Abstract
Background/Aim
Germline copy number variation (CNV) is a type of genetic variant that predisposes significantly to inherited cancers. Today, next-generation sequencing (NGS) technologies have contributed to multi gene panel analysis in clinical practice.
Materials and Methods
A total of 2,163 patients were screened for cancer susceptibility, using a solution-based capture method. A panel of 52 genes was used for targeted NGS. The capture-based approach enables computational analysis of CNVs from NGS data. We studied the performance of the CNV module of the commercial software suite SeqPilot (JSI Medical Systems) and of the non-commercial tool panelcn.MOPS. Additionally, we tested the performance of digital multiplex ligation-dependent probe amplification (digitalMLPA).
Results
Pathogenic/likely pathogenic variants (P/LP) were identified in 464 samples (21.5%). CNV accounts for 10.8% (50/464) of pathogenic variants, referring to deletion/duplication of one or more exons of a gene. In patients with breast and ovarian cancer, CNVs accounted for 10.2% and 6.8% of pathogenic variants, respectively. In colorectal cancer patients, CNV accounted for 28.6% of pathogenic/likely pathogenic variants.
Conclusion
In silico CNV detection tools provide a viable and cost-effective method to identify CNVs from NGS experiments. CNVs constitute a substantial percentage of P/LP variants, since they represent up to one of every ten P/LP findings identified by NGS multigene analysis; therefore, their evaluation is highly recommended to improve the diagnostic yield of hereditary cancer analysis.
Keywords: Hereditary cancer, CNVs, computational CNV analysis, MLPA, digital MLPA
The inheritance of pathogenic variants in one or more genes predispose to various cancer types, typically with early onset. All cancers are generated by the accumulation of variants and the resulting dysregulation of critical genes involved in the pathways that control cell proliferation, survival, and DNA maintenance (1). Comprehensive gene analysis should be performed, including methods efficiently detecting all types of variants such as single base alterations and minor insertions/deletions, as well as germline copy number variations (CNVs) (2).
CNVs are at least 50 base pair (bp) genetic variations in comparison to the reference genome and correspond to deletions, duplications, and complex multisite variants (3). CNV identification is currently recommended in comprehensive cancer genomic testing methodologies. There are various molecular techniques for CNV detection including array comparative genomic hybridization (array CGH), multiplex ligation-dependent probe amplification (MLPA) and quantitative real time PCR (RT-PCR) (4). MLPA is the most established and commonly used technology for detecting CNVs in one or a few cancer genes, but it is time-consuming and expensive in comparison to other techniques, and each gene requires a unique design.
Next-generation sequencing (NGS) is a method for detecting CNVs using the level of coverage of reads that have been matched to the human reference genome. The depth of coverage corresponds to the number of copies of a specific chromosomal region, and current molecular approaches are likely to be gradually displaced by these new sequencing technologies (5).
In this study, we investigated the performance of the CNV module of the commercial software suite SeqPilot (JSI Medical Systems) and the non-commercial utility panelcn.MOPS (6) to analyze NGS data as a screening approach for hereditary cancer genetic diagnostics testing, and the exon-level CNVs. The results were confirmed using the MLPA method. Furthermore, we assessed the effectiveness of digital multiplex ligation-dependent probe amplification (digitalMLPA), a novel technology that combines MLPA and NGS approaches for detecting cancer-related CNVs.
Materials and Methods
A total of 2,163 patients who were referred to Genekor’s Medical S.A (Athens, Greece) laboratory for genetic testing using a hereditary cancer panel were evaluated. Specifically, we tested 1,785 breast cancer patients, 267 ovarian cancer patients, and 111 colon cancer patients. Prior to molecular genetic testing, all patients signed an informed consent form, and authorization was granted for the anonymous use of their data for research purposes and/or scientific publications. Furthermore, demographic information, clinical and family history of patients, and pedigrees were ordered by clinicians at the time of testing.
According to the manufacturer’s instructions, genomic DNA was extracted from peripheral blood using the MagCore® Genomic DNA Whole Blood Kit (RBC Bioscience, New Taipei City, Taipei, Taiwan, ROC). A solution-based capture technique was used to analyze genes involved in hereditary cancer predisposition. As previously described (7), targeted NGS was performed with a panel of 52 genes (APC, ATM, ATR, AXIN2, BAP1, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CDK4, CDKN2A, CHEK2, EPCAM, FANCA, FANCL, FANCM, GALNT12, GEN1, HOXB13, MEN1, MLH1, MRE11, MSH2, MSH3, MSH6, MUTYH, NBN, NF1, NTHL1, PALB2, PMS2, POLD1, POLE, PPP2R2A, PTEN, RAD50, RAD51B, RAD51C, RAD51D, RET, RNF43, RPS20, SMAD4, SMARCA4, STK11, TP53, VHL) (Roche NimbleGen SeqCap EZ Choice, Pleasanton, CA, USA), and sample preparation was carried out in accordance with the manufacturer’s instructions in the SeqCap EZ Choice Library User’s Guide (Roche NimbleGen). Sequencing was carried out using the DNBSEQ-G400 technology (MGI Tech Co., Ltd., Beishan Industrial Zone, Shenzhen, PR China) and the commercially available software suite SeqNext version 4.4.0 (JSI Medical Systems GmbH, Ettenheim, Germany); sequence alterations were identified and interpreted in the context of a single clinically relevant transcript.
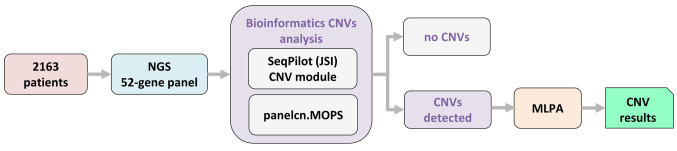
The presence of CNVs was investigated using the commercial computational algorithms SeqPilot (JSI medical systems GmbH) and panelcn.MOPS and MLPA. First, we used the SeqPilot and panelcn.MOPS algorithms to screen all genes of clinical interest, and then we used the MLPA (Multiplex Ligation-dependent Probe Amplification, MRC Holland, DL, Amsterdam, the Netherlands) approach to validate CNVs. Samples that failed quality control (QC) of the computational analysis of CNVs from NGS data by the two algorithms were analyzed by default with MLPA (Figure 1). Additionally, the samples of 381 of the 2163 patients included in this studied were analyzed using digitalMLPA.
Figure 1. Schematic of the study’s workflow. The capture-based method enabled computational analysis of copy number variations (CNVs) in nextgeneration sequencing (NGS) data.
The clinical significance of all identified CNVs was assessed using the American College of Medical Genetics and Genomics (ACMG) and Clinical Genome Resource (ClinGen) standards for CNV variant classification (8).
Computational analysis of CNVs from NGS data. The capture-based approach allowed the computational analysis of CNVs from NGS data. The CNV module of the software suite SeqPilot (JSI Medical Systems) and panelcn.MOPS were utilized for this purpose. Panelcn.MOPS detected CNVs ranging in size from a portion of a region of interest (ROI) to entire genes, which may include all ROIs studied in a given sample. Furthermore, panelcn.MOPS offers QC criteria not only for samples, but also for individual ROIs within a sample, which increases confidence in called CNVs.
MLPA. The MLPA reactions were carried out in accordance with the manufacturer’s instructions: Denatured genomic DNA was used to hybridize MLPA probes using the SALSA MLPA probe mix (BRCA1: P002; BRCA2: P045; CHEK2: P190; EPCAM, MSH6: P072, MLH1, MSH2: P003; MUTYH: P378; PALB2, RAD50, RAD51C, RAD51D: P260; TP53: P056, MRC Holland). The reactions, including the negative control samples, were carried out exactly as directed by the manufacturer. The SeqStudio Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA) was utilized for electrophoresis and the analysis was carried out using the Coffalyser.Net software.
DigitalMLPA. DNA from 381 patients was tested and validated in collaborate laboratories using a test version of the D001-B1 Hereditary Cancer Panel 1, which was recently produced by MRC Holland. The probemix included the following: a) 558 probes detecting copy number alterations in the following hereditary cancer genes: APC, ATM, BAP1, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CDK4, CDKN2A, CHEK2, EPCAM, MITF, MLH1, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, POLE, PTEN, RAD51C, RAD51D, GREM1, SMAD4, STK11 and TP53, b) Five mutant-specific probes that generate probe reads only when a particular mutation is present, c) Three wild type specific probes used to detect the wild type sequence of a specific mutation, and d) More than 120 control probes and fragments. The digitalMLPA processes were carried out as directed by the manufacturer, and sample-specific products from several reactions were pooled, diluted, denatured, and loaded onto an Illumina MiSeq V3 standard flow-cell for 115-bp single-read sequencing. After assessing the quality of the produced FASTQ files, readings were assigned to digitalMLPA probes and data were analyzed using in-house bioinformatics software (MRC Holland).
Results
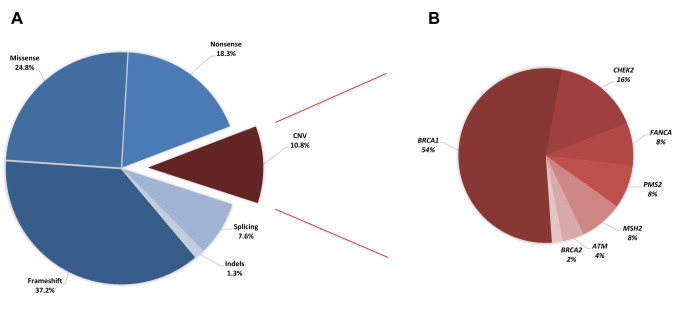
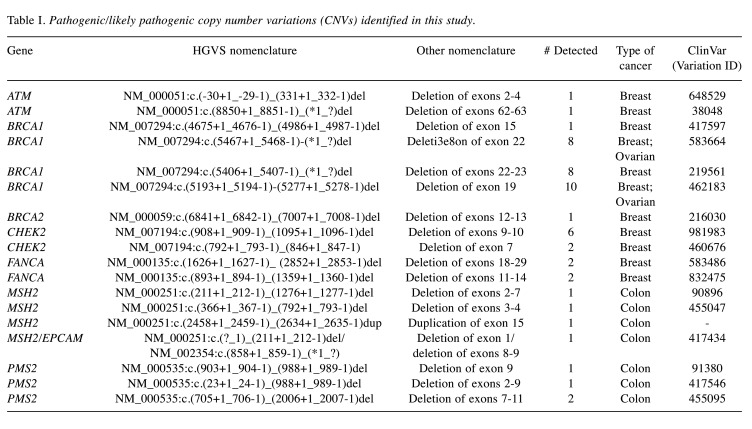
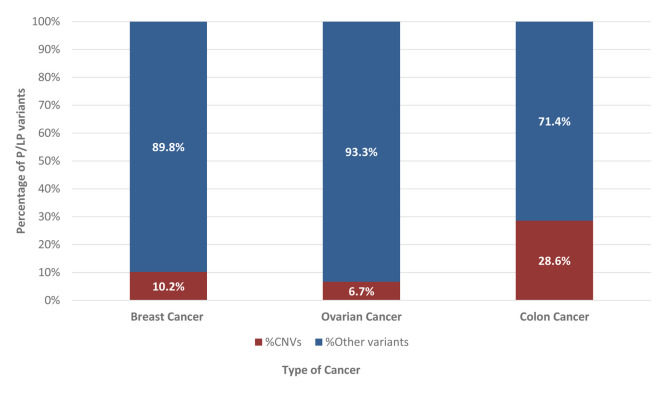
In 464 samples (21.5%), at least one pathogenic/likely pathogenic (P/LP) variant was found. CNVs accounted for 10.8% (50/464) of the observed P/LP variations, referring to the deletion/duplication of one or more exons of a gene. Seventy-two percent of the 50 CNVs found were in high-risk cancer genes (54% BRCA1, 2% BRCA2, 8% PMS2, 8% MSH2), 20% in moderate-risk genes (16% CHEK2, 4% ATM), and 8% in a low-risk gene (8% FANCA) (Figure 2). In this investigation, deletions were the most often found type of CNV. Furthermore, 46% (23/50) of detected CNVs involved a single exon, while the remaining 54% (27/50) involved several exons. Table I contains detailed information for all CNVs detected. P/LP variants were found in 362 (20.3%) and 74 (27.7%) patients with breast and ovarian cancer, respectively, while CNVs accounted for 10.2% (37/362) and 6.8% (5/74) of the clinically relevant cases, respectively. CNVs accounted for 28.6% of P/LP variations (8/28) in colorectal cancer patients (Figure 3).
Figure 2. Variant types identified in the 52 genes tested in this study. A) Distribution of variant types for the pathogenic/likely pathogenic variants identified in 464 individuals with positive findings. B) Distribution of genes with copy number variations (CNVs).
Table I. Pathogenic/likely pathogenic copy number variations (CNVs) identified in this study.
Figure 3. Percentage of pathogenic/ likely pathogenic (P/LP) copy number variations (CNVs) compared to all identified P/LP variants in the different types of cancer.
The deletion of exon 19 in the BRCA1 gene was the most often found CNV (20%). This variant is a large rearrangement that encompasses exon 19 of the BRCA1 gene, resulting in exon 19 deletion. Exon 19 encodes amino acid residues that are part of the BRCA1 C-Terminal domain (BRCT) domain, which is known to be crucial for BRCA1 protein activity. Furthermore, exon 9-10 deletions in the CHEK2 gene account for 12% of all CNVs found in our analysis. This variation involves a large genomic rearrangement that results in the deletion of CHEK2 exons 9-10. It is likely to result in a missing or disrupted protein product.
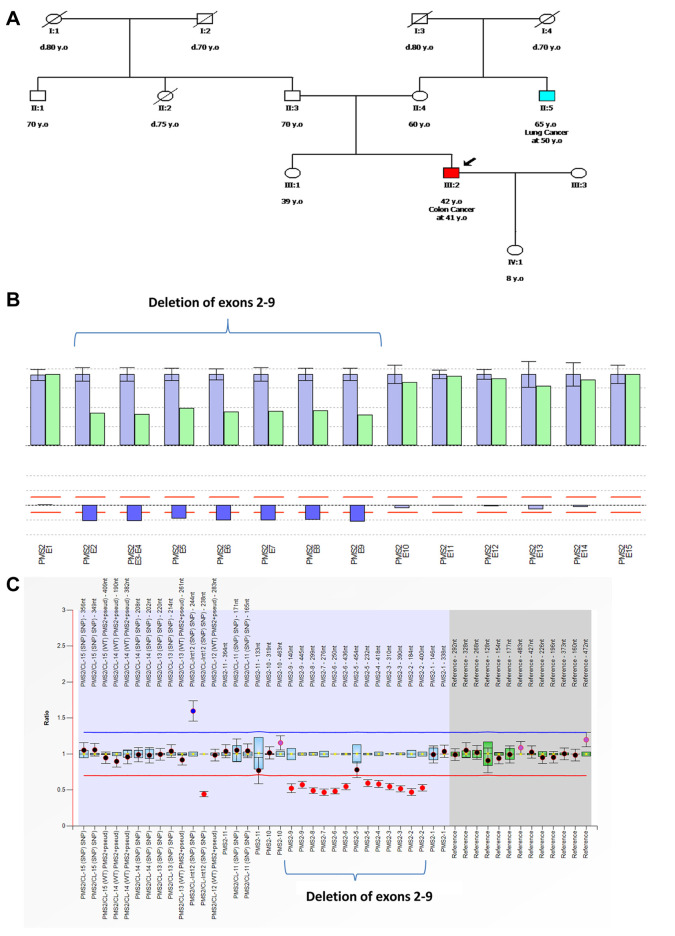
The CNVs were analyzed using the SeqPilot software suite’s CNV module (JSI Medical Systems) and panelcn.MOPS. Both algorithms were developed exclusively for CNV analysis of sequencing data, with 99-100% sensitivity and up to 100% specificity for the prediction of CNVs up to the level of a single gene exon. Figure 4 depicts an example of PMS2 deletion detection utilizing computational techniques and MLPA. A deletion of exons 2-9 in the PMS2 gene was identified in a 42-year-old individual with colon cancer. This variation involves a complete deletion of the region containing PMS2 exons 2-9. It generates an early translational stop signal, which is likely to result in a missing or damaged protein product.
Figure 4. A case of a patient with a pathogenic copy number variation (CNV). A) Patient pedigree with a deletion of exons 2-9 in the PMS2 gene. B) Detection of CNVs using next-generation sequencing utilizing JSI software was used to visualize the deletion of PMS2 germline exons 2-9. The bar plot depicts the relative coverage of each target ROI of the patient sample in green and the average relative target coverage of control samples in blue. Below each couple of bars is the ratio of the coverage of target ROIs of the patient sample versus the controls. Reds lines correspond to the 75% deletion limit (lower red dotted line) and the 135% duplication limit (upper red dotted line) for the calculated ratio. The average relative target coverage data are reported as means±standard deviations of control samples. C) Sample plots from Coffalyser.Net displaying probe ratios with 95% confidence intervals as error bars for all PMS2 exons. The proband’s MLPA analysis revealed heterozygous deletions of exons 2-9 in the PMS2 gene (SALSA MLPA probemix P008, MRC Holland).
The comparison of digitalMLPA data with traditional MLPA and computational analysis of CNVs from NGS data of 381 samples revealed 100% concordance. No CNV was detected in 99.2% of the samples. The CNVs identified in four patients were: a deletion of exons 9-10 in CHEK2, a loss of exon 19 in BRCA1, a deletion of exons 22-23 in BRCA1, and a deletion of exons 62-63 in ATM.
Discussion
Breast, colon, ovarian, and endocrine neoplasia are the most penetrant hereditary cancer syndromes, accounting for 5% to 10% of all cancers (9). Cancer genetic testing is an effective diagnostic test that promotes cancer prevention and early detection in people with genetic predisposition to cancer. The implementation of NGS technology has resulted in a revolution in diagnostic procedures; it allows for the simultaneous analysis of numerous genes and samples while reducing prices and turnaround time (10).
Nevertheless, accurate validation of bioinformatics algorithms for CNV identification from NGS data has been proven more difficult than for other types of variations such as point mutations or small deletions and insertions. CNVs have been found in hereditary breast/ovarian cancer, accounting for a small but clinically significant fraction of patients. While loss of function sequence alterations account for the majority of BRCA1 and BRCA2 variations, CNVs have been found to account for 3-15% of all BRCA1 and BRCA2 variants (11,12).
In this study, CNVs were found in 42 samples of patients with breast and ovarian cancer. CNVs were observed in the BRCA1/BRCA2 genes in 28 of the 42 samples (67%); CNVs were also found in the ATM (5%), CHEK2 (19%), and FANCA (10%) genes. The ATM gene is implicated in homologous recombination (HR) and is associated with a 15-40% increased risk of autosomal dominant breast cancer, a 5-10% increased risk of pancreatic cancer, and a 5%-10% increased risk of colorectal cancer in persons bearing a single P/LP variant (13,14). Monoallelic ATM P/LP pathogenic variants may also be related with ovarian cancer (3%) (15). The CHEK2 gene is implicated in HR and is linked to the Fanconi anemia (FA)-BRCA DNA damage repair pathway (16). Carriers of pathogenic frameshift CHEK2 variants have a 2- to 5-fold increased breast cancer risk compared to the general population (17). The FANCA gene is also implicated in HR and is linked to FA-A (autosomal recessive Fanconi anemia). Furthermore, P/LP variations in the FANCA gene are linked to breast, ovarian, and prostate cancer, with the estimated risk still unknown (18). Patients with P/LP germline variations in HR genes may benefit from platinum-based treatment and PARP inhibitor treatment, while therapeutic benefit has not been demonstrated for all HR genes (19,20).
CNV analysis is also required in patients with colorectal cancer, as it accounts for 28.6% of the P/LP variations identified in this study. Lynch syndrome (LS) is an autosomal dominant illness with significant penetrance that affects approximately 3% of colorectal cancer cases and is linked to inherited germline variants in genes involved for DNA mismatch repair (MMR) (MSH2, MLH1, MSH6, and PMS2). CNVs occur in 10% to 20% of LS cases, depending on the clinical and molecular criteria used to identify patient cohorts. MSH2 is the gene that most commonly shows deletions. MSH6 and MLH1 gross deletions/duplications have also been found, but at a lower frequency (21). The NCCN Guidelines for Colorectal Assessment advised genetic testing/counseling and management strategies for individuals with P/PL variations in MMR genes. Furthermore, the discovery of germline changes in MMR genes has therapeutic significance, as it may aid in the prediction of immunotherapy benefits (22).
There are numerous approaches for detecting CNVs, including aCGH array and MLPA, however both have limitations. In this study, we examined how two NGS CNV calling techniques could be used as a screening tool before MLPA validation in a hereditary cancer genetic diagnostic test. The CNV module of the SeqPilot software suite (JSI Medical Systems) and panelcn.MOPS demonstrated great specificity, as expected, because all CNVs discovered using these methods were later experimentally validated using the MLPA approach. These findings highlight the importance of incorporating CNV analysis into NGS panel testing on a regular basis, a technique that will significantly increase the efficiency of screening for hereditary cancer. Furthermore, finding CNVs in cancer patients improves diagnostic yield.
Appropriate genetic analysis enables proper clinical management and the development of relevant therapeutic strategies for patients with a positive finding. Similarly, it allows the individualization of cancer risk assessment for all carrier family members.
In conclusion, the integration of multiple CNV detection techniques (such as array CGH and MLPA, NGS and MLPA, qPCR, and digital MLPA) has the potential to provide accurate CNV analysis. In addition to SNV and indels, comprehensive genetic testing should also include CNV evaluation for all individuals with suspicion of inherited cancer, as CNVs account for up to one in ten P/LP variants detected by NGS.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
KA drafted the manuscript. KA, GP, KP, BM, NK, CD, AM, MR and NT carried out the DNA extraction and sequencing and contributed to the analysis and interpretation of the variant data. GNT performed the bio-informatics analysis. VV, CM, RI, SK, CC, IN, KP, MVA, EK, AP, SG, DZ, EL, AK, AK, CP, VO, ST, KK, TO, DTE, AC and AB provided the materials, demographic data and family history. EP and GN conceived of the study and participated in its design and coordination. All Authors read and approved the final manuscript.
References
- 1.Schmitt ML. Germline (Hereditary) risk. Clin J Oncol Nurs. 2021;25(6):21–22. doi: 10.1188/21.CJON.S2.21-22. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida R. Hereditary breast and ovarian cancer (HBOC): review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer. 2021;28(6):1167–1180. doi: 10.1007/s12282-020-01148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarrei M, Macdonald JR, Merico D, Scherer SW. A copy number variation map of the human genome. Nat Rev Genet. 2015;16(3):172–183. doi: 10.1038/nrg3871. [DOI] [PubMed] [Google Scholar]
- 4.Talevich E, Shain AH, Botton T, Bastian BC. CNVkit: Genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol. 2016;12(4):e1004873. doi: 10.1371/journal.pcbi.1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. 2016;17(6):333–351. doi: 10.1038/nrg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Povysil G, Tzika A, Vogt J, Haunschmid V, Messiaen L, Zschocke J, Klambauer G, Hochreiter S, Wimmer K. panelcn.MOPS: Copy-number detection in targeted NGS panel data for clinical diagnostics. Hum Mutat. 2017;38(7):889–897. doi: 10.1002/humu.23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agiannitopoulos K, Pepe G, Papadopoulou E, Tsaousis GN, Kampouri S, Maravelaki S, Fassas A, Christodoulou C, Iosifidou R, Karageorgopoulou S, Markopoulos C, Natsiopoulos I, Papazisis K, Vasilaki-Antonatou M, Venizelos V, Ozmen V, Tansan S, Kaban K, Eniu DT, Chiorean A, Nasioulas G. Clinical utility of functional RNA analysis for the reclassification of splicing gene variants in hereditary cancer. Cancer Genomics Proteomics. 2021;18(3):285–294. doi: 10.21873/cgp.20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riggs ER, Andersen EF, Cherry AM, Kantarci S, Kearney H, Patel A, Raca G, Ritter DI, South ST, Thorland EC, Pineda-Alvarez D, Aradhya S, Martin CL. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen) Genet Med. 2020;22(2):245–257. doi: 10.1038/s41436-019-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charron M, Kaiser B, Dauge A, Gallois H, Lapointe J, Dorval M, Nabi H, Joly Y. Integrating hereditary breast and ovarian cancer genetic counselling and testing into mainstream clinical practice: Legal and ethical challenges. Crit Rev Oncol Hematol. 2022;178:103797. doi: 10.1016/j.critrevonc.2022.103797. [DOI] [PubMed] [Google Scholar]
- 10.Ceyhan-Birsoy O, Jayakumaran G, Kemel Y, Misyura M, Aypar U, Jairam S, Yang C, Li Y, Mehta N, Maio A, Arnold A, Salo-Mullen E, Sheehan M, Syed A, Walsh M, Carlo M, Robson M, Offit K, Ladanyi M, Reis-Filho JS, Stadler ZK, Zhang L, Latham A, Zehir A, Mandelker D. Diagnostic yield and clinical relevance of expanded genetic testing for cancer patients. Genome Med. 2022;14(1):92. doi: 10.1186/s13073-022-01101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasickova P, Machackova E, Lukesova M, Damborsky J, Horky O, Pavlu H, Kuklova J, Kosinova V, Navratilova M, Foretova L. High occurrence of BRCA1 intragenic rearrangements in hereditary breast and ovarian cancer syndrome in the Czech Republic. BMC Med Genet. 2007;8:32. doi: 10.1186/1471-2350-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apessos A, Agiannitopoulos K, Pepe G, Tsaousis GN, Papadopoulou E, Metaxa-Mariatou V, Tsirigoti A, Efstathiadou C, Markopoulos C, Xepapadakis G, Venizelos V, Tsiftsoglou A, Natsiopoulos I, Nasioulas G. Comprehensive BRCA mutation analysis in the Greek population. Experience from a single clinical diagnostic center. Cancer Genet. 2018;220:1–12. doi: 10.1016/j.cancergen.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Jerzak KJ, Mancuso T, Eisen A. Ataxia-telangiectasia gene (ATM) mutation heterozygosity in breast cancer: a narrative review. Curr Oncol. 2018;25(2):e176–e180. doi: 10.3747/co.25.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaDuca H, Polley EC, Yussuf A, Hoang L, Gutierrez S, Hart SN, Yadav S, Hu C, Na J, Goldgar DE, Fulk K, Smith LP, Horton C, Profato J, Pesaran T, Gau CL, Pronold M, Davis BT, Chao EC, Couch FJ, Dolinsky JS. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med. 2020;22(2):407–415. doi: 10.1038/s41436-019-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lilyquist J, LaDuca H, Polley E, Davis BT, Shimelis H, Hu C, Hart SN, Dolinsky JS, Couch FJ, Goldgar DE. Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol Oncol. 2017;147(2):375–380. doi: 10.1016/j.ygyno.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moldovan GL, D’Andrea AD. How the Fanconi anemia pathway guards the genome. Annu Rev Genet. 2009;43:223–249. doi: 10.1146/annurev-genet-102108-134222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher S, Hughes E, Wagner S, Tshiaba P, Rosenthal E, Roa BB, Kurian AW, Domchek SM, Garber J, Lancaster J, Weitzel JN, Gutin A, Lanchbury JS, Robson M. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3(7):e208501. doi: 10.1001/jamanetworkopen.2020.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Valle J, Rofes P, Moreno-Cabrera JM, López-Dóriga A, Belhadj S, Vargas-Parra G, Teulé À, Cuesta R, Muñoz X, Campos O, Salinas M, de Cid R, Brunet J, González S, Capellá G, Pineda M, Feliubadaló L, Lázaro C. Exploring the role of mutations in Fanconi anemia genes in hereditary cancer patients. Cancers (Basel) 2020;12(4):829. doi: 10.3390/cancers12040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S, Messiou C, A’hern R, Tutt A, Ashworth A, Stone J, Carmichael J, Schellens JH, De Bono JS, Kaye SB. Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 20.Mateo J, Lord CJ, Serra V, Tutt A, Balmaña J, Castroviejo-Bermejo M, Cruz C, Oaknin A, Kaye SB, de Bono JS. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30(9):1437–1447. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valle L, De Voer RM, Goldberg Y, Sjursen W, Försti A, Ruiz-Ponte C, Caldés T, Garré P, Olsen MF, Nordling M, Castellvi-Bel S, Hemminki K. Update on genetic predisposition to colorectal cancer and polyposis. Mol Aspects Med. 2019;69:10–26. doi: 10.1016/j.mam.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Dal Buono A, Gaiani F, Poliani L, Correale C, Laghi L. Defects in MMR genes as a seminal example of personalized medicine: from diagnosis to therapy. J Pers Med. 2021;11(12):1333. doi: 10.3390/jpm11121333. [DOI] [PMC free article] [PubMed] [Google Scholar]