Abstract
BACKGROUND
Endotrophin, a collagen type VI-derived peptide, mediates metabolic dysregulation, inflammation, and fibrosis in animal models, but has not been studied in human heart failure (HF).
METHODS
We examined the association between circulating endotrophin and outcomes in participants suffering from HF with preserved ejection fraction (HFpEF) enrolled in the TOPCAT trial (n=205). Associations were validated in a participant-level meta-analysis (n=810) that included participants with HFpEF from the PHFS study (United States; n=174), PEOPLE cohort (New Zealand; n=168), a randomized trial of vasodilator therapy (United States; n=45), a cohort from Donostia University Hospital and University of Navarra (Spain; n=171), and the TRAINING-HF trial (Spain; n=47). We also assessed associations in HF with reduced ejection fraction in PHFS (n=1,642).
RESULTS
Plasma endotrophin levels at baseline were associated with risk of future death (standardized hazard ratio [HR] = 1.74; 95% confidence interval [CI]=1.36–2.24; P<0.001) and death or HF-related hospital admission (DHFA; standardized HR=2.11; 95% CI= 1.67–2.67; P<0.001) in TOPCAT. Endotrophin improved reclassification and discrimination for these outcomes beyond the MAGGIC risk score and NT-proBNP (N-terminal pro b-type natriuretic peptide). Findings were confirmed in the participant-level meta-analysis. In participants with HF with reduced ejection fraction in PHFS, endotrophin levels were associated with death (standardized HR=1.82; 95% CI=1.66–2.00; P<0.001) and DHFA (standardized HR=1.40; 95% CI=1.31–1.50; P<0.001), but the strength of the latter association was substantially lower than for the MAGGIC risk score (standardized HR=1.93; 95% CI=1.76–2.12) and BNP (standardized HR=1.78; 95% CI=1.66–1.92).
CONCLUSIONS
Circulating endotrophin levels are independently associated with future poor outcomes in patients with HF, particularly in HFpEF. (Funded by Bristol Myers Squibb; Instituto de Salud Carlos III [Spain] and European Regional Development Fund; European Commission CRUCIAL project; and the U.S. National Institutes of Health National Heart, Lung, and Blood Institute.)
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a heterogenous syndrome with few therapeutic options. Identification of novel biomarkers for risk stratification in HFpEF may improve assessment of prognosis in clinical practice, identification of high-risk individuals for clinical trials, and discovery of underlying biologic processes and therapeutic targets.1,2
Tissue fibrosis3-5 seems to play an important role in HFpEF. During fibrosis, activation of fibroblasts increases the production and deposition of collagens and other extracellular matrix proteins. Endotrophin is a peptide derived from the collagen type VI α3-chain released during collagen type VI formation. Endotrophin has recently been identified as a mediator of systemic inflammation, insulin resistance, metabolic dysregulation in adipose tissue, and tissue fibrosis in animal models,6,7 but has not yet been investigated in HFpEF to the best of our knowledge.
We report a series of retrospective analyses of clinical trial or observational cohort data in which we could measure blood (serum or plasma) endotrophin levels and associate these levels with known clinical outcomes among participants with HF, and/or studies in which we could compare these levels between participants with HFpEF, participants with HF with reduced ejection fraction (HFrEF), and participants without HF. Our analyses addressed four issues: (1) the association of baseline circulating endotrophin with outcomes in HFpEF using data from the TOPCAT trial (Treatment of Preserved Cardiac Function HF with an Aldosterone Antagonist); (2) validation of these observations using data from five additional international prospective HFpEF cohorts; (3) assessment of whether endotrophin levels are increased in participants with HFpEF compared with participants with HFrEF and control participants without HF; and (4) we further examined whether the association between blood endotrophin levels and outcomes observed in HFpEF also extends to HFrEF.
Methods
For all analyses, measurements of endotrophin were performed using PRO-C6 (Nordic Bioscience, Herlev, Denmark), which detects a neoepitope released from the C-terminal pro-peptide of type VI collagen.8,9 Technical details about the assay, including the stability of the analyte in stored samples, can be found in the Supplementary Appendix and Table S1 provided with the full text of this article at evidence.nejm.org. F. Genovese, A. L. Reese-Petersen, D. G.K. Rasmussen and M. A. Karsdal are full-time employees and shareholders at Nordic Bioscience, the company that produces this assay. These authors contributed to all biosample analyses, the selection and analytic design of two studies included in the meta-analysis (Donostia University Hospital and University of Navarra and the TRAINING-HF trial), and to manuscript preparation, but did not have a role in clinical data accrual or primary data analyses.
PROGNOSTIC ROLE OF ENDOTROPHIN IN THE TOPCAT TRIAL
We used stored plasma samples from the TOPCAT trial that were obtained from the U.S. National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center. TOPCAT was a multicenter, randomized, double-blinded, placebo-controlled trial of spironolactone in 3,445 adults with HFpEF,10,11 as described in the Supplementary Appendix. Given the previously documented differences regarding participant characteristics, medication adherence, phenotypic subgroups, and response to randomized therapy in Russia and Georgia compared with the Americas,10,12-14 we restricted our analysis to participants from the Americas with available plasma samples (n=205).
Endotrophin was measured from baseline visit plasma samples. We examined the relationship between baseline endotrophin levels and trial outcomes, including all-cause death, the trial primary end point (composite of cardiovascular mortality, aborted cardiac arrest, or hospitalization for the management of HF); and death or HF-related hospital admission (DHFA).
EXTERNAL VALIDATION OF THE RELATIONSHIP BETWEEN ENDOTROPHIN AND OUTCOMES IN HFpEF
We validated the relationships identified in TOPCAT between endotrophin levels and outcomes in five additional cohort studies in which participants with HFpEF for whom blood from a single baseline visit was available for assay were prospectively identified, enrolled, and observed for adjudicated outcomes. Cohorts were selected on the basis of convenience/availability. We included participants with HFpEF from the Penn Heart Failure Study (PHFS; United States; n=174),15-18 the PEOPLE cohort (New Zealand; n=168),19 a randomized trial of vasodilator therapy in HFpEF (United States; n=45),20 an HFpEF cohort from Donostia University Hospital and University of Navarra (Leizarán, Spain; n=171), and participants from the TRAINING-HF trial (University of Valencia; Spain; n=47), which assessed the effect of inspiratory muscle training and functional electrical stimulation in HFpEF.21 All endotrophin measurements in these cohorts were performed in plasma, except for the Leizarán cohort and TRAINING-HF cohorts, which were performed in serum. Inclusion and exclusion criteria for these studies are presented in the Supplementary Appendix. We performed a participant-level meta-analysis of all available patient data from these cohorts (n=810) to assess the relationship between endotrophin and all cause-death and DHFA.
COMPARISONS BETWEEN HFpEF VERSUS HFrEF AND NON-HF CONTROL PARTICIPANTS
To assess whether endotrophin levels are increased in HFpEF versus HFrEF and participants without HF, we measured endotrophin in three separate observational cohort studies.
Study 3A
A prospective multicenter study was designed to establish a discovery biobank to compare biomarkers between participants (n=57) with adjudicated HFpEF (n=15), HFrEF (n=27), and hypertension without HF (n=15). Inclusion and exclusion criteria for this study are shown in Table S2. Endotrophin measurements were performed in plasma.
Study 3B
The University of Pennsylvania Deep HF Phenotyping study (n=59) was designed to assess various exercise-related cardiovascular detailed phenotypes between participants with HFpEF (n=20), hypertension (n=19), and normotensive controls (n=20), as previously described.22,23 This study does not overlap with PHFS. Endotrophin measurements were performed in plasma.
Study 3C
A prospective study (n=320) of participants with HFpEF (n=81) and HFrEF (n=59) and non-HF (n=180) control participants referred for a cardiac MRI study at the Philadelphia VA Medical Center was performed. These studies are described in more detail in the Supplementary Appendix. Endotrophin measurements were performed in serum.
ASSOCIATION OF ENDOTROPHIN LEVELS AND OUTCOMES IN HFrEF
To determine whether the prognostic value of endotrophin extends to HFrEF, we studied participants with HFrEF enrolled in the Penn Heart Failure Study (n=1,642). All measurements were performed at baseline (single time point). Details about this study have been previously published15-18 and are described in the Supplementary Appendix. Endotrophin measurements were performed in plasma.
STATISTICAL ANALYSIS
In analyses 1, 2, and 4, we used Cox proportional-hazards regression to assess relationships between endotrophin and outcomes. We built unadjusted Cox models and models adjusted for covariates selected a priori — the MAGGIC risk score24 plus NT-proBNP or BNP levels as available in each cohort.13,25-27 The MAGGIC risk score incorporates 13 clinical variables: age, sex, BMI, systolic blood pressure, left ventricular ejection fraction (LVEF), serum creatinine, current smoking, diabetes mellitus, chronic obstructive pulmonary disease, New York Heart Association (NYHA) class, HF duration >18 months, β-blocker use, and angiotensin-converting enzyme inhibitor use. This score was derived from a large international database of patients with HF that included those with both HFrEF and HFpEF24 and has been specifically validated in HFpEF.28
Endotrophin values and covariates were Box-Cox transformed to improve normality and converted into z scores within each cohort (i.e., mean centered at 0 and divided by the standard deviation). Hazard ratios are standardized — per unit change in the z score — for a more intuitive assessment and comparison of their relationship with outcomes. We assessed log-log plots, Schoenfeld and Martingale residuals to test the proportionality, and linearity assumptions in Cox proportional-hazards models. We also computed Kaplan–Meier survival curves for tertiles of endotrophin and compared them with the log-rank test.
In analysis 2, an individual patient data meta-analysis was performed using a one- and two-stage approach with Cox proportional-hazards models for the outcomes of all-cause death and DHFA, adjusted for the MAGGIC risk score or its individual components. The one-stage approach used mixed-effects modeling with a random study intercept, random endotrophin effects, and unstructured variance.29 The two-stage approach used random-effects meta-analysis with profile likelihood estimation of variance.30 We performed a sensitivity analysis using stratified baseline hazards rather than using a meta-analytic approach. Covariate data that were intermittently missing across studies were assumed to be missing at random and were addressed using multiple imputations with chained equations.
In analysis 3, endotrophin levels between HFpEF, HFrEF, and non-HF groups were compared within each study using ANOVA after log-transformation, as appropriate, to improve normality. All means and 95% confidence intervals (CIs) are expressed in the native (nontransformed) scale (i.e., as geometric means).
Statistical analyses were performed using Matlab (Matlab 2019b; Mathworks, Natick, MA) and Stata (StataCorp, College Station, Texas). Other than for the primary data from TOPCAT, the widths of the reported CIs have not been adjusted for multiplicity, and the inferences drawn from the analyses may not be reproducible and should not be considered clinically directive.
Results
Table S3 summarizes information on the broader population affected by HF and assesses the representativeness of our study populations.
ANALYSIS 1: PROGNOSTIC ASSOCIATIONS OF ENDOTROPHIN IN THE TOPCAT TRIAL
A comparison of TOPCAT participants enrolled in the Americas with available plasma samples versus without is shown in Table S4. Most characteristics did not differ between the subsets, but participants with available samples demonstrated a slightly higher prevalence of White race, hypertension, atrial fibrillation, and statin use, and a slightly lower prevalence of chronic obstructive pulmonary disease.
Table S5 shows characteristics of the TOPCAT study participants included in this study stratified by tertiles of plasma endotrophin levels. Participants with higher plasma endotrophin exhibited progressively lower glomerular filtration rate, higher prevalence of NYHA class III/IV symptoms, diabetes mellitus, and higher use of glucose-lowering agents.
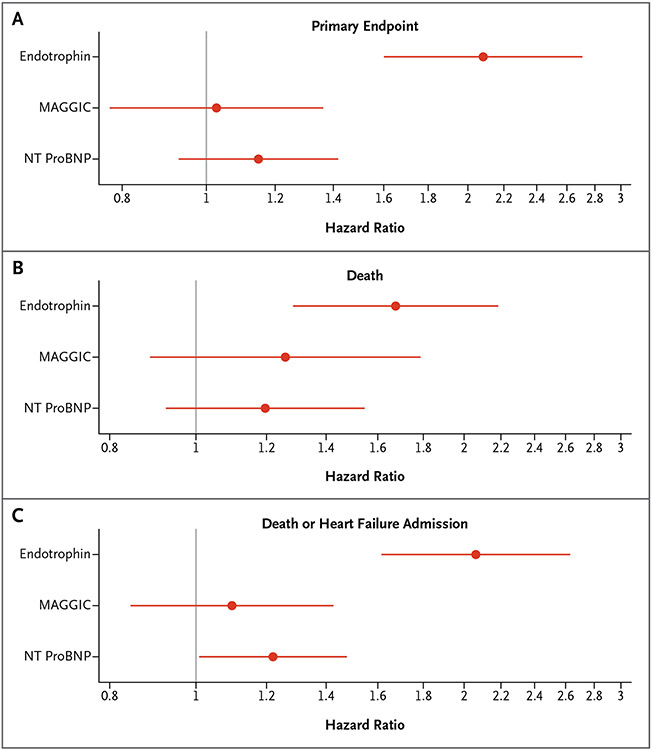
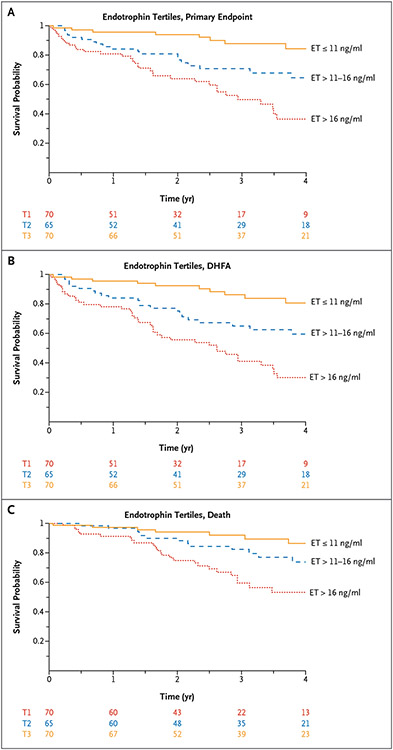
In unadjusted analyses (Fig. 1 and Table S6), plasma endotrophin was associated with the TOPCAT primary end point (standardized hazard ratio [HR]=2.10; 95% CI=1.62–2.71; P<0.001), all-cause death (standardized HR=1.74; 95% CI=1.36–2.24; P<0.001), and DHFA (standardized HR=2.11; 95% CI=1.67–2.67; P<0.001). Figure 2A-2C show Kaplan–Meier survival curves corresponding to tertiles of endotrophin for the various outcomes.
Figure 1. Multivariable Cox Proportional-Hazards Models in the TOPCAT trial.
Multivariable Cox proportional-hazards models that include endotrophin, NT-proBNP, and MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) risk score as predictors of (Panel A) the primary end point, (Panel B) death or heart failure–related hospital admission, and (Panel C) death in the Treatment of Preserved Cardiac Function HF with an Aldosterone Antagonist trial. Each of the three models included all three predictors.
Figure 2. Kaplan–Meier Survival Curves in the TOPCAT trial.
Kaplan–Meier survival curves for tertiles of plasma endotrophin (ET) for (Panel A) the primary end point, (Panel B) all-cause death or heart failure–related hospital admission (DHFA), and (Panel C) all-cause death in the Treatment of Preserved Cardiac Function HF with an Aldosterone Antagonist trial.
Similarly, endotrophin was associated with incident outcomes in models that adjusted for the MAGGIC risk score (Fig. 1 and Table S6). HRs for plasma endotrophin with respect to the primary end point, DHFA, or death did not appreciably change after adjustment for the MAGGIC risk score or for the MAGGIC risk score plus NT-proBNP (Fig. 1). When adjusted for endotrophin levels, NT-ProBNP levels were no longer associated with TOPCAT’s primary end point or with death and were only weakly associated with DHFA, whereas the MAGGIC risk score was no longer associated with any of the three studied outcomes. We note the relatively low number of events in these analyses, which could have resulted in false-negative findings regarding associations between NT-ProBNP and/or MAGGIC risk score and outcomes.
For the primary end point, Harrel’s C-statistic was 0.70 (95% CI=0.64–0.77) in a model that included endotrophin alone compared with 0.63 (95% CI=0.61–0.66) in a model that included MAGGIC risk score alone. Similarly, for DHFA, Harrel’s C-statistic was 0.71 (95% CI=0.64–0.77) in a model that included endotrophin alone compared with 0.62 (95% CI=0.59–0.65) in a model that included MAGGIC risk score plus NT-ProBNP without endotrophin. Information on various other model comparisons is shown in Table S7. The addition of endotrophin resulted in a net reclassification improvement index of 0.44 for death and 0.79 for DHFA.
ANALYSIS 2: RELATIONSHIP BETWEEN ENDOTROPHIN AND OUTCOMES IN HFpEF
Table 1 summarizes characteristics of the study populations included in the meta-analysis. Table S8 presents these characteristics stratified by tertiles of endotrophin. In general, higher endotrophin levels were associated with higher serum creatinine and tended to be associated with higher prevalence of diabetes. In PHFS, higher endotrophin was also associated with older age, a higher BMI, higher prevalence of NYHA class III/IV symptoms, and a higher LVEF.
Table 1.
General Characteristics of Study Participants Included in Participant-Level Meta-Analysis (Analysis 2).*
| Study† | TOPCAT | PHFS | PEOPLE | Vasodilator HFpEF Trial |
Leizarán | University of Valencia |
Overall |
|---|---|---|---|---|---|---|---|
| No. | 205 | 174 | 168 | 45 | 171 | 47 | 810 |
| Age — yr‡ | 72±10 | 61±15 | 77±10 | 64±9 | 75±9 | 74±9 | 71±12 |
| Male sex | 112 (55) | 84 (48) | 83 (49) | 34 (76) | 75 (44) | 20 (43) | 408 (50) |
| LVEF — % | 58±7 | 61±8 | 66±8 | 62±8 | 66±9 | 67±10 | 63±9 |
| SBP — mm Hg | 125±14 | 129±22 | 130±23 | 136±23 | 149±24 | 130±14 | 132±22 |
| BMI — kg/m2 | 34–7 | 33±10 | 30±7 | 37±7 | 31±5 | 32±5 | 32±8 |
| Cr — mg/dl | 1.2±0.3 | 1.4±1.0 | 1.3±0.5 | 1.5±1.1 | 1.0±0.3 | 1.2±0.5 | 1.2±0.6 |
| NYHA class 3/4 | 0 (0) | 63 (36) | 61 (36) | § | 87 (51) | 11 (23) | 222 (27) |
| Smoking | 11 (5) | 12 (7) | 9 (5) | 8 (18) | § | 3 (6) | 43 (5) |
| Diabetes | 95 (46) | 63 (36) | 62 (37) | 30 (67) | 0 (0) | 21 (45) | 271 (33) |
| COPD | 22 (11) | 55 (32) | § | 10 (22) | 14 (8) | 4 (9) | 105 (13) |
| β blocker | 171 (83) | 120 (69) | 122 (73) | 26 (58) | 68 (40) | 41 (87) | 548 (68) |
| ACEI or ARB | 153 (75) | 119 (68) | 125 (74) | 32 (71) | 142 (83) | 9 (19) | 580 (72) |
| DHFA — n | 72 (35) | 74 (43) | 67 (40) | 14 (31) | 130 (76) | 18 (38) | 375 (46) |
| Median time to censoring or DHFA event — yr | 2.6±1.5 | 3.6±2.9 | 1.5±0.7 | 3.8±1.9 | 3.9±2.1 | 2.6±1.2 | 2.9±2.1 |
| Death — n | 46 (22) | 41 (24) | 41 (24) | 9 (20) | 81 (47) | 11 (23) | 229 (28) |
| Median time to censoring or death — yr | 2.9±1.4 | 4.4±2.8 | 1.7±0.5 | 4.3±1.8 | 4.9±1.4 | 3±0.8 | 3.5±2 |
ACEI denotes angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; Cr, serum creatinine; LVEF, left ventricular ejection fraction; n, number of participants who reached the end point; NYHA, New York Heart Association class; PHFS, Penn Heart Failure Study; SBP, systolic blood pressure; and TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial.
TOPCAT and the Vasodilator HFpEF Trial were randomized controlled trials. All other studies were observational prospective cohort studies.
Data are given as mean±SD or no. (%) unless otherwise noted.
Data were missing and therefore imputed.
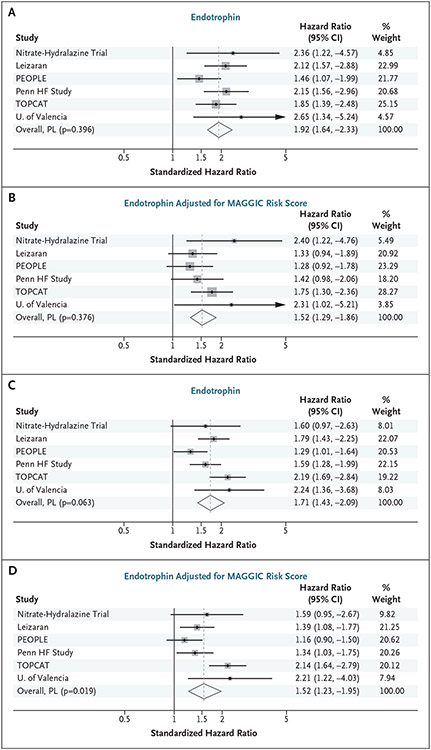
Endotrophin levels were consistently associated with all-cause death in all HFpEF cohorts (Fig. 3A and 3B). A one-stage participant-level meta-analysis of all cohorts (Table S9) showed an association with death (standardized HR= 1.92; 95% CI=1.66–2.22). Endotrophin remained associated with death after adjustment for MAGGIC risk score (standardized HR=1.54; 95% CI=1.32–1.80) and after additional adjustment for NT-proBNP (standardized HR=1.54; 95% CI=1.22–1.70). Results of two-stage meta-analysis showed similar results in unadjusted analyses (Fig. 3A), after adjustment for MAGGIC risk score (Fig. 3B), and after additional adjustment for NT-proBNP (Fig. S2A). Analyses related to the risk of DHFA, sensitivity analyses, and various comparisons of metrics of model discrimination and reclassification are shown in Tables S9, S10, and S11 and Figures S1 and S2.
Figure 3. Forest Plot of Participant-Level Meta-Analysis.
Forest plot of our participant-level (PL) meta-analysis assessing the relationship between (Panels A and B) endotrophin and death and (Panels C and D) death or heart failure–related hospital admission in six heart failure (HF) with preserved ejection fraction cohort studies in unadjusted analyses (Panels A and C) and models adjusted for MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) risk score (Panels B and D). A hazard ratio >1 indicates a higher risk of death with higher endotrophin levels. PEOPLE denotes, Prospective Evaluation of Outcome in Patients with Heart Failure with Preserved Left Ventricular Ejection Fraction; TOPCAT, Treatment of Preserved Cardiac Function HF with an Aldosterone Antagonist trial.
ANALYSIS 3: COMPARISONS BETWEEN HFpEF VERSUS HFrEF AND NON-HF
General characteristics of individuals included in the three studies for this analysis are shown in Tables S12-S15. Figure S3A-S3C shows comparisons of endotrophin levels between participants with HFpEF and HFrEF and control participants without HF in the various studies. In our multicenter biobanking study (Study 3A), endotrophin levels were 13.6 ng/ml (95% CI=10.6–16.6) in participants with HFpEF, 11.7 ng/ml (95% CI=9.9–13.4) in participants with HFrEF, and 8.9 ng/ml (95% CI=7.1–10.6) in participants without HF. In the University of Pennsylvania deep phenotyping study (Study 3B), endotrophin levels were 13.4 ng/ml (95% CI=10.9–15.9) in participants with HFpEF, 8.52 ng/ml (95% CI=6.88–10.2) in hypertensive participants without HF, and 8.97 ng/ml (95% CI=7.28–10.65) in normotensive controls. In the VA study (Study 3C), endotrophin levels were 9.6 ng/ml (95% CI=8.79–10.41) in participants with HFpEF, 5.59 ng/ml (95% CI=5.28–5.9) in participants without HF, and 6.58 ng/ml (95% CI= 5.92–7.23) in participants with HFrEF. In contrast to the substantially higher levels observed in HFpEF relative to HFrEF in the studies mentioned above, in PHFS, which included predominantly individuals with advanced HF, participants with HFpEF exhibited mean levels of 11.4 ng/ml (95% CI= 10.6–12.3) compared with 11.2 ng/ml (95% CI=10.9–11.5) in participants with HFrEF (Fig. S3D).
ANALYSIS 4: PROGNOSTIC ASSOCIATIONS OF ENDOTROPHIN IN HFrEF IN PHFS
Table S15 shows general characteristics of PHFS participants with HFrEF included in PHFS. Table S16 shows these characteristics stratified by tertiles of plasma endotrophin. Higher endotrophin levels were associated with older age, a higher proportion of African American participants, lower diastolic blood pressure, higher prevalence of diabetes mellitus, history of coronary revascularization procedures, atrial fibrillation/flutter, NYHA class III/IV symptoms, lower estimated glomerular filtration rate, LVEF, and various differences in medication use.
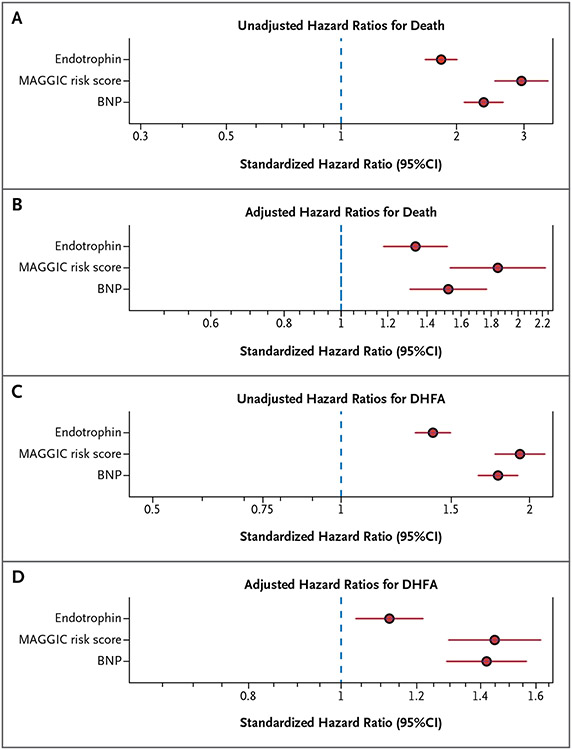
This section deals with the relationship between plasma endotrophin and outcomes among participants with HFrEF enrolled in PHFS. During a median follow-up time of 4.48 years, 445 participants died and 917 reached the end point (DHFA). Endotrophin levels were associated with death (standardized HR=1.82; 95% CI=1.66–2.00; Fig. 4A). After adjustment for MAGGIC risk score and BNP (Fig. 4B), the relationship was attenuated and adjusted standardized HR decreased to 1.34 (95% CI= 1.18–1.52). Endotrophin was also associated with the risk of DHFA in this cohort, both in unadjusted analyses (standardized HR=1.40; 95% CI= 1.31–1.50; Fig. 4C) and after adjustment for MAGGIC risk score and BNP (standardized HR=1.12; 95% CI=1.03–1.22; Fig. 4D). However, compared with endotrophin, associations with DHFA were stronger for MAGGIC risk score (standardized HR=1.93; 95% CI=1.76–2.12) and BNP (standardized HR=1.78; 95% CI=1.66–1.92) in unadjusted analyses (Fig. 4C). This was also true in an adjusted model (Fig. 4D) that included endotrophin (standardized HR=1.12; 95% CI=1.03–1.22), MAGGIC risk score (adjusted standardized HR=1.45; 95% CI=1.30–1.62), and BNP (HR=1.42; 95% CI= 1.29–1.56).
Figure 4. Relationship Between Endotrophin and Outcomes in Heart Failure with Reduced Ejection Fraction (n=1642) in Analysis 4.
Cox proportional-hazards models that include endotrophin (n = 1,642), MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) risk score, and BNP as predictors of death (Panels A and B) and of death or heart failure–related hospitalization (DHFA; Panels C and D). Panels A and C present unadjusted standardized hazard ratios, whereas Panels B and D present multivariable models that include endotrophin, MAGGIC risk score, and BNP. Multivariable models (Panels B and D) are based on participants for whom complete covariate data were available (n = 1337; 761 first DHFA events; 346 deaths).
Discussion
We performed analyses using extant samples from a randomized clinical trial, TOPCAT, and additional randomized trial or cohort studies, assessing the relationship between baseline circulating levels of endotrophin and clinical outcomes in patients with HF. First, we measured endotrophin using samples collected at baseline among participants enrolled in the TOPCAT trial. Our analysis demonstrated that levels of endotrophin in the blood, a bioactive peptide produced during collagen type VI formation, are strongly and independently associated with the risk of the primary trial end point, all-cause death and DHFA. Endotrophin levels alone were more strongly associated with outcomes in HFpEF than the MAGGIC risk score. We validated these findings in a meta-analysis of six studies in which endotrophin levels at study onset were measured and related to subsequent death and DHFA independently of MAGGIC risk score and NTproBNP. In three additional studies, we demonstrated that endotrophin levels were increased in patients with HFpEF compared with normotensive control participants, hypertensive control participants, and participants with HFrEF, although endotrophin levels did not differ between HFpEF and HFrEF in another distinct cohort of patients with advanced HF (PHFS). Among participants with HFrEF in this large cohort, we demonstrated that plasma endotrophin levels were independently associated with the risk of death as well and DHFA, although in contrast to our findings in TOPCAT (HFpEF population), they were less strongly associated with DHFA compared with MAGGIC risk score and NT-proBNP. Our studies demonstrate that, among patients with HFpEF, endotrophin levels are higher than in normal or hypertensive control participants without HF, and that, within cohorts of participants with HFpEF, higher levels are associated with a subsequent higher likelihood of all-cause death or the composite outcome of all-cause death or hospitalization for HF.
The search for systemic circulating biomarkers of tissue fibrosis in HFpEF represents an area of great interest, both for purposes of risk stratification and to identify individual patients with specific underlying biologic abnormalities. We found that endotrophin exhibited such an association with HF outcomes over a broad range of absolute risk in HFpEF, which prominently exceeded that associated with the MAGGIC risk score, and was associated with higher values of Harrel’s C index compared with base models that included MAGGIC risk score alone or the MAGGIC risk score plus NT-proBNP. Of interest, the prognostic information provided by endotrophin was largely independent of MAGGIC risk score and NT-proBNP. In contrast, the MAGGIC risk score and NT-proBNP added little to the risk prediction by endotrophin levels; therefore, endotrophin appears to be a promising biomarker for risk prediction in patients with HFpEF.
An important question is the source of excess circulating endotrophin in patients with HF as the beaded microfibril type VI collagen is found in virtually all tissues.31 Potential sources include myocardial, skeletal muscle, kidney, lung, vascular, liver, and adipose tissue. Circulating endotrophin was indeed associated with a lower estimated glomerular filtration rate in multiple cohorts included in our studies, suggesting a potential renal source. Endotrophin may also be secreted by adipose tissue, where it can trigger fibrosis and metabolic dysfunction,6 or may be related to myocardial fibrosis. Endotrophin, in fact, may have multiple sources and its levels are influenced by the systemic overall burden of comorbidities.32 Additional studies are needed to better understand the source of excess endotrophin and its effects on various tissues in HFpEF.
Strengths of our study include the comprehensive nature of our analyses; the use of multiple cohorts in multiple countries on three different continents, with consistent findings supporting the generalizability of our findings; the prospective enrollment of participants with HFpEF and HFrEF using strict selection criteria and careful event adjudication in the various studies; and the use of endotrophin, a biomarker specific for a collagen VI–derived matrikine. Weaknesses include the use of a subpopulation of TOPCAT participants with available samples (as opposed to the entire trial cohort), the retrospective nature of endotrophin measurements in frozen samples, and the observation that the results from the PHFS cohort were not fully concordant with those from other cohorts.
Of note, the set of prospective studies and analyses presented herein focus on the discrimination for incident outcomes provided by circulating endotrophin levels in HF. Discrimination is necessary but not sufficient for understanding the role of endotrophin in clinical practice. Additional studies will be required to characterize the calibration and specific utility of this biomarker in various clinical scenarios. This process should also involve novel assay development for easy and wide implementation in the clinical setting. Similarly, future studies should investigate changes over time in circulating endotrophin, including changes with decompensation, hospitalization for HF, and recovery after HF exacerbations.
In summary, circulating levels of endotrophin, a biomarker of collagen type VI formation with bioactive effects relevant to the pathophysiology of HF, is increased in HFpEF and is independently associated with outcomes in the populations of patients with HFpEF we studied. Although our data suggest that endotrophin represents a promising prognostic biomarker for risk stratification in HFpEF, it has yet to be used for the selection of more homogenous higherrisk populations for randomized trials of HF therapies. Whether antagonizing the biologic actions of endotrophin with pharmacologic interventions is a viable therapeutic approach for HF and whether reductions of endotrophin with treatment can help track the response to therapeutic interventions are areas for future research.
Supplementary Material
Acknowledgments
This study was funded by a University of Pennsylvania research grant from Bristol Myers Squibb to Dr. Chirinos; Instituto de Salud Carlos III (Spain) grants (CB16/11/00483 and PI18/01469 co-financed by ERDF funds) to Dr. Díaz and Dr. González, respectively; a European Commission CRUCIAL project grant (agreement 848109) to Dr. González; and a U.S. National Institutes of Health National Heart, Lung, and Blood Institute grant (K23-HL130551) to Dr. Zamani.
Footnotes
Disclosures
Author disclosures and other supplementary materials are available at evidence.nejm.org.
References
- 1.Pandey A, Shah SJ, Butler J, et al. Exercise intolerance in older adults with heart failure with preserved ejection fraction: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;78:1166–1187. DOI: 10.1016/j.jacc.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah SJ, Borlaug BA, Kitzman DW, et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute working group summary. Circulation 2020;141:1001–1026. DOI: 10.1161/CIRCULATIONAHA.119.041886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–559. DOI: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshihisa A, Sato Y, Yokokawa T, et al. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail 2018;5:262–270. DOI: 10.1002/ehf2.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 2014;113:1211–1216. DOI: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun K, Park J, Gupta OT, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun 2014;5:3485. DOI: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Gu X, Zhang N, Kolonin MG, An Z, Sun K. Divergent functions of endotrophin on different cell populations in adipose tissue. Am J Physiol Endocrinol Metab 2016;311:E952–E963. DOI: 10.1152/ajpendo.00314.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun S, Henriksen K, Karsdal MA, et al. Collagen type III and VI turnover in response to long-term immobilization. PLoS One 2015;10:e0144525. DOI: 10.1371/journal.pone.0144525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heumüller SE, Talantikite M, Napoli M, et al. C-terminal proteolysis of the collagen VI a3 chain by BMP-1 and proprotein convertase(s) releases endotrophin in fragments of different sizes. J Biol Chem 2019;294:13769–13780. DOI: 10.1074/jbc.RA119.008641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. DOI: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. DOI: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 12.de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT — new insights into regional variation. N Engl J Med 2017;376:1690–1692. DOI: 10.1056/NEJMc1612601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen JB, Schrauben SJ, Zhao L, et al. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail 2020;8:172–184. DOI: 10.1016/j.jchf.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeffer MA, Claggett B. Behind the scenes of TOPCAT — bending to inform. NEJM Evid 2022;1(1). DOI: 10.1056/EVIDctcs2100007. [DOI] [PubMed] [Google Scholar]
- 15.Basuray A, French B, Ky B, et al. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation 2014;129:2380–2387. DOI: 10.1161/CIRCULATIONAHA.113.006855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ky B, French B, Ruparel K, et al. The vascular marker soluble fms-like tyrosine kinase 1 is associated with disease severity and adverse outcomes in chronic heart failure. J Am Coll Cardiol 2011;58:386–394. DOI: 10.1016/j.jacc.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ky B, Kimmel SE, Safa RN, et al. Neuregulin-1β is associated with disease severity and adverse outcomes in chronic heart failure. Circulation 2009;120:310–317. DOI: 10.1161/CIRCULATIONAHA.109.856310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kannan L, Shaw PA, Morley MP, et al. Thyroid dysfunction in heart failure and cardiovascular outcomes. Circ Heart Fail 2018;11:e005266. DOI: 10.1161/CIRCHEARTFAILURE.118.005266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santhanakrishnan R, Ng TP, Cameron VA, et al. The Singapore Heart Failure Outcomes and Phenotypes (SHOP) study and Prospective Evaluation of Outcome in Patients with Heart Failure with Preserved Left Ventricular Ejection Fraction (PEOPLE) study: rationale and design. J Card Fail 2013;19:156–162. DOI: 10.1016/j.cardfail.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Zamani P, Akers S, Soto-Calderon H, et al. Isosorbide dinitrate, with or without hydralazine, does not reduce wave reflections, left ventricular hypertrophy, or myocardial fibrosis in patients with heart failure with preserved ejection fraction. J Am Heart Assoc 2017;6:e004262. DOI: 10.1161/JAHA.116.004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palau P, Domínguez E, López L, et al. Entrenamiento de la musculatura inspiratoria y la electroestimulación muscular funcional en el tratamiento de la insuficiencia cardiaca con función sistólica conservada: estudio TRAINING-HF. Rev Esp Cardiol (Engl Ed) 2019;72:288–297. DOI: 10.1016/j.recesp.2018.01.024.29551699 [DOI] [Google Scholar]
- 22.Zamani P, Proto EA, Wilson N, et al. Multimodality assessment of heart failure with preserved ejection fraction skeletal muscle reveals differences in the machinery of energy fuel metabolism. ESC Heart Fail 2021;8:2698–2712. DOI: 10.1002/ehf2.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamani P, Proto EA, Mazurek JA, et al. Peripheral determinants of oxygen utilization in heart failure with preserved ejection fraction: central role of adiposity. JACC Basic Transl Sci 2020;5:211–225. DOI: 10.1016/j.jacbts.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pocock SJ, Ariti CA, McMurray JJV, et al. Predicting survival in heart failure: a risk score based on 39,372 patients from 30 studies. Eur Heart J 2013;34:1404–1413. DOI: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 25.Prenner SB, Kumar A, Zhao L, et al. Effect of serum albumin levels in patients with heart failure with preserved ejection fraction (from the TOPCAT trial). Am J Cardiol 2020;125:575–582. DOI: 10.1016/j.amjcard.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chirinos JA, Orlenko A, Zhao L, et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2020;75:1281–1295. DOI: 10.1016/j.jacc.2019.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chirinos JA, Zhao L, Jia Y, et al. Reduced apolipoprotein M and adverse outcomes across the spectrum of human heart failure. Circulation 2020;141:1463–1476. DOI: 10.1161/CIRCULATIONAHA.119.045323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich JD, Burns J, Freed BH, Maurer MS, Burkhoff D, Shah SJ. Meta-Analysis Global Group in Chronic (MAGGIC) heart failure risk score: validation of a simple tool for the prediction of morbidity and mortality in heart failure with preserved ejection fraction. J Am Heart Assoc 2018;7:e009594. DOI: 10.1161/JAHA.118.009594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med 2000;19:1793–1819. DOI: . [DOI] [PubMed] [Google Scholar]
- 30.Cornell JE, Mulrow CD, Localio R, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med 2014;160:267–270. DOI: 10.7326/M13-2886. [DOI] [PubMed] [Google Scholar]
- 31.Lamandé SR, Bateman JF. Collagen VI disorders: insights on form and function in the extracellular matrix and beyond. Matrix Biol 2018;71-72:348–367. DOI: 10.1016/j.matbio.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Staunstrup LM, Bager CL, Frederiksen P, et al. Endotrophin is associated with chronic multimorbidity and all-cause mortality in a cohort of elderly women. EBioMedicine 2021;68:103391. DOI: 10.1016/j.ebiom.2021.103391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.