 1990), with the highest incidence of disease in the working‐age population (Suttorp‐Schulten 1996). In low‐income countries, uveitis accounts for 2.4% to 24% of legal blindness. Individual estimates are not available for the various causes of infectious uveitis, including onchocerciasis, the fifth‐leading cause of blindness worldwide (Durrani 2004; Suttorp‐Schulten 1996). A recent, large, retrospective analysis of medical chart records (over a 12‐month period) by Gritz and colleagues in California, reported the incidence of uveitis to be 52.4 per 100,000 person‐years, which was three times higher than previous estimates (Gritz 2004). Posterior uveitis alone accounts for approximately 15% to 22% of uveitis cases in the United States, and leads to approximately 10% of legal blindness in the United States (Suttorp‐Schulten 1996).
Description of the intervention
Corticosteroids are the mainstay acute treatment for all anatomical subtypes of non‐infectious uveitis. They can be administered orally, topically with drops or ointments, by periocular (around the eye) or intravitreal (inside the eye) injection, or by surgical implantation (Haupert 2000). Corticosteroids are immunosuppressant medications that reduce inflammation and macular edema (retinal swelling), a principal cause of reduced vision in uveitis. Treatment of posterior uveitis represents a particular therapeutic challenge, because topical steroids rarely reach therapeutic concentrations in the vitreous, thus, people with posterior uveitis often require administration of oral corticosteroids or local steroid injection (Jaffe 2006). These therapeutic modalities may lead to several complications, including cataract formation and elevated intraocular pressure. The systemic morbidity associated with oral steroids includes hyperglycemia (high blood sugar or frank diabetes mellitus), myopathy (muscle damage), secondary infections, impaired wound healing, mental status changes (ranging from mood changes to psychosis), and adrenal suppression (hormone problems). Periocular and intravitreal steroid injections also have limitations: they provide only short‐term control, often requiring repeated injections every three to six months to control inflammation, and the injection procedure may be complicated by globe perforation, retinal tears, hemorrhage, endophthalmitis (infection of the eye), ptosis (drooping lid), and fibrosis (Haupert 2000; Jager 2004). In addition to systemic corticosteroids, systemic immunomodulatory therapies, including methotrexate, azathioprine, mycophenolate mofetil, cyclosporine, adalimumab, infliximab, and alkylating agents, such as cyclophosphamide, are used to treat uveitis.
Currently, there is no standardized algorithm for the use of systemic immunosuppressive therapies for non‐infectious uveitis, and most specific agents are used off‐label for this indication. Many of these therapies can have serious side effects, including increased susceptibility to infection and certain types of cancers, as well as bone marrow suppression (low blood counts, poor blood clotting, decreased ability to fight infection). While these therapies require close monitoring, their long‐term side effect profiles may be more favorable than corticosteroids. Except for cyclosporine, which is approved for dry eye syndrome but not commonly used to treat uveitis, none of these therapies are available for local administration to the eye.
How the intervention might work
Several clinical trials have investigated the efficacy of a technology that involves corticosteroid delivery via an intravitreal sustained‐release implant (Callanan 2008; Jaffe 2000a; Lowder 2011b; Williams 2009). An intravitreal corticosteroid implant has the theoretical advantage of maintaining an adequate, relatively stable concentration of corticosteroids for several months or years, without repeated intravitreal injection and its inherent risks. Such an implant may decrease or eliminate the need for systemic immune suppression.
The first corticosteroid implant for uveitis to be approved by the U.S. Food and Drug Administration (FDA) was the fluocinolone acetonide (FA) sustained‐release implant (Retisert, Bausch & Lomb Inc., Rochester, NY [Callanan 2008; Kempen 2011; Pavesio 2010]). The FDA also approved a short‐acting biodegradable dexamethasone intravitreal steroid implant for macular edema caused by retinal vein occlusions and diabetes mellitus, along with non‐infectious uveitis affecting the posterior segment (NIPU; Ozurdex, Allergan Inc., Irvine, CA [Haller 2010; Lowder 2011; Taylor 2010]). There is also a non‐biodegradable FA implant (Yutiq, Eyepoint Pharmaceuticals Inc., Watertown, MA), which the FDA approved for the treatment of non‐infectious posterior uveitis (Jaffe 2019). While such implants may reduce the overall systemic impact of corticosteroids, the increased intraocular exposure may cause higher rates of cataract and glaucoma (Bollinger 2011; Goldstein 2007a; Kempen 2011; Pavesio 2010). These risks must be weighed against their potential benefits.
Why it is important to do this review
This review is needed to enable decision makers (policymakers, clinicians, and people with uveitis) to weigh the benefits and risks of steroid implants when choosing the best option for the treatment of uveitis. These implants are expensive; in 2006, the 0.59 mg FA implant (Retisert) cost approximately USD 20,000, the 0.18 mg FA implant cost USD 10,000, and the dexamethasone 0.7‐mg dexamethasoneimplant cost USD 1500 (Mohammad 2007).
Objectives
To determine the efficacy and safety of steroid implants in people with chronic non‐infectious posterior uveitis, intermediate uveitis, and panuveitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) that compared a corticosteroid implant with a sham procedure or standard‐of‐care therapy.
Types of participants
We included studies that enrolled participants with better than hand‐motion vision and a history of chronic posterior uveitis, intermediate uveitis, or panuveitis (one eye with a history of recurrent non‐infectious uveitis affecting the posterior segment), who required systemic corticosteroids for more than one month, or multiple sub‐Tenon’s capsule corticosteroid injections. We included studies with both active and quiescent disease.
We excluded studies that enrolled participants with infectious uveitis.
The review protocol initially planned to only include studies that enrolled participants 18 years of age or older (Brady 2013). Authors of the previous version eliminated the age restriction (Brady 2016); which we continued for the current update.
Types of interventions
We included trials comparing fluocinolone acetonide or dexamethasone intravitreous implants with standard‐of‐care therapy (for example systemic steroids, intravitreal steroids, disease‐modifying antirheumatic drugs), or sham injection. For trials that tested against standard‐of‐care therapy, the implants were used alongside traditional topical or systemic anti‐inflammatory therapies, as long as the dosage was stable at the time of enrollment, reflecting the fact that these medications are used both as monotherapy and add‐on therapy.
Types of outcome measures
Critical outcome
The critical outcome was the proportion of participants (or eyes) with a recurrence of uveitis at six months, or at the primary efficacy time point defined by the included trial. The definition of recurrence included any of the following:
Increase in vitreous haze by two or more steps above baseline;
Increase in anterior chamber cell by two or more steps above baseline;
Clinical indication to add or increase dose of systemic anti‐inflammatory medication to control inflammation.
Important outcomes
Important outcomes assessed at six months, or at the primary efficacy time point of the trial, included:
Mean difference in best‐corrected distance visual acuity (BCVA), measured by the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart, Snellen chart, or Snellen equivalent;
Mean difference in quality of life (QoL) scores, measured by any validated measures presented, e.g. National Eye Institute Visual Functioning Questionnaire (NEI‐VFQ), 36‐Item Short Form Health Survey (SF‐36);
-
Adverse events: we assessed the proportion of participants (or eyes) who experienced the following conditions through to the end of the trial period:
Cataract formation or progression, or participants with phakic eyes that required cataract extraction surgery;
Elevated intraocular pressure (IOP) > 10 mmHg over baseline, or receiving intervention (eye drops or surgery);
Endophthalmitis;
Retinal tear or retinal detachment;
Systemic adverse events related to steroid or immunomodulatory therapy.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; which contains the Cochrane Eyes and Vision Trials Register; 2021, Issue 11) in the Cochrane Library (searched 16 November 2021; Appendix 1), MEDLINE Ovid, MEDLINE Ovid In‐Process and Other Non‐Indexed Citations, MEDLINE Ovid Daily, OLDMEDLINE Ovid (January 1946 to 16 November 2021; Appendix 2), PubMed (1948 to 16 November 2021; Appendix 3), Embase (January 1980 to 16 November 2021; Appendix 4), Latin American and Caribbean Health Sciences Literature Database (LILACS; 1982 to 16 November 2021; Appendix 5), the metaRegister of Controlled Trials (mRCT; searched 16 November 2021; Appendix 6), ClinicalTrials.gov (www.clinicaltrials.gov; searched 16 November 2021; Appendix 7), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/search/en; searched 16 November 2021; Appendix 8). We did not use any date or language restrictions in the electronic search for studies.
Searching other resources
We searched the reference lists of included studies, review articles, and guidelines to identify additional studies. We did not search meeting abstracts for the American Academy of Ophthalmology, the American Academy of Optometry, or the Association for Research in Vision and Ophthalmology, because these conference proceedings are included in CENTRAL.
Data collection and analysis
Selection of studies
Review authors worked in pairs to independently review the titles and abstracts of all records identified through the electronic searches, using the web‐based review management software Covidence. For studies that appeared to meet the inclusion criteria, or for which the information provided in the title and abstract were insufficient for us to make a clear decision, we obtained the full‐text reports. Two review authors independently assessed the full‐text reports to determine whether the studies met the inclusion criteria. We resolved any disagreement at either stage of screening by discussion. All publications from studies meeting the inclusion criteria underwent an assessment of risk of bias and data extraction. We recorded studies that were excluded after screening the full‐text report or subsequent stages of the review process in the Characteristics of excluded studies table, with reasons for exclusion documented.
Data extraction and management
Two review authors independently extracted the data for study design, participant characteristics, and the critical and important outcomes onto electronic data collection forms, developed by Cochrane Eyes and Vision in Covidence. We resolved discrepancies by discussion. We also contacted the trial investigator or corresponding author of eligible trials to request additional information if the reporting of methods or results was unclear. If the investigator or author did not reply within two weeks, we extracted the relevant information available to us from trial registers or published full‐text reports.
For each included study, we put the following characteristics into RevMan Web 2022: year of publication, country from which participants were recruited, and source of study funding; details of the participants, including demographic characteristics and inclusion criteria; details of the type of intervention; details of the outcomes reported, including adverse events, and the method of assessment and time intervals. We extracted continuous variables as means, standard deviations, or the associated 95% confidence intervals (CI); dichotomous variables as number of participants (or eyes) for which the outcome was measured. Specifically, for changes in BCVA that were measured in ETDRS letters, we converted letters into logMAR units before meta‐analysis (Ferris 1982). In some studies, we were only able to extract numerical data from figures, by applying a free, web‐based software suggested in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Li 2021; WebPlotDigitizer 2021).
Assessment of risk of bias in included studies
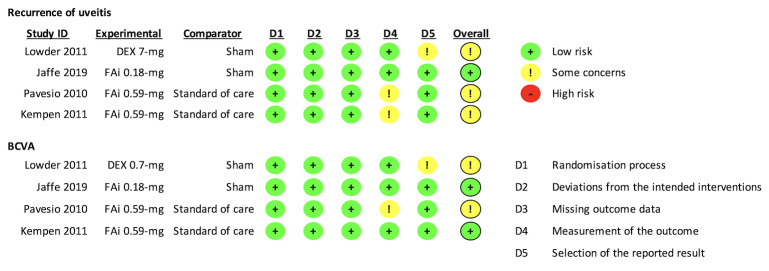
For the current update, we applied Cochrane's RoB 2 tool for risk assessment (Higgins 2021). Two review authors independently assessed the risk of bias for two outcomes: recurrence of uveitis and BCVA. We resolved disagreements on the RoB assessment by discussion within the author team.
We examined and reported on five domains.
Bias arising from the randomization process
Bias introduced by deviations from intended interventions
Bias due to missing outcome data
Bias in outcome measurement
Bias in selective reporting of outcome data
For each outcome specified for risk of bias assessment, we judged each domain as having low, high, or some concerns about risk of bias in accordance with signaling questions, for each included study that reported the outcome. At the study level, we provided an overall assessment on the risk of bias as:
Low, if we judged all domains to be at low risk of bias;
Some concerns, if we judged one or more domains to have some concerns, and none were at high risk;
High, if we judged one or more domains at high risk, or if we judged multiple domains to have some concerns (Higgins 2021).
Measures of treatment effect
For continuous outcomes (visual acuity and quality of life scores), we calculated mean differences (MD) with 95% CIs. For dichotomous outcomes, we calculated risk ratios (RR) with 95% CIs for proportions of participants (or eyes) with recurrence of uveitis. For prespecified adverse events, we reported RRs for proportion of eyes, to accommodate eye‐level data reported by Kempen 2011; the other three trials included only one study eye per participant.
Unit of analysis issues
The unit of analysis was a single eye for the majority of outcomes: recurrence rate of posterior uveitis, intermediate uveitis, or panuveitis; visual acuity; elevated intraocular pressure requiring intervention; reduction of cystoid macular edema; need for additional therapeutic modalities to control inflammation; cataract formation; cataract extraction; endophthalmitis; retinal tear, or retinal detachment.
The unit of analysis was the person for quality of life outcomes and potential systemic complications of therapy.
Dealing with missing data
We used imputed data reported and described by the trial investigators in the full‐text reports; we did not impute missing data ourselves. We contacted trial investigators for missing data. Since trial investigators did not respond (Pavesio 2010), or were unable to provide additional data (Kempen 2011), we extracted data available from the published report. For outcomes for which point estimates of the two comparison groups and P values were reported, we derived the between‐group standard deviation assuming Student t distribution, as suggested in Chapter 6 of the Cochrane Handbook (Li 2021).
Assessment of heterogeneity
We assessed the included trials for both clinical and methodological diversity by examining characteristics of the trial design, eligibility of trial participants, intervention and comparator differences, and outcome definitions. We assessed statistical heterogeneity using the I2 statistic, and considered the following thresholds when interpreting I2 values (Deeks 2021):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We assessed selective outcome reporting by comparing the outcomes specified in the study protocol or the methods section of the study report with the data reported in the study results, as guided by relevant signaling questions in the RoB 2 tool (Higgins 2021).
Data synthesis
We synthesized data from the included trials both qualitatively and quantitatively, according to the guidelines in Chapter 10 of the Cochrane Handbook (Deeks 2021). We calculated a summary risk ratio for dichotomous outcomes, and a summary mean difference for continuous outcomes, using random‐effects models if there were three or more trials reporting on the same outcome; otherwise, we used fixed‐effects models. When there was evidence of considerable clinical, methodological, or statistical heterogeneity across trials, we did not combine the data but described them qualitatively.
Subgroup analysis and investigation of heterogeneity
We did not conduct subgroup analyses because of the small number of included studies and methodologic heterogeneity.
Sensitivity analysis
We did not perform sensitivity analysis by age or clinical heterogeneity as planned in the protocol because of the small number of included trials (Brady 2013).
Summary of findings and assessment of the certainty of the evidence
We developed summary of findings tables, which included the assumed risk and corresponding risk for the following outcomes, based on the risk across control groups in the included studies:
Proportion of participants (or eyes) with recurrence of uveitis
Mean difference in BCVA
Mean difference in quality of life scores
Proportion of participants (or eyes) with cataract formation/progression or surgery
Proportion of participants (or eyes) with elevated IOP > 10 mmHg over baseline or receiving intervention
Proportion of participants (or eyes) with endophthalmitis
Proportion of participants (or eyes) with retinal tear or retinal detachment
We graded the overall certainty of the evidence for each outcome using the GRADE classification (Schünemann 2013). We assessed the certainty of evidence for each outcome as high, moderate, low, or very low, according to (1) high risk of bias; (2) indirectness of evidence; (3) unexplained heterogeneity or inconsistency of results; (4) imprecision; (5) high probability of publication bias, as described in Chapter 14 of the Cochrane Handbook (Schünemann 2021).
Results
Description of studies
Results of the search
In the 2016 version of the review, the review authors screened 2741 records, excluded 46 full‐text reports, and included two studies (Kempen 2011; Pavesio 2010). While updating the literature search in November 2021, we identified 1952 titles and abstracts, four of which we found by screening the Characteristics of excluded studies in the 2016 review. Overall, we screened 31 full‐text records for eligibility. We excluded seven studies (eight reports) with reasons, listed in the Characteristics of excluded studies table; three studies were ongoing trials, and we included two new trials (20 reports) in the current review (Jaffe 2019; Lowder 2011). See Figure 1. In total, we included four trials for evidence synthesis in this review. We described the individual included trials in the Characteristics of included studies table.
1.
Flow diagram for study selection for the 2022 update
Included studies
Types of studies
All four included studies were randomized controlled trials (RCTs) with a parallel‐group design, conducted among participants with a diagnosis of chronic non‐infectious uveitis affecting the posterior segment. Each participant was assigned randomly to the intervention or comparator group in all trials. Two trials randomized participants to either intraocular corticosteroid implant or standard‐of‐care systemic therapy (Kempen 2011; Pavesio 2010), while the other two trials randomized participants to either intraocular corticosteroid implant or sham procedure (Jaffe 2019; Lowder 2011).
Three of the four trials had two study arms. Lowder 2011 had three study arms (0.7 mg dexamethasone [DEX] implant, 0.35 mg DEX implant, and sham injection), however, we did not include data from the 0.35 mg DEX implant arm in our analysis, as this implant has never been commercially available. The included trials were all multicenter, international trials. Studies were published between 2010 and 2019. All studies reported industry funding or free intervention implants from the industry (Kempen 2011), and all trials reported information on trial registration with publicly available study protocols.
Types of participants
The four trials enrolled a total of 683 participants (907 eyes), with 129 to 240 participants enrolled per study. The percentage of female participants ranged from 48.5% to 71%; the age of participants (when reported) ranged from 12 to 74 years. All included trials enrolled participants with a clinically similar diagnosis of non‐infectious posterior uveitis, but with slightly different study populations: Pavesio 2010 enrolled participants who had clinically quiet non‐infectious posterior uveitis, while the other three trials enrolled participants who had active non‐infectious posterior uveitis in the study eye at the time of randomization (Jaffe 2019; Kempen 2011; Lowder 2011).
For participants with unilateral disease, the affected eye was the study eye. However, each study handled participants with bilateral disease differently. Pavesio 2010 chose the more severely affected eye as the study eye; Lowder 2011 treated the right eye as the study eye; Jaffe 2019 used the more severely affected eye in asymmetric bilateral disease, and the right eye in symmetric bilateral disease as the study eye; Kempen 2011 treated both eyes as study eyes in bilateral disease.
Types of interventions
Pavesio 2010 and Kempen 2011 used 0.59 mg fluocinolone acetonide (FA) intravitreal implant for their intervention group. These two trials used comparable standard‐of‐care systemic therapy comparison groups. The 0.59 mg FA implant could slowly release medication for approximately 30 months. Lowder 2011 used the 0.7 mg DEX implant for their intervention group, which would release medication for approximately three months. Jaffe 2019 used the 0.18 mg FA intravitreal implant for their intervention group, which could release medication for approximately 36 months.
Participants in the standard‐of‐care systemic therapy groups in Pavesio 2010 and Kempen 2011 were initially treated with oral corticosteroids, to which systemic immunomodulatory therapy was added if the uveitis recurred during tapering of corticosteroids. Both Lowder 2011 and Jaffe 2019 used similar procedures for participants in the sham procedure group, during which a blunt needle was applied against the sclera to mimic the injection procedure, thereby masking the participant.
We stratified the analysis by control treatment. Comparison 1 (corticosteroid implant versus sham) included data from Lowder 2011 and Jaffe 2019. Comparison 2 (corticosteroid implant versus standard‐of‐care systemic therapy) included data from Pavesio 2010 and Kempen 2011.
Types of outcomes
Critical outcomes
Recurrence of uveitis
Lowder 2011 and Jaffe 2019 both reported on this critical outcome at six months. Jaffe 2019 also reported on this outcome at 12 and 36 months post‐treatment. Pavesio 2010 reported only at 12 and 24 months post‐treatment. Kempen 2011 did not report on the recurrence of uveitis, but rather the proportion of eyes with 'residual active uveitis' at each study visit, and the percentage of eyes with control of uveitis at 24‐month follow‐up, which was also included in our analysis as a surrogate indicator for recurrence of uveitis.
Uveitis recurrence and activity were defined by clinical parameters (anterior chamber cells, vitreous haze, or decrease in visual acuity, or a combination) by Pavesio 2010, Kempen 2011, and Jaffe 2019. For Lowder 2011, we inferred data on recurrence of uveitis from 'the need for anti‐inflammatory rescue medication', reported by the authors.
Important outcomes
Mean difference in BCVA
Lowder 2011 reported change in BCVA from baseline at 6 months, Jaffe 2019 reported this at 12 months, and Kempen 2011 at 12 and 24 months. Pavesio 2010 reported the mean change in BCVA at each visit through to 24 months, along with the proportion of participants with improved visual acuity (defined as more than 15 letters on ETDRS chart from baseline).
Mean difference in QoL scores
Both Kempen 2011 and Lowder 2011 (via a sub‐analysis paper, Lightman 2013) reported on quality of life outcomes. Kempen 2011 used three different instruments to measure quality of life: the NEI‐VFQ, the SF‐36, and the EuroQoL questionnaire (EuroQoI 1990). The EuroQoL questionnaire included a visual analogue scale (VAS) for overall health‐related quality of life, and an EQ‐5D health utility index (Kempen 2011). Data were presented as mean changes from baseline to 12 months and 24 months, which we included in our analysis. Lightman 2013 also used the NEI‐VFQ, presenting data at 8 weeks, 16 weeks, and 26 weeks. We included data from this paper at six months.
Adverse events
Proportion of participants (or eyes) with cataract formation or progression, or participants with phakic eyes who required cataract extraction surgery
Two of the four trials reported the number of phakic eyes with cataract progression (Lowder 2011; Pavesio 2010); the other two reported on the incidence of cataract formation in initially non‐phakic eyes (Kempen 2011; Jaffe 2019). All four trials reported the number of phakic eyes that required cataract extraction after the intervention.
Cataract progression: Pavesio 2010 reported a total of 106 phakic eyes in their study, and defined cataract progression as a change of two grades or more in lens opacity. Kempen 2011 reported the number of phakic eyes that underwent surgery by group, during the 24‐month study period. Lowder 2011 reported a total of 117 phakic eyes; 47 of which had cataracts at the time of enrollment; formation and progression of cataracts were identified by biomicroscopy evaluation. Jaffe 2019 reported a total of 63 phakic eyes and the number of eyes that underwent cataract extraction surgeries during the first 12 months of the study.
Cataract formation: Kempen 2011 defined cataract formation as the identification of cataract by biomicroscopy evaluation at two consecutive visits, and reported incident cataract formation in 54 at‐risk eyes. In a post‐hoc analysis, Jaffe 2019 also compared risks for cataract formation among 90 study (at‐risk) eyes.
Proportion of participants (or eyes) with elevated intraocular pressure (IOP) > 10 mmHg over baseline or receiving intervention (eye drops or surgery)
All four trials reported on either or both outcomes, but used different threshold values for IOP elevation. Pavesio 2010 reported on IOP elevation of 10 mmHg or more from baseline; Kempen 2011 reported on IOP elevation of 10 mmHg or more from baseline and an absolute IOP of 30 mmHg or more; Lowder 2011 reported absolute IOP of 25 mmHg or more, and 35 mmHg or more; Jaffe 2019 reported on mean IOP and mean IOP change from baseline, along with IOP elevation of 12 mmHg or more from baseline, and absolute IOP higher than 25 mmHg and 30 mmHg.
Proportion of participants (or eyes) with endophthalmitis
All four trials measured infectious endophthalmitis clinically, by a biomicroscopy examination at each study visit.
Proportion of participants (or eyes) with retinal tear or retinal detachment
All four trials evaluated retinal tear and retinal detachment clinically, by biomicroscopy and indirect ophthalmoscopy examination during the trial period.
Proportion of participants with systemic adverse events related to steroid or immunomodulatory therapy
Only one trial reported systemic adverse events that could be considered to be related to steroid therapy, up to 24 months after treatment, such as hyperlipidemia diagnosis requiring treatment, hypertension diagnosis requiring treatment, diabetes mellitus, osteoporosis, white blood cell count less than 2500/mL, elevated liver enzymes, cancer diagnosis, and death (Kempen 2011).
Excluded studies
After the full‐text assessment, we excluded seven studies (eight reports; see Characteristics of excluded studies): three were non‐RCTs or dose‐response trials (Ciulla 2021; Cornish 2018; Errera 2019); two enrolled non‐uveitis participants (Couret 2020; NCT04976777); one compared different implant applicators (NCT02748512); Callanan 2020 was withdrawn from publication.
We identified three new ongoing studies (ChiCTR1900026160; NCT05070728; NCT05101928). We have no trials awaiting classification.
Risk of bias in included studies
We assessed the risk of bias using the RoB 2 tool for two outcomes we specified before data extraction, recurrence of uveitis and mean improvement in BCVA. Three of the four trials reported on both outcomes; Kempen 2011 did not specify recurrence of uveitis as a trial outcome, but reported the proportion of eyes with residual active uveitis at each study visit. As this outcome also evaluated control of inflammation, albeit in a broader manner, we included Kempen 2011 in our risk of bias assessment for recurrence of uveitis.
For recurrence of uveitis, we judged only one trial to be at low risk (25%) across all domains assessed; we had some concerns that the other three trials might have some bias due to biased outcome measurement or selective reporting (Figure 2). For mean improvement in BCVA, we considered two trials at low risk (50%) in all domains, whereas we had some concerns for the other two, in either biased outcome measurement or reporting (Figure 2).
2.
Risk of bias summary: review authors' judgments about each risk of bias domain for each trial that reported recurrence of uveitis and BCVA
Bias arising from the randomization process
We judged all four trials at low risk of bias arising from the randomization process for both uveitis recurrence and visual acuity outcomes.
Bias due to deviations from the intended intervention
We judged all four trials at low risk of bias in this domain for both uveitis recurrence and visual acuity outcomes.
Bias due to missing outcome data
We judged all four trials at low risk of bias for missing outcome data for both uveitis recurrence and visual acuity outcomes.
Bias in measurement of the outcome
We judged two of the four trials at low risk of measurement bias for both uveitis recurrence and visual acuity outcomes (Jaffe 2019; Lowder 2011).
We had some concerns of risk of measurement bias of uveitis recurrence, as not all investigators were masked to the treatment received by participants in two trials (Kempen 2011; Pavesio 2010). We judged that Kempen 2011 was at low risk of bias for visual acuity, but we had some concerns for Pavesio 2010's measurement of visual acuity, as it was unclear whether participants and assessors were masked during BCVA measurements.
Bias in selection of the reported result
We judged three of the four included trials at low risk of bias in this domain for both uveitis recurrence and visual acuity (Jaffe 2019; Kempen 2011; Pavesio 2010). We had some concerns for Lowder 2011's measurement of both uveitis recurrence and visual acuity, as there was neither a study protocol nor an analytic plan for the evaluation of potential risks.
Effects of interventions
Comparison 1. Corticosteroid implant versus sham procedure
Critical outcome
Proportion of participants (or eyes) with recurrence of uveitis
Both Lowder 2011 and Jaffe 2019 evaluated the proportion of participants who had a recurrence of uveitis at six months, when comparing those who received a corticosteroid implant with those who underwent a sham procedure. Lowder 2011 used a short‐acting (three‐month) corticosteroid implant, while Jaffe 2019 used a long‐acting (36‐month) implant. Combined results at the six‐month primary time point suggested that corticosteroid implants may decrease the risk of uveitis recurrence by 60% (risk ratio [RR] 0.40, 95% confidence interval [CI] 0.30 to 0.54; P = 0.04, I2 = 77%; 2 trials, 282 participants; Analysis 1.1; Figure 3; low‐certainty evidence) when compared with sham injection. Results were similar for the secondary time points, at 12 and 36 months, according to a single‐study estimate from Jaffe 2019 (Analysis 1.1). We downgraded the certainty of evidence for risk of bias (‐1) and imprecision (‐1).
1.1. Analysis.

Comparison 1: Steroid implant vs sham procedure, Outcome 1: Proportion of participants with recurrence of uveitis
3.

Forest plot of comparison 1: Steroid implant versus sham procedure, outcome: 1.1 Proportion of participants with recurrence of uveitis.
Important outcomes
Mean difference in BCVA
For the six‐month primary time point, a single‐study estimate from Lowder 2011 suggested that corticosteroid implants may lead to a greater improvement in BCVA ([mean difference] MD 0.15 logMAR, 95% CI 0.06 to 0.24; 1 trial, 153 participants; Analysis 1.2; low‐certainty evidence) than a sham injection. Results were comparable for the secondary time points of 12 and 36 months, according to the single‐study estimates from Jaffe 2019 (Analysis 1.2; Figure 4). We downgraded the certainty of the evidence for risk of bias (‐1) and imprecision (‐1).
1.2. Analysis.

Comparison 1: Steroid implant vs sham procedure, Outcome 2: Improvement in BCVA [logMAR]
4.

Forest plot of comparison 1: Steroid implant versus sham procedure, outcome: 1.2 Improvement in BCVA in logMAR.
Mean difference in quality of life scores
Only one study reported on quality of life scores (Lowder 2011, via a sub‐analysis in Lightman 2013). They used the NEI‐VFQ questionnaire to assess changes in participant‐reported quality of life over the study period, with a suggested minimal clinically important difference (MCID) of four to six points (Suner 2009). The single‐study estimates suggested that the corticosteroid implants resulted in little or no differences in the NEI‐VFQ scores (MD 2.85, 95% CI ‐3.64 to 9.34; 1 trial, 146 participants; Analysis 1.3; moderate‐certainty evidence) compared with sham injection. We downgraded the certainty of the evidence for imprecision (‐1).
1.3. Analysis.

Comparison 1: Steroid implant vs sham procedure, Outcome 3: Mean difference in quality of life scores
Adverse events
Proportion of participants (or eyes) with cataract formation, progression, or participants with phakic eyes who required cataract extraction surgery
Cataract formation: Jaffe 2019 reported on the implant‐associated risk of new cataract formation in 90 initially aphakic eyes. The single‐study estimates suggested that a corticosteroid implant may increase the risk of cataract formation compared with a sham procedure (RR 2.69, 95% CI:1.17 to 6.18; 1 trial, 90 eyes; Analysis 2.1; low‐certainty evidence).
Cataract progression: Lowder 2011 reported the risk of cataract progression in 117 phakic eyes, and suggested that corticosteroid implants may not increase cataract progression (RR 2.00, 95% CI 0.65 to 6.12; 1 trial, 117 eyes; Analysis 2.2; low‐certainty evidence) when compared with sham injection. This finding was comparable to the combined estimates for risks of 180 phakic eyes that underwent cataract extraction surgery during the trial period (RR 2.98, 95% CI: 0.82 to 10.81; 2 trials, 180 eyes; Analysis 2.3; low‐certainty evidence; Figure 5).
2.1. Analysis.

Comparison 2: Steroid implant vs sham procedure: ocular adverse events, Outcome 1: Proportion of eyes with cataract formation
2.2. Analysis.

Comparison 2: Steroid implant vs sham procedure: ocular adverse events, Outcome 2: Proportion of eyes with cataract progression
2.3. Analysis.

Comparison 2: Steroid implant vs sham procedure: ocular adverse events, Outcome 3: Proportion of eyes that underwent cataract surgery
5.

Forest plot of comparisons 1 and 3, outcome: 2.3 Proportion of participants or eyes that underwent cataract surgery. Trials in comparison 1 and Pavesio 2010 in comparison 3 included one study eye per participant; Kempen 2011 in comparison 3 reported eye‐level outcome data.
We downgraded the certainty of the evidence for indirectness (‐1) and imprecision (‐1).
Proportion of participants (or eyes) with elevated intraocular pressure (IOP) > 10 mmHg over baseline or receiving intervention (eye drops or surgery)
Elevated IOP: combined results suggested a corticosteroid implant may increase the risk of elevated IOP by 2.81 times of that in the sham group (95% CI 1.42 to 5.56; 2 trials, 282 participants; Analysis 2.4; moderate‐certainty evidence).
Elevated IOP requiring intervention: when compared with sham injection, steroid implants may increase the risk of requiring IOP‐lowering topical medication by 1.85 times (95% CI 1.05 to 3.25; 2 trials, 282 participants; Analysis 2.5; moderate‐certainty evidence). However, steroid implants likely resulted in comparable risks of elevated IOP that required IOP‐lowering surgery between the two comparison groups (RR 0.72, 95% CI 0.13 to 4.17; 2 trials, 282 participants; Analysis 2.6; moderate‐certainty evidence; Figure 6).
2.4. Analysis.

Comparison 2: Steroid implant vs sham procedure: ocular adverse events, Outcome 4: Proportion of eyes with elevated IOP
2.5. Analysis.

Comparison 2: Steroid implant vs sham procedure: ocular adverse events, Outcome 5: Proportion of eyes receiving IOP‐lowering medications
2.6. Analysis.

Comparison 2: Steroid implant vs sham procedure: ocular adverse events, Outcome 6: Proportion of eyes that underwent IOP‐lowering surgery
6.

Forest plot of comparisons 1 and 3, outcome: 2.6 Proportion of participants or eyes that underwent IOP‐lowering surgery. Trials in comparison 1 and Pavesio 2010 in comparison 3 included one study eye per participant; Kempen 2011 in comparison 3 reported eye‐level outcome data.
We downgraded the certainty of the evidence for imprecision (‐1).
Proportion of participants (or eyes) with endophthalmitis
Combined results suggested that steroid implants probably do not increase the risk of endophthalmitis over the sham procedure (RR 0.47, 95% CI 0.10 to 2.30; 2 trials; 280 participants; Analysis 2.7; moderate‐certainty evidence). We downgraded the certainty of the evidence for imprecision (‐1).
2.7. Analysis.

Comparison 2: Steroid implant vs sham procedure: ocular adverse events, Outcome 7: Proportion of eyes with endophthalmitis
Proportion of participants (or eyes) with retinal tear or retinal detachment
Combined results suggested that corticosteroid implants likely do not increase the risk of retinal tear or retinal detachment over the sham procedure (RR 1.11, 95% CI 0.21 to 5.75; 2 trials, 280 participants; Analysis 2.8; moderate‐certainty evidence). We downgraded the certainty of the evidence for imprecision (‐1).
2.8. Analysis.

Comparison 2: Steroid implant vs sham procedure: ocular adverse events, Outcome 8: Proportion of eyes with retinal tear or detachment
Proportion of participants with systemic adverse events related to steroid or immunomodulatory therapy
Neither trial provided usable data for this outcome. Lowder 2011 reported that "there were no notable changes from baseline in any vital signs or physical findings"; Jaffe 2019 reported that "approximately half of the participants in both treatment groups experienced a non‐ocular adverse event during the first 12 months of study", yet the study authors did not specify whether, or how many of these events were related to steroid or immunotherapy.
Comparison 2. Corticosteroid implant versus standard‐of‐care therapy
Critical outcomes
Proportion of participants (or eyes) with recurrence of uveitis
Pavesio 2010 evaluated the proportion of participants who had a recurrence of uveitis at 6 and 24 months, comparing those who received a corticosteroid implant with those who received standard‐of‐care systemic therapy, whereas Kempen 2011 evaluated the proportion of participants who had residual uveitis activity. Both trials used a long‐acting (30‐month) implant. Based on combined estimates at the 24‐month primary time point, corticosteroid implants were likely to decrease the risk of recurrence of uveitis by 54% (RR 0.46, 95% CI 0.35 to 0.60; 2 trials, 619 eyes; Analysis 3.1; Figure 7; low‐certainty evidence). Results were similar when including inferred cases of recurrence reported by Pavesio 2010 (Analysis 3.2), or when considering data reported at six months (Analysis 3.2).
3.1. Analysis.

Comparison 3: Steroid implant vs standard‐of‐care, Outcome 1: Proportion of eyes with recurrence of uveitis
7.

Forest plot of comparison 3: Steroid implant versus standard‐of‐care, outcome: 3.1 Proportion of eyes with recurrence of uveitis. Pavesio 2010 included one study eye per participant whereas Kempen 2011 included one or two affected eyes into the trial and reported at the eye level.
3.2. Analysis.

Comparison 3: Steroid implant vs standard‐of‐care, Outcome 2: Proportion of eyes with recurrence of uveitis; sensitivity analysis
We downgraded the certainty of the evidence for risk of bias (‐1) and indirectness (‐1), due to a different outcome definition in Kempen 2011.
Important outcomes
Mean difference in BCVA
Both Kempen 2011 and Pavesio 2010 reported the mean improvement in BCVA at 12 and 24 months. Based on combined estimates at the 24‐month primary study time point, steroid implants may have little to no effects on improving BCVA compared with standard‐of‐care therapies (MD 0.05, 95% CI ‐0.02 to 0.12; 2 trials, 619 eyes; Analysis 3.3; Figure 8; low‐certainty evidence). Findings were similar for the 12‐month secondary time point, but was significant for minimal BCVA improvement in the steroid implant group at the 6‐month secondary time point (Analysis 3.3). We downgraded the certainty of the evidence for risk of bias (‐1) and imprecision (‐1).
3.3. Analysis.

Comparison 3: Steroid implant vs standard‐of‐care, Outcome 3: Improvement in BCVA [logMAR]
8.

Forest plot of comparison 3: Steroid implant versus standard‐of‐care, outcome: 3.3 Improvement in BCVA in logMAR. Pavesio 2010 included one study eye per participant whereas Kempen 2011 included one or two affected eyes into the trial and reported at the eye level.
Mean difference in quality of life scores
Only one trial reported on quality of life (QoL) scores, using the NEI‐VFQ25 questionnaire, the SF‐36 physical functioning and mental well‐being subscales (general health‐related quality of life), the EuroQoL EQ‐VAS scores, and the EuroQoL EQ‐5D scores (Kempen 2011). The reported MCID for each of these QoL scales is: four to six points for the NEI‐VFQ25 (Mangione 2001); three to five points for the SF‐36 physical and mental subscales (Hays 2001); seven points for the EuroQoL (Pickard 2007); and 0.06 to 0.07 points for the EuroQoL‐5D (Pickard 2007).
The single‐study (N = 232) estimate suggested that the corticosteroid implant may increase the NEI‐VFQ25 score by 4.64 points (95% CI 0.13 to 9.15; Analysis 3.4) more than standard‐of‐care. Results of the two SF‐36 subscales were similarly improved in the implant group, compared with the control group. However, there was no evidence of differences in EuroQoL EQ‐VAS or EuroQoL EQ‐5D scores between the two groups, either clinically or statistically (Analysis 3.4). In general, corticosteroid implants likely increased participants' quality of life (moderate‐certainty evidence). We downgraded the certainty of evidence for imprecision (‐1).
3.4. Analysis.

Comparison 3: Steroid implant vs standard‐of‐care, Outcome 4: Mean difference in quality of life scores
Adverse events
Proportion of participants (or eyes) with cataract formation, progression, or participants with phakic eyes that required cataract extraction surgery
Cataract formation: neither of the two trials reported on this outcome.
Cataract progression: combined results of 24‐month follow‐up data suggested that a corticosteroid implant may increase the risk of cataract progression in phakic eyes by 2.71 times of those receiving standard‐of‐care (RR 2.71, 95% CI 2.06 to 3.56; 2 trials, 210 eyes; Analysis 2.2; low‐certainty evidence). Steroid implants may increase the risk of phakic eyes that underwent surgery by 2.98 times (RR 2.98, 95% CI 2.33 to 3.79; 2 trials, 371 eyes; Analysis 2.3; low‐certainty evidence; Figure 5). We downgraded the certainty of the evidence for indirectness (‐1) and imprecision (‐1).
Proportion of participants (or eyes) with elevated IOP > 10 mmHg from baseline or receiving intervention (eye drops or surgery)
Elevated IOP: evidence from combined results suggested that corticosteroid implants likely increased participants' risk of elevated IOP (> 10 mmHg from baseline) by 3.64 times over those in the standard‐of‐care group (RR 3.64, 95% CI 2.71 to 4.87; 2 trials, 605 eyes; Analysis 2.4; moderate‐certainty evidence).
Elevated IOP requiring intervention: when compared to standard‐of‐care, evidence also showed that steroid implants likely resulted in two times higher risk of IOP elevation that required topical medication (RR 3.04, 95% CI 2.36 to 3.91; 2 trials, 544 eyes; Analysis 2.5; moderate‐certainty evidence), or four times higher risk of requiring surgical intervention (RR 5.43, 95% CI 3.12 to 9.45; 2 trials, 599 eyes; Analysis 2.6; moderate‐certainty evidence; Figure 6).
We downgraded the certainty of the evidence for imprecision (‐1).
Proportion of participants (or eyes) with endophthalmitis
Based on combined results, evidence suggested that corticosteroid implants may or may not increase the risk of post‐injection endophthalmitis compared with standard‐of‐care (RR 7.30, 95% CI 0.91 to 58.72; 2 trials, 607 at‐risk eyes; Analysis 2.7; moderate‐certainty evidence). We downgraded the certainty of the evidence for imprecision (‐1).
Proportion of participants (or eyes) with retinal tear or retinal detachment
Evidence based on combined results suggested that steroid implants probably did not increase the risk for retinal tear or retinal detachment compared with standard‐of‐care (RR 2.07, 95% CI 0.51 to 8.40; 2 trials, 606 at‐risk eyes; Analysis 2.8; moderate‐certainty evidence). We downgraded the certainty of the evidence for imprecision (‐1).
Proportion of participants with systemic adverse events related to steroid or immunomodulatory therapy
Pavesio 2010 reported the risks of overall non‐ocular treatment‐related adverse events for the FA implant group (0%) and the standard‐of‐care therapy group (25.7%). They also reported the risks of non‐ocular severe adverse events for the implant group (0%) and the standard‐of‐care group (4.1%), without detailing the specific events that were considered to be treatment‐related.
Kempen 2011 reported the risks they considered potential systemic complications for steroid or immunosuppressive therapy separately.
Potential complications of steroid therapy: the incidence rates of hyperlipidemia (≥ 160 mg/mL); hyperlipidemia requiring treatment; diabetes; or bone osteopenia, porosis, or fracture were comparable between the two groups. The incidence of hypertension, defined by either elevated systolic (≥ 160 mmHg) or diastolic blood pressure (≥ 100 mmHg) was lower in the implant group (2.9 events per 100 person‐years) than in the control group (10.3 events per 100 person‐years, P for hazard ratio = 0.030). Nevertheless, the risk of new hypertension that required treatment was similar in both groups.
Potential complications of immunosuppressive therapy: no evidence suggested that the incidence of leukocytopenia (≤ 2500 cells/μL), thrombocytopenia (≤ 100,000/μL), anemia (≤ 10 g/dL), elevated liver enzymes, or serum creatinine levels was different in the two comparison groups.
Overall, the evidence suggested that corticosteroid implants may not increase the risks of systemic adverse events when compared with standard‐of‐care therapy.
Discussion
Summary of main results
In this update, we reported outcome data from four randomized controlled trials (RCTs) that compared local corticosteroid implants against either sham injection or standard‐of‐care systemic therapy in the treatment of non‐infectious uveitis affecting the posterior segment (NIPU). We analyzed data separately, based on the comparator therapy.
Two trials compared corticosteroid implants with sham injection. One trial evaluated a short‐acting implant (0.7 mg dexamethasone) that released corticosteroid for approximately three months, while the other evaluated a long‐acting implant (0.18 mg fluocinolone acetonide [FA]) that released corticosteroid for approximately 36 months. Low‐certainty evidence suggested that these corticosteroid implants were likely to reduce the risk of uveitis recurrence and to improve best‐corrected distance visual acuity (BCVA) at the six‐month primary time point compared with sham injection. Low‐certainty evidence showed higher rates of local adverse events in the corticosteroid implant groups for cataract formation, with higher risks of intraocular pressure (IOP) elevation, and the need for IOP‐lowering medications in the corticosteroid implant group. The relatively short follow‐up period for participants in Lowder 2011 may have limited the ability to detect cataract progression. Single‐study estimates for quality of life showed comparable changes at six months between the two comparison groups.
Two trials compared corticosteroid implants with standard‐of‐care systemic therapy. Both studies evaluated a long‐acting surgically‐placed implant (0.59 mg FA) that released corticosteroid for approximately 30 months. Low‐certainty evidence suggested that these corticosteroid implants may reduce the risk of uveitis recurrence and probably improve BCVA at the 24‐month primary time point compared with standard‐of‐care therapy. Low‐certainty evidence also showed higher risks of local adverse events in the corticosteroid implant groups for cataract formation and cataract progression, with higher risks of IOP elevation, and the need for medical or surgical interventions to lower IOP after receiving the steroid implants. Single‐study estimates from Kempen 2011 reported a lower incidence of hypertension in the implant group, but suggested comparable rates of diabetes, osteoporosis, blood count abnormalities, liver function, or serum creatinine abnormalities.
Overall completeness and applicability of evidence
In all the included trials, the majority of participants were described as White, potentially decreasing the applicability to non‐White populations. In this update, we continued the broadened eligibility criteria to include participants under 18 years of age. Both trials comparing 0.59 mg FA to standard‐of‐care evaluated slightly different populations; Pavesio 2010 enrolled participants with inactive uveitic disease, whereas Kempen 2011 enrolled participants with active disease. Since the primary outcome was evaluated at 24 months, we considered that the influence of this baseline difference on treatment effects was clinically trivial.
The corticosteroid implants evaluated in these four trials (0.59 mg FA, 0.7 mg dexamethasone, and 0.18 mg FA) are three of the four implants that are approved for the treatment of NIPU. The fourth implant, 0.19 mg FA, is thought to have essentially the same characteristics as the 0.18 mg FA, but is indicated for diabetic macular edema (Testi 2019).
Both comparators of sham therapy and standard‐of‐care systemic therapy provided useful data and evidence from different clinical perspectives. By comparing with sham therapy, the evidence provided baseline effectiveness and adverse effects of the corticosteroid implants per se. The comparator of standard‐of‐care systemic therapy provided clinically useful data for both severe, active disease, and controlled disease that was treated initially with either local or systemic therapy.
The outcome measures of uveitis recurrence and BCVA were useful, as these are commonly used clinical parameters that are followed for people with NIPU. Despite the relative paucity of evidence on quality of life outcomes, these outcomes were also of high clinical importance, as this information can assist in the shared decision‐making process of which anti‐inflammatory therapy to initiate. Kempen 2011 reported on various participant‐reported quality of life outcomes. The 0.59 mg FA implant was found to result in higher scores compared to standard‐of care therapy in visual functioning, and both physical and mental quality of life. However, it is important to note that although these results were statistically significant, the differences were just at or below the minimal clinically important differences, suggesting that these might not be meaningful improvements in quality of life.
Certainty of the evidence
We downgraded outcomes in this review due to imprecision of results, and risk of bias associated with biased outcome measurement or selective reporting. Specifically, we had some concerns about the reporting of the recurrence of uveitis by three trials, and BCVA by two trials. While the nature of the interventions and the comparators, particularly in the standard‐of‐care group, made complete masking impossible, unmasked assessors or data analysts might have predisposed trials to be at risk of bias in ascertaining or analyzing the outcome data.
Potential biases in the review process
We performed an extensive literature search in multiple electronic databases and trial registries, and handsearched reference lists of the included trials and the excluded studies of the previous review. We used standard Cochrane methodological procedures to avoid potential biases in the review process. We reported all outcomes that were specified in the protocol for this review, or reported that no data were available for specified outcomes.
Agreements and disagreements with other studies or reviews
We identified two recently published reviews on a similar topic (Abdulla 2022; Logan 2016). In Logan 2016, the authors discussed the 0.59 mg FA used in Kempen 2011, and the 0.7 mg DEX implants used in Lowder 2011. The authors also summarized case reports and series describing the successful use of the implant in the treatment of various uveitic diseases. They concluded that the 0.59 mg FA implant was a useful treatment for NIPU, but it was not necessarily superior to systemic therapy, and carried risks of ocular side effects. In regard to the 0.7 mg DEX implant, the authors also described a comparative case series comparing this implant to the 0.59 mg FA implant. Not surprisingly, the 0.7 mg DEX implant had a shorter time to re‐treatment compared to the 0.59 mg FA implant. They also summarized retrospective studies that found the 0.7 mg DEX implant was useful in the treatment of uveitic macular edema.
In Abdulla 2022, the authors discussed the 0.7 mg DEX implant, the 0.18 mg FA implant (they combined these data with data from the 0.19 mg FA implant), and the 0.59 mg FA implant. They also summarized data reported by Lowder 2011 and Jaffe 2019. They spent comparatively little time discussing the 0.59 mg FA implant, as they argued it had "largely been superseded" by the 0.18 and 0.19 mg FA implants. However, they did not discuss the potential differences arising from the fact that the 0.18 mg FA implant releases three‐to‐four fold less FA than the 0.59 mg FA implant; and while the 0.59 mg FA implant has a fairly steady release rate over three years, the 0.18 mg FA implant releases the drug at a higher rate for the first 12 months and then at a lower rate for the remaining lifespan of the implant (Modugno 2021).
Authors' conclusions
Implications for practice.
Our confidence is limited that local corticosteroid implants are superior to sham therapy or standard‐of‐care therapy in reducing the risk of uveitis recurrence in people with non‐infectious uveitis.
The included trials exhibited heterogeneity in design, such as the expected dose and product duration of the steroid implants, and in study outcomes, such as the definitions for, and frequency of quantifying core outcomes. Taken together, clinicians and people with chronic non‐infectious uveitis must anticipate the possibility of an increased risk of post‐implant surgery for cataract progression, or high intraocular pressure (or both), when considering steroid implants as part of a clinical management plan.
Implications for research.
With expanded eligibility criteria, we were able to combine efficacy and safety data reported by the included trials. However, high certainty evidence to guide clinical decisions is expected to be informed by trials that
recruit people with different types of uveitis (chronic posterior uveitis, intermediate uveitis, panuveitis), and then report type‐specific treatment effects;
standardize core outcome measures, such as recurrence of uveitis and time points for efficacy and safety outcomes;
measure and report person‐important outcomes, such as quality of life or visual function‐related outcomes.
Given the increased risks for local adverse effects of corticosteroid implants, future trials also need to incorporate participants' perspectives in evaluating the benefit‐harm utility of corticosteroid implants as a first‐line or second‐line treatment option for people with chronic non‐infectious uveitis. Results of the three ongoing trials may contribute to the growing evidence of fluocinolone acetonide and dexamethasone, particularly on the treatment benefits. However, future trials that examine head‐to‐head comparisons of implants with varied drug‐releasing rates over time can also provide information on how these steroid implants may achieve persistent anti‐inflammatory control without increasing short‐ or long‐term adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 2 August 2023 | New citation required but conclusions have not changed | Errors in text, axis labels, and Summary of Finding tables corrected; conclusions unchanged. |
| 2 August 2023 | Amended | Errors in text, axis labels, and Summary of Finding tables corrected; conclusions unchanged. |
History
Protocol first published: Issue 4, 2013 Review first published: Issue 2, 2016
| Date | Event | Description |
|---|---|---|
| 10 January 2022 | New citation required and conclusions have changed | Inclusion criteria expanded, but did not affect search strategies. Two new trials identified and included for synthesis. |
| 16 November 2021 | New search has been performed | New search performed; included one new term, 'Yutiq', which is a newer equivalent to 'Retisert*' |
Risk of bias
Risk of bias for analysis 1.1 Proportion of participants with recurrence of uveitis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.1.1 Primary efficacy time point: 6 months | ||||||||||||
| Jaffe 2019 | Low risk of bias | "An independent statistical group generated the randomization code, which was accessed for participant assignment through a central interactive voice response system". | Low risk of bias | At each site, one unmasked investigator administered study treatment and performed day 1 assessments, and one masked investigator performed all study assessments after day 1. All other study personnel and participants were masked to treatment assignment and remained so throughout the study. The primary efficacy analysis was pre‐specified, analyzed, and reported as in the analytic plan. | Low risk of bias | All participants were followed through month 6. | Low risk of bias | To avoid attributing an inflammatory response to the implantation procedure, assessments for uveitis recurrence began after day 7. The clinical definition of uveitis recurrence used in the study was pre‐specified according to FDA requirements (per study protocol). "...one masked investigator performed all study assessments after day 1. All other study personnel and participants were masked to treatment assignment and remained so throughout the study." | Low risk of bias | Data reported and analyzed for this outcome followed the pre‐specified analytic plan at pre‐specified study visits. | Low risk of bias | The trial was judged to be at low risk across all domains assessed. |
| Lowder 2011 | Low risk of bias | Randomization was performed centrally (using an interactive voice/web resposne system) by the study sponsor and was stratified by baseline vitreous haze. There were no notable between‐group differences in any demographic or baseline characteristic. | Low risk of bias | "Patients were masked with regard to study treatment". "The treatment investigator performed the implant placement and other treatment procedures ... and did not collect efficacy information... the key efficacy variables were collected and evaluated by follow‐up invesetigators who were also masked with regard to study treatment." | Low risk of bias | Nearly all participants randomized were analyzed for this outcome. Four and five patients in the DEXA implant (4/77=5%) and sham group (5/76=7%) were lost to follow‐up and their data for this outcome were imputed by "last observation carry forward", which was reasonable and appropriately analyzed/reported. | Low risk of bias | "The key efficacy variables were collected and evaluated by follow‐up investigators who were also masked with regard to study treatment." | Some concerns | There were no study protocol or analytic plan for evaluation. The current outcome variable (% patients requiring rescue medication) was chosen to reflect the review outcome measure "% participants with recurrence". It was not pre‐specified as primary or secondary outcomes by the study authors. It was likely that the choice of analytic approach and its reporting was affected by the statistical significance. | Some concerns | The trial was considered to be at low risk of bias in all domains, except that there were some concerns about potential selective reporting of results. |
| Subgroup 1.1.2 Other time point: 12 months | ||||||||||||
| Jaffe 2019 | Low risk of bias | "An independent statistical group generated the randomization code, which was accessed for participant assignment through a central interactive voice response system". | Low risk of bias | At each site, one unmasked investigator administered study treatment and performed day 1 assessments, and one masked investigator performed all study assessments after day 1. All other study personnel and participants were masked to treatment assignment and remained so throughout the study. The primary efficacy analysis was pre‐specified, analyzed, and reported as in the analytic plan. | Low risk of bias | All participants were followed through month 6. | Low risk of bias | To avoid attributing an inflammatory response to the implantation procedure, assessments for uveitis recurrence began after day 7. The clinical definition of uveitis recurrence used in the study was pre‐specified according to FDA requirements (per study protocol). "...one masked investigator performed all study assessments after day 1. All other study personnel and participants were masked to treatment assignment and remained so throughout the study." | Low risk of bias | Data reported and analyzed for this outcome followed the pre‐specified analytic plan at pre‐specified study visits. | Low risk of bias | The trial was judged to be at low risk across all domains assessed. |
| Subgroup 1.1.3 Other time point: 36 months | ||||||||||||
| Jaffe 2019 | Low risk of bias | "An independent statistical group generated the randomization code, which was accessed for participant assignment through a central interactive voice response system". | Low risk of bias | At each site, one unmasked investigator administered study treatment and performed day 1 assessments, and one masked investigator performed all study assessments after day 1. All other study personnel and participants were masked to treatment assignment and remained so throughout the study. The primary efficacy analysis was pre‐specified, analyzed, and reported as in the analytic plan. | Low risk of bias | All participants were followed through month 6. | Low risk of bias | To avoid attributing an inflammatory response to the implantation procedure, assessments for uveitis recurrence began after day 7. The clinical definition of uveitis recurrence used in the study was pre‐specified according to FDA requirements (per study protocol). "...one masked investigator performed all study assessments after day 1. All other study personnel and participants were masked to treatment assignment and remained so throughout the study." | Low risk of bias | Data reported and analyzed for this outcome followed the pre‐specified analytic plan at pre‐specified study visits. | Low risk of bias | The trial was judged to be at low risk across all domains assessed. |
Risk of bias for analysis 1.2 Improvement in BCVA [logMAR].
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.2.1 Primary efficacy time point: 6 months | ||||||||||||
| Lowder 2011 | Low risk of bias | Randomization was performed centrally (using an interactive voice/web resposne system) by the study sponsor and was stratified by baseline vitreous haze. There were no notable between‐group differences in any demographic or baseline characteristic. | Low risk of bias | Patients were masked with regard to study treatment. "The treatment investigator performed the implant placement and other treatment procedures ... and did not collect efficacy information ...the key efficacy variables were collected and evaluated by follow‐up invesetigators who were also masked with regard to study treatment." | Low risk of bias | Nearly all participants randomized were analyzed for this outcome. Four and five patients in the DEXA implant (4/77=5%) and sham group (5/76=7%) were lost to follow‐up and their data for this outcome were imputed by "last observation carry forward", which was reasonable and appropriately analyzed/reported. | Low risk of bias | BCVA was measured using ETDRS protocol. The key efficacy variables were collected and evaluated by follow‐up investigators who were also masked with regard to study treatment. | Some concerns | There were no study protocol or analytic plan for evaluation. All measurements at each pre‐specified study visit were reported and analyzed. | Some concerns | The trial was judged to be at low risk in all domains, except that there were some concerns in selective reporting of the results. |
| Subgroup 1.2.2 Other time point: 12 months | ||||||||||||
| Jaffe 2019 | Low risk of bias | "An independent statistical group generated the randomization code, which was accessed for participant assignment through a central interactive voice response system". | Low risk of bias | At each site, one unmasked investigator administered study treatment and performed day 1 assessments, and one masked investigator performed all study assessments after day 1. All other study personnel and participants were masked to treatment assignment and remained so throughout the study. BCVA assessed at month 12 was pre‐specified as secondary outcome. | Low risk of bias | Data for all participants were reported for month 12. | Low risk of bias | BCVA was measured using standard ETDSR protocol. All other study personnel and participants were masked to treatment assignment and remained so throughout the study. | Low risk of bias | Analysis and reporting of this outcome followed the study protocol and the analytic plan. | Low risk of bias | The trial was considered as low risk for all domains assessed. |
| Subgroup 1.2.3 Other time point: 36 months | ||||||||||||
| Jaffe 2019 | Low risk of bias | "An independent statistical group generated the randomization code, which was accessed for participant assignment through a central interactive voice response system". | Low risk of bias | At each site, one unmasked investigator administered study treatment and performed day 1 assessments, and one masked investigator performed all study assessments after day 1. All other study personnel and participants were masked to treatment assignment and remained so throughout the study. BCVA assessed at month 12 was pre‐specified as secondary outcome. | Low risk of bias | Data for all participants were reported for month 12. | Low risk of bias | BCVA was measured using standard ETDSR protocol. All other study personnel and participants were masked to treatment assignment and remained so throughout the study. | Low risk of bias | Analysis and reporting of this outcome followed the study protocol and the analytic plan. | Low risk of bias | The trial was considered as low risk for all domains assessed. |
Risk of bias for analysis 3.1 Proportion of eyes with recurrence of uveitis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.1.1 Primary efficacy time point: 24 months | ||||||||||||
| Kempen 2011 | Low risk of bias | "Patients were randomized to implant or systemic therapy…Randomization (1:1 ratio) was by vari‐ able length, permuted blocks within 2 strata (clinical center, inter‐ mediate vs posterior or panuveitis), with assignments produced by Stata 11.0. After data entry confirmed a subject’s eligibility and stratum, the study Web site revealed the next treatment assignment." Baseline demographic and clin‐ ical characteristics were distributed similarly between groups (Table 1). | Low risk of bias | Patients were not masked. Clinicians and coordinators were not masked. | Low risk of bias | Outcome data for all participants randomized were analyzed; missing indicators were used in longitudinal models for comparing treatment effects. | Some concerns | Definitions for uveitis recurrence were not reported but uveitis activity was asessed by clinician at each study visit. Since clinicians were not masked to the treatment received by the participants, the outcome measurement could have differed between groups. | Low risk of bias | Data for this outcome were analyzed and reported as pre‐specified in the trial registry record. | Some concerns | There was some concern due to the inability to mask outcome assessors for uveitis activity. |
| Pavesio 2010 | Low risk of bias | "Subjects were allocated to receive either an implant or standardized therapy as determined by a randomization code with treatment randomization numbers assigned by a centrally administered randomization procedure." "Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | Although participants were aware of their assigned intervention, "...efforts were made to avoid selection bias when allocating the study treatment. Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | "A total of 146 patients were enrolled and randomized...Six subjects randomized to the FA implant group discontinued before receiving treatment...and were excluded from the intent‐to‐treat population. A total of 131 subjects completed the 2‐year visit." | Some concerns | Outcome assessors were unmasked to the treatment received by the participants when documenting uveitis recurrence. | Low risk of bias | All of the prespecified outcomes from the methods section were reported in the results section. | Some concerns | The trial was considered at low risk for four domains but might have some concerns in biased measurement of the outcome because the outcome assessors were unmasked. |
| Subgroup 3.1.2 Other time point: 6 months | ||||||||||||
| Pavesio 2010 | Low risk of bias | "Subjects were allocated to receive either an implant or standardized therapy as determined by a randomization code with treatment randomization numbers assigned by a centrally administered randomization procedure." "Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | Although participants were aware of their assigned intervention, "...efforts were made to avoid selection bias when allocating the study treatment. Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | "A total of 146 patients were enrolled and randomized...Six subjects randomized to the FA implant group discontinued before receiving treatment...and were excluded from the intent‐to‐treat population. A total of 131 subjects completed the 2‐year visit." | Some concerns | Outcome assessors were unmasked to the treatment received by the participants when documenting uveitis recurrence. | Low risk of bias | All of the prespecified outcomes from the methods section were reported in the results section. | Some concerns | The trial was considered at low risk for four domains but might have some concerns in biased measurement of the outcome because the outcome assessors were unmasked. |
| Kempen 2011 | Low risk of bias | "Patients were randomized to implant or systemic therapy…Randomization (1:1 ratio) was by vari‐ able length, permuted blocks within 2 strata (clinical center, inter‐ mediate vs posterior or panuveitis), with assignments produced by Stata 11.0. After data entry confirmed a subject’s eligibility and stratum, the study Web site revealed the next treatment assignment." Baseline demographic and clin‐ ical characteristics were distributed similarly between groups (Table 1). | Low risk of bias | Patients were not masked. Clinicians and coordinators were not masked. | Low risk of bias | Outcome data for all participants randomized were analyzed; missing indicators were used in longitudinal models for comparing treatment effects. | Some concerns | Definitions for uveitis recurrence were not reported but uveitis activity was asessed by clinician at each study visit. Since clinicians were not masked to the treatment received by the participants, the outcome measurement could have differed between groups. | Low risk of bias | Data for this outcome were analyzed and reported as pre‐specified in the trial registry record. | Some concerns | There was some concern due to the inability to mask outcome assessors for uveitis activity. |
Risk of bias for analysis 3.2 Proportion of eyes with recurrence of uveitis; sensitivity analysis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.2.1 Primary efficacy time point: 24 months | ||||||||||||
| Kempen 2011 | Low risk of bias | "Patients were randomized to implant or systemic therapy…Randomization (1:1 ratio) was by vari‐ able length, permuted blocks within 2 strata (clinical center, inter‐ mediate vs posterior or panuveitis), with assignments produced by Stata 11.0. After data entry confirmed a subject’s eligibility and stratum, the study Web site revealed the next treatment assignment." Baseline demographic and clin‐ ical characteristics were distributed similarly between groups (Table 1). | Low risk of bias | Patients were not masked. Clinicians and coordinators were not masked. | Low risk of bias | Outcome data for all participants randomized were analyzed; missing indicators were used in longitudinal models for comparing treatment effects. | Some concerns | Definitions for uveitis recurrence were not reported but uveitis activity was asessed by clinician at each study visit. Since clinicians were not masked to the treatment received by the participants, the outcome measurement could have differed between groups. | Low risk of bias | Data for this outcome were analyzed and reported as pre‐specified in the trial registry record. | Some concerns | There was some concern due to the inability to mask outcome assessors for uveitis activity. |
| Pavesio 2010 | Low risk of bias | "Subjects were allocated to receive either an implant or standardized therapy as determined by a randomization code with treatment randomization numbers assigned by a centrally administered randomization procedure." "Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | Although participants were aware of their assigned intervention, "...efforts were made to avoid selection bias when allocating the study treatment. Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | "A total of 146 patients were enrolled and randomized...Six subjects randomized to the FA implant group discontinued before receiving treatment...and were excluded from the intent‐to‐treat population. A total of 131 subjects completed the 2‐year visit." | Some concerns | Outcome assessors were unmasked to the treatment received by the participants when documenting uveitis recurrence. | Low risk of bias | All of the prespecified outcomes from the methods section were reported in the results section. | Some concerns | The trial was considered at low risk for four domains but might have some concerns in biased measurement of the outcome because the outcome assessors were unmasked. |
| Subgroup 3.2.2 Other time point: 6 months | ||||||||||||
| Pavesio 2010 | Low risk of bias | "Subjects were allocated to receive either an implant or standardized therapy as determined by a randomization code with treatment randomization numbers assigned by a centrally administered randomization procedure." "Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | Although participants were aware of their assigned intervention, "...efforts were made to avoid selection bias when allocating the study treatment. Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | "A total of 146 patients were enrolled and randomized...Six subjects randomized to the FA implant group discontinued before receiving treatment...and were excluded from the intent‐to‐treat population. A total of 131 subjects completed the 2‐year visit." | Some concerns | Outcome assessors were unmasked to the treatment received by the participants when documenting uveitis recurrence. | Low risk of bias | All of the prespecified outcomes from the methods section were reported in the results section. | Some concerns | The trial was considered at low risk for four domains but might have some concerns in biased measurement of the outcome because the outcome assessors were unmasked. |
| Kempen 2011 | Low risk of bias | "Patients were randomized to implant or systemic therapy…Randomization (1:1 ratio) was by vari‐ able length, permuted blocks within 2 strata (clinical center, inter‐ mediate vs posterior or panuveitis), with assignments produced by Stata 11.0. After data entry confirmed a subject’s eligibility and stratum, the study Web site revealed the next treatment assignment." Baseline demographic and clin‐ ical characteristics were distributed similarly between groups (Table 1). | Low risk of bias | Patients were not masked. Clinicians and coordinators were not masked. | Low risk of bias | Outcome data for all participants randomized were analyzed; missing indicators were used in longitudinal models for comparing treatment effects. | Some concerns | Definitions for uveitis recurrence were not reported but uveitis activity was asessed by clinician at each study visit. Since clinicians were not masked to the treatment received by the participants, the outcome measurement could have differed between groups. | Low risk of bias | Data for this outcome were analyzed and reported as pre‐specified in the trial registry record. | Some concerns | There was some concern due to the inability to mask outcome assessors for uveitis activity. |
Risk of bias for analysis 3.3 Improvement in BCVA [logMAR].
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.3.1 Primary efficacy time point: 24 months | ||||||||||||
| Pavesio 2010 | Low risk of bias | "Subjects were allocated to receive either an implant or standardized therapy as determined by a randomization code with treatment randomization numbers assigned by a centrally administered randomization procedure." "Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | Treatment allocation was masked to both the investiga‐ tor and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject. "... some assessments, including fluorescein angiography, fundus photography, and laboratory parameters, were masked". | Low risk of bias | Nearly all participants randomized were analyzed for this outcome. Six subjects randomized to the FA implant group discontinued before receiving treatment because of administrative problems (n = 3), consent withdrawal (n = 2), or AEs (n = 1) and were excluded from the intent‐to‐treat population (130/146). | Some concerns | Visual acuity outcome assessors were aware of the intervention received by study participants. | Low risk of bias | Results were analyzed according the pre‐specified outcome of >15 letter improvement on ETDRS charts from baseline. | Some concerns | The trial was judged to be at low risk in four domains but with some concerns in biased outcome measurement due to unmasking of outcome assessors. |
| Kempen 2011 | Low risk of bias | "Patients were randomized to implant or systemic therapy…Randomization (1:1 ratio) was by vari‐ able length, permuted blocks within 2 strata (clinical center, inter‐ mediate vs posterior or panuveitis), with assignments produced by Stata 11.0. After data entry confirmed a subject’s eligibility and stratum, the study Web site revealed the next treatment assignment." Baseline demographic and clin‐ ical characteristics were distributed similarly between groups (Table 1). | Low risk of bias | Intention‐to‐treat analysis was used. | Low risk of bias | "...255 patients were enrolled...Among patients randomized, 232 completed visual acuity measurement at the 24 month follow‐up visit. Overall, 4415 of 4790 study visits (92%) were completed for the primary outcome through 24 months." | Low risk of bias | "Study‐certified visual acuity examiners measured best‐corrected visual acuity as the number of letters read from standard logarithmic visual acuity charts; change in this measure from baseline to 24 months was the primary outcome." "Other than at the 1 and 3 month visits, when post‐operative signs were expected to be visible, visual acuity examiners were masked." | Low risk of bias | The pre‐specified outcome was reported as the result. | Low risk of bias | The trial was judged to be at low across all domains. |
| Subgroup 3.3.2 Other time point: 12 months | ||||||||||||
| Pavesio 2010 | Low risk of bias | "Subjects were allocated to receive either an implant or standardized therapy as determined by a randomization code with treatment randomization numbers assigned by a centrally administered randomization procedure." "Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | Treatment allocation was masked to both the investiga‐ tor and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject. "... some assessments, including fluorescein angiography, fundus photography, and laboratory parameters, were masked". | Low risk of bias | Nearly all participants randomized were analyzed for this outcome. Six subjects randomized to the FA implant group discontinued before receiving treatment because of administrative problems (n = 3), consent withdrawal (n = 2), or AEs (n = 1) and were excluded from the intent‐to‐treat population (130/146). | Some concerns | Visual acuity outcome assessors were aware of the intervention received by study participants. | Low risk of bias | Results were analyzed according the pre‐specified outcome of >15 letter improvement on ETDRS charts from baseline. | Some concerns | The trial was judged to be at low risk in four domains but with some concerns in biased outcome measurement due to unmasking of outcome assessors. |
| Kempen 2011 | Low risk of bias | "Patients were randomized to implant or systemic therapy…Randomization (1:1 ratio) was by vari‐ able length, permuted blocks within 2 strata (clinical center, inter‐ mediate vs posterior or panuveitis), with assignments produced by Stata 11.0. After data entry confirmed a subject’s eligibility and stratum, the study Web site revealed the next treatment assignment." Baseline demographic and clin‐ ical characteristics were distributed similarly between groups (Table 1). | Low risk of bias | Intention‐to‐treat analysis was used. | Low risk of bias | "...255 patients were enrolled...Among patients randomized, 232 completed visual acuity measurement at the 24 month follow‐up visit. Overall, 4415 of 4790 study visits (92%) were completed for the primary outcome through 24 months." | Low risk of bias | "Study‐certified visual acuity examiners measured best‐corrected visual acuity as the number of letters read from standard logarithmic visual acuity charts; change in this measure from baseline to 24 months was the primary outcome." "Other than at the 1 and 3 month visits, when post‐operative signs were expected to be visible, visual acuity examiners were masked." | Low risk of bias | The pre‐specified outcome was reported as the result. | Low risk of bias | The trial was judged to be at low across all domains. |
| Subgroup 3.3.3 Other time point: 6 months | ||||||||||||
| Pavesio 2010 | Low risk of bias | "Subjects were allocated to receive either an implant or standardized therapy as determined by a randomization code with treatment randomization numbers assigned by a centrally administered randomization procedure." "Treatment allocation was masked to both the investigator and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject." | Low risk of bias | Treatment allocation was masked to both the investiga‐ tor and the subject through the use of an interactive voice response system that informed the investigator of the treatment group only after confirmation of inclusion of the subject. "... some assessments, including fluorescein angiography, fundus photography, and laboratory parameters, were masked". | Low risk of bias | Nearly all participants randomized were analyzed for this outcome. Six subjects randomized to the FA implant group discontinued before receiving treatment because of administrative problems (n = 3), consent withdrawal (n = 2), or AEs (n = 1) and were excluded from the intent‐to‐treat population (130/146). | Some concerns | Visual acuity outcome assessors were aware of the intervention received by study participants. | Low risk of bias | Results were analyzed according the pre‐specified outcome of >15 letter improvement on ETDRS charts from baseline. | Some concerns | The trial was judged to be at low risk in four domains but with some concerns in biased outcome measurement due to unmasking of outcome assessors. |
| Kempen 2011 | Low risk of bias | "Patients were randomized to implant or systemic therapy…Randomization (1:1 ratio) was by vari‐ able length, permuted blocks within 2 strata (clinical center, inter‐ mediate vs posterior or panuveitis), with assignments produced by Stata 11.0. After data entry confirmed a subject’s eligibility and stratum, the study Web site revealed the next treatment assignment." Baseline demographic and clin‐ ical characteristics were distributed similarly between groups (Table 1). | Low risk of bias | Intention‐to‐treat analysis was used. | Low risk of bias | "...255 patients were enrolled...Among patients randomized, 232 completed visual acuity measurement at the 24 month follow‐up visit. Overall, 4415 of 4790 study visits (92%) were completed for the primary outcome through 24 months." | Low risk of bias | "Study‐certified visual acuity examiners measured best‐corrected visual acuity as the number of letters read from standard logarithmic visual acuity charts; change in this measure from baseline to 24 months was the primary outcome." "Other than at the 1 and 3 month visits, when post‐operative signs were expected to be visible, visual acuity examiners were masked." | Low risk of bias | The pre‐specified outcome was reported as the result. | Low risk of bias | The trial was judged to be at low across all domains. |
Acknowledgements
We thank Lori Rosman, Information Specialist for Cochrane Eyes and Vision US, who created and executed the electronic search strategies.
We also thank Renee Wilson, Assistant Managing Editor for CEV US; Anupa Shah, Managing Editor for CEV, for support and guidance in preparation of this review.
We would like to acknowledge the contributions of Ehsan Rahimy (Thomas Jefferson University, PA), Rahul Reddy (Associated Retinal Consultants, AZ), Sunir Garg (Thomas Jefferson University, PA), and Johnny Tang (Center for Eye Health, MA) to the previous version of the review (Brady 2016).
We would also like to thank the following peer reviewers for their comments: Dr. Claudia Castiblanco (Albert Einstein College of Medicine, NY) and Dr. Umar Mian (Albert Einstein College of Medicine, NY) for the review manuscript.
This review update was managed by CEV@US and was signed off for publication by Drs. Tianjing Li and Gianni Virgili.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Uveitis] explode all trees #2 uveiti* #3 MeSH descriptor: [Panuveitis] explode all trees #4 Panuveitis #5 MeSH descriptor: [Ophthalmia, Sympathetic] explode all trees #6 (Ophthalm* near/2 Sympathetic) #7 MeSH descriptor: [Pars Planitis] explode all trees #8 "Pars Planitis" #9 MeSH descriptor: [Panophthalmitis] explode all trees #10 Panophthalmiti* #11 MeSH descriptor: [Uveomeningoencephalitic Syndrome] explode all trees #12 (Uveomeningoencephaliti* or "Vogt Koyanagi Harada" or VKH or fuch or "Harada disease" or "harada syndrome" or "vogt koyanagi disease") #13 MeSH descriptor: [Behcet Syndrome] explode all trees #14 (behcet* or "triple symptom complex") #15 MeSH descriptor: [Iridocyclitis] explode all trees #16 (Iridocycliti* or (Heterochromic NEXT/1 Cycliti*) or "anterior scleritis") #17 MeSH descriptor: [Iritis] explode all trees #18 Iriti* #19 Choroiditis #20 (choroiditi* or retinochoroiditi* or chorioretinitis) #21 ((Blau* NEXT/1 syndrome) or "familial juvenile systemic granulomatosis" or "Jabs disease") #22 ((Reiter* NEXT/1 disease) or (reiter* NEXT/1 syndrome) or "conjunctivo urethro synovial" or "urethrooculosynovial syndrome" or uroarthritis) #23 (uveoretinitis or "uveo retinitis") #24 vitritis* #25 MeSH descriptor: [Retinitis] explode all trees #26 retinitis or neuroretinitis #27 {OR #1‐#26} #28 MeSH descriptor: [Fluocinolone Acetonide] explode all trees #29 (fluocinolone* OR adermina OR alfabios OR alvadermo OR aplosyn OR capex OR cervicum OR cinolon OR clofeet OR "Co Fluocin" OR Cortiespec OR cortilona OR cremisona OR cynozet OR "derma‐smooth/fs" OR "Derma Smooth FS" OR "derma‐smoothe/fs" OR dermalar OR dermoflam OR dermoran OR dermotic OR "df 277" OR df277 OR esacinone OR Fluortriamcinolone OR flozet OR fluciderm OR Flucinar OR "flucinolone acetonide" OR flulone OR "flunolone‐v" OR fluocet OR Fluocid OR "fluocinolon acetonid" OR "fluocinonide acetonide" OR fluoderm OR Fluodermo OR fluolar OR fluonid OR fluonide OR fluotrex OR fluquinol OR flurosyn OR flusonlen OR fluzon OR fusalar OR Flusolgen OR Gelidina OR iluvien OR inoderm OR jellin OR Jellisoft OR lluvien OR localyn OR luci OR medidur OR neosynalar OR "nsc 92339" OR nsc92339 OR "ot 401" OR ot401 OR otoken OR psoranide OR radiocin OR retisert OR "rs 1401 at" OR "rs 1401at" OR "rs1401 at" OR rs1401at OR supralan OR synalar OR synandone OR Synamol OR synemol OR synotic OR syntopic OR trisyn OR yutiq OR "67‐73‐2") #30 MeSH descriptor: [Dexamethasone] explode all trees #31 (Dexamethasone* OR adrecort OR adrenocot OR "aeroseb dex" OR "aeroseb‐d" OR aflucoson OR aflucosone OR alfalyl OR anaflogistico OR aphtasolon OR arcodexan OR arcodexane OR artrosone OR auxiron OR azium OR bidexol OR "bisu ds" OR calonat OR cebedex OR cetadexon OR colofoam OR corsona OR corsone OR cortastat OR cortidex OR cortidexason OR cortidrona OR cortidrone OR cortisumman OR "dacortina fuerte" OR "dacortine fuerte" OR dalalone OR danasone OR "de‐sone la" OR decacortin OR decadeltosona OR decadeltosone OR decaderm OR decadion OR decadran OR decadron OR decadronal OR decadrone OR decaesadril OR decagel OR decaject OR decalix OR decameth OR decamethasone OR decasone OR decaspray OR decasterolone OR decdan OR decilone OR decofluor OR dectancyl OR dekacort OR delladec OR deltafluoren OR deltafluorene OR dergramin OR deronil OR desacort OR desacortone OR desadrene OR desalark OR desameton OR desametone OR desigdron OR "dexa cortisyl" OR "dexa dabrosan" OR "dexa korti" OR "dexa scherosan" OR "dexa scherozon" OR "dexa scherozone" OR "dexa‐p" OR "dexacen 4" OR dexachel OR dexacort OR dexacortal OR dexacorten OR dexacortin OR dexacortisyl OR dexadabroson OR dexadecadrol OR dexadrol OR dexagel OR dexagen OR dexahelvacort OR dexakorti OR dexalien OR dexalocal OR dexame OR dexamecortin OR dexameson OR dexamesone OR dexametason OR dexametasone OR dexameth OR dexamethason OR dexamethazon OR dexamethazone OR dexamethonium OR dexamonozon OR dexan OR dexane OR dexano OR dexapot OR dexascheroson OR dexascherozon OR dexascherozone OR dexason OR dexasone OR dexinoral OR dexionil OR dexmethsone OR dexona OR dexone OR dexpak OR dextelan OR dextenza OR dextrasone OR dexycu OR dezone OR dibasona OR doxamethasone OR esacortene OR "ex s1" OR exadion OR exadione OR firmalone OR "fluormethyl prednisolone" OR fluormethylprednisolon OR fluormethylprednisolone OR fluormone OR fluorocort OR fluorodelta OR fluoromethylprednisolone OR fortecortin OR gammacorten OR gammacortene OR grosodexon OR grosodexone OR hemady OR hexadecadiol OR hexadecadrol OR hexadiol OR hexadrol OR isnacort OR "isopto dex" OR isoptodex OR isoptomaxidex OR "lokalison f" OR loverine OR luxazone OR marvidione OR maxidex OR mediamethasone OR megacortin OR mephameson OR mephamesone OR metasolon OR metasolone OR "methazon ion" OR "methazone ion" OR methazonion OR methazonione OR methylfluorprednisolone OR "metisone lafi" OR mexasone OR millicorten OR millicortenol OR "mk 125" OR mk125 OR mymethasone OR neoforderx OR neofordex OR nisomethasona OR novocort OR "nsc 34521" OR nsc34521 OR "oftan‐dexa" OR opticorten OR opticortinol OR oradexan OR oradexon OR oradexone OR orgadrone OR ozurdex OR pidexon OR policort OR posurdex OR "predni f tablinen" OR "predni‐f" OR "prednisolone f" OR prodexona OR prodexone OR sanamethasone OR santenson OR santeson OR sawasone OR solurex OR "solurex la" OR spoloven OR sterasone OR thilodexine OR triamcimetil OR vexamet OR visumetazone OR visumethazone OR "50‐02‐2") #32 MeSH descriptor: [Drug Implants] explode all trees #33 MeSH descriptor: [Drug Delivery Systems] explode all trees #34 (Device* or implant* or shunt* or valve* or tube*) #35 {OR #28‐#34} #36 #27 and #35
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Uveitis/ 13. uveiti*.tw. 14. exp Panuveitis/ 15. Panuveitis.tw. 16. exp Ophthalmia, Sympathetic/ 17. (Ophthalm* adj2 Sympathetic).tw. 18. exp Pars Planitis/ 19. Pars Planitis.tw. 20. exp Panophthalmitis/ 21. Panophthalmiti*.tw. 22. exp Uveomeningoencephalitic Syndrome/ 23. (Uveomeningoencephaliti* or Vogt Koyanagi Harada or VKH or fuch or Harada disease or harada syndrome or vogt koyanagi disease).tw. 24. exp Behcet Syndrome/ 25. (behcet* or triple symptom complex).tw. 26. exp Iridocyclitis/ 27. (Iridocycliti* or Heterochromic Cycliti* or anterior scleritis).tw. 28. exp Iritis/ 29. Iriti*.tw. 30. exp Choroiditis/ 31. (choroiditi* or retinochoroiditi* or chorioretinitis).tw. 32. ((Blau* adj1 syndrome) or familial juvenile systemic granulomatosis or Jabs disease).tw. 33. ((Reiter* adj1 disease) or (reiter* adj1 syndrome) or conjunctivo urethro synovial or urethrooculosynovial syndrome or uroarthritis).tw. 34. (uveoretinitis or uveo retinitis).tw. 35. vitritis*.tw. 36. exp Retinitis/ 37. (retinitis or neuroretinitis).tw. 38. or/12‐37 39. exp Fluocinolone Acetonide/ 40. (fluocinolone* or adermina or alfabios or alvadermo or aplosyn or capex or cervicum or cinolon or clofeet or "Co Fluocin" or Cortiespec or cortilona or cremisona or cynozet or "derma‐smooth/fs" or "Derma Smooth FS" or "derma‐smoothe/fs" or dermalar or dermoflam or dermoran or dermotic or "df 277" or df277 or esacinone or Fluortriamcinolone or flozet or fluciderm or Flucinar or "flucinolone acetonide" or flulone or "flunolone‐v" or fluocet or Fluocid or "fluocinolon acetonid" or "fluocinonide acetonide" or fluoderm or Fluodermo or fluolar or fluonid or fluonide or fluotrex or fluquinol or flurosyn or flusonlen or fluzon or fusalar or Flusolgen or Gelidina or iluvien or inoderm or jellin or Jellisoft or lluvien or localyn or luci or medidur or neosynalar or "nsc 92339" or nsc92339 or "ot 401" or ot401 or otoken or psoranide or radiocin or retisert or "rs 1401 at" or "rs 1401at" or "rs1401 at" or rs1401at or supralan or synalar or synandone or Synamol or synemol or synotic or syntopic or trisyn or yutiq or "67‐73‐2").tw,rn. 41. exp Dexamethasone/ 42. (Dexamethasone* or adrecort or adrenocot or "aeroseb dex" or "aeroseb‐d" or aflucoson or aflucosone or alfalyl or anaflogistico or aphtasolon or arcodexan or arcodexane or artrosone or auxiron or azium or bidexol or "bisu ds" or calonat or cebedex or cetadexon or colofoam or corsona or corsone or cortastat or cortidex or cortidexason or cortidrona or cortidrone or cortisumman or "dacortina fuerte" or "dacortine fuerte" or dalalone or danasone or "de‐sone la" or decacortin or decadeltosona or decadeltosone or decaderm or decadion or decadran or decadron or decadronal or decadrone or decaesadril or decagel or decaject or decalix or decameth or decamethasone or decasone or decaspray or decasterolone or decdan or decilone or decofluor or dectancyl or dekacort or delladec or deltafluoren or deltafluorene or dergramin or deronil or desacort or desacortone or desadrene or desalark or desameton or desametone or desigdron or "dexa cortisyl" or "dexa dabrosan" or "dexa korti" or "dexa scherosan" or "dexa scherozon" or "dexa scherozone" or "dexa‐p" or "dexacen 4" or dexachel or dexacort or dexacortal or dexacorten or dexacortin or dexacortisyl or dexadabroson or dexadecadrol or dexadrol or dexagel or dexagen or dexahelvacort or dexakorti or dexalien or dexalocal or dexame or dexamecortin or dexameson or dexamesone or dexametason or dexametasone or dexameth or dexamethason or dexamethazon or dexamethazone or dexamethonium or dexamonozon or dexan or dexane or dexano or dexapot or dexascheroson or dexascherozon or dexascherozone or dexason or dexasone or dexinoral or dexionil or dexmethsone or dexona or dexone or dexpak or dextelan or dextenza or dextrasone or dexycu or dezone or dibasona or doxamethasone or esacortene or "ex s1" or exadion or exadione or firmalone or "fluormethyl prednisolone" or fluormethylprednisolon or fluormethylprednisolone or fluormone or fluorocort or fluorodelta or fluoromethylprednisolone or fortecortin or gammacorten or gammacortene or grosodexon or grosodexone or hemady or hexadecadiol or hexadecadrol or hexadiol or hexadrol or isnacort or "isopto dex" or isoptodex or isoptomaxidex or "lokalison f" or loverine or luxazone or marvidione or maxidex or mediamethasone or megacortin or mephameson or mephamesone or metasolon or metasolone or "methazon ion" or "methazone ion" or methazonion or methazonione or methylfluorprednisolone or "metisone lafi" or mexasone or millicorten or millicortenol or "mk 125" or mk125 or mymethasone or neoforderx or neofordex or nisomethasona or novocort or "nsc 34521" or nsc34521 or "oftan‐dexa" or opticorten or opticortinol or oradexan or oradexon or oradexone or orgadrone or ozurdex or pidexon or policort or posurdex or "predni f tablinen" or "predni‐f" or "prednisolone f" or prodexona or prodexone or sanamethasone or santenson or santeson or sawasone or solurex or "solurex la" or spoloven or sterasone or thilodexine or triamcimetil or vexamet or visumetazone or visumethazone or "50‐02‐2").tw,rn. 43. exp Drug Implants/ 44. exp Absorbable Implants/ 45. exp Drug Delivery Systems/ 46. (Device* or implant* or shunt* or valve* or tube*).tw. 47. or/39‐46 48. 11 and 38 and 47 49. remove duplicates from 48
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. uveiti*[tw] OR Panuveitis[tw] OR (Ophthalm*[tw] AND Sympathetic[tw]) OR Pars Planitis[tw] OR Panophthalmiti*[tw] OR Uveomeningoencephaliti*[tw] OR Vogt Koyanagi Harada[tw] OR VKH[tw] OR fuch[tw] OR Harada disease[tw] OR harada syndrome[tw] OR vogt koyanagi disease[tw] OR behcet*[tw] OR triple symptom complex[tw] OR Iridocycliti*[tw] OR Heterochromic Cycliti*[tw] OR anterior scleritis[tw] OR Iriti*[tw] OR choroiditi*[tw] OR retinochoroiditi*[tw] OR chorioretinitis[tw] OR Blau* syndrome[tw] OR familial juvenile systemic granulomatosis[tw] OR Jabs disease[tw] OR Reiter* disease[tw] OR reiter* syndrome[tw] OR conjunctivo urethro synovial[tw] OR urethrooculosynovial syndrome[tw] OR uroarthritis[tw] OR uveoretinitis[tw] OR uveo retinitis[tw] OR vitritis*[tw] OR retinitis[tw] OR neuroretinitis[tw] 3. (fluocinolone*[tw] OR adermina[tw] OR alfabios[tw] OR alvadermo[tw] OR aplosyn[tw] OR capex[tw] OR cervicum[tw] OR cinolon[tw] OR clofeet[tw] OR "Co Fluocin"[tw] OR Cortiespec[tw] OR cortilona[tw] OR cremisona[tw] OR cynozet[tw] OR "derma‐smooth/fs"[tw] OR "Derma Smooth FS"[tw] OR "derma‐smoothe/fs"[tw] OR dermalar[tw] OR dermoflam[tw] OR dermoran[tw] OR dermotic[tw] OR "df 277"[tw] OR df277[tw] OR esacinone[tw] OR Fluortriamcinolone[tw] OR flozet[tw] OR fluciderm[tw] OR Flucinar[tw] OR "flucinolone acetonide"[tw] OR flulone[tw] OR "flunolone‐v"[tw] OR fluocet[tw] OR Fluocid[tw] OR "fluocinolon acetonid"[tw] OR "fluocinonide acetonide"[tw] OR fluoderm[tw] OR Fluodermo[tw] OR fluolar[tw] OR fluonid[tw] OR fluonide[tw] OR fluotrex[tw] OR fluquinol[tw] OR flurosyn[tw] OR flusonlen[tw] OR fluzon[tw] OR fusalar[tw] OR Flusolgen[tw] OR Gelidina[tw] OR iluvien[tw] OR inoderm[tw] OR jellin[tw] OR Jellisoft[tw] OR lluvien[tw] OR localyn[tw] OR luci[tw] OR medidur[tw] OR neosynalar[tw] OR "nsc 92339"[tw] OR nsc92339[tw] OR "ot 401"[tw] OR ot401[tw] OR otoken[tw] OR psoranide[tw] OR radiocin[tw] OR retisert[tw] OR "rs 1401 at"[tw] OR "rs 1401at"[tw] OR "rs1401 at"[tw] OR rs1401at[tw] OR supralan[tw] OR synalar[tw] OR synandone[tw] OR Synamol[tw] OR synemol[tw] OR synotic[tw] OR syntopic[tw] OR trisyn[tw] OR yutiq[tw] OR "67‐73‐2"[tw] OR "67‐73‐2"[rn]) 4. (Dexamethasone*[tw] OR adrecort[tw] OR adrenocot[tw] OR "aeroseb dex"[tw] OR "aeroseb‐d"[tw] OR aflucoson[tw] OR aflucosone[tw] OR alfalyl[tw] OR anaflogistico[tw] OR aphtasolon[tw] OR arcodexan[tw] OR arcodexane[tw] OR artrosone[tw] OR auxiron[tw] OR azium[tw] OR bidexol[tw] OR "bisu ds"[tw] OR calonat[tw] OR cebedex[tw] OR cetadexon[tw] OR colofoam[tw] OR corsona[tw] OR corsone[tw] OR cortastat[tw] OR cortidex[tw] OR cortidexason[tw] OR cortidrona[tw] OR cortidrone[tw] OR cortisumman[tw] OR "dacortina fuerte"[tw] OR "dacortine fuerte"[tw] OR dalalone[tw] OR danasone[tw] OR "de‐sone la"[tw] OR decacortin[tw] OR decadeltosona[tw] OR decadeltosone[tw] OR decaderm[tw] OR decadion[tw] OR decadran[tw] OR decadron[tw] OR decadronal[tw] OR decadrone[tw] OR decaesadril[tw] OR decagel[tw] OR decaject[tw] OR decalix[tw] OR decameth[tw] OR decamethasone[tw] OR decasone[tw] OR decaspray[tw] OR decasterolone[tw] OR decdan[tw] OR decilone[tw] OR decofluor[tw] OR dectancyl[tw] OR dekacort[tw] OR delladec[tw] OR deltafluoren[tw] OR deltafluorene[tw] OR dergramin[tw] OR deronil[tw] OR desacort[tw] OR desacortone[tw] OR desadrene[tw] OR desalark[tw] OR desameton[tw] OR desametone[tw] OR desigdron[tw] OR "dexa cortisyl"[tw] OR "dexa dabrosan"[tw] OR "dexa korti"[tw] OR "dexa scherosan"[tw] OR "dexa scherozon"[tw] OR "dexa scherozone"[tw] OR "dexa‐p"[tw] OR "dexacen 4"[tw] OR dexachel[tw] OR dexacort[tw] OR dexacortal[tw] OR dexacorten[tw] OR dexacortin[tw] OR dexacortisyl[tw] OR dexadabroson[tw] OR dexadecadrol[tw] OR dexadrol[tw] OR dexagel[tw] OR dexagen[tw] OR dexahelvacort[tw] OR dexakorti[tw] OR dexalien[tw] OR dexalocal[tw] OR dexame[tw] OR dexamecortin[tw] OR dexameson[tw] OR dexamesone[tw] OR dexametason[tw] OR dexametasone[tw] OR dexameth[tw] OR dexamethason[tw] OR dexamethazon[tw] OR dexamethazone[tw] OR dexamethonium[tw] OR dexamonozon[tw] OR dexan[tw] OR dexane[tw] OR dexano[tw] OR dexapot[tw] OR dexascheroson[tw] OR dexascherozon[tw] OR dexascherozone[tw] OR dexason[tw] OR dexasone[tw] OR dexinoral[tw] OR dexionil[tw] OR dexmethsone[tw] OR dexona[tw] OR dexone[tw] OR dexpak[tw] OR dextelan[tw] OR dextenza[tw] OR dextrasone[tw] OR dexycu[tw] OR dezone[tw] OR dibasona[tw] OR doxamethasone[tw] OR esacortene[tw] OR "ex s1"[tw] OR exadion[tw] OR exadione[tw] OR firmalone[tw] OR "fluormethyl prednisolone"[tw] OR fluormethylprednisolon[tw] OR fluormethylprednisolone[tw] OR fluormone[tw] OR fluorocort[tw] OR fluorodelta[tw] OR fluoromethylprednisolone[tw] OR fortecortin[tw] OR gammacorten[tw] OR gammacortene[tw] OR grosodexon[tw] OR grosodexone[tw] OR hemady[tw] OR hexadecadiol[tw] OR hexadecadrol[tw] OR hexadiol[tw] OR hexadrol[tw] OR isnacort[tw] OR "isopto dex"[tw] OR isoptodex[tw] OR isoptomaxidex[tw] OR "lokalison f"[tw] OR loverine[tw] OR luxazone[tw] OR marvidione[tw] OR maxidex[tw] OR mediamethasone[tw] OR megacortin[tw] OR mephameson[tw] OR mephamesone[tw] OR metasolon[tw] OR metasolone[tw] OR "methazon ion"[tw] OR "methazone ion"[tw] OR methazonion[tw] OR methazonione[tw] OR methylfluorprednisolone[tw] OR "metisone lafi"[tw] OR mexasone[tw] OR millicorten[tw] OR millicortenol[tw] OR "mk 125"[tw] OR mk125[tw] OR mymethasone[tw] OR neoforderx[tw] OR neofordex[tw] OR nisomethasona[tw] OR novocort[tw] OR "nsc 34521"[tw] OR nsc34521[tw] OR "oftan‐dexa"[tw] OR opticorten[tw] OR opticortinol[tw] OR oradexan[tw] OR oradexon[tw] OR oradexone[tw] OR orgadrone[tw] OR ozurdex[tw] OR pidexon[tw] OR policort[tw] OR posurdex[tw] OR "predni f tablinen"[tw] OR "predni‐f"[tw] OR "prednisolone f"[tw] OR prodexona[tw] OR prodexone[tw] OR sanamethasone[tw] OR santenson[tw] OR santeson[tw] OR sawasone[tw] OR solurex[tw] OR "solurex la"[tw] OR spoloven[tw] OR sterasone[tw] OR thilodexine[tw] OR triamcimetil[tw] OR vexamet[tw] OR visumetazone[tw] OR visumethazone[tw] OR "50‐02‐2"[tw] OR "50‐02‐2"[rn]) 5. Device*[tw] OR implant*[tw] OR shunt*[tw] OR valve*[tw] OR tube[tw] OR tubes[tw] 6. #3 OR #4 OR #5 7. #2 AND #6 8. #1 AND #7 9. MEDLINE[sb] 10. #8 NOT #9
Appendix 4. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'uveitis'/exp #34 uveiti*:ab,ti #35 'autoimmune uveitis'/exp #36 'behcet disease'/exp #37 behcet*:ab,ti OR 'triple symptom complex':ab,ti #38 'blau syndrome'/exp #39 (blau* NEXT/1 syndrome):ab,ti OR 'familial juvenile systemic granulomatosis':ab,ti OR 'jabs disease':ab,ti #40 'choroiditis'/exp #41 choroiditi*:ab,ti OR chorioiditi*:ab,ti #42 'chorioretinitis'/exp #43 retinochoroiditi*:ab,ti OR chorioretiniti*:ab,ti #44 'vogt koyanagi syndrome'/exp #45 uveomeningoencephaliti*:ab,ti OR 'vogt koyanagi harada':ab,ti OR vkh:ab,ti OR fuch:ab,ti OR 'harada disease':ab,ti OR 'harada syndrome':ab,ti OR 'vogt koyanagi disease':ab,ti #46 'intermediate uveitis'/exp #47 'pars planitis':ab,ti #48 'iridocyclitis'/exp #49 iridocycliti*:ab,ti OR (heterochromic NEXT/1 cycliti*):ab,ti OR 'anterior scleritis':ab,ti #50 'iritis'/exp #51 iriti*:ab,ti #52 'kirisawa uveitis'/exp #53 'reiter syndrome'/exp #54 (reiter* NEXT/1 disease):ab,ti OR (reiter* NEXT/1 syndrome):ab,ti OR 'conjunctivo urethro synovial':ab,ti OR 'urethrooculosynovial syndrome':ab,ti OR uroarthritis:ab,ti #55 'sympathetic ophthalmia'/exp #56 (ophthalm* NEXT/2 sympathetic):ab,ti #57 'uveoretinitis'/exp #58 uveoretinitis:ab,ti OR 'uveo retinitis':ab,ti #59 'vitritis'/exp #60 vitritis*:ab,ti #61 panuveitis:ab,ti #62 panophthalmiti*:ab,ti #63 'retinitis'/exp #64 retinitis:ab,ti OR neuroretinitis:ab,ti #65 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 #66 'fluocinolone acetonide'/exp #67 (fluocinolone* OR adermina OR alfabios OR alvadermo OR aplosyn OR capex OR cervicum OR cinolon OR clofeet OR "Co Fluocin" OR Cortiespec OR cortilona OR cremisona OR cynozet OR "derma‐smooth/fs" OR "Derma Smooth FS" OR "derma‐smoothe/fs" OR dermalar OR dermoflam OR dermoran OR dermotic OR "df 277" OR df277 OR esacinone OR Fluortriamcinolone OR flozet OR fluciderm OR Flucinar OR flulone OR "flunolone‐v" OR fluocet OR Fluocid OR "fluocinolon acetonid" OR "fluocinonide acetonide" OR fluoderm OR Fluodermo OR fluolar OR fluonid OR fluonide OR fluotrex OR fluquinol OR flurosyn OR flusonlen OR fluzon OR fusalar OR Flusolgen OR Gelidina OR iluvien OR inoderm OR jellin OR Jellisoft OR lluvien OR localyn OR luci OR medidur OR neosynalar OR "nsc 92339" OR nsc92339 OR "ot 401" OR ot401 OR otoken OR psoranide OR radiocin OR retisert OR "rs 1401 at" OR "rs 1401at" OR "rs1401 at" OR rs1401at OR supralan OR synalar OR synandone OR Synamol OR synemol OR synotic OR syntopic OR trisyn OR yutiq OR "67‐73‐2"):ab,ti,rn,tn #68 'dexamethasone'/exp #69 (Dexamethasone* OR adrecort OR adrenocot OR "aeroseb dex" OR "aeroseb‐d" OR aflucoson OR aflucosone OR alfalyl OR anaflogistico OR aphtasolon OR arcodexan OR arcodexane OR artrosone OR auxiron OR azium OR bidexol OR "bisu ds" OR calonat OR cebedex OR cetadexon OR colofoam OR corsona OR corsone OR cortastat OR cortidex OR cortidexason OR cortidrona OR cortidrone OR cortisumman OR "dacortina fuerte" OR "dacortine fuerte" OR dalalone OR danasone OR "de‐sone la" OR decacortin OR decadeltosona OR decadeltosone OR decaderm OR decadion OR decadran OR decadron OR decadronal OR decadrone OR decaesadril OR decagel OR decaject OR decalix OR decameth OR decamethasone OR decasone OR decaspray OR decasterolone OR decdan OR decilone OR decofluor OR dectancyl OR dekacort OR delladec OR deltafluoren OR deltafluorene OR dergramin OR deronil OR desacort OR desacortone OR desadrene OR desalark OR desameton OR desametone OR desigdron OR "dexa cortisyl" OR "dexa dabrosan" OR "dexa korti" OR "dexa scherosan" OR "dexa scherozon" OR "dexa scherozone" OR "dexa‐p" OR "dexacen 4" OR dexachel OR dexacort OR dexacortal OR dexacorten OR dexacortin OR dexacortisyl OR dexadabroson OR dexadecadrol OR dexadrol OR dexagel OR dexagen OR dexahelvacort OR dexakorti OR dexalien OR dexalocal OR dexame OR dexamecortin OR dexameson OR dexamesone OR dexametason OR dexametasone OR dexameth OR dexamethason OR dexamethazon OR dexamethazone OR dexamethonium OR dexamonozon OR dexan OR dexane OR dexano OR dexapot OR dexascheroson OR dexascherozon OR dexascherozone OR dexason OR dexasone OR dexinoral OR dexionil OR dexmethsone OR dexona OR dexone OR dexpak OR dextelan OR dextenza OR dextrasone OR dexycu OR dezone OR dibasona OR doxamethasone OR esacortene OR "ex s1" OR exadion OR exadione OR firmalone OR "fluormethyl prednisolone" OR fluormethylprednisolon OR fluormethylprednisolone OR fluormone OR fluorocort OR fluorodelta OR fluoromethylprednisolone OR fortecortin OR gammacorten OR gammacortene OR grosodexon OR grosodexone OR hemady OR hexadecadiol OR hexadecadrol OR hexadiol OR hexadrol OR isnacort OR "isopto dex" OR isoptodex OR isoptomaxidex OR "lokalison f" OR loverine OR luxazone OR marvidione OR maxidex OR mediamethasone OR megacortin OR mephameson OR mephamesone OR metasolon OR metasolone OR "methazon ion" OR "methazone ion" OR methazonion OR methazonione OR methylfluorprednisolone OR "metisone lafi" OR mexasone OR millicorten OR millicortenol OR "mk 125" OR mk125 OR mymethasone OR neoforderx OR neofordex OR nisomethasona OR novocort OR "nsc 34521" OR nsc34521 OR "oftan‐dexa" OR opticorten OR opticortinol OR oradexan OR oradexon OR oradexone OR orgadrone OR ozurdex OR pidexon OR policort OR posurdex OR "predni f tablinen" OR "predni‐f" OR "prednisolone f" OR prodexona OR prodexone OR sanamethasone OR santenson OR santeson OR sawasone OR solurex OR "solurex la" OR spoloven OR sterasone OR thilodexine OR triamcimetil OR vexamet OR visumetazone OR visumethazone OR "50‐02‐2"):ab,ti,rn,tn #70 'drug delivery system'/exp #71 'drug implant'/exp #72 'biodegradable implant'/exp #73 device*:ab,ti OR implant*:ab,ti OR shunt*:ab,ti OR valve*:ab,ti OR tube*:ab,ti #74 #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 #75 #32 AND #65 AND #74
Appendix 5. LILACS search strategy
((Uveitis or Uveítis or Uveíte or MH:C11.941.879$ or Panuveitis or Panuveítis or Panuveíte or "Ophthalmia Sympathetic" or "Oftalmía Simpática" or "Oftalmia Simpática" or "Pars Planitis" or "Pars Planite" or "Panophthalmitis" or "Panoftalmitis" or "Panoftalmite" or MH:C01.252.354.900.675$ or MH:C01.539.375.354.900.675$ or MH:C01.539.375.450.900.675$ or MH:C01.703.343.900.675$ or MH:C11.294.354.900.675$ or MH:C11.294.450.900.675$ or "Uveomeningoencephalitic Syndrome" or "Síndrome Uveomeningoencefálico" or "Síndrome Uveomeningoencefálica" or MH:C10.114.843$ or MH:C10.228.228.553.900$ or MH:C20.111.258.925$ or Uveomeningoencephalitis or "Vogt Koyanagi Harada" or "Harada disease" or "harada syndrome" or "vogt koyanagi disease" or "Behcet syndrome" or "Síndrome de Behçet" or MH:C07.465.075$ or MH:C14.907.940.100$ or MH:C17.800.862.150$ or "triple symptom complex" or Iridocyclitis or Iridociclitis or Iridociclite or MH:C11.941.375.360$ or "Heterochromic Cyclitis" or MH:C11.941.160.478$ or chorioretinitis or Retinitis or Retinite or MH:C11.768.773$) AND (Fluocinolone or Fluocinolona or MH:D04.808.745.432.370$ or MH:D04.808.908.394$ or adermina or alfabios or alvadermo or aplosyn or capex or cervicum or cinolon or clofeet or "Co Fluocin" or Cortiespec or cortilona or cremisona or cynozet or "derma‐smooth/fs" or "Derma Smooth FS" or "derma‐smoothe/fs" or dermalar or dermoflam or dermoran or dermotic or "df 277" or df277 or esacinone or Fluortriamcinolone or flozet or fluciderm or Flucinar or "flucinolone acetonide" or flulone or "flunolone‐v" or fluocet or Fluocid or "fluocinolon acetonid" or "fluocinonide acetonide" or fluoderm or Fluodermo or fluolar or fluonid or fluonide or fluotrex or fluquinol or flurosyn or flusonlen or fluzon or fusalar or Flusolgen or Gelidina or iluvien or inoderm or jellin or Jellisoft or lluvien or localyn or luci or medidur or neosynalar or "nsc 92339" or nsc92339 or "ot 401" or ot401 or otoken or psoranide or radiocin or retisert or "rs 1401 at" or "rs 1401at" or "rs1401 at" or rs1401at or supralan or synalar or synandone or Synamol or synemol or synotic or syntopic or trisyn or yutiq or "67‐73‐2" or Dexamethasone or Dexametasona or MH:D04.808.745.432.769.344$ or MH:D04.808.908.238$ or MH:D26.255.210.315$ or MH:D27.720.280.210.315$ or MH:E07.695.025$ or Dexamethasone or adrecort or adrenocot or "aeroseb dex" or "aeroseb‐d" or aflucoson or aflucosone or alfalyl or anaflogistico or aphtasolon or arcodexan or arcodexane or artrosone or auxiron or azium or bidexol or "bisu ds" or calonat or cebedex or cetadexon or colofoam or corsona or corsone or cortastat or cortidex or cortidexason or cortidrona or cortidrone or cortisumman or "dacortina fuerte" or "dacortine fuerte" or dalalone or danasone or "de‐sone la" or decacortin or decadeltosona or decadeltosone or decaderm or decadion or decadran or decadron or decadronal or decadrone or decaesadril or decagel or decaject or decalix or decameth or decamethasone or decasone or decaspray or decasterolone or decdan or decilone or decofluor or dectancyl or dekacort or delladec or deltafluoren or deltafluorene or dergramin or deronil or desacort or desacortone or desadrene or desalark or desameton or desametone or desigdron or "dexa cortisyl" or "dexa dabrosan" or "dexa korti" or "dexa scherosan" or "dexa scherozon" or "dexa scherozone" or "dexa‐p" or "dexacen 4" or dexachel or dexacort or dexacortal or dexacorten or dexacortin or dexacortisyl or dexadabroson or dexadecadrol or dexadrol or dexagel or dexagen or dexahelvacort or dexakorti or dexalien or dexalocal or dexame or dexamecortin or dexameson or dexamesone or dexametason or dexametasone or dexameth or dexamethason or dexamethazon or dexamethazone or dexamethonium or dexamonozon or dexan or dexane or dexano or dexapot or dexascheroson or dexascherozon or dexascherozone or dexason or dexasone or dexinoral or dexionil or dexmethsone or dexona or dexone or dexpak or dextelan or dextenza or dextrasone or dexycu or dezone or dibasona or doxamethasone or esacortene or "ex s1" or exadion or exadione or firmalone or "fluormethyl prednisolone" or fluormethylprednisolon or fluormethylprednisolone or fluormone or fluorocort or fluorodelta or fluoromethylprednisolone or fortecortin or gammacorten or gammacortene or grosodexon or grosodexone or hemady or hexadecadiol or hexadecadrol or hexadiol or hexadrol or isnacort or "isopto dex" or isoptodex or isoptomaxidex or "lokalison f" or loverine or luxazone or marvidione or maxidex or mediamethasone or megacortin or mephameson or mephamesone or metasolon or metasolone or "methazon ion" or "methazone ion" or methazonion or methazonione or methylfluorprednisolone or "metisone lafi" or mexasone or millicorten or millicortenol or "mk 125" or mk125 or mymethasone or neoforderx or neofordex or nisomethasona or novocort or "nsc 34521" or nsc34521 or "oftan‐dexa" or opticorten or opticortinol or oradexan or oradexon or oradexone or orgadrone or ozurdex or pidexon or policort or posurdex or "predni f tablinen" or "predni‐f" or "prednisolone f" or prodexona or prodexone or sanamethasone or santenson or santeson or sawasone or solurex or "solurex la" or spoloven or sterasone or thilodexine or triamcimetil or vexamet or visumetazone or visumethazone or "50‐02‐2" or "Drug Delivery Systems" or "Sistemas de Liberación de Medicamentos" or "Sistemas de Liberação de Medicamentos" or MH:E02.319.300$ or Device$ or implant$ or shunt$ or valve$ or tube or tubes))
Appendix 6. metaRegister of Controlled Trials search strategy
(uveitis OR panuveitis OR choroiditis OR pars planitis OR panophthalmitis OR uveomeningoencephalitic OR behcet OR iridocyclitis OR iritis OR retinitis) AND (fluocinolone OR dexamethasone OR retisert* OR device* OR implant* OR shunt* OR valve* OR tube*)
Appendix 7. ClinicalTrials.gov search strategy
(uveitis OR panuveitis OR choroiditis OR pars planitis OR panophthalmitis OR uveomeningoencephalitic OR behcet OR iridocyclitis OR iritis OR retinitis) AND (fluocinolone OR dexamethasone OR retisert OR device OR implant OR shunt OR valve OR tube)
Appendix 8. ICTRP search strategy
uveitis AND fluocinolone OR uveitis AND dexamethasone OR uveitis AND retisert OR uveitis AND device OR uveitis AND implant OR uveitis AND shunt OR uveitis AND valve OR uveitis AND tube OR panuveitis AND fluocinolone OR panuveitis AND dexamethasone OR panuveitis AND retisert OR panuveitis AND device OR panuveitis AND implant OR panuveitis AND shunt OR panuveitis AND valve OR panuveitis AND tube OR choroiditis AND fluocinolone OR choroiditis AND dexamethasone OR choroiditis AND retisert OR choroiditis AND device OR choroiditis AND implant OR choroiditis AND shunt OR choroiditis AND valve OR choroiditis AND tube OR pars planitis AND fluocinolone OR pars planitis AND dexamethasone OR pars planitis AND retisert OR pars planitis AND device OR pars planitis AND implant OR pars planitis AND shunt OR pars planitis AND valve OR pars planitis AND tube OR panophthalmitis AND fluocinolone OR panophthalmitis AND dexamethasone OR panophthalmitis AND retisert OR panophthalmitis AND device OR panophthalmitis AND implant OR panophthalmitis AND shunt OR panophthalmitis AND valve OR panophthalmitis AND tube
uveomeningoencephalitic AND fluocinolone OR uveomeningoencephalitic AND dexamethasone OR uveomeningoencephalitic AND retisert OR uveomeningoencephalitic AND device OR uveomeningoencephalitic AND implant OR uveomeningoencephalitic AND shunt OR uveomeningoencephalitic AND valve OR uveomeningoencephalitic AND tube OR behcet AND fluocinolone OR behcet AND dexamethasone OR behcet AND retisert OR behcet AND device OR behcet AND implant OR behcet AND shunt OR behcet AND valve OR behcet AND tube OR iridocyclitis AND fluocinolone OR iridocyclitis AND dexamethasone OR iridocyclitis AND retisert OR iridocyclitis AND device OR iridocyclitis AND implant OR iridocyclitis AND shunt OR iridocyclitis AND valve OR iridocyclitis AND tube OR iritis AND fluocinolone OR iritis AND dexamethasone OR iritis AND retisert OR iritis AND device OR iritis AND implant OR iritis AND shunt OR iritis AND valve OR iritis AND tube OR retinitis AND fluocinolone OR retinitis AND dexamethasone OR retinitis AND retisert OR retinitis AND device OR retinitis AND implant OR retinitis AND shunt OR retinitis AND valve OR retinitis AND tube
Data and analyses
Comparison 1. Steroid implant vs sham procedure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion of participants with recurrence of uveitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Primary efficacy time point: 6 months | 2 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.30, 0.54] |
| 1.1.2 Other time point: 12 months | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.30, 0.51] |
| 1.1.3 Other time point: 36 months | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.57, 0.79] |
| 1.2 Improvement in BCVA [logMAR] | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 Primary efficacy time point: 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 Other time point: 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.3 Other time point: 36 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Mean difference in quality of life scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Steroid implant vs sham procedure: ocular adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion of eyes with cataract formation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Proportion of eyes with cataract progression | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 Sham procedure | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.65, 6.12] |
| 2.2.2 Standard‐of‐care | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [2.06, 3.56] |
| 2.3 Proportion of eyes that underwent cataract surgery | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Sham procedure | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.82, 10.81] |
| 2.3.2 Standard‐of‐care | 2 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [2.33, 3.79] |
| 2.4 Proportion of eyes with elevated IOP | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 Sham procedure | 2 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [1.42, 5.56] |
| 2.4.2 Standard‐of‐care | 2 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.64 [2.71, 4.87] |
| 2.5 Proportion of eyes receiving IOP‐lowering medications | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 Sham procedure | 2 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.05, 3.25] |
| 2.5.2 Standard‐of‐care | 2 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [2.36, 3.91] |
| 2.6 Proportion of eyes that underwent IOP‐lowering surgery | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.6.1 Sham procedure | 2 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.13, 4.17] |
| 2.6.2 Standard‐of‐care | 2 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.43 [3.12, 9.45] |
| 2.7 Proportion of eyes with endophthalmitis | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 Sham procedure | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.10, 2.30] |
| 2.7.2 Standard‐of‐care | 2 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.30 [0.91, 58.72] |
| 2.8 Proportion of eyes with retinal tear or detachment | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.8.1 Sham procedure | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.21, 5.75] |
| 2.8.2 Standard‐of‐care | 2 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.51, 8.40] |
Comparison 3. Steroid implant vs standard‐of‐care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proportion of eyes with recurrence of uveitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Primary efficacy time point: 24 months | 2 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.35, 0.60] |
| 3.1.2 Other time point: 6 months | 2 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.35, 0.59] |
| 3.2 Proportion of eyes with recurrence of uveitis; sensitivity analysis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 Primary efficacy time point: 24 months | 2 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.27, 0.51] |
| 3.2.2 Other time point: 6 months | 2 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.35, 0.59] |
| 3.3 Improvement in BCVA [logMAR] | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.3.1 Primary efficacy time point: 24 months | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.02, 0.12] |
| 3.3.2 Other time point: 12 months | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.06, 0.08] |
| 3.3.3 Other time point: 6 months | 2 | 619 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.01, 0.11] |
| 3.4 Mean difference in quality of life scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.1 NEI‐VFQ25 (range 0 to 100); MCID: 4 to 6 points | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.2 SF‐36 (physical component); MCID: 3 to 5 points | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.3 SF‐36 (mental component); MCID: 3 to 5 points | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.4 EuroQoL (VAS, range 0 to 100); MCID: 7 points | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.5 EuroQoL‐5D (range 0.00 to 1.00); MCID: 0.06 to 0.07 points | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jaffe 2019.
| Study characteristics | |
| Methods |
Study design: parallel‐group, randomized controlled trial Study duration: August 2013 to October 2019 Unit of randomization: person (one eye per person)* Masking of participants, treatment allocator, outcome assessor, or data analyzor: participants, outcome assessors, and other study personnel were masked Study visits and time points: screening (day ‐30 to 0), days 1, 7, 28; months 2, 3, 6, 9, 12, 18, 24, 30, 36 Follow‐up duration: 3 years Planned treatment duration (for standard‐of‐care arm): NA Numbers of participants lost or excluded after randomization: none lost to follow‐up by the primary efficacy time points at month 6; one and two participants in the FAi and sham group discontinued through month 12 How missing data were handled: "a recurrence event was imputed if, for a previously nonrecurrent study eye, the study eye was treated with a prohibited local or systemic medication, or the participant had a missing ophthalmic assessment at the 6‐ or 12‐month visit". Power and sample size calculation: "The study sample size of 120 participants (80 and 40 patients for FA insert and sham treatment groups, respectively) was calculated based on the primary end point; treatment groups were not sized to detect statistically significant differences in secondary end points." *Note: "The affected eye in unilateral uveitis, the more seriously affected eye in bilateral uveitis, and the right eye in equally affected, symmetrical uveitis were identified as the study eyes". |
| Participants |
Countries: 6 countries (USA, Germany, Hungary, India, Israel, UK) Setting: multicenter (33 clinical sites [in publication], 34 sites [on clinicaltrials.gov]) Interventions
Age, mean ± SD (range): 48.3 ± 13.9 Female, n (%): 50 (57.5%) Predominant race/ethnicity, n (%): White, 60 (69.0%) Diagnosis of posterior uveitis, n (%): 87 (100%) Number using systemic treatment at baseline, n (%): 44 (50.6%) Participants (eyes) randomized: 87 Participants (eyes) analyzed for efficacy outcome(s): 87 Participants (eyes) analyzed for safety outcome(s): 87
Age, mean ± SD (range): 48.3 ± 13.7 Female, n (%): 29 (69.0%) Predominant race/ethnicity, n (%): White, 26 (61.9%) Diagnosis of posterior uveitis, n (%): 42 (100%) Number using systemic treatment at baseline, n (%): 21 (50.0%) Participants (eyes) randomized: 42 Participants (eyes) analyzed for efficacy outcome(s): 42 Participants (eyes) analyzed for safety outcome(s): 42
Age, mean ± SD (range): 48.3 ± 13.8 Female, n (%): 79 (61.2%) Predominant race/ethnicity, n (%): White, 86 (66.7%) Diagnosis of posterior uveitis, n (%): 129 (100%) Number using systemic treatment at baseline, n (%): 65 (50.4%) Participants (eyes) randomized: 129 Participants (eyes) analyzed for efficacy outcome(s): 129 Participants (eyes) analyzed for safety outcome(s): 129 Inclusion criteria
Exclusion criteria
Baseline comparison "Overall, average disease duration was greater in the FA insert group when compared to the sham group (7.8 vs 5.6 years, respectively), and the proportion of FA insert group participants with disease duration greater than 5 years was nearly twice that observed in the sham group. A lower proportion of FA insert than sham injection study eyes (45% vs 50%, respectively) had a vitreous haze severity of 1/2+." |
| Interventions |
|
| Outcomes |
Primary outcome of the study:
Secondary outcomes of the study: treatment group comparisons through 12 months
|
| Notes |
Funding sources: "Supported by EyePoint Pharmaceuticals, Inc., Watertown, Massachusetts; and the National Institutes of Health, Bethesda, Maryland (S.F.). The sponsor participated in the design of the study, study conduct, data collection, data management, data analysis and interpretation, and preparation and review of the manuscript."
Declaration of interest:
GJJ: consultant ‐ AbbVie, Inc. (North Chicago, IL), Alcon Laboratories (Fort Worth, TX), Novartis Pharma AG (Basel, Switzerland), Neurotech USA, Inc. (Lincoln, RI), Heidelberg Engineering (Heidelberg, Germany). C.S.F.: Consultant ‐ Abbott Laboratories (Lake Bluff, IL), Alcon Laboratories (Fort Worth, TX), Allergan (Dublin, Ireland), Bausch & Lomb
(Rochester, NY), EyeGate Pharmaceuticals, Inc. (Waltham, MA), Genentech, Inc. (South San Francisco, CA), Inotech Bioscience (Rockville, MD), Inspire Pharmaceuticals, Inc. (Durham, NC), Ista Pharmaceuticals, Inc. (Irvine, CA), LUX Biosciences, Inc. (Jersey City, NJ), Merrimack Pharmaceuticals, Inc. (Cambridge, MA), Novartis Pharmaceuticals Corporation (East Hanover, NJ), Sirion Therapeutics, Inc. (Tampa, FL), Therakine (Hermosa Beach, CA)
CEP: consultant ‐ Allergan (Dublin, Ireland), Alimera Sciences (Alpharetta, GA), EYEVENSYS (Paris, France), Servier Laboratories (Suresnes, France), Santen Pharmaceutical Co., Ltd. (Japan), AbbVie, Inc.(North Chicago, IL)
DAP: employee ‐ EyePoint Pharmaceuticals, Inc. (Watertown, MA)
GER: employee ‐ EyePoint Pharmaceuticals, Inc. (Watertown, MA) Trial registry: NCT02746991 (clinicaltrials.gov) Publication language: English Contact information: Glenn J. Jaffe, MD; Department of Ophthalmology, Duke University |
Kempen 2011.
| Study characteristics | |
| Methods |
Study design: parallel‐group, randomized controlled trial (superiority trial) Study duration: December 2005 to December 2008 Unit of randomization: "Patients were randomized to implant or systemic therapy; patients with bilateral uveitis were assigned to receive implants in each eye meeting eligibility criteria".* Masking of participants, treatment allocator, outcome assessor, or data analyzor: "Other than at the 1‐ and 3‐month visits, when postoperative signs were expected to be visible, visual acuity examiners were masked". "Reading Center image evaluations for ocular sequelae of uveitis and of therapy, and glaucoma assessments all were masked. Patients, clinicians, and coordinators were not masked". Study visits and time points: "Patients completed study visits at baseline, 1 month, 3 months, and then every 3 months for at least 24 months (contiguous visit windows)." Follow‐up duration: 24 months for efficacy outcomes Planned treatment duration (for standard‐of‐care arm): "Most cases had active inflammation at baseline and received 1 mg/kg/day up to 60 mg/day of prednisone until either the uveitis was controlled or 4 weeks had elapsed". Numbers of participants lost or excluded after randomization: 4 in each group did not complete the 2‐year follow‐up ; 7 and 8 in the FAi and SOC group were lost prior to 2‐year follow‐up How missing data was handled: "All available visit information was incorporated into the model, with missing data indicators used to maintain the data structure". Power and sample size calculation: "By assuming bilateral disease in 67% of patients, a between‐eye correlation of 0.4, a standard deviation of 16 letters’ change over 2 years, and a 2‐sided type 1 error rate of 0.05, a sample size of 250 provided 91% power (assuming 10% crossover) to detect a treatment difference of 7.5 standard Early Treatment of Diabetic Retinopathy Study letters’ change in visual acuity from baseline to 24 months, a difference similar to that which drove widespread use of expensive new retinal treatments in other trials that tested them. One interim analysis using the O’Brien‐Fleming‐spending function was conducted; the nominal type 1 error rate was 0.049 for the final analysis." *Note: "For participants with unilateral disease (N = 31), the affected eye was the study eye. For participants with asymmetric bilateral disease (N = 224), both eyes were study eyes". |
| Participants |
Countries: 3 countries (Australia, United Kingdom, United States) Setting: multicenter (23 sites) Interventions
Age, mean ± SD (range): 46 ± 15 years Female, n (%): 91 (71%) Predominant race/ethnicity, n (%): White 72 (56%) Diagnosis of posterior uveitis, n (%): 79 (61%) Number using systemic treatment at baseline, n (%): NR Participants (eyes) randomized: 129 (245 eyes) Participants (eyes) analyzed for efficacy outcome(s): 129 (245 eyes) Participants (eyes) analyzed for safety outcome(s): 118
Age, mean ± SD (range): 47 ± 15 years Female, n (%): 100 (79%) Predominant race/ethnicity, n (%): White 70 (56%) Diagnosis of posterior or panuveitis, n (%): 79 (63%) Number using systemic treatment at baseline, n (%): NR Participants (eyes) randomized: 126 (234 eyes) Participants (eyes) analyzed for efficacy outcome(s): 126 (234 eyes) Participants (eyes) analyzed for safety outcome(s): 114
Age, mean ± SD (range): 46.3 ± 15.0 years Female, n (%): 191 (75%) Predominant race/ethnicity, n (%): White 142 (57%) Diagnosis of posterior or panuveitis, n (%): 158 (62%) Number using systemic treatment at baseline, n (%): NR Participants (eyes) randomized: 255 (479 eyes) Participants (eyes) analyzed for efficacy outcome(s): 255 (479 eyes) Participants (eyes) analyzed for safety outcome(s): 232 Inclusion criteria:
Exclusion criteria:
Baseline comparison: "Baseline demographic and clinical characteristics were distributed similarly between groups (Table 1)" |
| Interventions |
"Most cases had active inflammation at baseline and received 1 mg/kg/day up to 60 mg/day of prednisone until either the uveitis was controlled or 4 weeks had elapsed. After control was achieved, prednisone was tapered per study guidelines. Cases already suppressed at baseline began by tapering from their initial prednisone dose. Immunosuppression was indicated for (1) failure to initially control inflammation using corticosteroids; (2) corticosteroid‐sparing in cases consistently reactivating before reaching a prednisone dose of 10 mg/day; and (3) specific high‐risk uveitis syndromes. When indicated, clinicians selected the approved immunosuppressant most suitable for each patient; administration and monitoring for toxicity followed guidelines. Uveitis experts regularly monitored treatment regimens for protocol compliance at site visits." |
| Outcomes |
Primary outcome of the study
Secondary outcomes of the study
|
| Notes |
Funding sources: National Eye Institute, Research to Prevent Blindness, Paul and Evanina Mackall Foundation. Bausch and Lomb provided "support to the study in the form of a donation of a limited number of fluocinolone implants to patients who were … uninsured or otherwise unable to pay for the implants" Declaration of interest "Dr Kempen is a consultant for Alcon Laboratories, Allergan Pharmaceutical Corporation, Lux Biosciences Inc, and Sanofi Pasteur SA. Dr Jabs is a consultant for Abbott Laboratories, Alcon Laboratories, Allergan Pharmaceutical Corporation, Corcept Therapeutics, Genentech Inc, Genzyme Corporation, GlaxoSmithKline, Novartis Pharmaceutical Corporation, Roche Pharmaceuticals, and Applied Genetic Technologies Corporation. Dr Louis is a consultant for Bristol‐Myers Squibb, Medtronic Inc, and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr Thorne is a consultant for Heron Evidence Ltd, and Allergan. Drs Altaweel, Holbrook, and Sugar have no conflicts of interest." Trial registry: NCT00132691 (clinicaltrials.gov) Publication language: English Contact information: John H. Kempen, MD, PhD; Department of Ophthalmology, University of Pennsylvania |
Lowder 2011.
| Study characteristics | |
| Methods |
Study design: parallel‐group, randomized controlled trial Study duration: August 2006 to October 2008 Unit of randomization: person (one eye per person)* Masking of participants, treatment allocator, outcome assessor, or data analyzor: "Patients were masked with regard to study treatment, and the key efficacy variables were collected and evaluated by follow‐up investigators who were also masked with regard to study treatment". Study visits and time points: baseline, day 1, day 7, week 3, week 6, week 8, week 12, week 16, week 20, week 26 Follow‐up duration: 26 weeks Planned treatment duration (for standard‐of‐care arm): NA Numbers of participants lost or excluded after randomization: "4 and 5 participants in the DEX implant ‐ 0.7 mg and sham group discontinued after randomization, respectively; 2 in the implant group discontinued because of adverse events" How missing data were handled: "Any missing data from weeks 2 through 26 were imputed using the last observation carried forward method". Power and sample size calculation: "A sample size of 73 patients for each treatment group was determined to have a 93% power to detect a between‐group difference of 23% (DEX implant minus sham) in the proportion of patients with a vitreous haze score of 0 (assuming 10% of patients in the sham group would have a vitreous haze score of 0)". *Note: "Only 1 eye was designated as the study eye. If both eyes were eligible for the study, the right eye was designated as the study eye". |
| Participants |
Countries: 18 countries (USA, Australia, Austria, Brazil, Canada, Czech Republic, France, Germany, Greece, India, Israel, South Korea, Poland, Portugal, South Africa, Spain, Switzerland, UK) Setting: multicenter (46 sites) Interventions
Age, mean ± SD (range): 44 ± 14.8 Female, n (%): 46 (59.7%) Predominant race/ethnicity, n (%): White, 47 (61%) Diagnosis of posterior uveitis, n (%): 14 (18%) Number using systemic treatment at baseline, n (%): 20 (26%) Participants (eyes) randomized: 77 Participants (eyes) analyzed for efficacy outcome(s): 77 Participants (eyes) analyzed for safety outcome(s): 77
Age, mean ± SD (range): 44 ± 15.0 Female, n (%): 51 (67%) Predominant race/ethnicity, n (%): White, 46 (61%) Diagnosis of posterior uveitis, n (%): 18 (24%) Number using systemic treatment at baseline, n (%): 18 (24%) Participants (eyes) randomized: 76 Participants (eyes) analyzed for efficacy outcome(s): 76 Participants (eyes) analyzed for safety outcome(s): 76
Age, mean ± SD (range): 44 ± 14.9 (calculated) Female, n (%): 97 (63.4%) Predominant race/ethnicity, n (%): White, 93 (60.8%) Diagnosis of posterior uveitis, n (%): 32 (20.9%) Number using systemic treatment at baseline, n (%): 38 (24.8%) Participants (eyes) randomized: 153 Participants (eyes) analyzed for efficacy outcome(s): 153 Participants (eyes) analyzed for safety outcome(s): 153 Inclusion criteria
Exclusion criteria
Baseline comparison: "There were no notable between‐group differences in any demographic or baseline characteristic". |
| Interventions |
All patients were treated with a topical ophthalmic antibiotic 4 times daily starting 3 days prior to the day of their study procedure (day 0) and continuing for 3 days after the procedure. |
| Outcomes |
Primary outcome of the study
Secondary outcomes of the study: other outcome measures included
|
| Notes |
Funding sources: Allergan, Inc. "participated in the design of the study, data analysis, and interpretation and supervised the preparation of the manuscript and approved the final version." Declaration of interest: "Drs Robinson, Schiffman, Li, Cui, and Whitcup are employees of Allergan, Inc." Trial registry: NCT00333814 (clinicaltrials.gov) Publication language: English Contact information: Careen Lowder, MD; Cleveland Clinic Cole Eye Institute Contact efforts: emailed enquiry about numbers of participants who required at least one IOP‐lowering medications in the sham group |
Pavesio 2010.
| Study characteristics | |
| Methods |
Study design: parallel‐group, randomized controlled trial Study duration: April 2002 to August 2005 Unit of randomization: person (one eye per person)* Masking of participants, treatment allocator, outcome assessor, or data analyzor: open‐label study Study visits and time points: "Subjects in the implant treatment group returned to the study site on day 2, whereas subjects in both treatment groups returned to the study site on week 1 (± 2 days), weeks 4, 8, 12, 18, 24, 30, and 34 (± 1 week), at 1 year, and then every 3 months (± 1 month) thereafter, through 3 years for safety and efficacy assessments." Follow‐up duration: 24 months for efficacy outcomes; 36 months for safety and efficacy outcomes Planned treatment duration (for standard‐of‐care arm): 6 months before tapering or altering Numbers of participants lost or excluded after randomization: 6 randomzied to the FAi group discontinued before receiving the implant; another 8 did not complete the 2‐year follow‐up (5 in the implant group) How missing data were handled: 6 participants who did not receive the implant were excluded from the (modified) ITT analysis Power and sample size calculation: "A total enrollment of 150 subjects was planned based on the results of a previous study that suggested that the 1‐year recurrence rate for SOC would be approximately 30%, and based on the expectation that the implant would reduce this rate to 10% at 1 year. A sample size of 75 subjects per treatment was determined to have 85% power to detect a difference with respect to the primary end point in a 2‐tailed test (α = 0.05)". *Note: "For participants with unilateral disease, the affected eye was the study eye. For participants with asymmetric bilateral disease, the study eye was the more severely affected eye". |
| Participants |
Countries: 10 countries (France, Germany, Israel, Italy, Portugal, Saudi Arabia, Spain, Switzerland, Turkey, United Kingdom) Setting: multicentre (37 sites) Interventions
Age, mean ± SD (range): 40.4 ± 14.4 (12 to 75) years Female, n (%): 32 (48.5%) Predominant race/ethnicity, n (%): White 60 (90.9%) Diagnosis of posterior uveitis, n (%): NR Number using systemic treatment at baseline, n (%): NR Participants (eyes) randomized: 72 Participants (eyes) analyzed for efficacy outcome(s): 66 Participants (eyes) analyzed for safety outcome(s): 66
Age, mean ± SD (range): 43.1 ± 13.5 (18 to 70) years Female, n (%): 50 (67.6%) Predominant race/ethnicity, n (%): White 64 (86.5%) Diagnosis of posterior uveitis, n (%): NR Number using systemic treatment at baseline, n (%): NR Participants (eyes) randomized: 74 Participants (eyes) analyzed for efficacy outcome(s): 74 Participants (eyes) analyzed for safety outcome(s): 74
Age, mean ± SD (range): 41.8 ± 13.9 (12 to 75) years (calculated) Female, n (%): 82 (58.6%) Predominant race/ethnicity, n (%): 124 (88.6%) Diagnosis of posterior uveitis, n (%): 41 (29.4%) (calculated) Number using systemic treatment at baseline, n (%): 140 (100%) Participants (eyes) randomized: 146 Participants (eyes) analyzed for efficacy outcome(s): 140 Participants (eyes) analyzed for safety outcome(s): 140 Inclusion criteria
Exclusion criteria
Baseline comparison: "Subject demographics are shown in Table 2. The 2 treatment groups were similar in age and race, but there was a statistically significant difference between the treatment groups in gender, with 67.6% of SOC subjects being female versus 48.5% of implant subjects being female (P = 0.02). Baseline values for VA, IOP, and anterior chamber flare, anterior chamber cells, vitreous haze, and incidence of cataracts were similar between the implant and SOC groups". |
| Interventions |
"The SOC group received prednisolone or an equivalent corticosteroid alone, or an immunosuppressive agent was added to the therapy and the corticosteroid dose was reduced. Levels considered acceptable for therapy with steroids alone were 0.2 mg/kg daily (or 15 mg/day for the average weight). When inflammation could not be controlled with this level of corticosteroid, immunosuppressive agents were added. With the use of an immunosuppressive agent, the objective was to reduce steroid use to 0.1 mg/kg daily of prednisolone equivalent after 4 to 6 weeks of combination therapy. Approved immunosuppressants included cyclosporine A, methotrexate, cyclophosphamide, mycophenolate mofetil, azathioprine, and tacrolimus. If an immunosuppressive agent was not recommended, subjects were managed by maintaining systemic steroids at a higher level (0.2 mg/kg daily of prednisolone equivalent) or by increasing the steroids in case of inflammation. This regimen was followed by a slow taper to a minimal dose of 0.2 mg/kg daily (10 mg/day for subjects whose weight was 50 kg). After 6 months, if the disease was controlled, the treatment doses were tapered according to the standard guideline of each investigational site." |
| Outcomes |
Primary outcomes of the study
Secondary outcomes of the study
|
| Notes |
Funding source: Bausch and Lomb Inc. Declaration of interest: Of the 5 study authors, lead author is a consultant for Bausch and Lomb Inc, and 3 authors are employees of Bausch and Lomb Inc. Trial registry: NCT00468871 (clinicaltrials.gov) Publication language: English Contact information: Carlos E. Pavesio, MD, FRCOphth; Moorfields Eye Hospital, London |
AE: adverse event DEX: dexamethasone FA: fluocinolone acetonide FAi: fluocinolone acetonide intravitreal (implant/insert) IOP: intraocular pressure MUST: Multicenter Uveitis Steroid Treatment NA: not applicable NR: not reported NIPU: non‐infectious posterior uveitis SD: standard deviation VA: visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acharya 2004 | Ineligible design: not an RCT |
| ACTRN12605000485639 | Ineligible comparator: dose‐comparing trial |
| Anonymous 1995 | Ineligible design |
| Ansari 2010 | Ineligible design: not an RCT |
| Arcinue 2013 | Ineligible design: not an RCT |
| Bollinger 2009 | Ineligible study population |
| Callanan 2008 | Ineligible comparator: a dose‐comparing trial |
| Callanan 2020 | This article was withdrawn at the request of the author(s), editor, or both |
| Campochiaro 2013 | Ineligible study population |
| Cano‐Parra 2006 | Ineligible design: not an RCT |
| Ciulla 2021 | Ineligible study population |
| Cornish 2018 | Ineligible design: not an RCT |
| Couret 2020 | Ineligible study population |
| Eng 2007 | Ineligible study population |
| Ermakova 2003 | Ineligible design: not an RCT |
| Errera 2019 | Ineligible design: observational study |
| Galor 2007 | Ineligible design: not an RCT |
| Garg 2006 | Ineligible design: not an RCT |
| Goldstein 2007 | Ineligible design: a pooled analysis of 3 dose‐comparing trials |
| Jaffe 2000a | Ineligible design: an interventional case series |
| Jaffe 2000b | Ineligible design: a case report |
| Jaffe 2016 | Ineligible comparator: a dose‐comparing study |
| Kim 2011 | Ineligible design: not an RCT |
| Kuppermann 2007 | Ineligible study population |
| Mercante 2007 | Ineligible study population |
| Mustakallio 1973 | Ineligible study population |
| NCT02309385 | Ineligible study population |
| NCT02482129 | Ineligible study population |
| NCT02517619 | Ineligible study population |
| NCT02748512 | Ineligible intervention |
| NCT04976777 | Ineligible study population |
| Neger 1996 | Ineligible study population |
| Novack 2008 | Ineligible design: not an RCT |
| Ram 2013 | Ineligible study population |
| Sangwan 2015 | Ineligible comparator: a dose‐comparing trial |
| Taylor 2012 | Ineligible study design: case series |
| Wen 1991 | Ineligible intervention |
| Williams 2009 | Ineligible study population |
FA: fluocinolone acetonide FAi: fluocinolone acetonide intravitreal (implant/insert) mg: milligram RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1900026160.
| Study name | A phase iii, multicenter, randomized, double‐blind, controlled, safety and efficacy study for a fluocinolone acetonide intravitreal (FAi) insert in subjects with chronic non‐infectious uveitis affecting the posterior segment of the eye |
| Methods | Randomzied parallel‐group controlled trial |
| Participants |
Age limitation: not reported Gender: both Inclusion criteria
Exclusion criteria
|
| Interventions | Target sample size: 100 in experimental and 50 in control group
|
| Outcomes | Primary outcomes
Secondary outcomes: not reported |
| Starting date | 01 October 2019 (expected) |
| Contact information | Contact person: Changdong Liu, Dan Jia; Ocumension Tehrapeutics (Shanghai) Co., Ltd., Shanghai, China Email: dan.jia@ocumension.com (Dan Jia replied that the trial is still ongoing). |
| Notes | Sources of financial support: fully self‐raised Date of last update: 30 September 2019 |
NCT05070728.
| Study name | Safety and efficacy of an injectable fluocinolone acetonide intravitreal insert (FAi) |
| Methods | Randomized parallel‐group controlled trial |
| Participants |
Age limitation: 18 years and older Gender: all Inclusion criteria
Exclusion criteria
|
| Interventions | Target sample size: 60 in total
|
| Outcomes | Primary outcome:
|
| Starting date | 17 November 2021 |
| Contact information | Contact person: Dario Paggiarino, MD Email: dpaggiarino@eyepointpharma.com |
| Notes | Sponsors and collaborators: EyePoint Pharmaceuticals, Inc. |
NCT05101928.
| Study name | Ozurdex monotherapy trial (OM) |
| Methods | randomized parallel‐group controlled trial |
| Participants |
Age limitation: 18 years and older Sex: all Inclusion criteria
Exclusion criteria
|
| Interventions | Target sample size: 84 in total
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | 1 December 2021 (expected) |
| Contact information | Contact person: Melanie Lalonde, PhD Email: mlalonde@ohri.ca |
| Notes | Sponsors and collaborators: Ottawa Hospital Research Institute |
DEX: dexamethasone FA: fluocinolone acetonide FAi: fluocinolone acetonide intravitreal (implant/insert) OM: Ozurdex monotherapy
Differences between protocol and review
For the updated search, we modified the search strategies by including one term, 'Yutiq', which is a newer equivalent to 'Retisert*' used in the original search strategies.
To allow for evaluations of effectiveness and adverse effects of corticosteroid implants compared with 'no treatment', we also decided to include trials that used sham procedures as the comparator. We further performed separate comparisons and subgroup analyses by comparator to explore differential effects of steroid implants when compared with sham versus with standard care.
For this update, we also decided to use the new version of the risk of bias tool, RoB 2, to assess risk of bias in two outcomes: the proportion of recurrence of uveitis (the primary outcome) and mean changes in best corrected visual acuity (BCVA).
Before data extraction, we decided not to include "treatment‐associated systemic adverse events" in the Summary of Finding tables because we did not expect to encounter sufficient numbers of events reported during the trial period.
We extended the time points for collecting relevant review outcomes to 36 months, as reported by the included trials.
We did not perform subgroup analysis by age, or clinical heterogeneity, as planned in the review protocol, because of the small number of trials included. We did not perform sensitivity analysis by excluding trials of high risk of bias or trials that were sponsored by industry either, due to the small number of included trials.
Contributions of authors
For the update, all review authors reviewed and agreed upon the eligibility criteria, participated in study selection, data abstraction, data analysis, results interpretation, drafted portions of the review; commented critically on drafts regarding intellectual content, and approved the final version for publication.
Sources of support
Internal sources
-
None, Other
No internal source of support.
External sources
-
National Eye Institute, National Institutes of Health, USA
Cochrane Eyes and Vision Group US Project, supported by grant UG1EY020522 (PI: Tianjing Li, MD, MHS, PhD)
-
Public Health Agency, UK
This review was supported by the HSC Research and Development (R&D) Division of the Public Health Agency which funds the Cochrane Eyes and Vision editorial base at Queen's University Belfast.
-
Queen's University of Belfast, UK
Gianni Virgili, Co‐ordinating Editor for Cochrane Eyes and Vision’s work is funded by the Centre for Public Health, Queen’s University of Belfast, Northern Ireland
Declarations of interest
Amit Reddy: no financial disclosures
Su‐Hsun Liu: reports a grant UG1 EY020522 from the National Eye Institute, National Institutes of Health, USA; payment to institution
Christopher J Brady: No financial conflicts of interest
Pamela C Sieving: reports no financial conflicts. She is a special volunteer for the National Eye Institute, National Institutes of Health, USA: this is an unpaid position in which she performs occasional literature searches and analysis of scholarly impact for NEI staff.
Alan G Palestine: serves as a Co‐investigator for Cochrane Eyes and Vision US Satellite, which is support by grant UG1 EY020522 from the National Eye Institute, National Institutes of Health, USA.
Edited (no change to conclusions)
References
References to studies included in this review
Jaffe 2019 {published data only}
- CTRI/2014/07/004726. To assess the safety & efficacy of fluocinolone acetonide intravitreal (FAi) insert in patients with chronic, non-infectious uveitis affecting the posterior segment of the eye. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2014/07/004726 (first received 10 July 2014).
- CTRI/2014/12/005337. A research to study the effect of fluocinolone acetonide intravitreal (FAi) insert in patients with inflammation of middle layer of the eye. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2014/12/005337 (first registered 30 December 2014).
- Cai CX, Skalak C, Keenan RT, Grewal DS, Jaffe GJ. Time to disease recurrence in noninfectious uveitis following long-acting injectable fluocinolone acetonide implant. Graefe's Archive for Clinical and Experimental Ophthalmology 2020;258(5):1023-30. [DOI] [PubMed] [Google Scholar]
- EUCTR2013-001810-14-GB. A clinical study of the FAI insert in patients with uveitis in the back of the eye that is not caused by an infection. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2013-001810-14-GB (first received 19 August, 2013).
- Jaffe GJ, Foster CS, Pavesio CE, Paggiarino DA, Riedel GE. Effect of an injectable fluocinolone acetonide insert on recurrence rates in chronic noninfectious uveitis affecting the posterior segment: twelve-month results. Ophthalmology 2019;126(4):601‐10. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, Paggiarino D, Riedel G. An injectable fluocinolone acetonide intravitreal insert decreases the incidence of recurrence in patients with chronic non-infectious uveitis affecting the posterior segment of the eye: 12-month results. Investigative Ophthalmology & Visual Science 2017;58(8):1232. [Google Scholar]
- Jaffe GJ, Pavesio CE. Effect of a fluocinolone acetonide insert on recurrence rates in noninfectious intermediate, posterior, or panuveitis: three-year results. Ophthalmology 2020;127(10):1395‐404. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ. Treatment of non-infectious uveitis that affects the posterior segment with a single intravitreal fluocinolone acetonide insert (FAi) - 3-year results. Investigative Ophthalmology & Visual Science 2019;60(9):3858. [Google Scholar]
- NCT01694186. Safety and efficacy of an injectable fluocinolone acetonide intravitreal insert. clinicaltrials.gov/show/NCT01694186 (first submitted 24 September 2012).
- NCT02746991. Safety and efficacy study of a fluocinolone acetonide intravitreal (FAi) insert in subjects with chronic non-infectious posterior uveitis. clinicaltrials.gov/show/NCT02746991 (first received 16 April 2016).
- Nguyen QD, Patel K, Paggiarino DA. Controlling posterior segment uveitic recurrences: results from a phase 3 study of 0.18 mg fluocinolone acetonide intravitreal insert (FAi) in subjects with chronic non-infectious uveitis affecting the posterior segment. Investigative Ophthalmology & Visual Science 2018;59(9):418. [Google Scholar]
- Nguyen QD, Patel K, Paggiarino DA. Minimizing recurrences of ocular inflammation and need for adjunctive treatment of non-infectious posterior uveitis (NIPU) during the 2 years following treatment with a single 0.18 mg fluocinolone acetonide intravitreal insert (FAi). Investigative Ophthalmology & Visual Science 2019;60(9):3513. [Google Scholar]
- Sharma S. Course of non-infectious uveitis affecting the posterior segment: fellow-eye data from a 3-year study of the fluocinolone acetonide intravitreal insert. Investigative Ophthalmology & Visual Science 2020;61(7):5364. [Google Scholar]
- Shrivastav A, Mayor R, Agarwal M, Venkatesh R, Singh S. Re: Jaffe, et al: Injectable fluocinolone acetonide long-acting implant for noninfectious intermediate uveitis, posterior uveitis, and panuveitis: 2-year results. Ophthalmology 2017;124(8):e62. [DOI] [PubMed] [Google Scholar]
- Singer MA, Krambeer C, Paggiarino D. IOP elevation in patients treated with fluocinolone acetonide insert for chronic noninfectious uveitis affecting the posterior segment. Ophthalmic surgery lasers and imaging retina 2021;52(7):387‐90. [DOI] [PubMed] [Google Scholar]
Kempen 2011 {published data only}
- Frick KD, Drye LT, Kempen JH, Dunn JP, Holland GN, Latkany P, et al. Associations among visual acuity and vision- and health-related quality of life among patients in the multicenter uveitis steroid treatment trial. Investigative Ophthalmology & Visual Science 2012;53(3):1169-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, Holbrook JT, Ansari H, Alexander J, Burke A, Reed SB, et al. Risk of elevated intraocular pressure and glaucoma in patients with uveitis: results of the multicenter uveitis steroid treatment trial. Ophthalmology 2013;120(8):1571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook JT, Kempen JH, Prusakowski NA, Altaweel MM, Jabs DA. Challenges in the design and implementation of the multicenter uveitis steroid treatment (MUST) trial — lessons for comparative effectiveness trials. Clinical Trials 2011;8(6):736-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Louis TA, Sugar EA, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology 2011;118(10):1916-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Sugar EA. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. American Journal of Ophthalmology 2010;149(4):550-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group, Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Louis TA, Sugar EA, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology 2012;119(2):212. [DOI: 10.1016/j.ophtha.2011.07.027] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multicenter Uveitis Steroid Treatment Trial Follow-up Study Research Group. Quality of life and risks associated with systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, or panuveitis: fifty-four-month results of the multicenter uveitis steroid treatment trial and follow-up study. Ophthalmology 2015;122(10):1976-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar EA, Holbrook JT, Kempen JH, Burke AE, Drye LT, Thorne JE, et al. Cost-effectiveness of fluocinolone acetonide implant versus systemic therapy for noninfectious intermediate, posterior, and panuveitis. Ophthalmology 2014;121(10):1855-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins-Netzer O, Lightman S, Drye L, Kempen J, Holland G, Rao NA, et al. Outcome of treatment of uveitic macular edema: the multicenter uveitis steroid treatment trial 2-year results. Ophthalmology 2015;122(11):2351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowder 2011 {published data only}
- Lightman S, Belfort R Jr, Naik RK, Lowder C, Foster CS, Rentz AM, et al. Vision-related functioning outcomes of dexamethasone intravitreal implant in noninfectious intermediate or posterior uveitis. Investigative Ophthalmology & Visual Science 2013;54(7):4864-70. [DOI] [PubMed] [Google Scholar]
- Lowder C, Belfort R Jr, Lightman S, Foster CS, Robinson MR, Schiffman RM, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Archives of Ophthalmology 2011;129(5):545-53. [DOI] [PubMed] [Google Scholar]
- NCT00333814. A study of the safety and efficacy of a new treatment for non-infectious intermediate or posterior uveitis. clinicaltrials.gov/show/NCT00333814 (first received 6 June 2006).
- Naik RK, Rentz AM, Foster CS, Lightman S, Belfort R Jr, Lowder C, et al. Normative comparison of patient-reported outcomes in patients with noninfectious uveitis. JAMA Ophthalmology 2013;131(2):219-25. [DOI] [PubMed] [Google Scholar]
- Saraiya NV, Patel SS, Goldstein DA. A report of high intraocular pressure with the dexamethasone intravitreal implant. Archives of Ophthalmology 2011;129(12):1638-9. [DOI] [PubMed] [Google Scholar]
Pavesio 2010 {published data only}
- Muller B, Orlic N, Pleyer U. Intravitreal fluocinolone-implant (Retisert) in posterior non-infectious uveitis — intermediate results after 34 weeks [Intravitreales fluocinolon-implantat (retisert) bei posteriorer nichtinfektiöser uveitis – zwischenanalyse nach 34 wochen]. Klinische Monatsblatter fur Augenheilkunde 2004;221(Suppl 7):S8. [Google Scholar]
- NCT00468871. Safety and efficacy of fluocinolone acetonide intravitreal implant vs standardized therapy. clinicaltrials.gov/show/NCT00468871 (first received 3 May 2007).
- Pavesio C, Zierhut M, Bairi K, Comstock TL, Usner DW. Evaluation of an intravitreal fluocinolone acetonide implant versus standard systemic therapy in noninfectious posterior uveitis. Ophthalmology 2010;117(3):567-75. [DOI] [PubMed] [Google Scholar]
- Pavesio CE, Bairi K. Evaluation of an intravitreal fluocinolone acetonide implant vs. standard systemic therapy in noninfectious posterior uveitis. American Academy of Ophthalmology 2006:Poster 194. [DOI] [PubMed]
References to studies excluded from this review
Acharya 2004 {published data only}
- Acharya N, Young L. Sustained-release drug implants for the treatment of intraocular disease. International Ophthalmology Clinics 2004;44(3):33-9. [DOI] [PubMed] [Google Scholar]
ACTRN12605000485639 {published data only}
- ACTRN12605000485639. A multicenter, randomized, double-masked controlled study to evaluate the safety and efficacy of an intravitreal fluocinolone acetonide (0.59 and 2.1 mg) implant in patients with non-infectious uveitis affecting the posterior segment of the eye. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12605000485639 (first received 23 September 2005).
Anonymous 1995 {published data only}
- Anonymous. Good results from eye implant study. Treatment Review 1995;16:4. [PubMed] [Google Scholar]
Ansari 2010 {published data only}
- Ansari H, Kempen JH. Proof of concept for combined insertion of fluocinolone acetonide and glaucoma drainage implants for eyes with uveitis and glaucoma. American Journal of Ophthalmology 2010;149(5):699-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arcinue 2013 {published data only}
- Arcinue CA, Ceron OM, Foster CS. A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. Journal of Ocular Pharmacology and Therapeutics 2013;29(5):501-7. [DOI] [PubMed] [Google Scholar]
Bollinger 2009 {published data only}
- Bollinger KE, Smith SD. Prevalence and management of elevated intraocular pressure after placement of an intravitreal sustained-release steroid implant. Current Opinion in Ophthalmology 2009;20(2):99-103. [DOI] [PubMed] [Google Scholar]
Callanan 2008 {published data only}
- Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Archives of Ophthalmology 2008;126(9):1191-201. [DOI] [PubMed] [Google Scholar]
- Ibrahim M, Nguyen QD. Treatment of posterior uveitis with a fluocinolone acetonide implant. Evidence-Based Ophthalmology 2009;10(4):224-6. [Google Scholar]
- Jaffe GJ, Martin D, Callanan D, Levy B. Fluocinolone acetonide intravitreal implant to treat posterior uveitis: 2-year results of a multicenter clinical trial. Investigative Ophthalmology & Visual Science 2005;46(13):ARVO E-abstract 2386. [Google Scholar]
- Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology 2006;113(6):1020-7. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, Martin DF, Callanan D, Levy B, Comstock T. Fluocinolone acetonide intravitreal implant to treat posterior segment uveitis: 3-year results of a multicenter clinical trial. Investigative Ophthalmology & Visual Science 2006;47(13):ARVO E-abstract 1523. [Google Scholar]
- Sheppard JD Jr, Nguyen QD, Usner DW, Comstock TL. Post-cataract outcomes in patients with noninfectious posterior uveitis treated with the fluocinolone acetonide intravitreal implant. Clinical Ophthalmology 2012;6(1):79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S, Nussenblatt RB. Fluocinolone acetonide for the treatment of uveitis: weighing the balance between local and systemic immunosuppression. Archives of Ophthalmology 2008;126(9):1287-9. [DOI] [PubMed] [Google Scholar]
Callanan 2020 {published data only}
- Callanan D, Nguyen QD, Suhler EB, Paggiarino D, Riedel GE. Reduced risk of recurrence of non-infectious posterior segment uveitis after 0.18 mg fluocinolone acetonide insert: randomized trial. American Journal of Ophthalmology 2020 Feb 20 [Epub ahead of print]. [DOI: 10.1016/j.ajo.2020.02.011] [DOI] [PubMed]
Campochiaro 2013 {published data only}
- Campochiaro PA, Nguyen QD, Hafiz G, Bloom S, Brown DM, Busquets M, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology 2013;120(3):583-7. [DOI] [PubMed] [Google Scholar]
Cano‐Parra 2006 {published data only}
- Cano-Parra J, Diaz-Llopis M. New drugs in the treatment of noninfectious uveitis [Nuevos fármacos en el tratamiento de las uveítis no infecciosas]. Archivos de la Sociedad Espanola de Oftalmologia 2006;81(12):671-3. [DOI] [PubMed] [Google Scholar]
Ciulla 2021 {published data only}
- Ciulla TA, Kapik B, Grewal DS, Ip MS. Visual acuity in retinal vein occlusion, diabetic, and uveitic macular edema: central subfield thickness and ellipsoid zone analysis. Ophthalmology Retina 2021;5(7):633-47. [DOI] [PubMed] [Google Scholar]
Cornish 2018 {published data only}
- Cornish EE, McCluskey PJ. Dexamethasone intravitreal implant for macular oedema and uveitis. Medicine Today 2018;20(1):38-41. [Google Scholar]
Couret 2020 {published data only}
- Couret C, Poinas A, Volteau C, Riche VP, Le Lez ML, Errera MH, et al. Comparison of two techniques used in routine care for the treatment of inflammatory macular oedema, subconjunctival triamcinolone injection and intravitreal dexamethasone implant: medical and economic importance of this randomized controlled trial. Trials 2020;21(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eng 2007 {published data only}
- Eng K, Kertes P. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema: commentary. Evidence-Based Ophthalmology 2007;8(3):170-2. [DOI] [PubMed] [Google Scholar]
Ermakova 2003 {published data only}
- Ermakova NA. Efficacy of corticosteroids and cyclosporin in the treatment of retinal vasculitis in patients with Behcet's disease. Advances in Experimental Medicine and Biology 2003;528:563-5. [DOI] [PubMed] [Google Scholar]
Errera 2019 {published data only}
- Errera MH, Westcott M, Benesty J, Falah S, Smadja J, Ores R, et al. A comparison of the dexamethasone implant (Ozurdex®) and inferior fornix-based sub-tenon triamcinolone acetonide for treatment of inflammatory ocular diseases. Ocular Immunology and Inflammation 2019;27(2):319-29. [DOI] [PubMed] [Google Scholar]
Galor 2007 {published data only}
- Galor A, Margolis R, Kaiser PK, Lowder CY. Vitreous band formation and the sustained-release, intravitreal fluocinolone (Retisert) implant. Archives of Ophthalmology 2007;125(6):836-8. [DOI] [PubMed] [Google Scholar]
Garg 2006 {published data only}
- Garg S. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Evidence-Based Ophthalmology 2006;7(4):216-7. [DOI] [PubMed] [Google Scholar]
Goldstein 2007 {published data only}
- Goldstein DA, Godfrey DG, Hall A, Callanan DG, Jaffe GJ, Pearson PA, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Archives of Ophthalmology 2007;125(11):1478-85. [DOI] [PubMed]
- Viola F, Staurenghi G, Ratiglia R. Uveitis treated with fluocinolone acetonide implants. Archives of Ophthalmology 2009;127(1):115-6. [DOI] [PubMed] [Google Scholar]
Jaffe 2000a {published data only}
- Jaffe GJ, Ben-Nun J, Guo H, Dunn JP, Ashton P. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology 2000;107(11):2024-33. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, McCallum RM, Branchaud B, Skalak C, Butuner Z, Ashton P. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology 2005;112(7):1192-8. [DOI] [PubMed] [Google Scholar]
Jaffe 2000b {published data only}
- Jaffe GJ, Pearson PA, Ashton P. Dexamethasone sustained drug delivery implant for the treatment of severe uveitis. Retina 2000;20:402-3. [DOI] [PubMed] [Google Scholar]
Jaffe 2016 {published data only}
- Jaffe GJ, Lin P, Keenan RT, Ashton P, Skalak C, Stinnett SS. Injectable fluocinolone acetonide long-acting implant for noninfectious intermediate uveitis, posterior uveitis, and panuveitis: two-year results. Ophthalmology 2016;123(9):1940-8. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim SJ. Diagnosis and management of noninfectious pediatric uveitis. International Ophthalmology Clinics 2011;51(1):129-45. [DOI] [PubMed] [Google Scholar]
Kuppermann 2007 {published data only}
Mercante 2007 {published data only}
- Mercante AR, Huang SS, Reddy R. Cystoid macular edema in non-infectious uveitis treated with fluocinolone acetonide intravitreal implant: 3-year results of a multi-center clinical trial. Investigative Ophthalmology & Visual Science 2007;48(13):ARVO E-Abstract 280. [Google Scholar]
Mustakallio 1973 {published data only}
- Mustakallio A, Kaufman HE, Johnston G, Wilson RS, Roberts MD, Harter JC. Corticosteroid efficacy in postoperative uveitis. Annals of Ophthalmology 1973;5(6):719-30. [PubMed] [Google Scholar]
NCT02309385 {unpublished data only}
- NCT02309385. Safety and efficacy study of DSP-visulex for the treatment of anterior uveitis. clinicaltrials.gov/show/NCT02309385 (first received 1 December 2014).
NCT02482129 {unpublished data only}
- NCT02482129. Proof of concept study to evaluate safety and efficacy of LME636 in the treatment of acute anterior uveitis. clinicaltrials.gov/show/NCT02482129 (first received 25 June 2015).
NCT02517619 {unpublished data only}
- NCT02517619. Safety and efficacy of iontophoretic dexamethasone phosphate ophthalmic solution in non-infectious anterior uveitis (EGP-437-006). clinicaltrials.gov/show/NCT02517619 (first received 5 August 2015).
NCT02748512 {published data only}
- NCT02748512. Utilization and safety of the Mk ii inserter and the safety of the FAi insert in non-infectious uveitis. clinicaltrials.gov/show/NCT02748512 (first received 21 April 2016).
NCT04976777 {published data only}
- NCT04976777. A study to evaluate an updated dexamethasone intravitreal (into the eye) applicator in adult participants with macular edema due to diseases of the retina. clinicaltrials.gov/show/NCT04976777 (first received 15 July 2021).
Neger 1996 {published data only}
- Neger RE. The eyes have it, too. Positively Aware: the Monthly Journal of the Test Positive Aware Network 1996;7(3):10-1. [PubMed] [Google Scholar]
Novack 2008 {published data only}
- Novack GD. Clinical indications for ophthalmic corticosteroids. Ocular Surface 2008;6(4):199-202. [DOI] [PubMed] [Google Scholar]
Ram 2013 {published data only}
- Ram J, Gupta A, Gupta V. Intraoperative dexamethasone implant in uveitis patients with cataract undergoing phacoemulsification. Ocular Immunology and Inflammation 2013;21(6):462-7. [DOI] [PubMed] [Google Scholar]
Sangwan 2015 {published data only}
- Hall J. Comment on: "Use of the fluocinolone acetonide intravitreal implant for the treatment of noninfectious posterior uveitis: 3-year results of a randomized clinical trial in a predominantly Asian population". Ophthalmology and Therapy 2015;4(1):65‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan VS, Paul HA, Usner DW. Safety and efficacy of an intravitreal fluocinolone acetonide implant in subjects with noninfectious uveitis. American Academy of Ophthalmology 2007:Poster 199.
- Sangwan VS, Pearson PA, Paul H, Comstock TL. Use of the fluocinolone acetonide intravitreal implant for the treatment of noninfectious posterior uveitis: 3-year results of a randomized clinical trial in a predominantly Asian population. Ophthalmology and Therapy 2015;4(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scassellati Sforzolini B. Reply to Hall J: "Use of the fluocinolone acetonide intravitreal implant for the treatment of noninfectious posterior uveitis: 3-year results of a randomized clinical trial in a predominantly Asian population". Ophthalmology and Therapy 2015;4(1):67‐68. [DOI: 10.1007/s40123-015-0030-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2012 {published data only}
- Taylor SR, Tomkins-Netzer O, Joshi L, Morarji J, McLoone E, Lightman S. Dexamethasone implant in pediatric uveitis. Ophthalmology 2012;119(11):2412. [DOI] [PubMed] [Google Scholar]
Wen 1991 {published data only}
- Wen S, Gao R, Hu Z. Comparison of Chinese traditional therapy combined with Western medicine and Western medicine alone in the treatment of uveitis. Eye Science 1991;7(4):205-8. [PubMed] [Google Scholar]
Williams 2009 {published data only}
- Williams G A, Kuppermann B D, Blumenkranz M S, Haller J A, Whitcup S M, Weinberg D V. Treatment of inflammatory macular edema with a dexamethasone posterior-segment drug delivery system. American Academy of Ophthalmology 2004:Poster 87.
- Williams GA, Haller JA, Kuppermann BD, Blumenkranz MS, Weinberg DV, Chou C, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. American Journal of Ophthalmology 2009;147(6):1048-54. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1900026160 {published data only}
- ChiCTR1900026160. A phase III, multi-center, randomized, double-blind, controlled, safety and efficacy study for a fluocinolone acetonide intravitreal (FAi) insert in subjects with chronic non-infectious uveitis affecting the posterior segment of the eye. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1900026160 (first received 24 September 2019).
NCT05070728 {published data only}
- NCT05070728. Safety and efficacy of an injectable fluocinolone acetonide intravitreal insert (FAi). clinicaltrials.gov/show/NCT05070728 (first received 6 October 2021).
NCT05101928 {published data only}
- NCT05101928. Ozurdex monotherapy trial. clinicaltrials.gov/show/NCT05101928 (first received 21 October 2021).
Additional references
Abdulla 2022
- Abdulla D, Ali Y, Menezo V, Taylor SRJ. The use of sustained release intravitreal steroid implants in non-infectious uveitis affecting the posterior segment of the eye. Ophthalmology and Therapy 2022;Jan:1-9. [DOI: 10.1007/s40123-022-00456-4] [DOI] [PMC free article] [PubMed]
Bollinger 2011
- Bollinger K, Kim J, Lowder CY, Kaiser PK, Smith SD. Intraocular pressure outcome of patients with fluocinolone acetonide intravitreal implant for noninfectious uveitis. Ophthalmology 2011;118(10):1927-31. [DOI] [PubMed] [Google Scholar]
Callanan 2008
- Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Archives of Ophthalmology 2008;126(9):1191-201. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 23 Feb 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2021
- Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Durrani 2004
- Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica 2004;218(4):223-36. [DOI] [PubMed] [Google Scholar]
EuroQoI 1990
- The EuroQol Group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. [DOI] [PubMed] [Google Scholar]
Ferris 1982
- Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. American Journal of Ophthalmology 1982;94(1):91-6. [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;4(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Goldstein 2007a
- Goldstein DA, Godfrey DG, Hall A, Callanan DG, Jaffe GJ, Pearson PA, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Archives of Ophthalmology 2007;125(11):1478-85. [DOI] [PubMed] [Google Scholar]
Gritz 2004
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California epidemiology of uveitis study. Ophthalmology 2004;111(3):491-500. [DOI] [PubMed] [Google Scholar]
Haller 2010
- Haller JA, Bandello F, Belfort Jr R, Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117(6):1134-46. [DOI] [PubMed] [Google Scholar]
Haupert 2000
- Haupert CL, Jaffe GJ. New and emerging treatments for patients with uveitis. International Ophthalmology Clinics 2000;40(2):205-20. [DOI] [PubMed] [Google Scholar]
Hays 2001
- Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Annals of Medicine 2001;33:350-7. [DOI] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Jaffe 2006
- Jaffe GJ, Martin DF, Callanan D, Levy B, Comstock T. Fluocinolone acetonide intravitreal implant to treat posterior segment uveitis: 3-year results of a multi-center clinical trial. Investigative Ophthalmology & Visual Science 2006;47(13):ARVO E-abstract 1523. [Google Scholar]
Jager 2004
- Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreous injection: a comprehensive review. Retina 2004;24(5):676-98. [DOI] [PubMed] [Google Scholar]
Li 2021
- Li T, Higgins JPT, Deeks JJ. Chapter 5: Collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Lightman 2013
- Lightman S, Belfort R Jr, Naik RK, Lowder C, Foster CS, Rentz AM, et al. Vision-related functioning outcomes of dexamethasone intravitreal implant in noninfectious intermediate or posterior uveitis. Investigative Ophthalmology & Visual Science 2013;54(7):4864-70. [DOI] [PubMed] [Google Scholar]
Logan 2016
- Logan SA, Weng CY, Carvounis PE. Intravitreal steroid implants in the management of retinal disease and uveitis. International Ophthalmology Clinics 2016;56(4):127-49. [DOI] [PubMed] [Google Scholar]
Mangione 2001
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Archives of Ophthalmology 2011;119(7):1050-8. [DOI] [PubMed] [Google Scholar]
Modugno 2021
- Modugno RL, Testi I, Pavesio C. Intraocular therapy in noninfectious uveitis. Journal of Ophthalmic Inflammation and Infection 2021;11(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammad 2007
- Mohammad DA, Sweet BV, Elner SG. Retisert: is the new advance in treatment of uveitis a good one? Annals of Pharmacotherapy 2007;41(3):449-54. [DOI] [PubMed] [Google Scholar]
Nussenblatt 1990
- Nussenblatt RB. The natural history of uveitis. International Ophthalmology 1990;14(5-6):303-8. [DOI] [PubMed] [Google Scholar]
Pickard 2007
- Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health and Quality of Life Outcomes 2007;5(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.19.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Suner 2009
- Suner IJ, Kokame GT, YU E, Ward J, Dolan C, Bressler NM. Responsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Investigative Ophthalmology & Visual Science 2009;50(8):3629-35. [DOI] [PubMed] [Google Scholar]
Suttorp‐Schulten 1996
- Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. British Journal of Ophthalmology 1996;80(9):844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2010
- Taylor SR, Isa H, Joshi L, Lightman S. New developments in corticosteroid therapy for uveitis. Ophthalmologica 2010;224(Suppl 1):46-53. [DOI] [PubMed] [Google Scholar]
Testi 2019
- Testi I, Pavesio C. Preliminary evaluation of YUTIQ™ (fluocinolone acetonide intravitreal implant 0.18 mg) in posterior uveitis. Therapeutic Delivery 2019;10(10):621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
WebPlotDigitizer 2021 [Computer program]
- WebPlotDigitizer. Rohatgi A, Version 4.5. Pacifica, California, USA: Ankit Rohatgi, 2021. Available from: automeris.io/WebPlotDigitizer.
Williams 2009
- Williams GA, Haller JA, Kuppermann BD, Blumenkranz MS, Weinberg DV, Chou C, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. American Journal of Ophthalmology 2009;147(6):1048-54. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brady 2013
- Brady CJ, Villanti A, Reddy R, Sieving PC, Garg SJ, Tang J. Corticosteroid implants for chronic non‐infectious uveitis. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD010469. [DOI: 10.1002/14651858.CD010469] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brady 2016
- Brady CJ, Villanti AC, Law HA, Rahimy E, Reddy R, Sieving PC, et al. Corticosteroid implants for chronic non-infectious uveitis. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD010469. [DOI: 10.1002/14651858.CD010469.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]