Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver an opinion on 3’‐Sialyllactose (3’‐SL) sodium salt as a novel food (NF) pursuant to Regulation (EU) 2015/2283. The NF is mainly composed of the human identical milk oligosaccharide (HiMO) 3’‐SL but also containing D‐lactose, sialic acid and a small fraction of other related oligosaccharides resulting in a fully characterised mixture of carbohydrates. The NF is produced by fermentation with a genetically modified strain of Escherichia coli K‐12 DH1. The information provided on the manufacturing process, composition and specifications of the NF does not raise safety concerns. The applicant intends to add the NF in a variety of foods, including infant and follow‐on formula, foods for infants and toddlers, foods for special medical purposes and food supplements. The target population is the general population. The anticipated daily intake of 3’‐SL from the NF at the maximum proposed use levels is unlikely to exceed the intake level of naturally occurring 3’‐SL in breastfed infants on a body weight basis. The intake of 3’‐SL in breastfed infants on a body weight basis is expected to be safe also for other population groups. The intake of other carbohydrate‐type compounds structurally related to 3’‐SL is also considered of no safety concern. Food supplements are not intended to be used if other foods with added NF (as well as breast milk, milk, fermented milk‐based products and selected cheeses retaining milk sugar (e.g. curd cheese) for infants and young children) are consumed on the same day. The Panel concludes that the NF is safe under the proposed conditions of use for the proposed target populations.
Keywords: 3’‐Sialyllactose, 3’‐SL , human milk oligosaccharide, HMO, HiMO , novel food, safety
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
On 28 February 2019, the company Glycom A/S submitted a request to the European Commission in accordance with Article 10 of Regulation (EU) 2015/22831 to place 3’‐Sialyllactose on the Union market as a novel food.
The novel food is intended to be used as an ingredient in dairy products and analogues, beverages, bakery wares, foods for special groups, foods for special medical purposes, foods for diet replacement for weight control, and in food supplements. The target population is the general population.
In accordance with Article 10(3) of Regulation (EU) 2015/2283, the European Commission asks the European Food Safety Authority to provide a scientific opinion by carrying out the assessment for 3’‐Sialyllactose sodium salt as a novel food.
2. Data and methodologies
2.1. Data
The safety assessment of this novel food (NF) is based on data supplied in the application and information submitted by the applicant following EFSA requests for supplementary information.
During the assessment, the Panel identified additional data which were not included in the application (Goehring et al., 2014 ; Gorbach, 1978 , Rudloff et al., 1996, 2012; Zeng et al., 2018).
Administrative and scientific requirements for NF applications referred to in Article 10 of Regulation (EU) 2015/2283 are listed in the Commission Implementing Regulation (EU) 2017/24692.
A common and structured format on the presentation of NF applications is described in the EFSA guidance on the preparation and presentation of an NF application (EFSA NDA Panel, 2016). As indicated in this guidance, it is the duty of the applicant to provide all available (proprietary, confidential and published) scientific data, including both data in favour and not in favour to supporting the safety of the proposed NF.
This NF application includes a request for protection of proprietary data in accordance with Article 26 of Regulation (EU) 2015/2283. Data claimed to be proprietary by the applicant include:
Detailed description of the production process – raw materials and schematic overview of the processing (section of the dossier 2.b.1.1‐4).
Annexes to the dossier which relate to the identity, the production process, production microorganism, composition and specifications of the NF (annex I ‘NMR analytical report’, annex II ‘production strain data’, annex III ‘production strain certificates’, annex IV ‘raw materials and processing aids’, annex V ‘certificate of analysis and batch data’, annex VI ‘analytical methods and validation report’, annex VII ‘stability reports’, annex VIII ‘laboratory accreditation certificates’).
‘Intakes assessment report’ (annex X to the dossier).
-
Unpublished toxicological study reports:
-
○
3’‐Sialyllactose – Bacterial Reverse Mutation Test (Unpublished study report, 2019a); In vitro Mammalian Cell Micronucleus Test (Unpublished study report, 2019b); 14‐Day Toxicity Study in the Neonatal Rat (Unpublished study report, 2019c); 90‐Day Toxicity Study in the Neonatal Rat (Unpublished study report, 2019d)
-
○
6’‐sialyllactose – Bacterial Reverse Mutation Test (Unpublished study report, 2018a); In vitro Mammalian Cell Micronucleus Test (Unpublished study report, 2018b); 14‐Day Toxicity Study in the Neonatal Rat (Unpublished study report, 2018c); 90‐Day Toxicity Study in the Neonatal Rat (Unpublished study report, 2018d)
-
•
Appendix B.3 to the dossier referring to the summary table of statistically significant observations in toxicity studies with 3’‐Sialyllactose.
-
○
2.2. Methodologies
The assessment follows the methodology set out in the EFSA guidance on NF applications (EFSA NDA Panel, 2016) and the principles described in the relevant existing guidance documents from the EFSA Scientific Committee. The legal provisions for the assessment are laid down in Article 11 of Regulation (EU) 2015/2283 and in Article 7 of the Commission Implementing Regulation (EU) 2017/2469.
This assessment concerns only risk that might be associated with consumption of the NF under the proposed conditions of use and is not an assessment of the efficacy of 3’‐Sialyllactose (3’‐SL) sodium salt with regard to any claimed benefit.
3. Assessment
3.1. Introduction
The NF primary constituent is the sodium salt of 3’‐Sialyllactose, henceforth named ‘3’‐SL sodium salt’. The trisaccharide 3’‐Sialyllactose (3’‐SL) is composed of the monomers glucose, galactose and N‐acetylneuraminic acid (NANA, hereinafter also referred to as ‘sialic acid’). It is one of the sialylated (acidic) oligosaccharides in the oligosaccharide fraction of human milk (HMOs – human milk oligosaccharides). The 3’‐SL in the NF is obtained by fermentation and is isolated as a purified ingredient in the sodium salt form. The NF is intended to be used in foods for infants and young children (including the use as human‐identical milk oligosaccharides (HiMO) in infant formulas (IF) and follow‐on formulas), foods for special medical purposes, total diet replacements for weight control, food supplements, beverages and in a variety of other foods (e.g. dairy products, bakery wares). The target population is the general population.
Other HiMOs, namely LNnT, 2’‐FL, DFL and LNT produced by chemical synthesis or by fermentation with derivatives of E. coli K‐12, have been previously assessed (EFSA NDA Panel, 2015, 2019b,b).
The applicant indicated that according to Regulation (EU) 2015/2283, this NF falls under the following categories:
‘food with a new or intentionally modified molecular structure, where that structure was not used as, or in, a food within the Union before 15 May 1997’;
‘food consisting of, isolated from or produced from microorganisms, fungi or algae.’
3.2. Identity of the NF
The NF is a powdered mixture mainly composed of 3’‐SL sodium salt, but also containing D‐lactose, sialic acid and a small fraction of other related saccharides resulting in a fully characterised mixture of carbohydrates (about 95% in representative batches, see Table 1). It is produced by fermentation with the genetically modified strain of Escherichia coli K12 DH1 MDO. The main component is the sodium salt of Neu5Ac‐(α2‐3)‐Gal‐(β1‐4)‐Glc (3’‐SL) in which sodium N‐acetylneuraminate is linked through an α‐(2‐3) bond to D‐galactose and linked through a β‐(1‐4) bond to the reducing end of d‐glucose, which is in equilibrium between the α‐ and β‐anomeric forms. 3’‐Sialyllactose is a constitutional isomer of 6’‐Sialyllactose (6’‐SL), which contains the same monosaccharide moieties, with the linkage between Neu5Ac and d‐galactose being α‐(2‐6) instead of α‐(2‐3).
Table 1.
Batch‐to‐batch analysis for five batches of NF
| Parameters | Batches | Mean ± SD | ||||
|---|---|---|---|---|---|---|
| CPN5115 1000516 FD | CPN5115 1000616 FD | CPN5115 1000816 FD | CPN5115 1000916 FD | CPN5115 1001016 FD | ||
| physicochemical properties | ||||||
| pH (20°C, 5% solution) | 5.3 | 4.8 | 5.4 | 5.6 | 5.8 | 5.4 ± 0.4 |
| Composition | ||||||
| HIMS (w/w % dry matter)a | 91.5 | 92.0 | 93.4 | 92.2 | 92.9 | 92.4 ± 0.8 |
| 3’‐SL sodium salt (w/w % dry matter) | 90.3 | 91.0 | 91.0 | 89.8 | 90.9 | 90.6 ± 0.5 |
| d‐Lactose [w/w %] | 0.63 | 0.51 | 1.86 | 1.80 | 1.41 | 1.24 ± 0.64 |
| Sialic acid [w/w %] | 0.48 | 0.43 | 0.44 | 0.56 | 0.52 | 0.49 ± 0.5 |
| 3’‐sialyl‐lactulose [w/w %] | 1.70 | 1.61 | 1.86 | 1.52 | 1.46 | 1.63 ± 0.16 |
| Sum of other carbohydrates [w/w %] | 1.08 | 1.04 | 0.80 | 0.59 | 0.73 | 0.8 ± 0.2 |
| Total carbohydrates (dry matter) [w/w %]b | 94.2 | 94.6 | 96.0 | 94.3 | 95.0 | 94.8 ± 0.6 |
| Water [w/w %] | 3.20 | 3.15 | 2.40 | 2.51 | 2.40 | 2.73 ± 0.41 |
| Sodium [w/w %] | 3.07 | 3.06 | 3.03 | 2.89 | 3.01 | 3.01 ± 0.07 |
| Chloride by IC [w/w %] | < 0.005 | < 0.004 | < 0.020 | 0.013 | 0.016 | 0.012 ± 0.006 |
| Residual proteins (Bradford method) [w/w %] | 0.0063 | 0.0050 | < LoR | < LoR | < LoR | |
| Microbiological parameters | ||||||
| Aerobic mesophilic total plate count [CFU/g] | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 |
| Enterobacteriaceae [in 10 g] | Absent | Absent | Absent | Absent | Absent | Absent |
| Salmonella spp. [in 25 g] | Absent | Absent | Absent | Absent | Absent | Absent |
| Cronobacter (Enterobacter) sakazakii [in 10 g] | Absent | Absent | Absent | Absent | Absent | Absent |
| Listeria monocytogenes [in 25 g] | Absent | Absent | Absent | Absent | Absent | Absent |
| Bacillus cereus [CFU/g] | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 |
| Yeasts [CFU/g] | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 |
| Moulds [CFU/g] | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 |
| Residual endotoxins [EU/mg] | 0.053 | 0.124 | 0.016 | 0.053 | 0.081 | 0.065 ± 0.04 |
HiMS: human identical milk saccharides, it includes 3’‐SL sodium salt, D‐lactose and sialic acid; CFU: colony forming units; 3’‐SL: 3’‐sialyllactose; IC ion chromatography; ± SD: standard deviation; LoR (limit of reporting) 0.0050 w/w %; EU: endotoxin units.
Sum of 3’‐SL sodium salt, lactose and sialic acid.
Sum of all carbohydrates.
3’‐SL sodium salt is characterised by the chemical formula C23H38NO19Na; molecular mass: 655.53 Da; CAS No 1128596‐80‐5; IUPAC name: N‐Acetyl‐α‐d‐neuraminyl‐(2→3)‐β‐d‐galactopyranosyl‐(1?4)‐d‐glucose, sodium salt.
The structure has been confirmed by monodimensional (1D) and two‐dimensional (2D) 1H and 13C Nuclear Magnetic Resonance Spectroscopy (NMR). 1H NMR spectra show chemical shifts fully corresponding to those reported in the literature for 3’‐SL (Urashima et al., 2018). By Nuclear Overhauser Effect Spectroscopy (NOESY), some intra‐ and inter‐residual correlations were shown. All correlations for carbons involved in glycosidic linkages were evidenced in the Hetero Single Quantum Coherence (HSQC) spectrum and the interglycosidic correlations showing the positions of the glycosidic linkages, were confirmed by the Heteronuclear Multiple Bond Correlation (HMBC) spectrum. Taken together, NMR data confirm that the glycosidic linkage between N‐acetylneuraminic acid (Neu5Ac) C2 and the adjacent Galactose (Gal H3 and H3’) is α‐(2‐3). An inter‐residual correlation was also observed between Gal H1 and Glc C4 in the HMBC spectrum, this together with the chemical shifts and the coupling constants of the Gal H1 and Glc H1 signals are indicating the 1‐4 connection between the Gal and Glc units and that the pyranose configuration is β for the Gal unit and that the reducing end glucose is in equilibrium between the α‐ and the β‐anomeric forms. Also, the mass fragmentation pattern of 3’‐SL in MS/MS spectrometry has been provided and is consistent with the structure proposed (Figure 1).
Figure 1.
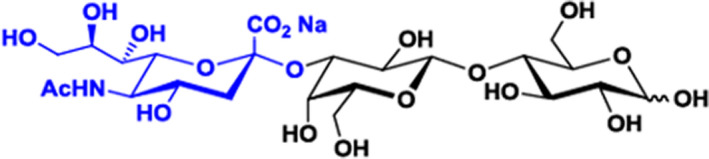
Structure of 3’‐SL sodium salt
The 3’‐SL produced by the described microbial fermentation has been shown to be chemically and structurally identical to its naturally occurring counterpart present in human milk oligosaccharides by mono‐ and two‐dimensional NMR and is therefore considered as HiMO.
3.3. Production process
According to the data provided by the applicant, the NF is produced pursuant to Good Manufacturing Practice (GMP) and Hazard Analysis Critical Control Points (HACCP) principles. The production process is comparable to those of the already authorised HiMOs such as LNnT (lacto‐N‐neotetraose), 2’‐FL (fucosyllactose), 2’‐FL/DFL (difucosyllactose) and LNT (lacto‐N‐tetraose) also previously assessed by EFSA (2'FL/DFL and LNT, EFSA NDA Panel, 2019b,b).
The manufacturing process can be broadly divided into two stages. In the first stage, d‐lactose and d‐glucose are used for production of 3’‐SL by the adapted cellular metabolism of the producing microorganism, which uses D‐glucose as an energy source, and d‐lactose as a substrate for 3’‐SL biosynthesis. At the end of the production process, the production microorganism is entirely removed from the medium by filtration. The second stage of the process includes several purification steps, after which the acidic 3’‐SL solution is alkalised with NaOH with formation of the 3’‐SL sodium salt to obtain the NF as a dried powdered mixture.
The microorganism used in the fermentation process for the production of the NF is a genetically modified derivative of the parental strain E. coli K‐12 DH1 (F‐ ʎ‐ gyrA96 recA1 relA1 endA1 thi‐1 hsdR17 supE44), that was obtained by the applicant from the German Collection of Microorganisms and Cell cultures (DSMZ) and deposited under DSM No. 4235. The derivative producing strain has been deposited in the DSMZ in Braunschweig, Germany.
The parental strain E. coli K‐12 DH1 is derived from E. coli K‐12 by forced random mutagenesis. The whole genomes of E. coli K‐12 and of other derivative strains, including E. coli K‐12 DH1, were sequenced and compared with other E. coli strains. The results indicate that E. coli K‐12 and its derivatives show genomic differences compared to pathogenic strains (Blattner et al., 1997; Lukjancenko et al., 2010). Although the species E. coli was considered not suitable for qualified presumption of safety (QPS) status (EFSA BIOHAZ Panel, 2018), E. coli K‐12 is considered as a safe and non‐pathogenic or toxigenic microorganism widely used for biotechnological applications (Gorbach, 1978; OECD, 1986; Muhldorfer and Hacker, 1994; U.S. EPA, 1997).
The applicant provided a detailed description of the genetic modification steps applied to the parental strain E. coli K‐12 DH1 to obtain the derivative strain used to produce the NF.
The absence of viable cells of the producing microorganism in the NF was demonstrated by testing five batches of the NF for bacteria from the Enterobacteriaceae family according to internationally recognised methods (ISO 21528‐1:2004, MSZ ISO 21528‐2:2007). The specification for Enterobacteriaceae set at ‘absent in 10 g’ ensures absence in the final product of the microorganism, since E. coli belongs to the Enterobacteriaceae family. Furthermore, the absence of residual proteins is confirmed by an adaptation of the Bradford protein method which has been developed to quantify proteins down to 0.005 w/w% level. No residual protein was detected in the final method above the 0.01 w/w% specification.
In addition, no residual DNA from the production organism was detected in five independent batches of the NF using three quantitative polymerase chain reaction (PCR) assays targeting short subsequences of specific inserted genes as well as a short subsequence of the 23S rRNA subunit of E. coli. Upon EFSA's request, the applicant provided data to demonstrate the validity of the method in accordance to the EFSA guidance on microorganisms used as production organisms (EFSA FEEDAP Panel, 2018).
The Panel considers that the production process is sufficiently described and does not raise safety concerns.
3.4. Compositional data
The NF contains 3’‐SL sodium salt as the primary ingredient (around 90% w/w dry matter as sodium salt). The remainder is a mixture of substances such as d‐lactose, sialic acid, 3’‐sialyl‐lactulose and other carbohydrates.
The Panel therefore notes that although the main component of the NF is 3’‐SL sodium salt, other fractions (lactose, sialic acid and 3’‐sialyl‐lactulose) are present in different amounts in the NF. Lactose is the most prevalent molecule in human breast milk (approximately 7 g/100 mL). Sialic acid is an endogenous human and ubiquitous nutritional monosaccharide (Röhrig et al., 2017, EFSA NDA Panel, 2017) while 3’‐sialyl‐lactulose is derived from 3’‐SL by isomerisation of the terminal glucose moiety into fructose mainly under alkaline conditions during the production process (Zeng et al., 2018). In representative batches of the NF, the amount of lactose was below 2% while the content of each of the other two saccharides was below 1% or ranging between 1% and 2% for sialic acid and 3’‐sialyl‐lactulose, respectively.
With regard to the physico‐chemical properties, the NF can be described as white to off‐white amorphous powder or agglomerate. It is readily soluble in aqueous solutions (max. 500 mg/mL, 25°C).
The applicant provided results of batch‐to‐batch compositional analysis for five batches of the NF (Table 1). 3’‐SL and other minor impurities have been analysed by high performance anion exchange chromatography coupled to pulsed amperometric detection (HPAEC‐PAD) using ‘in house’ validated methods as well as analysis by certified external international laboratories.
The microbiological purity of batches of the NF has been assessed for non‐pathogenic microorganisms (bacteria, yeasts and moulds) as general hygiene indicators, as well as for selected food‐borne pathogens.
The Panel considers that the information provided on the composition of the NF is sufficient and does not raise safety concerns.
3.4.1. Stability
Stability of NF
The bulk stability of the powdered NF produced by fermentation has been investigated in two representative batches, in an ongoing 5‐year study under real‐time conditions (25°C, 60% relative humidity (RH)) and an ongoing 2‐year study under accelerated conditions (40°C, 75% RH). Results were provided for both studies for 12 months.
Upon EFSA's request for additional information, the applicant provided interim results of the same two batches of the NF up to 24 months for both the real‐time stability (sensory testing and microbiological purity limited to 12 months) and accelerated stability studies. Results on composition, sensory testing and microbiological parameters indicate that there is no appreciable degradation of NF ingredients, no changes in impurity profile and no alterations in the microbiological quality of the NF. Additionally, 5‐year retest data were performed on a single batch and confirmed the stability of 3’‐SL when protected from light and stored at room temperature and ambient humidity.
On the basis of these data and of the accelerated stability studies at 40°C, the reaction rate of isomerisation was calculated and extrapolated by the Arrhenius analysis to 25°C (which is the temperature of storage of the NF), indicating, according to the applicant, a shelf‐life of 5 years for the NF.
In addition, results for stress stability studies for the NF have been provided by the applicant. The NF in the solid state was heated in an oven at 80°C for 28 days under two different humidity conditions. The results show stability of 3’‐SL level and a slight increase of lactose and isomerisation to 3’‐sialyl‐lactulose that although slightly more marked at higher humidity were both lower than 5%.
Based on the data available, the Panel considers that the NF is expected to be stable for at least 24 months when stored at room temperature in the solid state.
Stability of NF under the intended conditions of use
The NF was stable for a month in water solutions at neutral pH and 35°C, while in acidic conditions (pH 3 at 35°C), it was almost completely hydrolysed to lactose and sialic acid (about 50% each one). Under alkaline conditions (pH 9), isomerisation to 3’‐sialyl‐lactulose was observed (up to approximately 13%).
When oxidative stress was applied to a 3’‐SL solution for one day, no degradation was observed neither in the presence of 0.1% H2O2 nor in the presence of 0.1 M equivalent of ACVA (4,4’‐azobis (cyanovaleric acid)).
The stability of the NF in the powdered IF has been investigated following 12 months storage at different temperatures (4–37°C) and the target concentration of 0.26 g/100 g (dry matter) was found to be constant over time.
No other stability data for the NF in real food matrices have been provided. However, the applicant made reference to studies conducted with other HiMS (human identical milk saccharides) (i.e. 2’‐FL, LNnT and sialic acid) in real food matrices (e.g. yoghurts, ready‐to‐drink flavoured milk) where no significant loss of the added HiMO has been observed. In its previous assessments for these HiMOs, the NDA Panel concluded that ‘the data provide sufficient information with respect to the stability of the NF’ (EFSA NDA Panel, 2015, 2017).
The Panel considers that the available data provide sufficient information with respect to the stability of the NF in the food matrices at neutral pH, when stored at room temperature under proper storage conditions.
3.5. Specifications
The specifications of the NF as proposed by the applicant are presented in Table 2. The parameters include the main components of the NF mixture of HiMS, predominantly 3’‐SL sodium salt, D‐lactose, sialic acid and the isomer 3’‐sialyl‐lactulose. The sum of relevant HiMS has been introduced by the applicant as a parameter to ensure a highly consistent product quality. The 3’‐SL sodium salt content is specifically set at a minimum of 88 w/w % (dry matter).
Table 2.
Specifications of the NF
| Description: 3’‐Sialyllactose sodium salt (3’‐SL) is a purified, white to off‐white powder or agglomerates to that is produced by a microbial process and contains limited levels of lactose and sialic acid | ||
|---|---|---|
| Source: A genetically modified strain of Escherichia coli K‐12 DH1 MDO | ||
| Parameter | Specification | Method |
| Sum of HiMSa (w/w % dry matter) | ≥ 90.0 | HPAEC/PAD |
| 3’‐SL sodium salt (w/w % dry matter) | ≥ 88.0 | HPAEC/PAD |
| D‐Lactose [w/w %] | ≤ 5.0 | HPAEC/PAD |
| Sialic acid [w/w %] | ≤ 1.5 | HPAEC/PAD |
| 3’‐sialyl‐lactulose [w/w %] | ≤ 5.0 | HPAEC/PAD |
| Sum of other carbohydrates [w/w %] | ≤ 3.0 | HPAEC/PAD |
| pH (20°C, 5% solution) | 4.5–6.0 | Eur. Ph. 9.2 2.2.3 (07/2016:20203) |
| Water [w/w %] | ≤ 8.0 | Karl‐Fisher |
| Sodium [w/w %] | 2.5–4.5 | EN 13805:2002; EPA‐6010C:2007 |
| Chloride by IC [w/w %] | ≤ 1.0 | ISO10304‐2:1999 |
| Residual protein [w/w %] | ≤ 0.01 | Bradford assay (UV spectroscopy)b |
| Microbiological parameters | ||
| Aerobic mesophilic total plate count | ≤ 1,000 CFU/g | ISO 4833‐1:2014 |
| Enterobacteriaceae | ≤10 CFU/g | ISO 21528‐1:2004, ISO 21528‐2:2007 |
| Salmonella spp. | Absent in 25 g | ISO 6579:2006 |
| Yeasts | ≤ 100 CFU/g | ISO 21527‐2:2008 |
| Moulds | ≤ 100 CFU/g | ISO 21527‐2:2008 |
| Residual endotoxins | ≤ 10 EU/mg | Eur. Ph. 2.6.14 |
3’‐SL sodium salt: 3’‐Sialyllactose sodium salt; CFU: colony‐forming units; EU: endotoxin units; Eur. Ph.: European Pharmacopoeia; HiMO: Human‐identical Milk Oligosaccharides; HPLC: high‐performance liquid chromatography; IC: ion chromatography; CAD: charged aerosol detection; PAD: pulsed amperometric detection; HPAEC: high‐performance anion exchange chromatography; KF: Karl‐Fischer; RT: retention time; ISO: international organization for standardization; UV: ultraviolet.
Sum of HiMS: 3’‐SL sodium salt, D‐lactose and sialic acid.
LOR (limit of reporting) = 17 mg/kg.
Another parameter called ‘sum of other carbohydrates’ has been introduced, to include other carbohydrates individually present in low concentrations, up to a total amount of 3% w/w.
Microbiological parameters for Listeria monocytogenes, Cronobacter (Enterobacter) sakazakii and Bacillus cereus are monitored through internal specifications.
Analyses were performed using internationally recognised methods or newly developed and validated analytical protocols at Glycom's Research & Development Department and confirmed by accredited laboratories.
The Panel considers that the information provided on the specification of the NF is sufficient.
3.6. History of use of the NF and/or of its source
3.6.1. History of use of the NF
The NF does not have a history of use.
3’‐SL has also been detected in domestic farm animal milk, albeit generally at lower concentrations as compared to human milk. Oligosaccharides in bovine milk are 20 times less concentrated than in human milk; however, sialylated oligosaccharides accounted for approximately up to 80% of the total oligosaccharide pools. 3’‐SL is the most represented oligosaccharide in bovine milk and its concentration is estimated to be ranging from 47 to 55 mg/L and over 1 g/L in bovine colostrum (Aldredge et al., 2013; Urashima et al., 2013; Albrecht et al., 2014), therefore approximately six times lower than the breast milk levels.
3.6.2. Consumption of oligosaccharides constituent of the NF in breast milk
Human breast milk contains a family of structurally related oligosaccharides, known as HMOs and representing the third largest fraction of solid components. The highest concentrations of HMOs occur in human colostrum (20–25 g/L), and concentrations between 5 and 20 g/L occur in mature human milk (Thurl et al., 2010; Bode, 2012; Urashima et al., 2018). HMOs’ concentration and composition vary across mothers and over the course of lactation. 3’‐SL belongs to the subfraction of ‘acidic’ HMOs, which is characterised by the presence of sialic acids, and the whole subfraction accounts for 1.5–3.3 g/L (Thurl et al., 2010; Rijnierse et al., 2011; Bode, 2012).
There are two naturally occurring sialyllactoses which are constitutional isomers with a minimal structural difference: the oligosaccharide backbone can be sialylated by α‐(2‐3) or α‐(2‐6) linkages, resulting in 3’‐SL or 6’‐SL, respectively. The two forms have been shown to have similar functions and biological roles (Tarr et al., 2015).
Several publications on HMOs and 3’‐SL in human milk have been provided by the applicant.
The highest concentration of 3’‐SL and other HMOs in human milk is reported in colostrum, and the concentration is depending on the stage of lactation (Asakuma et al., 2007; Austin et al., 2016; Coppa et al., 1999, 2011; Kunz et al., 2017; McGuire et al., 2017; Spevacek et al., 2015; Thurl et al., 2010, 2017). Thurl et al. (2017) summarised the findings from 21 studies and reported that the content of 3’‐SL in milk from mothers who delivered at term ranged from 0.14 to 0.24 g/L (average 0.19 g/L). It was noted that the range of 3’‐SL was slightly wider (ranging from 0.21 to 0.36 g/L, average 0.29 g/L) in mothers who delivered preterm.
Based on the mean and the highest reported occurrence levels of 3’‐SL in human milk from mothers who had a preterm delivery as reported by Thurl et al. (2017), and considering the average and high daily intake of breast milk (800 mL and 1,200 mL, respectively) for infants from 0 to 6 months (EFSA NDA Panel, 2013), the daily intake levels of 3’‐SL from human milk for a 6.7 kg body weight (bw) infant (EFSA Scientific Committee, 2012) has been calculated (Table 3). This default body weight used by the NDA Panel is for an infant of 3–6 months of age, who is more likely than younger infants to consume these volumes of human milk.
Table 3.
Estimated daily intake levels of 3’‐SL from human milk (800 and 1,200 mL) for infants of 6.7 kg bw, based on mean and high concentration of 0.29 g/L and 0.36 g/L, respectively, of 3’‐SL measured in human milk from mothers who had a preterm delivery (Thurl et al., 2017)
| Daily intake levels (mg/kg bw) from 800 mL of human milk | Daily intake levels (mg/kg bw) from 1,200 mL of human milk | |||
|---|---|---|---|---|
| Mean concentration | High concentration | Mean concentration | High concentration | |
| 3’‐SL | 35 | 43 | 52 | 64 |
bw: body weight.
3.7. Proposed uses and use levels and anticipated intake
3.7.1. Target population
The target population proposed by the applicant is the general population.
3.7.2. Proposed uses and use levels
The NF is intended to be added to a variety of foods, at the maximum use levels as indicated in Table 4. The Panel notes that for the category ‘Food for special medical purposes’, the applicant did not propose either maximum use levels or maximum intake levels.
Table 4.
Proposed Food Uses and maximum use Levels of 3’‐SL
| EU food category number | Food category name | Proposed Maximum use level |
|---|---|---|
| 1 | Dairy products and analogues | |
| 1.1 | Unflavoured pasteurised and unflavoured sterilised (including UHT) milk | 0.25 g/L |
| 1.2/1.3 | Unflavoured fermented milk‐based products |
0.25 g/L beverages 0.5 g/kg products other than beverages |
| 1.4 | Flavoured fermented milk‐based products including heat‐treated products |
0.25 g/L beverages 2.5 g/kg products other than beverages |
| 7 | Bakery wares | |
| 7.2 | Fine bakery wares. Cereal bars only | 2.5 g/kg |
| 13 | Foods for Special Groups (FSG) | |
| 13.1 | Foods for infants and young children | |
| 13.1.1 | Infant formula as defined in Regulation (EU) No 609/2013 | 0.2 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer |
| 13.1.2 | Follow‐on formula as defined in Regulation (EU) No 609/2013 | 0.15 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer |
| 13.1.3 | Processed cereal‐based food and baby food for infants and young children as defined in Regulation (EU) No 609/2013 | 0.15 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer |
| 1.25 g/kg for products other than beverages | ||
| 13.1.4 | Milk‐based drinks and similar products intended for young children | 0.15 g/L in the final product ready for use, marketed as such or reconstituted as instructed by the manufacturer |
| 1.25 g/kg for products other than beverages | ||
| 13.2 | Foods for special medical purposes as defined in Regulation (EU) No 609/2013 | |
| 13.2 | Foods for special medical purposes as defined in Regulation (EU) No 609/2013 | On case‐by‐case basis |
| 13.3 | Total diet replacement for weight control as defined in Regulation (EU) No 609/2013 | |
| 13.3 | Total diet replacement for weight control as defined in Regulation (EU) No 609/2013 |
0.5 g/L beverages 5 g/kg products other than beverages |
| 14 | Beverages | |
| 14.1.4 | Flavoured drinks (excluding cola‐type drinks) | 0.25 g/L |
UHT: ultra‐high temperature.
The applicant also intends to market the NF as food supplement, at the maximum daily intake of 0.5 g for individuals above 3 years of age or at a maximum level of 0.2 or 0.15 g/day when intended for infants (up to 11 months) or young children (12–35 months), respectively.
The applicant stated that food supplements are not intended to be used if other foods with added NF or breast milk are consumed on the same day.
The Panel notes that because of the levels of 3’‐SL in cow milk, food supplements intended for infant and young children should also not be used if milk, fermented milk‐based products and selected cheeses retaining milk sugar (e.g. curd cheese) are also consumed on the same day.
3.7.3. Anticipated intake of the NF
Anticipated intake of the NF from the consumption of infant formula in infants up to 16 weeks of age
IF is expected to be the only food consumed by infants aged 0–16 weeks who are not breastfeed. A high consumption of IF has been estimated to be 260 mL/kg bw per day for infants aged 0–16 weeks (EFSA Scientific Committee, 2017). Based on the maximum proposed use level of the NF (0.2 g/L in IF), the high intake of the NF from IF alone is estimated for an infant of 6.7 kg to be 52 mg/kg bw per day.
The Panel notes that the anticipated daily intake of the NF from the consumption of IF (only) does not exceed the estimated high daily intake of 3’‐SL in breast‐fed infants per kg/bw (Table 3).
Anticipated intake of 3’‐SL from the proposed uses and use levels of the NF
The applicant estimated the daily intake of the NF by using the EFSA Food Additive Intake model (FAIM) tool (FAIM 2.0, 2017) and compared the intake to surveys conducted in the UK (NDNS (National Diet and Nutrition Survey) and its complement DNSIYC (Diet and Nutrition Survey of Infants and Young Children)). However, considering that the food categories in the FAIM tool, which are based on Regulation (EC) 1333/20083, do not allow a precise matching with the food categories proposed for the NF, EFSA performed an assessment of the anticipated daily intake of the NF, at the maximum proposed use levels of the NF, using individual data from EU dietary surveys (EFSA, 2011) and by applying the FoodEx2 classification (Table 5). The range of mean and 95th percentile anticipated daily intake of the NF for all subjects, among the EU dietary surveys, is presented in Table 6.
Table 5.
FoodEx2 categories and maximum use levels of the NF used in the refined estimate of the anticipated daily intake of the NF using individual data from EU dietary surveys
| CODE | FoodEx2 level | Food category | Maximum use level of the NF mg/100 g |
|---|---|---|---|
| A02LV | 5 | Cow milk | 25 |
| A0CXA | 5 | European buffalo milk | 25 |
| A02MC | 5 | Sheep milk | 25 |
| A02MB | 4 | Goat milk | 25 |
| A02MV | 3 | Butter milk | 25 |
| A02NQ | 4 | Yoghurt drinks | 25 |
| A02NR | 4 | Probiotic milk‐like drinks | 25 |
| A02NV | 5 | Kefir | 25 |
| A02NE | 4 | Yoghurt | 50 |
| A00EY | 4 | Cereal bars | 250 |
| A00EZ | 4 | Cereal bars plain | 250 |
| A00FA | 4 | Cereal bars mixed | 250 |
| A03PZ | 4 | Infant formulae, powder | 163 |
| A03QE | 4 | Infant formulae, liquid | 20 |
| A03QK | 4 | Follow‐on formulae, powder | 121 |
| A03QQ | 4 | Follow‐on formulae, liquid | 15 |
| A03QZ | 3 | Cereals with an added high protein food which have to be reconstituted | 75 |
| A03QY | 3 | Simple cereals which have to be reconstituted | 75 |
| A0BZF | 3 | Cereals with added high protein food reconstituted | 15 |
| A0BZE | 3 | Simple cereals for infants and children reconstituted | 15 |
| A03RA | 3 | Biscuits, rusks and cookies for children | 125 |
| A03RC | 2 | Ready‐to‐eat meal for infants and young children | 125 |
| A03RB | 3 | Pasta for children | 125 |
| A03RN | 3 | Fruit and vegetable juices and nectars specific for infants and young children | 15 |
| A03RP | 3 | Special food for children's growth | 15 |
| A03RT | 4 | Total daily diet replacement for weight reduction | 500 |
| A0EQN | 5 | Soft drinks with minor amounts of fruits or flavours | 25 |
| A03EA | 5 | Soft drink with fruit juice (fruit content below the minimum for nectars) | 25 |
| A03EX | 5 | Soft‐drink, flavoured, no fruit | 25 |
Table 6.
Ranges among EU surveys of the estimated daily intake of the NF (mg/kg bw), based on the individual food intake data from the EFSA Comprehensive Food Consumption Database
| Age groups | Number of EU dietary surveys | Estimated daily intake of the NF – all subjects (mg/kg bw) | |
|---|---|---|---|
| Range of means (lowest and highest) among EU dietary surveys | Range of 95th percentile (lowest and highest) among EU dietary surveys | ||
| Infants (up to 11 months) | 11 | 8–36 | 19–71 |
| Young children or toddlers (12–35 months) | 14 | 5–20 | 13–70 |
| Other children(3–9 years) | 19 | 2–8 | 5–14 |
| Adolescents (10–17 years) | 18 | 0–3 | 2–7 |
| Adults (18–64 years) | 19 | 0–2 | 1–4 |
| Elderly (≥ 65 years) | 18 | 0–1 | 1–3 |
| Pregnant women | 2 | 1‐2 | 2–4 |
| Lactating women | 2 | 1‐2 | 3‐4 |
bw: body weight.
The refined anticipated daily intake of the NF for each population group from each EU dietary survey is available in the excel file annexed to this scientific opinion (it can be found in the online version of this output under ‘Supporting information’: https://doi.org/10.2903/j.efsa.2020.6098).
The Panel notes that while the estimates for the mean intake are within the natural intake, the highest estimated 95th percentile intake (i.e. 71 mg/kg bw) on the basis of 11 dietary surveys covered by the EFSA Food Consumption Database, is slightly above (approximately 10%) the high estimate for 3’‐SL from human milk (i.e. 64 mg/kg bw) in infants and young children.
Considering the conservative assumption underlying this type of intake assessment, in particular, assuming that all foods of the proposed food categories consumed are added with the NF at the maximum proposed use levels and that 3’‐SL sodium salt accounts for about 90% of the NF, the Panel considers that it is unlikely that infants and young children would exceed the high intake levels estimated for 3’‐SL intake from human milk.
3.7.4. Anticipated intake of 3’‐SL from food supplements
The applicant has proposed a maximum daily intake of 0.5 g of the NF as food supplements for individuals above 3 years of age or at a maximum level of 0.2 or 0.15 g/day when intended for infants (up to 11 months) or young children (12–35 months), respectively. Food supplements are not intended to be used if other foods with added 3’‐SL are consumed on the same day. For infants and young children, food supplements are not intended to be used if breast milk or other foods with added NF or milk, fermented milk‐based products and selected cheeses retaining milk sugar (e.g. curd cheese) are consumed on the same day.
The maximum daily intake from food supplements of the NF (i.e. 0.5 g/day) results in a maximum daily intake ranging from 7 to 22 mg/kg bw in the general population. The maximum dose of 0.2 g/day in infants (bw of 6.7 kg) and 0.15 g/day in young children (bw of 11.9 kg) results in a maximum intake of 30 or 13 mg/kg bw, respectively (default body weight values from EFSA Scientific Committee (2012)).
The Panel notes that the maximum daily intake of 3’‐SL from the use of NF as food supplements (i.e. from 150 mg to 0.5 g/day) for any population category does not exceed the estimated high daily intake of 3’‐SL from human milk calculated for infants on a body weight basis (Table 3).
3.7.5. Combined intake from the NF and other sources
The Panel notes that the NF is not authorised for use in food categories other than those proposed for the NF under assessment. Additional sources for the oligosaccharides contained in the NF will be human breast milk, cow's milk, fermented milk‐based products and selected cheeses retaining milk sugar (e.g. curd cheese). Therefore, food supplements intended for infant and young children are not to be used if breast milk or other foods with added NF or milk, fermented milk‐based products and selected cheeses retaining milk sugar (e.g. curd cheese) are consumed on the same day.
3.8. Absorption, distribution, metabolism and excretion (ADME)
There are no data submitted for the NF.
HMOs, including 3’‐SL, are considered ‘non‐digestible oligosaccharides’ (EFSA NDA Panel, 2014) since they do not undergo any significant digestion in the upper gastrointestinal tract (Brand‐Miller et al., 1995, 1998; Engfer et al., 2000; Gnoth et al., 2000; Chaturvedi et al., 2001; Rudloff and Kunz, 2012).
Brand‐Miller et al. (1995, 1998) reported that HMOs, consumed as a load (a purified oligosaccharide fraction from human milk), are fermented in the colon by intestinal microbiota. Chaturvedi et al. (2001) and Coppa et al. (2001) reported that 97% and 40–50%, respectively, of the ingested HMOs are excreted unchanged in faeces of breastfed infants. Furthermore, approximately 1–2% of the ingested amounts of HMOs is excreted unchanged in the infants’ urine (Rudloff et al., 1996; Goehring et al., 2014; Kunz et al., 2017; EFSA NDA Panel, 2019b,b).
It has been observed that an infant, who is breastfed, may receive up to 150 mg of individual oligosaccharides from each feed and that an amount up to 3 mg per day is found in the urine (Rudloff and Kunz, 2012; Rudloff et al., 2012).
Based on information available on HMOs, the Panel considers that limited digestion of the NF occurs in the upper gastrointestinal tract and that only small amounts are expected to be absorbed. Moreover, there are no indications that the absorption of 3’‐SL or other components of the NF may differ from that of similar components in human milk.
3.9. Nutritional information
The NF is mainly composed by the non‐digestible oligosaccharide 3’‐SL.
The Panel considers that consumption of the NF at the proposed use levels is not nutritionally disadvantageous.
The Panel notes that the NF, being a sodium salt, may contribute to the daily sodium intake.
In its Opinion on DRVs for sodium, the NDA Panel has provided advice on levels of sodium intake that are considered safe and adequate4 for population groups aged 1 year and older (EFSA NDA Panel, 2019a). Considering the maximum sodium content in the NF of 4.5%, the intake of sodium from the NF is expected to represent up to 3% (up to 38 mg sodium/day) of the sodium intake of 1.1 g/day considered as safe and adequate for toddlers (1–3 years). For other children and adults, the intake of sodium from the NF can represent up to 2% of the sodium intake levels considered as safe and adequate for these age groups.
As for infants up to the age of 6 months consuming IF, the maximum sodium intake from the NF would be approximately 16 mg per day considering a daily intake of IF of 260 mL/kg. This corresponds to about 13% of the daily sodium intake of exclusively breast‐fed infants (120 mg sodium/day during the first 6 months; EFSA NDA Panel, 2019c).
For older infants aged 7–11 months, the Panel established an adequate intake (AI)5 of 200 mg/day (EFSA NDA Panel, 2019c). In this age group, the maximum sodium intake from the NF is estimated to be 28 mg sodium/day, which corresponds to 14% of the AI.
3.10. Toxicological information
The list of toxicological studies, which were provided and claimed proprietary by the applicant, is reported in Table 7. These studies were conducted with the NF (batch CPN51151000516FD), which was characterised by 90.3% w/w dry matter of 3’‐SL sodium salt (with a total of 91.5% dry matter of HiMSs).
Table 7.
List of toxicological studies with the NF provided by the applicant
| Test material | Reference | Type of study |
|---|---|---|
| NF (90.3% w/w dry matter 3’‐SL sodium salt) | Unpublished study report (2019a) | Bacterial reverse mutation test (Ames test) |
| Unpublished study report (2019b) | In vitro mammalian Cell Micronucleus Test | |
| Unpublished study report (2019c) | 14‐day DRF repeated dose oral toxicity study in neonatal rats | |
| Unpublished study report (2019d) | 90‐day GLP repeated dose oral toxicity study in neonatal rats |
DRF: dose range finding.
The applicant also provided information on toxicological studies conducted with enzymatically produced 3’‐SL (Kim et al., 2018) and the Panel considers that these toxicological studies can provide supporting evidence for the safety assessment of the NF (see Section 3.10.3). The Panel noted that toxicological studies were also conducted with 6’‐SL, the constitutional isomer of 3’‐SL, which was obtained by microbial fermentation using genetically modified strains of E. coli K12 DH1 MDO.
The Panel also noted that under acidic conditions, the NF will be hydrolysed to lactose and sialic acid. The amount of sialic acid potentially formed (at the maximum level and intake i.e. infants at 95th percentile (Table 6), it would result in 1.1 mg/kg bw) is lower than the intake based on natural levels in human milk (i.e. 11 mg/kg bw (EFSA NDA Panel, 2017)). Under alkaline conditions, 3’‐sialyl‐lactulose would also be formed although the content would remain very low (up to 3.6 mg/kg bw) and considered not having any laxative effect (suggested use in infants to treat constipation is 2.5 mL syrup/day corresponding to approximately 1.65 g lactulose/day).
3.10.1. Genotoxicity
The potential genotoxicity of the NF was investigated in a bacterial reverse mutation test and an in vitro mammalian cell micronucleus test (Unpublished study report, 2019a). These studies were conducted in compliance with Organisation for Economic Co‐operation and Development (OECD) principles of Good Laboratory Practice (GLP) (OECD, 1998a) and in accordance with the OECD test guidelines No 471 and 487 of 1997 and 2016, respectively.
The in vitro assessment of the mutagenic potential of the NF was performed with histidine‐dependent auxotrophic mutants of Salmonella typhimurium, strains TA1535, TA1537, TA98 and TA100, and a tryptophan‐dependent mutant of Escherichia coli, strain WP2 uvrA (pKM101), that were exposed to the NF diluted in water at concentrations up to 5000 μg/plate either in the presence or absence of liver microsomal fractions (S9). No substantial, reproducible or dose‐related increases in revertant colony numbers over control counts were observed with any of the strains following exposure to 3’‐SL at any concentration (irrespective of the presence or absence of S9). No evidence of toxicity was obtained following exposure to the NF. Therefore, the NF was shown to be non‐mutagenic at concentrations up to 5,000 μg/plate, in the absence or presence of metabolic activation.
In the in vitro mammalian cell micronucleus test, concentrations of LNT up to 2,000 μg/mL were tested to assess the potential of 3’‐SL to cause an increase in the induction of micronuclei in in vitro cultured human peripheral blood lymphocytes in the presence or absence of metabolic activation (S9 fraction). No statistically significant increases in the number of binucleate cells containing micronuclei both after 3‐h treatment in the presence of S9 mix or following 20‐h treatment in the absence of S9 were recorded. The NF did not show any evidence of clastogenicity or aneugenicity in the absence and presence of metabolic activation.
Based on the results of these studies, the Panel considers that there are no concerns regarding genotoxicity of the NF.
3.10.2. Repeated dose toxicity studies
The applicant provided a 14‐day repeated dose oral toxicity study where groups of eight Crl:CD(SD) neonatal rats/sex were given water (control), 4,000 and 5,000 mg/kg bw per day of 3’‐SL sodium salt by oral gavage starting from day 7 of age (Unpublished study report, 2019c). There were no deaths or any variations in clinical signs, body weight or macroscopic pathology attributable to the NF. Clinical pathology parameters were not assessed.
In the 90‐day study groups of 10 Crl:CD(SD) neonatal rats/sex were given water (control), 1,000, 3,000 or 5,000 mg/kg bw per day of NF expressed as 3’‐SL sodium salt by oral gavage starting from day 7 of age once daily for at least 90 days, until the day before necropsy (Unpublished study reports, 2018d). An additional control group was treated with oligofructose powder (a non‐digestible oligosaccharide permitted in infant nutrition) at 5,000 mg/kg per day, to compare any effects related to the general fibre‐like characteristics at the same high dose. Five additional rats/sex for control groups and high dose of NF were observed over a 4‐week recovery period.
This study has been designed based upon the OECD TG408 (OECD, 1998ab), but has been adapted (i.e. use of juvenile animals) to consider the requirements for toxicity testing of new chemical entities for use in the paediatric population (as was suggested by US FDA, 2006 and EMEA, 2008). There were no test item‐related deaths or any variations in clinical signs, functional observation battery tests, macroscopic and microscopic examinations attributable to the NF.
Statistically significant changes have been observed in some parameters (summary results can be found in the online version of this output under ‘Supporting information’: https://doi.org/10.2903/j.efsa.2020.6098). However, the findings observed were of low magnitude, often without dose correlation, sometimes occurring only in one gender or at the end of recovery period and are considered by the Panel as overall not biologically relevant. The Panel also notes that this specific experimental design, with treatment of animals by gavage prior to weaning, may result in high variability in several parameters.
The author of the study concluded that under the experimental condition applied the high dose of 5,000 mg/kg bw per day of 3’‐SL is considered as the No Observed Adverse Effect Level (NOAEL). The Panel agrees with this conclusion.
3.10.3. Toxicological studies conducted with 3’‐SL enzymatically synthetised
The applicant reported that in studies conducted following ‘Toxicological Principles for the Safety Assessment of Food Ingredients’ (FDA ‘Redbook 2000’) and in accordance with GLP principles, 3’‐SL (purity of 98.8%) did not elicit adverse effects in any of the tested doses or concentrations. No signs of mutagenicity, clastogenicity or aneugenicity in genotoxicity studies (Bacterial mutation assay, chromosomal aberrations assay and in vivo micronucleus) were noted. In the 28‐day and 90‐day oral study in the rat (weaned rats of standard age), the highest dose of 2,000 mg/kg bw per day was identified as a NOAEL. The authors concluded that ‘The 3’‐SL sodium salt…shows toxicity profiles that are comparable to other carbohydrates and HMOs’ (Kim et al., 2018).
Table 8.
List of toxicological studies conducted with 3’‐SL produced by enzymatic synthesis
| Test material | Reference | Type of study | Experimental details |
|---|---|---|---|
| 3’‐SL (98.8%) | Kim et al. (2018) | Bacterial Reverse Mutation Test | 5, 10, 50, 100, 250, 500, 1,000, 2,500 and 5,000 μg 3’‐SL mL; ± S9 |
| In vitro chromosome aberration test (CHL) | 5, 10, 50, 100, 250, 500, 1,000, 2,500 and 5,000 μg 3’‐SL/mL; ± S9 | ||
| In vivo mammalian Cell Micronucleus Test | 500, 1,000 and 2,000 mg 3’‐SL/kg bw | ||
| Acute Toxicity Study in the SD Rat | 5 SD rats/sex per group; 5,000, 10,000, 15,000 and 20,000 mg 3’‐SL/kg bw per day | ||
| 28‐day GLP repeated dose oral toxicity study in the SD rat | 10 SD rats/sex per group; 500, 1,000, 2,000 mg 3’‐SL/kg bw per day | ||
| 90‐day GLP repeated dose oral toxicity study in the SD rat | 10 SD rats/sex per group; 500, 1,000, 2,000 mg 3’‐SL/kg bw per day |
CHL: Chinese hamster lung; SD: Sprague‐Dawley.
The Panel considers the information on 3’‐SL when produced by enzymatic synthesis as supportive for the assessment of the NF.
3.10.4. Human data
No human intervention studies with 3’‐SL have been provided by the applicant and no reference to human data was made.
3.11. Allergenicity
The protein content in the NF is low as indicated in the specifications (Table 2).
In addition, the applicant has assessed the allergenic potential of introduced proteins as a result of the genetic modification of the E. coli K‐12 host (which itself is recognised as non‐allergenic) using the search algorithms provided by the Allergen Online tool (version 17) of the University of Nebraska (FARRP, 2017). No sequence alerts for potential allergenicity were identified.
The Panel considers that the likelihood of allergenic reactions to the NF is low.
4. Discussion
The NF is mainly constituted of the sodium salt of a HiMO, 3’‐SL and other minor fractions (e.g. sialic acid and 3’‐sialyl‐lactulose). The NF is obtained by microbial fermentation with a genetically modified strain of E. coli K‐12 DH1 MDO. The information provided on the manufacturing process, composition and specifications of the NF, including the absence of DNA from the producing microorganisms, does not raise safety concerns.
The applicant intends to add the NF to a variety of foods, including IF and follow‐on formula, foods for infants and young children, foods for special medical purposes and food supplements. The target population is the general population.
The Panel notes that under acidic condition, the NF will be hydrolysed to lactose and sialic acid and it will not meet the specifications. The same would apply for 3’‐sialyl‐lactulose formed under alkaline conditions.
The Panel considers that there are no concerns regarding genotoxicity of the NF. The highest dose of 5,000 mg/kg bw per day used in the 90‐day oral toxicity study conducted in neonatal rats was considered as the NOAEL. When comparing the NOAEL from the 90‐day toxicity study with the estimated exposure per population category (at 95th percentile; Table 6), the margins of exposure are ranging from 50 to 800. It is also noted that with substances of this nature, the maximum feasible doses that can be used in subchronic studies (e.g. because of risk of nutritional imbalance) are only able to ensure a relatively low safety margin with respect to the highest estimated daily intakes in the intended population.
Considering that 3’‐SL is a naturally occurring oligosaccharide present in human milk, the history of human exposure to 3’‐SL concerns breast‐fed infants. 3’‐SL levels are relatively low in the breast milk and the most abundant acidic HMO is its constitutional isomer 6’‐SL. The Panel notes that an assessment of 3’‐SL when enzymatically produced has been published (Kim et al., 2018). The Panel also noted that other HiMOs (2’‐FL and DFL, LNT) produced by fermentation using the same E. coli strain and genotype have been recently assessed (EFSA NDA Panel, 2019b,b).
The Panel notes that the anticipated daily intake of 3’‐SL in the NF from the consumption of IF only, in infants up to 16 weeks of age, does not exceed the highest intake level of 3’‐SL in breastfed infants on a body weight basis. The anticipated daily intake of the NF for the proposed uses at their respective maximum use levels in the other population categories is unlikely to exceed the highest intake level of 3’‐SL in breastfed infants on a body weight basis. Thus, since the intake in breastfed infants on a body weight basis is expected to be safe also for other population groups, the Panel considers that the intake of the NF for the proposed uses at their respective maximum use levels can be considered safe. The maximum daily intake of the 3’‐SL as a food supplement at the proposed maximum levels (i.e. from 150 mg to 500 mg/day) for the respective population categories also does not exceed the highest intake level of 3’‐SL in breastfed infants per kg bw. Food supplements are not intended to be used if other foods with added NF (as well as breast milk, milk, fermented milk‐based products and selected cheeses retaining milk sugar (e.g. curd cheese) for infants and young children) are consumed on the same day.
In terms of foods for special medical purposes, the applicant did not propose maximum use levels and the Panel considers that the maximum use levels of the NF should not lead to higher daily intakes than those estimated on the basis of the maximum levels specified for the proposed food uses or the maximum daily intake proposed for food supplements.
It is finally noted that, as with other oligosaccharides, which are natural components of human milk, the safety assessment is mainly based on the comparison between the natural intake in breastfed infants and the estimated intake as NF. The same considerations apply for lactose and other mono‐ and oligosaccharides (i.e. sialic acid) that are only present as a very small fraction in the NF and considered of no safety concern. 3’‐sialyl‐lactulose that is not a natural milk component is characterised by very low concentrations not having any laxative effect.
5. Conclusions
The Panel concludes that the NF, composed of 3’‐SL sodium salt and other structurally related mono‐ and oligosaccharides, is safe under the proposed conditions of use, including the use as a food supplement. The target population is the general population.
Food supplements are not intended to be used if other foods with added NF (as well as breast milk, milk, fermented milk‐based products and selected cheeses retaining milk sugar (e.g. curd cheese) for infants and young children) are consumed on the same day.
The Panel could not have reached the conclusions on the safety of the NF under the proposed conditions of use without the following data claimed as proprietary by the applicant:
annexes to the dossier which relate to the identity, the production process, composition and specifications of the NF (see annexes indicated in Section 2.1).
bacterial reverse mutation test (unpublished study report, 2019a), in vitro micronucleus test (Unpublished study report, 2019b) and 90‐day oral toxicity study with the NF (Unpublished study report, 2019d) including the summary table of the statistically significant observations in the 90‐day study (Appendix B.3 to the dossier).
Steps taken by EFSA
Letter from the European Commission to the European Food Safety Authority with the request for a scientific opinion on the safety of 3’‐SL sodium salt as a novel food. Ref. Ares (2019)3757466, dated 12/06/2019.
On 12/06/2019, a valid application from the European Commission on 3’‐SL as NF, which was submitted by Glycom A/S, was made available to EFSA by the European Commission through Commission e‐submission portal (NF 2019/0881) and the scientific evaluation procedure started.
On 12/09/2019 and on 16/12/2019, EFSA requested the applicant to provide additional information to accompany the application and the scientific evaluation was suspended.
On 02/12/2019 and 20/01/2020, additional information was provided by the applicant and the scientific evaluation was restarted.
During its meeting on 25/03/2020, the NDA Panel, having evaluated the data, adopted a scientific opinion on the safety of 3’‐SL sodium salt as a NF pursuant to Regulation (EU) 2015/2283.
Abbreviations
- 2’‐FL
2’‐Fucosyllactose
- 3’‐SL
3’‐Sialyllactose
- 6’‐SL
6’‐Sialyllactose
- ACVA
4,4’‐azobis (cyanovaleric acid)
- ADME
absorption, distribution, metabolism and excretion
- bw
body weight
- CAD
charged aerosol detection
- CFU
colony forming units
- Da
Daltons
- DFL
difucosyllactose
- DSMZ
German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen)
- EMEA
European Medicines Agency
- EU
endotoxin units
- Eur.Ph.
European pharmacopeia
- FAIM
Food Additive Intake Model
- FDA
US Food and Drug Administration
- Gal
Galactose
- Glc
Glucose
- GLP
Good Laboratory Practice
- GMP
Good Manufacturing Practice
- HACCP
Hazard Analysis Critical Control Points
- HiMS
human identical milk saccharides
- HiMO
human identical milk oligosaccharides
- HMO
human milk oligosaccharide
- HPAEC
high performance anion exchange chromatography
- HPLC/CAD
high performance liquid chromatography/charged aerosol detection
- HMBC
heteronuclear multiple bond correlation
- HSQC
heteronuclear single quantum coherence
- IC
Ion chromatography
- IF
infant formula
- ISO
international organization for standardisation
- K‐F
Karl Fischer
- LNT
lacto‐N‐tetraose
- LNnT
lacto‐N‐neotetraose
- LOR
limit of reporting
- MS
mass spectrometry
- NANA
N acetyl neuraminic acid
- NDA
The EFSA Panel on Nutrition, Novel Foods and Food Allergens
- NDNS
National Diet and Nutrition Survey
- Neu5Ac
N‐acetylneuraminic acid
- NF
novel food
- NOAEL
no observed adverse effect level
- NOESY
Nuclear Overhauser Effect Spectroscopy
- NMR
nuclear magnetic resonance spectroscopy
- OECD
Organisation for Economic Co‐operation and Development
- PAD
pulsed amperometric detection
- PCR
Polymerase chain reaction
- QPS
qualified presumption of safety
- RH
relative humidity
- RT
retention time
- UHT
Ultra‐high temperature
- UV
ultraviolet
Supporting information
Summary results of the 90‐day repeated dose toxicity study in the rat with the NF
Daily intake of the NF (mg/kg bw)
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Castenmiller J, De Henauw S, Hirsch‐Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Engel KH, Frenzel T, Heinonen M, Marchelli R, Neuhäuser‐Berthold M, Poulsen M, Schlatter JR, van Loveren H, Colombo P and Knutsen HK, 2020. Scientific Opinion on the safety of 3’‐Sialyllactose (3’‐SL) sodium salt as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2020;18(5):6098, 22 pp. 10.2903/j.efsa.2020.6098
Requestor: European Commission following an application by Glycom A/S
Question number: EFSA‐Q‐2019‐00204
Panel members: Dominique Turck, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, John Kearney, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Acknowledgements: The Panel wishes to thank Petra Gergelova and Roman Švejstil for the support provided to this scientific opinion.
Adopted: 25 March 2020
Notes
Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) N0 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 (2013/0435 (COD). OJ L 327, 11.12.2015, p. 1–22.
Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, pp. 64–71.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives (Text with EEA relevance) OJ L 354, 31.12.2008, p. 16–33.
These levels of sodium intake are called ‘safe’ because it takes account of the evidence describing the relationship between sodium intake and CVD risk in the general population and ‘adequate’ in line with the definition of an Adequate Intake.
An Adequate Intake (AI) is the value set when there is not enough data to calculate an average requirement for a nutrient. An AI is the average level of intake of a nutrient, based on observations or experiments, that is assumed to be adequate.
References
- Albrecht S, Lane JA, Marino K, Al Busadah KA, Carrington SD, Hickey RM and Rudd PM, 2014. A comparative study of free oligosaccharides in the milk of domestic animals. British Journal of Nutrition, 111, 1313–1328. [DOI] [PubMed] [Google Scholar]
- Aldredge DL, Geronimo MR, Hua S, Nwosu CC, Lebrilla CB and Barile D, 2013. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology, 23, 664–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakuma S, Akahori M, Kimura K, Watanabe Y, Nakamura T, Tsunemi M, Arai I, Sanai Y and Urashima T, 2007. Sialyl oligosaccharides of human colostrum: changes in concentration during the first three days of lactation. Bioscience, biotechnology and biochemistry, 71, 1447–1451. [DOI] [PubMed] [Google Scholar]
- Austin S, De Castro CA, Benet T, Hou Y, Sun H, Thakkar SK, Vinyes‐Pares G, Zhang Y and Wang P, 2016. Temporal change of the content of 10 oligosaccharides in the milk of Chinese urban mothers. Nutrients, 8, 346. 10.3390/nu8060346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner FR, Plunkett G 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado‐Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B and Shao Y, 1997. The complete genome sequence of Escherichia coli K‐12. Science, 277, 1453–1462. [DOI] [PubMed] [Google Scholar]
- Bode L, 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology, 22, 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand‐Miller JC, McVeagh P, McNeil Y and Gillard B, 1995. Human milk oligosaccharides are not digested and absorbed in the small intestine of young infants. Proceedings of the Nutrition Society of Australia, 19, 44. [Google Scholar]
- Brand‐Miller JC, McVeagh P, McNeil Y and Messer M, 1998. Digestion of human milk oligosaccharides by healthy infants evaluated by the lactulose hydrogen breath test. The Journal of Pediatrics, 133, 95–98. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Warren CD, Buescher CR, Pickering LK and Newburg DS, 2001. Survival of human milk oligosaccharides in the intestine of infants. In Newberg DS (ed). In: Bioactive components of human milk. (Advances in experimental medicine and biology, volume 501). Springer Science+Business Media, New York, NY. pp. 315–323. [DOI] [PubMed] [Google Scholar]
- Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A and Gabrielli O, 1999. Oligosaccharides in human milk during different phases of lactation. Acta Paediatrica, suppl, 430, 89–94. [DOI] [PubMed] [Google Scholar]
- Coppa GV, Pierani P, Zampini L, Bruni S, Carloni I and Gabrielli O, 2001. Characterization of oligosaccharides in milk and faeces of breast‐fed infants by high‐performance anion‐exchange chromatography. Advances in Experimental Medicine and Biology, 501, 307–314. [DOI] [PubMed] [Google Scholar]
- Coppa GV, Gabrielli O, Zampini L, Galeazzi T, Ficcadenti A, Padella L, Santoro L, Soldi S, Carlucci A, Bertino E and Morelli L, 2011. Oligosaccharides in 4 different milk groups, Bifidobacteria, and Ruminococcus obeum. Journal of Pediatric Gastroenterology and Nutrition, 53, 80–87. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2018. Statement on the update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA 7: suitability of taxonomic units notified to EFSA until September 2017. EFSA Journal 2018;16(1):5131, 43 pp. 10.2903/j.efsa.2018.5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2018. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2013. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA Journal 2013;11(10):3408, 103 pp. 10.2903/j.efsa.2013.3408 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2014. Scientific Opinion on the essential composition of infant and follow‐on formulae. EFSA Journal 2014;12(7):3760, 106 pp. 10.2903/j.efsa.2014.3760 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015. Scientific opinion on the safety of lacto‐N‐neotetraose as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFSA Journal 2015;13(7):4183, 32 pp. 10.2903/j.efsa.2015.4183 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016. Guidance on the preparation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA Journal 2016;14(11):4594, 24 pp. 10.2903/j.efsa.2016.4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2017. Scientific Opinion on the safety of synthetic N‐acetyl‐D‐neuraminic acid as a novel food pursuant to Regulation (EC) No 258/97. EFSA Journal 2017;15(7):4918, 28 pp. 10.2903/j.efsa.2017.4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2019a. Safety of lacto‐N‐tetraose (LNT) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2019b;17(12):5907, 27 pp. 10.2903/j.efsa.2019.5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2019b. Safety of 2’‐fucosyllactose/difucosyllactose mixture as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2019;17(6):5717, 23 pp. 10.2903/j.efsa.2019.5717 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens), 2019c. Scientific Opinion on the dietary reference values for sodium. EFSA Journal 2019;17(9):5778, 191 pp. 10.2903/j.efsa.2019.5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age (Question no EFSA‐Q‐2016‐00489, adopted: 26 April 201). EFSA Journal 2017;15:4849, 58 pp. 10.2903/j.efsa.2017.4849. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMEA , 2008. Guideline on the need for non‐clinical testing in juvenile animals of pharmaceuticals for paediatric indications. Ref. EMEA/CHMP/SWP/169215/2005.
- Engfer MB, Stahl B, Finke B, Sawatzki G and Daniel H, 2000. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. The American Journal of Clinical Nutrition, 71, 1589–1596. [DOI] [PubMed] [Google Scholar]
- FAIM (Food Additives Intake Model) 2.0, 2017. Available online http://www.efsa.europa.eu/en/applications/
- FARRP (Food Allergy Research and Resource Program), 2017. AllergenOnline version 17: home of the FARRP allergen protein database. University of Nebraska‐Lincoln, Food Allergy Research and resource Program (FARRP), Lincoln, NE. Available online: http://www.allergenonline.org/ [Accessed: 18 January 2017].
- Gnoth MJ, Kunz C, Kinne‐Saffran E and Rudloff S, 2000. Human milk oligosaccharides are minimally digested in vitro . The Journal of Nutrition, 130, 3014–3020. [DOI] [PubMed] [Google Scholar]
- Goehring KC, Kennedy AD, Prieto PA and Buck RH, 2014. Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PLoS ONE, 9, e101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach SL, 1978. Recombinant DNA: an infectious disease perspective. Journal of Infectious Diseases, 137, 615–623. Available online: http://www.fasebj.org/content/30/1_Supplement/671.4?relatedurls=yes&legid=fasebj;30/1_Supplement/671.4 [DOI] [PubMed] [Google Scholar]
- Kim D, Gurung RB, Seo W, Lee AW and Woo J, 2018. Toxicological evaluation of 3’‐sialyllactose sodium salt. Regulatory Pharmacology and Toxicology, 94, 83–90. [DOI] [PubMed] [Google Scholar]
- Kunz C, Meyer C, Collado MC, Geiger L, Garcıa‐Mantrana I, Bertua‐Rıos B, Martınez‐Costa C, Borsch C and Rudloff S, 2017. Influence of gestational age, secretor, and lewis blood group status on the oligosaccharide content of human milk. JPGN, 64, 789–798. [DOI] [PubMed] [Google Scholar]
- Lukjancenko O, Wassenaar TM and Ussery DW, 2010. Comparison of 61 sequenced Escherichia coli genomes. Microbial Ecology, 60, 708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, Kamau‐Mbuthia EW, Kamundia EW, Mbugua S, Moore SE, Prentice AM, Kvist LJ, Otoo GE, Brooker SL, Price WJ, Shafii B, Placek C, Lackey KA, Robertson B, Manzano S, Ruız L, Rodrıguez JM, Pareja RG and Bode L, 2017. What's normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. The American Journal of Clinical Nutrition, 105, 1086–1100[plus supplementary data]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhldorfer I and Hacker J, 1994. Genetic aspects of Escherichia coli virulence. Microbial Pathogenesis, 16, 171–181. [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 1986. Recombinant DNA safety considerations [“Blue Book”]. Available online: https://www.oecd.org/sti/biotech/40986855.pdf
- OECD (Organisation for Economic Co‐operation and Development), 1997. Test No. 471: Bacterial reverse mutation test. In: OECD guidelines for the testing of chemicals, Section 4: Health effects, 11 pp.
- OECD (Organisation for Economic Co‐operation and Development), 1998a. OECD Principles of good laboratory practice (as revised in 1997). OECD series on principles of good laboratory practice and compliance monitoring, number 1, ENV/MC/CHEM(98)17, 41 pp.
- OECD (Organisation for Economic Co‐operation and Development), 1998b. Test No. 408: Repeated dose 90‐day oral toxicity study in rodents. In: OECD guidelines for the testing of chemicals, Section 4: Health effects, 10 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2016. Test No. 487: In vitro mammalian cell micronucleus test. In: OECD guidelines for the testing of chemicals, Section 4: Health effects, 29 pp.
- Rijnierse A, Jeurink PV, van Esch BCAM, Garssen J and Knippels LMJ, 2011. Food‐derived oligosaccharides exhibit pharmaceutical properties. European Journal of Pharmacology, 668, S117–S123. [DOI] [PubMed] [Google Scholar]
- Röhrig CH, Choi SSH and Baldwin N, 2017. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Critical Reviews in Food Science and Nutrition, 57, 1017–1038. 10.1080/10408398.2015.1040113 [DOI] [PubMed] [Google Scholar]
- Rudloff S and Kunz C, 2012. Milk oligosaccharides and metabolism in infants. American Society for Nutrition. Advances in Nutrition, 3, 398S–405S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudloff S, Pohlentz G, Diekmann L, Egge H and Kunz C, 1996. Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta Paediatrica, 85, 598–603. [DOI] [PubMed] [Google Scholar]
- Rudloff S, Pohlentz G, Borsch C, Lentze M and Kunz C, 2012. Urinary excretion of in vivo 13C‐labelled milk oligosaccharides in breastfed infants. British Journal on Nutrition, 107, 957–963. [DOI] [PubMed] [Google Scholar]
- Spevacek AR, Smilowitz JT, Chin EL, Underwood MA, German JB and Slupsky CM, 2015. Infant maturity at birth reveals minor differences in the maternal milk metabolome in the first month of lactation. Journal of Nutrition, 145, 1698–1708[plus supplemental tables]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr AJ, Jeffrey D. Galley JD, Fisher S, Chichlowski M, Berg BM and Bailey MT, 2015. The prebiotics 3′Sialyllactose and 6′Sialyllactose diminish stressor‐induced anxiety‐like behavior and colonic microbiota alterations: evidence for effects on the gut‐brain axis. Brain, Behavior, and Immunity, 50, 166–177. 10.1016/j.bbi.2015.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurl S, Munzert M, Henker J, Boehm G, Muller‐Werner B, Jelinek J and Stahl B, 2010. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. British Journal of Nutrition, 104, 1261–1271. [DOI] [PubMed] [Google Scholar]
- Thurl S, Munzert M, Boehm G, Matthews C and Stahl B, 2017. Systematic review of the concentrations of oligosaccharides in human milk. Nutrition Reviews, 75, 920–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unpublished study report , 2018a. 6’‐O‐sialyllactose, sodium salt: bacterial reverse mutation test. [Confidential]. (Study No: SS49JP).
- Unpublished study report , 2018b. 6’‐O‐Sialyllactose, sodium salt: in vitro micronucleus test in human lymphocytes. [Confidential]. (Study No: KX89DV).
- Unpublished study report , 2018c. 6’‐O‐Sialyllactose, sodium salt (6’‐SL): 14‐Day Dose Range Finding Study in the Neonatal Crl:CD(SD) Rat by Oral (Gavage) Administration. [Confidential]. (Study No: DD50XF).
- Unpublished study report , 2018d. 6’‐O‐sialyllactose, sodium salt (6’‐SL): 90‐day toxicity study in the neonatal Crl:CD(SD) Rat by Oral (Gavage) administration followed by a 4‐week recovery period. [Confidential]. (Study No: PK34SR).
- Unpublished study report , 2019a. (amended). 3’‐O‐sialyllactose, sodium salt: bacterial reverse mutation test. [Confidential]. (Study No: LG34PH).
- Unpublished study report , 2019b. (amended). 3’‐O‐Sialyllactose, sodium salt: In vitro Micronucleus Test in Human Lymphocytes. [Confidential]. (Study No: JW24VG).
- Unpublished study report , 2019c. (amended). Lacto‐N‐tetraose (LNT) and 3’‐O‐Sialyllactose (3’‐SL): 14‐day dose range finding study in the neonatal Crl:CD(SD) rat by oral (gavage) administration. [Confidential]. (Study No: LQ48WR).
- Unpublished study report , 2019d. (amended). 3’‐O‐sialyllactose (3’‐SL): 90‐day toxicity study in the neonatal Crl:CD(SD) rat by oral (gavage) administration followed by a 4‐week recovery period. Final report: [Confidential]. (Study No: NY76DJ).
- Urashima T, Taufik E, Fukuda K and Asakuma S, 2013. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Bioscience, Biotechnology, and Biochemistry, 77, 455–466. [DOI] [PubMed] [Google Scholar]
- Urashima T, Yamaguchi E, Ohshima T, Fukuda K and Saito T, 2018. Chemical structures of oligosaccharides in milk of the raccoon (Procyon lotor). Glycoconjugate Journal, 35, 275–286. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency), 1997. Escherichia coli K‐12 final risk assessment: attachment I–final risk assessment of Escherichia coli K‐12 derivatives. Available online: xxx [Accessed: 27 September 2012].
- US FDA , 2006. Guidance for industry: nonclinical safety evaluation of paediatric drug products. CDER February 2006.
- Zeng J, Hu Y, Jia T, Zhang R, Su T, Sun J, Gao J, Li G, Cao M and Song M, 2018. Chemoenzymatic synthesis of sialylated lactuloses and their inhibitory effects on Staphylococcus aureus. PLoS ONE, 13, e0199334. 10.1371/journal.pone.0199334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary results of the 90‐day repeated dose toxicity study in the rat with the NF
Daily intake of the NF (mg/kg bw)


