Abstract
A 32-year-old man was admitted for the evaluation of proteinuria (5.69 g/day). A light microscopic examination showed markedly dilated glomerular capillary loops with vacuolated areas in many glomeruli, and vacuolated areas were seen on peritubular capillaries in the tubulointerstitium. When electron microscopy specimens prepared by pre-fixation with glutaraldehyde and post-fixation with osmium tetroxide were used for oil red staining, the deposition was confirmed on the affected areas. A genetic analysis of apoE showed that the lipoprotein glomerulopathy was due to apoE-Sendai (Arg145Pro, p.R163P) heterozygosity, which was found in not only the patient but also his mother and twin brother.
Keywords: lipoprotein glomerulopathy, oil red staining, apolipoprotein E
Introduction
Lipoprotein glomerulopathy (LPG), a primary disorder of lipid metabolism characterized by thrombus-like material containing lipoproteins in the glomeruli, was first reported in 1989 in Japan (1); the majority of subsequent reports were from East Asia, particularly Japan and China. Clinically, the disease is characterized by mild to nephrotic-range massive proteinuria, and chronic renal failure is found in about half of patients.
Light microscopy shows that collapsed glomerular capillaries with segmental sclerosis coexist with enlarged capillary lumina with pale-stained, mesh-like substances, which we refer to as “lipoprotein thrombi.” Immunofluorescence microscopy shows no significant deposition of complement or immunoglobulins; electron microscopy shows a layered structure that resembles a fingerprint and is composed of granules and vacuoles in the dilated capillary lumen; and biochemical findings show high plasma apoE (more than twice the upper limit of normal). A DNA sequencing analysis of the apoE gene showed that LPG involves 15 polymorphic variants, the most common of which are apoE-Sendai and apoE-Kyoto (2-6).
Although lipoprotein glomerulopathy was named as such because it features lipoprotein thrombi localized in the glomerulus, we report a case in which lipoprotein thrombi were found not only in the glomerular capillaries but also in the peritubular capillaries within the tubulointerstitium, as shown by staining electron microscopy specimens with the lipid stain oil red.
Case Report
A 32-year-old Japanese man was admitted to our hospital for the evaluation of proteinuria, which had first been noted at a medical examination when he was 29 years old. His twin brother and grandmother also had a history of proteinuria.
On admission, the patient was 168 cm tall and weighed 66.0 kg. His blood pressure was 112/78 mm Hg, and his body temperature was 36.2°C. Heart and breath sounds were normal. Edema was not present in the extremities, and no xanthomas or corneal opacities were observed. The laboratory findings were as follows: total protein, 5.4 g/dL; albumin, 3.3 g/dL; serum creatinine, 0.70 mg/dL; estimated glomerular filtration rate, 106 mL/min/1.73 m2. The 24-hour urinary protein excretion was 5.69 g, and the urine sediment contained 1 to 4 red blood cells per high-power field. A kidney biopsy was performed to evaluate proteinuria.
Serum lipid data are shown in Table 1.
Table.
Lipid Data.
| Lipid | Value | Reference range |
|---|---|---|
| Total cholesterol (mg/dL) | 284 | 122-220 |
| Low-density lipoprotein cholesterol (mg/dL) | 161 | 60-119 |
| High-density lipoprotein cholesterol (mg/dL) | 61 | 35-70 |
| Triglyceride (mg/dL) | 209 | 30-150 |
| Apo A1 (mg/dL) | 187 | 119-155 |
| Apo B (mg/dL) | 134 | 73-109 |
| Apo E (mg/dL) | 13.8 | 2.7-4.3 |
| Lipoprotein fractionation α (LDL)(%) | 29 | 27-52 |
| Lipoprotein fractionation preβ (VLDL)(%) | 21 | 6-27 |
| Lipoprotein fractionation β (HDL)(%) | 50 | 35-52 |
| Hyperlipidemia WHO classification | IIa |
A kidney biopsy
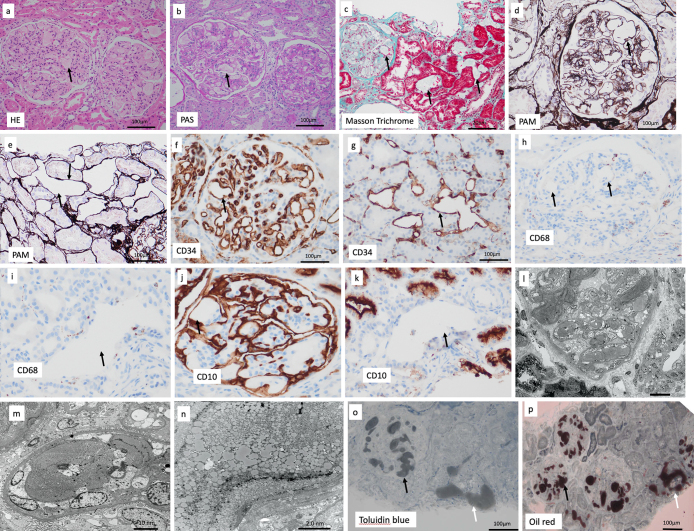
A light microscopic examination showed segmental sclerosis in 10 out of 26 glomeruli but no global sclerosis. Collapsed glomerular capillaries with segmental sclerosis coexisted with markedly dilated glomerular capillary loops that showed vacuolated areas in many glomeruli. The contents of the vacuolated areas were pale pink on periodic acid Schiff and hematoxylin and eosin staining (Fig. 1a, b), but the inner structure of the affected areas could not be identified even with periodic acid methenamine silver and Masson Trichrome staining (Fig. 1c, d). Vacuolated areas were found also in the tubulointerstitium (Fig. 1e).
Figure 1.
Kidney biopsy findings. a-d: Collapsed glomerular capillaries with segmental sclerosis coexisted with marked dilatation of glomerular capillary loops that were seen as vacuolated areas (arrow). c, e: Vacuolated areas were seen in the tubulointerstitium (arrow). f, g: Vacuolated areas in the glomeruli and interstitium were surrounded by walls positive for CD34 (which stains vascular walls; arrow). h, i: Vacuolated areas in the glomeruli and interstitium were negative for CD68 (which stains macrophages; arrow). j, k: CD10 (which stains proximal tubules and glomerular capillaries) was positive in glomerular capillaries (arrow; j) and negative in the wall of the vacuolated area in the interstitium (arrow; k). l-n: Electron microscopy. o: Affected areas of intraglomerular and peritubular capillaries were stained gray (arrow) by toluidine blue staining. p: Affected areas of intraglomerular and peritubular capillaries were stained brown (arrow) by oil red staining. a: Hematoxylin and Eosin staining (original magnification ×400). b: Periodic acid Schiff staining (original magnification ×400). C: Masson trichrome staining (original magnification ×200). d, e: Periodic acid methenamine silver staining (original magnification ×400). f, g: CD34 staining (original magnification ×400). h, i: CD68 staining (original magnification ×400). j, k: CD10 staining (original magnification ×400). o: Toluidine blue staining (original magnification ×200). p: Oil red staining (original magnification ×100).
Immunofluorescence microscopy showed no staining for immunoglobulins or complement, and electron microscopy showed that the affected glomerular capillaries were filled with variably sized vacuolated particles (Fig. 1l, n). Foot process effacement was partially observed in the epithelial cells of the damaged glomerulus.
The vacuolated areas in the glomeruli and tubulointerstitium were surrounded by walls positive for CD34 (which stains vascular walls) (Fig. 1f, g), but the areas themselves were negative for CD68 (which stains macrophages) (Fig. 1h, i). CD10 (which stains proximal tubules and glomerular capillaries) was positive in the vacuolated areas of the glomerular capillaries (Fig. 1j) but negative in the wall of the vacuolated areas in the tubulointerstitium (Fig. 1k). These results showed that the vacuolated areas were localized at the intraglomerular capillary and peritubular capillary of the tubulointerstitium. The area of fibrosis in the tubulointerstitium of the cortex sampled was poor at about 10%, but fibrosis in the tubulointerstitium was strong in the glomerulus and associated peritubular capillary area, where deposits were abundant.
Oil red staining
Oil red staining was performed to determine whether these lesions corresponded to LPG, but staining of formalin-fixed paraffin specimens was insufficient. In conventional light microscopy paraffin sections, most lipids leach from the tissue because of the organic solvents used in the preparation process and are seen only as vacuoles during microscopy. Oil red staining of fresh-frozen specimens was also insufficient. Therefore, electron microscopy specimens prepared by pre-fixation with glutaraldehyde and post-fixation with osmium tetroxide were used for oil red staining because of the lipid-eluting properties of the fixatives (7). Intraglomerular and peritubular capillaries in the affected areas were stained gray by toluidine blue staining (Fig. 1o) and positive brown with oil red staining (Fig. 1p).
Liquid chromatography-mass spectrometry (LC-MS/MS)
LC-MS/MS combines high-performance LC with a triple quadrupole MS (MS/MS). In tissue sections from the kidney biopsy, the above-mentioned lesions in the glomeruli and tubules were sectioned by laser microdissection, and LC-MS/MS was performed on the protein solution (organic components) extracted from them, according to the previously reported method (8). The level of apoE was markedly higher than that of the other apolipoproteins, including apo B-100, apo A1, apo(a), and apo C11 (Table 2).
Table 2.
Liquid Chromatography-mass Spectrometry. Apolipoprotein E (apoE) was Markedly Increased Compared with Other Apolipoproteins, Including Apo B-100, Apo A1, Apo (a), and Apo C11.
| normal cpntrol | this case | Fold (Case/Con) | ||||
|---|---|---|---|---|---|---|
| ApoE | not detected | 265 | >100↑↑ | |||
| ApoB-100 | not detected | 78 | >100↑↑ | |||
| Apolipoprotein A-I | not detected | 30 | >100↑ | |||
| Apolipoprotein (a) | not detected | 14 | >100↑ | |||
| Apolipoprotein C-II | not detected | 11 | >100↑ | |||
| Immunoglobulin heavy constant gamma 1 | not detected | 20 | >100↑ | |||
| Immunoglobulin kappa constant | not detected | 22 | >100↑ | |||
| C3 | not detected | 56 | >100↑↑ | |||
| C4-A | not detected | 31 | >100↑ | |||
| C4-B | not detected | 34 | >100↑ | |||
| Fibrinection | 6 | 195 | 32.5 |
ApoE genetic analyses
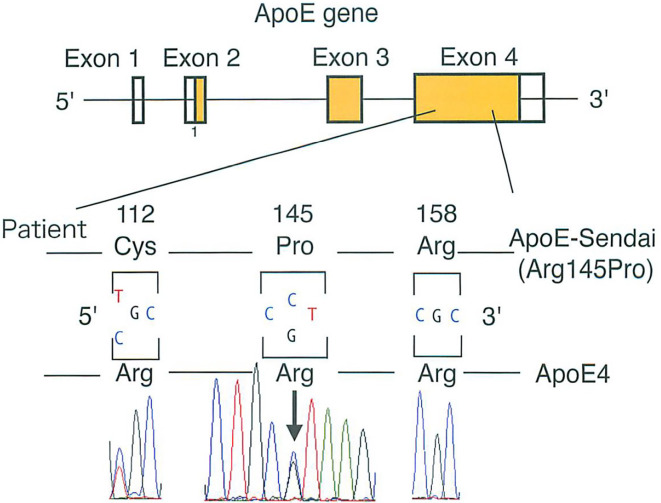
ApoE genetic analyses showed that the apoE gene had a E2/E4 phenotype and a ε3/4 genotype, which corresponds to the Sendai mutation. Therefore, the patient was diagnosed with LPG due to apoE-Sendai (Arg145Pro, p.R163P) heterozygosity (Fig. 2).
Figure 2.
A genetic analysis of apolipoprotein E.
The same genetic form was found also in the patient's asymptomatic mother and his symptomatic twin brother and grandmother.
Clinical course
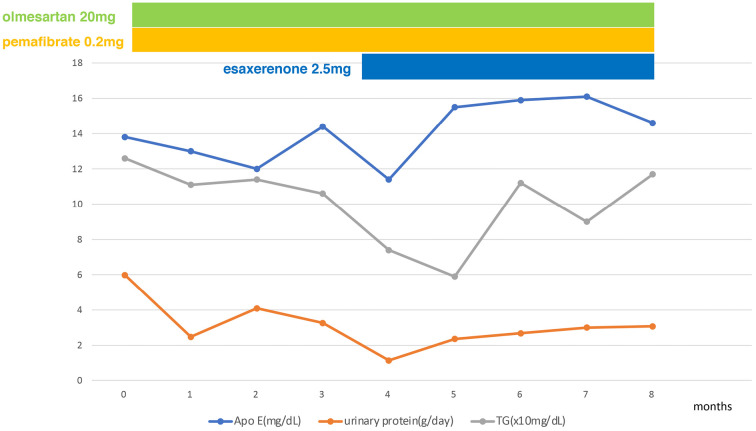
After the kidney biopsy, treatment was started with the angiotensin II receptor blocker (olmesartan), pemafibrate and a mineral corticoid receptor blocker (esaxerenone). Ten months later, the patient's urinary protein level had decreased to 3.0 g daily, his serum creatinine level was 0.87 mg/dL, and his serum albumin level was 4.0 mg/dL. The serum ApoE and triglyceride levels remain unchanged (Fig. 3).
Figure 3.
Clinical course (changes in ApoE protein and urinary protein after the kidney biopsy). Olmesartan (angiotensin II receptor antagonist, 20 mg daily), pemafibrate (triglyceride-lowering drug) (0.2 mg daily), esaxerenone (nonsteroidal anti-mineralocorticoid) (2.5 mg daily), triglyceride (TG).
Discussion
We presented a case of proteinuria due to LPG. The findings of LPG in the glomeruli and the results of genetic analyses corresponded to those previously reported as apoE-Sendai (2-6). In many earlier cases, oil red staining was performed using formalin-fixed paraffin specimens or fresh-frozen sections from kidney biopsies of patients with LPG, and positive staining was seen in the glomeruli. However, in the present case, these methods were insufficient for confirming the localization of lipoprotein thrombi. Therefore, we used electron microscopy sections of kidney biopsy specimens for oil red staining, a novel approach that has not been previously described. The use of these sections improved the sensitivity of lipid staining and revealed that deposits were present not only in the glomerulus but also in the tubulointerstitium. The deposits were positive for CD10 staining in the glomerular capillaries but negative in the tubulointerstitium and positive for CD34 staining in the glomeruli and tubulointerstitium. Taken together, these two results showed that lipoprotein thrombi were present not only in the glomerular capillaries but also in the peritubular capillaries of tubulointerstitium.
The fact seems to be that previous studies have not paid much attention to tubulointerstitial lesions, although investigators have noticed the presence of vacuolated lesions in the tubulointerstitium in this disease. Saito et al. examined six cases of LPG and reported that glomerular lesions were characteristic, whereas tubulointerstitial lesions were nonspecific, and lipoprotein thrombi were noted in some venules in one case. No further detailed study of tubular lesions has yet been performed (1). Finn et al. also summarized past papers and noted that lipoprotein thrombi were found only in glomeruli, and deposits in the tubulointerstitium were foam cells rather than lipoprotein thrombi (6). Interstitial foam cells are occasionally observed in various renal diseases, and they have been reported to belong to the monocyte/macrophage lineage or be the phenotypical transformation of macrophages and to be associated with heavy proteinuria and hyperlipidemia (9). Since this deposit was negative for CD68 in our case, we speculate that it was not directly related to macrophages.
Thus, the present case is the first report of lipoprotein thrombi in the tubulointerstitium.
We speculate that the findings in this case imply that lipoproteins leak only from the glomerulus through the efferent arteriole into the peritubular capillaries of the tubules, or perhaps the deposits were formed in this contiguous vasculature, resulting in extensive tissue damage to the nephrons and tubulointerstitium in that area. CD68 staining was negative in the areas with the deposits, indicating that macrophages are not involved in LPG; consequently, this finding distinguishes LPG from other glomeruloscleroses in which foamy macrophages are seen (2,4,5,6).
Fukunaga et al. reported a 20-year-old woman in whom apoE-related glomerular disease resembling membranous nephropathy was diagnosed because of elevated plasma apoE levels and the detection of the apoE-Toyonaka genotype; a large amount of apoE was shown in deposits in the glomeruli using tandem MS. However, the authors described this case as a new type of LPG and not a typical case (10). The present report describes the first case involving quantitative LC-MS/MS of apoE in intraglomerular deposits of a patient with typical LPG.
LPG is an inherited renal disease that is mainly diagnosed by a kidney biopsy in the presence of low nephrotic levels of proteinuria and a decreased renal function without hematuria. It is thought that lipoprotein deposition associated with apoE in the glomerular lumen causes secondary FSGS lesions, resulting in hypertension and proteinuria. This disease differs from other lipid diseases in the lack of extrarenal lesions. Since ApoE is closely related to triglyceride, fenofibrate, a triglyceride-lowering drug, has been reported to be effective in reducing proteinuria and preserving the renal function (11). The present case suggests that the entire nephron may be affected, as the peritubular capillaries were also affected by deposits contiguous with the deposit-affected glomerulus. Treatment with fenofibrate and antihypertensive drugs was initiated in our patient, and proteinuria was successfully reduced, although the follow-up period is still short. Although the mode of inheritance has not been previously described, the fact that similar cases were described in the present and previous reports in both sexes and in siblings, the parents' generation, and the grandparents' generation suggests an autosomal dominant mode of inheritance.
In conclusion, we experienced a typical case of LPG that previously would have been classified as apoE-Sendai. Studies that used conventional staining methods with paraffin sections or fresh-frozen sections reported finding lipoprotein thrombi in the glomerular capillaries. In the present study, we performed oil red staining with electron microscopy and found that the lipoprotein thrombi were localized not only in the glomerular capillaries but also in the peritubular capillaries. In addition, we used LC-MS/MS to confirm the presence of lipoprotein thrombi, which consisted of apoE.
This investigation was conducted in accordance with the Declaration of Helsinki. The patient gave his informed consent for this case report to be published.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Mochizuki S, Moriya T, Naganuma H, Nagasawa T, Ueno Y, Sato H, Sasano H, Saito T for their advice in performing oil red staining.
The following related papers are published here. (Significance of fat stains in serial sections from Epon-embedded tissue samples for electron microscopy in renal diseases. 5(4): 240-245.)
References
- 1. Saito T, Sato H, Kudo K, et al. Lipoprotein glomerulopathy: glomerular lipoprotein thrombi in a patient with hyperlipoproteinemia. Am J Kidney Dis 13: 148-153, 1989. [DOI] [PubMed] [Google Scholar]
- 2. Saito T, Oikawa S, Sato H, Sato T, Ito S, Sasaki J. Lipoprotein glomerulopathy: significance of lipoprotein and ultrastructural features. Kidney Int Suppl 71: S37-S41, 1999. [DOI] [PubMed] [Google Scholar]
- 3. Matsunaga A, Saito T. Apolipoprotein E mutations: a comparison between lipoprotein glomerulopathy and type III hyperlipoproteinemia. Clin Exp Nephrol 18: 220-224, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Saito T, Matsunaga A, Fukunaga M, Nagahama K, Hara S, Muso E. Apolipoprotein E-related glomerular disorders. Kidney Int 97: 279-288, 2020. [DOI] [PubMed] [Google Scholar]
- 5. Rovin BH, Roncone D, McKinley A, Nadasdy T, Korbet SM, Schwartz MM. APOE Kyoto mutation in European Americans with lipoprotein glomerulopathy. N Engl J Med 357: 2522-2524, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Finn LS. Lipoprotein glomerulopathy. In: Heptinstall's Pathology of the Kidney. 7th ed. Jennette JC, Olson JL, Schwartz MM, Silva FG, Eds. Wolters Kluwer, Philadelphia, 2015: 1225-1228. [Google Scholar]
- 7. Ubara Y, Kawaguchi T, Nagasawa T, et al. ; Committee of Practical Guide for Kidney Biopsy 2020. Kidney biopsy guidebook 2020 in Japan. Clin Exp Nephrol 25: 325-364, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawata N, Kang D, Aiuchi T, et al. Proteomics of human glomerulonephritis by laser microdissection and liquid chromatography-tandem mass spectrometry. Nephrology (Carlton) 25: 351-359, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kurihara I, Saito T, Soma J, et al. Clinicopathological characteristics of interstitial foam cells in membranous nephropathy. Kidney Int Suppl 71: S144-S146, 1999. [DOI] [PubMed] [Google Scholar]
- 10. Fukunaga M, Nagahama K, Aoki M, et al. Membranous nephropathy-like apolipoprotein E deposition disease with apolipoprotein E toyonaka (Ser197Cys) and a homozygous apolipoprotein E2/2. Case Rep Nephrol Dial 8: 45-55, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu Z, Huang S, Wu Y, et al. Hereditary features, treatment, and prognosis of the lipoprotein glomerulopathy in patients with the APOE Kyoto mutation. Kidney Int 85: 416-424, 2014. [DOI] [PubMed] [Google Scholar]