Abstract
A 73-year-old woman with myelodysplastic syndrome and diabetes mellitus, chronic renal failure and paroxysmal atrial fibrillation, received a diagnosis of facial cellulitis and was treated by antibiotics. However, her symptoms deteriorated. Facial magnetic resonance imaging (MRI) showed orbital cellulitis. She had weakness of visual acuity requiring changing the antibiotics. She also underwent steroid pulse treatment. Her symptoms temporarily improved, but she became comatose and died. Results of a molecular analysis of the residual cerebrospinal fluid indicated Rhizopus species infection. For immunocompromised hosts with refractory orbital cellulitis, mucormycosis should be considered as a differential diagnosis, and appropriate treatment should be promptly performed.
Keywords: immunocompromised host, orbital cellulitis, mucormycosis
Introduction
Orbital cellulitis (OC) is an infection of the soft tissue behind the orbital septum (1). It manifests with erythema and edema of the eyelids, vision loss, a fever, headache, proptosis, chemosis, and diplopia (2). It is considered one of the most important ophthalmic emergencies, as delays in its diagnosis and treatment can lead to serious ocular and systemic morbidity and mortality (1,2). OC is caused most commonly by extension of sinus disease into the orbit, especially in young patients (1). However, it is also induced by infection of the eyelids or face and even due to hematogenous spread from distant locations (2).
Mucormycosis is a difficult-to-diagnose rare disease with high morbidity and mortality (3). Incidentally, all-cause mortality rates for mucormycosis range from 40% to 80%, with rates varying depending on the underlying conditions and sites of infection (4). Mucormycosis is mainly caused by Rhizopus species, Mucor species, Cunninghamella species, Lichthemia species, and Rhizomucor species (5). However, the diagnosis is often delayed, and the disease tends to progress rapidly. Urgent surgical and medical intervention is lifesaving (3).
We herein report a case of a fatal Rhizopus species infection after facial injury in a patient with myelodysplastic syndrome (MDS) and diabetes mellitus (DM).
Case Report
This case study was approved by the Ethics Committee of Juntendo Shizuoka Hospital. The approval number was 298. Informed consent was obtained from the family of the deceased patient.
A 73-year-old woman with DM (no insulin treatment), MDS requiring multiple transfusions (every 2 weeks over 2 years), chronic renal failure and paroxysmal atrial fibrillation was transported to the emergency room at our hospital due to difficulty moving. Her history included auto-sensitization dermatitis and right hip joint operation. She had also fallen down and injured the roof of her nose two weeks before arrival. The wound did not heal spontaneously, so she had undergone treatment by a plastic surgeon one week earlier; however, the wound had pus, so she received a prescription of cefaclor four days before her arrival.
On arrival, her vital signs were as follows: consciousness, clear; blood pressure, 166/85 mmHg; heart rate, 100 beats per minute; respiratory rate, 30 breaths per minute; percutaneous saturation of oxygen under room air, 94%; and body temperature, 37.8°C. Her face had redness, swelling and pain (Fig. 1) in addition to pain at the left side of the thorax and back. An electrocardiogram showed normal findings. The venous blood gas findings were as follows: pH, 7.37; pCO2 37 mmHg; pO2, 14.9 mmHg; HCO3-, 31 mmol/L; base excess, -2.9 mmol/L; and lactate, 1.8 mmol/L. A rapid antigen detection test for group A streptococcus was negative. A simple urine qualitative examination showed 3+ for protein without leukocytosis. The main results of a biochemical blood analysis on arrival are shown in Table 1.
Figure 1.
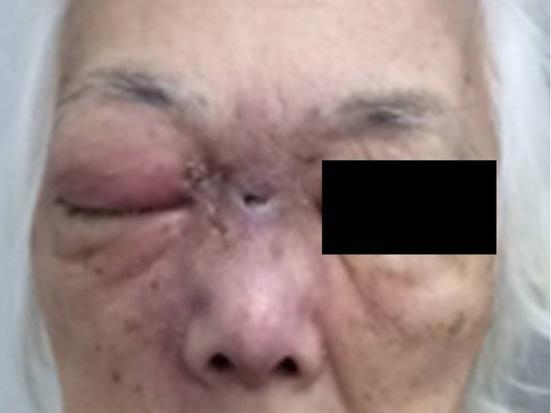
The patient’s face on arrival. The right side of the face showed redness and swelling.
Table 1.
Results of a Biochemical Analysis on Arrival.
| White blood cell count | 3,100 | /μL | |
| Neutrophil | 1,739 | /μL | |
| Hemoglobin | 8.3 | g/dL | |
| Platelet | 40.0×104 | /μL | |
| Total protein | 9.0 | g/dL | |
| Albumin | 3.2 | g/dL | |
| Glucose | 137 | mg/dL | |
| HbA1C | 7.2 | % | |
| Aspartate aminotransferase | 19 | U/L | |
| Alanine aminotransferase | 22 | U/L | |
| Glutamyl transpeptidase | 37 | IU/L | |
| Creatinine phosphokinase | 15 | U/L | |
| Amylase | 137 | mg/dL | |
| Blood urea nitrogen | 32 | mg/dL | |
| Creatinine | 1.14 | mg/dL | |
| Sodium | 132 | mEq/L | |
| Potassium | 5.2 | mEq/L | |
| Chloride | 99 | mEq/L | |
| Ferritin | 10,774 | ng/mL | |
| C-reactive protein | 12.5 | mg/dL | |
| Lactate dehydrogenase | 282 | IU/L | |
| Prothrombin time international normalized ratio | 1.37 | ||
| Activated partial thromboplastin time | 33.8 | s | |
| Fibrinogen | 569 | mg/dL | |
| Fibrin degradation products | 4.0 | μg/mL |
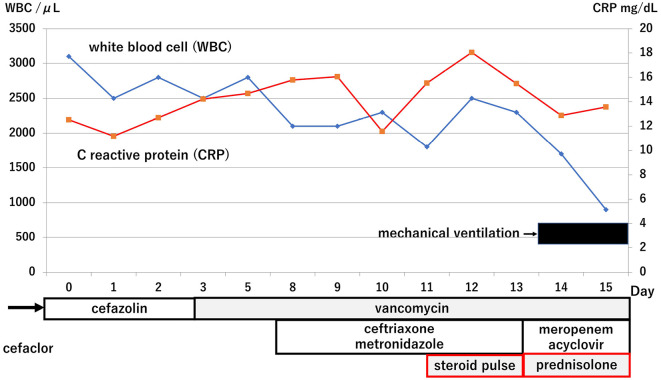
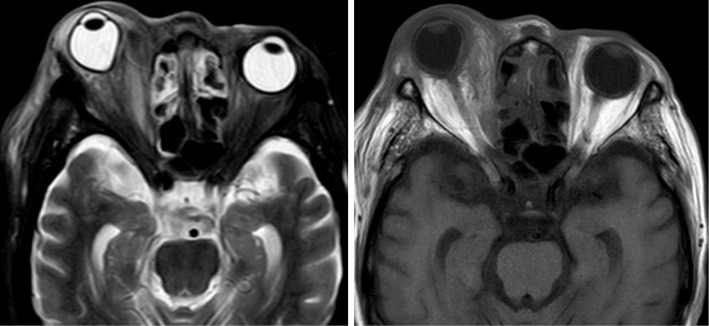
Whole-body computed tomography indicated right facial skin edema and swelling of the mucosa at the sinuses. She received a diagnosis of facial cellulitis with sinusitis and was treated by antibiotics. The time course of treatment and the main laboratory findings are shown in Fig. 2. Antibiotics and steroid infusion were changed (cefazolin to vancomycin on day 3) because her symptoms deteriorated (Fig. 2), with severe eye pain and exophthalmos. Her facial redness and swelling improved gradually after the antibiotics were changed, but severe eye pain and red exophthalmos remained. Facial magnetic resonance imaging (MRI) on day 6 showed orbital cellulitis with deformity of right eye ball due to swelling of musculus orbicularis oculi (Fig. 3). She had weakness of visual acuity on day 7, so she received ceftriaxone and metronidazole in addition to previous antibiotics. As the serum ferritin concentration increased from around 5,000 ng/mL before trauma to around 10,000 ng/mL after trauma, and the patient had a high antinuclear antibody titer (×640), steroid pulse treatment (1 g of methylprednisolone per day for 3 days) was performed on day 11 based on a diagnosis of hemophagocytic syndrome or orbital autoimmune disease (5-7).
Figure 2.
Time course of treatment and main laboratory results. The antibiotics therapy was switched without antifungal drugs. However, her condition ultimately deteriorated. Steroid pulse, 1 g of methylprednisolone per day for 3 days. Prednisolone, 50 mg per day.
Figure 3.
Facial magnetic resonance imaging (MRI) findings on day 6. MRI showed right orbital cellulitis with deformity of the right eyeball due to swelling of the musculus orbicularis oculi (left, short inversion time inversion recovery image; right, T1-weighed image).
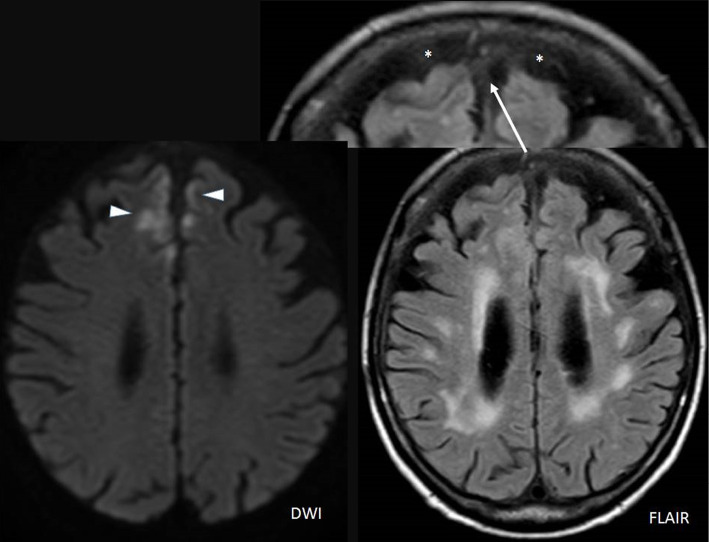
On day 11, her eye pain had decreased, and her appetite had improved. However, on day 12, her left visual acuity decreased, and emergency MRI of the face and brain revealed acute inflammatory or ischemic signal changes at the bilateral frontal lobes, abnormal signal changes in the cerebrospinal fluid (CSF) at the frontal side (Fig. 4), and persistent right exophthalmos. She became comatose on day 13 and underwent tracheal intubation with mechanical ventilation. A spinal tap showed an increased level of protein and normal range of glucose and leukocyte counts [protein, 128 mg/dL; glucose, 71 mg/dL; leukocytes, 6/μL (monocytes, 5; neutrophils, 1/μL)]. As beta-D-glucan was negative, meropenem and acyclovir were added in addition to vancomycin on day 13. However, her comatose state did not improve, and she became complicated with unstable circulation, dying on day 16. Her family did not consent to an autopsy.
Figure 4.
Facial magnetic resonance imaging (MRI) findings on day 12. MRI of the face and brain revealed acute inflammatory or ischemic signal changes at the bilateral frontal lobes (triangle, diffusion-weighted imaging) and abnormal signal changes in the cerebrospinal fluid at the frontal side (*).
The results of all cultures of sputum, urine, blood, and CSF, as well as C10 and C11 monoclonal IgM antibodies for cytomegalovirus using serum and polymerase chain reaction (PCR) for the herpes simplex virus using CSF from day 13 were negative. All culture results are shown in Table 2. Eye discharge culture showed Staphylococcus epidermis, which was sensitive to various antibiotics; however, this finding suggested that S. epidermis was not the originating bacteria. The molecular analysis [amplification of fungal deoxyribonucleic acid (DNA) by PCR combined with DNA sequencing] of residual CSF at the National Institute of Infectious Disease indicated Rhizopus species infection (Supplementary material). She was therefore posthumously diagnosed with fatal cutaneous-(rhino-)orbital-cerebral mucormycosis.
Table 2.
Results of Cultures of All Specimens and Minimum Inhibitory Concentration of Antibiotics for Staphylococcus epidermis on Day 3.
| Specimen | Day 0 | Day 3 | Day 11 |
|---|---|---|---|
| Sputum | Could not examine | Negative | |
| Urine | Negative | Negative | |
| Blood | Negative | Negative | |
| Nasal cavity | Negative | ||
| Cerebral spinal fluid | Negative | ||
| Eye discharge | Staphylococcus epidermis* |
| Antibiotics* | Minimum inhibitory concentration |
|---|---|
| Methyl-phenyl-isoxazolyl penicillin | ≤0.12 |
| Ampicillin | ≤0.12 |
| Cefazolin | ≤0.5 |
| Cefmetazole | ≤1 |
| Imipenem | ≤0.25 |
| Vancomycin | ≤1 |
Discussion
This is a rare case of cutaneous-(rhino-)orbital-cerebral mucormycosis in a patient with MDS and DM. Cutaneous mucormycosis, while less common than sinonasal or pulmonary infections, can cause widespread tissue necrosis after seemingly innocuous encounters (8). The most common location of cutaneous mucormycosis is the extremities, and extensive infection has been reported after trauma or orthopedic procedures (8). Rarely, facial traumatic wounds result in occurrence of cutaneous mucormycosis (9). We were unable to find any report wherein facial trauma infection progressed to fatal orbital-cerebral mucormycosis.
Rhino-orbital-cerebral mucormycosis is a common infection among mucormycosis subtypes (10). Because sporangiospores can be deposited in the nasal turbinate and paranasal sinuses in immunocompromised patients (10), sinus mucormycosis may extend into the orbit, extraocular muscles, and optic nerve. The brain may be seeded by invasion of the ethmoidal and orbital veins, which drain into the cavernous sinuses. However, mucosal edema and fluid collection at the sinuses in the present case improved after antibiotic treatment, so the possibility of a sinus route may have been minimized. The most common risk factors of cutaneous mucormycosis are DM and hematological malignancies, including MDS (9,11). In addition, steroid treatment, renal failure and iron overload are also risk factors, similar to the situation in the present patient (10,12).
The early diagnosis of mucormycosis is vital for obtaining a survival outcome. However, a diagnosis of mucormycosis is still difficult to establish. Imaging (computed tomography), classic microbiological tools (a direct examination, culture, and fungal biomarkers), and histopathology are the pillars of the diagnostic work-up of mucormycosis (13,14). However, none of the currently available classic diagnostic tests provides sufficient sensitivity and specificity on its own; the optimal approach therefore relies on a combination of multiple diagnostic strategies, including imaging and fungal biomarkers (13,14). Fortunately, mucormycosis might be suspected based on the results of direct microscopy of clinical specimens. Culture of specimens is also strongly recommended for genus and species identification, although the sensitivity of culture is low. In addition, there are no useful serological markers for detecting mucormycosis (15). Unfortunately, we were unable to obtain any positive results from cultures. We consulted ophthalmologists to obtain a biopsy specimen from the infected area; however, the ophthalmologists refused due to concerns about exacerbating the infection. A molecular analysis of the CSF at the National Institute of Infectious Diseases indicated Rhizopus species infection, which was very useful for diagnosing mucormycosis in the present case. Molecular methods, such as PCR combined with DNA sequencing, are useful for identifying the causative pathogen; however, this is not a commercial examination, so it is not easy to employ routinely in clinical settings at present. The development of a PCR assay for diagnosing mucormycosis may lead to further advances in the field (13,14).
In this case, the patient was treated for indigenous skin bacteria and was resistant to antibiotics therapy; thus, antibiotics therapy was switched as needed. However, fungal orbital cellulitis (invasive mold disease, such as mucormycosis and aspergillosis) should be strongly suspected in patients with an immunocompromised status due to aging, DM, and/or MDS with hyperferritinemia - like the present case (16,17) - and broad-spectrum antimicrobial therapy, including appropriate antifungal drugs, should be provided at the first treatment (1-3), even if β-D-glucan or culture tests are negative. The poorest prognosis is observed in patients with hematological malignancies or disseminated disease, especially to the central nervous system, as in the present patient (18). However, the implementation of first-line treatment with liposomal amphotericin B in the present case might have obtained a survival outcome. Steroid treatment may have provided short relief of pain in the present case; however, this was retrospectively considered undesirable and likely to promote deterioration due to the development of mucormycosis when performed in the absence of appropriate antifungal drugs.
Conclusion
This is a rare case of cutaneous-(rhino-)orbital-cerebral mucormycosis in a patient with MDS and DM. Mucormycosis should be considered as a differential diagnosis for immunocompromised hosts with refractory orbital cellulitis. Amplification of fungal DNA by PCR in collaboration with a specialized facility may be an important method for diagnosing mucormycosis.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported in part by a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from the Promotion and Mutual Aid Corporation for Private Schools of Japan.
Supplementary Material
Results of amplification of fungal DNA by PCR combined with DNA sequencing.
References
- 1. Alsulaiman HM, Al-Faky Y. Microbiology and outcome of pediatric orbital cellulitis in a Tertiary Eye Care Center in Saudi Arabia after the routine administration of Haemophilus influenzae Type B vaccine. Saudi J Ophthalmol 35: 239-243, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsirouki T, Dastiridou AI, Ibánez Flores N, et al. Orbital cellulitis. Surv Ophthalmol 63: 534-553, 2018. [DOI] [PubMed] [Google Scholar]
- 3. Kontoyiannis DP, Lewis RE. 259-Agents of mucormycosis and entomophthoramycosis. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th ed. Mandel GL, Bennet JE, Dolin R, Eds. Elsevier, Amsterdam, 2010: 3257-3270. [Google Scholar]
- 4. Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. ; Mucormycosis ECMM MSG Global Guideline Writing Group.. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19: e405-e421, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodrigues Morais J, Rodrigues Santos R, Pires Costa P, Fonseca T, Farinha F. Refractory orbital myositis in systemic lupus erythematosus a role for rituximab. Eur J Case Rep Intern Med 8: 003038, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin WV, Saumur M, Al-Mohtaseb Z. Scleritis, keratitis, and orbital cellulitis: isolated ocular manifestation of systemic lupus erythematosus. Lupus 27: 1985-1988, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Kim YR, Kim DY. Current status of the diagnosis and treatment of hemophagocytic lymphohistiocytosis in adults. Blood Res 56: S17-S25, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hammoudi DAS, Morar MM, Garbuzov A, Urias D, Katira KM. Lower extremity salvage in a diabetic patient with cutaneous mucormycosis and COVID-19 after open patella fracture. Ochsner J 22: 163-168, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hubbard DC 2nd, Fleenor JW, Su MG, Tsai JH. A 47-year-old man with a necrotic wound after trauma. Digit J Ophthalmol 27: 33-37, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gamaletsou MN, Sipsas NV, Roilides E, Walsh TJ. Rhino-orbital-cerebral mucormycosis. Curr Infect Dis Rep 14: 423-434, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Skiada A, Drogari-Apiranthitou M, Pavleas I, Daikou E, Petrikkos G. Global cutaneous mucormycosis: a systematic review. J Fungi (Basel) 8: 194, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. MacDonald ML, Weiss PJ, Deloach-Banta LJ, Comer SW. Primary cutaneous mucormycosis with a Mucor species: is iron overload a factor? Cutis 54: 275-278, 1994. [PubMed] [Google Scholar]
- 13. Lamoth F, Calandra T. Early diagnosis of invasive mould infections and disease. J Antimicrob Chemother 72: i19-i28, 2017. [DOI] [PubMed] [Google Scholar]
- 14. Dannaoui E. Recent developments in the diagnosis of mucormycosis. J Fungi (Basel) 8: 457, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Son HJ, Sung H, Park SY, et al. Diagnostic performance of the (1-3)-β-D-glucan assay in patients with Pneumocystis jirovecii compared with those with candidiasis, aspergillosis, mucormycosis, and tuberculosis, and healthy volunteers. PLoS One 12: e0188860, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsirouki T, Dastiridou AI, Ibánez Flores N, et al. Orbital cellulitis. Surv Ophthalmol 63: 534-553, 2018. [DOI] [PubMed] [Google Scholar]
- 17. Beer T, Vadakara J. Etiologies and short-term mortality in patients with ultraelevated serum ferritin. South Med J 108: 574-578, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Lanternier F, Dannaoui E, Morizot G, French Mycosis Study Group, et al. A global analysis of mucormycosis in France: the RetroZygo Study (2005-2007). Clin Infect Dis 54 (Suppl 1): S35-S43, 2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of amplification of fungal DNA by PCR combined with DNA sequencing.