Abstract
BACKGROUND
The deleterious effects of discontinuation of digoxin on outcomes in ambulatory patients with chronic heart failure with reduced ejection fraction (HFrEF) receiving angiotensin-converting enzyme (ACE) inhibitors are well-documented.
OBJECTIVES
To determine the relationship between digoxin discontinuation and outcomes in hospitalized patients with HFrEF receiving more contemporary guideline-directed medical therapies (GDMT) including beta-blockers and mineralocorticoid receptor antagonists (MRAs).
METHODS
Of the 11,900 hospitalized patients with HFrEF (EF ≤45%) in Medicare-linked OPTIMIZE-HF, 3,499 received pre-admission digoxin, which was discontinued in 721 patients. Using propensity scores for digoxin discontinuation, estimated for each of the 3,499 patients, we assembled a matched cohort of 698 pairs of patients, balanced on 50 baseline characteristics (mean age, 76 years; mean EF, 28%; 41% women; 13% African American; 65% on beta-blockers).
RESULTS
Four-year post-discharge, digoxin discontinuation was associated with significantly higher risks of HF readmission (HR, 1.21; 95% CI, 1.05–1.39; p=0.007), all-cause readmission (HR, 1.16; 95% CI, 1.04–1.31; p=0.010), and the combined endpoint of HF readmission or all-cause mortality (HR, 1.20; 95% CI, 1.07–1.34; p=0.002), but not all-cause mortality (HR, 1.09; 95% CI, 0.97–1.24; p=0.163). Discontinuation of digoxin was associated with a significantly higher risk of all 4 outcomes at 6-month and 1-year post-discharge. At 30 days, digoxin discontinuation was associated with higher risks of all-cause mortality (HR, 1.80; 95% CI, 1.26–2.57; p=0.001) and the combined endpoint (HR, 1.36; 95% CI, 1.07–1.71; p=0.007) but not of HF readmission (HR, 1.19; 95% CI, 0.90–1.59; p=0.226) or all-cause readmission (HR, 1.03; 95% CI, 0.84–1.26; p=0.778).
CONCLUSIONS
Among hospitalized older patients with HFrEF on more contemporary GDMT, discontinuation of chronic digoxin therapy was associated with poor outcomes.
Keywords: Digoxin discontinuation, Heart failure, Mortality, Readmission, Reduced ejection fraction
Condensed Abstract
The deleterious effects of discontinuation of digoxin on outcomes in ambulatory patients with chronic heart failure with reduced ejection fraction (HFrEF) not receiving more contemporary guideline-directed medical therapy (GDMT) are well-known. In the current propensity score-matched study, we demonstrate that discontinuation of chronic digoxin therapy is associated with poor outcomes in hospitalized patients with HFrEF receiving GDMT. These findings highlight the negative outcomes associated with digoxin discontinuation in hospitalized patients with HFrEF receiving contemporary GDMT including beta-blockers and mineralocorticoid receptor antagonists.
Introduction
Heart failure (HF) is a leading cause of hospital admission and readmission (1). Digoxin is approved by the United States Food and Drug Administration for the treatment of mild to moderate HF to reduce the risk of HF-related hospitalizations and emergency care. According to the American College of Cardiology Foundation / American Heart Association (ACCF/AHA) HF guideline, digoxin may be used, unless contraindicated, to decrease hospitalizations due to worsening HF in patients with HF with reduced ejection fraction (HFrEF) (2). In the Digitalis Investigation Group (DIG) trial, the largest randomized controlled trial (RCT) of digoxin in HF, digoxin reduced the risk of all-cause and HF hospitalizations in patients with HFrEF but had no effect on all-cause mortality (3–5). The lack of a mortality benefit of digoxin in the DIG trial, taken together with the emergence of other evidence-based guideline-directed medical therapies (GDMT) with proven efficacy and effectiveness in lowering the risks of both all-cause mortality and hospitalization, has led to a dramatic decline in the use of digoxin in patients with HFrEF (6).
Both a lower rate of initiation of digoxin therapy and a higher rate of discontinuation of chronic digoxin therapy may lead to the underutilization of digoxin. The efficacy and effectiveness of digoxin in lowering the risk of admission and readmission in patients with HFrEF are well established (3–5,7–10). However, less is known about the effect of discontinuation of digoxin in patients with HFrEF. Findings from the Randomized Assessment of the effect of Digoxin on Inhibitors of the Angiotensin-Converting Enzyme (RADIANCE) and Prospective Randomized Study of Ventricular Failure and the Efficacy of Digoxin (PROVED) trials suggest that discontinuation of digoxin therapy increased the risk of adverse outcomes in ambulatory patients with chronic HFrEF (11,12). A post hoc analysis of the DIG trial demonstrated that discontinuation of digoxin is associated with poor outcomes in ambulatory patients with chronic HFrEF (13). Patients in these studies were receiving angiotensin-converting enzyme (ACE) inhibitors, but not beta-blockers or mineralocorticoid receptor antagonists (MRAs). The objective of the current study is to examine the relationship between discontinuation of chronic digoxin therapy and outcomes in a propensity score-matched cohort of hospitalized patients with HFrEF receiving more contemporary GDMT.
Methods
Data Source and Study Population
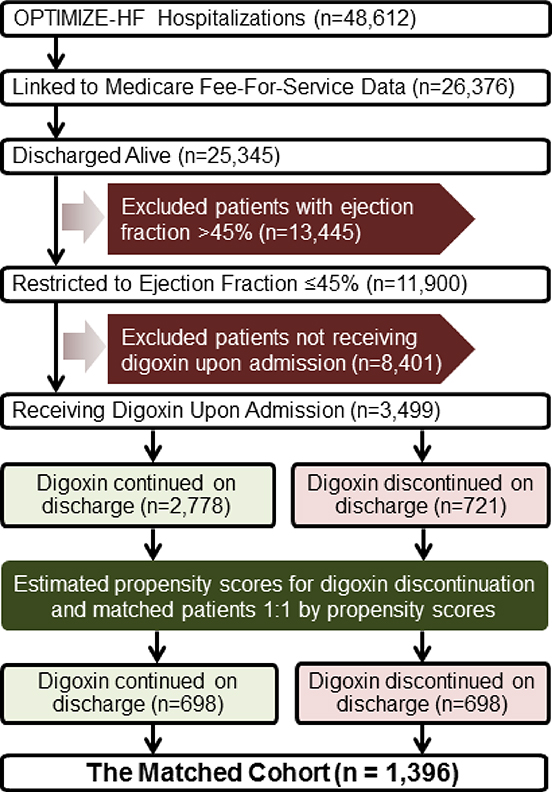
The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) is a national web-based registry of acute HF based on 48,612 HF hospitalizations occurring in 259 hospitals across 48 states between March 1, 2003 and December 31, 2004 (14–18). The registry contains extensive data on demographics, patient and hospital characteristics, quality of care, and short-term outcomes in a small subset of patients. We obtained information about long-term outcomes through probabilistic linking of OPTIMIZE-HF with the Medicare data (19). The Medicare-linked OPTIMIZE-HF data included 25,345 unique patients who were discharged alive, of whom 11,900 had HF with left ventricular EF ≤45% (Figure 1). We used this cutoff for EF as this was used in the DIG Main trial to define HFrEF (3). We excluded 8,401 patients not receiving digoxin prior to hospital admission. Thus, our study cohort consisted of 3,499 patients who were receiving digoxin prior to hospital admission. In 721 of these patients, digoxin was discontinued prior to hospital discharge.
Figure 1: Assembly of the Study Cohort.
Flow chart displaying assembly of a matched cohort of patients with heart failure with reduced ejection fraction, by discontinuation of digoxin therapy (OPTIMIZE-HF = Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure).
Assembly of a Balanced Cohort
We used propensity score matching to assemble a cohort in which patients with continuation and discontinuation of chronic digoxin therapy would be balanced on key measured baseline characteristics. A notable advantage of using the propensity score approach is that as in a randomized controlled trial, the process of cohort assembly is outcome-blinded and the balance in measured baseline characteristics can be displayed in a tabular form (20,21). To achieve this goal, we first calculated propensity scores for each of the 3,499 patients using a non-parsimonious multivariable logistic regression model (22–24). In the model, discontinuation of digoxin was the dependent variable and 50 baseline characteristics listed in the Online Figure were used as covariates. Using a matching algorithm described elsewhere (25), we matched 698 or 97% of the 721 patients whose digoxin was discontinued with another 698 patients whose digoxin was continued, but had the same probability or propensity scores for discontinuation.
Propensity score models are sample-specific adjusters and are not intended to be used for out-of-sample prediction or estimation of coefficients, and measures of fitness and discrimination are not important for the assessment of the model’s effectiveness (25,26). As such, we assessed our propensity score model by estimating pre- and post-match between-group absolute standardized differences for the 50 measured baseline characteristics used in our propensity score model (22–24). Absolute standard difference values <10% suggest inconsequential residual imbalance and a value of 0% indicates no residual imbalance. We also used the same logistic regression model used for the estimation of propensity scores to estimate multivariable-adjusted odds ratios (OR) for digoxin discontinuation associated with each of the 50 baseline characteristics.
Assembly of a Sensitivity Cohort
Because worsening kidney function during hospitalization is one of the common reasons why digoxin may be discontinued in the inpatient setting, we assembled a sensitivity cohort that accounted for acute kidney injury (AKI). Data on both admission and discharge serum creatinine levels were available on 2,971 of the 3,499 patients, of whom 418 (14%) had AKI defined as a rise of serum creatinine from admission to discharge by ≥0.03 mg/dL. Digoxin was discontinued in 21% (622/2,971) of the patients. We then calculated the probability (propensity scores) of digoxin discontinuation for each of the 2,971 patients using the same regression model described above, thus assembling 602 pairs of patients. In the model, we replaced discharge serum creatinine with admission serum creatinine and AKI so that patients will be balanced on AKI in the matched cohort
Outcomes Data
We examined the following outcomes: HF readmission, all-cause readmission, all-cause mortality, and the combined endpoint of HF readmission or all-cause mortality. All outcomes were examined at 30 days, 6 months, 1 year and 4 years after hospital discharge. Data on all outcome events and times to events were collected from the Medicare data (19).
Statistical Analyses
Descriptive analyses between group baseline characteristics were compared using the Pearson chi-square and Wilcoxon rank sum tests, as appropriate. All outcome analyses were conducted using matched data. Cox regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) associated with digoxin discontinuation and time to events. We assessed the assumption of the proportional hazard by visual examinations of the log (minus log) curves. Kaplan-Meier survival analysis was used to generate survival plots by digoxin discontinuation. For readmission events within any given time, we used time to event within that time (e.g. 30 days for 30-day readmission) for patients who had an event, and for those without a readmission, we censored by time to death or end of follow-up (e.g. 30 days for 30-day readmission), whichever occurred first. For mortality events, we used time to death or time to censoring at study end. Subgroup analyses were conducted to determine the homogeneity of the association of digoxin discontinuation and the combined endpoint of HF readmission or all-cause mortality in the matched cohort.
To examine if significant associations observed in our matched data could be explained away by an unmeasured baseline characteristic, we conducted formal sensitivity analyses using Rosenbaum’s approach (27). From the 698 pairs of matched patients, we identified pairs in which we could directly compare survival times within each pair to determine whether one member of the pair clearly had a longer survival or event-free survival time than the other member. We then tested whether, in the absence of a hidden bias, patients in the digoxin discontinuation group had shorter survival time than their matched counterparts. A significant sign-score test provides strong evidence of a relationship between discontinuation of digoxin and time to a particular event. The sign-score test is used to calculate “sensitivity bounds” for a hypothetical unmeasured confounder to determine how much it would need to increase the odds of digoxin discontinuation to explain away its significant associations with outcomes. Our sensitivity analysis assumes that the potential unmeasured confounder is a binary baseline characteristic that is a near perfect predictor of the outcomes, which is also not strongly correlated with any of 50 baseline characteristics used in our propensity score model. However, sensitivity analysis cannot determine if such an unmeasured confounder exists. All statistical tests were 2-tailed, and a p value <0.05 was considered significant. All statistical analyses were conducted using IBM SPSS Statistics for Windows software, version 24 (IBM, Armonk, New York).
Results
The 1,396 matched patients had a mean (±standard deviation) age of 76 (±11) years and left ventricular ejection fraction of 28 (±9) percent; 41% were female, and 13% were African American. Before matching, patients in the digoxin discontinuation group were older, had higher left ventricular ejection fraction and fewer received guideline directed medical therapies for HFrEF (Table 1). After propensity score-matching, absolute standardized differences for all 50 baseline characteristics were <10%, suggesting inconsequential residual bias (Online Figure 1).
Table 1.
Baseline Characteristics of Hospitalized Older Patients with Heart Failure with Reduced Ejection Fraction by Discontinuation of Digoxin
| Before propensity score-matching (n=3,499) |
After propensity scorematching (n=1,396) |
|||||
|---|---|---|---|---|---|---|
| Digoxin discontinuation |
Digoxin discontinuation |
|||||
| No (n=2,778) | Yes (n=721) | P value | No (n=698) | Yes (n=698) | P value | |
| Age (years) | 75 (±11) | 76 (±11) | 0.047 | 76 (±11) | 76 (±11) | 0.827 |
| Women | 1,086 (39%) | 290 (40%) | 0.580 | 287 (41%) | 279 (40%) | 0.663 |
| African American | 389 (14%) | 105 (15%) | 0.700 | 84 (12%) | 103 (15%) | 0.135 |
| Left ventricular ejection fraction (%) | 26 (±9) | 28 (±10) | <0.001 | 28 (±9) | 28 (±10) | 0.973 |
| Smoker in past 1 year | 359 (13%) | 81 (11%) | 0.223 | 83 (12%) | 80 (11%) | 0.803 |
| Past medical history | ||||||
| Prior heart failure | 2,621 (94%) | 683 (95%) | 0.691 | 658 (94%) | 662 (95%) | 0.637 |
| Prior HF hospitalization in 6 months | 602 (22%) | 161 (22%) | 0.702 | 154 (22%) | 153 (22%) | 0.948 |
| Hypertension | 1,805 (65%) | 465 (64%) | 0.809 | 459 (66%) | 449 (64%) | 0.575 |
| Myocardial infarction | 872 (31%) | 220 (31%) | 0.651 | 228 (33%) | 215 (31%) | 0.455 |
| Coronary revascularization | 1,097 (39%) | 281 (39%) | 0.801 | 278 (40%) | 268 (38%) | 0.583 |
| Diabetes mellitus | 1,135 (41%) | 304 (42%) | 0.525 | 302 (43%) | 292 (42%) | 0.588 |
| Stroke/transient ischemic attack | 430 (15%) | 101 (14%) | 0.327 | 103 (15%) | 100 (14%) | 0.820 |
| Peripheral vascular disease | 438 (16%) | 107 (15%) | 0.541 | 106 (15%) | 104 (15%) | 0.881 |
| Atrial fibrillation | 1,271 (46%) | 324 (45%) | 0.696 | 313 (45%) | 314 (45%) | 0.957 |
| Ventricular arrhythmia | 299 (11%) | 69 (10%) | 0.352 | 60 (9%) | 67 (10%) | 0.515 |
| Implantable cardioverter defibrillator | 341 (12%) | 86 (12%) | 0.800 | 88 (13%) | 82 (12%) | 0.623 |
| Bi-ventricular pacemaker | 230 (8%) | 50 (7%) | 0.236 | 54 (8%) | 49 (7%) | 0.609 |
| Chronic obstructive pulmonary disease | 813 (29%) | 179 (25%) | 0.018 | 165 (24%) | 176 (25%) | 0.493 |
| Anemia | 407 (15%) | 94 (13%) | 0.270 | 93 (13%) | 92 (13%) | 0.937 |
| Depression | 251 (9%) | 70 (10%) | 0.577 | 64 (9%) | 65 (9%) | 0.926 |
| Admission findings | ||||||
| Dyspnea at rest | 1,183 (43%) | 291 (40%) | 0.281 | 300 (43%) | 284 (41%) | 0.385 |
| Dyspnea on exertion | 1,751 (63%) | 433 (60%) | 0.142 | 417 (60%) | 421 (60%) | 0.827 |
| Orthopnea | 838 (30%) | 165 (23%) | <0.001 | 156 (22%) | 165 (24%) | 0.567 |
| Paroxysmal nocturnal dyspnea | 503 (18%) | 95 (13%) | 0.002 | 87 (12%) | 94 (13%) | 0.577 |
| Jugular venous pressure elevation | 948 (34%) | 250 (35%) | 0.782 | 226 (32%) | 242 (35%) | 0.364 |
| Third heart sound | 378 (14%) | 92 (13%) | 0.552 | 83 (12%) | 88 (13%) | 0.683 |
| Pulmonary rales | 1,709 (62%) | 423 (59%) | 0.162 | 415 (59%) | 411 (59%) | 0.828 |
| Peripheral edema | 1,687 (61%) | 467 (65%) | 0.047 | 459 (66%) | 452 (65%) | 0.694 |
| Pulse (beats per minute) | 85 (±20) | 83 (±20) | 0.014 | 82 (±19) | 83 (±20) | 0.538 |
| Systolic blood pressure (mmHg) | 133 (±28) | 134 (±30) | 0.537 | 133 (±29) | 134 (±30) | 0.706 |
| Diastolic blood pressure (mmHg) | 74 (±17) | 72 (±18) | 0.006 | 71 (±17) | 72 (±18) | 0.553 |
| Laboratory findings | ||||||
| Admission serum sodium (mEq/L) | 137 (±10) | 136 (±13) | 0.349 | 136 (±10) | 136 (±13) | 0.941 |
| Admission hemoglobin (g/dL) | 13 (±3) | 12 (±2) | 0.040 | 12 (±2) | 12 (±2) | 0.795 |
| Admission serum BNP (pg/mL)* | 1,208 (734) | 1,222 (749) | 0.435 | 1,186 (883) | 1,223 (759) | 0.363 |
| Discharge serum creatinine (mg/dL) | 1.6 (±0.9) | 1.7 (±1.0) | 0.008 | 1.7 (±1.2) | 1.7 (±1.0) | 0.729 |
| Discharge medications | ||||||
| ACE inhibitors or ARBs | 2,001 (72%) | 436 (60%) | <0.001 | 449 (64%) | 429 (61%) | 0.268 |
| Beta-blockers | 2,027 (73%) | 450 (62%) | <0.001 | 457 (65%) | 444 (64%) | 0.467 |
| Aldosterone antagonists | 559 (20%) | 106 (15%) | 0.001 | 105 (15%) | 106 (15%) | 0.940 |
| Loop diuretics | 2,354 (85%) | 513 (71%) | <0.001 | 507 (73%) | 510 (73%) | 0.857 |
| Nitrates | 795 (29%) | 170 (24%) | 0.007 | 157 (22%) | 170 (24%) | 0.411 |
| Amlodipine | 118 (4%) | 43 (6%) | 0.050 | 42 (6%) | 40 (6%) | 0.820 |
| Other calcium channel blockers | 189 (7%) | 39 (5%) | 0.176 | 43 (6%) | 39 (6%) | 0.649 |
| Anti-arrhythmics | 483 (17%) | 127 (18%) | 0.886 | 135 (19%) | 123 (18%) | 0.408 |
| Hospital length of stay (days)* | 4 (4) | 5 (5) | <0.001 | 5 (5) | 5 (5) | 0.332 |
| Hospital characteristics | ||||||
| Region | ||||||
| Midwest | 958 (34%) | <0.001 | 244 (35%) | 0.49 | ||
| Northeast | 439 (16%) | 79 (11%) | ||||
| South | 928 (33%) | 236 (34%) | ||||
| West | 453 (16%) | 139 (20%) | ||||
| Size (number of beds)* | 375 (233) | 375 (230) | 0.136 | 375 (220) | 375 (230) | 0.198 |
| Academic center | 1,410 (51%) | 353 (49%) | 0.390 | 365 (52%) | 345 (49%) | 0.284 |
Values are n (%), mean ± standard deviation, or median (interquartile range) when indicated by an asterisk; p values comparing medians are based on nonparametric independent sample median test.
ACE = angiotensin-converting Enzyme; ARB = angiotensin receptor blocker; BNP = B-type natriuretic peptide; HF = heart failure
4-Year Outcomes
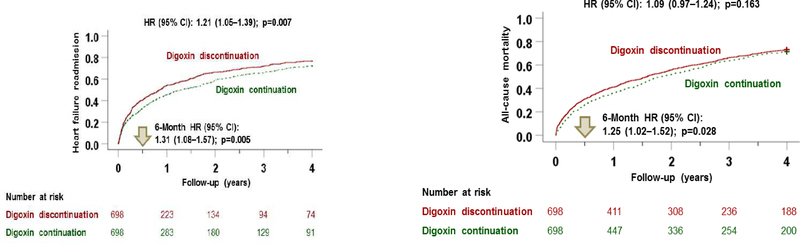
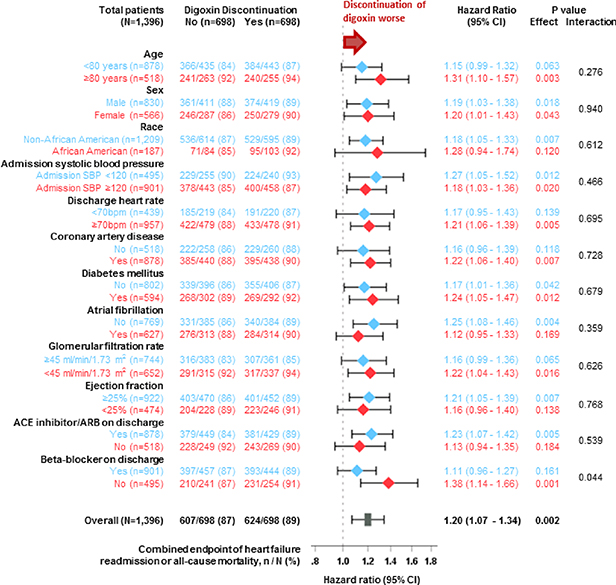
Digoxin discontinuation was associated with significantly higher risks of HF readmission (HR, 1.21; 95% CI, 1.05–1.39; p=0.007), all-cause readmission (HR, 1.16; 95% CI, 1.04–1.31; p=0.010), and the combined endpoint of HF readmission or all-cause mortality (HR, 1.20; 95% CI, 1.07–1.34; p=0.002), but not with all-cause mortality (HR, 1.09; 95% CI, 0.97–1.24; p=0.163; Table 2, Central Illustration). The association between digoxin discontinuation and the combined endpoint of HF readmission or all-cause mortality at 4 years was homogeneous among various clinically relevant subgroups of patients, except by beta-blocker use at hospital discharge (Figure 2). HRs (95% CIs) for 4-year combined endpoint associated with digoxin discontinuation in the subgroups receiving and not receiving beta-blockers were 1.11 (0.96–1.27; p=0.161) and 1.38 (1.14–1.66; p=0.001), respectively (p for interaction, 0.044; Figure 2).
Table 2.
Outcomes in 1,396 Propensity Score-Matched Hospitalized Older Patients with Heart Failure (HF) with Reduced Ejection Fraction by Digoxin Discontinuation at Hospital Discharge
| Events (%) for digoxin discontinuation at hospital discharge |
Hazard ratio associated with digoxin discontinuation (95% CI); p value | ||
|---|---|---|---|
| No (n=698) | Yes (n=698) | ||
| 30-day outcomes | |||
| HF readmission | 89 (13%) | 101 (14%) | 1.19 (0.90–1.59); p=0.226 |
| All-cause readmission | 195 (28%) | 193 (28%) | 1.03 (0.84–1.26); p=0.778 |
| All-cause mortality | 47 (7%) | 82 (12%) | 1.80 (1.26–2.57); p=0.001 |
| HF readmission or all-cause mortality | 131 (19%) | 171 (24%) | 1.36 (1.09–1.71); p=0.007 |
| 6-month outcomes | |||
| HF readmission | 205 (29%) | 246 (35%) | 1.31 (1.08–1.57); p=0.005 |
| All-cause readmission | 377 (54%) | 409 (59%) | 1.18 (1.03–1.36); p=0.019 |
| All-cause mortality | 181 (26%) | 215 (31%) | 1.25 (1.02–1.52); p=0.028 |
| HF readmission or all-cause mortality | 321 (46%) | 377 (54%) | 1.28 (1.10–1.48); p=0.001 |
| 1-year outcomes | |||
| HF readmission | 276 (40%) | 317 (45%) | 1.28 (1.09–1.51); p=0.003 |
| All-cause readmission | 472 (68%) | 483 (69%) | 1.15 (1.02–1.31); p=0.028 |
| All-cause mortality | 251 (36%) | 287 (41%) | 1.21 (1.02–1.43); p=0.028 |
| HF readmission or all-cause mortality | 415 (59%) | 475 (68%) | 1.27 (1.11–1.45); p<0.001 |
| 4-year outcomes | |||
| HF readmission | 391 (56%) | 407 (58%) | 1.21 (1.05–1.39); p=0.007 |
| All-cause readmission | 595 (85%) | 579 (83%) | 1.16 (1.04–1.31); p=0.010 |
| All-cause mortality | 498 (71%) | 510 (73%) | 1.09 (0.97–1.24); p=0.163 |
| HF readmission or all-cause mortality | 607 (87%) | 624 (89%) | 1.20 (1.07–1.34); p=0.002 |
Central Illustration: Kaplan-Meier Plots for Outcomes by Digoxin Discontinuation.
This study assessed the relationship of discontinuation of chronic digoxin therapy with heart failure readmission (top panel) and all-cause mortality (middle panel) in 698 pairs of propensity score-matched patients with heart failure with reduced ejection fraction. During 4 years of follow-up, digoxin discontinuation was associated with a significantly higher risk of all outcomes, compared with patients whose chronic digoxin therapy was continued. CI = confidence interval; HR = hazard ratio.
Figure2: Forest Plots for Subgroup Analyses of Combined Outcome by Digoxin Discontinuation.
In all variables analyzed, patients in the digoxin discontinuation group had a lower risk of the combined endpoint of heart failure readmission or all-cause mortality compared to patients whose chronic digoxin therapy was continued. ACE = angiotensin-converting enzyme, ARB = angiotensin receptor blocker, CI = confidence interval, SBP = systolic blood pressure. Note: Results of subgroup analyses need to be interpreted with caution as they may be false positive due to multiple comparisons and false negative due to inadequate power.
Findings from our sensitivity analyses demonstrate that the significant 4-year associations of digoxin discontinuation with all-cause readmission and the combined endpoint were insensitive to unmeasured confounders. Of the 698 matched pairs, in 580 pairs we were able to determine which patient within a matched pair had a shorter 4-year total readmission-free survival, and in 55% (318/580) of those pairs, they belonged to the digoxin discontinuation group (sign-score test p, 0.020). A hidden baseline characteristic would need to increase the odds of digoxin discontinuation by 3% to explain away this association. For the combined endpoint, in 682 pairs we were able to determine which member of the pair had a shorter time to event and in 55% (378/682) of those pairs, they belonged to the digoxin discontinuation group (sign-score test p, 0.005). A hidden baseline characteristic would need to increase the odds of digoxin discontinuation by 7% to explain away this association.
6-Month and 1-Year Outcomes
Digoxin discontinuation was associated with higher risks of HF readmission, all-cause readmission, all-cause mortality and the combined endpoint of HF readmission or all-cause mortality at 6 months and 1 year after hospital discharge (Table 2). Of the 698 matched pairs, in 325 pairs we were able to determine which patient within a matched pair had a shorter 6-month HF readmission-free survival, and in 56% (182/325) of those pairs, they belonged to the digoxin discontinuation group (sign-score test p, 0.031). A hidden baseline characteristic would need to increase the odds of digoxin discontinuation by 2% to explain away this association.
30-Day Outcomes
Digoxin discontinuation had no association with HR or all-cause readmissions at 30-day post-discharge (Table 2). Digoxin discontinuation was associated with a higher risk of all-cause mortality (HR, 1.80; 95% CI, 1.26–2.57; p=0.001; Table 2). In 120 of the 698 matched pairs, we were able to determine which patient within a matched pair had a shorter survival, and in 65% (78/120) of those pairs, they belonged to the digoxin discontinuation group (sign-score test p, 0.001). A hidden baseline characteristic would need to increase the odds of digoxin discontinuation by 22% to explain away this association. Digoxin discontinuation was also associated with a higher risk of the combined endpoint (HR, 1.36; 95% CI, 1.09–1.71; p=0.007; Table 2).
Findings from the Sensitivity Cohort
The 1,204 matched patients had a mean (±standard deviation) age of 76 (±11) years and left ventricular ejection fraction of 28 (±10) percent; 40% were female, and 15% were African American and were balanced on 51 baseline characteristics including admission and discharge serum creatinine and in-hospital AKI (Online Table 1). Digoxin discontinuation was associated with significantly higher risks of HF readmission (HR, 1.26; 95% CI, 1.08–1.46; p=0.003), all-cause readmission (HR, 1.15; 95% CI, 1.02–1.30; p=0.026), and the combined endpoint of HF readmission or all-cause mortality (HR, 1.22; 95% CI, 1.08–1.38; p=0.001), but not with all-cause mortality (HR, 1.12; 95% CI, 0.98–1.28; p=0.098; Online Table 2).
Predictors of Discontinuation of Digoxin
Among the 3,499 pre-match patients with HFrEF who were on digoxin at the time of hospital admission, discharge prescriptions of ACE inhibitors or angiotensin receptor blocker (ARBs) (OR, 0.64; 95% CI, 0.53–0.77; p<0.001), beta-blockers (OR, 0.68; 95% CI, 0.57–0.82; p<0.001), loop diuretics (OR, 0.52; 95% CI, 0.43–0.64; p<0.001) and nitrates (OR, 0.76; 95% CI, 0.62–0.94; p=0.011) were associated with lower odds of digoxin discontinuation. The odds of digoxin discontinuation were also lower among patients with orthopnea (OR, 0.77; 95% CI, 0.61–0.97; p=0.026), anemia (OR, 0.72; 95% CI, 0.56–0.94; p=0.016), and chronic obstructive pulmonary disease (OR, 0.81; 95% CI, 0.66–0.99; p=0.038), but higher among those with lower extremity edema (OR, 1.32; 95% CI, 1.10–1.60; p=0.003). Interestingly, in-hospital AKI was not associated with digoxin discontinuation (OR, 0.89; 95% CI, 0.68–1.17; p=420).
Discussion
Findings from our study demonstrate that discontinuation of chronic digoxin therapy in hospitalized older patients with HFrEF is associated with a significantly higher risk of poor outcomes. The associations with readmissions became significant at 6 months and lasted for 4 years but were not significant during the first 30 days after hospital discharge. In contrast, the association with mortality was significant at 30 days but disappeared after the first year. The risk of the combined endpoint of HF readmission or all-cause mortality was significantly higher in the group with digoxin discontinuation throughout the entire follow-up. To the best of our knowledge, this is the first report of adverse outcomes associated with discontinuation of chronic digoxin therapy in a propensity score-matched cohort of hospitalized older patients with HFrEF, receiving contemporary GDMT including ACE inhibitors/ARBs, beta-blockers and MRAs.
The elucidation of mechanistic explanations of the association between discontinuation of digoxin and poor outcomes is outside the scope of the current study. Digoxin is known for its efficacy and effectiveness in lowering the risk of HF and all-cause readmissions (3–5,7–9). It has been suggested that the positive inotropic effect of digoxin on cardiac performance may be attenuated during long-term therapy and may not be related to clinical outcomes (11,28,29). The clinical benefit of digoxin is often attributed to its ability to suppress neurohormones, specifically those in the sympathetic nervous system (30–36). In the RADIANCE and PROVED trials, the two major randomized controlled trials of digoxin withdrawal, significantly more patients in the digoxin discontinuation group had worsening functional capacity and symptoms, and a reduction in ejection fraction (11,12). Although neurohormonal parameters were not measured in these trials, indirect evidence from a significant increase in heart rate and body weight in the digoxin discontinuation groups suggested that digoxin discontinuation was associated with activation of the sympathetic neurohormonal system (11,12). Plasma noradrenaline concentration has been shown to be significantly higher among patients with HF with higher New York Heart Association class symptoms (37). The neurohormonal activation would be expected to be even higher in hospitalized patients with decompensated HF, which may in turn explain a more pronounced relationship between digoxin discontinuation and adverse outcomes in these patients. It is also possible that the higher risk of readmission in the digoxin discontinuation group may in part be explained by the negative rebound effect from digoxin withdrawal. However, unlike other cardiovascular drugs such as beta-blockers, the rebound syndrome phenomenon is less well documented for digoxin (38,39). Furthermore, findings from the studies of initiation of digoxin therapy suggest that evidence for clinical effectiveness of digoxin in lowering the risk of hospitalization (8–10)
Considering the observational nature of the current study, we also explored the roles of confounding due to bias, such as indication bias. Findings from the AHA’s Get With The Guidelines (GWTG) HF registry (6) and the pre-match data from the current study suggest that fewer patients in the digoxin discontinuation group were receiving ACE inhibitors or ARBs, beta-blockers, and MRAs. Although we were able to balance the use of these drugs in our propensity score-matched cohort, matching may not balance the underlying reasons for their underuse in the first place. Persistence of those reasons during follow-up may result in subsequent underuse of those drugs, which in turn may contribute to poor outcomes. These drugs are known for their proven efficacy and effectiveness in lowering the risks of both all-cause mortality and hospitalization. If the underuse of ACE inhibitors or ARBs, beta-blockers and MRAs in the digoxin discontinuation group confounded the worse outcomes in that group, then they would be expected to increase the risk of mortality. However, in our study digoxin discontinuation had no relationship with 4-years mortality, suggesting that results of our study are unlikely to be explained by the underuse of other neurohormonal antagonists. A higher comorbidity burden may also explain worse outcomes. However, patients in our matched cohort were balanced on 50 baseline characteristics including prior HF hospitalizations.
HF remains a leading cause for hospital admission and readmission for older adults, and digoxin has been shown to lower both risks without adversely affecting mortality (1,3,4,7–9). Digoxin is an inexpensive and relatively safe drug at low doses and recommended for patients with HFrEF who remain symptomatic despite optimal guideline directed medical therapies (2). The use of digoxin has declined substantially in recent years (6), which is likely due to both lower rates of initiation as well as higher rates of discontinuation. The effect of discontinuation of digoxin therapy on outcomes is well documented in the PROVED and the RADIANCE trials (11,12). However, both PROVED and the RADIANCE included ambulatory patients with HFrEF from an earlier era of HF therapy limited to use of ACE inhibitors, had small sample sizes, and used intermediate and softer endpoints. To the best of our knowledge, the current study is the first to examine the relationship between the discontinuation of digoxin therapy and harder endpoint in relatively large sample of hospitalized patients with decompensated HFrEF. More importantly, the use of GDMT in our study was similar to that in more contemporary HFrEF populations (6). Considering the recent decline in the use of digoxin, a drug known for its efficacy and effectiveness in reducing the risk of hospitalization (3,4,6,8,10), the findings of the current study are important as they suggest that in hospitalized patients with HFrEF receiving contemporary GDMT including beta-blockers and mineralocorticoid receptor antagonists, the discontinuation of digoxin therapy may be associated with worse post-discharge outcomes.
Limitations
Our study had several limitations. Despite our use of propensity score-matched balanced cohorts, bias due to imbalances in unmeasured baseline characteristics is possible. Findings from our sensitivity analyses suggest that the associations observed could be sensitive to an unmeasured confounder. However, sensitivity analysis cannot determine if such an unmeasured confounder exists. For an unmeasured binary baseline characteristic to be a confounder, it will need to be a near perfect predictor of the outcome being studied and also could not be strongly related to the 50 baseline characteristics used in our logistic regression model to estimate propensity scores, which is an unlikely possibility. The medical, surgical and device management of HFrEF have evolved since OPTIMIZE-HF and the current study is based on fee-for-service Medicare beneficiaries, which may limit generalizability. We did not have data on digoxin use during follow-up. If digoxin therapy was restarted in a large proportion of patients in the digoxin discontinuation group or digoxin was discontinued in the digoxin therapy group, that could potentially attenuate between-group difference and underestimate true associations. Similarly, we did not have data on the use and uptitration of other HF drugs during follow-up, a differential use of which may influence outcomes. Results of subgroup analyses need to be interpreted with caution as they may be false positive due to multiple comparisons and false negative due to inadequate power (40).
Conclusions
Among hospitalized older patients with HFrEF receiving more contemporary GDMT including ACE inhibitors/ARBs, beta-blockers and MRAs, discontinuation of chronic digoxin therapy was associated with poor outcomes. Findings from this study suggest that it may be premature to abandon the use of digoxin in patients with HFrEF.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Patient Care
In hospitalized older patients with HFrEF receiving guideline-directed medical management, discontinuation of chronic digoxin therapy is associated with poor outcomes.
Translational Outlook
Prospective studies are needed to confirm the effect of digoxin discontinuation on clinical outcomes in patients with HFrEF managed with contemporary therapy and determine responsible mechanisms.
Acknowledgments
Funding: Dr. Ali Ahmed was in part supported by the National Institutes of Health through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the National Heart, Lung, and Blood Institute (NHLBI). OPTIMIZE-HF was sponsored by GlaxoSmithKline, but played no role in the design, conduct, analyses or interpretation of the current study.
Abbreviations
- ACE
Angiotensin-converting enzyme
- ARBs
Angiotensin receptor blockers
- CI
Confidence interval
- HF
Heart failure
- HFrEF
Heart failure with reduced ejection fraction
- MRAs
Mineralocorticoid receptor antagonists
Footnotes
Disclosures: Dr. Fonarow reports consulting with Abbott, Amgen, Bayer, Janssen, Medtronic, Novartis, and was the Principle Investigator of OPTIMIZE-HF. None of the other authors report any conflicts of interest related to this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs.
Tweet: Digoxin discontinuation is linked to poor outcomes among hospitalized patients with HFrEF receiving contemporary GDMT.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009;360:1418–28. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 3.The Digitalis Investigation Group Investigators. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525–33. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Rich MW, Love TE et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006;27:178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Patel K, Filippatos G et al. Effect of oral digoxin in high-risk heart failure patients: a pre-specified subgroup analysis of the DIG trial. Eur J Heart Fail 2013;15:551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel N, Ju C, Macon C et al. Temporal Trends of Digoxin Use in Patients Hospitalized With Heart Failure: Analysis From the American Heart Association Get With The Guidelines-Heart Failure Registry. JACC Heart Fail 2016;4:348–56. [DOI] [PubMed] [Google Scholar]
- 7.Bourge RC, Fleg JL, Fonarow GC et al. Digoxin reduces 30-day all-cause hospital admission in older patients with chronic systolic heart failure. Am J Med 2013;126:701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed A, Bourge RC, Fonarow GC et al. Digoxin use and lower 30-day all-cause readmission for Medicare beneficiaries hospitalized for heart failure. Am J Med 2014;127:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam PH, Bhyan P, Arundel C et al. Digoxin use and lower risk of 30-day all-cause readmission in older patients with heart failure and reduced ejection fraction receiving beta-blockers. Clin Cardiol 2018;41:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qamer SZ, Malik A, Bayoumi E et al. Digoxin use and outcomes in patients with heart failure with reduced ejection fraction. Am J Med (in press) 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Packer M, Gheorghiade M, Young JB et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med 1993;329:1–7. [DOI] [PubMed] [Google Scholar]
- 12.Uretsky BF, Young JB, Shahidi FE, Yellen LG, Harrison MC, Jolly MK. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. J Am Coll Cardiol 1993;22:955–62. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed A, Gambassi G, Weaver MT, Young JB, Wehrmacher WH, Rich MW. Effects of discontinuation of digoxin versus continuation at low serum digoxin concentrations in chronic heart failure. Am J Cardiol 2007;100:280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonarow GC, Abraham WT, Albert NM et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J 2004;148:43–51. [DOI] [PubMed] [Google Scholar]
- 15.Fonarow GC, Stough WG, Abraham WT et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007;50:768–77. [DOI] [PubMed] [Google Scholar]
- 16.Bayoumi E, Lam PH, Dooley DJ et al. Spironolactone and Outcomes in Older Patients with Heart Failure and Reduced Ejection Fraction. Am J Med 2019;132:71–80 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam PH, Gupta N, Dooley DJ et al. Role of High-Dose Beta-Blockers in Patients with Heart Failure with Preserved Ejection Fraction and Elevated Heart Rate. Am J Med 2018;131:1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mujib M, Patel K, Fonarow GC et al. Angiotensin-converting enzyme inhibitors and outcomes in heart failure and preserved ejection fraction. Am J Med 2013;126:401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Kilgore ML, Arora T et al. Design and rationale of studies of neurohormonal blockade and outcomes in diastolic heart failure using OPTIMIZE-HF registry linked to Medicare data. Int J Cardiol 2013;166:230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology 2001;2:169–188. [Google Scholar]
- 21.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 22.Ahmed A, Husain A, Love TE et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J 2006;27:1431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed A, Rich MW, Zile M et al. Renin-angiotensin inhibition in diastolic heart failure and chronic kidney disease. Am J Med 2013;126:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed A, Fonarow GC, Zhang Y et al. Renin-angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med 2012;125:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed MI, White M, Ekundayo OJ et al. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J 2009;30:2029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor Observational Studies. New York: Springer-Verlag, 2002:105–170. [Google Scholar]
- 28.Taggart AJ, Johnston GD, McDevitt DG. Digoxin withdrawal after cardiac failure in patients with sinus rhythm. J Cardiovasc Pharmacol 1983;5:229–34. [DOI] [PubMed] [Google Scholar]
- 29.Fleg JL, Gottlieb SH, Lakatta EG. Is digoxin really important in treatment of compensated heart failure? A placebo-controlled crossover study in patients with sinus rhythm. Am J Med 1982;73:244–50. [DOI] [PubMed] [Google Scholar]
- 30.Slatton ML, Irani WN, Hall SA et al. Does digoxin provide additional hemodynamic and autonomic benefit at higher doses in patients with mild to moderate heart failure and normal sinus rhythm? J Am Coll Cardiol 1997;29:1206–13. [DOI] [PubMed] [Google Scholar]
- 31.Gheorghiade M, Hall V, Lakier JB, Goldstein S. Comparative hemodynamic and neurohormonal effects of intravenous captopril and digoxin and their combinations in patients with severe heart failure. J Am Coll Cardiol 1989;13:134–42. [DOI] [PubMed] [Google Scholar]
- 32.Gheorghiade M, Ferguson D. Digoxin. A neurohormonal modulator in heart failure? Circulation 1991;84:2181–6. [DOI] [PubMed] [Google Scholar]
- 33.Krum H, Bigger JT Jr., Goldsmith RL, Packer M. Effect of long-term digoxin therapy on autonomic function in patients with chronic heart failure. J Am Coll Cardiol 1995;25:289–94. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson DW, Berg WJ, Sanders JS, Roach PJ, Kempf JS, Kienzle MG. Sympathoinhibitory responses to digitalis glycosides in heart failure patients. Direct evidence from sympathetic neural recordings. Circulation 1989;80:65–77. [DOI] [PubMed] [Google Scholar]
- 35.Covit AB, Schaer GL, Sealey JE, Laragh JH, Cody RJ. Suppression of the renin-angiotensin system by intravenous digoxin in chronic congestive heart failure. Am J Med 1983;75:445–7. [DOI] [PubMed] [Google Scholar]
- 36.Torretti J, Hendler E, Weinstein E, Longnecker RE, Epstein FH. Functional significance of Na- K-ATPase in the kidney: effects of ouabain inhibition. Am J Physiol 1972;222:1398–405. [DOI] [PubMed] [Google Scholar]
- 37.Sigurdsson A, Amtorp O, Gundersen T, Nilsson B, Remes J, Swedberg K. Neurohormonal activation in patients with mild or moderately severe congestive heart failure and effects of ramipril. The Ramipril Trial Study Group. Br Heart J 1994;72:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nattel S, Rangno RE, Van Loon G. Mechanism of propranolol withdrawal phenomena. Circulation 1979;59:1158–64. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt TA, Holm-Nielsen P, Kjeldsen K. No upregulation of digitalis glycoside receptor (Na,K-ATPase) concentration in human heart left ventricle samples obtained at necropsy after long term digitalisation. Cardiovasc Res 1991;25:684–91. [DOI] [PubMed] [Google Scholar]
- 40.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet 2005;365:176–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.