Abstract
A novel virus named SARS-CoV-2 emerged from Wuhan, China in late 2019. Since then, the virus has quickly spread worldwide, leading the WHO to declare it as a pandemic; by the end of April 2020, the number of cases exceeded 3 million. Due to the high infectivity rate, SARS-CoV-2 is difficult to contain, making disinfectant protocols vital, especially for essential, highly trafficked areas such as hospitals, grocery stores, and delivery centers. According to the CDC, best practices to slow the spread rely on good hand hygiene, including proper handwashing practices as well as the use of alcohol-based hand sanitizers (ABHS). However, they provide warning against sanitizing products containing benzalkonium chloride (BAC), which has sparked fear in both the scientific community as well as the general public as BAC, a common quaternary ammonium compound (QAC), is ubiquitous in soaps and cleaning wipes, as well as hospital sanitation kits. This viewpoint aims to highlight the outdated and incongruous data in the evaluation of BAC against the family of known coronaviruses, as well as points to the need for further evaluation of the efficacy of QACs against coronaviruses.
Graphical Abstract
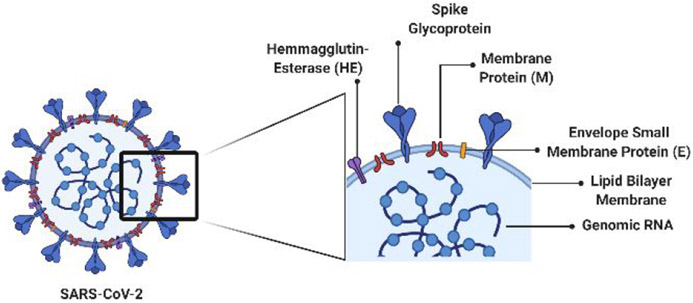
In the late months of 2019 leading into January of 2020, a novel virus emerged in Wuhan, China and was given the name Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2; Figure 1); the associated disease was termed COVID-19 (CO-rona-VI-rus D-isease 2019, Figure 1).1-3 The virus quickly spread across Europe and into North and South America, leading the World Health Organization (WHO) to declare it as a pandemic on March 11, 2020, with over 118,000 infections in over 114 countries at that time.4 Seven weeks later, the number of cases exceeded 3 million worldwide.5 SARS-CoV-2 is a part of the family of viruses known as Coronaviridae, which have led to previous coronavirus outbreaks: a SARS-CoV outbreak in 2002 and a Middle East Respiratory Syndrome (MERS)-CoV outbreak from 2012-2015.3,6-9 These three viral outbreaks have yielded similar symptoms, including shortness of breath, fever, coughing, and headaches. Although Coronaviridae is not new to the human population, SARS-CoV-2 is a larger threat as it has a much greater reproduction number (R0) than previous coronavirus strains, meaning that the number of cases generated by one infected person is higher than previous outbreaks.9-13 This, coupled with longer incubations times, latent infections, and delayed symptoms, makes the virus difficult to contain.
Figure 1.
Cartoon representation of SARS-CoV-2 viral structure.
Because of this, there is an urgent need for appropriate disinfection protocols to slow the spread of COVID-19. According to the Centers for Disease Control and Prevention (CDC), the most effective way to reduce COVID-19 infectivity rates is to practice appropriate hand hygiene, specifically employing proper handwashing with warm water and soap for a minimum of 20 seconds and avoiding touching one’s face.14 In addition, the CDC advocates for the use of alcohol-based hand sanitizers (ABHS), but provides warning about products containing benzalkonium chloride (BAC), stating:
“Benzalkonium chloride, along with both ethanol and isopropanol, is deemed eligible by the FDA for use in the formulation of healthcare personnel hand rubs. However, available evidence indicates benzalkonium chloride has less reliable activity against coronavirus than either of the alcohols.”13
As BAC and related disinfectants are ubiquitous, found not only in soaps, but also cleaning wipes and hospital sanitation kits, knowing their efficacy against SARS-CoV-2 is imperative to address the spread of the virus. The CDC goes on to reference a recent review, published in the Journal of Hospital Infection by Kampf, et al., that tabulated the results of previous studies investigating how long viruses within the same family (MERS-CoV, SARS-CoV, and endemic human coronavirus) can exist on surfaces ranging from steel and plastic to disposable gowns and surgical gloves.15 Overall, it was reported that this class of virus can persist on inanimate objects with a wide range of times from 2–8h (paper and aluminum) to ≤5d (plastic, ceramic, and others). Since the publication of this and other reports, the scientific community has debated the length of time SARS-CoV-2 can persist on inanimate objects, with some reporting SARS-CoV-2 RNA on surfaces after >14 days.10,16-18
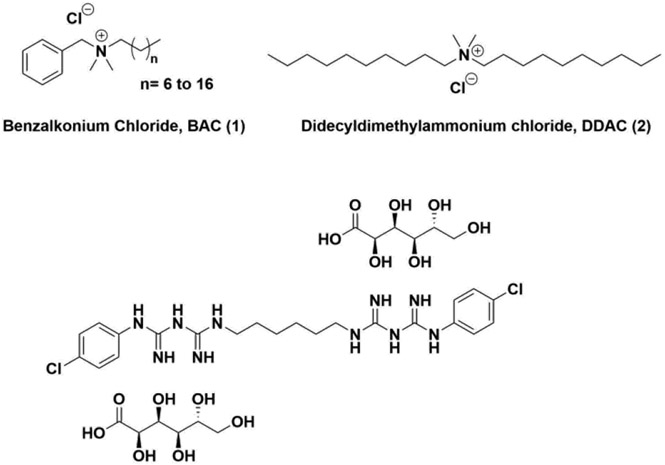
With survival times varying amongst surface types, guidelines for best disinfectant practices are vital, most notably for essential work locations including hospitals, grocery stores, and delivery centers that together face higher traffic and risk rate than other locations. Currently, the CDC offers limited guidelines for surface disinfectants in the face of COVID-19. However, both the scientific community and general public have deferred to the article mentioned above for guidance in best practices, as it also includes a table of previous reports on the ability of common disinfectants to eradicate this family of viruses.15 The disinfectants in their literature review include two alcohols (ethanol and propanol), sodium hypochlorite, peroxides, aldehydes (formaldehyde and glutardialdehyde), two different types of quaternary ammonium compounds (BAC, 1 and didecyldimethyl ammonium chloride, 2; Figure 2), and several others. It is from this data table that the CDC provided its recommendations on ABHS and regarded BAC, a common quaternary ammonium compound (QAC), as ineffective at killing the virus. The pertinent data was based on a suspension-based assay found in an article by Wood and Payne in 1998 (Table 1, ref. 19).
Figure 2.
Chemical structures of QACs used.
Table 1.
Ability of different QACs to inactivate viral loadings based upon literature findings.
| QAC | Conc. (%w/v) | Type of Assay | Virus Tested | Exposure Time |
Reduced Viral Loading? (Y/N;log10) |
Ref. |
|---|---|---|---|---|---|---|
| BAC | 0.04 | QCT | HCoV | 1 min | N; 3.0 | |
| BAC, HCl | 0.04 (pH= 1.0) | QCT | HCoV | 1 min | Y; >3.0 | 20 |
| BAC, EtOH | 0.04, 70 | QCT | HCoV | 1 min | Y; >3.0 | |
| BAC | 0.2 | Suspension | HCoV | 10 min | N; 0.0 | 19 |
| BAC | 1 | Suspension | SARS-CoV | 5–30min | Y; reduced growth; RNA still detectable by RT-PCR | 29 |
| Mikrobac Forte (BAC) | 0.5 | Suspension | SARS-CoV | 30, 60min | Y; ≥6.13 | 31 |
| Kohrsolin FF (BAC) | 0.5 | Suspension | SARS-CoV | 30, 60min | Y; ≥3.75 | |
| BAC | 0.01 | Suspension | TGEV | 5 min | Y; ≥3.0 | |
| CG | 0.008 | QCT | HCoV 229E | 5min | N; <3.0 | |
| CG, EtOH | 0.008, 70 | QCT | HCoV229E | 5min | Y; ≥3.0 | 30 |
| Mix of BAC/CG | 0.066 | QCT | HCoV 229E | 10 min | Y; 4.0 | |
| DDAC | 0.0025 | Suspension | CCoV | 3 d | Y; >4.0 | 28 |
| BAC | 0.00175 | Suspension | CCoV | 3 d | N; 3.0 | |
| BAC, EtOH | 0.1, 79 | Suspension | MHV | 30 s | Y; ≥3.0 | 6 |
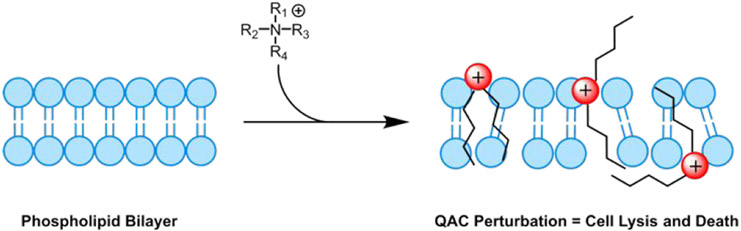
Beyond the use of BAC as a hand sanitizing agent, Kampf and coworkers also provided one example of BAC as a surface disinfectant from an article by Sattar, et al. in 1989 with an active concentration of 0.04% w/v on a steel surface, showing it to also be ineffective at reducing the viral loading of HCoV (endemic human coronavirus) after 1 min exposure (Table 1).15,20 Combining this with the suspension assay result has sparked debate and worry as QACs, specifically BAC, are the active ingredient found in many household disinfecting wipes and sprays, as well as an additive in various soaps and non-alcohol-based hand sanitizers. This is due to their ability to eradicate surface bacteria and common viruses such as influenza by disrupting their phospholipid membrane (Figure 3).21-27
Figure 3.
Mode of action of QACs against both bacterial and viral phospholipid membranes. Red spheres represent positively charged nitrogen atoms.
Despite the current reluctance of the CDC to endorse a BAC-based hand sanitizer against COVID-19, the research backing the stance is neither current nor uniformly asserted. In particular, the disinfectant concentrations in the reports discussed herein are inconsistent, with broad ranges of applied QACs from 0.00175-1.5% w/v (Table 1, Figure 4). Additionally, the type of analysis varied widely. Most studies utilized suspension-based assays in which the virus was suspended in minimal media, but the contents of the media varied with concentrations and types of organic loading, added salt concentrations, as well as added antibiotics. For surface evaluations, some analyses utilized quantitative carrier tests (QCTs) with stainless steel discs as carriers of viral loads where others simply described evaluating surface contamination but lacked details for reproducibility. It is the goal of this viewpoint to shed light on more recent data regarding the activity of BAC against coronaviruses, point out the disparities between available research, as well as draw attention to the need for further research into QACs. There is a need to not only broaden our understanding of the effectiveness of BAC against SARS-CoV-2, but also to design and develop improved QACs for future infection control.
Figure 4.
Graph displaying reduction in viral load by various QACs. Data obtained from Table 1. Note that QAC concentration is displayed in log10 for clarity. Data points that are listed as viral load reduction of >3.0, or <3.0 were given values of 3.2 and 2.8 respectively to aid in clarity. Additionally, data from Ansaldi, et al. was omitted as it did not contain viral load reduction values. Outlier from data set (Wood, et al.), used for CDC reference is marked in red.
Based on with another article briefly mentioned in the Kampf, et al., the work performed by Pratelli in Zoonoses and Public Health analyzed the effectiveness of QACs against Canine Coronavirus (CCoV), another family member within Coronaviridae.28 Although these results pertain to CCoV, it is within the same family as SARS-CoV-2 and should aid in evaluating the efficacy of QACs. Pratelli utilized a suspension-based assay, suspending cell cultures in Eagle’s minimal essential medium and supplementing with 10% fetal bovine serum. The report found that DDAC was able to reduce the viral loading of CCoV by >4.0 log10 after 3-day exposure (Table 1). Conversely, BAC was unable to reduce the viral loading (3.0 log10), but the author does note that both agents caused significant morphological damage to the virus.
Within a 2004 article by Ansaldi, et al. in the Journal of Preventive Medicine and Hygiene, the authors analyzed the efficacy of the same group of disinfectants described by Kampf, et al. against SARS-CoV within a 1 mL salt solution (concentration or identity of salt not specified) with a standard concentration of cell-culture.29 Each replicate was exposed to 2 mL solutions of disinfectant with recommended disinfectant concentrations, e.g. BAC=1% w/v. Interestingly, it was found that BAC did not significantly damage the cell monolayer but was able to reduce the replication of SARS-CoV after 5-minute exposure (Table 1). However, after 30-minute exposure to BAC, Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) found SARS-CoV RNA still present on the surface. This result was consistent against each type of disinfectant analyzed (ethanol, BAC, sodium hypochlorite, etc.) as well as against three types of viruses tested (Influenza, Respiratory Syncytial Virus, and SARS-CoV). Therefore, it begs the question of whether intact RNA alone is an infective agent of SARS-CoV, or if the reduction of cell replication from BAC is adequate. This question remains unanswered and makes it difficult to appropriately evaluate efficacy of disinfectants across analyses.
Additionally, two articles from 1989 and 2009 present discrepancies regarding BAC alone as well as within provided commercial formulations. For example, within the 2009 article by Dellano, et al. the authors analyzed effectiveness of common household disinfectants (one being Lysol with 0.1% w/v of BAC and ≤70% ethanol) against Murine Hepatitis Virus (MHV, a surrogate vector with structural similarities to SARS, but lower biosafety level).6 They found that the BAC formulations were effective at inactivating this class of virus after only 30-second exposure (Table 1). This result was echoed in the previous 1989 article by Sattar, et al. when analyzing BAC and ethanol mixtures.20 Additionally, Sattar, et al. analyzed toilet bowl cleaners (with a solution of 0.04% w/v of BAC and hydrochloric acid, pH=1.0). This formulation was again proven to be effective at inactivating coronaviruses although this lower concentration of BAC was only able to affect a 3-log reduction (99.9%) in viral load in the absence of additives. Both articles point to the difficulty proving if combinatorial formulations lead to efficacy, or if the QACs aid in the viral reduction.
In addition to BAC, other commonly employed QACs such as chlorhexidine gluconate (CG, 3, Figure 2) were also examined in a 2004 editorial by Schmidt, et al.30 It was found therein that CG at a concentration of 0.008% w/v was unable to reduce the viral loading of HCoV229E, but when added to 70% ethanol reduced the viral load by 3 log10 in QCT (Table 1). In addition, the article also examined the effectiveness of combinatorial QACs (presumably CG and BAC) at a 0.066% w/v in QCTs, which proved effective after 10-minute exposure resulting in viral reduction of 4.0 log10. The only example of BAC efficacy alone in this article was with 0.01% w/v against transmissible gastroenteritis virus (TGEV), a common coronavirus known to infect pigs. Within the suspension assay, BAC reduced the viral load by 3 log10. These analyses again display the incongruity of the reports of QAC disinfectant efficacy between research laboratories.
A distinct inconsistency regarding the effectiveness of BAC is evident in a 2005 article, again by Kampf in the Journal of Hospital Infection.31 Contrary to the 2020 review, this earlier report evaluated the effectiveness of four commercially available ABHS, three surface disinfectants (two of which contained BAC at 0.5% w/v), and one instrument disinfectant (containing glutaraldehyde) against SARS-CoV. Utilizing a suspension-based assay with three different types of organic loads (0.3% serum albumin, 10% fecal calf serum, and 0.3% serum albumin/ 0.3% sheep erythrocytes), the study showed that each disinfectant evaluated was effective at significantly lowering the viral load across all types of organic loadings. Specifically looking at the BAC data, one formulation (Mikrobac forte) reduced the viral loading by ≥6.13 log10 while the other (Kohrsolin FF) reduced the viral loading by ≥3.75 log10 in comparison to ≥4.3 with ABHS, all of which had 30-60 minute exposure times (Table 1).
Through this brief literature analysis, it is apparent that the current research on the effectiveness of BAC, and more broadly QACs, is inconsistently presented across a select few reports from the past decade. Beyond the incongruity between the assays employed, we also hypothesize that the variability in results may be due to the solution of disinfectant, specifically variations in concentrations analyzed; we note that common household wipes are sold with a ~0.3% w/v concentration of a mixture of BAC structures. Within our own research efforts, we have found that outdated solutions of BAC have increased rates of micelle formations, rendering high levels of variability in effectiveness between assays (unpublished results). Furthermore, it appears that the reluctance of the CDC in endorsing BAC as a disinfectant against SARS-CoV-2 is based upon an outlier in one paper (Figure 4).
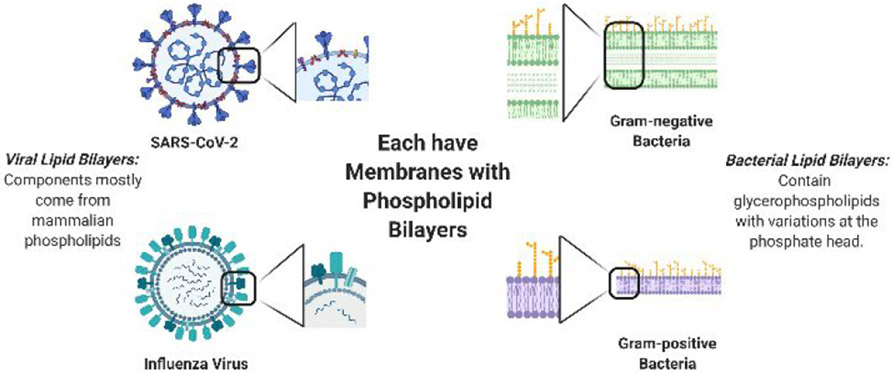
Additionally, the question remains if SARS-CoV-2 RNA is an infective agent on its own. It is plausible that QACs are successful in eradicating infectivity if only the RNA is detected after treatment. It has frequently been confirmed that QACs are effective against influenza viruses as well as both Gram-positive and Gramnegative bacterial strains.21-27 In comparing the outer membranes between these and SARS-CoV-2 (Figure 5), we postulate that QACs should be effective in decreasing the viral load for disinfection procedures against COVID-19 as both contain relatively similar phospholipid bilayers. Furthermore, a newer generation of multi-cationic QACs have recently been developed and garner further studies against these emerging viruses.27
Figure 5.
Comparing the viral envelopes (membranes) to that of bacterial membranes. Note that both influenza and SARS-CoV-2 have phospholipid membranes similar to that of mammalian phospholipids due to method of infectivity and replication.
These findings, coupled with the dangerous trend of inappropriately misusing disinfectants, warrants an urgent need to establish consistency in how we analyze the effectiveness of QACs against the family of coronaviruses to allow factual recommendations for use of disinfectants. This pandemic serves as an opportunity for enhanced antiseptics, and more specifically QAC development, as commercially available disinfectants have room for improvement both with formulation, and concentration, as well as effectiveness against both viral and bacterial contagions.
ACKNOWLEDGMENT
This work was funded by the National Institute of General Medical Sciences (GM119426).
Footnotes
Conflict of Interest
W.M.W. and K.P.C.M. have intellectual property claims on the multi-QAC compounds and their use as antiseptics.
REFERENCES
- (1).Jiang S, Shi Z, Shu Y, Song J, Gao GF, Tan W, and Guo D. A distinct name is needed for the new coronavirus. Lancet. 2020, 395, 949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Guanier j. Three Emerging Coronaviruses in Two Decades The Story of SARS, MERS, and Now COVID-19. Am J Clin Pathol. 2020, 153, 420–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Gorbalenya AE, Baker SC, Baric RS et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol, 2020, 5, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).“WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 – 11 March 2020.” World Health Organization, Mar. 2020, www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Date accessed: 27 Apr. 2020 [Google Scholar]
- (5).The New York Times. “Coronavirus Map: Tracking the Global Outbreak.” The New York Times, The New York Times, 28 Apr.2020, www.nvtiines.coin/interactive/2020/world/coronavirus-maps.html. Date accessed: 28 Apr. 2020 [Google Scholar]
- (6).Dellanno C, Vega Q, and Boesenberg D The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Am J Infect Control, 2009, 37, 649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Rezabakhsh A, Ala A, and Khodaei SH Novel Coronavirus (COVID-19): A New Emerging Pandemic Threat. J. Res Clin Med, 2020, 8, 5. [Google Scholar]
- (8).Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe Acute Respiratory Syndrome- related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2., Nat Microbiol., 2020, 5, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ceccarelli M, Berretta M, M., Venanzi Rullo E, Nunnari G, and Cacopardo B Editorial – Differences and similarities between Severe Acute Respiratory Syndrome (SARS)-CoronaVirus (CoV) and SARS-CoV-2. Would a rose by another name smell as sweet? Eur Rev Med Pharmacol Sci, 2020, 24, 2781–2783. [DOI] [PubMed] [Google Scholar]
- (10).Lai CC, Shih TP, Ko WC, Tang HJ, and Hsueh PR Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wu JT, Leung K, and Leung GM, Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study., Lancet, 2020, 395, 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Liu Y, Eggo RM, and Kucharski AJ, Secondary attack rate and superspreading events for SARS-CoV-2., Lancet, 2020, 395, 10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lai A, Bergna A, Acciarri C, Galli G, and Zehender G Early phylogenetic estimate of the effective reproduction number of SARS-CoV-2., J Med Virol, 2020, 92, 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Centers for Disease Control and Prevention. “Frequently Asked Questions about Hand Hygiene for Healthcare Personnel Responding to COVID-2019.” Centers for Disease Control and Prevention, 18 Mar. 2020, www.cdc.gov/coronavirus/2019-ncov/hcp/hand-hygiene-faq.html. Date accessed: 23 Apr. 2020 [Google Scholar]
- (15).Kampf G, Todt D, Pfaender S, and Steinmann E, Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents., J Hosp Infect, 2020, 104, 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).van Doremalen N, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, and Munster VJ, Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1., N Engl J Med, 2020, 382, 1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wei Xiang Ong S, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, and Marimuthu K, Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient., JAMA, 2020, 323, 1610–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chin A, Chu J, Perera M, Hui K, Yen H, Chan M, Peiris M, and Poon L, Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe, 2020, In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wood A and Payne D, The action of three antiseptics/disinfectants against enveloped and non-enveloped viruses., J Hosp Infect, 1998, 38, 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sattar SA, Springthorpe VS, Karim Y, and Loro P Chemical disinfection of non-porous inanimate surfaces experimentally contaminated with four human pathogenic viruses., Epidem. Inf, 1989, 102, 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Nemoto M, Bannai H, Tsujimura K, Yamanaka T, and Kondo T, Comparison of the Virucidal Effects of Disinfectant Agents Against Equine Influenza A Virus., J. Equine Vet. Sci, 2014, 34, 715–718. [Google Scholar]
- (22).Gerba CP Quaternary Ammonium Biocides: Efficacy in Application., Appl. Environ. Microbiol, 2015, 81, 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wessels S and Ingmer H Modes of action of three disinfectant active substances: a review., Regul. Toxicol. Pharmacol, 2013, 67, 456–167. [DOI] [PubMed] [Google Scholar]
- (24).Tuladhar E, de Koning MC, Fundeanu I, Beumer R, and Duizera E Residual Viral and Bacterial Contamination of Surfaces after Cleaning and Disinfection., Appl. Environ. Microbiol, 2012, 78, 2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Morrison KR, Allen R, Minbiole KPC, and Wuest WM More QACs, more questions: recent advances in structure activity relationships and hurdles in understanding resistance mechanism., Tet Lett, 2019, 60, 150935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Jennings MC, Minbiole KPC, and Wuest WM Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance., ACS Infectious Diseases, 2015. 1, 288. [DOI] [PubMed] [Google Scholar]
- (27).Jennings MC, Buttaro BA, Minbiole KPC, Wuest WM Bioorganic Investigation of Multicationic Antimicrobials to Combat QAC-Resistant Staphylococcus aureus., ACS Infectious Diseases, 2015. 1, 304. [DOI] [PubMed] [Google Scholar]
- (28).Pratelli A., Action of Disinfectants on Canine Coronavirus Replication In Vitro., Zoonoses Public Health., 2007, 54, 383–386. [DOI] [PubMed] [Google Scholar]
- (29).Ansaldi F, Banfi F, Morelli P, Valle L, Durando P, Sticchi L, Contos S, Gasparini R, and Crovari P, SARS-CoV, influenza A and syncitial respiratory virus resistance against common disinfectants and ultraviolet irradiation., J Prev Med Hyg, 2004, 45, 5–8. [Google Scholar]
- (30).Schmidt A, Weber O, and Wolff MH, (Eds.). 2005. Coronaviruses with Special Emphasis on First Insights Concerning SARS. New York: Birkhäuser Basel. [Google Scholar]
- (31).Rabenau HF, Kampf G, Cinatl J, and Doerr HW, Efficacy of various disinfectants against SARS coronavirus., J Hosp Infect, 2005, 61, 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]