Abstract
Objectives:
To analyze the number, epidemiology and circumstances of needlestick and sharps injuries (NSSI) and exposures to body fluids and to identify further preventive measures to improve the occupational safety of health care workers (HCW).
Material and Methods:
Setting: German university tertiary-care referral center. Retrospective study based on injury documentation sheets of the hospital's staff and faculty health service and, if given, on reports by continuity doctors and by the accident and emergency department in January 2014–June 2016.
Results:
Altogether, 567 injuries were registered with a significant decrease of cases over the study period. The majority of accidents occurred in the operating theater (35%). Stress, time pressure, overstrain, carelessness and distraction were found to be the main reasons for injuries. At least 30% of the cases were preventable, mainly by wearing personal protective equipment (PPE), by proper disposal of an item and by early replacement of overfilled sharps containers (SC). In 20% of the cases involving an item, the injury was caused by a safety-engineered device (SED). Almost one-third of these injuries were attributable to an improper use of the SED.
Conclusions:
Despite many efforts made to reduce their number, NSSI still occur. Health care workers and students should be offered regular trainings to be sensitized to this topic and to learn the appropriate use of SED. Moreover, organizational measures must be taken, such as the provision of suitable PPE and safe SC. Strategies need to be established to improve the working conditions and reduce the stress level of HCW.
Keywords: occupational safety, sharps injuries, care workers, needlestick injuries, exposures to body fluids, safety-engineered device
INTRODUCTION
Needlestick and sharps injuries (NSSI) are defined as skin injuries caused by objects that are contaminated with potentially contagious material [1]. In addition, the skin and mucous membranes can be exposed to possibly infectious body fluids (BF) by splashes. Needlestick and sharps injuries are counted among the most frequent work-related accidents of health care workers (HCW) [2]. According to a review by Elseviers et al. [3], the incidence of NSSI ranges 1.4–9.5/100 HCW/year. In Germany there were roughly 500 000 NSSI/year occurring before the introduction of safety-engineered devices (SED) [4]. In 2015 around 51 000 NSSI were reported to the German Professional Association for Health Service and Welfare [2].
Needlestick and sharps injuries hold the risk of occupational infection which has been described for >60 different pathogens [5]. Transmission of the hepatitis B virus (HBV), the hepatitis C virus (HCV) and the human immunodeficiency virus (HIV) play a leading role with 0.42 HBV, 0.05–1.3 HCV, and 0.04–0.32 HIV infections per 100 NSSI [3].
The psychological consequences accompanying NSSI constitute another relevant aspect. As reported by Sohn et al. [6], having experienced a NSSI leads to higher levels of stress, anxiety and depression, in particular when the index patient (IP) is known to have a chronic infection [7].
The costs caused by a single NSSI lie between EUR 110 [8] and EUR 272 [9]. They result from blood tests, vaccination against HBV, post-exposure prophylaxis and psychiatric support (when the IP is HIV-positive) [9], excluding potential expenses for treatment of transmitted infections and for loss of working hours.
Aiming to create a safe work environment and reduce the number of NSSI, Council Directive 2010/32/EU was passed by the European Union in May 2010 [10] and transferred into German law by a revision of the TRBA 250 (Technical Rules for Biological Agents) in March 2014 [1].
This study was conducted at the University Medical Center Hamburg-Eppendorf (UKE), the largest hospital in Hamburg with approx. 14 142 employees (including trainees and temporary workers) and 3388 students [11]. The authors aimed to examine the number and epidemiology of NSSI taking place at the UKE to get an understanding of how and why such injuries occur despite the implementation of the above-mentioned political measures and to identify further preventive potential in terms of improving the occupational safety of HCW.
MATERIAL AND METHODS
All reported NSSI as well as blood and body fluid exposures (BBFE) are documented at the hospital's staff and faculty health service. The study was conducted retrospectively by collecting data from the injury documentation sheets which consist of 2 parts. The first section comprises personal data (age, occupation, department, immunity to HBV), details of the IP (where available) and information about the injury (date, time, type of device, place, cause) which each need to be filled in and ticked respectively; the second part is a free-text field. If given, reports by continuity doctors and by the accident and emergency department were also used for data collection. All injuries that occurred in January 2014–June 2016 were included; those that had not occurred at the UKE but were still documented were excluded from the study.
The authors consulted with the Ethics Committee of the Hamburg Medical Association, which considered ethical approval to be unnecessary but requested for reasons of data protection the anonymization of names and dates of birth. Nominal variables were created and encoded based on a literature research using PubMed and the analysis of the documentation sheets with emphasis on the written comments.
An injury was graded as preventable if the question of how it could have been prevented could clearly be answered naming a plausible solution. Injuries attributable to an unexpected movement, harmful behavior of the patient, an unfortunate incident, unsafe or defect equipment and those that happened despite all safety precautions that had been taken, were graded as probably unavoidable. The use of an SED was considered to be improper if the injury occurred despite the safety mechanism (SM) being constantly active, if it happened during the activation of the SM or if the SM was incompletely or not activated at all. If an injury occurred during the activation of the SM, the activation status was categorized as incompletely activated.
The data was entered into a Microsoft Excel file. Statistical analyses were conducted using Microsoft Excel 2010 and IBM SPSS Statistics v. 22. Standard descriptive methods were used to determine frequency distributions. Furthermore, a correlation analysis was performed in order to investigate the relationship between the number of injuries and the course of the study period. Pearson correlation coefficient (r) was used to assess this relationship. A negative coefficient indicates a decrease in NSSI while a positive coefficient indicates an increase in NSSI. Differences between different groups could not be analyzed using additional statistical tests due to the very small frequencies in some of the subgroups.
The building of homogenous and comparable, albeit small, subgroups outweighed the approach to build big enough subgroups to perform statistical analysis.
RESULTS
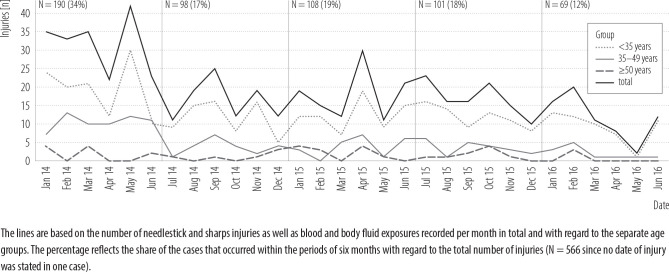
During the study period, 586 cases were registered, of which 19 were excluded for not having occurred at the UKE. Most of the injuries were percutaneous (85%), followed by eye and/or mouth contaminations (12%) and exposures of non-intact or intact skin to BF (2%). Looking at the age distribution of the persons injured, the age was <35 years in 68% of cases, 35–49 years in 25% and ≥50 years in 7%. In the course of the study period, there was a significant drop in the total number of injuries (r = –0.66, p < 0.001). The distribution of the cases, with regard to the age groups and the time of injury, is presented in Figure 1. Table 1 shows the distribution of injuries by profession, device, place and activity.
Figure 1.
Frequency distribution of the needlestick and sharps injuries in the course of the study period, January 2014–June 2016, University Medical Center Hamburg-Eppendorf, Germany
Table 1.
Distribution of injury events with regard to profession, device, place and activity, January 2014–June 2016, University Medical Center Hamburg-Eppendorf, Germany
| Variable | Injury event (N = 567) | |
|---|---|---|
| n | % | |
| Profession | ||
| doctor | 215 | 38 |
| nurse | 113 | 20 |
| medical assistant in the operating theater or functional diagnostic area | 104 | 18 |
| other profession with patient contact | 36 | 6 |
| medical student | 30 | 5 |
| laboratory and research staff | 25 | 4 |
| dentist | 16 | 3 |
| other profession without patient contact | 14 | 2 |
| student of dentistry | 11 | 2 |
| not available | 3 | 0.5 |
| Device | ||
| hollow-bore needle | 236 | 42 |
| body fluid or solid body material | 82 | 14 |
| solid needle | 76 | 13 |
| knife/scalpel | 70 | 12 |
| surgical instrument | 27 | 5 |
| dental instrument | 24 | 4 |
| needle (not further specified) | 11 | 2 |
| unknown | 11 | 2 |
| other | 23 | 4 |
| not available | 7 | 1 |
| Place of injury | ||
| operating theater | 197 | 35 |
| ward | 137 | 24 |
| out-patient department | 61 | 11 |
| intensive care unit | 43 | 8 |
| laboratory | 39 | 7 |
| angiography/endoscopy/radiology | 38 | 7 |
| accident and emergency department | 13 | 2 |
| other | 27 | 5 |
| not available | 12 | 2 |
| Activity | ||
| with direct patient contact | 256 | 45 |
| surgical activity and dissection | 99 | 39 |
| all kinds of body puncture | 87 | 34 |
| other | 70 | 27 |
| without direct patient contact | 228 | 40 |
| tidying up, cleaning and disposal | 129 | 57 |
| other | 99 | 43 |
| not clearly assessable | 78 | 14 |
| not available | 5 | 1 |
Cause of injury, preventability and use of personal protective equipment
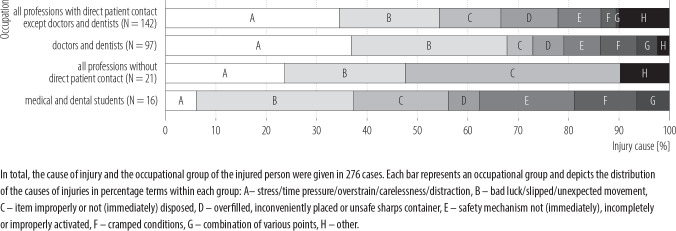
The cause of injury could be derived from the documentation sheets in 49% of cases. Stress, time pressure, over-strain, carelessness and distraction were the main reasons for injuries (33%), followed by bad luck (24%) and the item being improperly or not (immediately) disposed of (13%). The distribution of the causes of injury with respect to the occupational groups is shown in Figure 2.
Figure 2.
Distribution of the causes of injury with regard to the occupational groups, January 2014–June 2016, University Medical Center Hamburg-Eppendorf, Germany
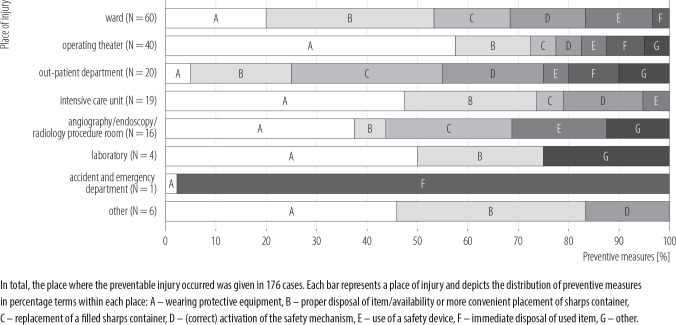
A statement regarding the preventability of the injury could be made in 46% of cases, of which a good third would probably not have been avoidable (34%) compared to almost two-thirds that were likely preventable (66%). There were more avoidable cases without direct patient contact (44%) than with (21%). Cleaning, tidying up and disposing were the major activities (56%) involved in preventable cases. Members of the housekeeping staff were mainly affected by avoidable injuries (91%), followed by the nursing staff (52%). Most of the preventable cases occurred in the intensive care unit (ICU) (44%), on the ward (44%) and in the areas where angiography, endoscopy and radiology are performed (42%). Looking at how these injuries could have been avoided, wearing personal protective equipment (PPE) comes in first place (33%), disposing of an item properly and having a (conveniently placed) sharps container (SC) at one's disposal tie for second (23%), and replacing an overfilled SC third (13%). Bearing in mind the aim of developing preventive strategies, Figure 3 presents ways in which injuries could have been prevented in the different hospital areas.
Figure 3.
Measures how the preventable injuries could have been avoided with regard to the place of injury, January 2014–June 2016, University Medical Center Hamburg-Eppendorf, Germany
Information regarding the use of PPE could be derived from the documentation sheets in 56% of cases. No gloves were worn in at least 4% of cases, even though their use would have been appropriate. No safety goggles and/or surgical mask were used in 75% of the cases in which an eye and/or mouth contamination occurred, even though a splash of BF or solid body material into the face could have theoretically been expected. Surgical and dissecting activities (33%) as well as activities connected with the use of vascular access devices (inserting, flushing, removing and taking a blood sample) (31%) were predominantly concerned. These splash injuries that happened due to a lack of precautionary facial protection measures primarily occurred in the ICU (16%), secondly in the angiography, endoscopy and radiology areas (13%), and thirdly in the operating theater (11%).
Use of safety-engineered device
Taking into account only the injuries involving an item (85%), it was used by the person who injured himself in 59% of the incidents, whereas it was used by somebody else in 28%. Just looking at the latter injuries, the majority of these occurred when the item was no longer being used for its purpose (64%), while it was held in the injurer's hand in 31% at the time of the injury. On the whole, there were more injuries occurring after than during the use of an item (Table 2). Injuries occurring after disposal took place especially in areas where angiography, endoscopy and radiology are performed (20%) and in the ICU (18%).
Table 2.
Time of injury regarding the use and disposal of item, January 2014–June 2016, University Medical Center Hamburg-Eppendorf, Germany
| Time of injury | IInjuries involving an item (N = 485) | |
|---|---|---|
| n | % | |
| During use of item | 178 | 37 |
| After use of item | 229 | 47 |
| before disposal | 91 | 40 |
| during disposal | 76 | 33 |
| after disposal | 35 | 15 |
| item does not need to be disposed | 7 | 3 |
| could not be assessed | 20 | 9 |
| Not assessable | 78 | 16 |
An SED was used in 20% of the cases involving an item. Safety mechanisms that need to be activated manually were most frequent (80%), followed by passively triggered mechanisms (18%) and such which are constantly active (2%). Most of the injuries occurred before the activation of the SM (Table 3). Injuries occurring after the activation were caused by the use of defective devices (50%) and an incomplete activation of the SM (42%). Altogether, 30% of the injuries involving an SED were attributable to an improper use (Table 4).
Table 3.
Activation of safety mechanism of safety-engineered device (SED), January 2014–June 2016, University Medical Center Hamburg-Eppendorf, Germany
| Variable | IInjuries involving a SED (N = 97) | |
|---|---|---|
| n | % | |
| Time of injury with regard to activation of safety mechanism | ||
| before activation | 51 | 53 |
| during activation | 13 | 13 |
| after activation | 12 | 12 |
| safety mechanism constantly active (i.e. blood culture adapter) | 2 | 2 |
| could not be assessed | 19 | 20 |
| Activation status of safety mechanism at the time of injury | ||
| not activated | 57 | 59 |
| incompletely activated | 18 | 19 |
| fully activated | 1 | 1 |
| safety mechanism constantly active (i.e. blood culture adapter) | 2 | 2 |
| could not be assessed | 19 | 20 |
Table 4.
Causes for preventable needlestick and sharps injuries with safety-engineered devices (SED), January 2014–June 2016, University Medical Center Hamburg-Eppendorf, Germany
| Cause | Preventable injuries with SED (N = 29) | |
|---|---|---|
| n | % | |
| Improperly activated | 9 | 31 |
| Not activated at all | 7 | 24 |
| Incompletely activated | 5 | 17 |
| Not immediately activated | 3 | 10 |
| Improper use of intravenous lines | 2 | 7 |
| Improper use of blood culture adapter | 2 | 7 |
| Manual activation of a passively triggered safety mechanism that did not work | 1 | 3 |
DISCUSSION
Against the backdrop of NSSI/BBFE causing considerable health-related and financial burdens [3,5,6,8,9], efforts have been made over recent years to reduce the number of injuries. Nevertheless, exposures of HCW to potentially infectious body material still occur. In this study the unpreventable cases made up almost 16%, closely corresponding to results described by Wicker et al. [7]. In contrast, just over 30% of cases happened needlessly. Preventable injuries were found to lie between 30.9% [12] and 55.2% [7] in other studies. It is essential to understand the mechanisms leading to this kind of NSSI/BBFE so that measures can be adopted to lower the number of injuries to a minimum.
Personal protective equipment
Personal protective equipment protects the skin and mucous membranes from direct contact with potentially infectious BF. In addition, the use of gloves decreases the volume of BF transferred through an NSSI [12,13] and might thus to some extent reduce the risk of infection [2]. In this study, one-third of the avoidable cases could have been prevented by wearing appropriate PPE, indicating a lack of adherence to basic precautions. Such has also been described in other studies where compliance with the use of PPE lay between only 5% [14] and 35% [15]. Although information regarding the use of gloves could be determined in only 44% of the cases, wearing gloves seems to be practiced for the most part at the UKE. Gershon et al. [16] have shown similar results, whereas compliance with glove use was found to be clearly lower in other studies [5,15,17,18]. The use of gloves in this study was notably neglected after having finished an activity, that is when cleaning up, activating the SM of an SED and disposing of used items. By contrast, the inadequate usage of facial protection equipment represents a major problem with 75% of splash injuries to the eye(s) and/or mouth that could have been prevented by wearing protective eyewear and/or a surgical mask. The use of protective eyewear ranges in the literature between 1.6% [15] and 79% [19], that of a surgical mask between 4.1% [15] and 55.5% [16].
Coinciding with results by Nelsing et al. [15], the authors discovered that surgical and dissecting activities as well as activities connected to the use of vascular access devices pose a particular risk for facial splash injuries, stressing the urgency of a more natural use of PPE when such activities are performed. In general, it seems that HCW are more likely to wear PPE the higher the risk of infection is estimated [17,20,21]. According to other studies, major reasons for noncompliance with the usage of PPE appear to be interference with working skills [15,21,22], being impractical [21,22] and time-consuming [14,21,22]. Many HCW underestimate the risk of eye contamination despite wearing spectacles [15] and some do not use a PPE because nobody does it and they fear being a target of ridicule [22].
Waste disposal
Taking into consideration only the cases involving an item, the majority of injuries occurred after its use. Of these, injuries before disposal were most frequent, followed by NSSI that either happened during or after disposal. Focusing on the item-related avoidable cases alone, 91% occurred after use: 30% before, 35% during and 30% after disposal. In other studies, NSSI taking place after the use of an item but before its disposal made up between 9.4% [23] and 31% [18], those happening during disposal between 5% [8] and 46.6% [24]. Having disposed of an item improperly, not having disposed of it immediately or not having disposed of it at all was the third most frequent cause of injury in this study. In the literature, rates of improper disposal range between 6.2% [5] and 28% [18].
Coinciding with results of other studies [18,25–27], the authors of this manuscript could show that the housekeeping staff was at the highest risk of receiving an NSSI as a consequence of improper disposal. Eighty-two percent of the injured cleaning staff suffered from an injury due to a sharp being disposed of in a garbage bag or being left on the floor. What makes these cases even worse is the fact that the IP is usually not known [25]. Health care workers must be made aware that irresponsible behavior in terms of sharps disposal can have severe impacts on other hospital staff. On the other hand, there were cases recorded where SC were available but injuries occurred nonetheless. For the most part, these container-associated sharps injuries (CASI) were attributable to overfilled SC. The SC was inconveniently placed in three cases and it was punctured by the item in one case.
In a study by Floret et al. [5], the number of CASI lay in 6.7–9.1%. Dulon et al. [8] have shown a CASI rate of 13.5% based on the same reasons as in this study. There is no doubt that SC are essential in terms of safety, however, they need to be placed, used and designed in a way that does not increase the risk of injury. As reported by Grimmond et al. [28], >90% of CASI are related to container design. Sharps containers with a large aperture, a deep atrium and a passive overfill protection allowing for one-handed deposit and that are situated close-at-hand seemed to reduce the risk of obtaining a CASI [29].
In another study by Grimmond and Naisoro [29], the rate of CASI could be significantly decreased by replacing small transportable SC with SC that had a large capacity and were constantly placed in the room where the sharps were used. Bearing these results in mind, SC presently used at the UKE should undergo an evaluation and the placement of SC in each hospital room should be considered. In the end, every HCW must be made aware of his responsibility to replace a full SC so that overfilling will not even occur.
Safety-engineered device
In Germany the use of SED for activities going along with a high infectious potential became obligatory in August 2007 [30]. In accordance with the TRBA 250, the activation of the SM must either work automatically or be performed one-handed right after use. The completed activation of the SM must be clearly recognizable [1]. On the one hand, it seems that the introduction of SED had a lowering effect on the total number of NSSI [5,31–33]; on the other, the proportion of injuries associated with SED has risen [31,34,35]. In this study, 20% of the item-related cases were caused by SED compared to rates of 9.8% [31] to 44% [14] described in the literature. In accordance with these results, several studies have shown that the majority of SED-associated injuries occur before the activation of the device [31,36] or when the SM is not activated [14,35,37]. In this study 82% of the avoidable SED-related injuries could have been prevented by an (immediate, complete or proper) activation of the safety feature. Dulon et al. [2,8] have shown similar results and identified a lack of practical experience as the major cause of failure when SED were used. Being stressed and/or overworked seems to further increase insecurities in terms of correct SED usage [2]. Just as described by other authors [2,8], SED with SM which need to be activated manually made up the principal share in this study. At the same time, the risk of obtaining an NSSI appears to be highest when using manually activated SED and lowest when using SED with passively triggered features; semiautomatic devices lie in between [31]. Where applicable, a more comprehensive provision of automatic SED should thus be considered.
CONCLUSIONS
All in all, 2 factors seem to be decisive in view of reducing NSSI/BBFE:
-
–
taking organizational measures (e.g. providing suitable PPE, SED and SC with a low risk of obtaining a CASI),
-
–
offering regular trainings for HCW and students.
As presented in a meta-analysis by Tarigan et al. [38], training interventions on standard precautions led to a reduction of NSSI rate by 34%, introducing SED (and SC) reduced the rate of NSSI by 49%. The protective effect of combining training with the use of SED was shown to be even higher. In the 2011/2012 winter semester a tutorial was introduced at the UKE giving students the opportunity to practice the correct performance of blood sampling and intravenous line insertion. It is conceivable that this might have contributed to some extent to the above-mentioned significant decrease in injuries over the study period especially in the age group of those <35 years. However, it remains unclear why the number of cases was comparatively high in the first half-year of 2014 and what exactly led to the conspicuously sudden drop hereafter. Same as described by other authors [2,7,8,24], stress, time pressure, overstrain, carelessness and distraction were the main causes for injury in this study. It is therefore important to develop strategies to improve the working conditions and reduce workloads.
Limitations
This study only depicts the NSSI/BBFE that happened at a single German university hospital and is based on self-reported cases alone. It therefore most likely does not take into account the entire number of injuries that occurred during the study period. Underreporting rates described in the literature come up to 90% [4]. Perceiving the risk of infection to be low and a lack of time appear to be the main reasons for underreporting [36,39]. The results of this study might be distorted by the reporting behavior, which needs to be borne in mind when interpreting them. Due to the fact that the study was conducted retrospectively, only the information given on the documentation sheets could be used for data collection. Especially with regard to the free-text fields, it was not always possible to gain complete information and despite the precise definition of the variables, a certain scope for interpretation remained.
Concluding general implications for practice
Despite the implementation of precautionary measures, experiencing a NSSI/BBFE remains a relevant risk concerning all occupational groups and areas of work in the hospital.
Health care workers, students and trainees should thus be regularly sensitized for this topic and made aware of the potential risks that can lead to an injury. In this connection, it is favorable that regular trainings are offered by employers, company doctors and medical faculties. Beyond this, printed and online newsletters, start pages of frequently used work-related computer programs and posters positioned at places in the hospital where they easily catch the eye, could be used to draw attention to the topic.
The working environment should encourage HCW and students to practice an open error culture that allows identifying risk potentials based on which solutions can be developed.
Structural measures such as provision of PPE, SED and safe disposal conditions need to be ensured. At the same time, understaffing should be avoided as far as possible to decrease the workload for each HCW and thus reduce the risk of stress related injuries.
In conclusion, NSSI/BBFE will probably never be completely avoidable; nonetheless, the implementation of above mentioned measures can contribute to a reduction of their number and like this to a diminution of consequences concerning health and financial aspects.
REFERENCES
- 1.TRBA 250 (Technical Rules for Biological Agents). Federal Institute for Occupational Safety and Health; website. [cited 2019 Feb 27]. Available from: https://www.baua.de/DE/Angebote/Rechtstexte-und-TechnischeRegeln/Regelwerk/TRBA/pdf/TRBA-250.pdf.blob=publicationFile&v=4. [Google Scholar]
- 2.Dulon M, Lisiak B, Wendeler D, Nienhaus A.. Workers' Compensation Claims for Needlestick Injuries Among Healthcare Personnel in Hospitals, Doctors' Surgeries and Nursing Institutions. Gesundheitswesen 2017. [DOI] [PubMed] [Google Scholar]
- 3.Elseviers MM, Arias-Guillen M, Gorke A, Arens HJ.. Sharps injuries amongst healthcare workers: review of incidence, transmissions and costs. J Ren Care 2014; 40:150–6. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann F, Kralj N, Beie M.. Needle stick injuries in health care - frequency, causes und preventive strategies. Gesundheitswesen 2002; 64:259–66. [DOI] [PubMed] [Google Scholar]
- 5.Floret N, Ali-Brandmeyer O, L'Hériteau F, Bervas C, Barquins--Guichard S, Pelissier G,. et al. Sharp Decrease of Reported Occupational Blood and Body Fluid Exposures in French Hospitals, 2003-2012: Results of the French National Network Survey, AES-RAISIN. Infect Control Hosp Epidemiol 2015; 36:963–8. [DOI] [PubMed] [Google Scholar]
- 6.Sohn JW, Kim BG, Kim SH, Han C.. Mental health of healthcare workers who experience needlestick and sharps injuries. J Occup Health 2006; 48:474–9. [DOI] [PubMed] [Google Scholar]
- 7.Wicker S, Stirn AV, Rabenau HF, von Gierke L, Wutzler S, Stephan C.. Needlestick injuries: causes, preventability and psychological impact. Infection 2014; 42:549–52. [DOI] [PubMed] [Google Scholar]
- 8.Dulon M, Lisiak B, Wendeler D, Nienhaus A.. Causes of needlestick injuries in three healthcare settings: analysis of accident notifications registered six months after the implementation of EU Directive 2010/32/EU in Germany. J Hosp Infect 2017; 95:306–11. [DOI] [PubMed] [Google Scholar]
- 9.Glenngard AH, Persson U.. Costs associated with sharps injuries in the Swedish health care setting and potential cost savings from needle-stick prevention devices with needle and syringe. Scand J Infect Dis 2009; 41:296–302. [DOI] [PubMed] [Google Scholar]
- 10.Council Directive 2010/32/EU. EUR-Lex website. [cited 2019 Feb 27]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/.uri=CELEX:32010L0032.
- 11.UKE website. [cited 2021 Nov 28]. Available from: https://www.uke.de/allgemein/presse/zahlen-fakten/index.html
- 12.Mast ST, Woolwine JD, Gerberding JL.. Efficacy of gloves in reducing blood volumes transferred during simulated needlestick injury. J Infect Dis 1993; 168:1589–92. [DOI] [PubMed] [Google Scholar]
- 13.Lefebvre DR, Strande LF, Hewitt CW.. An enzyme-mediated assay to quantify inoculation volume delivered by suture needlestick injury: two gloves are better than one. J Am Coll Surg 2008; 206:113–22. [DOI] [PubMed] [Google Scholar]
- 14.Green-McKenzie J, McCarthy RB, Shofer FS.. Characterisation of occupational blood and body fluid exposures beyond the Needlestick Safety and Prevention Act. Journal of infection prevention 2016; 17:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelsing S, Nielsen TL, Nielsen JO.. Noncompliance with universal precautions and the associated risk of mucocutaneous blood exposure among Danish physicians. Infect Control Hosp Epidemiol 1997; 18:692–8. [DOI] [PubMed] [Google Scholar]
- 16.Gershon RR, Vlahov D, Felknor SA, Vesley D, Johnson PC, Delclos GL,. et al. Compliance with universal precautions among health care workers at three regional hospitals. Am J Infect Control 1995; 23:225–36. [DOI] [PubMed] [Google Scholar]
- 17.Kinlin LM, Mittleman MA, Harris AD, Rubin MA, Fisman DN.. Use of gloves and reduction of risk of injury caused by needles or sharp medical devices in healthcare workers: results from a case crossover study. Infect Control Hosp Epidemiol 2010; 31:908–17. [DOI] [PubMed] [Google Scholar]
- 18.Kevitt F, Hayes B.. Sharps injuries in a teaching hospital: changes over a decade. Occup Med (Lond) 2015; 65:135–8. [DOI] [PubMed] [Google Scholar]
- 19.Hasak JM, Novak CB, Patterson JMM, Mackinnon SE.. Prevalence of Needlestick Injuries, Attitude Changes, and Prevention Practices Over 12 Years in an Urban Academic Hospital Surgery Department. Ann Surg 2018; 267:291–6. [DOI] [PubMed] [Google Scholar]
- 20.Hettiaratchy S, Hassall O, Watson C, Wallis D, Williams D.. Glove usage and reporting of needlestick injuries by junior hospital medical staff. Ann R Coll Surg Engl 1998; 80:439–41. [PMC free article] [PubMed] [Google Scholar]
- 21.Scheller B, Wicker S, Rabenau HF, Marzi I, Wutzler S.. Risk estimation of blood-borne infections by emergency room personnel. Unfallchirurg 2016; 119:575–80. [DOI] [PubMed] [Google Scholar]
- 22.Wicker S, Wutzler S, Schachtrupp A, Zacharowski K, Scheller B.. Occupational exposure to blood in multiple trauma care. Anaesthesist 2015; 64:33–8. [DOI] [PubMed] [Google Scholar]
- 23.Wicker S, Jung J, Allwinn R, Gottschalk R, Rabenau HF.. Prevalence and prevention of needlestick injuries among health care workers in a German university hospital. Int Arch Occup Environ Health 2008; 81:347–54. [DOI] [PubMed] [Google Scholar]
- 24.Wicker S, Ludwig AM, Gottschalk R, Rabenau HF.. Needlestick injuries among health care workers: occupational hazard or avoidable hazard. Wien Klin Wochenschr 2008; 120:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rymer W, Gladysz A, Filipowski H, Zubkiewicz-Zarebska A, Tuminska A, Knysz B.. Risk of occupational exposure to the HBV infection in non-clinical healthcare personnel. Med Pr 2016; 67:301–10. [DOI] [PubMed] [Google Scholar]
- 26.Ream PS, Tipple AF, Salgado TA,. et al. Hospital housekeepers: Victims of ineffective hospital waste management. Arch Environ Occup Health 2016; 71:273–80. [DOI] [PubMed] [Google Scholar]
- 27.Ream PS, Tipple AF, Barros DX, Souza AC, Pereira MS.. Biological risk among hospital housekeepers. Arch Environ Occup Health 2016; 71:59–65. [DOI] [PubMed] [Google Scholar]
- 28.Grimmond T, Bylund S, Anglea C,. et al. Sharps injury reduction using a sharps container with enhanced engineering: a 28 hospital nonrandomized intervention and cohort study. Am J Infect Control 2010; 38:799–805. [DOI] [PubMed] [Google Scholar]
- 29.Grimmond T, Naisoro W.. Sharps injury reduction: a six-year, three-phase study comparing use of a small patientroom sharps disposal container with a larger engineered container. Journal of infection prevention 2014; 15:170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittmann A, Zylka-Menhorn V.. Arbeitsschutz: Verletzungssichere Instrumente für Kliniken und Praxen obligatorisch. Dtsch Arztebl International 2007;104(10):A-624/B-549/C-528. [Google Scholar]
- 31.Tosini W, Ciotti C, Goyer F,. et al. Needlestick injury rates according to different types of safety engineered devices: results of a French multicenter study. Infect Control Hosp Epidemiol 2010; 31:402–7. [DOI] [PubMed] [Google Scholar]
- 32.Frickmann H, Schmeja W, Reisinger E,. et al. Risk Reduction of Needle Stick Injuries Due to Continuous Shift from Unsafe to Safe Instruments at a German University Hospital. European journal of microbiology & immunology 2016; 6: 227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambers A, Mustard CA, Holness DL, Nichol K, Breslin FC.. Barriers to the Adoption of Safety Engineered Needles Following a Regulatory Standard: Lessons Learned from Three Acute Care Hospitals. Healthc Policy 2015; 11:90–101. [PMC free article] [PubMed] [Google Scholar]
- 34.Kanamori H, Weber DJ, DiBiase LM,. et al. Impact of Safety-Engineered Devices on the Incidence of Occupational Blood and Body Fluid Exposures Among Healthcare Personnel in an Academic Facility, 2000-2014. Infect Control Hosp Epidemiol 2016; 37:497–504. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell AH, Parker GB, Kanamori H, Rutala WA, Weber DJ.. Comparing non-safety with safety device sharps injury incidence data from two different occupational surveillance systems. J Hosp Infect 2017; 96:195–8. [DOI] [PubMed] [Google Scholar]
- 36.Kessler CS, McGuinn M, Spec A, Christensen J, Baragi R, Hershow RC.. Underreporting of blood and body fluid exposures among health care students and trainees in the acute care setting: a 2007 survey. Am J Infect Control 2011; 39:129–34. [DOI] [PubMed] [Google Scholar]
- 37.Black L. Chinks in the armor: percutaneous injuries from hollow bore safety-engineered sharps devices. Am J Infect Control 2013; 41:427–32. [DOI] [PubMed] [Google Scholar]
- 38.Tarigan LH, Cifuentes M, Quinn M, Kriebel D.. Prevention of needle-stick injuries in healthcare facilities: a meta-analysis. Infect Control Hosp Epidemiol 2015; 36: 823–9. [DOI] [PubMed] [Google Scholar]
- 39.Voide C, Darling KE, Kenfak-Foguena A, Erard V, Cavassini M, Lazor-Blanchet C.. Underreporting of needlestick and sharps injuries among healthcare workers in a Swiss University Hospital. Swiss Med Wkly 2012; 142:w13523. [DOI] [PubMed] [Google Scholar]