Abstract
OBJECTIVES:
Inotropic support is commonly used in patients with cardiogenic shock (CS). High-quality data guiding the use of dobutamine or milrinone among this patient population is limited. We compared the efficacy and safety of these two inotropes among patients with low cardiac output states (LCOS) or CS.
DATA SOURCES:
MEDLINE, Embase, and Cochrane Central Register of Controlled Trials were searched up to February 1, 2023, using key terms and index headings related to LCOS or CS and inotropes.
DATA EXTRACTION:
Two independent reviewers included studies that compared dobutamine to milrinone on all-cause in-hospital mortality, length of ICU stay, length of hospital stay, and significant arrhythmias in hospitalized patients.
DATA SYNTHESIS:
A total of eleven studies with 21,084 patients were included in the meta-analysis. Only two randomized controlled trials were identified. The primary outcome, all-cause mortality, favored milrinone in observational studies only (odds ratio [OR] 1.19 (95% CI, 1.02–1.39; p = 0.02). In-hospital length of stay (LOS) was reduced with dobutamine in observational studies only (mean difference –1.85 d; 95% CI –3.62 to –0.09; p = 0.04). There was no difference in the prevalence of significant arrhythmias or in ICU LOS.
CONCLUSIONS:
Only limited data exists supporting the use of one inotropic agent over another exists. Dobutamine may be associated with a shorter hospital LOS; however, there is also a potential for increased all-cause mortality. Larger randomized studies sufficiently powered to detect a difference in these outcomes are required to confirm these findings.
Keywords: cardiogenic shock, dobutamine, milrinone
KEY POINTS
Question: Is there a difference in efficacy or safety between dobutamine and milrinone among patients with cardiogenic shock (CS)?
Findings: In this systematic review and meta-analysis of all available literature no clear significant difference between dobutamine and milrinone. A signal exists for increased mortality with dobutamine among patients with CS.
Meaning: Limited differences exist between dobutamine and milrinone in the treatment of CS. The link between Dobutamine and worsened mortality warrants further investigation.
Cardiogenic shock (CS) is a state of low cardiac output resulting in clinical, biochemical, and hemodynamic manifestations of end-organ hypoperfusion (1, 2). It may develop as a consequence of acute myocardial infarction, severe valvular heart disease, myocarditis, Takotsubo’s cardiomyopathy, decompensated pre-existing cardiomyopathies, uncontrolled tachyarrhythmias, pulmonary embolism, among others (3, 4). Despite advancements in therapeutic options for CS, short-term outcomes have remained largely unchanged for several decades, with mortality up to 40–50% (5, 6). Although there have been considerable advances in available mechanical circulatory support, studies to date have failed to show a meaningful improvement in clinical outcomes, with emergency revascularization as the only therapy shown to reduce the risk of death among patients with myocardial infarction complicated by CS (7–10). The use of vasopressor and inotropic support in patients with CS remains the mainstay of treatment, with several governing bodies supporting inotrope use in the management of both acute heart failure with reduced cardiac output and ST-elevation myocardial infarction complicated by CS (11–13). Dobutamine and milrinone are both approved inotropic agents in North America for use in CS.
The recently published Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock (DOREMI) trial compared dobutamine to milrinone in the management of patients with CS, and no difference in clinical outcomes was observed (14). Before the publication of this trial, observational data was primarily used to guide decision-making on the choice of inotrope in CS. We previously reported a comparison of all available data comparing the efficacy and safety of dobutamine to milrinone among hospitalized patients (15). In this systematic review and meta-analysis, we sought to update this comparison with the inclusion of the DOREMI trial.
METHODS
Systematic Review
We report this review according to the reporting structure suggested by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for randomized controlled trials (RCTs) and the Meta-analysis of Observational Studies in Epidemiology guidelines for observational studies (Supplemental Fig. 2, http://links.lww.com/CCX/B237) (16). The review protocol used for our initial systematic review and meta-analysis was used for this update (15).
We sought to assess studies of hospitalized patients with CS who were treated with inotropic therapy, with the aim of comparing dobutamine to milrinone. The primary outcome of interest was all-cause in-hospital mortality. Secondary efficacy outcomes included all-cause mortality at 1 year, length of stay (LOS) in the ICU, and LOS in hospital. Secondary safety endpoints included atrial and ventricular arrhythmias associated with symptoms and/or requiring anti-arrhythmic therapy.
Search Strategy, Study Selection, and Data Collection
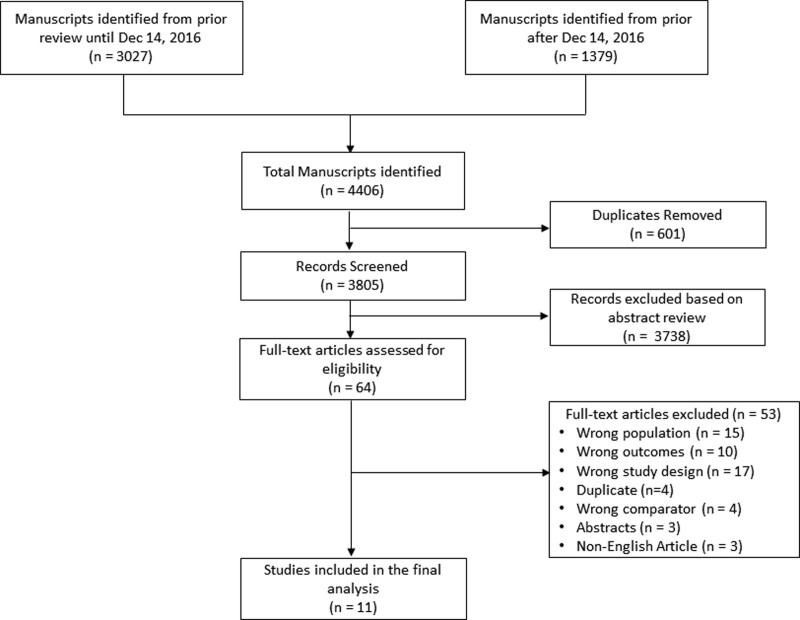
The systematic review search consisted of three updates of a previously reported review (15). The updates were conducted on July 6, 2020, November 19, 2021, and February 1, 2023. Searches were conducted in MEDLINE, the Cochrane Central Register of Controlled Trials, and Embase via Ovid (see Supplemental Tables for full details, http://links.lww.com/CCX/B237). The results were exported to Covidence (Melbourne, Australia) and duplicates were eliminated using the platform’s duplicate identification feature (Fig. 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of literature search.
We included all RCTs, prospective cohort studies, or case-control studies that evaluated dobutamine or milrinone in the management of patients with CS and included one of the following endpoints: in-hospital mortality, long-term mortality (within 1-year following hospital discharge), in-hospital LOS, ICU LOS, or arrhythmic events. We excluded abstracts, case series, case reports, narrative reviews, studies suspected of reporting outcomes of the same patients, and studies evaluating patients with acute decompensated heart failure not meeting the Society of Cardiovascular Angiography and Interventions definitions of CS. When we encountered multiple studies evaluating the same population, only the most recent or the source with the largest sample size was selected for inclusion.
Abstracts were screened for inclusion by two investigators. Conflicts were resolved through consensus or adjudicated by a third independent investigator when consensus was not reached. We collected study data including inotrope administration strategy (route of administration, dosing, duration), indication for inotrope use, in-hospital mortality, in-hospital LOS, ICU LOS, symptomatic arrhythmias, and discharge from the hospital. Baseline characteristics of patients were also collected when reported. Where no baseline characteristics were available authors were contacted to obtain primary data. Study characteristics, specifically year, author, single or multicenter study, study design, and number of patients were also included.
Risk of Bias
We evaluated the risk of bias using the Newcastle-Ottawa Scale for observational studies and the Cochrane risk-of-bias tool for randomized trials (17, 18). Quality of included studies was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation guidelines (19, 20).
Statistical Analysis
The results of the included studies are summarized individually and evaluated collectively. Continuous variables were represented as mean and sd or median and interquartile range, as appropriate. Categorical variables were represented as proportions (%). Random-effects meta-analysis was performed for efficacy and statistical heterogeneity was evaluated using the I2 statistic. Odds ratios (ORs) with 95% CI and mean differences were calculated for efficacy measures. p value of less than 0.05 was considered statistically significant. All analyses were performed using RevMan, v5.3 (Cochrane Collaboration, Copenhagen, Denmark) software.
RESULTS
Study Selection and Characteristics
The published literature regarding the relative safety and efficacy of milrinone versus dobutamine for CS has been previously published assessing all studies from inception to December 14, 2016 (15). A total of 4,406 articles were identified through our search strategy and screened for inclusion (3,027 articles before December 14, 2016, and 1,379 articles after December 14, 2016). Sixty-four full-text articles were reviewed for eligibility, where 53 studies were excluded because they included the wrong population, assessed the wrong outcomes, were the wrong studies, had the wrong comparator, were duplicated, were abstracts, or were of non-English articles.
A total of 11 studies involving 21,084 patients were included in the final analysis. 9 of the included studies were cohort studies with only two RCTs. The majority of studies included were of very low or low quality, predominantly due to the observational nature of the data.
All-Cause In-Hospital Mortality
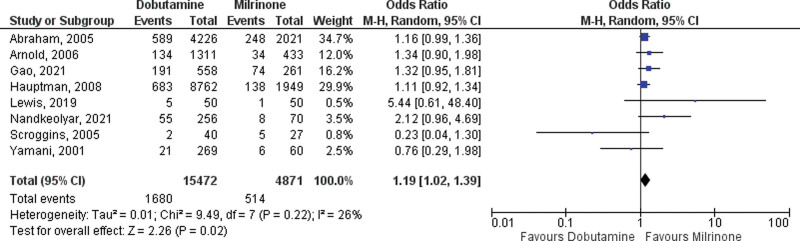
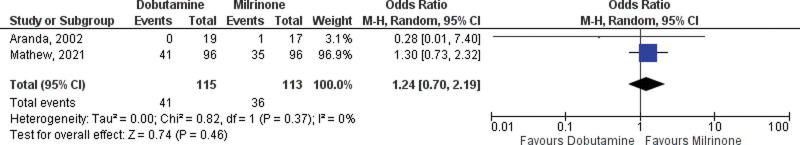
A total of 10 studies reporting in-hospital mortality in CS treated with dobutamine or milrinone were included in the primary analysis, two RCTs, and eight observational studies (14, 21–29). A total of 4,984 were treated with milrinone, and 15,587 patients were treated with dobutamine. The pooled OR for in-hospital mortality among RCTs was not different between the two drugs (OR 1.24; 95% CI, 0.70–2.19; Fig. 2). The pooled OR in observational studies for in-hospital all-cause mortality favored milrinone with an OR of 1.19 (95% CI, 1.02–1.39; p = 0.02; Fig. 3). There was little heterogeneity with I2 values of 26% (p = 0.22) and 0% (p = 0.46).
Figure 2.
Forest plot of in-hospital mortality with dobutamine vs milrinone inotrope therapy (observational studies). M-H = Mantel-Haenzel Analysis.
Figure 3.
Forest plot of in-hospital mortality with dobutamine vs milrinone inotrope therapy (randomized studies). M-H = Mantel-Haenzel Analysis.
A funnel plot for in-hospital mortality was produced (Supplemental Fig. 1, http://links.lww.com/CCX/B237). Although only a small number of studies are included in the analysis, visual interpretation does not demonstrate asymmetry suggestive of significant publication bias.
ICU and In-hospital Length of Stay
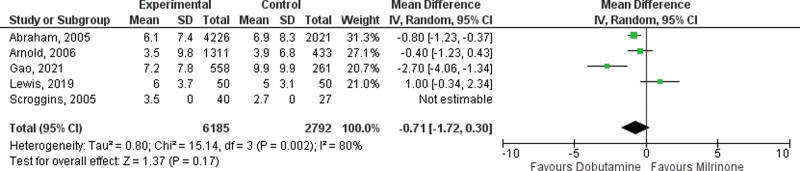
Five observational studies and one RCT reported ICU LOS (14, 21, 23, 24, 26, 28). There was no difference in length of ICU stay with milrinone, compared with dobutamine in either observational data (mean difference –0.71 d; 95% CI, –1.72 to 0.30 d; p = 0.17; Fig. 4) or randomized data (mean difference 1.00 d; 95% CI, –0.28 to 2.28 d; Fig. 5). There was considerable heterogeneity with an I2 value of 82% (p = 0.0002) among observational studies.
Figure 4.
Forest plot of ICU length of stay (observational studies) with dobutamine vs milrinone inotrope therapy.
Figure 5.
Forest plot of ICU length of stay (randomized studies) with dobutamine vs milrinone inotrope therapy.
Length of hospital stay was reported in eight observational studies and two RCTs (14, 21–26, 30, 31). Length of hospital stay was longer among patients treated with dobutamine, when compared with milrinone in observational studies (mean difference –1.85 d; 95% CI –3.62 to –0.09; p = 0.04). There was no difference in hospital LOS among RCTs (mean difference –0.69 d; 95% CI, –5.15 to 3.78). There was considerable heterogeneity with an I2 value of 80% (p = 0.0006) (Supplemental Figs. 3 and 4, http://links.lww.com/CCX/B237).
Incidence of Clinically Significant Arrhythmias
We identified a total of two observational studies and one RCT that reported the incidence of arrhythmias (14, 22, 26, 29). There was no difference in arrhythmic episodes between those treated with dobutamine and those treated with milrinone in either observational studies (OR 2.22; 95% CI, 0.99–4.98; p = 0.05) or RCT data (OR 1.11; 95% CI, 0.66–1.87). There was moderate heterogeneity among observational studies with an I2 value of 56% (p = 0.13), and no heterogeneity among RCT data I2 value of 0% (p = 0.59) (Supplemental Figs. 5 and 6, http://links.lww.com/CCX/B237).
DISCUSSION
In this systematic review and meta-analysis, we compared the use of dobutamine and milrinone in hospitalized patients with CS. Our review confirms that the majority of the available data are of poor methodological quality and mostly retrospective in nature, with only two RCTs performed on the data. Although no difference in all-cause mortality is seen in randomized data between milrinone and dobutamine, there is a trend toward improved survival with milrinone which is supported by improved mortality in observational data. No difference in ICU LOS or clinically significant arrhythmias was noted between the two agents. Based on the available data, no strong recommendations can be made with regard to the preferential use of one inotrope over another in the management of hospitalized patients, but these findings may suggest larger studies looking at mortality alone might be warranted.
In observational data, a reduction in all-cause in-hospital mortality was associated with milrinone. Furthermore, of these observational studies—three of the 11 included studies are of low or very low methodologic quality. Although it maybe tempting to infer benefit, the lack of robust data makes drawing firm conclusions difficult. Indeed, our analysis of only RCTs did not support this—however, a 20% relative reduction is noted and the wide CIs do not rule out a clinically meaningful difference (14, 22). Importantly, in the largest included randomized control trial, the DOREMI study—there were no differences in Vasoactive-Inotropic Score (32), response to beta-blocker (33), rapidity of lactate clearance (34), in patients with and without AMI (35), or sex differences (36)—all suggesting no important differences in response to therapy or in surrogate markers. A subgroup of the DOREMI study comparing RV to LV failure as the cause of CS is submitted for publication. Given the current evidence although there maybe differences in patients in whom milrinone is selected there remains no definitive evidence one agent is superior to the other.
Although no difference in ICU LOS was noted between the two agents, a small difference in in-hospital LOS was found in observational studies. This may be reflective of the patient population chosen for each inotrope among the observational studies included. Milrinone has been used more extensively as a long-term therapy in patients with end-stage heart failure and as a result, is more likely to be used in patients requiring longer hospital admissions for comprehensive heart failure work-up (37). Furthermore, despite milrinone being a more expensive drug than dobutamine it remains more likely to be used among patients who are candidates for advanced support including heart transplantation (22). The possibility remains that dobutamine results in early mortality among patients leading to shorter hospital admissions, or conversely results in more rapid recovery; however, these findings need to be validated in larger prospective trials.
Finally, with regard to safety outcomes, there was no difference in clinically significant arrhythmia. Arrhythmias are common among patients with CS with up to 45% of patients experiencing atrial arrhythmias and up to 15% experiencing ventricular arrhythmias (14). Although dobutamine has been postulated to be more arrhythmogenic due to its mechanism of action on beta-1 and beta-2 receptors—no difference is detected in this study. Furthermore, in the two randomized studies which examined this question, there was no difference between the two agents (14, 22). Thus, although it is tempting to infer a better risk profile with milrinone based on mechanism of action the data does not support this tenet.
Our analysis includes both randomized and observational data owing predominantly to the lack of rigorous studies examining dobutamine and milrinone in the treatment of CS. The trend toward improved mortality with milrinone seen in RCT data is further supported by the same trend seen in all available studies examining the topic. This consistent trend across observational and randomized data warrants further investigation with an adequately powered study to explore a mortality benefit with milrinone over dobutamine among patients with CS.
Although inotropes remain an important part of our armamentarium, previous descriptions support the lack of significant differences between the two agents in patients with CS. A comparison of these agents in 36 patients awaiting cardiac transplantation showed no difference in in-hospital mortality, along with similar rates of symptomatic sustained or nonsustained arrhythmias (22). Two further observational studies also showed no difference in in-hospital mortality (26, 29). Furthermore, a comparative study of chronic dobutamine or milrinone infusions in patients with heart failure showed no difference in all-cause mortality (38). Dobutamine and milrinone are two of the most commonly used inotropic agents in CS with the most robust data. Limited evidence for the use of alternate agents, including dopamine, norepinephrine, and levosimendan exists (39, 40). How these agents fit in the clinician’s armamentarium for the treatment of CS remains undetermined and requires dedicated studies. Inotropic therapy in patients with CS remains a contentious topic. Most available studies are observational with only two randomized trials comparing dobutamine and milrinone. This systematic review and meta-analysis support the hypothesis that there is minimal difference between dobutamine and milrinone among patients with CS. These results further beg the question of whether inotropic therapy portends benefit over systematic cardiac critical care in the absence of inotropy to patients in CS. Inotropic therapy versus placebo therapy has never been addressed by an RCT, through the CAPITAL DOREMI 2 trial (NCT05267886) which aims to assess this question and is currently enrolling patients.
Limitations
Our study has several limitations. First, most randomized data comes from a single RCT (14) with the remainder from observational data, as such inherent biases exist in all observational studies confounding the results found and obfuscating our ability to make firm recommendations on inotrope choice. Second, the lack of long-term outcomes in the included studies precludes the ability to make any conclusions on long-term outcomes. Furthermore, significant heterogeneity among the included patients exists with some studies including only postcardiac surgery patients, and those awaiting transplant, with other studies including all-comers with CS regardless of etiology. The use of inotropes as a bridge on and off of mechanical circulatory support further confounds the results. Although this reflects “real-life” practice, the generalizability of this analysis is relatively limited. Further dedicated studies among specific phenotypes and etiologies of CS are required to aid in the choice of inotropic agents in specific populations.
CONCLUSIONS
Based on the current data available, there is a consistent trend toward a reduction in in-hospital mortality with milrinone use in CS when compared with dobutamine. Data from large randomized clinical trials are needed to confirm or refute this observation.
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Baran DA, Grines CL, Bailey S, et al. : SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019; 94:29–37 [DOI] [PubMed] [Google Scholar]
- 2.Thiele H, Ohman EM, de Waha-Thiele S, et al. : Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur Heart J 2019; 40:2671–2683 [DOI] [PubMed] [Google Scholar]
- 3.Berg DD, Bohula EA, Morrow DA: Epidemiology and causes of cardiogenic shock. Curr Opin Crit Care 2021; 27:401–408 [DOI] [PubMed] [Google Scholar]
- 4.Berg DD, Bohula EA, Van Diepen S, et al. : Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes 2019; 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah M, Patnaik S, Patel B, et al. : Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol 2018; 107:287–303 [DOI] [PubMed] [Google Scholar]
- 6.Hochman JS, Sleeper LA, Webb JG, et al. : Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 1999; 341:625–634 [DOI] [PubMed] [Google Scholar]
- 7.Burkhoff D, Cohen H, Brunckhorst C, et al. ; TandemHeart Investigators Group: A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J 2006; 152:469.e1–469.e8 [DOI] [PubMed] [Google Scholar]
- 8.Ouweneel DM, Eriksen E, Sjauw KD, et al. : Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017; 69:278–287 [DOI] [PubMed] [Google Scholar]
- 9.Thiele H, Zeymer U, Thelemann N, et al. : Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: Long-term 6-year outcome of the Randomized IABP-SHOCK II Trial. Circulation 2018; 139:395–403 [DOI] [PubMed] [Google Scholar]
- 10.Schrage B, Ibrahim K, Loehn T, et al. : Impella Support for acute myocardial infarction complicated by cardiogenic shock. Circulation 2019; 139:1249–1258 [DOI] [PubMed] [Google Scholar]
- 11.Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37:2129–2200 [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation: 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239 [DOI] [PubMed] [Google Scholar]
- 13.O’Gara PT, Kushner FG, Ascheim DD, et al. : 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140 [DOI] [PubMed] [Google Scholar]
- 14.Mathew R, Di Santo P, Jung RG, et al. : Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N Engl J Med 2021; 385:516–525 [DOI] [PubMed] [Google Scholar]
- 15.Mathew R, Visintini SM, Ramirez FD, et al. : Efficacy of milrinone and dobutamine in low cardiac output states: Systematic review and meta-analysis. Clin Invest Med 2019; 42:E26–E32 [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. : The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G., BS, O’Connell D., Peterson J., Welch V., Losos M., Tugwell P.. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. January 4, 2021. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 12, 2023
- 18.Higgins JP, Altman DG, Gotzsche PC, et al. ; Cochrane Bias Methods Group: The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balshem H, Helfand M, Schunemann HJ, et al. : GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64:401–406 [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Vist G, et al. : GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol 2011; 64:407–415 [DOI] [PubMed] [Google Scholar]
- 21.Abraham WT, Adams KF, Fonarow GC, et al. ; ADHERE Scientific Advisory Committee and Investigators: In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol 2005; 46:57–64 [DOI] [PubMed] [Google Scholar]
- 22.Aranda JM, Jr, Schofield RS, Pauly DF, et al. : Comparison of dobutamine versus milrinone therapy in hospitalized patients awaiting cardiac transplantation: A prospective, randomized trial. Am Heart J 2003; 145:324–329 [DOI] [PubMed] [Google Scholar]
- 23.Arnold LM, Crouch MA, Carroll NV, et al. : Outcomes associated with vasoactive therapy in patients with acute decompensated heart failure. Pharmacotherapy 2006; 26:1078–1085 [DOI] [PubMed] [Google Scholar]
- 24.Gao F, Zhang Y: Inotrope use and intensive care unit mortality in patients with cardiogenic shock: An analysis of a large electronic intensive care unit database. Front Cardiovasc Med 2021; 8:696138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauptman PJ, Swindle J, Burroughs TE, et al. : Resource utilization in patients hospitalized with heart failure: Insights from a contemporary national hospital database. Am Heart J 2008; 155:978–985.e1 [DOI] [PubMed] [Google Scholar]
- 26.Lewis TC, Aberle C, Altshuler D, et al. : Comparative effectiveness and safety between milrinone or dobutamine as initial inotrope therapy in cardiogenic shock. J Cardiovasc Pharmacol Ther 2019; 24:130–138 [DOI] [PubMed] [Google Scholar]
- 27.Nandkeolyar S, Doctorian T, Fraser G, et al. : Predictors of in-hospital mortality in cardiogenic shock patients on vasoactive or inotropic support. Clin Med Insights Cardiol 2021; 15:11795468211049449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scroggins N, Edwards M, Delgado R, 3rd. Increased cost effectiveness with nesiritide vs. milrinone or dobutamine in the treatment of acute decompensated heart failure. Congest Heart Fail 2005;11:311–314. [DOI] [PubMed] [Google Scholar]
- 29.Yamani MH, Haji SA, Starling RC, et al. : Comparison of dobutamine-based and milrinone-based therapy for advanced decompensated congestive heart failure: Hemodynamic efficacy, clinical outcome, and economic impact. Am Heart J 2001; 142:998–1002 [DOI] [PubMed] [Google Scholar]
- 30.King JB, Shah RU, Sainski-Nguyen A, et al. : Effect of inpatient dobutamine versus milrinone on out-of-hospital mortality in patients with acute decompensated heart failure. Pharmacotherapy 2017; 37:662–672 [DOI] [PubMed] [Google Scholar]
- 31.Mazurek J: Dobutamine versus milrinone in left ventricular failure. J Card Fail 2010; 16:S73 [Google Scholar]
- 32.Parlow S, Di Santo P, Mathew R, et al. ; CAPITAL DOREMI investigators: The association between mean arterial pressure and outcomes in patients with cardiogenic shock: Insights from the DOREMI trial. Eur Heart J Acute Cardiovasc Care 2021; 10:712–720 [DOI] [PubMed] [Google Scholar]
- 33.Di Santo P, Mathew R, Jung RG, et al. ; CAPITAL DOREMI investigators: Impact of baseline beta-blocker use on inotrope response and clinical outcomes in cardiogenic shock: A subgroup analysis of the DOREMI trial. Crit Care 2021; 25:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marbach JA, Stone S, Schwartz B, et al. : Lactate clearance is associated with improved survival in cardiogenic shock: A Systematic Review and Meta-Analysis of Prognostic Factor Studies. J Card Fail 2021; 27:1082–1089 [DOI] [PubMed] [Google Scholar]
- 35.Jung RG, Di Santo P, Mathew R, et al. : Implications of myocardial infarction on management and outcome in cardiogenic shock. J Am Heart Assoc 2021; 10:e021570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prosperi-Porta G, Motazedian P, Di Santo P, et al. ; CAPITAL DOREMI investigators: No sex-based difference in cardiogenic shock: A post-hoc analysis of the DOREMI trial. J Cardiol 2022; 80:358–364 [DOI] [PubMed] [Google Scholar]
- 37.Harhash AA, Cassuto J, Hussein A, et al. : Safety of outpatient milrinone infusion in end-stage heart failure: ICD-level data on atrial fibrillation and ventricular tachyarrhythmias. Am J Med 2020; 133:857–864 [DOI] [PubMed] [Google Scholar]
- 38.Gorodeski EZ, Chu EC, Reese JR, et al. : Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail 2009; 2:320–324 [DOI] [PubMed] [Google Scholar]
- 39.Schumann J, Henrich EC, Strobl H, et al. : Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev 2018; 1:CD009669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Backer D, Biston P, Devriendt J, et al. ; SOAP II Investigators: Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.