Abstract
Injuries of various types occur commonly in the lives of humans and other animals, and lead to a pattern of persistent pain and recuperative behaviour that allows safe and effective recovery. Here, we propose a control-theoretic framework to explain the adaptive processes in the brain that drive physiological post-injury behaviour. We set out an evolutionary and ethological view on how animals respond to injury, illustrating how the behavioural state associated with persistent pain and recuperation may be just as important as phasic pain in ensuring survival. Adopting a normative approach, we suggest that the brain implements a continuous optimal inference of the current state of injury from diverse sensory and physiological signals. This drives the various effector control mechanisms of behavioural homeostasis, which span modulation of ongoing motivation and perception to drive rest and hyper-protective behaviours. However, an inherent problem with this is that these protective behaviours may partially obscure information about whether injury has resolved. Such information-restriction may seed a tendency to aberrantly or persistently infer injury, and so promote transition to pathological chronic pain states.
Introduction
Injury is an inevitable consequence of life in a hazardous and competitive world, and animals’ ubiquitous capability for tissue healing provides a passage to recovery that has been an essential feature of life across species throughout evolution1. However, animals are especially vulnerable during the recovery period, both because of the myriad of complex ways in which an injury might affect physiological and behavioural functions, and because external threats may be greater in the context of reduced functionality. In humans, one of the defining components of the injured state is pain, but pain is universally unpleasant and its role in helping to guide recovery remains unclear.
In health, acute phasic pain - for instance after touching a hot saucepan - provides a valuable and effective signal that informs of imminent harm, enabling us to rapidly respond to the cause of the threat, and learn from the experience to avoid it in the future2. But if a true injury is actually sustained - for instance you physically burn yourself - a different pattern of pain ensues. One aspect of this is a general amplification of evoked phasic pain around the injured region, which enhances protective behaviour and derives from a combination of peripheral and central sensitization3. Another aspect is a spontaneous background pain that persists at rest, and is typically associated with a persistent sense of fatigue, lowered mood and anxiety4. The functional role of this background pain is less clear, and leads to a question as to whether persistent pain and its associated symptoms are part of an adaptive physiological response to an injury. If so, it should be possible to understand these symptoms in an evolutionary context and formalise their behavioural function.
In this Perspective, we set out the evolutionary framework for considering how animals across species respond to injury, and how such behaviours may improve adaptive fitness. We then show how this can be used to inform and constrain computational models of post-injury behavioural homeostasis, and formulate a control-theoretic approach for persistent pain. We argue that that the very nature of adaptive control creates a fundamental problem for the system: that is, the behaviour used to protect against pain makes it difficult to know if an injury has resolved (information restriction). From the control perspective, resolution of persistent pain and injury is equivalent to a finely tuned yet difficult inference problem, and maladaptive emotional and behavioural responses may lead to an inherent risk of augmentation and persistence into a chronic pain-like state.
Ecological and evolutionary perspectives.
Nociception is an evolutionary necessity, as important as the ability to identify sources of energy or food, and to reproduce5-7. Responses to injury vary enormously across the animal kingdom (Supplemental FIG. 1a). Sessile animals without nervous systems respond via molecular and cellular changes that prioritise tissue regeneration and repair, and these responses are highly conserved among all animals8. The molecular detectors for aversive control, for example, the transient receptor potential superfamily of receptors, have ancient homologues involved in thermal, mechanical and chemical sensation9.10, evident in non-animal species from microbes to algae11-15.
Over the course of evolution, injury is likely one of the earliest arising and most persistent features of an animal’s sensory experience. Among extant animal species, there is abundant evidence that injuries of many kinds are common, arising from accidents and inanimate dangers, predator-prey interactions, parasites and infections, and within-species interactions and conflicts16-19 (Supplemental FIG. 1b). The emergence of sensory neurons tuned to injury and noxious sensation (known as nociceptors) has occurred multiple times, with nociceptors described in nearly all extant animal phyla20,21. Even animals with very small nervous systems tend to dedicate neurons to the detection and response to injury22. This seemingly simple behaviour illustrates a basic control principle: sensors can detect environmental events and conditions before actual damage occurs, and use the signal to create a window of opportunity for evasive responses. This capacity for predictive control was extended early in brain evolution with development of the apparatus for associative learning (e.g. Pavlovian conditioning), which allows the sensed threat to be acted on as soon as it is predicted23. Homologues for the neural architectures and modulators involved in the sophisticated predictive aversive learning seen in mammals clearly exist in comparatively simple brains such as Drosophila24.
Irrespective of nervous system size or complexity, effective avoidance of bodily damage relies on two core principles: accurate, reliable and early detection of potential harm, and eliciting the right response in action. Depending on the context, optimal responses to the threat of injury might be offensive or defensive, and this complexity in selecting the optimal response to an acute nociceptive stimulus has led to a broad repertoire of highly context- and species-specific responses25. Meanwhile, larger brains also allow much more complicated instrumental behaviour - the ability to choose and reinforce actions supported by action-outcome learning, and through to cognitive architectures that allow an internal representation of the state of the external and internal environment26. Such an active sensing-action cycle is common and crucial for fitness and survival across animals.
Despite considerable sophistication in the capacity for avoidance, however, animals still frequently fail to avoid harm. Once an injury is incurred, the landscape of selection pressures acting on the brain shifts radically from circuits promoting injury-avoidance behaviour, onto those driving sustained recuperative, protective behaviour (Supplemental FIG. 1c). Importantly, failure to avoid injury comprises only a small proportion of the total fitness costs associated with injury. A major proportion of the cost is influenced by post-injury behavioural choices. In the post-injury phase, the adaptive value of long-lasting pain is apparent both as a potential means of driving recuperative behaviours during the healing period, and as a powerful enhancer of contextual memory of the painful event.
Given the frequency of injury occurrences and their obvious fitness costs, it is ultimately likely that behaviours that occur as a direct result of injuries are adaptive7,27. However, empirical tests of this pervasive hypothesis are uncommon, partly because the neural mechanisms driving these more complex behaviours are poorly understood in comparison to those driving immediate avoidance. What is clear is that in the aftermath of an injury, there is a dynamic transition from the acute defensive stage to a more prolonged recuperative stage, including whole-body behaviours such as rest, immobility, vigilance; and injury-directed behaviours such as wound guarding or grooming. This observation leads to the question regarding the role of persistent pain in driving recuperative behaviour. These complex behaviours are influenced by various homeostatic and autonomic systems, but evidence for the role of pain sensing to drive ecologically-relevant hypervigilance and recuperative behaviours can be found in studies of laboratory rodents 28, where provision of analgesic drugs acting directly on pain pathways restores normal behaviours 29-31. Interestingly, only a few invertebrate animals – not surprisingly, those with the most complex brains such as decapod crustaceans and cephalopod molluscs - have been shown to engage in recuperative and wound-directed behaviour, and is where evidence for vertebrate-like, persistent pain experience is strongest 32-35 (BOX 1).
BOX 1. Comparative studies reveal distinct ecological functions of acute and prolonged pain.
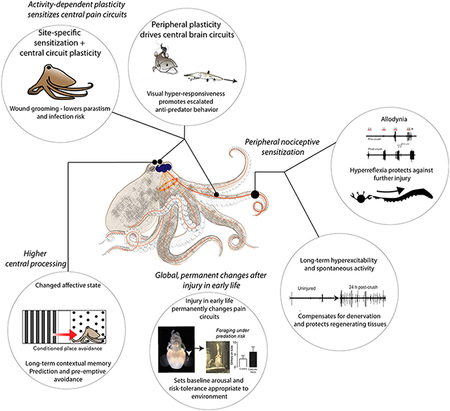
Shared selection pressures have led to many conserved features of nociception and nociceptive plasticity among evolutionarily distant species. Nociceptors function at the molecular, cellular and sometimes circuit level with surprising similarity across clades, however, relatively few studies have attempted to identify how such similar cellular processes may drive similar injury-induced behaviors, and what function these behaviors may serve. Recent work in cephalopod molluscs (octopus, cuttlefish and squid) suggests that both acute and prolonged pain may have adaptive functions162-164 (see Box Figure). Multiple studies of nociception and pain-like states in various cephalopod taxa now demonstrate many similarities between behavioral and neural responses to injury in cephalopods and those found in mammals164-166. Cephalopods have peripheral nociceptors that undergo intrinsic changes after injury to tissue they innervate, including acute reduction in activation threshold, increased firing rate and increased after discharge. Studies of acute post-injury behavior indicate that this primary nociceptive plasticity is necessary and sufficient to drive hypervigilance and hyper-reactivity to perceived threats, directing attention toward potential predators and investing energy into prolonged escape behaviors162,163,167. In some cephalopod taxa, short-term nociceptive plasticity also drives protective and wound-directed behaviors, similar in appearance and function to those seen in mammals and driven by complex (although still largely unexplored) central brain networks34. Likewise, long-lasting changes in behavior are driven by similar long-term or permanent changes to nociceptors, higher-order pain networks, and cognitive circuits in cephalopods as in mammals162,163, promoting contextual memory and optimizing future behavioral choices. Spontaneous firing of nociceptors persists in cephalopods for multiple days after tissue injury, which may produce tonic or persistent pain experience34. Permanent changes to nociceptive networks in cephalopods after injury in early life appear to optimise response thresholds to tolerate persistent danger164, suggesting an ecological role for these conserved patterns of long-lasting pain plasticity in mammals and humans.
One further important determinant of the nature and duration of recuperative behaviour is sociality, and whether the social environment facilitates extended recuperatory behaviours, which range from group protection of the injured member, to food provisioning, and to shared parental care. If we presume that persistent pain restricts the range of normal behaviour to those only that facilitate predator avoidance and wound healing, then recuperative behaviour (as hypothesised to be promoted by persistent pain) can only be adaptive in animals either whose physiology allows for extended periods of low nutrition, or whose social group can buffer the risks of starvation and isolation 27. Thus, animals who have high energy demand or lack social groups to share resources, are less likely to benefit from extended recuperative behaviour. In an optimality model, it can be shown that animals should direct allocation of limited post-injury resources to combating an immediate survival threat and away from recuperation and maintenance, up until the cost of accumulated damage from neglecting recuperative and nutritive needs increases mortality36. It follows that animals under greater pressure to allocate resources to maintenance (such as those with high energy demands), must limit recuperative behavior to ensure survival. Sociality is one mechanism that provides an escape from this tradeoff, as it permits individuals to access resources provided by in-group members (food, shelter and co-operative wound-care, but also time spent scanning for predators and predator deterrent behaviors) without limiting recuperation. Social animals have evolved pain-signalling strategies, such as vocalisation and facial expression, to elicit help from their in-group members. However, these pain experiences are only adaptive when the sender and receiver both benefit from the sender’s survival. Therefore, outward signals of pain in social animals are constrained by the risk of eavesdropping37, which can be appreciated, somewhat paradoxically, by observing injury-feigning behaviour in birds (“the broken wing trick”) that is highly effective at luring predators away from their chicks 38.
Overall, this ecology and evolutionary perspective illustrates the clear functional distinction in the roles of acute phasic vs. persistent pain, and promotes the idea that persistent pain may be as important in preserving the survival of injured animals as is acute phasic pain. Because the healing phase may vary in different species and vary from different types of injuries, this perspective also provides a framework for addressing the important question of whether our current clinical classification on acute vs. chronic pain fails to consider that persistent pain (which is typically considered pathological) can be a normal, adaptive and vitally important response to injury that may be a necessary aspect of experience-dependent behavioural optimisation39. In other words, persistent pain arises as part of normal functioning of the pain system optimised over the course of evolution by natural selection, and is not necessarily an inherent pathological state. However, understanding the mechanism of persistent pain may help us comprehend how it may extend to be a pathological state in certain circumstances.
Formalising models of persistent pain
The temporal sequence of events transitioning from acute phasic nociception (associated with active defensive and rapid learning) to a tonic persistent injury state (associated with recuperative and protective behaviours) illustrates the fundamental ecological necessity of the pain system. The importance of this notion was highlighted by Wall40, developed in the ‘perceptual-defensive-recuperative’ (PDR) model41, and later adopted in the ‘adaptive hypothesis’ for injury-related behaviour42. In terms of adaptive control, evolutionary assumptions are key to constraining the space of solutions that brains adopt to achieve pain-associated behavioural functions43,44. Within this approach lies two conceptual dichotomies. The first is between normativity (the objective function of the behaviour or why animals behave in a certain way) and statistical optimality (how information is processed in a mathematically ideal way). The second is between computational and algorithmic levels of analysis, which distinguishes what problem the brain solves, from how it solves it45. By its very nature, acute phasic pain appeals to a notion of adaptive control, where the control signal is aimed to optimize a cost-to-go function. Once one assumes that nociceptive afferents deliver an at least partially reliable signal of impending tissue damage, then the brain can use this as a cost function, both in terms of driving immediate defensive responses and by shaping future responses through learning. In this context, evolution tunes both the nociceptive signal and the learning system 46,47. This has been central to contemporary models of phasic pain processing, in which pain feeds into a control loop that comprises three core processes: stimulus or saliency detection, action selection, and action execution (FIG. 1a)2. This is operated in a hierarchy, interleaved with value learning and motor learning in the sensory-motor loop, supervised by top-down meta-learning that regulates the control process.
FIGURE 1. A control perspective for injury and pain.
a) Schematic diagram of hierarchical control loop for nociception, action selection and action execution, and metacontrol. At the heart of this are two control loops: one for action selection, and one for action control. Sitting above this is a meta-control loop which monitors and modulates the ‘lower’ control loops. This illustrates the computational difference between pain and nociception: with nociception reflecting the sensory signal communicating afferent information, and pain representing the control signal that governs learning and response execution2.
b) A general flowchart of closed-loop control for inferring the latent injured or pain state . The system receives bottom-up input , subject to an endogenous control input u(t), and produces a behavioral output. . The behavioral output also produces feedback (such as an efference copy of action) to influence the bottom-up or top-down processes. The controllability of the plant (dashed box) induces two different outcomes: high level of controllability leads to adaptive emotional and behavioral responses, followed by pain recovery, whereas low perceived controllability leads to maladaptive emotional and behavioral responses which causes chronic pain.
c) An implementation view of Bayesian inference through a feedforward architecture, where neural firing rates representing belief distributions are encoded independently and summed toward an output. The generative model that produces a Bayesian prediction estimate from multisensory inputs through a nonlinear mapping can be implemented by a recurrent neural network of excitatory and inhibitory neurons98-100.
A control systems approach to persistent pain and post-injury behaviour
To understand the core computations in a hierarchical control process as outlined in FIG. 1a, we can formulate persistent pain as an optimal control problem (BOX 2). Put simply, control theory aims to develop control strategies and apply control signals to achieve system optimality and stability. In physiology, for instance, the autonomic nervous system uses simple feedforward control to regulate heart rate and blood pressure, whereas the homeostatic control system may use feedback control to regulate body temperature. Let us consider a generic control diagram in the context for injury-state inference and action selection (FIG. 1b). The plant (dashed box) describes a continuous inference of the state of injury or recovery, which can be treated as a latent state of the system. The system receives sensory and physiological observations, , makes inference on the latent state, and uses feedback to minimise prediction errors. The endogenous input is operated on the plant to achieve the “optimal control” of the perceived injury or pain, with the control process happening at multiple levels in the body and the brain. Meta-control, operated as a hierarchy to regulate the control process (FIG. 1a), can bias the control signal. One example of meta-control is the homeostatic priorities that finds a balance between exploration and risk-seeking behaviors. Central to the control system, the plant produces an action or behavioural output , which may send an internal efference copy to predict, enhance or suppress the system nociceptive, autonomic, and endocrine signals in the feedback. A good example of this process has been demonstrated in a human self-reinforcing pain expectation experiment48, showing that a subject’s behavioural output (i.e., pain rating ) may be used to enhance the expected pain state . The discrepancy between the predicted and actual sensory feedback can be used as an indirect measure of “perceived controllability”, which is also related to the notion of ‘surprisal’ in active inference49. This perceived controllability may influence the control process (through meta-control or modifying the cost-rate function). Together, optimising and inferring based on a control approach provides a computational module for the hierarchical learning described in FIG. 1a.
BOX 2. Control theory and inference.
Control theory deals with the control of dynamical systems, and aims to develop a model governing the application of system inputs to drive the system to a desired state (setpoint), while achieving control stability and optimality. One simple dynamical system is a continuous-time linear time-invariant (LTI) system for a latent state variable and observed variable .
where the eigenvalues of matrix determine the response rates of associated modes as they are excited by the control signal ; , and are weighting matrices. Without loss of generality, is assumed to be normalized around the desired state . The LTI system is controllable if and only if it can be driven from any initial state to any desired final state in finite time168. The setpoint can be either constant or time-varying. There is a duality between control and estimation169,170, and uncontrollability also implies unpredictability.
A control strategy can be categorized as feedforward and feedback control. While feedforward control “ballistic control”) is simple, fast and energy efficient, feedback control can handle uncertainties of the system and change control signals via error feedback. Feedforward and feedback control can be combined to optimize performance. A feedforward control example is the autonomic nervous system that regulates involuntary physiological processes such as heart rate, blood pressure and respiration, whereas the homeostatic control system is a good example for negative feedback control.
Optimal control theory is naturally linked to reinforcement learning (RL): the control emphasizes cost minimization, whereas RL emphasizes reward maximization. In this setup, let denote the observed state, denotes the action to be optimized, and denotes a cost-rate function of state-action pair . The goal of optimal control is to find the policy for taking a dynamical system from one state to another in the presence of some constraints:
where describes a known deterministic or stochastic dynamical system, and are fixed, and is a constant penalizing the total duration in computation. The cost-rate function can be modified to accommodate additional internal or external cost. For instance, the original cost function may reduce risk-aversion behaviors and lead to a sampling bias, so a penalizing term may help promote exploration or risk-seeking action. Another possibility is to find a balance between minimising short-term cost and maximizing long-term reward. The dynamical system, if stochastic, can be represented by a probabilistic graphical model; the search for the optimal policy is equivalent to an inference problem171. While control answers the question “which action leads to the optimum future”, inference answers the question “which action is taken given the future is optimal”. In a special case where is a hidden discrete variable and is an observed discrete variable, the inference or planning is given by a Welch-Baum algorithm for the hidden Markov model172. Generalizations of optimal control have been discussed elsewhere173-175.
This control perspective of injury and persistent pain leads to a series of central questions: i) How does the brain recognise and represent the state of injury; ii) What action or modulatory processes reflect the effector arm of such a control system; and iii) How the outcome of this control is sensed and used to close the control loop. Despite growing effort of characterizing complex pain-related behaviours50,51, relatively few experimental studies have explicitly probed physiological injury behaviours. Because of the control-inference duality, the control-theoretic framework can shed light on understanding persistent pain and physiological injury behaviour.
Injury state representation.
The brain has multiple modes of information relevant to the nature and extent of an injury that can be used to optimise injury inference. In addition to tonic nociceptor firing, patterns of non-nociceptor somatosensory afferent (e.g., haptic and mechanical sensation), autonomic afferents, immune signals, as well as other exteroceptive sensory information (e.g., seeing blood) convey discriminative information. Injury can be also inferred from changes in sensory feedback from actions and motor commands which may not be clear at rest, for example touch and probing behaviours to identify the presence or absence of peripheral sensitization, which occurs at least in part through brain-independent processes. It can also include sensing mechanical disability (e.g., joint instability) from exploratory actions to identify abnormalities in non-nociceptive feedback. Finally, other physiological signals, such as information arising from circulating inflammatory mediators and endocrine signals inform the brain about probable tissue insult52,53. In a Bayesian framework, therefore, an internal representation of self-injury should integrate and rationalise these multiple sources of information to infer the site, nature and severity of an injury, alongside prior information from existing beliefs.
This multimodal inference process expands the domain of inputs that are conventionally considered to have a core role in signalling injury beyond tonic nociceptor firing. This doesn’t necessarily imply that tonic nociceptor firing is not required for perceiving injury, but it does imply that it may be insufficient in the absence of other concordant sensory information. In the proposed framework, these individual afferent sensory pathways sit beneath injury state representations in an inference hierarchy, so are inherently interpreted in the context of this higher-level representation. That is, a higher-level belief that the body is injured acts as a prior on the processing of the lower-level modalities. Such inferential hierarchies provide a mechanism for understanding various multisensory integration effects in pain perception54,55.
Control output.
The suite of effector mechanisms spans three distinct domains. First, widespread neuromodulatory influences (for example, through dopaminergic and serotonergic signalling) across a broad range of motivated behaviours can implement the adaptive changes required given the inferred state of injury56. Current normative computational models of reward and motivation allow a clear prediction of how recuperative behaviour can be achieved given changes in homeostatic value function57. Specifically, this may involve modulation of reward learning in multiple ways: reducing reward sensitivity, increasing relief-seeking, reducing exploration and novelty seeking for non-injury relevant outcomes, increasing effort costs, and changing risk and ambiguity preferences in line with changed homeostatic priorities58. Additionally, explicitly protective behaviours can be facilitated through increased punishment sensitivity and increased punishment generalisation, as well as by enhancing innate defensive responses. Many of the global, non-regional responses are also seen in the context of inflammatory illness behaviours, which are also associated with fatigue, lowered mood, and increased anxiety, and now typically considered as adaptive responses in this context59-61.
Second, endogenous modulation can tune pain sensitivity. In the acute phase, stress-related analgesia occurs, and reflects the reduction in pain that occurs in the context of an acute stressor or danger, and is assumed to facilitate evasive action by suppressing the potentially intrusive interference of pain responses62,63. Stress-induced analgesia is widespread among diverse animal clades with differing levels of neural complexity, suggesting deep evolutionary conservation 64,65 and strong ongoing selection pressure66,67 on the behavioral outputs of stress-induced analgesia; most commonly, suppression of pain behavior in the context of acute predatory threat. However, once this has subsided, peripheral and central sensitization directly increase pain responsivity in the injury site, by increasing the sensitivity to pain (hyperalgesia) and expanding the type of stimuli that cause pain to non-nociceptors (allodynia) 68. In mammals, this further drives protective action to specific injured body parts, which can elicit hyper-protective responses to further injury or increased hypersensitivity to phasic pain 69, and can change the sensory feedback to induce the reorganisation of a sensorimotor map 70. This is associated with neural plasticity and supraspinal reorganisation71,72. For instance, the state of central sensitization leads to structured change in functional connectivity among dorsal horn neuronal populations73. In contrast, post-injury recovery is accompanied by supraspinal control of stepping and reorganisation of descending and propriospinal connections74.
Third, injury states can direct the physiological responses appropriate to the specific nature of the injury, including autonomic, endocrine, and immune responses. These incorporate general stress responses, and may also include coordination of an injury-specific physiological response to regulate local tissue homeostasis75.
Closed-loop control.
A central part of control is to infer whether the injury has resolved, as opposed to persisted or worsened. However, inference may become difficult because behaviours that are associated with sensitising pain and avoiding further damage paradoxically restrict or degrade the information required to continually sense injury. For example, avoidant behaviours are negatively correlated with the information acquisition about what is being avoided - for instance continuing to not move an injured limb makes it difficult to know whether moving it still hurts. This is a form of exploration-exploitation dilemma: exploration involves accepting short-term risk of exacerbating one's pain in order to get information that allows one to better establish the current state or resolution of the injury; once this is determined, the information can be exploited, such as by returning to normality if the injury has been resolved76. Also, centrally mediated hyperalgesia and allodynia inherently amplify the signal that led to them in the first place; this can potentially obscure the presumed magnitude of the afferent nociceptive injury signal, making it difficult to know whether or not it is still required. In a control-theoretic account, to infer the state of peripheral injury, the brain needs to account for both top-down modulatory effects and bottom-up sensory information, as well as efference copy of the associated actions and its induced responses. This efference copy is incorporated into the inference process, whilst also considering that some responses are controlled by peripheral mechanisms, and some afferent signals may habituate over time. If the system operates optimally, then given any uncertainty in the posterior belief, the brain also needs to calculate the relative cost of incorrectly inferring injury resolution versus incorrectly inferring persistent injury. These costs also need to be calculated and incorporated into the controller. Hence in humans where recuperation costs may be relatively low, this will usually favour inferring persistent injury.
Meta-control.
Meta-control reflects a higher layer of control that regulates the control process 77,78. First, it may monitor and regulate control (FIG. 1a), and use the achieved controllability to optimise meta-parameters of control to improve performance. The perceived controllability may affect the meta-control, such as by modifying the cost-rate function to include an additional penalizing term. One example of meta-control is the homeostatic priorities that seeks balance between exploration and risk-seeking behaviours79. Second, it may also modify parameters according to factors outside of the control system, for instance threats of a different nature that might have some relevance, or other factors of homeostasis (for instance, sleep or energy homeostasis). Third, it may modulate the value function implicit within the control system: for example, the presence of conspecifics might increase the relative value of recuperation, given the safety they confer. This higher-level of meta-control may also relate to subjective perceptions of controllability with direct clinical relevance80.
Neural implementation
Hierarchical inference.
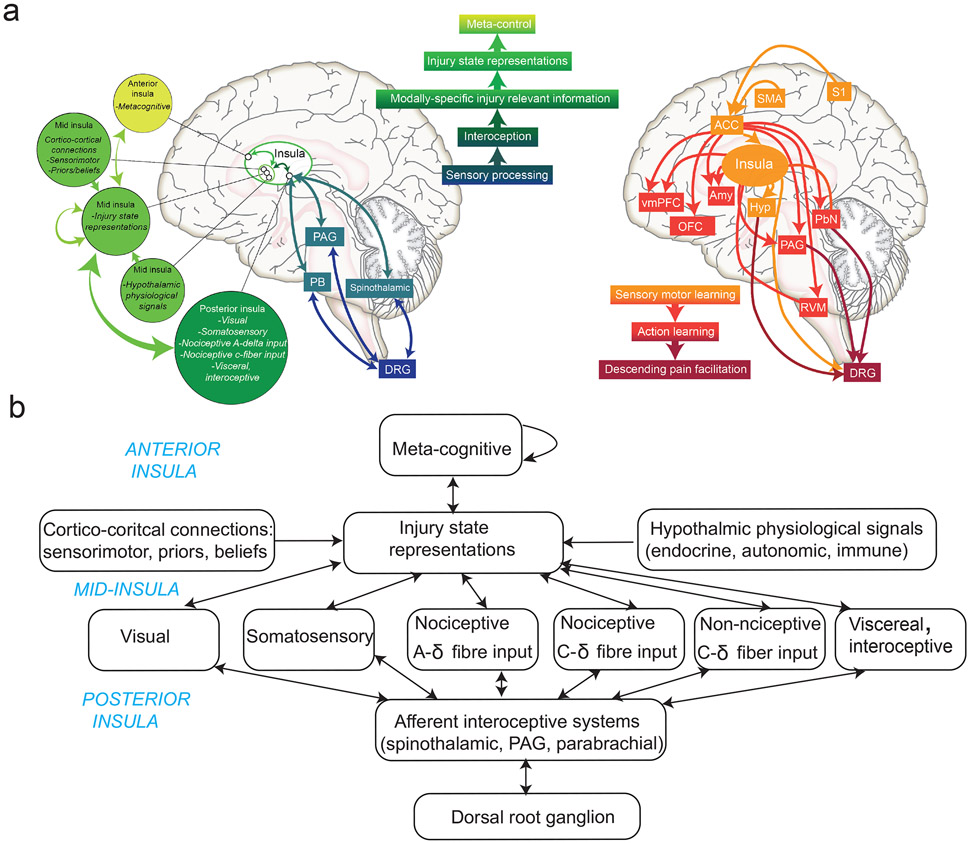
In terms of functional anatomy, injury inference appeals to regions that receive multisensory afferents with the capability to integrate these signals to compute the likelihood of peripheral injury. The insula cortex is well positioned in the brain for its anatomical and functional connectivity to other areas along the afferent and efferent pain pathways (FIG. 2a,b), suggesting its active role in injury state inference similar to that proposed in other interoceptive and visceral states81-83. Specifically, the insula receives afferent signals from the range of interoceptive and exteroceptive sensory inputs that carry injury-relevant information84,85. This includes signals related to nociception, autonomic change, intercoceptive C-fibre input (thermal change, pleasant touch, and itch)86, images of bodily injury87,88, immune activation89, and sensorimotor incongruence 90. The insula also receives direct connections from the medial and lateral thalamic nuclei, brainstem autonomic sites (including vagal afferents), parabrachial and amygdala nuclei, and hypothalamus91. It can therefore integrate the convergent bodily information required for inference of injury, alongside other physiological states92. This also fits with the structural anatomy of the insula, which involves multiple topographic representations through dorsal granular regions (often viewed as a primary interoceptive cortex), projecting to distinct dysgranular ‘stripes’ and to anterior agranular regions that are associated with higher-level functions93,94. Nevertheless, other brain regions are also capable of supporting higher-level multimodal inference. For instance, the ventromedial prefrontal cortex (VMPFC) has been proposed for self-referential models of physical health95, and the anterior cingulate cortex (ACC) also has a core integrative role that spans interoceptive, exteroceptive and motor domains in the context of pain96,97. Therefore, it is not impossible that these regions coordinate together to achieve integrative inference across a distributed pain network.
FIGURE 2. Neural implementation and representations of injury and persistent pain.
a) Information flows and afferent (left) and efferent (right) pathways for insula-centered injury-state inference and effector control. At the heart of this is an insula-centered hierarchy with successively higher latent abstractions of the injury state. Afferent pathways feed various inputs to the hub, from subcortical and cortico-cortical projections; and efferent routes can implement different types of responses. This includes the multiple afferent pathways that ascend the spinal cord to various brainstem nuclei, such as the parabrachial, periaqueductal gray (PAG), dorsal respiratory group (DRG), locus coeruleus (LC) and others, forming the bidirectional brainstem-subcortical network176,177.
b) Schematic illustration of an insula-hub perspective for injury state representations in more details, including the different types of sensory information important for inference. The anterior, mid, and posterior segments of the insula have distinct and complementary functional roles. Note that the injury state inference may be shared with broader cortical areas, including the ACC and VMPFC, which are omitted here.
Second, a key part of injury inference relates to the recognition of new phasic (acute) pain, especially as a result of motor movement or external events. This appeals to exteroceptive inference in the sensorimotor control loop – in which the occurrence or magnitude of pain exceeds that is normally expected as a result of a particular stimulus or movement. This, alongside other exteroceptive information that comes from vision, plus higher-level information in the form of explicit and semantic knowledge, also needs to be integrated with interoceptive information to conduct inference. Whilst the locus of higher-level injury representation is still unclear, we speculate that it is possible that it is encoded either in a localized region such as the anterior or mid-insula, or instead have a more distributed representation across the insula and ACC, as well as possibly including conceptual representations in regions such as the VMPFC, hippocampus, and linked with lower-level physiological representations in the hypothalamus. Such higher-order representations also relate to the concept of meta-control, which has not been well studied, but also thought to involve higher-order representations in the anterior insula, ACC, as well as ventromedial and dorsal prefrontal regions that are often associated with contingency knowledge, model-based decision-making, and subjective awareness95.
More mechanistically, injury-state level probabilistic inference sits above lower-level inference in individual modalities, and can be implemented through local recurrent neural circuits (FIG. 1c)98– 100 and spike-timing synaptic plasticity101-103. Algorithmic implementation of inference in the brain has been broadly discussed100,104. This view also parallels neural architectures suggested to implement predictive coding within cortical layers105 or between different cortical areas106.
Efferent control.
The multiplex nature of efferent control appeals to a broad network of regions implementing specific components of control. First, modulation of dorsal horn nociceptive neurons can be mediated by multiple opioidergic and monoaminergic descending pathways from the PAG and hypothalamus. These can implement descending facilitation, but also stress-related hypoalgesia when behaviourally required. These sites are likely controlled by a coordinated network, that includes the rostral anterior cingulate cortex (rACC), mid anterior cingulate cortex (mACC), VMPFC, and insula91,107.
Next, modulation of physiological function, including regulation of sleep, appetite, arousal, endocrine and immunological tone is likely mediated by hypothalamic pathways108-110. For instance, hypothalamic dynorphin/KOR signalling modulates arousal, vigilance and sleep architecture in a mouse model of neuropathic injury111. And other hypothalamic neuropeptide systems such orexin are involved in co-regulation of pain and sleep110,112. Parabrachial-lateral hypothalamic circuits mediate bidirectional effects of appetite and pain108,109, including appetite suppression during chronic pain113. Additionally, insula-hypothalamic circuits are implicated in integration and synchronisation of endocrine and immune activation during a range of stressors, including pain114.
The modulation of motivational behaviour during chronic pain is closely associated with midbrain, frontostriatal and amygdala-centred circuits. These behaviours include seeking relief, revaluing, and learning changing reward priorities, and similarly enhancing punishment sensitivity, and risk preferences, which may have neuromodulatory effects on motivational and value learning systems (including dopamine and serotonin neurotransmitters). For instance, tonic pain modulation of Pavlovian and instrumental reward and punishment valuation is apparent through the amygdala, cingulate and VMPFC responses in humans115,116, and mesolimbic circuits are directly implicated in rodent models58. These circuits have also been involved in pathological chronification of pain in human patients117. More broadly, modulation of mesolimbic monoaminergic neuromodulatory pathways may also have the capacity to enhance learning and hence cortical reorganization across widespread regions68.
Oscillatory Neurodynamics.
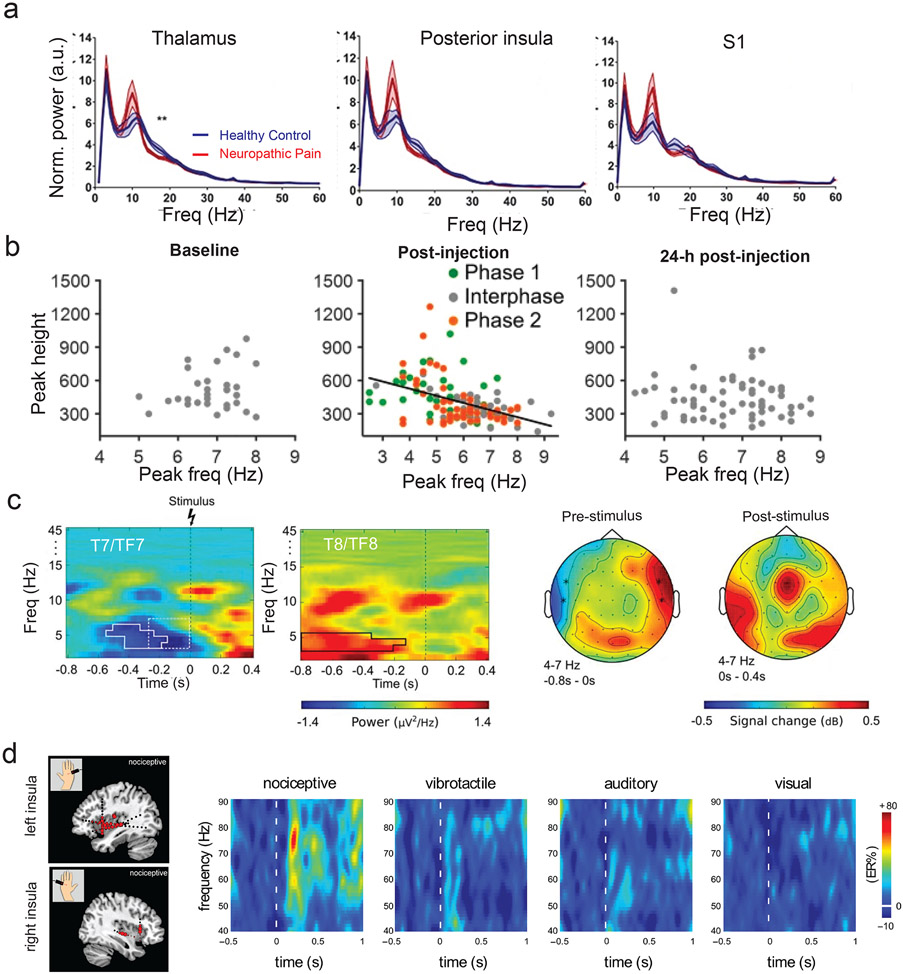
The afferent and efferent pain pathways (FIG. 2a,b) fit with a proposed role as components of an injury-based control plant. One possible idea is that they may be synchronized by neural oscillations recruited locally and propagated between long-range neural circuits118. Traveling waves may provide a possible mechanism for integrating and transferring information across cortical and subcortical areas at different frequencies119. Theta (4-9 Hz) rhythms are also known to be associated with the saliency, arousal, and alert states in the cortex and hippocampus120,121. Human and animal studies have reported abnormal or augmented 8-10 Hz oscillations in both pain and injury states across many pain-associated areas (FIG. 3a), including the insula, somatosensory, anterior cingulate, prefrontal, posterior parietal cortices122-126 , and sensory thalamus127. The dynamics of somatosensory cortical theta oscillations may also represent the status of post-injury or pain state128 (FIG. 3b). In healthy subjects, the pre-stimulus theta oscillations in the insula cortex modulate pain perception129 (FIG. 3c), whereas in chronic pain patients, post-stimulus theta oscillations are related to pain processing123,130. Time-dynamic pulse modulation of spinal cord simulation (SCS) in chronic pain-treated rats has shown reduced EEG theta power and spontaneous pain131. Further, modulation of theta power originated in the insula by a brain-computer interface (BCI) has shown that up-training theta activity leads to increased pain discrimination, whereas down-training theta activity has an opposite effect132. Recent human intracranial EEG recordings also showed that theta and beta oscillations are organized in the form of traveling waves along the anterior-posterior axis of the insula, suggesting frequency multiplexing used in insular information processing133. Put together, theta oscillations are a plausible mechanism by which an insula-centred hub coordinates activity with a broader persistent pain network. In addition to theta oscillations, it is not impossible that propagating waves at other oscillatory frequencies, such as alpha (9-13 Hz), beta (13-30 Hz) and gamma (30-50 Hz) bands, carry complementary information across various degrees of injury-to-pain states134-137 (FIG. 3d). Typically, higher frequency oscillations are confined to a small neuronal space or involve bottom-up processing, whereas very large networks are recruited during slow oscillations118. Our prediction is that low-frequency oscillations or traveling waves can efficiently transfer and mediate a large distributed persistent pain network.
FIGURE 3. Brain oscillations that encode post-injury or pain state.
a) Neuropathic patients showed augmented MEG theta/alpha power (8-10 Hz) in multiple brain regions (thalamus, posterior insula, and primary somatosensory cortex or S1) of the ascending nociceptive pathway compared to age-matched healthy control (adapted from REF125, Wolter Kuwer Health, Inc).
b) Noxious stimuli generated a reversible shift in the S1 LFP theta-peak frequency from a formalin-induced mouse model of pain. The scatter plots illustrate the association between theta-peak frequency and theta-peak height, where each dot represents a sample computed from a 2-min moving temporal window. This shift in theta-peak frequency was observed during nociceptive phases but not during the baseline or recovery period and was inversely correlated with instantaneous pain intensity. This result suggest that dynamics of theta oscillations may represent the ongoing status of injury or pain state (adapted from REF128, CC-BY-4.0).
c) Changes in pre-stimulus theta activity in the insula modulate human pain perception. Left: From EEG recordings of healthy subjects, time-frequency representations showed the difference between pain and non-pain trials averaged across two temporal electrodes (T7/TF7 vs T8/TF8). Solid outline indicates the significant pre-stimulus effect identified by permutation testing. EEG source localization revealed two generators at the bilateral insula cortex for the scalp theta power differences, especially at the contralateral insula site. Right: Comparison of brain topographies in relative power differences (dB) between pain and non-pain trials during the pre- and post-stimulus periods (adapted from REF129, Society for Neuroscience).
d) LFP gamma oscillations (40-90 Hz) are enhanced in response to phasic pain. Left: Magnitude of gamma-band oscillations elicited by nociceptive stimuli in the contralateral left or right insula from insular LFP recordings of epileptic patients. The size of circles represents the magnitude of post-stimulus change in the gamma band magnitude (150-300 ms). Right: Comparison of time-frequency representation of the changes in oscillatory power (40-90 Hz) elicited by nociceptive, vibrotactile, auditory and visual stimuli at the insula (adapted from REF134, Oxford University Press).
Although the exact nature of cortical control of autonomic, endocrine and immune functions remains less well understood, the insula-cingulate network may have a core central modulatory role in detecting saliency, regulating brain network transition and facilitating rapid access to the motor system138-140. For instance, sympathetic and parasympathetic effects are evident from stimulations of posterior-to-anterior insular regions (respectively) in epilepsy patients141, possibly through interactions with cingulate, amygdala and ventromedial hypothalamus142.
Chronic pain transition: an information restriction model
There are multiple models and theories proposed to understand chronic pain, and these arise from different approaches founded on biological and psychosocial risk factors, neural plasticity and learning, and molecular and circuit-level dysfunction143-145. Drawing on insights from these, the computational approach espoused here leads to a novel perspective in which chronic pain might sometimes arise from an internal state wherein injury is incorrectly inferred (in contrast to instances whereby there are persistent peripheral drivers of ongoing pain, and in which injury is correctly inferred). Consequently, this internal state drives subjective persistent pain and its associated behaviours including hypersensitivity to pain, fatigue, low mood, sleep disturbance, and anxiety. This class of behaviours may be adaptive in the context of ongoing physiological injury, but can be considered maladaptive in the context of chronic pain. From a control perspective, maladaptivity may also relate to low perceived controllability.
The control-as-inference framework presented here illustrates a key route to chronification via information restriction (FIG. 4a). At the heart of the model is the assumption that pain in some instances of chronic pain exceeds the amount that would be predicted on the basis of the peripheral tissue injury and nociceptive input, a situation that could arise if an injury has resolved or reduced without the central brain control system (‘control plant’) recognizing this. This will occur if the brain has incomplete or inaccurate information about the peripheral status of the injury, and the model illustrates several specific reasons why this might occur (FIG. 4b).
FIGURE 4. Information restriction model of chronic pain.
a) Illustration of pain through a timeline from injury through to recovery. The solid line reflects the normal, adaptive profile of pain; whereas the dashed line reflects the transition to chronic pain, mediated (at least partially, since there are other factors involved in chronic pain) by various mechanisms of information restriction.
b) Illustration of the four key mechanisms that seed information restriction, which effectively reduces the ability of the brain to recognize that an injury has resolved. This results in an internal model of a peripheral injury that is persistent, and which also continues to drive the physiological and behavioural responses appropriate to a state of true injury. We frame these mechanisms as ‘maladaptive’ to emphasize that the nature of the mechanism, when ‘ill-tuned’, leads to a sub-optimal outcome.
Maladaptive learning.
First, increased avoidance responses will reduce the access to information about whether particular actions and movements continue to still cause increased pain. For instance, an injury might have been initially inferred from acute pain occurring when walking on a damaged joint, but avoiding walking means that the individual cannot access information about whether the acute pain is lessened or absent. Second, this problem will be exaggerated by the parallel motivational factors which change the experienced environment, including altered reward learning (reduced exposure to rewards and novelty), and increased punishment sensitivity and aversive generalization (negative exposure). This change in valuation makes the outcomes of situations and actions seem worse than they previously were. This type of information restriction has strong parallels with the classical fear avoidance and learning model144,146-149, the maladaptive learning model150-153, and psychosocial models of chronic pain154.
Maladaptive model.
The Bayesian control-theoretic approach assumes that the brain uses a generative model for the control processes, both central and peripheral, to modulate the incoming input. That is, descending facilitation, central sensitization, and peripheral sensitization increase incoming pain signals, and therefore amplify the acute pain signals that helped to infer injury in the first place. To avoid a self-potentiating loop, the Bayesian model implies that the control plant has an efference copy of these processes so they can be considered. But many of these facilitatory processes are complex and not directly controlled by the brain, and any modulation of the incoming signal has the capacity to add noise, and hence increase uncertainty155-157. Consequently, increased uncertainty may inherently bias inferring a state of persistence injury, because the relative cost of under-estimating injury is higher than that of over-estimating it. This mechanism draws on existing chronic pain models of spinal-brain interactions143, and active inference158,159.
Maladaptive integration.
Lesions of the sensory system, spinothalamic system, autonomic system, nerve lesions, or amputation can lead to an obvious information loss. Because these lesions are likely to represent abnormalities that have not been encountered sufficiently often through evolution, the brain will not have an adaptive mechanism for factoring them in to internal models of the injury state, and so they will lead to persistent incongruent multisensory integration of information required to infer the absence or resolution of injury160. This persistent incongruent state is strongly related existing models of sensorimotor reorganisation as a factor contributing to chronic pain68.
Maladaptive priors.
Finally, incorrect or pessimistic beliefs and expectancies of persistent injury or reduced subjective controllability, including from other cognitive sources, will impact on higher-level meta-control and maintain injury representations156. This is because strongly held beliefs, in a Bayesian context, will downplay the importance of incoming afferent information, minimizing the degree of information restriction. This idea draws on models of chronic pain that highlight expectancy effects and the concept of chronic pain as a ‘self-fulfilling prophecy’ leading to persistence48,161.
In summary, a control-theoretic model of physiological pain provides multiple routes to aberrant pain persistence, in which information restriction interferes with inference of return to health, leading to perpetuation of both pain and its accompanying suite of pain-related behaviours. Importantly, these predictions are experimentally testable, and indeed there is already experimental evidence that speaks to many components of them. Importantly, a key feature of this conceptualization of chronic pain is that it highlights the potential of information-rich therapeutic strategies, such as cognitive and action-based approaches (including cognitive behavioural therapy and physiotherapy), and neurotechnologies designed to manipulate and augment information input to the brain (such as neurostimulation, neurofeedback and virtual reality).
Conclusion
Injury has been a prevalent problem throughout evolution, and the brain is likely to have evolved to develop optimized survival strategies. In this Perspective, we have set out how this process can be formalized in information processing terms, with a particular focus on optimal control and inference of injury states. The dominant behavioural phenomenon associated with injury is pain, and our proposed framework distinguishes the acute protective and defensive role of phasic pain, with the recuperative role of tonic injury state associated with persistent pain. in a hierarchical model of injury, processing of afferent pain information sits beneath a higher latent injury representation, which exploits cross-modal interactions. Using this information, the brain can then direct control behaviours appropriate to injury, implemented within neural hierarchies across a network of hypothalamic, cortical and subcortical hubs. Importantly, the control-theoretic view illustrates a key vulnerability: because the control behaviours can make it harder to tell whether the injury has recovered – namely, they inherently restrict new information, there may be a susceptibility to incorrectly inferring persistence of injury, manifest clinically as transition to chronic pain.
Supplementary Material
SUPPLEMENTAL FIGURE. Overview of injury-related behaviours.
a) A core classification of the different types of response to an injury, spanning recuperative, protective and physiological responses.
b) The spectrum of ecologically significant causes of injury that can occur, summarised across species, with different causes having different importance across species.
c) Timeline of how pain responses change following an injurious event, from immediate defensive behaviours through to recuperation and recovery.
Acknowledgments
We thank Pranav Mahajan for valuable feedback and comments. The work was partially supported by the US National Science Foundation (CBET-1835000 to Z.S.C. and IOS-2047331 to R.J.C.), the US National Institutes of Health (NS121776 and DA056394 to Z.S.C.), and the Wellcome trust (214251), Versus Arthritis (21357, 21192), the Institute of Information & Communications Technology Planning & Evaluation (IITP) (2019-0-01371) to B.S.
References
- 1.Bateson P. Assessment of pain in animals. Animal Behavior 42, 827–839 (1991). [Google Scholar]
- 2.Seymour B. Pain: a precision signal for reinforcement learning and control. Neuron, 101, 1029–1041 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ Central sensitization: implications for the diagnosis and treatment of pain. Pain, 152, S2–S15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melzack R. (1999). Pain–an overview. Acta Anaesthesiologica Scandinavica, 43, 880–884 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Broom DM The Evolution of Pain. (Cambridge Univ. Press, 2001). [Google Scholar]
- 6.Sneddon LU. Evolution of nociception and pain: evidence from fish models. Phil. Trans. R. Soc. B 374, 20190290 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonavita V & De Simone R Pain as an evolutionary necessity. Neurological Sciences, 32, 61–66 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Wu YC, Franzenburg S, Ribes M, & Pita L. Wounding response in porifera (sponges) activates ancestral signaling cascades involved in animal healing, regeneration, and cancer. Sci. Rep 12, 1307 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng G, Shi X & Kadowaki T Evolution of TRP channels inferred by their classification in diverse animal species. Molecular Phylogenetics and Evolution, 84, 145–157 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Himmel NJ and Cox DN Transient receptor potential channels: current perspectives on evolution, structure, function and nomenclature. Proceedings of the Royal Society B, 287, 20201309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL & Garrity PA Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature, 464, 597–600 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anishkin A & Kung C Microbial mechanosensation. Current Opinion in Neurobiology, 15, 397–405 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y, Idikuda V, Chowdhury S & Chanda B Activation of the archaeal ion channel MthK is exquisitely regulated by temperature. Elife, 9, e59055 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capasso L, Ganot P, Planas-Bielsa V, Tambutté S & Zoccola D Intracellular pH regulation: characterization and functional investigation of H+ transporters in Stylophora pistillata. BMC Molecular and Cell Biology, 22, 1–19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arias-Darraz L, Cabezas D, Colenso CK, Algegria-Arcos M, et al. A transient receptor potential ion channel in Chlamydomonas shares key features with sensory transduction-associated TRP channels in mammals. Plant Cell. 27, 177–188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer JJ & Byers J. As good as dead? Sublethal predation facilitates lethal predation on an intertidal clam. Ecology Letters 8, 160–166 (2005). [Google Scholar]
- 17.Wilbur HM & Semlitsch RD. Ecological consequences of tail injury in Rana tadpoles. Copeia, 1990(1), 18–24 (1990). [Google Scholar]
- 18.Bertilsson-Friedman P. Distribution and frequencies of shark-inflicted injuries to the endangered Hawaiian monk seal (Monachus schauinslandi). J. Zoology 268, 361–368 (2006). [Google Scholar]
- 19.Mukherjee S & Heithaus MR. Dangerous prey and daring predators: a review. Biol. Rev. Camb. Philos. Soc 88, 550–563 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Walters ET & Moroz LL. Molluscan memory of injury: evolutionary insights into chronic pain and neurological disorders. Brain Behav. Evol 74, 206–218 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters ET. Chronic pain, memory, and injury: Evolutionary clues from snail and rat nociceptors. Int J Comp Psychol, 22,127–140 (2009). [Google Scholar]
- 22.Wittenberg N & Baumeister R Thermal avoidance in Caenorhabditis elegans: An approach to the study of nociception. Proc. Natl. Acad. Sci. USA 96, 10477–10482 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackintosh NJ (Ed.). Animal Learning and Cognition. (Academic Press, 2013). [Google Scholar]
- 24.Kim H, Kim K & Yim J. Biosynthesis of drosopterins, the red eye pigments of Drosophila melanogaster. IURMB Life, 65, 334–340 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Bolles RC Species-specific defense reactions and avoidance learning. Psychological Review, 77, 32 (1970). [Google Scholar]
- 26.Gillan CM, Urcelay GP, & Robbins TW An associative account of avoidance. In Murphy RA & Honey RC (Eds.), The Wiley Handbook on the Cognitive Neuroscience of Learning (pp. 442–467). (Wiley-Blackwell, 2016). [Google Scholar]
- 27.Walters ET & Williams ACDC Evolution of mechanisms and behaviour important for pain. Phil. Trans. R. Soc. B 374, 20190275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lister KC, Bouchard SM, Markova T, Aternali A, et al. Chronic pain produces hypervigilance to predator odor in mice. Current Biology, 30, R866–R867 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Morgan MM & Ataras K. Morphine restores and naloxone-precipitated withdrawal depresses wheel running in rats with hindpaw inflammation. Pharmacology Biochemistry and Behavior 209, 173251 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G, Kim YH, Li H, et al. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat. Neurosci 20, 917–926 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walters ET. Nociceptors as chronic drivers of pain and hyperreflexia after spinal cord injury: an adaptive-maladaptive hyperfunctional state hypothesis. Front. Physiol 3, 309 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elwood RW. Discrimination between nociceptive reflexes and more complex responses consistent with pain in crustaceans. Philosophical Transactions of the Royal Society B, 374, 20190368 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alupay JS, Hadjisolomou SP, & Crook RJ Arm injury produces long-term behavioral and neural hypersensitivity in octopus. Neuroscience Letters, 558, 137–142 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Crook RJ. Behavioral and neurophysiological evidence suggests affective pain experience in octopus. iScience, 24, 102229 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birch J, Burn C, Schnell A, Browning H & Crump A Review of the evidence of sentience in Cephalopod molluscs and decapod crustaceans. The London School of Economics and Political Science 1–108 (2021). [Google Scholar]
- 36.McNamara JM & Buchanan KL. Stress, resource allocation, and mortality. Behavioral Ecology, 16, 1008–1018 (2005). [Google Scholar]
- 37.Kappesser J. The facial expression of pain in humans considered from a social perspective. Phil. Trans. R. Soc. B 374, 20190284 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ristau CA Aspects of the cognitive ethology of an injury-feigning bird, the piping plover. In Cognitive Ethology (pp. 111–146). (Psychology Press, 2013). [Google Scholar]
- 39.Santiago Vi. 2022. Painful Truth: The Need to Re-Center Chronic Pain on the Functional Role of Pain. Journal of Pain Research 15, 497–512 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wall PD On the relation of injury to pain. Pain, 6, 253–264 (1979). [DOI] [PubMed] [Google Scholar]
- 41.Bolles RC, & Fanselow MS A perceptual-defensive-recuperative model of fear and pain. Behavioral and Brain Sciences, 3, 291–323 (1980). [Google Scholar]
- 42.Walters ET Injury-related behavior and neuronal plasticity: an evolutionary perspective on sensitization, hyperalgesia, and analgesia. International Review of Neurobiology, 36, 325–427 (1994). [DOI] [PubMed] [Google Scholar]
- 43.Młynarski W, Hledík M, Sokolowski TR & Tkačik G Statistical analysis and optimality of neural systems. Neuron, 109, pp. 1227–1241 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Parker GA & Smith JM Optimality theory in evolutionary biology. Nature, 348, 27–33 (1990). [Google Scholar]
- 45.Marr D. Vision. (MIT Press, 1982). [Google Scholar]
- 46.Elfwing S, Uchibe E, Doya K, & Christensen HI Co-evolution of shaping rewards and meta-parameters in reinforcement learning. Adaptive Behavior, 16, 400–412 (2008). [Google Scholar]
- 47.Singh S, Lewis RL, Barto AG, & Sorg J Intrinsically motivated reinforcement learning: An evolutionary perspective. IEEE Trans. Autonomous Mental Development, 2, 70–82 (2010). [Google Scholar]
- 48.Jepma M, Koban L, van Doom J et al. Behavioural and neural evidence for self-reinforcing expectancy effects on pain. Nat Hum Behav 2, 838–855 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith R, Friston KJ, Whyte CJ. A step-by-step tutorial on active inference and its application to empirical data. J. Mathematical Psychology, 107, 102632 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuttle AH, Molinaro MJ, Jethwa JF, et al. A deep neural network to assess spontaneous pain from mouse facial expressions. Mol. Pain, 14, 1744806918763658 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones JM, Foster W, Twomey CR, Burdge J, Ahmed OM, Pereira TD, Wojick JA, Corder G, Plotkin JB, Abdus-Saboor I. A machine-vision approach for automated pain measurement at millisecond timescales. eLife 9, e57258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank MG, Fonken LK, Watkins LR, & Maier SF Microglia: Neuroimmune-sensors of stress. In Seminars in Cell & Developmental Biology (Vol. 94, pp. 176–185). (Academic Press, 2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grace PM, Hutchinson MR, Maier SF, & Watkins LR Pathological pain and the neuroimmune interface. Nature Reviews Immunology, 14, 217–231 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Senkowski D, Höfle M & Engel AK (2014). Crossmodal shaping of pain: a multisensory approach to nociception. Trends Cogn. Sci 18, 319–327 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Vastano R, Costantini M & Widerstrom-Noga E Maladaptive reorganization following SCI: The role of body representation and multisensory integration. Prog. Neurobiol 208, 102179 (2022). [DOI] [PubMed] [Google Scholar]
- 56.Grossman CD & Cohen JY. Neuromodulation and neurophysiology on the timescale of learning and decision-making. Annu. Rev. Neurosci 45, 317–337 (2022). [DOI] [PubMed] [Google Scholar]
- 57.Keramati M, & Gutkin B Homeostatic reinforcement learning for integrating reward collection and physiological stability. eLife, 3, e04811 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navratilova E, Porreca F Reward and motivation in pain and pain relief. Nat Neurosci 17, 1304–1312 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dantzer R, Heijnen CJ, Kavelaars A, Laye S, & Capuron L The neuroimmune basis of fatigue. Trends Neurosci. 37, 39–46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khandaker G, Harrison N, Bullmore E, & Dantzer R (Eds.). Textbook of Immunopsychiatry. (Cambridge Univ. Press, 2021). [Google Scholar]
- 61.Lopes PC, French SS, Woodhams DC, & Binning SA Sickness behaviors across vertebrate taxa: proximate and ultimate mechanisms. Journal of Experimental Biology, 224, jeb225847 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Butler RK, & Finn DP Stress-induced analgesia. Progress in Neurobiology, 88, 184–202 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Vachon-Presseau E. Effects of stress on the corticolimbic system: implications for chronic pain. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 87, 216–223 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Kavaliers M. Evidence for opioid and non-opioid forms of stress-induced analgesia in the snail, Cepaea nemoralis. Brain Research, 410, 111–115 (1987). [DOI] [PubMed] [Google Scholar]
- 65.Rodgers RJ, & Randall JI Defensive analgesia in rats and mice. The Psychological Record, 37, 335–347 (1987). [Google Scholar]
- 66.Marek P & Szacki J. Environmentally induced analgesia in wild mice: comparison with laboratory mice. Physiological Zoology 61, 330–332 (1988). [Google Scholar]
- 67.Saksida LM, Galea LAM, Kavaliers M. Predator-induced opioid and non-opioid mediated analgesia in young meadow voles: sex differences and developmental changes. Brain Research. 617, 214–219 (1993). [DOI] [PubMed] [Google Scholar]
- 68.Kuner R, & Flor H (2017). Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci, 18, 20–30. [DOI] [PubMed] [Google Scholar]
- 69.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiological Reviews, 89, 707–758 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Takeuchi Y, Osaki H, Yagasaki Y, Katayama Y, & Miyata M (2017). Afferent fiber remodeling in the somatosensory thalamus of mice as a neural basis of somatotopic reorganization in the brain and ectopic mechanical hypersensitivity after peripheral sensory nerve injury. eNeuro, 4, ENEURO.0345-16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jutzeler CR, Freund P, Huber E, Curt A, & Kramer JLK. Neuropathic pain and functional reorganization in the primary sensorimotor cortex after spinal cord injury. J. Pain, 16, 1256–1267 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Huynh V, Lütolf R, Rosner J, et al. Supraspinal nociceptive networks in neuropathic pain after spinal cord injury. Hum Brain Mapp. 42, 3733–3749 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Contreras-Hernández E, Chávez D, Hernández E, et al. Supraspinal modulation of neuronal synchronization by nociceptive stimulation induces an enduring reorganization of dorsal horn neuronal connectivity. J Physiol. 596, 1747–1776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Courtine G, Song B, Roy R et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med 14, 69–74 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martelli D, Yao ST, McKinley MJ, & McAllen RM Reflex control of inflammation by sympathetic nerves, not the vagus. Journal of Physiology, 592, 1677–1686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denrell J. (2007). Adaptive learning and risk taking. Psychological Review, 114, 177 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Wang JX Meta-learning in natural and artificial intelligence. Current Opinion in Behavioral Sciences, 38, 90–95 (2021). [Google Scholar]
- 78.Eppinger B, Goschke T, Musslick S. Meta-control: from psychology to computational neuroscience. Cogn. Affect. Behav. Neurosci 21, 447–452 (2021). [DOI] [PubMed] [Google Scholar]
- 79.Marković D, Goschke T, & Kiebel SJ Meta-control of the exploration-exploitation dilemma emerges from probabilistic inference over a hierarchy of time scales. Cognitive, Affective & Behavioral Neuroscience, 21, 509–533 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rudy TE, Kerns RD, & Turk DC (1988). Chronic pain and depression: toward a cognitive-behavioral mediation model. Pain, 35, 129–140. [DOI] [PubMed] [Google Scholar]
- 81.Barrett L, Simmons W Interoceptive predictions in the brain. Nat Rev Neurosci 16, 419–429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fermin AS, Friston K, Yamawaki S An insula hierarchical network architecture for active interoceptive inference. Royal Society Open Science, 9, 220226 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Namkung H, Kim S-H, Sawa A. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci 40, 200–207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Craig A. How do you feel — now? The anterior insula and human awareness. Nat. Rev. Neurosci 10, 59–70 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Gogolla N. The insular cortex. Curr. Biol 27, R580–R586 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Craig AD, Chen K, Bandy D, & Reiman EM Thermosensory activation of insular cortex. Nature Neurosci, 3, 184–190 (2000). [DOI] [PubMed] [Google Scholar]
- 87.Wright P, He G, Shapira NA, Goodman WK, & Liu Y Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport, 15, 2347–2351 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Zhou F, Zhao W, Qi Z et al. A distributed fMRI-based signature for the subjective experience of fear. Nat Commun 12, 6643 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koren T, Amer M, Krot M, Boshnak N, et al. Insular cortex neurons encode and retrieve specific immune responses. Cell, 184, 5902–5915 (2021). [DOI] [PubMed] [Google Scholar]
- 90.Katayama O, Nishi Y, Osumi M, Takamura Y, Kodama T, & Morioka S Neural activities behind the influence of sensorimotor incongruence on dysesthesia and motor control. Neuroscience Letters, 698, 19–26 (2019). [DOI] [PubMed] [Google Scholar]
- 91.Evrard HC The organization of the primate insular cortex. Frontiers in Neuroanatomy, 13, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gehrlach DA, Dolensek N, Klein AS, et al. Aversive state processing in the posterior insular cortex. Nat. Neurosci, 22, 1424–1437 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Krockenberger M, Saleh TO, Logothetis NK, & Evrard HC Connection “stripes” in the primate insula. https://www.biorxiv.org/content/10.1101/2020.11.03.361055v1.full (2020). [Google Scholar]
- 94.Uddin LQ, Nomi JS, Hebert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J. Clin. Neurophysiol 34, 300–306 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koban L, Gianaros PJ, Kober H et al. The self in context: brain systems linking mental and physical health. Nat Rev Neurosci 22, 309–322 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, & Davidson RJ The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci 12, 154–167 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meda KS, Patel T, Braz JM, Malik R, et al. Microcircuit mechanisms through which mediodorsal thalamic input to anterior cingulate cortex exacerbates pain-related aversion. Neuron, 102, 944–959 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rao RP. Bayesian computation in recurrent neural circuits. Neural Comput. 16, 1–38 (2004). [DOI] [PubMed] [Google Scholar]
- 99.Orhan A & Ma WJ Efficient probabilistic inference in generic neural networks trained with non-probabilistic feedback. Nat Commun 8, 138 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sohn H & Narain D. Neural implementations of Bayesian inference. Curr Opin Neurobiol. 70, 121–129 (2021). [DOI] [PubMed] [Google Scholar]
- 101.Pachitariu M, Petreska B, & Sahani M. Recurrent linear models of simultaneously-recorded neural populations. Adv. Neural Info. Proc. Syst (MIT Press, 2013). [Google Scholar]
- 102.Pecevski D, & Maass W. Learning probabilistic inference through spike-timing-dependence plasticity. eNeuro, 3, e0048–15.2016 1-35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aitchison L, Jegminat J, Menendez JA, et al. Synaptic plasticity as Bayesian inference. Nature Neurosci. 24, 565–571 (2021). [DOI] [PubMed] [Google Scholar]
- 104.Pitkow X & Angelaki DE. Inference in the brain: statistics flowing in redundant population codes. Neuron, 94, 943–953 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bastos AM, Usrey WM, Adams RA, et al. Canonical microcircuits for predictive coding. Neuron, 76, 695–711 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song Y, Yao M, Kemprecos H, Byrne A, et al. Predictive coding models for pain perception. J. Comp. Neurosci 49, 107–127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bannister K. Descending pain modulation: influence and impact. Current Opinion in Physiology, 11, 62–66 (2019). [Google Scholar]
- 108.Wright H, Li X, Fallon NB, Giesbrecht T, et al. (2015). Heightened eating drive and visual food stimuli attenuate central nociceptive processing. Journal of Neurophysiology, 113, 1323–1333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wright H, Li X, Fallon NB, Crookall R, et al. (2016). Differential effects of hunger and satiety on insular cortex and hypothalamic functional connectivity. European Journal of Neuroscience, 43, 1181–1189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou W, Cheung K, Kyu S, Wang L, et al. Activation of orexin system facilitates anesthesia emergence and pain control. Proc. Natl. Acad. Sci. USA, 115, E10740–E10747 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ito H, Navratilova E, Vagnerova B, Watanabe M, et al. Chronic pain recruits hypothalamic dynorphin/kappa opioid receptor signalling to promote wakefulness and vigilance. Brain. DOI: 10.1093/brain/awac153 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ahmadi-Soleimani SM, Mianbandi V, Azizi H, et al. (2020). Coregulation of sleep-pain physiological interplay by orexin system: An unprecedented review. Behavioural Brain Research, 391, 112650 (2020). [DOI] [PubMed] [Google Scholar]
- 113.Phua SC, Tan YL, Kok AMY, et al. A distinct parabrachial–to–lateral hypothalamus circuit for motivational suppression of feeding by nociception. Science Advances, 7, eabe4323 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schiller M, Ben-Shaanan TL, & Rolls A Neuronal regulation of immunity: why, how and where? Nature Reviews Immunology, 21, 20–36 (2021). [DOI] [PubMed] [Google Scholar]
- 115.Seymour B, O'doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, & Dolan R Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat. Neurosci, 8, 1234–1240 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Zhang S, Mano H, Lee M, Yoshida W, Kawato M, Robbins TW, & Seymour B The control of tonic pain by active relief learning. eLife, 7, e31949 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baliki M, Petre B, Torbey S et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 15, 1117–1119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buzsaki G & Draguhn A. Neuronal oscillations in cortical networks. Science, 304, 1926–1929 (2004). [DOI] [PubMed] [Google Scholar]
- 119.Muller L, Chavane F, Reynolds J et al. Cortical travelling waves: mechanisms and computational principles. Nat Rev Neurosci 19, 255–268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Buzsaki G. Theta oscillations in the hippocampus. Neuron, 33, 325–340 (2002). [DOI] [PubMed] [Google Scholar]
- 121.Tendler A & Wagner S. Different types of theta rhythmicity are induced by social and fearful stimuli in a network associated with social memory. eLife, 4, e03614 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stern J, Jeanmonod D & Sarnthein J Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage 31, 721–731 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Sarnthein J, Stern J, Aufenberg C, Rousson V, & Jeanmonod D Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain 129, 55–64 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Kisler LB, Kim JA, Hemington KS, et al. Abnormal alpha band power in the dynamic pain connectome is a marker of chronic pain with a neuropathic component. Neuroimage Clin. 26, 102241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim JA, Bosma RL, Hemington KS, Rogachov A, et al. Neuropathic pain and pain interference are linked to alpha-band slowing and reduced beta-band magnetoencephalography activity within the dynamic pain connectome in patients with multiple sclerosis. Pain 160, 187–197 (2019). [DOI] [PubMed] [Google Scholar]
- 126.Tan LL, Oswald MJ, & Kuner R. Neurobiology of brain oscillations in acute and chronic pain. Trends Neurosci. 44, 629–642 (2021). [DOI] [PubMed] [Google Scholar]
- 127.Leblanc BW, Lii TR, Silverman AE, Alleyne RT & Saab CY Cortical theta is increased while thalamocortical coherence is decreased in rat models of acute and chronic pain. Pain 155, 773–782 (2014). [DOI] [PubMed] [Google Scholar]
- 128.Iwamoto S, Tamura M, & Nawano M. Dynamics of neuronal oscillations underlying nociceptive response in the mouse primary somatosensory cortex. Sci. Rep 11, 1667 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taesler P & Rose M Prestimulus theta oscillations and connectivity modulate pain perception. J. Neurosci 36, 5026–5033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walton KD, Dubois M, & Llinas RR Abnormal thalamocortical activity in patients with complex regional pain syndrome (CRPS) type I. Pain 150, 41–51 (2010). [DOI] [PubMed] [Google Scholar]
- 131.Edhi MM, Heijmans L, Vanent KN et al. Time-dynamic pulse modulation of spinal cord stimulation reduces mechanical hypersensitivity and spontaneous pain in rats. Sci Rep 10, 20358 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Taesler P & Rose M The modulation of neural insular activity by a brain computer interface differentially affects pain discrimination. Sci Rep 11, 9795 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Das A, Myers J, Mathura R, Shofty B, Metzger BA, Bijanki K, Wu C, Jacobs J, Sheth SA. Spontaneous neuronal oscillations in the human insula are hierarchically organized traveling waves. eLife 11, e76702 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liberati G, Klöcker A, Algoet M, Mulders D, Safronova MM, Santos SF, Vaz JGR, Raftopoulos C, Mouraux A. Gamma-band oscillations preferential for nociception can be recorded in the human insula. Cerebral Cortex, 28, 3650–3664 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gélébart J, Garcia-Larrea L, Frot M. Amygdala and anterior insula control the passage from nociception to pain, Cerebral Cortex, 2022;,bhac290, 10.1093/cercor/bhac290 [DOI] [PubMed] [Google Scholar]
- 136.Zhang H, Watrous AJ, Patel A, Jacobs J. Theta and alpha oscillations are traveling waves in the human neocortex. Neuron, 98, 1269–1281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bahramisharif A, van Gerven MA, Aarnoutse EJ, et al. Propagating neocortical gamma bursts are coordinated by traveling alpha waves. J Neurosci. 33, 18849–18854 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function 214, 655–667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 18, 499–500 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang Z, Tarnal V, Vlisides PE, et al. Anterior insula regulates brain network transitions that gate conscious access. Cell Rep. 35, 109081 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chouchou F, Mauguière F, Vallayer O, Catenoix H, et al. How the insula speaks to the heart: Cardiac responses to insular stimulation in humans. Human Brain Mapping, 40, 2611–2622 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schiller M, Ben-Shaanan TL, & Rolls A Neuronal regulation of immunity: why, how and where? Nature Reviews Immunology, 21, 20–36 (2021). [DOI] [PubMed] [Google Scholar]
- 143.Apkarian AV, Baliki MN, & Geha PY Towards a theory of chronic pain. Progress in Neurobiology, 87, 81–97 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, & Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin. J. Pain, 28, 475–483 (2012). [DOI] [PubMed] [Google Scholar]
- 145.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, & Turk DC The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological Bulletin, 133, 581 (2007). [DOI] [PubMed] [Google Scholar]
- 146.Meulders A. From fear of movement-related pain and avoidance to chronic pain disability: a state-of-the-art review. Current Opinion in Behavioral Sciences, 26, 130–136 (2019). [Google Scholar]
- 147.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, & Vlaeyen JW The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. Journal of Behavioral Medicine, 30, 77–94 (2007). [DOI] [PubMed] [Google Scholar]
- 148.Thapa T, Graven-Nielsen T & Schabrun SM Aberrant plasticity in musculoskeletal pain: a failure of homeostatic control? Experimental Brain Research, 239, 1317–1326 (2021). [DOI] [PubMed] [Google Scholar]
- 149.Zaman J, Vlaeyen JW, Van Oudenhove L, Wiech K, & Van Diest I Associative fear learning and perceptual discrimination: a perceptual pathway in the development of chronic pain. Neuroscience & Biobehavioral Reviews, 51, 118–125 (2015). [DOI] [PubMed] [Google Scholar]
- 150.Flor H. Maladaptive plasticity, memory for pain and phantom limb pain: review and suggestions for new therapies. Expert Review of Neurotherapeutics, 8, 809–818 (2008). [DOI] [PubMed] [Google Scholar]
- 151.Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, & Svensson P (1996). The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain, 64, 231–240 (1996). [DOI] [PubMed] [Google Scholar]
- 152.Nees F, & Becker S Psychological processes in chronic pain: influences of reward and fear learning as key mechanisms–behavioral evidence, neural circuits, and maladaptive changes. Neuroscience, 387, 72–84 (2018). [DOI] [PubMed] [Google Scholar]
- 153.Neugebauer V, Mazzitelli M, Cragg B, Ji G, Navratilova E, & Porreca F Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology, 170, 108052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, & Wasan AD The role of psychosocial processes in the development and maintenance of chronic pain. Journal of Pain, 17, T70–T92 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Review of Neurotherapeutics, 12, 577–585 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bushnell MC, Čeko M, & Low LA Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci 14, 502–511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chapman CR, & Vierck CJ The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. Journal of Pain, 18, 359 (2017). [DOI] [PubMed] [Google Scholar]
- 158.Friston K. Computational psychiatry: from synapses to sentience. Mol Psychiatry (2022). 10.1038/s41380-022-01743-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tabor A, & Burr C Bayesian learning models of pain: a call to action. Current Opinion in Behavioral Sciences, 26, 54–61 (2019). [Google Scholar]
- 160.Walters ET Adaptive mechanisms driving maladaptive pain: how chronic ongoing activity in primary nociceptors can enhance evolutionary fitness after severe injury. Philosophical Transactions of the Royal Society B, 374, 20190277 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fields HL How expectations influence pain. Pain, 159, S3–S10 (2018). [DOI] [PubMed] [Google Scholar]
- 162.Crook RJ, Lewis T, Hanlon RT & Walters ET Peripheral injury produces long-term sensitization of responses to tactile and visual stimuli in squid, Loligo pealei. J. Exp. Biol 214, 3173–3185 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Crook RJ, Dickson KD, Hanlon RT & Walters ET Nociceptive sensitization reduces predation risk. Curr. Biol 24, 1121–1125 (2014). [DOI] [PubMed] [Google Scholar]
- 164.Howard R, Lopes L, Lardie C, Perez PV & Crook RJ Early-life injury produces life-long neural hyperexcitability, cognitive deficit and altered defensive behavior in squid. Euprymna scolopes. Phil. Trans. R. Soc. B 374, 281–289 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Crook RJ, Hanlon RT & Walters ET Squid have nociceptors that display long term sensitization and spontaneous activity after bodily injury. J. Neurosci 33, 10021–10026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bazarini S & Crook RJ Environmental estrogen exposure disrupts sensory processing and nociceptive plasticity in the cephalopod. Euprymna scolopes. J. Exp. Biol 223, jeb218008 (2020). [DOI] [PubMed] [Google Scholar]
- 167.Oshima M, di Pauli von Treuheim T, Carroll J, Hanlon RT, Walters ET, & Crook RJ Peripheral injury alters schooling decisions in injured squid. Behavioural Processes, 128, 89–95 (2016). [DOI] [PubMed] [Google Scholar]
- 168.Kalman RE. Mathematical description of linear dynamical systems. SIAM J. Series A: Control, 1, 152–192 (1963). [Google Scholar]
- 169.Pavon M & Wets RJ-B. The duality between estimation and control from a variational viewpoint: the discrete time case. Mathematical Programming Study, 18, 1–11 (1982). [Google Scholar]
- 170.Todorov E. Optimal control theory. In Bayesian Brain. (MIT Press, 2006). [Google Scholar]
- 171.Levine S. Reinforcement learning and control as probabilistic inference: tutorial and review. https://arxiv.org/abs/1805.00909 (2018). [Google Scholar]
- 172.Attias H. Planning by probabilistic inference. Proceedings of the Ninth International Workshop on Artificial Intelligence and Statistics, PMLR R4:9-16 (2003). [Google Scholar]
- 173.Todorov E. Efficient computation of optimal actions. Proc. Natl. Acad. Sci. USA, 106, 11478–11483 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tschantz A, Seth AK, & Buckley CL. Learning action-oriented models through active inference. PLoS Comput Biol 16, e1007805 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Millidge B, Tschantz A, Seth AK, Buckley CL. On the relationship between active inference and control as inference. In Verbelen T, Lanilos P, Buckley CL, De Boom C, eds. Active Inference, pp. 3–11, Springer; (2020). [Google Scholar]
- 176.Kato F, Sugimura YK, & Takahashi Y. Pain-associated neural plasticity in the parabrachial to central amygdala circuit: pain changes the brain, and the brain changes the pain. Adv. Exp. Med. Biol 1099, 157–166 (2018). [DOI] [PubMed] [Google Scholar]
- 177.Yeh LF, Watanabe M, Sulkes-Cuevas J, & Johansen JP Dysregulation of aversive signaling pathways: a novel circuit endophenotype for pain and anxiety disorders. Current Opin. Neurobiol 48, 37–44 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE. Overview of injury-related behaviours.
a) A core classification of the different types of response to an injury, spanning recuperative, protective and physiological responses.
b) The spectrum of ecologically significant causes of injury that can occur, summarised across species, with different causes having different importance across species.
c) Timeline of how pain responses change following an injurious event, from immediate defensive behaviours through to recuperation and recovery.