Abstract
Background
Clinician adherence to guideline recommendations in the pharmacological therapy of schizophrenia is important for favorable patient outcomes. To evaluate whether prescriptions followed the guidelines for pharmacological therapy of schizophrenia, we recently developed a summary indicator of multiple quality indicators: the individual fitness score (IFS). It is unclear whether adherence to the guidelines is related to patient outcomes. Here, we investigated correlations between the IFS values and psychotic symptoms in patients with schizophrenia.
Methods
We assessed whether patients’ current prescriptions adhered to the guideline recommendations using the IFS in 47 patients with treatment-resistant schizophrenia (TRS) and 353 patients with non-TRS (total n = 400), respectively. We investigated correlations between the IFS and total scores and scores on the 5 subscales of the Positive and Negative Syndrome Scale (PANSS). Furthermore, we explored correlations between over 2-year longitudinal changes in IFS values and changes in psychotic symptoms in some patients (n = 77).
Results
We found significant negative correlation between the IFS and PANSS total score in all patients with schizophrenia (β = −0.18, P = 9.80 × 10−5). The IFS was significantly and nominally negatively correlated with the PANSS total score in patients with non-TRS (Spearman’s rho = −0.15, P = 4.40 × 10−3) and patients with TRS (rho = −0.37, P = .011), respectively. The IFS was also significantly and nominally negatively correlated with several factors, such as the negative and depressed factors, in patients with non-TRS and patients with TRS, respectively (P < .05). Furthermore, the change in IFS values was marginally negatively correlated with the changes in PANSS total scores and scores on the positive and depressed factors (P < .05).
Conclusions
These findings suggest that efforts to improve clinician adherence to guideline recommendations for pharmacological therapy of schizophrenia, as assessed by the IFS, may lead to better outcomes in patients with schizophrenia.
Keywords: Guidelines, pharmacological therapy, schizophrenia, PANSS, individual fitness score
Significance Statement.
We investigated relationships between clinician adherence to guideline recommendations for pharmacological therapy of schizophrenia and psychotic symptoms in patients with schizophrenia. The adherence to guidelines was assessed using a summary indicator of multiple quality indicators: the individual fitness score (IFS). Higher IFS values, indicating better adherence to guideline recommendations, were associated with lower levels of psychotic symptoms in patients. Improvements in IFS values over a 2-year period might be correlated with improvements in psychotic symptoms.
INTRODUCTION
Schizophrenia is a chronic psychiatric disorder characterized by a combination of positive symptoms (such as hallucinations, delusions, and disorganized thinking), negative symptoms (such as lack of motivation and social withdrawal), and/or cognitive impairments. Pharmacotherapy is a crucial part of the treatment strategy for schizophrenia, along with psychotherapy and social support. The primary goal of pharmacotherapy for schizophrenia is to reduce symptoms and improve quality of life (QoL).
Antipsychotic medications are the core of pharmacotherapy for schizophrenia. There are 2 main classes of antipsychotic medications: first-generation (typical) antipsychotics (FGAs) and second-generation (atypical) antipsychotics (SGAs). Both classes of antipsychotics are effective in treating positive symptoms of schizophrenia, but SGAs are often preferred due to their lower incidence of extrapyramidal side effects, such as parkinsonism and tardive dyskinesia (Leucht et al., 2009). SGAs are also effective in treating negative symptoms and cognitive deficits in schizophrenia (Woodward et al., 2005; Zhang et al., 2013; Siskind et al., 2016). However, SGAs are associated with metabolic side effects, such as weight gain, hyperlipidemia, and diabetes (Roerig et al., 2011; Volpato et al., 2013; Guenette et al., 2014; Jeon and Kim, 2017). Clozapine is an SGA that is reserved for treatment-resistant schizophrenia (TRS). It is highly effective in improving positive symptoms, negative symptoms, and cognitive impairments (Siskind et al., 2016). However, clozapine is associated with increased risks of hypersalivation, agranulocytosis, and myocarditis.
In addition to antipsychotics, other medications, such as anticholinergics, benzodiazepines, antidepressants, and mood stabilizers, may also be used as adjunctive therapy in the treatment of specific symptoms of schizophrenia. Anticholinergics may be used to treat extrapyramidal side effects or akathisia associated with antipsychotics; benzodiazepines may be used to treat anxiety, agitation, or insomnia; antidepressants may be used to treat depression or anxiety; and mood stabilizers may be used to treat mood swings or aggression. However, these adjunctive medications have multiple potential risks, including dry mouth, constipation, urinary retention, sedation, cognitive impairments, medication dependence, and withdrawal symptoms (Leucht et al., 2009; Desmarais et al., 2012). Therefore, their use in patients with schizophrenia needs to be carefully considered.
Polypharmacy, the concurrent use of multiple medications, is common and a complex issue in the treatment of schizophrenia (Kishimoto et al., 2013; Bighelli et al., 2022). Polypharmacy in schizophrenia is often based on clinical experience rather than evidence-based practice. There is a lack of research on the safety and efficacy of polypharmacy. Several issues associated with polypharmacy in schizophrenia include increased risk of side effects, reduced QoL, drug‒drug interactions that reduce efficacy or increase toxicity, increased health care costs, and poor treatment adherence (Takeuchi et al., 2015; Heald et al., 2017; Aly El-Gabry et al., 2018; Pae et al., 2021). The American Psychiatric Association’s Practice Guideline and the National Institute for Health and Care Excellence guideline recommend that clinicians carefully evaluate the risks and benefits of polypharmacy vs monotherapy as the first-line treatment options whenever possible (Taylor and Perera, 2015; Keepers et al., 2020). Similarly, the Guidelines for Pharmacological Therapy of Schizophrenia published by the Japanese Society of Neuropsychopharmacology recommend that clinicians perform monotherapy, use the minimum effective dose of medication, and avoid unnecessary polypharmacy (Japanese Society of Neuropsychopharmacology, 2021). Thus, many guidelines emphasize monotherapy and the importance of minimizing the use of multiple medications due to the potential risks and uncertainties associated with polypharmacy (Hasan et al., 2012; Castle et al., 2017; Crockford and Addington, 2017; Japanese Society of Neuropsychopharmacology, 2021).
Adherence to guideline recommendations in the treatment of schizophrenia is important to the quality of treatments and patient QoL and outcomes. Adherence to guideline recommendations can vary depending on several factors, including the clinician’s experience and training, patient preferences, and availability of resources. While guidelines provide evidence-based recommendations and best practices, they may not always be followed in clinical practice due to various barriers, such as the lack of awareness or familiarity with the guidelines among clinicians, that is, a gap between the guidelines and actual clinical practice (evidence–practice gap). Thus, to disseminate the guidelines and standardize medical practice and improve adherence to guidelines, we provide regular training and education in the “Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)” project (Takaesu et al., 2019; Ichihashi et al., 2020; Iida et al., 2020; Numata et al., 2021) on the Guidelines for Pharmacological Therapy of Schizophrenia (Japanese Society of Neuropsychopharmacology, 2021). The EGUIDE project aimed to ensure the social implementation of treatment guidelines for schizophrenia by conducting training sessions and evaluating whether trained psychiatrists follow these guidelines.
Assessing adherence to guideline recommendations in schizophrenia can be challenging, as it requires evaluating the extent to which clinical practice aligns with the guideline. Several methods can be used to assess adherence, including medical record reviews; surveys assessing clinician knowledge, attitudes, and practices; and clinical performance feedback regarding adherence to guideline recommendations and patient outcomes (Owen et al., 2000; Dickey et al., 2006; Stiles et al., 2009; Drosos et al., 2020; Jin et al., 2021). Based on these methods, we can assess quality indicators (QIs), such as the percentage of patients who received appropriate medication. However, we should assess several QIs according to multiple guideline recommendations; thus, a single indicator summarizing multiple QIs (with values ranging from 0 to 100 points) can facilitate evaluation of adherence to guideline recommendations. Recently, we developed an individual fitness score (IFS) in each patient to evaluate whether pharmacological treatments (prescriptions) in each clinician adhere to the Guidelines for Pharmacological Therapy of Schizophrenia (see Table 1 in the paper by Inada et al., 2022) and confirmed the validity of the IFS using a survey of the prescriptions at admission and discharge among EGUIDE project participants (Inada et al., 2022). However, a combined evaluation of clinician adherence and patient outcomes is needed. It is not known whether higher IFS values (indicating greater adhering to guideline recommendations in schizophrenia) are related to better patient outcomes.
Table 1.
Demographic Characteristics of Patients With Treatment-Resistant Schizophrenia and Non-TRS
| TRS (n = 47) | non-TRS (n = 353) | z or χ2 | P | |
|---|---|---|---|---|
| Age, y | 39.4 (11.3) | 36.3 (13.0) | −1.9 | .051 |
| Sex, M/F (% male) | 26/21 (55.3) | 179/174 (50.7) | 0.2a | .55 |
| Age at onset, y | 21.3 (7.9) | 24.4 (10.7) | 2.2 | .027 |
| Education, y | 13.0 (2.1) | 14.0 (2.5) | 2.8 | 5.47 × 10 −3 |
| Estimated premorbid IQ | 97.6 (11.9) | 101.8 (9.8) | 2.0 | .046 |
| Duration of illness, y | 18.0 (9.9) | 11.8 (9.6) | −4.3 | 1.96 × 10 −5 |
| PANSS: total score | 102.5 (21.8) | 78.5 (21.2) | −6.5 | 7.76 × 10 −11 |
| Positive score | 14.9 (3.3) | 11.1 (3.7) | −6.3 | 3.50 × 10 −10 |
| Negative score | 22.7 (5.0) | 17.0 (5.5) | −6.5 | 1.14 × 10 −10 |
| Disorganized score | 10.6 (3.0) | 8.0 (2.5) | −5.5 | 3.86 × 10 −8 |
| Excited score | 12.0 (4.0) | 8.8 (3.2) | −5.1 | 2.83 × 10 −7 |
| Depressed score | 8.2 (2.6) | 7.3 (2.7) | −2.3 | .022 |
| Individual fitness score | 51.8 (44.1) | 60.6 (36.0) | 1.4 | .18 |
| Total CP-eq dosage (mg/d) | 1153.7 (453.4) | 491.4 (460.0) | −8.4 | 3.83 × 10 −17 |
| Atypical CP-eq dosage (mg/d) | 1118.1 (422.2) | 452.7 (437.3) | −8.7 | 4.69 × 10 −18 |
| Typical CP-eq dosage (mg/d) | 35.6 (137.6) | 38.7 (160.1) | 0.1 | .89 |
| BPD-eq dosage (mg/d) | 0.5 (1.2) | 0.8 (1.5) | 1.6 | .11 |
| Imipramine-eq dosage (mg/d) | 3.3 (22.8) | 5.9 (23.2) | 1.5 | .15 |
| Diazepam-eq dosage (mg/d) | 4.2 (7.8) | 6.5 (11.2) | 1.5 | .14 |
| DIEPSS total score | 1.3 (1.0) | 0.5 (0.7) | −6.0 | 1.70 × 10 −9 |
Abbreviations: BPD-eq, biperiden equivalents (of total antiparkinsonian drugs); CP-eq, chlorpromazine equivalents (of antipsychotics); DIEPSS, Drug-Induced Extrapyramidal Symptoms Scale; IQ, intelligence quotient; PANSS, Positive and Negative Syndrome Scale; TRS, treatment-resistant schizophrenia. Complete demographic information was not obtained for all patients (estimated premorbid IQ in TRS, n = 27; non-TRS, n = 249, DIEPSS score in TRS, n = 46; non-TRS, n = 313). Means (SDs) are shown.
a χ 2 test. P values <.05 are shown in bold.
We hypothesized that higher IFS values (indicating better adherence to recommended pharmacological therapy for schizophrenia) would be correlated with lower psychotic symptoms in patients with schizophrenia. In this study, we investigated correlations of IFS values with total scores and scores on 5 subscales of the Positive and Negative Syndrome Scale (PANSS) in patients with schizophrenia (n = 400). Furthermore, we explored correlations of changes in IFS values over a 2-year period with changes in PANSS total scores and subscale scores in patients with schizophrenia, although the sample size (n = 77) was limited.
METHODS
Participants
Patients with schizophrenia (n = 467) were recruited from both the outpatient and inpatient populations at Osaka University Hospital. All patients were not biologically related within the second degree of kinship and were of Japanese descent. Each patient had been diagnosed by at least 2 trained psychiatrists according to the criteria of the “Diagnostic and Statistical Manual of Mental Disorders,” fourth edition (DSM-IV), based on the Structured Clinical Interview for the DSM-IV.
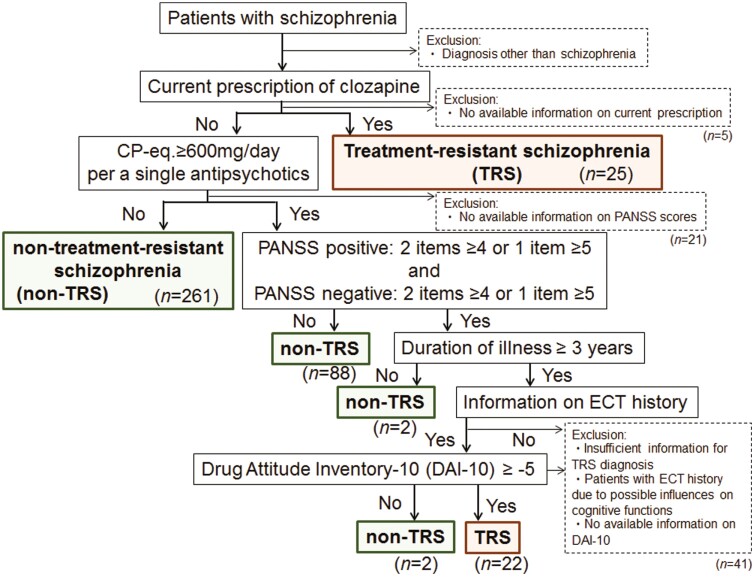
To assess whether patients had TRS or nontreatment-resistant schizophrenia (non-TRS), we used a flowchart prepared with reference to a previous study (Howes et al., 2017) in Figure 1. Patients were divided into TRS and non-TRS groups based on a current prescription of clozapine, current positive and negative symptom severity assessed by the PANSS (Kay et al., 1987), duration of illness, electroconvulsive therapy (ECT) history, and current drug adherence assessed by the Drug Attitude Inventory-10 (Nielsen et al., 2012) (Figure 1). The IFS in each patient was designed to evaluate whether pharmacotherapy in each clinician adhered to the treatment guidelines for schizophrenia without considering individual patient characteristics, such as the presence or absence of comorbidities, except for TRS and non-TRS (Inada et al., 2022). However, to exclude the possibility that neurological or medical conditions might affect the central nervous system and thus psychiatric symptoms, patients with neurological or medical conditions, such as atypical headaches, head trauma with loss of consciousness, chronic lung disease, kidney disease, chronic hepatic disease, thyroid disease, active cancer, cerebrovascular disease, epilepsy, seizures, substance-related disorders, or intellectual disability, were further excluded from this study. Finally, 47 patients with TRS (26 males/21 females, mean age ± SD: 39.4 ± 11.3 years) and 353 patients with non-TRS (179 males/174 females, mean age ± SD: 36.3 ± 13.0 years) were included (Table 1). Patients with TRS had younger age at onset, lower estimated premorbid IQ, fewer years of education, higher PANSS total scores and subscale scores, took higher chlorpromazine equivalent (CP-eq) dosages, and had higher total scores on the Drug-Induced Extrapyramidal Symptoms Scale than patients with non-TRS (P < .05; Table 1). Among 400 patients with schizophrenia at Time 2 (T2, at present), we retrospectively extracted prescriptions from more than 2 years earlier (at Time 1, T1) as well as the PANSS total scores and subscale scores among 77 patients with schizophrenia. Demographic characteristics at T2 between 77 followed and 323 not followed patients with schizophrenia are shown in supplementary Table 1. In addition, clinical characteristics and IFS between 77 patients at T1 and T2 are shown in supplementary Table 2.
Figure 1.
Flowchart of the method used to classify patients with schizophrenia as having treatment-resistant schizophrenia (TRS) or non-TRS schizophrenia. This flowchart displays the identification of TRS. Patients who did not meet the criteria of TRS or in whom a diagnosis of TRS could not be determined (e.g., due to the improvement of symptoms when taking a high chlorpromazine equivalent [CP-eq] dosage or with better medication adherence [drug attitude]) were treated as those with non-TRS. DAI-10, 10-item version of Drug Attitude Inventory; ECT, electroconvulsive therapy; PANSS, Positive and Negative Syndrome Scale.
Written informed consent was obtained from all participants after the procedures had been thoroughly explained. This study was performed in accordance with the Declaration of Helsinki from the World Medical Association and was approved by the Research Ethics Committees of Osaka University and the National Center of Neurology and Psychiatry (B2022-044).
Measurement of IFS
To assess the degree to which prescriptions for patients with schizophrenia adhered to the Guidelines for Pharmacological Therapy of Schizophrenia, we utilized the IFS (see Table 1 in the paper by Inada et al., 2022). The IFS ranges from 0 to 100, with higher scores indicating better adherence to the guideline recommendations of pharmacological therapy (Inada et al., 2022).
Briefly, an IFS of 100 is given for complete adherence to the guidelines; this value is assumed, and values are deducted for any nonrecommended treatment. The IFS was developed separately for non-TRS and TRS because the treatment strategies differ between non-TRS and TRS. For non-TRS, SGA monotherapy with an appropriate dose is given 100 points. Values are deducted for any concomitant use of nonrecommended medications. For the concomitant use of antipsychotics, the score is deducted for excessive high-dose prescriptions; 25 points are deducted for exceeding the appropriate dose, and 50 points are deducted for exceeding the dose by more than 1.5 times. For multiple-drug combinations, 25 points are deducted for 2-drug combinations, and 50 points are deducted for use of 3 or more drugs; these deductions are larger for higher doses. As the guidelines recommend the use of SGAs rather than FGAs, 5 points are deducted for the use of FGAs. Furthermore, concomitant use of psychotropic drugs, such as antidepressants, is penalized; 15 points are deducted for concomitant use of 1 drug, 35 points are deducted for 2 drugs, and 55 points are deducted for 3 or more drugs. A total 80 points are deducted for the use of dopaminergic agents because their effects oppose those of antipsychotic agents, and their concomitant use is not rational. For TRS, treatment with clozapine or ECT is recommended. Thus, a large deduction (60 points) was made if none of these treatments were used. If the total score is less than zero, the score is set to zero.
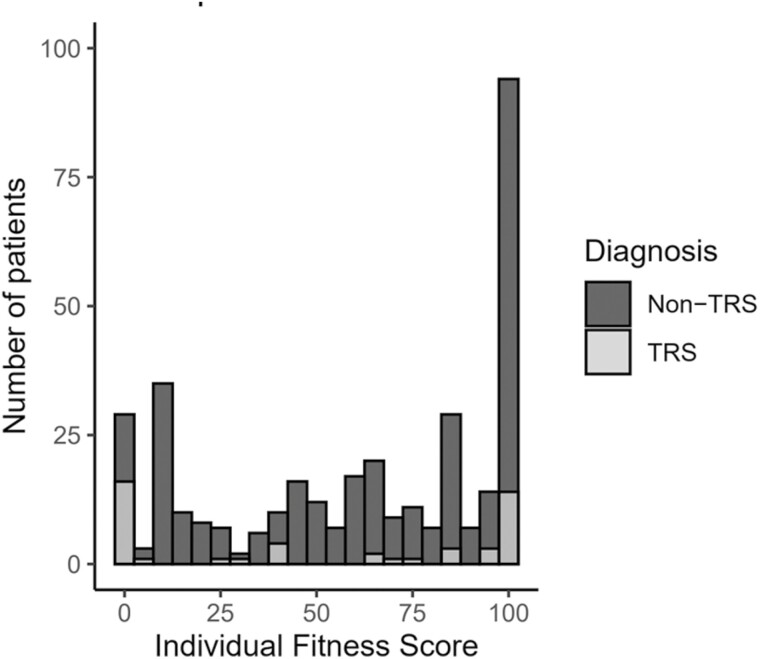
The distributions of IFS values in patients with TRS and non-TRS are shown in Figure 2. Although the IFS values ranged from 0 to 100 in patients with TRS and non-TRS and there was no difference in the IFS values between the 2 groups, the IFS values among the TRS group were distributed mostly at 0 or 100.
Figure 2.
Distribution of individual fitness score (IFS) values in patients with treatment-resistant schizophrenia (TRS) and non-TRS.
Assessments of Psychotic Symptoms
To assess the psychotic symptoms of schizophrenia, we used the PANSS total score and scores on the 5 subscales: positive, negative, disorganized/concrete, excited, and depression/anxiety factors (Kay et al., 1987; Wallwork et al., 2012).
Statistical Analysis
All statistical analyses were performed using IBM SPSS 28.0 (IBM Japan, Tokyo, Japan). Since we assumed that the IFS values and PANSS scores were not normally distributed using Kolmogorov-Smirnov test (P < .05), we performed a nonparametric test in this study. Differences in continuous variables, such as age and years of education, were compared between patients with TRS and patients with non-TRS using Mann-Whitney U tests. Differences in categorical variables, such as sex, were compared between groups using Pearson χ2 test. Differences in clinical characteristics and IFS were compared between patients at more than 2 years earlier (T1) and at present (T2) using paired t tests. Influence of the IFS on PANSS total scores in whole patients with schizophrenia was assessed using a linear regression analysis with PANSS scores as a dependent variable, the IFS as an independent variable, and the diagnosis of TRS or non-TRS as a covariate. Correlations of IFS values with PANSS total scores and subscale scores were assessed using Spearman correlation analyses in patients with TRS and non-TRS, respectively. To explore correlations of changes in IFS values over 2 years with changes in PANSS total scores and scores on the 5 subscales in patients with schizophrenia, we performed a linear regression analysis with ΔPANSS total score (T2 − T1) as a dependent variable, ΔIFS (T2 − T1) as an independent variable, and the TRS diagnosis as a covariate. The nominal significance level was set at a 2-tailed P < .05 for all statistical tests. A Bonferroni-corrected P value threshold of <.01 (α = .05/5 subscales) was used to avoid type I error.
RESULTS
Relationships of IFS Values With PANSS Total Scores and Scores on the Five Subscales in Patients With Schizophrenia
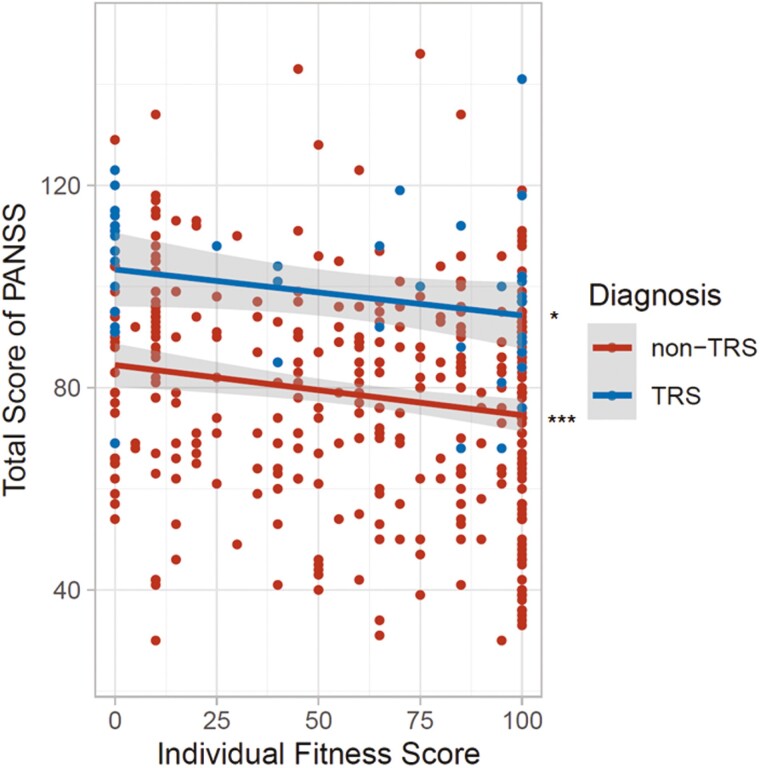
We first investigated the correlation between IFS values and PANSS total scores in whole patients with schizophrenia and found significant negative correlation between IFS values and PANSS total scores in whole patients with schizophrenia (β = −0.18, P = 9.80 × 10−5). As the IFS was calculated by separate formulas for TRS and non-TRS (see Table 1 in the paper by Inada et al., 2022), we next investigated the correlation between IFS values and PANSS total scores in patients with non-TRS and patients with TRS, respectively (Figure 3). IFS values were significantly and nominally negatively correlated with PANSS total scores in patients with non-TRS (Spearman rho = −0.15, P = 4.40 × 10−3) and in patients with TRS (rho = −0.37, P = .011), respectively. Higher IFS values (indicating better adherence to recommended pharmacological therapy for schizophrenia) were correlated with lower psychotic symptoms in patients with schizophrenia.
Figure 3.
The relationship between the individual fitness score (IFS) and total score on the Positive and Negative Syndrome Scale (PANSS) in patients with schizophrenia. A higher IFS indicates better clinician adherence to the recommended pharmacological therapy for schizophrenia, and a lower PANSS total score indicates milder psychotic symptoms in patients with schizophrenia. Abbreviations: non-TRS, nontreatment-resistant schizophrenia; TRS, treatment-resistant schizophrenia. *P < .05, ***P < .001.
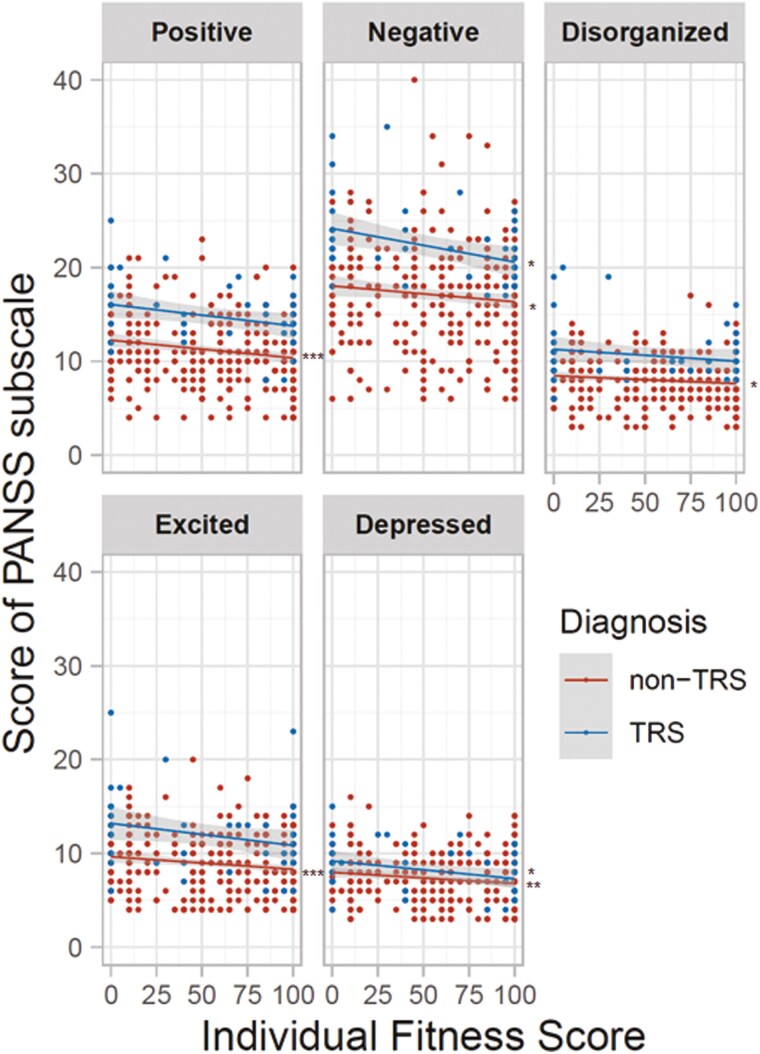
We further investigated correlations between IFS values and scores on the 5 PANSS subscales (positive, negative, disorganized/concrete, excited, and depressed symptoms) in patients with schizophrenia (Figure 4). We found several significant and nominal negative correlations between IFS values and PANSS subscale scores in patients with non-TRS and patients with TRS, respectively. In patients with non-TRS, higher IFS values were correlated with lower positive symptoms (rho = −0.17, P = 1.27 × 10−3), negative symptoms (rho = −0.12, P = .022), disorganized/concrete symptoms (rho = −0.12, P = .019), excited symptoms (rho = −0.14, P = 7.42 × 10−3), and depressed symptoms (rho = −0.16, P = 2.40 × 10−3). Although the number of patients with TRS was limited, higher IFS values were also correlated with lower negative symptoms (rho = −0.36, P = .012) and depressed symptoms (rho = −0.31, P = .031).
Figure 4.
Relationships of individual fitness score (IFS) values with scores on the 5 Positive and Negative Syndrome Scale (PANSS) subscales in patients with schizophrenia. From upper left to lower right: positive symptoms, negative symptoms, disorganized/concrete symptoms, excited symptoms, and depressed symptoms. A lower subscale score indicates lower levels of each symptom in patients with schizophrenia. *P < .05, **P < .01, ***P < .001.
Relationships of Changes in IFS Values Over 2 Years With Changes in PANSS Total Scores and Scores on the Five Subscales in Patients With Schizophrenia
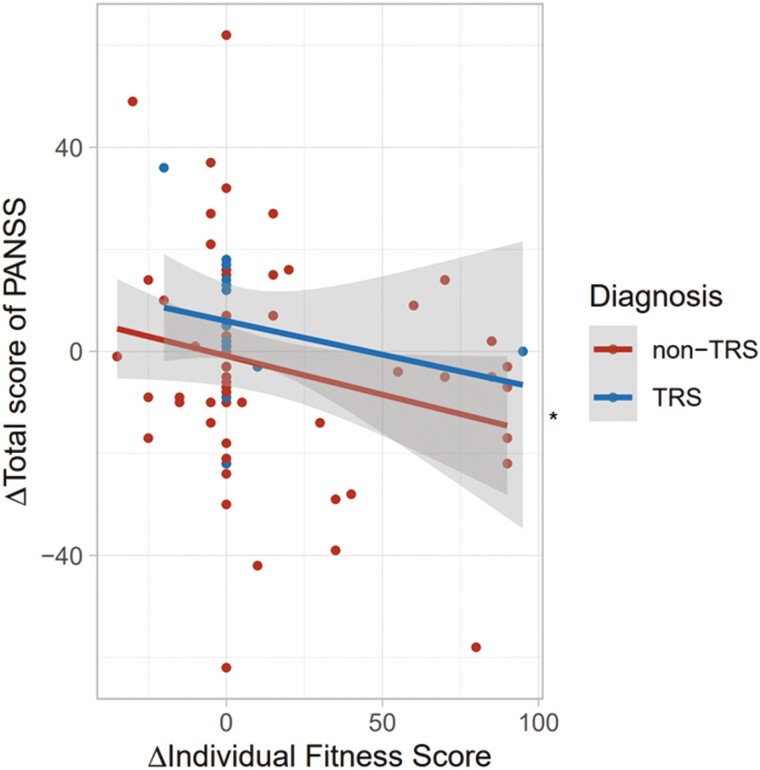
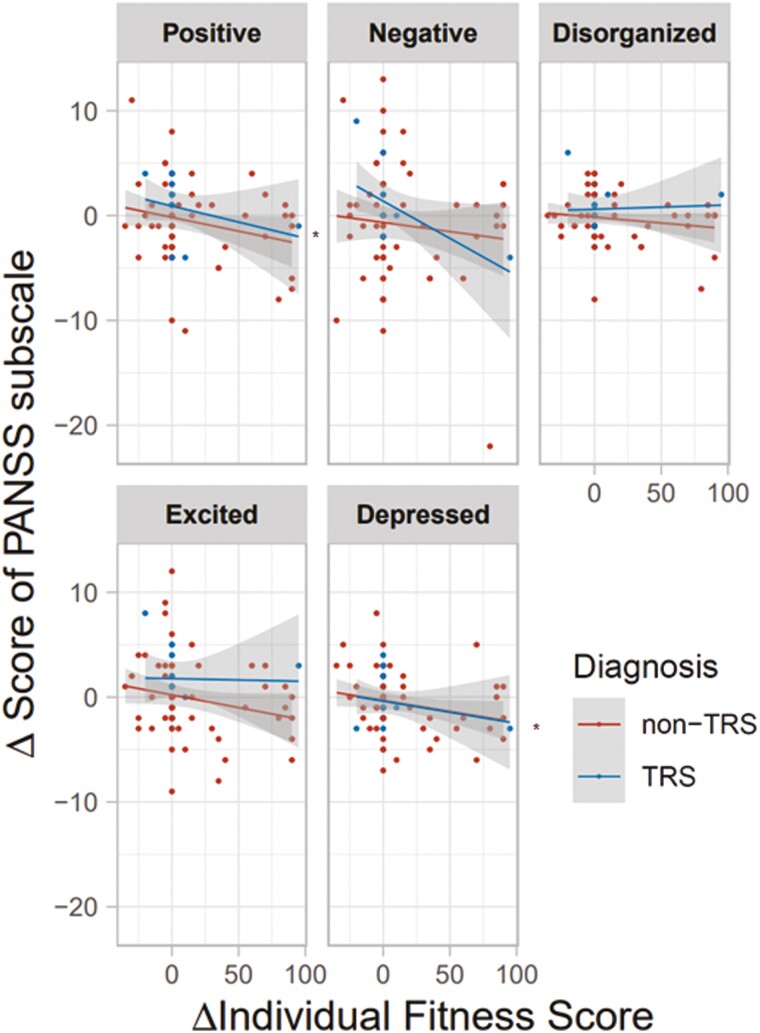
Patients at T2 had higher IFS values (t = 3.3, P = 1.28 × 10−3) and higher atypical CP-eq dosage (t = 2.4, P = .019) than patients at T1 (supplementary Table 2). The method to calculate the IFS could be partly related to the severity of the individuals with schizophrenia. Thus, we next explored correlations of changes in IFS values over 2 years with changes in PANSS total scores and scores on the 5 subscales in patients with schizophrenia (Figures 5 and 6). The change in the IFS (ΔIFS) was marginally negatively correlated with the change in PANSS total score (ΔPANSS total score) (Figure 5; β = −0.23, P = .041). Furthermore, the change in the IFS was marginally negatively correlated with the changes in positive symptoms (β = −0.24, P = .040) and depressed symptoms (β = −0.24, P = .037) (Figure 6). We further explored whether higher IFS values at T1 were correlated with greater improvement in PANSS scores. However, there was no significant relationship between the IFS values at T1 and improvement in PANSS scores (β = −0.08, P = .47). Thus, higher IFS values would not necessarily predict greater improvement in PANSS scores. These findings suggest that the longitudinal improvement in IFS values might be correlated with improvements in psychotic symptoms.
Figure 5.
Relationship of changes in individual fitness score (IFS) values over 2 years (T2-T1) and changes in PANSS total score (T2 − T1) in patients with schizophrenia. ΔIFS (T2-T1) = IFS at Time 1 (T1) – IFS at Time 2 (T2); ΔPANSS total score (T2-T1) = PANSS total score at T1—PANSS total score at T2. The PANSS total score at T1 (over 2 years earlier) was retrospectively extracted from the PANSS total score at T2 (at present). As the number of patients with TRS was limited, the diagnosis of TRS or non-TRS was included as a covariate in a linear regression analysis with ΔPANSS total score as a dependent variable and ΔIFS as an independent variable. *P < .05.
Figure 6.
Relationships of changes in individual fitness score (IFS) values over 2 years (T2-T1) and changes in the 5 PANSS subscale scores (T2 − T1) in patients with schizophrenia. *P < .05.
DISCUSSION
This is the first study, to our knowledge, to investigate the relationship between clinician adherence to guideline recommendations for pharmacological therapy of schizophrenia (assessed using a summary indicator of multiple QIs: the IFS) and patient outcomes. We found that higher IFS values, indicating better adherence to guideline recommendations, were associated with milder psychotic symptoms in both non-TRS and TRS patients. Furthermore, improvements in the IFS over a 2-year period were marginally correlated with improvements in psychotic symptoms. These findings suggest that adherence to guideline recommendations for pharmacological therapy of schizophrenia, as measured by the IFS, is associated with better outcomes in patients with schizophrenia. We highlight the importance of adherence to guidelines in the treatment of schizophrenia and suggest that efforts to improve adherence among clinicians may lead to better patient outcomes.
The IFS is a single indicator reflecting scores on multiple Qis, with potential scores in the range of 0 to 100 points. These QIs encompass a variety of factors, including SGA monotherapy with appropriate doses, concomitant use of antipsychotics, concomitant use of other psychotropic drugs such as antidepressants and treatments with clozapine or ECT for TRS. We assessed clinician adherence to guideline recommendations for pharmacological therapy of schizophrenia in patients with schizophrenia. Several previous studies have investigated the relationship between adherence to guideline recommendations for pharmacological therapy of schizophrenia and outcomes in patients (Owen et al., 2000; Dickey et al., 2006; Stiles et al., 2009; Drosos et al., 2020; Jin et al., 2021). Clinician adherence to guideline-recommended antipsychotic doses was associated with better outcomes in patients with schizophrenia (Owen et al., 2000), and clinician adherence to guideline recommendations decreased lifetime costs and improved health impacts (Jin et al., 2021). However, the methods (medical record reviews, surveys, and clinical performance feedback) used to assess adherence to guidelines among clinicians have varied among studies (Owen et al., 2000; Dickey et al., 2006; Bollini et al., 2008; Stiles et al., 2009; Drosos et al., 2020; Jin et al., 2021), and no studies have investigated a single indicator summarizing multiple QIs. These studies, along with the current study, highlight the importance of adhering to guideline recommendations in the treatment of schizophrenia. Our findings suggest that better clinician adherence to various guideline recommendations, not just 1 recommendation, may lead to better patient outcomes.
Our analyses involved correlations between IFS values and PANSS scores without any covariates. These findings might have been affected by several demographic variables, such as age, sex, years of education, and duration of illness. Thus, we further investigated whether IFS values were correlated with these demographic variables. Only duration of illness was nominally and negatively correlated with IFS values in patients with non-TRS (rho = −0.14, P = .010) but not in those with TRS (rho = 0.07, P = .62), indicating that longer duration of illness was related to lower IFS in patients with non-TRS. Therefore, because the duration of illness might have affected our findings, we reevaluated the correlations between IFS values and PANSS scores with duration of illness as a covariate. Even after including duration of illness as a covariate, our findings remained significant (P < .05).
We found that lower IFS values were associated with more severe psychotic symptoms at T2 (at present) in 400 patients with schizophrenia. However, these findings might have been affected by patients who were difficult to treat for a long time rather than clinician adherence to guideline recommendations for pharmacological therapy of schizophrenia among clinicians. Thus, we explored correlations between changes in IFS values over 2 years (T2 − T1) and changes in psychotic symptoms (T2 − T1), although the sample sizes at T1 were relatively small (n = 77, 77/400: 19.3%). In comparisons in clinical characteristics and IFS between patients at T1 and T2, patients at T2 had nominally higher atypical CP-eq dosage than patients at T1, while patients at T2 had lower depressed symptoms, lower typical CP-eq dosage, BPD-eq dosage, Imipramine-eq dosage, and Diazepam-eq dosage than patients at T1, although these longitudinal changes were not statistically significant. In the exploratory correlation analysis, we found that longitudinal improvements in IFS values might be associated with longitudinal improvements in psychotic symptoms. These findings support that IFS values were affected by several QIs and suggest that improvements in clinician adherence to guideline recommendations for pharmacological therapy of schizophrenia may lead to improvements in patient outcomes.
There are several limitations to consider when interpreting our findings. The patient’s prescription was determined by consultation with 2 or more psychiatrists at the university hospital or by psychiatrists at the referral clinic/hospital. Thus, our patient’s prescriptions in this study were affected by not a psychiatrist but many psychiatrists. As we did not have correct information of the number of treating psychiatrists, we could not adjust for the effect of the psychiatrists related to the patient’s prescription. Further study in other institutes or hospitals with all psychiatrists related to prescriptions as a covariate is needed to confirm our findings. As sample sizes of patients with TRS (n = 47) and for analyses of 2-year longitudinal changes (n = 77) were relatively small, the significance levels were marginal (0.01 < P < .05) due to a lack of statistical power. Further research using larger sample sizes is needed to confirm our findings. Using a flowchart prepared with reference to a previous study (Howes et al., 2017), we divided patients with schizophrenia into TRS and non-TRS groups based on current available information. However, this classification might have been insufficient. Although the definitions of TRS are inconsistent among previous studies (Howes et al., 2017), classification based on consensus definitions for TRS is needed. Guidelines may not account for all the individual factors that influence the course of the illness and response to treatment. Although the guideline recommendations should be the first consideration, clinicians may need to use their clinical judgment and adjust treatment plans based on the patient’s response and side effects; thus, treatment plans may deviate from the guidelines.
In conclusion, we found that greater clinician adherence to guideline recommendations for pharmacological therapy of schizophrenia, as assessed by the IFS, was associated with milder psychotic symptoms in patients with both non-TRS and TRS. Additionally, improvements in the IFS over a 2-year period were marginally correlated with improvements in PANSS scores. The IFS, a single indicator that summarizes multiple QIs, appears to be a useful tool for assessing clinician adherence to guideline recommendations for pharmacological therapy of schizophrenia. These findings suggest that efforts to improve clinician adherence to guidelines may lead to better outcomes in patients with schizophrenia. To resolve gaps between the schizophrenia guideline recommendations and current clinical practice, further regular training, and education, such as in our EGUIDE project, is needed.
Supplementary Material
Acknowledgments
We thank all the individuals who participated in this study for their cooperation. This work was supported by the Japan Agency for Medical Research and Development (AMED) under grant numbers JP18dm0307002, JP21dk0307103, JP21uk1024002, JP21wm0425012, and JP22dk0307112; by the Japan Society for the Promotion of Science (JSPS) KAKENHI under grant number JP20H03611; and by an Intramural Research Grant (3-1) for Neurological and Psychiatric Disorders of NCNP.
Contributor Information
Fumitoshi Kodaka, Department of Psychiatry, The Jikei University School of Medicine, Tokyo, Japan.
Kazutaka Ohi, Department of Psychiatry, Gifu University Graduate School of Medicine, Gifu, Japan.
Yuka Yasuda, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Japan; Life Grow Brilliant Mental Clinic, Medical Corporation Foster, Osaka, Japan.
Michiko Fujimoto, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Japan; Department of Psychiatry, Osaka University, Graduate School of Medicine, Osaka, Japan.
Hidenaga Yamamori, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Japan; Department of Psychiatry, Osaka University, Graduate School of Medicine, Osaka, Japan; Community Health Care Organization Osaka Hospital, Osaka, Japan.
Naomi Hasegawa, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Japan.
Satsuki Ito, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Japan.
Kentaro Fukumoto, Department of Neuropsychiatry, School of Medicine, Iwate Medical University, Iwate, Japan.
Junya Matsumoto, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Japan.
Kenichiro Miura, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Japan.
Norio Yasui-Furukori, Department of Psychiatry, Dokkyo Medical University School of Medicine, Mibu, Japan.
Ryota Hashimoto, Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Kodaira, Japan.
Interest Statement
The authors declare that they have no conflicts of interest.
Data Availability
Our data are not publicly available because they contain information that could compromise research participant privacy/consent.
References
- Aly El-Gabry DM, Abdel Aziz K, Okasha T, Azzam H, Okasha A (2018) Antipsychotic polypharmacy and its relation to metabolic syndrome in patients with schizophrenia: an Egyptian study. J Clin Psychopharmacol 38:27–33. [DOI] [PubMed] [Google Scholar]
- Bighelli I, Rodolico A, Siafis S, Samara MT, Hansen WP, Salomone S, Aguglia E, Cutrufelli P, Bauer I, Baeckers L, Leucht S (2022) Antipsychotic polypharmacy reduction versus polypharmacy continuation for people with schizophrenia. Cochrane Database Syst Rev 8:Cd014383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollini P, Pampallona S, Nieddu S, Bianco M, Tibaldi G, Munizza C (2008) Indicators of conformance with guidelines of schizophrenia treatment in mental health services. Psychiatr Serv 59:782–791. [DOI] [PubMed] [Google Scholar]
- Castle DJ, Galletly CA, Dark F, Humberstone V, Morgan VA, Killackey E, Kulkarni J, McGorry P, Nielssen O, Tran NT, Jablensky A (2017) The 2016 Royal Australian and New Zealand College of Psychiatrists guidelines for the management of schizophrenia and related disorders. Med J Aust 206:501–505. [DOI] [PubMed] [Google Scholar]
- Crockford D, Addington D (2017) Canadian Schizophrenia Guidelines: schizophrenia and other psychotic disorders with coexisting substance use disorders. Can J Psychiatry 62:624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarais JE, Beauclair L, Margolese HC (2012) Anticholinergics in the era of atypical antipsychotics: short-term or long-term treatment? J Psychopharmacol 26:1167–1174. [DOI] [PubMed] [Google Scholar]
- Dickey B, Normand SL, Eisen S, Hermann R, Cleary P, Cortés D, Ware N (2006) Associations between adherence to guidelines for antipsychotic dose and health status, side effects, and patient care experiences. Med Care 44:827–834. [DOI] [PubMed] [Google Scholar]
- Drosos P, Brønnick K, Joa I, Johannessen JO, Johnsen E, Kroken RA, Stain HJ, Hegelstad WTV, Larsen TK (2020) One-year outcome and adherence to pharmacological guidelines in first-episode schizophrenia: results from a consecutive cohort study. J Clin Psychopharmacol 40:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenette MD, Chintoh A, Remington G, Hahn M (2014) Atypical antipsychotic-induced metabolic disturbances in the elderly. Drugs Aging 31:159–184. [DOI] [PubMed] [Google Scholar]
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Möller HJ; World Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Schizophrenia (2012) World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry 13:318–378.22834451 [Google Scholar]
- Heald A, Livingston M, Yung A, De Hert MA (2017) Prescribing in schizophrenia and psychosis: increasing polypharmacy over time. Hum Psychopharmacol 32:e2579. [DOI] [PubMed] [Google Scholar]
- Howes OD, et al. (2017) Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry 174:216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi K, et al. (2020) Prescription patterns in patients with schizophrenia in Japan: first-quality indicator data from the survey of “Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)” project. Neuropsychopharmacol Rep 40:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H, et al. (2020) Unmet needs of patients with major depressive disorder - Findings from the “Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)” project: a nationwide dissemination, education, and evaluation study. Psychiatry Clin Neurosci 74:667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada K, et al. (2022) Development of individual fitness score for conformity of prescriptions to the “Guidelines for Pharmacological Therapy of Schizophrenia”. Neuropsychopharmacol Rep 42:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japanese Society of Neuropsychopharmacology (2021) Japanese Society of Neuropsychopharmacology: “Guideline for Pharmacological Therapy of Schizophrenia”. Neuropsychopharmacol Rep 41:266–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SW, Kim YK (2017) Unresolved issues for utilization of atypical antipsychotics in schizophrenia: antipsychotic polypharmacy and metabolic syndrome. Int J Mol Sci 18:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Tappenden P, MacCabe JH, Robinson S, McCrone P, Byford S (2021) Cost and health impacts of adherence to the National Institute for Health and Care Excellence schizophrenia guideline recommendations. Br J Psychiatry 218:224–229. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987) The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, Servis M, Walaszek A, Buckley P, Lenzenweger MF, Young AS, Degenhardt A, Hong SH; (Systematic Review) (2020) The American Psychiatric Association Practice Guideline for the treatment of patients with schizophrenia. Am J Psychiatry 177:868–872. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Watanabe K, Uchida H, Mimura M, Kane JM, Correll CU (2013) Antipsychotic polypharmacy: a Japanese survey of prescribers’ attitudes and rationales. Psychiatry Res 209:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM (2009) Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373:31–41. [DOI] [PubMed] [Google Scholar]
- Nielsen RE, Lindström E, Nielsen J, Levander S (2012) DAI-10 is as good as DAI-30 in schizophrenia. Eur Neuropsychopharmacol 22:747–750. [DOI] [PubMed] [Google Scholar]
- Numata S, et al. (2021) Improvements in the degree of understanding the treatment guidelines for schizophrenia and major depressive disorder in a nationwide dissemination and implementation study. Neuropsychopharmacol Rep 41:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen RR, Thrush CR, Kirchner JE, Fischer EP, Booth BM (2000) Performance measurement for schizophrenia: adherence to guidelines for antipsychotic dose. Int J Qual Health Care 12:475–482. [DOI] [PubMed] [Google Scholar]
- Pae CU, Han C, Bahk WM, Lee SJ, Patkar AA, Masand PS (2021) Consideration of long-acting injectable antipsychotics for polypharmacy regimen in the treatment of schizophrenia: put it on the table or not? Clin Psychopharmacol Neurosci 19:434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerig JL, Steffen KJ, Mitchell JE (2011) Atypical antipsychotic-induced weight gain: insights into mechanisms of action. CNS Drugs 25:1035–1059. [DOI] [PubMed] [Google Scholar]
- Siskind D, McCartney L, Goldschlager R, Kisely S (2016) Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry 209:385–392. [DOI] [PubMed] [Google Scholar]
- Stiles PG, Boothroyd RA, Dhont K, Beiler PF, Green AE (2009) Adherence to practice guidelines, clinical outcomes, and costs among Medicaid enrollees with severe mental illnesses. Eval Health Prof 32:69–89. [DOI] [PubMed] [Google Scholar]
- Takaesu Y, et al. (2019) Improvement of psychiatrists’ clinical knowledge of the treatment guidelines for schizophrenia and major depressive disorders using the “Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)” project: a nationwide dissemination, education, and evaluation study. Psychiatry Clin Neurosci 73:642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Suzuki T, Remington G, Uchida H (2015) Antipsychotic polypharmacy and corrected QT interval: a systematic review. Can J Psychiatry 60:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M, Perera U (2015) NICE CG178 psychosis and schizophrenia in adults: treatment and management - an evidence-based guideline? Br J Psychiatry 206:357–359. [DOI] [PubMed] [Google Scholar]
- Volpato AM, Zugno AI, Quevedo J (2013) Recent evidence and potential mechanisms underlying weight gain and insulin resistance due to atypical antipsychotics. Braz J Psychiatry 35:295–304. [DOI] [PubMed] [Google Scholar]
- Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D (2012) Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res 137:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zald DH (2005) A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol 8:457–472. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU (2013) Efficacy and safety of individual second-generation vs first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol 16:1205–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our data are not publicly available because they contain information that could compromise research participant privacy/consent.