Everyone needs social interactions, not only humans but also most species. Positive social interaction feedback is a reward, promoting more social behaviors and acting as a stress buffer. On the contrary, negative social interactions such as school bullying may become a type of stress that leads to mental illnesses including anxiety and depression disorders. Chronic social defeat stress (CSDS) is a widely accepted social trauma model in rodents based on repeated social attacks, following which the defeated mice can be classified as susceptible (SUS) and resilient (RES) according to their social performance. SUS mice rather than RES mice show significant social avoidance, anhedonia, weight loss, and endocrine disruption [1]. At present, the CSDS model is commonly used in the study of anxiety and depression, but its social effects are rarely studied. Li and colleagues’ work sheds light on how social defeats affect responses to social information, especially social reward [2].
Recent studies have suggested that social avoidance caused by chronic social failure is controlled by the suppression of mesolimbic dopaminergic reward centers: the ventral tegmental area (VTA) and the nucleus accumbens (NAc) [3, 4]. Besides reward deficiency, the mice being attacked have fear-enhancing phenotypes associated with the activity of the oxytocin pathway in the lateral septum (LS) [5]. Similarly, the activation of GABAergic neurons in the LS by acute stress causes hyperalgesia and anxiety-like behaviors [6]. However, most studies have focused on interactions between social defeat stress and mental abnormalities, such as depression-like behaviors. It is not clear how social trauma affects social rewards at the cellular and circuitry levels. Recently published in Nature, Li et al. revealed that a population of LS neurotensin (NTLS) neurons are hyperactivated following CSDS, gating the susceptibility to the stress and occluding social reward effects.
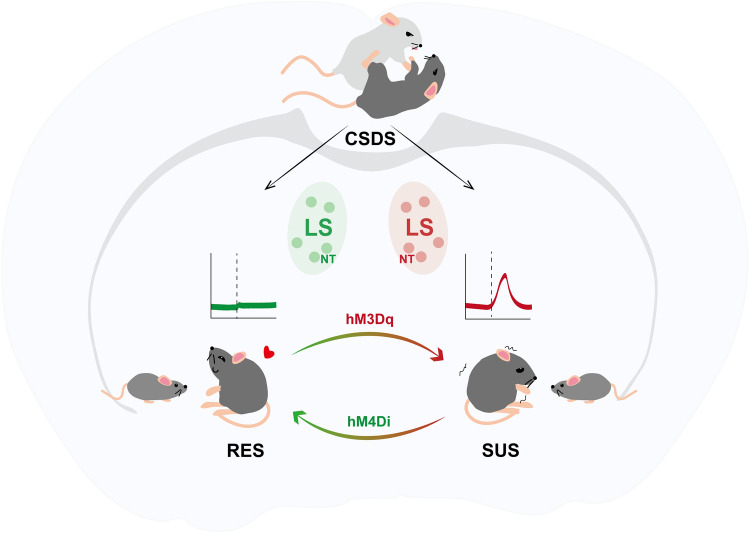
Based on the social interaction ratio, mice that underwent the CSDS were divided into RES and SUS groups, with SUS mice exhibiting social avoidance even toward non-threatening juvenile mice. At the same time, RES mice were more willing to take social interactions with juveniles which were recognized as rewards to them. Taking the learning and memory process into consideration, Li et al. found no deficits in non-social cognitive tests, supporting the conclusion that CSDS induces a specific disruption in social rewards but not in general reward processing. To characterize the neuronal types mediating the susceptibility, Li et al. performed ex vivo high-throughput screening of Fos with iDISCO+ and ClearMap assay on samples collected post-exposure to CSDS and found over 94% colocalization between Nt and Fos in SUS mice, while there was very limited overlap with Crhr2+, Oxtr+, Sst+, and Drd3+ neurons which were all functionally expressed in the LS. Moreover, NT neurons were specifically enriched in the lateral-ventral part of the LS, where the Fos accumulated. These findings confirmed that NT neurons that accumulated in the LS were highly activated in SUS mice, implying a role of NTLS neurons in gating social reward and social avoidance (Fig. 1).
Fig. 1.
NTLS neurons in socially defeated mice gate social reward processing. Li et al. showed that NTLS neurons in SUS mice were hyperactivated during the juvenile-paired session. Similar phenotypes were induced in RES mice by activating NTLS neurons with hM3Dq. In parallel, inhibition of NTLS neurons with hM4Di in SUS mice reversed the decreased social interaction that resulted from CSDS.
The LS has been demonstrated to be a nexus for mood and motivation, where NT neurons are enriched to modulate neuroendocrine activity and feeding behaviors [7]. However, it is not well-understood whether NTLS neurons respond to CSDS and how they control the social reward processing. Li et al. aimed to determine the association of NTLS hyperactivation with CSDS susceptibility in vivo. Combining fiber photometry with Nt-Cre transgenic mice, Li et al. examined the real-time NTLS neuronal activity of CSDS mice during their social interactions with juveniles, finding significantly higher fluorescent Ca2+ activity in SUS mice rather than RES or control (CTRL) mice. Notably, analogous elevated neuronal activity was observed when unstressed CTRL mice were experiencing tail pinch and social attack, either in female or male mice. These findings suggest that SUS mice may take juveniles as social threats as a result of the NTLS neuronal hyperactivation.
Next, to establish a causal relationship between the activity of NTLS neurons and changes in social behaviors after CSDS, Li et al. bidirectionally manipulated NTLS neuronal activity using the designer receptors exclusively activated by designer drugs (DREADDs) strategy. As demonstrated above, higher activity of NTLS neurons was related to the social reward deficits in SUS mice. In order to mimic the hyperactivation state, Li et al. activated NTLS neurons with hM3Dq tools in RES mice following CSDS. As expected, SUS-like phenotypes were induced in hM3Dq-RES mice, represented by reduced interaction durations in multiple behavioral tests. In parallel, inhibition of NTLS neurons with hM4Di in SUS mice reversed the decreased social interaction that resulted from CSDS. Moreover, manipulation of NTLS neurons had no significant effects on the general preference test without a social context. Consistent effects were found in mice of both sexes, verifying the modulation of NTLS neurons in social behavioral changes induced by CSDS. To further assess the actions of NTLS neurons in responding to stress, Li et al. replaced CSDS with non-social stressors such as chronic restraint stress, finding that anxiety-like behaviors rather than social interactions were modified. Overall, Li et al. showed that NTLS neuronal activity could be a bridge between social stress and social reward.
The LS provides widespread innervations to those brain regions regulating distinct behaviors. Li et al. took advantage of viral tracing and chemogenetic neuro-control to dissect the NTLS downstream targets mediating social behaviors. Among multiple regions identified, they mainly focused on the NAc, the anterior hypothalamic nucleus (AHN), the lateral hypothalamus, and the periaqueductal grey (PAG), based on their well-known social and reward involvement. With little overlap visualized on the application of triple stains to the NAc, AHN, and PAG, they demonstrated that LS neurons separately project to these regions, which was further confirmed by retrograde viral tracing. Li et al. also examined the functions of these NTLS circuits with optogenetics, showing that activating the NTLS→AHN or NTLS→NAc circuit, rather than the NTLS→PAG circuit reduces the duration of social investigation, while none of them had significant effects on social avoidance.
Previously, it had been shown that acute stress can activate LS GABAergic neurons to induce anxiety-like behaviors [6]. Therefore, LS hyperactivation in the social defeat model is not an entirely new concept. Nonetheless, Li and colleagues’ study is remarkable as it reveals that NTLS neurons in SUS mice are hyperactivated in response to CSDS, resulting in mistakenly perceiving juveniles as threats rather than rewards. Their study provides theoretical support for the clinical use of radiological examination to screen individuals with high susceptibility to social stress. It is also significant in offering potential targets for the medical diagnosis and treatment of social deficits accompanying post-traumatic stress disorder.
Meanwhile, several intriguing questions are raised. First, the NT is a neuropeptide known to modulate GABAergic neurons by increasing GABA release [8]. Yet, the GABA and NT signaling works in different modes: GABA is released at the basal activity level, while NT is recruited once neurons are over-activated and act like a brake to suppress over-reaction [7]. In the future, it will be necessary to dissect the actions of GABA and NT separately. This could be addressed by respectively blocking GABA or NT signaling with pharmacology or CRISPR/Cas9 techniques, followed by behavioral analysis to determine the individual effects of GABA and NT on the performance of mice, including susceptibility to social stress, perception of social reward, and anxiety-like behaviors. Second, according to cleared whole-brain Fos mapping, the pedunculopontine nucleus (PPN) and the mediodorsal nucleus of the thalamus (MD) are also highly activated in SUS mice. Since the PPN and MD play roles in reward and decision-making [9], it will be interesting to figure out whether the hyperactivity of these brain regions contributes to the deficits in social reward processing. Last, we human beings share a response to social stress similar to rodents; they can also be divided into SUS and RES subpopulations [10]. It is important to figure out why resilient people can minimize the adverse effects of social stress and how we can find ways to take precautions. Unfortunately, the reason why NTLS neurons are not hyperactivated in RES mice has not been examined. It will be interesting to investigate whether NTLS neurons in RES mice are suppressed by upstream inhibitory input or just blunted by an elevated threshold. However, either hypothesis needs further evidence from neural circuit mapping and electrophysiological assays.
In conclusion, this study broadens our understanding of the LS neural circuits underlying social reward deficits after social defeat stress, providing new insights for the clinical treatment of post-traumatic stress disorder and related psychiatric diseases.
Acknowledgements
This highlight article was supported by the National Natural Science Foundation of China (32100792 and 32200825), the Shandong Natural Science Foundation (ZR202111040168), and the Zhejiang Natural Science Foundation (LQ21C090003).
References
- 1.Golden SA, Covington HE, III, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Durand-de Cuttoli R, Aubry AV, Burnett CJ, Cathomas F, Parise LF, et al. Social trauma engages lateral septum circuitry to occlude social reward. Nature. 2023;613:696–703. doi: 10.1038/s41586-022-05484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Yao Y, Gao G, Liu K, Shi X, Cheng M, Xiong Y, et al. Projections from D2 neurons in different subregions of nucleus accumbens shell to ventral Pallidum play distinct roles in reward and aversion. Neurosci Bull. 2021;37:623–640. doi: 10.1007/s12264-021-00632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzmán YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, et al. Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci. 2013;16:1185–1187. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Pan X, Zhou Y, Wu Z, Ren K, Liu H, et al. Lateral septum-lateral hypothalamus circuit dysfunction in comorbid pain and anxiety. Mol Psychiatry. 2023;28:1090–1100. doi: 10.1038/s41380-022-01922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Chen G, Zhong J, Jiang S, Lai S, Xu H, et al. A circuit from lateral septum neurotensin neurons to tuberal nucleus controls hedonic feeding. Mol Psychiatry. 2022;27:4843–4860. doi: 10.1038/s41380-022-01742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrie KA, Schmidt D, Bubser M, Fadel J, Carraway RE, Deutch AY. Neurotensin activates GABAergic interneurons in the prefrontal cortex. J Neurosci. 2005;25:1629–1636. doi: 10.1523/JNEUROSCI.3579-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty S, Kolling N, Walton ME, Mitchell AS. Critical role for the mediodorsal thalamus in permitting rapid reward-guided updating in stochastic reward environments. Elife. 2016;5:e13588. doi: 10.7554/eLife.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci USA. 2020;117:3326–3336. doi: 10.1073/pnas.1914655117. [DOI] [PMC free article] [PubMed] [Google Scholar]