Abstract
Background.
Preconditioning deceased organ donors with calcineurin inhibitors (CNIs) may reduce ischemia–reperfusion injury to improve transplant outcomes.
Methods.
We searched MEDLINE, EMBASE, Cochrane Library, and conference proceedings for animal models of organ donation and transplantation, comparing donor treatment with CNIs with either placebo or no intervention, and evaluating outcomes for organ transplantation. Reviewers independently screened and selected studies, abstracted data, and assessed the risk of bias and clinical relevance of included studies. Where possible, we pooled results using meta-analysis; otherwise, we summarized findings descriptively.
Results.
Eighteen studies used various animals and a range of CNI agents and doses and evaluated their effects on a variety of transplant outcomes. The risk of bias and clinical applicability were poorly reported. Pooled analyses suggested benefit of CNI treatment on early graft function in renal transplants (3 studies; serum creatinine: ratio of means [RoM] 0.54; 95% confidence interval [CI], 0.34-0.86) but not for liver transplants (2 studies; serum alanine transaminase: RoM 0.61; 95% CI, 0.30-1.26; and serum aspartate aminotransferase: RoM 0.58; 95% CI, 0.26-1.31). We found no reduction in graft loss at 7 d (2 studies; risk ratio 0.54; 95% CI, 0.08-3.42). CNI treatment was associated with reduced transplant recipient levels of interleukin-6 (4 studies; RoM 0.36; 95% CI, 0.19-0.70), tumor necrosis factor-alpha (5 studies; RoM 0.36; 95% CI, 0.12-1.03), and cellular apoptosis (4 studies; RoM 0.30; 95% CI, 0.19-0.47).
Conclusions.
Although this compendium of animal experiments suggests that donor preconditioning with CNIs may improve early kidney graft function, the limited ability to reproduce a true clinical environment in animal experiments and to assess for risk of bias in these experiments is a serious weakness that precludes current clinical application.
INTRODUCTION
Organ transplantation saves lives every day; however, the lifesaving potential is limited, in part, by the viability of transplant grafts. With organ demand universally exceeding supply, organs accepted for transplantation increasingly push the boundaries of suitability in terms of donor age and comorbidities.1 Ischemia–reperfusion injury (IRI), an unavoidable consequence of transplantation, represents the cellular dysfunction and cell death that occur when blood flow is restored to a transplant graft.2 At the time of retrieval, arterial clamping causes ischemia, which leads to ATP depletion, mitochondrial failure, osmotic disequilibrium, and cell membrane decay.3 Ischemia amplifies genetic transcription of destructive inflammatory cytokines. The restoration of oxygen supply on transplantation catalyzes the production of reactive oxygen species and recruits inflammatory cells into the graft, accelerating cell death and tissue necrosis.4 This response precipitates inflammation within the graft, contributing to early graft dysfunction,5 which is directly associated with acute and chronic disease in the transplant graft and graft rejection and graft loss.5,6
Interventions that attenuate IRI can improve graft viability. Clinical trials support many of these interventions, including specific operative procedures at the time of organ procurement (eg, preservation solution, normothermic regional perfusion),7,8 ex situ treatments (eg, pulsatile machine perfusion, ex vivo lung perfusion),9-11 and posttransplant therapies (nitric oxide, mannitol).12,13 However, interventions directed specifically to the donor provide a unique opportunity to precondition organs against IRI. In theory, the upstream nature of donor interventions maximizes the opportunity for prophylaxis against IRI for all organ transplants and represents a new frontier for optimizing graft viability.
Emerging evidence suggests that donor preconditioning with calcineurin inhibitors (CNIs) can mitigate IRI. This is in addition to their key role in maintaining immunosuppression. CNIs (ie, cyclosporine, tacrolimus) are routinely administered posttransplantation to prevent graft rejection for kidneys, liver, lungs, heart, and pancreas.14-17 These agents have multiple immune and anti-ischemic effects that align well with many molecular pathways of IRI, potentially reducing the risk of IRI.18-21 Among these effects, CNIs bind to the calcineurin–calmodulin complex to prevent activation of the nuclear factor of activated T cells (NF-AT), thus inhibiting interleukin (IL)-2 production.22 Moreover, they inhibit endothelin-1 and inducible nitric oxide synthase, which improve microcirculation.22
Early animal studies suggested that donor calcineurin inhibition can reduce inflammatory cytokines posttransplantation,23 attenuate graft necrosis,24 and improve early graft function.25 However, CNIs have long been associated with putative side effects in recipients that may be associated with vascular injury (ie, arteriolar hyalinosis).26 Thus, it is important to systematically assess the animal literature related to CNI administration to donors before conducting clinical investigations.
MATERIALS AND METHODS
This systematic review was exempt from the Research Ethics Board approval.
Eligibility Criteria
We included published studies and abstracts from animal models of organ transplantations. Models of allotransplantation (ie, organs transplanted to a different animal of the same species) and autotransplantation (ie, organs retransplanted to the donor animal) were eligible because both models resulted in IRI. We included studies, for all organ types, that compared the administration of CNIs (ie, cyclosporine or tacrolimus) with placebo or with no intervention and reported on any of the following recipient outcomes: (1) early posttransplant graft function, (2) graft loss, (3) serum proinflammatory cytokines, and (4) graft histology. We accepted the range of authors’ definitions and measurements for each outcome. Considering a theoretical risk that any organ graft could conceivably carry a small amount of donor tacrolimus to the organ recipient, with an associated risk of adverse effects (eg, acute kidney injury) in that recipient, we reported kidney function in the nontransplanted organs of recipients whenever available.
Search Strategy
With the assistance of a medical librarian, we searched Cochrane Central, EMBASE, and MEDLINE from inception to December 2022 (File S1, SDC, http://links.lww.com/TXD/A555) and conference proceedings from the International Society of Organ Donation and Procurement, The Transplantation Society, the Canadian Society of Transplantation, American Transplant Congress, and European Society of Transplantation over the past 5 y. After importing references in Endnote (version X8.0.1), 2 reviewers independently and in duplicate screened titles and abstracts for eligibility and reviewed full-text articles in a second stage. We documented reasons for the exclusion of each study, where applicable.
Data Extraction
Two reviewers independently extracted data from each study using pretested and calibrated data collection forms. We recorded information on the animal models, study interventions (eg, specific CNI, dose, timing of administration), control interventions, and outcomes (eg, recipient vital status, graft function, anatomical and histological findings, serum inflammatory markers). We resolved disagreements through discussion. When available, we collected data on warm and cold ischemic times (CITs) because they are widely viewed as strong predictors of graft dysfunction.27,28
Clinical Relevance and Risk of Bias Assessments
To determine and describe the clinical relevance of each preclinical model, 2 reviewers applied in duplicate a published framework to evaluate the following items: (1) relevance of animal age, size, and comorbidities; (2) administration of the therapy before organ retrieval; and (3) supportive care for donors and recipients that mimics clinical care (ie, fluids, antibiotics).29 Reviewers rated each item as “yes,” “no,” or “unclear.”
We used the SYRCLE tool to assess the risk of bias in included studies.30 This system assesses 10 domains: (1) sequence generation (ie, methods used to generate the allocation sequence), (2) comparability of study groups at baseline, (3) allocation concealment, (4) random housing (ie, the extent to which intervention and control animals are randomly assigned to various laboratory spaces during the study), (5) investigator blinding, (6) random outcome assessments (ie, the random selection of animals for assessment of specific outcomes), (7) blinding of outcome assessments, (8) completeness of outcome data, (9) selective reporting of outcomes, and (10) other sources of bias. In terms of baseline characteristics, we compared animal sex, age, and CITs between groups. To assess selective outcome reporting, we compared outcomes described in the Methods section to those reported in the Results sections for each article or published protocol, if available. As other potential sources of bias, reviewers assessed industry funding and potential confounding treatments that differed between study groups. In duplicate, 2 reviewers rated each domain for each study as having “high,” “low,” or “unclear” risk on the overall risk of bias for each study. We resolved disagreements by consensus.31
Statistical Analysis
We calculated chance-corrected agreement for eligibility decisions using the κ statistic.31 We conducted descriptive analyses and reported means with SD or proportions, as appropriate. We pooled outcome data across studies, across the range of animal models, interventions, and outcomes measured. Specifically, we planned to pool data across animal types (eg, rats, pigs), across organ types (and also pooled for specific organs), and across interventions (eg, we included studies of tacrolimus and cyclosporine together). If an outcome was measured at >1 time interval, we analyzed the most protracted measurements. Where data were deemed too clinically heterogenous to pool, we provided narrative summaries.
Using RevMan (version 5.4)32 and R (version 4.0.5; package metaphor)33 software, we pooled continuous outcomes using the ratio of weighted means (RoM) with inverse variance method and reported with 95% confidence interval (CI).34 We used ratio because it eliminates the units of measurement in the outcomes to provide a more meaningful summary of treatment effects across measures than the use of a standardized mean difference.35 We pooled dichotomous outcomes using the risk ratio with a corresponding 95% CI. We applied Mantel–Haenszel random-effects models36 and measured statistical heterogeneity using the I2 statistic, visual inspection of the forest plots, and the chi-square test. There were too few studies to address publication bias.31
RESULTS
Study Selection
From 1267 citations, 18 studies were eligible for this review (Figure 1) and included descriptions of >500 animals (Table 1).23-25,37-51 κ agreement for study selection was 0.77. All studies were published between 1991 and 2020.
FIGURE 1.
PRISMA flow diagram. CNI, calcineurin inhibitor; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
TABLE 1.
Study characteristics grouped by organ type
| Author, year | Species (n) | Calcineurin inhibitorsa | Outcome | |||
|---|---|---|---|---|---|---|
| Agent | Dose | Timing of administration before organ retrieval | Time of measurement after transplant | All reported effects of CNI | ||
| Kidney | ||||||
| Abbasi Dezfouli, 202037 | Pigs(26) | CSA | 10 mg/kg | Retrieval | D 5 | Similar creatinine, BUN ↓tissue TNF-αSimilar cortical necrosis |
| Cicora, 201238 | Rats(24) | TAC | 0.3 mg/kg | 12 h | 24 h | ↓Creatinine, BUN, tubular necrosis, tissue TNF-α, IL-6, apoptosis |
| Cicora, 201123 | Rats(31) | TAC | 0.3 mg/kg | 12 h | 24 h | ↓ Creatinine, urea, tubular necrosis, apoptosis, tissue TNF-α, IL-6 |
| Martinez-Pilli, 201139 | Rats(25) | CSA | 5 mg/kg | 24 h and 6 h | 24 h | Similar creatinine, tubular necrosis, tissue TNF-α |
| Cicora, 201040 | Rats(30) | TAC | 0.3 mg/kg | 12 h to 6 h | 24h | ↓ Creatinine, urea, tubular necrosis, apoptosis |
| Shihab, 201041 | Rats(40) | CSA | 10 mg/kg | 7 d or 24 h | D | ↓ Creatinine, tubular necrosis |
| Shihab, 200925 | Rats(48) | TACCSA | 1 mg/kg10 mg/kg | 7 d or24 h | Day 3 | ↓ Creatinine, tubular necrosis |
| Reutze-Selke, 200342 | Rats(NR) | TAC | 0.3 mg | 24 h or 1 h | 6 mo | ↓ Proteinuria,glomerulosclerosis |
| Liver | ||||||
| Tarrab, 201243 | Rats(17) | CSA | 5 mg/kg | 24 h. | 4 h | Similar ALT, AST, bilirubin, necrosis |
| Hüser, 200944 | Rats(41) | TAC | 1 or0.01mg/kg | 3 d(daily) | D 3 | ↓ ALT, necrosis |
| Sasaki, 200445 | Rats(102) | TAC | 1 mg/kg | 4 d(daily) | 6 h and d 14 | ↓ ALT, LDH, plasma IL-6, TNF-αSimilar AST, graft loss |
| Kawano, 199646 | Rats(NR) | TAC | 1 mg/kg | 16 h | 24 h and d 7 | ↓ ASTSimilar graft loss |
| Yamanoi, 199447 | Pigs(14) | CSA | 10 mg/kg | 3 d(daily) | D 3 | ↓ ALT, LDH |
| Goto, 199148 | Rats(59) | CSA | 10 mg/kg | 3 d(daily) | D 7 | Similar graft loss |
| Lung | ||||||
| Bayer, 201349 | Rats(12) | TAC | 6.4 mg | 1 h | 4 h | ↑ Pao2Similar tissue IL-1,-2, -6,-18, TNF-α |
| Bowel | ||||||
| Oltean, 200750 | Rats(60) | TAC | 0.3 mg/kg | 6 h | 24 h | ↓ Tissue ICAM-1Similar necrosis |
| Oltean, 200651 | Rats(20) | TAC | 0.3 mg/kg | 6 h | 12 h | ↓ Plasma IL-6, necrosisSimilar TNF-α |
| Oltean, 200524 | Rats(14) | TAC | 0.3 mg/kg | 6 h | 6 h | ↓ Tissue ICAM-1, necrosis, apoptosisSimilar caspase-3 |
aAll groups are compared with the control group.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CNI, calcineurin inhibitor; CSA, cyclosporine; ICAM, intercellular adhesion molecule; IL, interleukin; LDH, lactate dehydrogenase; NR, not reported; Pao2, Po2; TAC, tacrolimus; TNF-α, tumor necrosis factor-alpha.
Study Characteristics
Most studies (16/18; 89%) used rat models of organ transplantation,23-25,38-46,48-51 whereas 2 studies used pigs (Table 1).37,47 The specific organ of interest varied and included kidney (8 studies),23,25,37-42 liver (6 studies),43-48 bowel (3 studies),24,50,51 and lung (1 study).49
Table 1 lists the CNI regimens. Eleven studies (61%) tested tacrolimus,23,24,38,40,42,44-46,49-51 6 studies tested cyclosporine37,39,41,43,47,48, and 1 tested both medications (Table 1).25 The range of doses was 5 to 10 mg/kg for cyclosporine25,37,39,41,43,47,48 and 0.01 to 1 mg/kg for tacrolimus.23-25,38,40,42,44-46,49-51 Eleven studies (61%) administered a single dose.24,25,38,40-43,46,48,50,51
The principal routes of administration were intravenous (8/18 studies; 44%),23,24,38,40,42,46,50,51 enteral (4 studies; 22%),37,39,43,48 intraperitoneal (2 studies; 11%),25,41 and intramuscular (2 studies; 11%).45,47 The timing of CNI therapy initiation varied between studies. CNIs were administered within 12 h of organ retrieval in 9 studies (50%)23,24,37,38,40,42,49-51 and between 1 and 7 d in 8 studies (44%).25,39,42,44,45,47,48
The timing of outcome measures ranged from 4 h to 7 d posttransplantation, although 1 study assessed transplant graft histology 6 mo after transplantation.42
Clinical Relevance
Table 2 summarizes the clinical relevance of these studies. Thirteen studies (72%) did not report animal age (ie, adult or pediatric), whereas the remainder studied only adult animals.23,24,37-40,44-46,48-51 Ten studies reported animal sex and studied exclusively males.23,25,37,38,40,41,43,45,48,49 None of the animal models included animals with comorbidities (eg, hypertension, diabetes). All but 1 study used an allotransplant model.23 According to the eligibility for this review, all studies administered the study drug to donors before organ retrieval. One study created a model of neurologically deceased donor animals.37 All studies included a CIT of >2 h. In kidney transplant studies, the mean (SD) CIT was 7.9 (9.5) h,23,25,37-39,41,42 and the mean (SD) warm ischemic time was 29 (15.6) min.25,37,39,41 In liver transplantation, the mean (SD) CIT was 9.4 (10.8) h,43-47 and warm ischemic time was 28.7 (27.2) min.43-45 Nine studies (50%) did not report supportive care for recipients.23,24,39,42,44,46,49-51 When reported, 3 studies (17%) administered fluids and antibiotics immediately after transplantation.37,47,48 No study administered immunosuppressive therapy in recipients.
TABLE 2.
Potential clinical relevance of preclinical model
| Relevance of model | Relevance of illness | Relevance of therapy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, year | Adult | Comorbidities | Size | >2 h of cold ischemic time | Hetero (vs auto) transplant | Therapy initiated before retrieval | CNI dose analogous to usual | Adequate supportive care | |
| Donor | Recipient | ||||||||
| Kidney | |||||||||
| Abbasi Dezfouli, 202037 | ⦸ | ⊗ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ |
| Cicora, 201238 | ⦸ | ⊗ | ⊗ | ⊕ | ⊗ | ⊕ | ⊕ | ⊕ | ⊗ |
| Cicora, 201123 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊗ |
| Martinez-Pilli, 201139 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⦸ |
| Cicora, 201040 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊕ | ⦸ | ⦸ |
| Shihab, 201041 | ⊕ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊗ | ⊕ | ⊗ |
| Shihab, 200925 | ⊕ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊗ | ⊕ | ⊗ |
| Reutze-Selke, 200342 | ⊕ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⦸ | ⊕ | ⦸ |
| Liver | |||||||||
| Tarrab, 201243 | ⊕ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊕ | ⊕ | ⊗ |
| Hüser, 200944 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊗ | ⦸ | ⦸ |
| Sasaki, 200445 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⦸ | ⊕ | ⊗ |
| Kawano, 199646 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊗ | ⦸ | ⦸ |
| Yamanoi, 199447 | ⊕ | ⊗ | ⊕ | ⊕ | ⊕ | ⊕ | ⦸ | ⊕ | ⊕ |
| Goto, 199148 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊗ | ⦸ | ⊕ |
| Lung | |||||||||
| Bayer, 201349 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⦸ | ⊕ | ⦸ |
| Bowel | |||||||||
| Oltean, 200750 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊕ | ⦸ | ⦸ |
| Oltean, 200651 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊕ | ⦸ | ⦸ |
| Oltean, 200524 | ⦸ | ⊗ | ⊗ | ⊕ | ⊕ | ⊕ | ⊕ | ⦸ | ⦸ |
CNI, calcineurin inhibitor; ⊕, yes; ⊗, no; ⦸, unclear.
Risk of Bias
Table 3 presents reviewers’ assessments of the risk of bias in individual studies. The risk of bias was judged as unclear for most of the domains due to insufficient reporting of the 10 SYRCLE items.
TABLE 3.
Risk of bias assessment
| Author, year | Random sequence | Baseline characteristics | Allocation concealed | Random housing | Blinding personnel | Random outcome assessment | Blinding outcome assessment | Incomplete outcome data | Selective outcome reporting | Other bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Kidney | ||||||||||
| Abbasi Dezfouli, 202037 | ⦸ | ⊕ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⊕ |
| Cicora, 201238 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⊕ |
| Cicora, 201123 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⊕ |
| Martinez-Pilli, 201139 | ⦸ | ⊕ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⦸ |
| Cicora, 201040 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ |
| Shihab, 200925 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ |
| Shihab, 201041 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⦸ |
| Reutze-Selke, 200342 | ⦸ | ⊕ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊗ | ⊗ |
| Liver | ||||||||||
| Tarrab, 201243 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⊕ |
| Hüser, 200944 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⦸ |
| Sasaki, 200445 | ⦸ | ⦸ | ⦸ | ⊕ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⊕ |
| Kawano, 199646 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⦸ |
| Yamanoi, 199447 | ⦸ | ⊕ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⊗ | ⊕ |
| Goto, 199148 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⊕ | ⦸ |
| Lung | ||||||||||
| Bayer, 201349 | ⦸ | ⊕ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ |
| Bowel | ||||||||||
| Oltean, 200750 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⦸ |
| Oltean, 200651 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⦸ |
| Oltean, 200524 | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⊕ | ⦸ |
⊕, low risk of bias; ⊗, high risk of bias; ⦸, unclear risk of bias.
Study Outcomes
For many of the continuous measures reported ahead, we observed substantial variations in means and SDs, suggesting important differences in the study animals, interventions, or outcome measurements. Given the limited details of study methods, and the relatively small number of studies to support any exploratory subgroup analyses, we elected to pool results where possible and to comment descriptively.
Graft Function
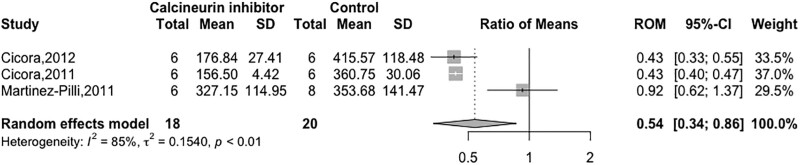
Seven studies (39%) measured serum creatinine levels in renal recipients at 1 d,23,38-40 3 d,25,41 and 5 d.37 Five of these studies reported reduced creatinine levels after the administration of CNIs.23,25,38,40,41 Three studies (N = 38) reported creatinine quantitatively, thus allowing for pooled analysis.38-40 The pooled estimate suggested a beneficial effect of CNIs on creatinine (RoM 0.54; 95% CI, 0.34-0.86; I2 = 85%; Figure 2). From the 4 studies not represented in the pooled estimate, 3 reported that posttransplant creatinine levels were lower among kidney recipients exposed to CNIs.23,25,41 Four studies assessed blood urea nitrogen levels or urea at 123,38,40 and 5 d37 of which 3 reported lower levels for the groups exposed to CNI.23,38,40
FIGURE 2.
Forest plot of the calcineurin inhibitor effect on serum creatinine. CI, confidence interval; ROM, ratio of weighted means; RR, risk ratio.
Five studies assessed serum aminotransferases with conflicting results.43-47 Aminotransferases were measured at 4 h,43 6 h,45 24 h,46 and 3 d.44,47 Three studies observed lower serum levels of alanine aminotransferase (ALT) in the CNI group,44, 46,47 whereas 1 study reported lower levels of aspartate aminotransferase (AST) without a difference of ALT.45 In 1 study, AST and ALT levels were similar between groups.43 The pooled estimate (2 studies; N = 67) suggested no difference in ALT (RoM 0.61; 95% CI, 0.30-1.26; I2 = 96%) and AST (RoM 0.58; 95% CI, 0.26-1.31; I2 = 50.2%) with donor pretreatment (Figure S2A, SDC, http://links.lww.com/TXD/A555).43,45 All 3 studies not included in the pooled analysis qualitatively reported lower serum aminotransferases level for liver recipients exposed to CNIs.44,46,47
For the single lung transplantation model, donor treatment with CNIs improved oxygenation (Pao2) as measured at 3 h posttransplantation (group CNI = 344.8 ± 235 mmHg versus group control = 61.3 ± 35.8 mmHg; P = 0.01).49
Graft Loss
Three studies (17%) reported liver graft loss between 746,48 and 14 d45 after transplantation. Two studies reported this quantitatively (N = 40), and when pooled, treatment with CNIs did not reduce graft loss (RR 0.54; 95% CI, 0.08-3.42; I2 = 75%; Figure S2B, SDC, http://links.lww.com/TXD/A555).45,48
Inflammatory Cytokines
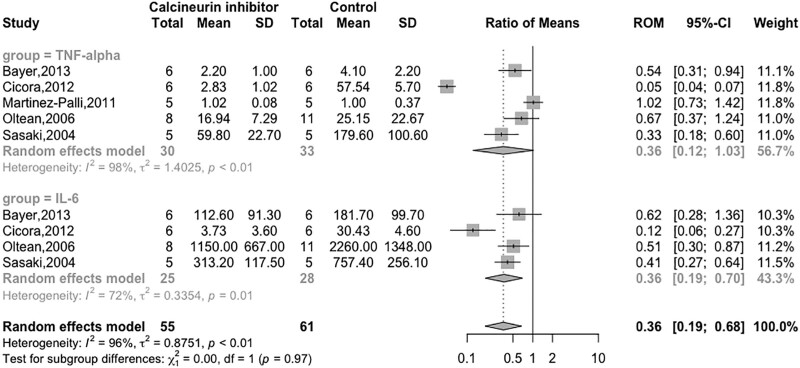
Nine studies evaluated inflammatory cytokines from kidney recipients (4 studies),23,37-39 bowel recipients (3 studies),24,50,51 liver recipients (1 study),45 and lung recipients (1 study).49 The most common time point measurement was 24 h,23,38,39,51 followed by 6 h,23,45 4 h,49 12 h,50 and 5 d.37 Seven studies measured cytokines in tissue23,24,37,39,40,49,50 and 2 studies in plasma.45,50
In kidney recipients exposed to CNIs, all studies reported significantly lower levels of tumor necrosis factor-alpha (TNF-α)23,37,38 and IL-6.38 In liver recipients, treatment of donors with CNIs lowered IL-6 and TNF-α,45 whereas in bowel recipients, there was a reduction in IL-6 but no difference in TNF-α.51 Results were similar between the 2 groups for the lung transplantation models.49
Pooled estimates of all organs suggested a reduction of IL-6 (N = 53; RoM 0.36; 95% CI, 0.19-0.70; I2 = 72%)38,45,49,51 and a nonsignificant reduction in TNF-α (N = 63; RoM 0.36; 95% CI, 0.12-1.03; I2 = 98%)38,39,45,49,51 in transplants from donors exposed to CNIs (Figure 3). Studies, not included in the pooled analysis, qualitatively reported reduced IL-623 and TNF-α.23,37
FIGURE 3.
Forest plot of calcineurin inhibitors on TNF-α and IL-6. An asterisk denotes studies reporting on plasma biomarker levels rather than tissue levels. CI, confidence interval; IL, interleukin; RoM, ratio of means; TNF-α, tumor necrosis factor-alpha.
Histology
Using a variety of tubular injury scales, 8 studies reported renal histology at 4 h,37 24 h,23,38,39,51 72 h,25,41 and 6 mo.42 In 5 of 8 studies, treatment with CNIs was associated with reduced renal necrosis.23,25,38,41,42 One study reported reduced liver necrosis with the administration of tacrolimus.44 Two out of 3 bowel studies reported improved graft structure with CNIs compared with grafts from untreated animals.24,51
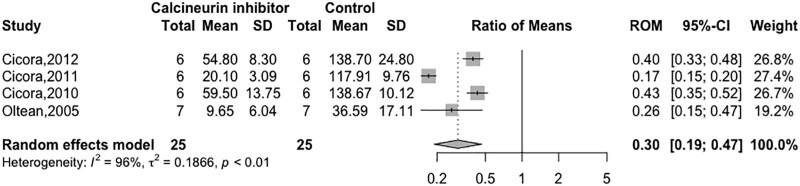
Four studies measured apoptosis at 624 and 24 h.23,38,40 In all studies, there was a reduction in the number of apoptotic nuclei in transplants exposed to CNIs. One study measured caspase-3 in transplanted organs with similar results at 6 h posttransplant (group control: 5.1 ± 4.86 versus group CNI: 3.06 ± 2.04 pmol released amido-4-methylcoumarin/mg protein minutes).24 Pooled analysis reported a reduction in apoptosis of transplanted organs when preexposed to CNIs (N = 50; RoM 0.30; 95% CI, 0.19-0.47; I2 = 96%; Figure 4).23,24,38,40
FIGURE 4.
Forest plot of calcineurin inhibitors on apoptosis. CI, confidence interval; RoM, ratio of means.
Safety
Only 1 study evaluated for adverse effects in native organs up to 12 h posttransplantation. In a study on bowel transplantation, liver function tests, creatinine levels, and blood urea nitrogen levels were all lower in recipients exposed to CNIs.51
DISCUSSION
This systematic review and meta-analysis of preclinical models, including 18 studies of >500 animals, observed general improvement in recipient levels of inflammatory cytokines, early graft function, and graft histology. The broad range of organs, dosing strategies, and evaluated outcomes support the robustness of qualitatively and quantitatively similar findings.
These results suggest that donor preconditioning with CNIs may be beneficial in the transplantation of some, if not all, organ types. This finding supports the consideration of human studies as a next step. An additional factor to support this intervention in donors is the mechanism of tacrolimus, which inhibits the opening of mitochondrial transition pores and limits cell destruction by inflammatory mechanisms and apoptosis.52 Recently, immunomodulation of tacrolimus has been associated with direct hemodynamic effects in lung donors. In a preclinical study of 18 neurologically deceased pigs, tacrolimus (2.5 mg/kg BID) compared with placebo reduced donor pulmonary artery pressure and pulmonary vascular resistance, thus reducing donor pulmonary edema.53
The translation of our findings to clinical care should proceed carefully. The absence of comorbidities (eg, hypertension, diabetes, hypercholesterolemia) and the predominance of males in animal models of ischemic preconditioning has been suggested as a possible explanation for translational failure in human studies.54,55
The closely related Cis-A-rein study (target N = 648 recipients; ClinicalTrials.gov NCT02907554) currently underway in France is a clinical trial investigating the effects of pretreating neurologically deceased donors with cyclosporine A (2.5 mg/kg 2 h before organ recovery, versus placebo), specifically with respect to rates of delayed renal graft function.56 Findings from this trial will allow a comparison of preclinical and clinical research findings, thus informing future clinical trials in organ donation and transplantation.
Limitations of our systematic review include the wide range of years of publication, the variability in animal models, the timing and duration of CNIs, and our restriction to models of transplantation, thus excluding other models of IRI. Current literature suggests that the techniques applied to induce IRI contribute to the limitation of the translation to human results.57 All studies used a transplantation model without immunosuppressive therapy posttransplantation, which might have fostered acute rejection and thus masked a benefit of donor preconditioning with CNIs. Moreover, inferences from our findings are limited by the inadequate reporting of study methods, which hinders our ability to assess the overall risk of bias. Previous groups have found that underreporting of methodological details in preclinical studies was associated with the overestimation of treatment effects.58-61 Pooled analyses must be interpreted cautiously because there is a high degree of heterogeneity, and the majority of included studies did not present study findings quantitatively; instead, they made qualitative statements about differences in findings between study groups. Finally, the number of studies reporting graft function and loss was small, raising concerns about selective outcome reporting.
Strengths of our systematic review include a comprehensive search, independent duplicate assessments of study eligibility, and the pooling of results across studies where possible. Where possible, we assessed the risk of bias and the clinical relevance of included studies. We reported outcomes (eg, graft function, graft loss) that are relevant in clinical transplantation.
In conclusion, this systematic review is limited by the possibly high risk of bias and low clinical relevance of the underlying studies. Nevertheless, across a broad range of CNI agents, doses, timing, animal models, and organ types, we observed a consistent finding of improved early posttransplant kidney graft function with donor CNI administration. This systematic review provides a rationale for supporting future clinical trials on the treatment of organ donors with CNIs in humans. Moreover, this report provides a current compendium of animal experiments in this field.
ACKNOWLEDGMENTS
The authors would like to acknowledge M. Samuel Lemaire-Paquette for performing the statistical analyses.
Supplementary Material
Footnotes
This research did not receive any dedicated funding from any agency in the public, commercial, or not-for-profit sectors. F.D., F.-M.C., M.C., and F.L. are recipients of Career Awards from the Fonds de la Recherche du Québec-Santé. A.F.T. holds a Canada Research Chair in Critical Care Neurology and Trauma.
P.S. received honoraria for advertisement boards from Astellas and Paladin Labs. M.Sl. receives a stipend for his role as a Hospital Donation Physician and Regional Medical Lead with the Trillium Gift of Life Network.
F.D., R.B., and M.O.M. conceptualized the study. F.D., W.R., D.A., R.B., and M.O.M. participated in the research design. F.D., W.R., D.A., and M.O.M. participated in article selection and data extraction. F.D., R.B., and M.O.M. conducted data analysis. C.I., G.J.B., K.E.A.B., H.C., F.-M.C., M.C., P.C., S.D., S.W.E., A.J.F., S.H., G.K., F.L., S.O., B.R., K.S., D.T., A.F.T., M.J.W., M.Sl., M.Se., and M.O.M. provided content expertise and participated in the data interpretation. All the authors participated in writing the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.D’Aragon F, Lamontagne F, Cook D, et al. ; Canadian Critical Care Trials Group and the Canadian Donation and Transplant Research Program. Variability in deceased donor care in Canada: a report of the Canada-DONATE cohort study. Can J Anaesth. 2020;67:992–1004. [DOI] [PubMed] [Google Scholar]
- 2.Requião-Moura LR, Durão Junior M de S, Matos ACC de, et al. Ischemia and reperfusion injury in renal transplantation: hemodynamic and immunological paradigms. Einstein (São Paulo). 2015;13:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponticelli C. Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transplant. 2014;29:1134–1140. [DOI] [PubMed] [Google Scholar]
- 4.Menke J, Sollinger D, Schamberger B, et al. The effect of ischemia/reperfusion on the kidney graft. Curr Opin Organ Transplant. 2014;19:395–400. [DOI] [PubMed] [Google Scholar]
- 5.Troppmann C, Gruessner AC, Gillingham KJ, et al. Impact of delayed function on long-term graft survival after solid organ transplantation. Transplant Proc. 1999;31:1290–1292. [DOI] [PubMed] [Google Scholar]
- 6.Foroutan F, Friesen EL, Clark KE, et al. Risk factors for 1-year graft loss after kidney transplantation: systematic review and meta-analysis. Clin J Am Soc Nephrol. 2019;14:1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.León Díaz FJ, Fernández Aguilar JL, Nicolás de Cabo S, et al. Combined flush with histidine-tryptophan-ketoglutarate and University of Wisconsin solutions in liver transplantation: preliminary results. Transplant Proc. 2018;50:539–542. [DOI] [PubMed] [Google Scholar]
- 8.Hessheimer AJ, de la Rosa G, Gastaca M, et al. Abdominal normothermic regional perfusion in controlled donation after circulatory determination of death liver transplantation: outcomes and risk factors for graft loss. Am J Transplant. 2022;22:1169–1181. [DOI] [PubMed] [Google Scholar]
- 9.Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med. 2018;6:357–367. [DOI] [PubMed] [Google Scholar]
- 10.Moers C, Smits JM, Maathuis MHJ, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7–19. [DOI] [PubMed] [Google Scholar]
- 11.Wight J, Chilcott J, Holmes M, et al. The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors. Health Technol Assess. 2003;7:1–94. [DOI] [PubMed] [Google Scholar]
- 12.Lang JD, Teng X, Chumley P, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lugo-Baruqui JA, Ayyathurai R, Sriram A, et al. Use of mannitol for ischemia reperfusion injury in kidney transplant and partial nephrectomies—review of literature. Curr Urol Rep. 2019;20:6. [DOI] [PubMed] [Google Scholar]
- 14.Chadban SJ, Ahn C, Axelrod DA, et al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. 2020;104:S11–S103. [DOI] [PubMed] [Google Scholar]
- 15.Costanzo MR, Dipchand A, Starling R, et al. ; International Society of Heart and Lung Transplantation Guidelines. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. [DOI] [PubMed] [Google Scholar]
- 16.Kandaswamy R, Stock PG, Gustafson SK, et al. OPTN/SRTR 2016 annual data report: pancreas. Am J Transplant. 2018;18:114–171. [DOI] [PubMed] [Google Scholar]
- 17.Gruessner RW, Bartlett ST, Burke GW, et al. Suggested guidelines for the use of tacrolimus in pancreas/kidney transplantation. Clin Transplant. 1998;12:260–262. [PubMed] [Google Scholar]
- 18.St Peter SD, Moss AA, Mulligan DC. Effects of tacrolimus on ischemia-reperfusion injury. Liver Transpl. 2003;9:105–116. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Yuan X, Luo Y, et al. Evaluating the effects of immunosuppressants on human immunity using cytokine profiles of whole blood. Cytokine. 2009;45:141–147. [DOI] [PubMed] [Google Scholar]
- 20.Lázaro Fernández A, Jado Rodríguez JC, Torres Redondo AM, et al. Anticalcineurinics: role of mitochondrial transition pore on nephrotoxicity. In: Gowder SJT, ed. Pharmacology and Therapeutics. IntechOpen; 2014. [Google Scholar]
- 21.Hausenloy DJ, Maddock HL, Baxter GF, et al. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. [DOI] [PubMed] [Google Scholar]
- 22.Pillans P. Experimental and clinical pharmacology: immunosuppressants—mechanisms of action and monitoring. Aust Prescr. 2006;29:99–101. [Google Scholar]
- 23.Cicora F, Lausada N, Vasquez DN, et al. Protective effect of immunosuppressive treatment before orthotopic kidney autotransplantation. Transpl Immunol. 2011;24:107–112. [DOI] [PubMed] [Google Scholar]
- 24.Oltean M, Olofsson R, Zhu C, et al. FK506 donor pretreatment improves intestinal graft microcirculation and morphology by concurrent inhibition of early NF-kappaB activation and augmented HSP72 synthesis. Transplant Proc. 2005;37:1931–1933. [DOI] [PubMed] [Google Scholar]
- 25.Shihab FS, Bennett WM, Andoh TF. Donor preconditioning with a calcineurin inhibitor improves outcome in rat syngeneic kidney transplantation. Transplantation. 2009;87:326–329. [DOI] [PubMed] [Google Scholar]
- 26.Einecke G, Reeve J, Halloran PF. Hyalinosis lesions in renal transplant biopsies: time-dependent complexity of interpretation. Am J Transplant. 2017;17:1346–1357. [DOI] [PubMed] [Google Scholar]
- 27.Quiroga I, McShane P, Koo DDH, et al. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant. 2006;21:1689–1696. [DOI] [PubMed] [Google Scholar]
- 28.Tennankore KK, Kim SJ, Alwayn IPJ, et al. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016;89:648–658. [DOI] [PubMed] [Google Scholar]
- 29.Lamontagne F, Briel M, Duffett M, et al. Systematic review of reviews including animal studies addressing therapeutic interventions for sepsis. Crit Care Med. 2010;38:2401–2408. [DOI] [PubMed] [Google Scholar]
- 30.Hooijmans CR, Rovers MM, de Vries RBM, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3. Cochrane; 2022. Available at https://training.cochrane.org/handbook .Accessed June 15, 2023. [Google Scholar]
- 32.The Cochrane Collaboration. Review Manager Web (RevMan Web). Available at https://revman.cochrane.org. Accessed June 15, 2023. [Google Scholar]
- 33.R Core Team. The R Project for Statistical Computing. Available at https://www.r-project.org. Accessed June 15, 2023. [Google Scholar]
- 34.Friedrich JO, Adhikari N, Herridge MS, et al. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142:510–524. [DOI] [PubMed] [Google Scholar]
- 35.Friedrich JO, Adhikari NKJ, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Methodol. 2008;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tufanaru C, Munn Z, Stephenson M, et al. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13:196–207. [DOI] [PubMed] [Google Scholar]
- 37.Abbasi Dezfouli S, Nikdad M, Ghamarnejad O, et al. Oral preconditioning of donors after brain death with calcineurin inhibitors vs. inhibitors of mammalian target for rapamycin in pig kidney transplantation. Front Immunol. 2020;11:1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cicora F, Roberti J, Vasquez D, et al. Preconditioning donor with a combination of tacrolimus and rapamacyn to decrease ischaemia-reperfusion injury in a rat syngenic kidney transplantation model. Clin Exp Immunol. 2012;167:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Palli G, Hirose R, Liu T, et al. Donor pre-treatment with everolimus or cyclosporine does not reduce ischaemia-reperfusion injury in a rat kidney transplant model. Nephrol Dial Transplant. 2011;26:1813–1820. [DOI] [PubMed] [Google Scholar]
- 40.Cicora F, Gonzalez P, Cicora P, et al. Beneficial effects of immunosuppressive drugs administered to donors in renal transplant: is the combined administration of rapamycin plus tacrolimus more effective than the use of each drug separately. Transplantation. 2010;90:875.20736897 [Google Scholar]
- 41.Shihab FS, Bennett WM, Andoh TF. Role of cellular cholesterol in pharmacologic preconditioning with cyclosporine in experimental kidney transplantation. Am J Nephrol. 2010;31:134–140. [DOI] [PubMed] [Google Scholar]
- 42.Reutzel-Selke A, Zschockelt T, Denecke C, et al. Short-term immunosuppressive treatment of the donor ameliorates consequences of ischemia/ reperfusion injury and long-term graft function in renal allografts from older donors. Transplantation. 2003;75:1786–1792. [DOI] [PubMed] [Google Scholar]
- 43.Tarrab E, Huet PM, Brault A, et al. Cyclosporin-A does not prevent cold ischemia/reperfusion injury of rat livers. J Surg Res. 2012;175:333–342. [DOI] [PubMed] [Google Scholar]
- 44.Hüser N, Doll D, Altomonte J, et al. Graft preconditioning with low-dose tacrolimus (FK506) and nitric oxide inhibitor aminoguanidine (AGH) reduces ischemia/reperfusion injury after liver transplantation in the rat. Arch Pharm Res. 2009;32:215–220. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki K, Miyake H, Kinoshita T, et al. Protective effect of FK506 and thromboxane synthase inhibitor on ischemia-reperfusion injury in non-heart-beating donor in rat orthotopic liver transplantation. J Med Invest. 2004;51:76–83. [DOI] [PubMed] [Google Scholar]
- 46.Kawano K, Bowers JL, Kim YI, et al. FK506 reduces oxidative hepatic injury following cold ischemic preservation and transplantation. Transplant Proc. 1996;28:1902–1903. [PubMed] [Google Scholar]
- 47.Yamanoi A, Kohno H, Chang T, et al. Beneficial effect of donor pretreatment with cyclosporin A in porcine liver transplantation. Surg Res Comm. 1994;15:229–236. [Google Scholar]
- 48.Goto S, Kim YI, Shimada T, et al. The effects of pretransplant cyclosporine therapy on rats grafted with twelve-hour cold-stored livers—with special reference to reperfusion injury. Transplantation. 1991;52:615–621. [DOI] [PubMed] [Google Scholar]
- 49.Bayer J, Das NA, Baisden CE, et al. Effect of inhaled tacrolimus on ischemia reperfusion injury in rat lung transplant model. J Thorac Cardiovasc Surg. 2013;146:1213–9; discussion 1219. [DOI] [PubMed] [Google Scholar]
- 50.Oltean M, Pullerits R, Zhu C, et al. Donor pretreatment with FK506 reduces reperfusion injury and accelerates intestinal graft recovery in rats. Surgery. 2007;141:667–677. [DOI] [PubMed] [Google Scholar]
- 51.Oltean M, Mera S, Olofsson R, et al. Transplantation of preconditioned intestinal grafts is associated with lower inflammatory activation and remote organ injury in rats. Transplant Proc. 2006;38:1775–1778. [DOI] [PubMed] [Google Scholar]
- 52.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. [DOI] [PubMed] [Google Scholar]
- 53.Belhaj A, Dewachter L, Hupkens E, et al. Tacrolimus prevents mechanical and humoral alterations in brain death–induced lung injury in pigs. Am J Respir Crit Care Med. 2022;206:584–595. [DOI] [PubMed] [Google Scholar]
- 54.McCafferty K, Forbes S, Thiemermann C, et al. The challenge of translating ischemic conditioning from animal models to humans: the role of comorbidities. Dis Model Mech. 2014;7:1321–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pitcher JM, Wang M, Tsai BM, et al. Preconditioning: gender effects. J Surg Res. 2005;129:202–220. [DOI] [PubMed] [Google Scholar]
- 56.Orban JC, Fontaine E, Cassuto E, et al. ; AzuRéa network. Effects of cyclosporine A pretreatment of deceased organ donors on kidney graft function (Cis-A-rein): study protocol for a randomized controlled trial. Trials. 2018;19:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lerink LJS, de Kok MJC, Mulvey JF, et al. Preclinical models versus clinical renal ischemia reperfusion injury: a systematic review based on metabolic signatures. Am J Transplant. 2022;22:344–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zwetsloot PP, Végh AMD, Jansen of Lorkeers SJ, et al. Cardiac stem cell treatment in myocardial infarction: a systematic review and meta-analysis of preclinical studies. Circ Res. 2016;118:1223–1232. [DOI] [PubMed] [Google Scholar]
- 59.van der Worp HB, Sena ES, Donnan GA, et al. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain. 2007;130:3063–3074. [DOI] [PubMed] [Google Scholar]
- 60.Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA. 2006;296:1731–1732. [DOI] [PubMed] [Google Scholar]
- 61.Macleod MR, O’Collins T, Horky LL, et al. Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab. 2005;25:713–721. [DOI] [PubMed] [Google Scholar]