Abstract
Energy production and storage has become a pressing issue in recent decades and its solutions bring new problems. This paper reviews the literature on the human and environmental risks associated with the production, use, and disposal of increasingly common lithium-ion batteries. Popular electronic databases were used for this purpose focused on the period since 2000. Assessment of the toxicological and environmental impact of batteries should then have a holistic scope to precede and guide the introduction of appropriate safety measures. In this short review the authors will try to touch upon this complex subject and point out some important issues related to an unprecedented development of lithium ion batteries-powered world. Given the multi-billion dollar business with the risks associated with the development of new technologies requires careful consideration of whether the balance of profits and losses is beneficial to humans and the planet. Int J Occup Med Environ Health. 2023;36(1):3–20
Keywords: occupational exposure, lithium, environment, technology, waste management, electric power supplies
INTRODUCTION
Drive to mobility and dependency on technology, which accompanies people most of the time, result in a growing need for portable power sources. Lithium-ion batteries (LIBs) are currently the most common technology used in portable electronics, electric vehicles as well as aeronautical, military, and energy storage solutions. European Commission estimates the lithium batteries market to be worth ca. EUR 500 million a year in 2018 and reach EUR 3–14 billion a year in 2025. This rapid growth is, to a large extent, driven by the growing needs of plug-in hybrid (PHEV) and electric vehicles (EVs), together with stationary storage industries [1]. In 2019 2.1 million electric cars were sold and this number is predicted to grow by a factor of 10 in 2040 [2].
Batteries produced in 2018 could store about 290 gigawatt-hours (GWh), while 2028 is anticipated to expand it to >2 terawatt-hour (TWh) [3]. Tesla predicts that a complete transition to electric-powered cars will require 10 TWh of battery capacity/year (ca. 100-fold growth from current status) and another 10 TWh/year (ca. 1600-fold growth) to fulfill the electricity consumption by other activities [4]. The dominating cell size/form factor is 18650 type, powering, e.g., Tesla S and X model EVs, which can store 10–13 watt-hour (Wh) of energy [5]. Coarse calculations then predict their production to rise up to 80 billion cells/year during the next 8 years. Concerning life span of 3–8 years batteries create new persistent waste stream, and earlier analyses predicted 25 billion units to become waste in 2020 in China only [6]. “The Global E-waste Monitor 2020” published by UNITAR reports 4.7 million metric tons (Mt) of small IT and telecommunication equipment waste produced globally in 2019 [7].
Taking into account that, e.g., 21.2% of mobile phone weight on averages is its battery [8] significant part of this waste stream consists of (often non-removable) batteries. If these devices 8.8% share in global e-waste production will not change, it will result in ca. 6.6 Mt of waste in 2030. Noteworthy is that rapport do not include cells from EVs or power storage solutions into any of mentioned e-waste types. It does state however that only 17.4% of e-waste in 2019 was collected and recycled in a documented way, which raises a question of the environmental fate of the remaining majority. These volumes illustrate a scale of mounting risks and challenges associated with a) sourcing raw materials, b) production, c) safety of use and d) recycling/repurposing of used batteries.
METHODS
Scoping literature review, used to identify the scope or coverage of a body of literature on a given topic is useful for examining emerging evidence when it is still unclear what other, more specific questions can be posed and valuably addressed by a more precise systematic review. Scoping literature review is conducted to explore more general research question. The following electronic databases were used for this purpose: Medline, Google Scholar, FreeFull PDF, MedNar, Science Research, World Wide Science, DOI. The literature review focused on the period since 2000. In some aspects older publications, e.g., reports on toxicity, were also used. The review concerned papers published in English. In this review, the authors will try to address a number of issues related to the unprecedented development of energy storage technology i.e., a world powered by lithium-ion batteries.
RESULTS
Lithium battery components
Lithium-ion cell consists of 3 main parts: cathode, anode and a separator, all immersed in the electrolyte. Additional elements include current collectors, made of aluminum for the cathode and copper for the anode, as well as the casing made of Fe-Ni alloy, aluminum or plastic [9]. Container material does not affect battery properties and consists of readily recyclable and stable compounds. Anode, cathode, separator and electrolyte are, on the other hand, crucial for the cell cycling (charging/ discharging) process. Their components, with an emphasis on metals building cathode, are also less indifferent to health and environment if not treated correctly.
Separator allows the flow of lithium ions between the cathode and anode, and prevent the flow of electrons (insulate against short circuiting) [10]. It is most commonly made of microporous polypropylene (PP), polyethylene (PE) or PE/PP membrane. Barrier made of these semi-crystaline polyolefins, or other materials (non-woven fabric mats, inorganic composites or their modifications) has to characterize with optimal permeability and mechanical strength to stop electrode particles and contain enough electrolyte [9]. Together with the separator, sandwiched between the 2 electrodes is lithium-containing electrolyte.
Lithium salts dissolved in the electrolyte are the primary source of positive lithium ions (Li+). Their movement between anode and cathode (through the electrolyte and separator) allows electron flow between positive and negative current collectors and so battery charging and discharging. Lithium, in the form of conductive LiPF6, Li[N(CF3 SO2)2], LiBF4, Li(CF3SO3), LiClO4 or LiAsF6 salts makes up for 1.2–2.0% of battery mass [9,11]. These solid/ crystalline substances are dissolved in low viscosity, high conductivity cyclic or linear carbonate, e.g., dimethyl carbonate, ethyl methyl carbonate, diethyl carbonate, propylene carbonate, ethylene carbonate or γ-butyrolactone, in case of liquid electrolytes. This type of electrolyte usually contains numerous additives, including Li deposition improvers (fluoroethylene carbonate), shutdown additives (cyclohexylbenzene or biphenyl), fire-retardants, overcharge protectors (anisole derivatives), surface-film-forming additives (vinylene or vinylethylene carbonates), cathode protection agents (dimethyl acetamide, silicones or tributylamine) or stabilizers protecting Li salt decomposition [7,9]. Additives also help to prevent decreasing of solvent liquidity (e.g., in low temperatures), which lowers Li-ion cell performance. Solid inorganic (lithium lanthanum zirconate, amorphous lithium phosphorous oxynitride sulfidic glasses) or polymer (solvent-free polyethylene oxide) electrolyte technologies, aided by different additives, start to penetrate the market, although the most popular still include liquid ones.
Anode (negative) and cathode (positive electrode) temporarily bind/release Li ions and their chemical characteristics strongly affects lithium-ion cell properties (energy density, capacity etc.). During discharge Li+ released from metallic lithium, stored between graphite layers of anode, travel to cathode and forms metal oxides. To fuse electrode material to respective collector, inert binder, like polyvinylidene fluoride (PVDF) is used. Most popular types of cathode and anode chemistries, together with some of their properties are listed in Table 1.
Table 1.
Li-ion batteries types according to cathode/anode material chemistry in 2020-2021 (based on [11,82,83])
| Active compound | Short name | Chemical formula | Commercialization year | Cycle life* | Application | Comment |
|---|---|---|---|---|---|---|
| Cathode | ||||||
| lithium cobalt oxide | LCO | LiCoO2 | 1991 | 500–1000 | mobile phones, tablets, laptops, cameras | very high specific energy; limited specific power; high cost due to Co use; currently less relevant |
| lithium iron phosphate | LFP | LiFePO4 | 1996 | >2000 | portable and stationary equipment needing high load currents and endurance | very flat voltage discharge curve; low capacity; one of safest Li-ions; used for special markets (primarily energy storage); elevated self-discharge |
| lithium manganese oxide | LMO | LiMn2O4 | 1999 | 300–700 | power tools, medical devices, electric powertrains (Nissan Leaf) | high power but less capacity; safer than LCO; commonly mixed with NMC to improve performance; currently less relevant |
| lithium nickel cobalt aluminum oxide | NCA | LiNiCoAlO2 | 1999 | 500 | medical devices, industrial, electric powertrain (Tesla model S) | shares similarities with Li-cobalt; serves as Energy Cell; good life span |
| lithium nickel manganese cobalt oxide | NMC | LiNixMnyCozO2** | 2008 | 1000–2000 | e-bikes, medical devices, EVs, industrial, energy storage (Tesla Powerwall and Powerpack) | provides high capacity and high power; serves as Hybrid Cell; dominant cathode chemistry |
| Anode | ||||||
| graphite | 1991 | dominant anode chemistry | low cost; high energy density | |||
| tin/cobalt alloy | 2005 | consumer electronics | better capacity than graphite | |||
| lithium titanate | LTO | Li4Ti5O12 | 2008 | 3000–7000 | UPS, electric powertrain (Mitsubishi i-MiEV, Honda Fit), solar-powered street lighting | long life, fast charge, wide temperature range; low specific energy; among safest Li-ion batteries; ability to ultra-fast charge; cost limiting to special applications |
| hard carbon | 2013 | home electronics | improved storage capacity | |||
| silicon/carbon | 2013 | smartphones | contains silicon nanowires |
Number of charge-discharge cycles is related to a number of conditions (e.g., depth of discharge or working temperature).
X, y and z values indicate proportions between Ni, Mn and Co, which in most cases sum up to 1; e.g., LiNi0.8Mn0.1Co0.1O2 is shortened to NMC811.
Graphite or other carbon forms (e.g., amorphous) are the most prevalent anode material. Lithium titanate (Li4Ti5O12, LTO), lithium alloys and lithium metal as well as lithium metal nitrides, transitional metal vanadates and nanocomposites (e.g., silicone nanowires) make their way into new designs and promise to improve their performance [9,12]. Binders allowing electrode layer formation may include carboxymethyl cellulose and styrene-butadiene rubber [13]. Anode accounts for a 15–30% of a battery weight, which includes a copper collector [14].
Most widespread cathode materials include transition metals' oxides and lithium iron phosphate [9]. Depending on cathode chemistry, during discharge lithium iron phosphate (LFP), lithium cobalt (LCO), lithium manganese (LMO), lithium nickel manganese cobalt (NMC) or lithium nickel cobalt aluminum (NCA) oxide are the end products of reduction half-reaction. Electrons (through external circuit) and lithium ions (through separator) are released from anode in oxidation half-reaction and combine in the cathode. Charging is a reverse of this process, where electrons are delivered via external circuitry. Active material, as in anode formation, is mixed with binder(s), e.g., PVDF [13]. Transition metals building cathodes account for up to 14% of battery mass (cathode type depending) [11] and strongly affect battery production cost (51%) and recycling cost-effectiveness [12].
They are, in parallel, the main source of (eco)toxicological biohazards, especially accounting for projected market growth, energy-hungry supply chains and waste management.
Sourcing raw materials
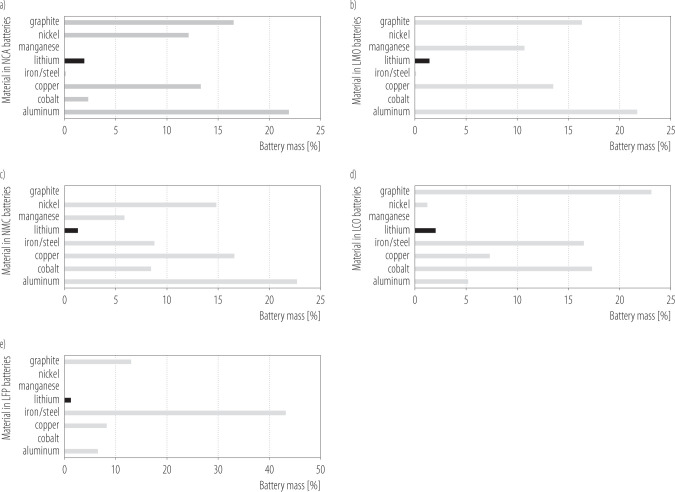
Aluminum (Al), iron/steel (Fe), copper (Cu), manganese (Mn), cobalt (Co), lithium (Li), nickel (Ni) and graphite are the raw materials crucial for electrodes (and electrolyte) production (Figure 1). With exception of iron and copper they were listed among 50 critical minerals by the US Geological Survey [15] indicating high economic and political importance. They are also classified as energy transition metals (ETMs), which status and demand for is rapidly growing due to their use in green energy technologies (incl. acquiring solar and wind energy as well as its storage). Uneven distribution of some of their ores, though, locate mining operations in regions with fragile economy and/or high corruption rates (Latin America and African countries, Russia, China) and accentuate environmental and social risks [16]. Concerning the severity of negative effects on environmental, social and governance dimensons Lèbre et al. [16] places mining of cobalt and manganese on second and third place. Copper scores eighth, nickel 10th, aluminum 12th while iron and lithium are 17th and 18th among 20 analysed commodities. Cobalt is a prime example of an ETM, which supply chain is considered not sustainable and harmful for environment and communities. It is present in only few LIBs types (LCO, NMC, NCA), which are however prevalent on the market. The main supplier of this rare (approx. 0.003% of earth crest) and valuable metal's ore is Democratic Republic of Congo (DRC), which in 2021 provided over 70% of world mine production (120 000 t), mainly as a byproduct of copper (and nickel) mining from sediment-hosted stratiform deposits [17]. The main cobalt refiner and consumer remains China, which allocates >80% of its production to rechargeable batteries industry. Growing demand, diminishing supplies and lack of substitutes in many technological processes places Co on the critical nonfuel minerals list [18]. In addition, it is considered as a “conflict resource” involving child labor and illicit export, which sponsor internal conflicts in already politically unstable DRC [19].
Figure 1.
Content of selected materials in batteries of a) lithium nickel cobalt aluminium (NCA), b) lithium manganese (LMO), c) lithium nickel manganese cobalt (NMC), d) lithium cobalt (LCO), e) lithium iron phosphate (LFP) (based on [84–86])
Lack of proper control over mines in this area and significant role of artisanal operations (15–20% of overall mining production) led to documented environmental pollution and health effects in surrounding communities. Studies from Congolese University of Lubumbashi have shown significantly elevated urinary levels of cobalt, as well as arsenic, cadmium, copper, lead and uranium in Katanga Copperbelt inhabitants living in the vicinity of mines or smelting plants. Measured urinary cobalt concentrations were 43-fold higher, in comparison to USA general population and the most affected group were children <14 years [20]. Following studies from the same group involved diggers working in the artisanal extraction areas and residents of the surrounding areas [21]. Significant raise of, correlated, Co levels in urine and blood were found in all studied groups. Stratification by age revealed 9.3-fold increase of urine and 7.5-fold of blood cobalt concentration in children, allied with 45 times elevated levels of oxidative stress marker – 8-hydroxydeoxyguanosine – in these children's blood samples. 8-Hydroxydeoxyguanosine is a product of guanine oxidation by reactive oxygen species, UV radiation or genotoxic agents. It is linked with aging, cancer development as well as memory formation, through 8-oxo-dG-dependent demethylation of CpG sites. Cobalt oxides absorbed from dust are distributed through the lymph and blood systems and primarily undergo uptake by liver and kidney as well as excretion in urine and feces. Following initial rapid removal, mainly renal, of metal ions (40% in the first 24 h and ca. 70% after a week) process decelerate, though, exposing tissues long term to remaining metal [22].
Physiologically cobalt is an important constituent of vitamin B12, although in higher doses it is acutely toxic. Soluble cobalt salts characterize with LD50 between 150–500 mg/kg [23] and are classified as possibly carcinogenic to humans (group 2B agents) by International Agency for Research on Cancer (IARC). Systemic effects of Co poisoning include hematological and endocrine (at blood conc. >300 µg/l), as well as neurological (vision and hearing) and cardiovascular symptoms at concentration >700 μg/l [24]. Occupational, environmental, dietary or medical (e.g., from metal-on-metal hip prostheses) exposure to high concentration of cobalt particles leads to elevation of free Co2+ ions in serum. Those, in turn, are involved in ROS generation and oxidative damage to DNA, proteins and lipids, DNA repair impairment, alteration of erythropoiesis and mitochondrial functions, disturbance of iodine intake by thyroid and deregulation of iron and calcium homeostasis [25]. Carcinogenic potential of cobalt is also associated with its ability to activate hypoxiainducible factor (HIF) and potentially inducing transcription of a number of genes promoting tumor growth [22]. Nickel constitutes ca. 80% of NCA and ca. 30% of NMC cathodes [26]. Accounting for a dominant market position of the latter and high energy density offered by the Ni-containing chemistries, their share in overall Li-ion batteries numbers is predicted to grow by 50% till year 2025. Despite battery production consume only ca. 5% of nickel production, this share will grow and increase process ecological costs. While Ni production constantly increases, its leading producers – Indonesia and Philippines – start to consider its environmental and human costs. In 2017 Philippines closed 23 (mostly nickel) mines to fight environmental degradation. Extracting Ni from low-grade ores (~1–2% nickel) renders it energy intensive process, which creates a conflict of interest between mining companies and diminishing tropical forest biodiversity in Indonesia and New Caledonia [27]. In comparison with other metals Ni is ranked 7th on a list of most damaging to human health and ecosystems and 9th for global warming potential [28]. Environmental contamination was documented also for nickel mines in Canada [29], Cuba [30], northwest Russia and Finland [31]. Emission of sulfur dioxide, leading to acid rain, as well as release of heavy metals and acidic mine drainage to soil and water were the main issues. Report published in 2020 by World Resources Institute [32] focused on the effects of legal and illegal mining operations (including Ni extraction), which span over 20% of the indigenous Amazon region. Its outcomes confirm not only pollution found in at least 30 Amazonian rivers flowing through Guyana, Colombia, Ecuador, Peru, Bolivia and Brazil but also erodes local communities, which are proven to protect their local environment.
Nickel plays crucial role in plants growth and development and is considered an essential nutrient for some microorganisms and animals [33]. Enzymes and cofactors containing Ni were documented in archaea, bacteria, algae, primitive eukaryotes and plants [34], although it is physiological role in higher animals is not fully resolved. Nickel is considered the most important sensitizer among metals, often resulting with contact dermatitis [35]. Exposure to poorly soluble Ni sulfides and oxides was linked to immunotoxicity, cardiovascular and respiratory tract disorders (including asthma, lung fibrosis and cancer), observed at different doses and exposure lengths [30]. Despite its widespread commercial use, e.g., in coins, stainless steel cooking utensils or taps, nickel salts and oxides are classified as class 1 carcinogen, while metallic nickel and its alloys as possibly cancerogenic (group 2B) by IARC [36]. Toxic effects of Ni on immune and respiratory system are closely linked to occupational inhalation from fossil fuel combustion or nickel-related manufacturing. This route affects most often metal refineries or plating industry workers, although people inhabiting areas in proximity of nickel mining, processing and recycling sites are also endangered [37]. Molecular mechanisms of nickel toxicity, despite not fully understood, connect to oxidative stress and mitochondrial dysfunction, resembling mechanisms of cobalt toxicity (see above). Initial impairment of mitochondrial membrane potential leads to decrease in mitochondrial ATP levels and mtDNA damage [38]. More specifically, Ni promotes shift of energy metabolism towards anaerobic glycolysis by stabilizing hypoxia-inducible factor-1α (HIF-1α) and inducing hypoxia-like response. Disturbed assembly of some iron–sulfur cluster proteins, like aconitase and mitochondrial complexes I, II and III, together with HIF-1α-dependent activation of plasminogen activator inhibitor (PAI-1), vascular endothelia growth factor (VEGF), and the CXC chemokine genes are some of the involved mechanisms. Neurons exposed to Ni produced more ROS, which resulted with lipid peroxidation, disturbed membrane integrity and reduction of antioxidant mechanisms capacity. These cellular alterations are linked to nickel role in the etiology of numerous mental disorders [39] and align with findings of its accumulation in the central nervous system [38].
From 82 000 tons of lithium produced globally in 2020 >70% will be used by for battery manufacturing. Five mines in Australia, 2 brine operations in Chile and Argentina, each, and 2 producers (1 brine, 1 mineral) in China deliver >95% of this number. Overproduction and Li price drop prevent most of these companies to expand in 2020 and some to cease production, nevertheless projected market growth will result in securing and opening new sources if necessary. U.S. Geological Survey monography [40] concerning lithium states no ecological or human safety issues related to the element, adding though that with the rise in its production and consumption these will certainly appear. Alarming example of this happening are the effects of intense lithium recovery from underground brines in one of the driest areas in the world, namely Salar de Atacama in Chile. Expansion of lithium evaporation operations in this part of the “lithium triangle” already creates conflict with local communities and pressure to fragile ecosystems. Extraction of Tibetan resources, e.g., from lithium salts-rich Chabyer Tsaka salt lake, similarly lacks proper attention to indigenous ecosystem and inhabitants [41]. Both hard rock mining and extraction from underground reservoirs are burdened with high ecological costs, including CO2 production (15 tones/tone of Li) for former or land (3124 m2/t of Li) and water (469 m3/t of Li) for the latter [42]. Development of efficient methods utilizing lithium-reach geothermal waters, though, opens new venues for “green lithium” extraction.
Lithium use is well established and effective therapy in bipolar disorder [43]. For that reason, data regarding its toxicity is widely available and its metanalysis indicates several side effects, associated with kidney, thyroid and parathyroid glands functioning, as well as possible teratogenicity [44]. Irreversible neuropathy, linked to cerebellar demyelination, was mentioned in few cases [45], although positive correlation was also suggested between lithium levels in drinking water and suicide risk reduction [46]. Lithium is predominantly absorbed in GI tract and excreted through kidneys [47] which may suggest lesser role of (occupational) inhalation as entry route. Even at therapeutic levels this metal can interfere with multiple enzymes, affecting also hematopoiesis, glycogen synthesis or embryonic development [48]. These and other actions of Li on the molecular level are attributed to its ability to interfere with phosphatidyl inositol (PI) second-messenger system, and related receptors activity, as well as to regulate protein kinase C (PKC) and arachidonic acid signaling cascades [49]. Toxicity towards human cardiomyocytes, in other studies, was connected with alteration in glycogen synthase kinase 3 beta signaling [50].
Risk associated with battery cell production
Depending on the level of production process automatization operators can be exposed to solvents, electrolytes or metal powders used in battery production process. Occupational safety regulations in many countries stipulate exposure limits for number of these substances (e.g., GESTIS – International limit values for chemical agents [51]). N-Methylpyrrolidone, used to dissolve the polyvinylidene fluoride (PVDF) binder during cathode coating, is an example of toxic compound which emission has to be controlled in the process [52]. N-Methylpyrrolidone is most commonly absorbed through the skin and is irritating towards eyes, lungs, respiratory system. It also has teratogenic effects and prolonged exposure, as with many organic solvents, may harm kidneys, liver and nervous system. Permissible exposure limit (PEL), set by Occupational US Occupational Safety and Health Administration (OSHA) is 1 ppm during 8-hour work day [53].
Other example of occupational risks include contact with metal powders, either during their production or forming active cathode material. Finely divided lithium, cobalt, manganese or nickel can create combustible/explosive mixtures with air (combustible dust) [54] and pose serious health threat when inhaled, especially chronically. Assessment of these health risks led to establishing occupational exposure limits (by different regulatory bodies) for these 4 metals, which are summarized in Table 2. In addition, lithium (especially in the powder form), being an alkali metal, is as well very reactive in contact with moisture, while nickel and aluminum (used e.g., for current collectors) are a reactive metal pair, which must be kept separated. Environment where these materials are stored and used then need to be properly ventilated, and working with them organized towards minimizing effect on operators. This is achieved, among others, locating number of cell assembly steps, from electrodes constituents mixing to electrolyte filling, in controlled conditions of ISO 8 or ISO 7 class clean rooms [13]. Environment with defined upper limits of suspended particles and number of per-hour air changes (as per ISO 14644 standard) is required predominantly to achieve contaminant free electrodes, albeit creates work environment safer as well for the operators.
Table 2.
Occupational exposure limits for active metal cathode components (based on [87])
| Organization | Exposure limit type | Active metal cathode component | |||
|---|---|---|---|---|---|
| lithium hydride | cobalt metal, dust and fume | manganese, compounds and fume | nickel, metal and insoluble compounds | ||
| OSHA | PEL-TWA (8-hour) | 0.025 mg/m3 | 0.1 mg/m3 | 1 mg/m3 | |
| PEL-STEL | |||||
| PEL-C | 5 mg/m3 | ||||
| NIOSH | REL-TWA (up to 10-hour) | 0.025 mg/m3 | 0.05 mg/m3 | 1 mg/m3 | 0.015 mg/m3 |
| REL-STEL | 3 mg/m3 | ||||
| REL-C | |||||
| ACGIH | TLV-TWA (8-hour) | 0.02 mg/m3 (inhalable particulate matter) | 0.02 mg/m3 (respirable particulate matter) 0.1 mg/m3 (inhalable particulate matter) | elemental: 1.5 mg/m3 (inhalable particulate matter) insoluble inorganic compounds (NOS): 0.2 mg/m3 (inhalable particulate matter) | |
| TLV-STEL | |||||
| TLV-C | 0.05 mg/m3 (inhalable particulate matter) | ||||
| Cal/OSHA (DOSH) | PEL-TWA (8-hour) | 0.020 mg/m3 | 0.2 mg/m3 | 0.5 mg/m3 (metal) | |
| 0.1 mg/m3 (insoluble compounds) | |||||
| PEL-STEL | 3 mg/m3 | ||||
| PEL-C | |||||
ACGIH – American Conference of Governmental Industrial Hygienists; Cal/OSHA – California Occupational Safety and Health Administration; DOSH – Division of Occupational Safety and Health; NIOSH – National Institute for Occupational Safety and Health.
C–ceiling (the concentration that should not be exceeded during any part of the working exposure); PEL – permissible exposure limit; REL – recommended exposure limits; STEL – short-term exposure (15-minute time-weighted average exposure that should not be exceeded at any time during a workday); TLV – threshold limit value; TWA – time-weighted average (over given period of time).
Another type of risk arising from battery manufacturing, although considered in a longer time perspective, is the CO2 gas emission. According to MIT researchers, manufacturing LIBs holding 80 kWh (capacity of e.g., Tesla Model 3 battery) can produce 2400–16 000 kg CO2 [55]. Lower estimates base on manufacturing located in Europe and U.S. while higher on locations in China and East Asia, and strongly corelate with energy mix involved. These values, however, include effects of raw materials mining and refining, electrode material production (e.g., coprecipitation and calcination of NMC powder) as well as cell production and battery assembly. Manufacturing is responsible for ca. 30% of the overall energy used (and so CO2 produced), predominantly for heating stages, and clean/dry environment preservation [56]. In respect to electric vehicles contribution to greenhouse gas (GHG) production during their lifecycle, EPA ascribe 18% of this value to batteries, assuming (among others) U.S. average grid emissions [57]. Interestingly, even with this component missing in gas cars, their overall GHGs emission is over 2 times greater than EVs with ~500 km (300 miles) range.
Risks during casual usage
Thermal runaway is one of the most recognized safety issues for lithium-ion batteries end users. It is a process of rapid self-heating, driven by internal exothermic reactions, which may end up in cell destruction, release of toxic gases and a high risk of fire or explosion [58]. This self-perpetuating process may be initiated by disruption of battery integrity (e.g., casing puncture), internal failure (e.g., short cutting) or its mistreatment (e.g., overheating or overcharging). Rise of cell temperature >70–90°C, state-of-charge depending, leads to protective SEI layer breakage. Exothermic decomposition of electrolyte and its reaction with intercalated lithium follows when temperature reaches >120°C. Sealing separator pores at this stage aims to prevent anode and cathode shortcutting, although with further temperature growth most separators loose integrity. Between 150–350°C products of cathode decomposition react with electrolyte and deliver oxygen, which promotes further temperature increase and PVDF binder exothermic reaction with lithiated carbon (>200°C). Temperatures during such events may exceed 600°C and melt aluminum current collectors and other casing elements. Regardless of cathode type during thermal runaway 18 650 cell releases around 1.2 l of gas per Ah of capacity [59]. This volume cannot be contained in standard enclosure and safety features, in a form of safety valves, aim to prevent uncontrolled pressure build up and explosion [60]. Physical injury and equipment damage are probable due to high temperature, corrosive nature of expelled materials as well as toxic nature of gases released. Garbage, recycle trucks and landfill fires ascribed to discarded batteries damage were documented. Fire accident involving cargo of batteries transported by UPS flight pushed U.S. Department of Transportation to establish regulations in this regard. While in 2006 some major laptop manufacturers had to recall a number of products after included batteries fires and explosions were reported [61].
Released during thermal events is typically a mixture of flammable, toxic, and corrosive volatiles, including carbon di- and monoxide, hydrogen, oxygen, short chain hydrocarbons (e.g., ethane, methane) and compounds containing fluorine [62].
Another group of irritant, harmful, toxic and/or flammable LIB elements are solvents. According to U.S. Department of Energy Protective Action Criteria (PAC) classification [63] even 250 ml of most of commonly used solvents, released in a confined space, can be harmful. This amount invokes level 2 Acute Exposure Level Guidelines (AEGLs) symptoms, i.e., irreversible or other serious, long-lasting, adverse health effects or an impaired ability to escape. For highly volatile electrolytes, e.g., diethyl carbonate, 1,2-dimethoxyethane, 1,3-dioxolane or 2-methyl-tetrahydrofuran this level of toxic effects may occur with volumes below 15 ml, evaporating into a 63 m3 space [64]. Assuming that electrolyte accounts for 11–15% of a 46 g lithium battery weight (exception is NMC chemistry, where it is <2%), three 18650 cells contain this volume. For reference, battery packs of Tesla's models S and X are built of >8000 of such cells, version dependent. Damage to battery casing in a closed space (storage facility, garage) then, may easily create life threatening conditions. In addition, evaporation of these volatile organic compounds in case of temperature rising is the primary risk factor for cell enclosure rupture.
Flame-retardant additives (e.g., fluorophosphates) present in solvents also contribute to vent gas toxicity.
The main source of hydrogen fluorine-containing compounds, however, are reactions including lithium salts and water. Residual water can be present in solvent itself or become available following cell damage. The effects include release of gaseous hydrogen fluoride (HF), phosphorus pentafluoride (PF5) and phosphoryl fluoride (POF3). Single publication suggests also pentafluoroarsenic and pentafluorophosphate presence in compromised batteries [65]. Considering PAC measures, 24 ppm (20 mg/m3) concentration of HF after 1 h exposure can lead to mentioned level 2 AEGLs health effects. This exposure level results from a release of ca. 20 ml of 1 mol LiPF6 electrolyte in 62 m3 room [64].
Disposal and recycling
Spent batteries final destination is a municipal solid waste landfill, waste-to-energy facility (e.g., waste incinerator) or specialized recycling facility. The first option is still the most probable one, unless local regulations are introduced. It also bears the most significant risk of leaching metals from stored cell cathodes into underlying groundwater or places where biosolids (produced during landfill leachate treatment) are applied [66]. Potential to release toxic elements to water supplies is the reason for most of the countries to require dumpsites to introduce liner and leachate treatment systems.
Lithium-ion batteries have potential to release number of metals with varying levels of toxicity to humans. While copper, manganese and iron, for example, are considered essential to our health, cobalt, nickel and lithium are trace elements which have toxic effects if certain levels are exceeded [67]. All LIBs can potentially release lithium, cobalt is another metal of concern and nickel, copper, as well as iron have genotoxic effects and can lead to premature aging [68]. Thallium, occasionally found in LIBs, is absorbed through skin and has relatively high toxic potential when compared with other elements at similar concentrations [65]. Number of studies aimed assessing the risks associated with permanent storage of lithium batteries in landfills [11]. Using laboratory-scale leaching models, cobalt, copper and nickel, released from studied cells, significantly exceeded regulatory threshold concentrations (RTC). In regards to chromium, lead and thallium only some among studied samples have shown above-RTC levels [69]. Findings of Shen et al. [50] show, on the example of lithium, that lack of proper LIBs utilization/recycling together with their growing numbers already poisons Yangtze and other Shanghai rivers. Elevated levels of this metal were also measured in Shanghai tap water. Higher amounts of Li are harmful for aquatic and terrestrial environments, while its concentration raising in food chains bring harm to humans and other animals.
Other cell elements are rarely treated as battery-specific risk factors, due to their stability and levels comparable to other waste streams. For example, carbon-based anode material in popular LIBs, including graphite, activated charcoal and some types of mesoporous carbon is inert and considered safe for the environment. Other types of mesoporous carbons however are IARC group 2B possible human carcinogens. Polypropylene and polyethylene separator materials are ubiquitous polymers, and dominating element of microplastic pollutants negatively affecting whole (predominantly marine) ecosystems. Polypropylene microparticles were also shown to stimulate immune response in human PBMC cells [70]. Toxicity of consumer plastic products lacks comprehensive assessment, despite number of concerns.
In relation to the problem of heavy and transition metals (Hg, Pb, As, Cu, Ni and Cr) leaching and accumulation of in the environment it is worth to mention phytoremediation. This method harness plants for contaminated soil, water and air clean up. Some plants (hyperaccumulators), possibly through long-term exposure, gained abilities to bioaccumulate, degrade or render harmless pollutants in a solar-powered, although long lasting process. Nickel, cobalt, iron, platinum, palladium and other heavy metals accumulated in aerial parts of the plants may then be recovered through metal phytomining [71]. Example of Alyssum bertolonii Desv. (Brassicaceae) show that some nickel hyperaccumulators are able to reach its content of 1%, translating to 10% metal in the ash [72]. Nickel was also found to create complexes with citric and methylated aldaric acid in latex derived from other hyperaccumulator, endemic tree from New Caledonia – Pycnandra acuminata [73]. Other studies indicate that some bacteria and fungi can be used to bioremediation of various heavy metals pollution [34].
Report from research agency MarketsandMarkets [74] asses that the global battery recycling market in 2020 was worth USD 17.2 billion and will grow to USD 23.2 billion in 2025. Concerning lithium-ion cells recycling specifically USD 1.5 billion in 2019 is believed to reach USD 12.2 billion in 2025 and USD 18.1 billion in 2030 [75]. Different factors however may be responsible for the development of spent LIBs recycling. Energy used during lithium-ion batteries raw materials extraction and transportation, often >20 000 nautical miles, exemplifies non-sustainable supply chain. These factors, in addition to a substantial CO2 production led manufacturers to invest in recycling of used batteries and shift towards the use of recovered materials. The cost of cobalt, nickel as well and production technology are the main elements affecting final battery production cost [76]. Availability and price of metals composing cathode determine profitability of lithium batteries recycling. Anodes are recycled mainly for the cupper collectors, although new chemistries may change that.
Eventuality of LIBs incineration with domestic waste stream produces incineration bottom ash (IBA), which contains recoverable metals. Specialized treatment trains are able to recover close to 90% of non-ferrous metals (from 4–50 mm particle size), which are the most valuable part of IBA. Interestingly, methods also exist for efficient metal extraction from IBA disposed of in landfills, which was practiced in the past. The scale of this approach is economically argued, and may become more significant in the future.
This approach aligns also with an idea to treat spent LIBs as “artificial minerals”, a source of metals which separation is much easier than from natural sources. Industrial scale extraction of these elements is currently based on 2-step process. Pretreatment, which includes deactivation, crushing and thermal/physical treatment result in separation of binder, plastics/aluminum (casing), electrolyte, steel, as well as smaller amounts of Co, Cu and Li2CO3. Subsequent are pyrometallurgical or hydrometallurgical processes, which in a step-wise manner delivers remaining metals. Drawbacks of the former include substantial energy required to render waste gases safe, as well as metal-rich slag byproduct not being cost effective for further processing [77]. Latter method involves the use of strong reductants and organic or inorganic acids as leaching agents. Number of proprietary processes involve both hydro- and pyrometallurgical elements [11] and depending on the (pre)treatment methodology, leaching and precipitation chemistry recovery rates of Li, Co, Mn, Ni (in pure or salt form) reach 100% in some cases, and >90% in most [78]. Growing size of this market and expected profitability propel studies in making recycling more efficient but also greener. Example are trials with bio-hydrometallurgical methods, which employ Acidithiobacillus ferrooxidans to produce sulfuric acid and ferric ions used for leaching [79]. Symptomatic for circular economy are also efforts to develop in-house recycling technologies and facilities by batteries manufacturers [80], leading to shortening supply chains, feeding-back/coupling know-how from production to recycling stage and vice versa.
CONCLUSIONS
The use of energy grows steadily for the last 65 years and will continue to do so, led strongly by the growth of Asian countries. Production and storage of this medium will have to follow demand, and use gradually more green and renewable resources. Batteries stand in the center of this process and adequate measures will have to accompany sourcing their substrates, developing better energy storage technologies and dealing with waste – destiny of each LIB after 3–10 years of usage. Efficiency of existing (and continuous search for improved) recycling methods allow us to reduce the energy required for crucial energy transition metals extraction. On the other hand, recovering and repurposing of valuable batteries ingredients is not necessarily (yet) a profitable business model, especially in the absence of regional regulations.
In this review the authors focus on human and environmental costs of LIBs, recognizing that these are 2 “branches of the same tree.” Life-cycle environmental impact assessments (LCAs), published by manufacturers or independent organizations have the same aim, although demonstrate as well challenges of such comprehensive task. Recent review by Porzio and Scown [81] highlights that future LCAs should include, for instance, progression of shrinking resources obtainment costs, extraction and mining taking place outside controlled industry environment (e.g., artisanal cobalt mining), scale effects achieved in big manufacturing facilities or dropping kilograms of battery mass as a functional unit for its capacity. Existing and new LCAs, with the use of growing amount of data, will be needed to build energetically sustainable future. Strain exerted on the environment with scaling production, as well as LIBs manufacturing conditions have to be controlled. Mining and processing sites located in less developed countries and managed by Chinese, American or European companies not always seem to comply with their homeland regulations. Extraction and processing of transition metals creates a high-risk environment, where, in most cases limits of exposure to occupational risks are regulated. These thresholds however are also challenged due to lack of transparency, limited supporting data and heavy involvement of industry.
The authors hope that the above summary of selected issues related to the whole “ecosystem” of lithium-ion batteries life-cycle is another voice in the important discussion. Balancing multi-billion business offering us life-easing solutions on one and sustainable, green future on the other scale will never be easy, mainly for that latter side.
Footnotes
Funding: this work was supported by Medical University of Gdansk (project No. ST-02-30022/0000731/01/304/304/0/2022 entitled “Purine metabolites in diseases,” project manager: Iwona Rybakowska) and by Medical University of Gdansk (project No. ST-02-30022/0000750/01/320/320/0/2022 entitled “Psychoactive substances,” project manager: Jacek Sein Anand).
REFERENCES
- 1.Tsiropoulos I, Tarvydas D, Lebedeva N. Li-ion batteries for mobility and stationary storage applications. Publications Office of the European Union. [Internet] The EU; 2018. [cited 2021 March 21]. Available from: 10.2760/87175. [DOI] [Google Scholar]
- 2.The International Energy Agency. Technology Roadmap – Electric and plug-in hybrid electric vehicles [Internet]. 2011;5 [cited 2021 May 12]. Available from: https://www.iea.org/reports/electric-and-plug-in-hybrid-electric-vehicles-roadmap.
- 3.Placek M. Estimated production capacity of tier 1 to 3 lithium-ion battery factories worldwide in 2018 with a forecast for 2023 and 2028 [Internet]. Statista; 2021. [cited 2021 June 30]. Available from: https://www.statista.com/statistics/1247625/global-production-capacity-of-lithium-ion-battery-factories. [Google Scholar]
- 4.Tesla [Internet]. Tesla; 2020. [cited 2021 April 19]. Battery Day presentation. Available from: https://www.tesla.com/2020shareholdermeeting. [Google Scholar]
- 5.Panasonic [Internet]. NCR18650BF; 2020. [cited 2020 April 15]. Panasonic Industrial Devices. Availaible from: https://na.industrial.panasonic.com/products/batteries/rechargeable-batteries/lineup/lithium-ion/series/90729/model/90735.
- 6.Zeng X, Li J, Ren Y. Prediction of various discarded lithium batteries in China. IEEE International Symposium on Sustainable Systems and Technology. 2012, 10.1109/ISSST.2012.6228021. [DOI] [Google Scholar]
- 7.Forti V, Baldé CP, Kuehr R, Bel G. The Global E-waste Monitor 2020: Quantities, flows and the circular economy potential. United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR) – co-hosted SCYCLE Programme, International Telecommunication Union (ITU) and International Solid Waste Association (ISWA), Bonn/Geneva/Rotterdam [Internet]. Bonn/Geneva/Rotterdam: United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR); 2020. [cited 2021 May 18]. Available from: https://ewastemonitor.info/gem-2020. [Google Scholar]
- 8.Tan Q, Dong Q, Liu L, Song Q, Liang Y, Li J. Potential recycling availability and capacity assessment on typical metals in waste mobile phones: A current research study in China. J Clean Prod. 2017;148:509–517. https://www.infona.pl/resource/bwmeta1.element.elsevier-eb559424-f2d6-359d-a35f-09a16bf8e5ec. [Google Scholar]
- 9.Kurzweil, P., Brandt, K.. Overview of Rechargeable Lithium Battery Systems. [In:] Electrochemical Power Sources: Fundamentals, Systems, and Applications. Elsevier; 2019. p. 47–82. [Google Scholar]
- 10.Li A, Yuen ACY, Wang W, De Cachinho Cordeiro IM, Wang C, et al. A Review on Lithium-Ion Battery Separators towards Enhanced Safety Performances and Modelling Approaches. Molecules. 2021;26(2):478. 10.3390/molecules26020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winslow KM, Laux SJ, Townsend TG. A review on the growing concern and potential management strategies of waste lithium-ion batteries. Resources, Conservation and Recycling. 2018;129:263–277. 10.1016/j.resconrec.2017.11.001. [DOI] [Google Scholar]
- 12.Houache MSE, Yim C-H, Karkar Z, Abu-Lebdeh Y. On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review. Batteries. 2022;8(7):70. 10.3390/batteries8070070. [DOI] [Google Scholar]
- 13.Heiner H, Kampker A, Lienemann C, Locke M, Offermanns C. Lithium-ion Battery Cell Production Process. DMA Battery Production [Internet]. 2019. [cited 20221 March 15]. Available from: https://www.pem.rwth-aachen.de/global/show_document.asp?id=aaaaaaaaabdqbtk.
- 14.Zeng X, Li J. Spent rechargeable lithium batteries in e-waste: Composition and its implications. Frontiers of Environmental Science and Engineering. 2014;8(5):792–796. 10.1007/s11783-014-0705-6. [DOI] [Google Scholar]
- 15.U.S. Geological Survey [Interet]. USGS; 2022. [cited 2022 June 18]. Burton J. Final List of Critical Minerals 2022. Available from: https://www.usgs.gov/news/national-news-release/us-geological-survey-releases-2022-list-critical-minerals.
- 16.Lèbre É, Stringer M, Svobodova K, Owen J R, Kemp D, Côte C, et al. The social and environmental complexities of extracting energy transition metals. Nature Communications. 2020;11(1). 10.1038/s41467-020-18661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Geological Survey [Internet]. Mineral Commodity Summaries. 2022. [cited 2022 June 20]. Available from: https://www.usgs.gov/centers/national-minerals-information-center/cobalt-statistics-and-information.
- 18.Hayes SM, McCullough EA. Critical minerals: A review of elemental trends in comprehensive criticality studies. Resources Policy. 2018;59:192–199. 10.1016/j.resourpol.2018.06.015. [DOI] [Google Scholar]
- 19.Prause L. Chapter 10 – Conflicts related to resources: The case of cobalt mining in the Democratic Republic of Congo. In: Bleicher A, Pehlken A, editors. The Material Ba sis of Energy Transitions. Academic Press, Elsevier; 2020. pp. 153–167. 10.1016/B978-0-12-819534-5.00010-6. [DOI] [Google Scholar]
- 20.Banza CLN, Nawrot TS, Haufroid V, Decrée S, De Putter T, et al. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environmental Research. 2009;109(6):745–752. 10.1016/j.envres.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Banza Lubaba Nkulu C, Casas L, Haufroid V, De Putter T, Saenen ND, Kayembe-Kitenge T, et al. Sustainability of artisanal mining of cobalt in DR Congo. Nature Sustainability. 2018; 1(9):495–504. 10.1038/s41893-018-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonsen LO, Harbak H, Bennekou P. Cobalt metabolism and toxicology-A brief update. Science of the Total Environment. 2012;432:210–215. 10.1016/j.scitotenv.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson JD, Beyersmann D. Cobalt and Cobalt Compounds [Internet]. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH Verlag GmbH and Co. KGaA. 2005. [cited 2021 March 5]. Available from: 10.1002/14356007.a07_281.pub2. [DOI] [Google Scholar]
- 24.Leyssens L, Vinck B, Van Der Straeten C, Wuyts F, Maes, L. Cobalt toxicity in humans – A review of the potential sources and systemic health effects. Toxicology. 2017;387:43–56. 10.1016/j.tox.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Paustenbach DJ, Tvermoes BE, Unice KM, Finley BL, Kerger BD. A review of the health hazards posed by cobalt. Critical Reviews in Toxicology. 2013;43(4):316–362. 10.3109/10408444.2013.779633. [DOI] [PubMed] [Google Scholar]
- 26.Nickel Institute [Internet]. Nickel energizing Batteries. 2018. [cited 2021 October 15]. Available from: https://www.nickelinstitute.org.
- 27.Moran D, Petersone M, Verones F. On the suitability of input-output analysis for calculating product-specific biodiversity footprints. Ecological Indicators. 2016;60:192–201. 10.1016/j.ecolind.2015.06.015. [DOI] [Google Scholar]
- 28.Nuss P, Eckelman MJ. Life Cycle Assessment of Metals: A Scientific Synthesis. PLoS ONE, 2014;9(7):e101298. 10.1371/journal.pone.0101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchinson TC, Whitby LM. The effects of acid rainfall and heavy metal particulates on a boreal Forest ecosystem near the sudbury smelting region of Canada. Water, Air, and Soil Pollution. 1977;7(4):421–438. 10.1007/BF00285542. [DOI] [Google Scholar]
- 30.González H, Ramírez M, Torres I. Impact of nickel mining and metallurgical activities on the distribution of heavy metals in sediments of Levisa, Cabonico and Nipe Bays, Cuba. Environmental Geochemistry and Health. 1997;19(2):57–62. 10.1023/A:1018490103105. [DOI] [Google Scholar]
- 31.Moiseenko TI, Kudryavtseva LP. Trace metal accumulation and fish pathologies in areas affected by mining and metallurgical enterprises in the Kola region, Russia. Environmental Pollution. 2001;114(2):285–297. 10.1016/S0269-7491(00)00197-4. [DOI] [PubMed] [Google Scholar]
- 32.World Resouces Institute [Internet] Release: New Study Reveals Mining in the Amazon Treatens 20% of Indigenous Lands. October 2020. [cited 2022 June 25]. Available from: https://www.wri.org/news/release-new-study-reveals-mining-amazon-threatens-20-indigenous-lands.
- 33.Rana SVS. Metals and apoptosis: Recent developments. Journal of Trace Elements in Medicine and Biology. 2008;22(4): 262–284. 10.1016/j.jtemb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A. Nickel: Human health and environmental toxicology. Int J Environ Res Public Health. 2020;17(3):679. 10.3390/ijerph17030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahlström MG, Thyssen JP, Menné T, Johansen JD. Prevalence of nickel allergy in Europe following the EU Nickel Directive – a review. Contact Dermatitis. 2017;77(4):193–200. 10.1111/cod.12846. [DOI] [PubMed] [Google Scholar]
- 36.Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F.. et al. A review of human carcinogens – part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10(5):453-454. 10.1016/s1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- 37.Grimsrud TK, Berge SR, Haldorsen T, Andersen A. Exposure to different forms of nickel and risk of lung cancer. Am J Epidemiol. 2002;156(12):1123–1132. 10.1093/aje/kwf165. [DOI] [PubMed] [Google Scholar]
- 38.Song X, Fiati Kenston SS, Kong L, Zhao J. Molecular mechanisms of nickel induced neurotoxicity and chemoprevention. Toxicology. 2017;392:47–54. 10.1016/j.tox.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Baj J, Forma A, Sitarz E, Karakuła K, Flieger W., et al. Beyond the mind-serum trace element levels in schizophrenic patients: A systematic review. Int J Mol Sci. 2020;21(24):1–52. 10.3390/ijms21249566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Geological Survey. Mineral Commodity Summaries 2021. Report. USGG; 2021, 10.3133/MCS2021. [DOI] [Google Scholar]
- 41.Free Tibet [Internet]. Free Tibet; 2021. [cited 2021 March 15]. BYD in Tibet: the costs of lithium extraction. Available from: https://freetibet.org/lithium-tibet. [Google Scholar]
- 42.Early C. The new “gold rush” for green lithium [Internet]. BBC Future; 2020. [cited 2021 May 12]. Available from: https://www.bbc.com/future/article/20201124-how-geothermal-lithium-could-revolutionise-green-energy. [Google Scholar]
- 43.Geddes J, Goodwin G, Rendell J, Azorin J, Cipriani A, et al. Lithium plus valproate combination therapy versus mono-therapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. The Lancet. 2010;375(9712):385–395. 10.1016/S0140-6736(09)61828-6. [DOI] [PubMed] [Google Scholar]
- 44.McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR. Lithium toxicity profile: A systematic review and meta-analysis. The Lancet. 2012;379(9817):721–728. 10.1016/S0140-6736(11)61516-X. [DOI] [PubMed] [Google Scholar]
- 45.Keltner NL, Grant JS. Biological perspectives: Irreversible lithium-induced neuropathy: Two cases. Perspect Psychiatr Care. 2008;44(4):290–293. 10.1111/j.1744-6163.2008.00189.x. [DOI] [PubMed] [Google Scholar]
- 46.Vita A, De Peri L, Sacchetti E. Lithium in drinking water and suicide prevention: A review of the evidence. Int Clin Psychopharmacol. 2015;30(1):1–5. 10.1097/YIC.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 47.Freeman MP, Freeman SA. Lithium: Clinical Considerations in Internal Medicine. Am J Med. 2006;119(6):478–481. 10.1016/j.amjmed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Phiel CJ, Klein PS. Molecular targets of lithium action. Annu Rev Pharmacol Toxicol. 2001;41:789–813. 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- 49.Corbella B, Vieta E. Molecular targets of lithium action. Acta Neuropsychiatr. 2003;15(6):316–340. 10.1046/j.1601-5215.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 50.Shen J, Li X, Shi X, Wang W, Zhou H, et al. The toxicity of lithium to human cardiomyocytes. Environ Sci Eur. 2020;32(1):59. 10.1186/s12302-020-00333-6. [DOI] [Google Scholar]
- 51.GESTIS [Internet]. 2022. [cited May 2022]. International limit values for chemical agents, Available from: https://limitvalue.ifa.dguv.de.
- 52.Liu Y, Zhang R, Wang J, Wang Y. Current and future lithium-ion battery manufacturing. iScience 2021;24(102332). 10.1016/j.isci.2021.102332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.US Occupational Safety and Health Administration (OSHA) [Internet]. 2021. [cited May 2021]. N-methyl-2-pyrrolidinone. Available from: https://www.osha.gov/chemicaldata/875.
- 54.National Fire Protection Association [Internet]. NFPA; 2022. [cited 2022 June 15]. NFPA 484 Standard for Combustible Metal. Available from: https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=484.
- 55.Climate Portal [Internet]. Cambridge: Massachusetts Institute of Technology; 2022. [cited 2022 June 17]. Crawford I, Shao-Horn Y, Keith D. How much CO2 is emitted by manufacturing batteries? Available from: https://climate.mit.edu/ask-mit/how-much-co2-emitted-manufacturing-batteries. [Google Scholar]
- 56.IVL Swedish Environmental Research Institute [Intenet]. IVL; 2019. [cited 2021 February 16]. Emilsson E, Dahllöf L. Lithium-Ion Vehicle Battery Production – Status 2019 on Energy Use, CO2 Emissions, Use of Metals, Products Environmental Footprint, and Recycling. Available from: https://www.ivl.se/english/ivl/publications/publications/lithium-ion-vehicle-battery-production----status-2019-on-energy-use-co2-emissions-use-of-metals-products-environmental-footprint-and-recycling.html. [Google Scholar]
- 57.United States Environmental Protection Agency [Internet]. EPA; 2022. [cited 2022 July 15]. Electric Vehicle Myths. https://www.epa.gov/greenvehicles/electric-vehicle-myths#note6. [Google Scholar]
- 58.Pfrang A, Kriston A, Ruiz V, Lebedeva N, di Persio F. Safety of Rechargeable Energy Storage Systems with a focus on Li-ion Technology. [In:] Rodriguez-Martinez LM, Omar N, editors. Emerging Nanotechnologies in Rechargeable Energy Storage Systems. Elsevier Inc, 2017. p. 253–290. 10.1016/B978-0-323-42977-1.00008-X. [DOI] [Google Scholar]
- 59.Roth EP, Orendorff C J. How electrolytes influence battery safety. Electrochem Soc Interface. 2012;21(2):45–49. 10.1149/2.F04122if]. [DOI] [Google Scholar]
- 60.Finegan DP, Darcy E, Keyser M, Tjaden B, Heenan TM, et al. Identifying the Cause of Rupture of Li-Ion Batteries during Thermal Runaway. Adv Sci. 2018;5(1):1700369. 10.1002/advs.201700369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.U.S. Consumer Product Safety Commission [Internet]. Bethesda: U.S. Consumer Product Safety Commission; 2010. [cited 2021 February 23]. PC Notebook Computer Batteries Recalled Due to Fire and Burn Hazard. Available from:. https://web.archive.org/web/20130108181246/https://www.cpsc.gov/cpscpub/prerel/prhtml09/09035.html. [Google Scholar]
- 62.Mikolajczak C, Kahn M, White K, Long RT. Lithium-Ion Batteries Hazard and Use Assessment [Internet]. New York: Springer; 2011. [cited 2021 May 19]. Available from: https://books.google.pl/books?hl=en&lr=&id=V4IVCvgv558C&oi=fnd&pg=PR6&ots=QyKYmoFs09&sig=a3XEsU0xMdkup-kNlv7-pT33nLY&redir_esc=y#v=onepage&q&f=false. [Google Scholar]
- 63.Office of Environment, Health, Safety & Security [Internet]. U.S. Department of Energy; 2018. [cited April 15 2021]. Protective Action Criteria (PAC) with AEGLs, ERPGs, and TEELs. Available from: https://www.energy.gov/ehss/protective-action-criteria-pac-aegls-erpgs-teels. [Google Scholar]
- 64.Lebedeva NP, Boon-Brett L. Considerations on the Chemical Toxicity of Contemporary Li-Ion Battery Electrolytes and Their Components. J Electrochem Soc. 2016;163(6):A821–A830. 10.1149/2.0171606jes. [DOI] [Google Scholar]
- 65.Nan J, Han D, Zuo X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J Power Sources. 2005;152(1–2):278–284. 10.1016/j.jpowsour.2005.03.134. [DOI] [Google Scholar]
- 66.Dubey B, Townsend T, Solo-Gabriele H. Metal loss from treated wood products in contact with municipal solid waste landfill leachate. J Hazard Mater. 2010;175(1–3):558–568. 10.1016/j.jhazmat.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 67.Smith KS, Huyck HLO. An Overview of the Abundance, Relative Mobility, Bioavailability, and Human Toxicity of Metals. In: Plumlee GS, Logsdon MJ, editors. The Environmental Chemistry of Mineral Deposits, Reviews in Economic Geology. Vol. 6A. 1999. p. 29-70. [Google Scholar]
- 68.Mehta R, Templeton DM, O'Brien PJ. Mitochondrial involvement in genetically determined transition metal toxicity. II. Copper toxicity. Chem Biol Interact. 2006;163(1–2): 77–85. 10.1016/j.cbi.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 69.Kang DHP, Chen M, Ogunseitan OA. Potential environmental and human health impacts of rechargeable lithium batteries in electronic waste. Environ Sci Technol. 2013;47(10): 5495–5503, 10.1021/es400614y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang J, Choi D, Han S, Choi J, Hong J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci Total Environ. 2019;684:657–669. 10.1016/j.scitotenv.2019.05.071. [DOI] [PubMed] [Google Scholar]
- 71.Chaney RL, Angle JS, Baker Alan JM, Li YM. Method for phytomining of nickel, cobalt and other metals from soil [Internet]. 1999. [cited 2021 March 8]. Available from: https://www.researchgate.net/publication/43258437_Method_for_phytomining_of_nickel_cobalt_and_other_metals_from_soil.
- 72.Horie K, Mizuno N, Nosaka S. Characteristics of nickel accumulation in native plants growing in ultramafic rock areas in Hokkaido. Soil Sci Plant Nutr. 2000;46(4): 853–862. 10.1080/00380768.2000.10409151. [DOI] [Google Scholar]
- 73.Callahan DL, Roessner U, Dumontet V, De Livera AM, Doronila A, Baker AJ, et al. Elemental and metabolite profiling of nickel hyperaccumulators from New Caledonia. Phytochemistry. 2012;81:80–89. 10.1016/j.phytochem.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 74.MarketsandMarkets [Internet]. 2020a. [cited 2021 April 17]. Battery Recycling Market Global Forecast to 2025. Available from: https://www.marketsandmarkets.com/Market-Reports/battery-recycling-market-147696175.html.
- 75.MarketsandMarkets [Internet]. 2020b. [cited 2021 April 17]. Lithium-ion Battery Recycling Market Global Forecast to 2030. Available from: https://www.marketsandmarkets.com/Market-Reports/lithium-ion-battery-recycling-market-153488928.html.
- 76.OSTI.GOV [Internet]. Argonne: U.S. Department of Energy, Office of Scientific and Technical Information, 2012. [cited 2021 May 21]. Nelson PA, Gallagher KG, Bloom ID, Dees DW. Modeling the Performance and Cost of Lithium-Ion Batteries for Electric-Drive Vehicles. 2 ed. Available from: 10.2172/1209682. [DOI] [Google Scholar]
- 77.Georgi-Maschler T, Friedrich B, Weyhe R, Heegn H, Rutz M. Development of a recycling process for Li-ion batteries. J Power Sources. 2012;207:173–182. 10.1016/j.jpowsour.2012.01.152. [DOI] [Google Scholar]
- 78.Xiao J, Li J, Xu Z. Challenges to Future Development of Spent Lithium Ion Batteries Recovery from Environmental and Technological Perspectives. Envi Sci Technol. 2019 2020, 54(1):9–25. 10.1021/acs.est.9b03725. [DOI] [PubMed] [Google Scholar]
- 79.Xin B, Zhang D, Zhang X, Xia Y, Wu F, Chen S, Li L. Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria. Bioresour Technol. 2009; 100(24):6163–6169. 10.1016/j.biortech.2009.06.086. [DOI] [PubMed] [Google Scholar]
- 80.Norhtvolt [Internet]. Stockholm: Northvolt AB; 2022. [cited May 2022]. Closing the loop on batteries. Available from: https://northvolt.com/articles/revolt. [Google Scholar]
- 81.Porzio J, Scown CD. Life-Cycle Assessment Considerations for Batteries and Battery Materials. Adv Energy Mater. 2021; 11(33):2100771. 10.1002/aenm.202100771. [DOI] [Google Scholar]
- 82.Battery University [Internet]. Isidor Buchmann;2021. [cited 2021 May 15]. BU-205: Types of Lithium-ion Available from: https://batteryuniversity.com/learn/article/types_of_lithium_ion. [Google Scholar]
- 83.Wikipedia [Internet]. 2021. [cited 2021 June 14]. Lithium-ion battery. Available from:. https://en.wikipedia.org/wiki/Lithium-ion_battery.
- 84.Gaines L, Sullivan J, Burnham A, Belharouak I. Life-Cycle Analysis of Production and Recycling of Lithium Ion Batteries. Transp Res Rec. 2011;2252(1):57–65, 10.3141/2252-08. [DOI] [Google Scholar]
- 85.ProQuest [Internet]. Rochester Institute of Technology; 2016. [cited 2021 June 13]. Richa K. Sustainable management of lithium-ion batteries after use in electric vehicles. Available from: https://search.proquest.com/docview/1865634774?pq-origsite=gscholar. [Google Scholar]
- 86.Wang X, Gaustad G, Babbitt CW. Targeting high value metals in lithium-ion battery recycling via shredding and size-based separation. Waste Manag. 2016;51:204–213. 10.1016/j.wasman.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 87.Occupational Safety and Health Administration [Internet]. U.S. Department of Labour; 2022. [cited 2022 June 8]. Occupational Chemical Databases. Available from: https://www.osha.gov/chemicaldata. [Google Scholar]