Abstract
Follistatin-like protein 1 (FSTL1) has been demonstrated to participate in the pathogenesis of several neurological diseases. The current study informed the role of H3K27 acetylation-induced FSTL1 upregulation in Alzheimer’s disease (AD). Our investigation discovered the upregulated FSTL1 expression and enhanced autophagy activity in AD. FSTL1 knockdown successfully attenuated the injuries of Aβ1−42-challenged SH-SY5Y cells through the inhibition of autophagy activity. Besides, FSTL1 deficiency suppresses the inflammatory response and NF-κB signaling in AD. Moreover, it was found that p300 was recruited by transcriptional factor RUNX1 to stimulate the H3K27 acetylation in FSTL1 promoter region, which caused the upregulation of FSTL1 in AD. To summarize, p300 acted as a co-activator of RUNX1 to trigger the activation of FSTL1 in AD, resulting in the exacerbated injuries and inflammatory responses of Aβ1−42-induced SH-SY5Y cells.
Keywords: FSTL1, H3K27 acetylation, Autophagy, Inflammation, Alzheimer’s disease
Introduction
Alzheimer’s disease (AD), one of the most prevalent neurodegenerative diseases, is characterized by gradually impaired ability in learning and memory (Takeda et al. 2021). More than 29.8 million people suffered from AD worldwide and the number is continuously increasing (Cao et al. 2018). The neuropathological hallmark of AD is the accumulation of amyloid plaques and the neurofibrillary tangles in the neurons (Moore and Martin 2018). Currently, the levels of tau, phospho-tau, and Aβ peptides in the cerebrospinal fluid (CSF) represent the key biomarkers for AD diagnosis in clinical practice (Cucos et al. 2022). However, widespread use of the CSF biomarkers is limited due to the invasiveness of collection by lumbar puncture (Hu et al. 2021). Therefore, it is imperative to excavate the molecular mechanisms underlying the pathogenesis of AD and identify novel biomarkers.
Follistatin-like protein 1 (FSTL1) is a secreted glycoprotein belonging to the Follistatin family (Szabó et al. 2020). Growing evidence manifests that FSTL1 is involved in the pathological and physiological processes of neurological diseases through mediating certain signaling pathways. For example, Xiao et al. revealed that in chronic unpredictable mild stress (CUMS)-induced mice, FSTL1 knockdown considerably suppressed CUMS-stimulated depression- or anxiety-like symptoms, synaptic plasticity deficits, microglia activation in CUMS-induced mice, and the expression of indicated proteins in the TLR4/MyD88/NF-κB pathway, suggesting that FSTL1 deficiency may ameliorate depression through the inhibition of the TLR4/MyD88/NF-κB pathway (Xiao et al. 2022). In another case, recombinant FSTL1 attenuated the neuronal apoptosis and neurological deficits via the phosphorylation of Akt by enhancing the activity of DIP2A (Liang et al. 2014). Xiang et al. reported that mice with FSTL1 genetic knockdown exhibited interrupted synaptic transmission and impaired cognitive learning and memory, possibly involving multiple underlying signaling pathway (Xiang et al. 2020). Noticeably, a recent publication revealed that knockdown of FSTL1 could improve neural oscillation in Aβ1–42 induced AD model mice (Kumari et al. 2023). Nevertheless, the mechanism causing the upregulation of FSTL1 in AD and the possible molecular mechanism involved remain unclarified.
Autophagy, an essential degradation pathway in mammalian cells to clear abnormal protein aggregates, is responsible for protein homeostasis and neuronal health (Qi et al. 2021). The dysfunction of autophagy has been implicated in the pathogenesis of AD due to its pivotal role in the generation and metabolism of Aβ and the accumulation of tau (Li et al. 2017). Previous studies have revealed that autophagy is involved in the FSTL1-mediated pathogenesis of human diseases. For example, FSTL1 induces epithelial-mesenchymal transition (EMT) and airway remodeling by intensifying autophagy in asthma (Liu et al. 2017). FSTL1 aggravates cigarette smoke-induced chronic obstructive pulmonary disease through the regulation of autophagy (Liu et al. 2021a, b). FSTL1 enhances autophagy activity and reduces the injuries of cardiomyocytes under hypoxia/ischemia conditions (Yang et al. 2019). It remains unverified whether FSTL1 affects the development of AD by mediating autophagy activity.
The present work was designed to investigate the potential and molecular mechanism of FSTL1 in AD. We assume that H3K27 acetylation-induced FSTL1 upregulation facilitates neuronal damage and inflammation via the modulation of autophagy activity and NF-κB signaling in AD. Our findings may help to better understand the role of FSTL1 in AD as a potent therapeutic biomarker.
Materials and methods
Serum samples
Twenty-two pairs of AD patients and age-matched healthy subjects were recruited to collect the serum samples at the 904th Hospital of Joint Logistic Support Force of PLA. The samples were stored at − 80 °C until use. Protocol for this investigation was approved by the Ethics Committee of 904th Hospital of Joint Logistic Support Force of PLA (Approval No. 20,231,031,003). All participants signed a written consent form.
Cell culture and treatment
The human neuroblastoma cell line SH-SY5Y was purchased from the American Type Culture Collection (Manassas, USA) and maintained in DMEM culture medium (HyClone, USA) containing 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin under 5% CO2 at 37 °C. To establish the AD cell model, SH-SY5Y cells were incubated with 10 µM Aβ1−42 for 24 h. To stimulate or inhibit autophagy, SH-SY5Y cells were treated with 100 nM rapamycin (LC Laboratories, USA) for 1 h or 10 µM Chloroquine for 12 h, which are widely used activator and inhibitor for autophagy activities, respectively (Shao et al. 2022; Feng et al. 2018).
Cell transfection
shFSTL1 and its matched negative control (shNC), RUNX1 and p300 overexpression plasmids (oe-RUNX1 and oe-p300), and empty pcDNA3.1 vector as control were all purchased from Gene Pharma (Shanghai, China). These vectors were transfected into SH-SY5Y cells using Lipofectamine 2000 (Invitrogen, USA) following the manufacturer’s instructions. The cells were used for subsequent experiments 48 h after transfection.
RT-qPCR
The total RNA was isolated using the reagent TRIzol (Invitrogen, USA). cDNA was synthesized using a Takara cDNA synthesis kit. Quantitative RT-PCR was performed in a 7500 Real-Time PCR system using SYBR Premix Ex Taq kit (Both from Takara, China). Gene expression was normalized to GAPDH and analyzed using the 2−ΔΔCT method.
Western blot
The total protein was isolated using RIPA lysis buffer (Beyotime, China). Nuclear extracts only for NF-κB p65 analysis were obtained with a nuclear extraction buffer. Protein concentrations were quantified using the BCA method. 50 µg samples were loaded and separated using 10% SDS-PAGE and then transferred to PVDF membranes to be incubated with 5% non-fat milk for 2 h. After washing with PBS three times, the membranes were incubated with the primary antibodies against FSTL1, LC3, Bcl-2, cleaved caspase-3, Beclin-1, P62, IκBα, p-IκBα, NF-κB p65, and GAPDH overnight at 4 °C. Subsequently, the membranes were washed and incubated with HRP-conjugated secondary antibodies for another 2 h at room temperature. The protein bands were visualized using an enhanced chemiluminescence reagent (EMD Millipore, USA), and the intensity of bands was quantified by ImageJ 1.52a (National Institutes of Health, USA).
ELISA
The levels of TNF-α, IL-1β, and IL-6 in the supernatant of treated SH-SY5Y cells were determined using ELISA kits according to the manufacturer’s instructions.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed using an EZ-Magna ChIP kit (Millipore, USA) following previous steps (Zhang et al. 2021). Briefly, cells were fixed with 1% formaldehyde and then incubated with 0.125 M glycine to terminate the cross-linking. The cross-linked DNA was sonicated and then incubated with anti-H3K27ac antibody or IgG overnight at 4 °C (CST, USA). Antibody-protein complexes were precipitated with protein G beads. Purified DNA was analyzed using RT-qPCR.
Luciferase reporter assay
Transcription activity was analyzed by a luciferase reporter gene assay using the Dual-Luciferase reporter assay system according to the manufacturer’s instructions (Promega). The luciferase activity was normalized to that of the Renilla luciferase.
CCK-8 assay
Cell viability was evaluated with a CCK-8 kit. Briefly, cells (2 × 103 cells per well) were plated in 96-well plates and incubated at 37 °C. CCK-8 dilution was added to incubate with the cells at indicated time points, and then the absorbance at 450 nm was measured to determine cell proliferation.
Measurement of LDH release
Cellular cytotoxicity was evaluated using a commercial LDH kit (Biovision, China). In brief, treated SH-SY5Y cells (5 × 105 cells/well) were seeded into 96-well plates and cultured overnight at 37 °C. The supernatant of each well (50 µl) was transferred to another 96-well plate, and 100 mL LDH solution was added followed by a 30 min incubation in the dark. The absorbance of each well was determined at 490 nm using a microplate reader (Becton Dickinson, USA).
TUNEL
Cell apoptosis was analyzed using a TUNEL assay. Treated SH-SY5Y cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. TUNEL reaction mixture was subsequently incubated with the cells for 1 h Next, the TUNEL-stained cells were counterstained with DAPI (2 µg/ml; Beyotime Institute of Biotechnology). Images were acquired from five randomly selected fields of view using a fluorescence microscope.
ROS detection
To examine intracellular ROS levels, treated SH-SY5Y cells were collected and incubated at 37 °C with cell-permeable 2′,7′-dichlorofluorescin diacetate (DCFH-DA, 10 µM) for 30 min according to the manufacturer’s instructions. The level of ROS was determined by the FACSC Calibur flow cytometer (BD Biosciences).
Statistical analysis
Data are presented as the mean ± standard deviation (SD) and were analyzed using GraphPad Prism software. Each experiment was repeated no less than three times. Comparison between two groups was conducted using Student’s t-test; the comparison in multiple groups was analyzed by a one-way ANOVA followed with Tukey’s post hoc test. P < 0.05 was considered statistically significant.
Results
FSTL1 upregulation and increased autophagy activity were identified in AD
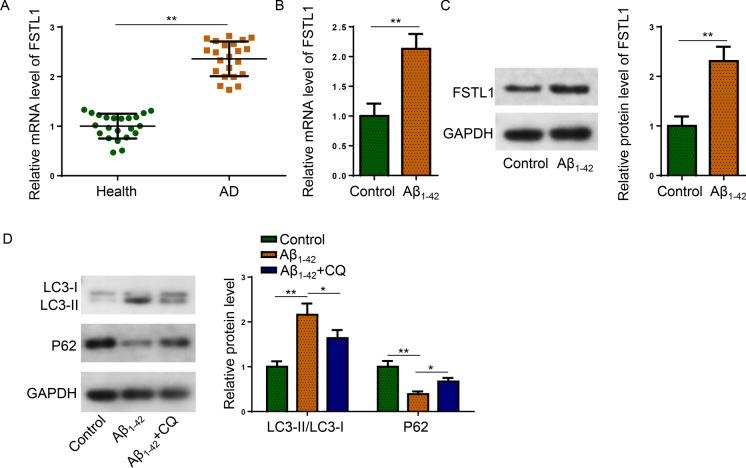
To evaluate the expression pattern of FSTL1 in AD, RT-qPCR detected that the mRNA level of FSTL1 was significantly elevated in AD patient serum samples (Fig. 1A). Besides, FSTL1 mRNA and protein levels were both elevated by Aβ1−42 treatment in SH-SY5Y cells (Fig. 1B, C). For the determination of autophagy activity, western blot showed that the LC3-II/LC3-I ratio was substantially elevated and P62 protein expression was remarkably suppressed by Aβ1−42 in SH-SY5Y cells, which was effectively reversed by the introduction of Chloroquine (Shao et al. 2022) (Fig. 1D). Taken together, abundant FSTL1 expression and enhanced autophagy activity were identified in AD.
Fig. 1.
FSTL1 expression and autophagy activity were increased in AD. A The mRNA expression of FSTL1 in serum samples were detected by RT-qPCR (Student’s t-test; **P < 0.01). B, C The mRNA and protein expression of FSTL1 were detected in Aβ1−42-treated and untreated SH-SY5Y cells (Student’s t-test; **P < 0.01). D Protein expression of LC3 and P62 were detected in untreated (Control), Aβ1−42-treated, or Aβ1−42 + Chloroquine co-treated SH-SY5Y cells (One-way ANOVA; *P < 0.05, **P < 0.01)
FSTL1 knockdown alleviated the injury of Aβ1−42-challenged SH-SY5Y cells
Next, the effect of FSTL1 on Aβ1−42-induced injuries of SH-SY5Y cells was investigated. FSTL1 was successfully silenced as indicated by the noticeably reduced mRNA and protein levels (Fig. 2A, B). Subsequently, the CCK-8 assay detected that cell proliferation impaired by Aβ1−42 treatment was markedly revived by FSTL1 depletion (Fig. 2C). TUNEL assay indicated that FSTL1 deficiency attenuated the apoptosis of SH-SY5Y cells enhanced by Aβ1−42 (Fig. 2D). Besides, FSTL1 knockdown abrogated the increase of cleaved caspase-3 expression and the decrease of Bcl-2 level in SH-SY5Y cells caused by Aβ1−42 (Fig. 2E). Moreover, the elevated LDH release and ROS production in Aβ1−42-induced SH-SY5Y cells were dramatically suppressed by silencing FSTL1 (Fig. 2F, G). In sum, the injuries of SH-SY5Y cells induced by Aβ1−42 were attenuated by FSTL1 knockdown.
Fig. 2.
Effect of FSTL1 on Aβ1−42-induced injuries in SH-SY5Y cells. Aβ1−42-challenged SH-SY5Y cells were transfected with shNC or shFSTL1. A The mRNA level expression of FSTL1 by RT-qPCR (Student’s t-test; ***P < 0.001). B The protein expression of FSTL1 was detected by western blot (Student’s t-test; **P < 0.01). C Cell proliferation was observed via CCK-8 assay (One-way ANOVA; *P < 0.05, **P < 0.01). D Cell apoptosis was evaluated using TUNEL assay (One-way ANOVA; *P < 0.05, **P < 0.01). E Protein expression of cleaved caspase-3, and Bcl-2 was detected (One-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001). F LDH release was detected by a commercial LDH kit (One-way ANOVA; **P < 0.01, ***P < 0.001). G Intracellular ROS production was analyzed using flow cytometry (One-way ANOVA; *P < 0.05, **P < 0.01)
FSTL1 exacerbated Aβ1−42-induced injuries in SH-SY5Y cells by enhancing autophagy
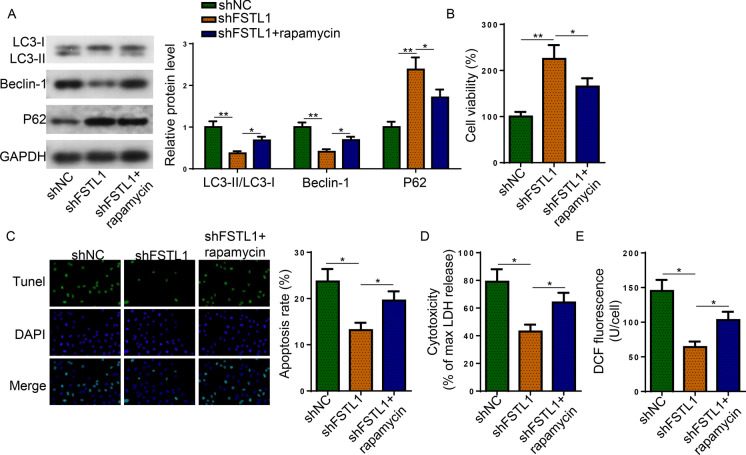
To determine the participation of autophagy in FSTL1-mediated AD progression, rapamycin was introduced to Aβ1−42-challenged SH-SY5Y cells following FSTL1 knockdown. The decrease of LC3-II:LC3-I ratio and Beclin-1 protein expression as well as the increase of P62 protein level caused by FSTL1 deficiency were abrogated by rapamycin (Fig. 3A). Furthermore, the protective effect of shFSTL1 on the injuries of Aβ1−42-challenged SH-SY5Y cells was abated by rapamycin (Fig. 3B–E). These results indicated that FSTL1 mediated Aβ1−42-induced injuries in SH-SY5Y cells through regulating autophagy activity.
Fig. 3.
FSTL1 induced autophagy to exacerbate Aβ1−42-induced injuries. Aβ1−42-challenged SH-SY5Y cells were treated with shNC, shFSTL1, or shFSTL1 + rapamycin. A The protein expression of LC3, Beclin-1, and P62 was detected. B–E The proliferation (B), apoptosis (C), LDH release (D), and ROS production (E) of treated cells were evaluated. (One-way ANOVA; *P < 0.05, **P < 0.01)
FSTL1 depletion suppressed the secretion of pro-inflammatory cytokines by deactivating NF-κB signaling
Neuroinflammation induced by Aβ is essential in the pathogenesis of AD (Huang et al. 2018). Herein, we assumed that FSTL1 promoted the pro-inflammatory effect of Aβ by enhancing the activity of NF-κB signaling. ELISA assay observed that FSTL1 knockdown significantly reduced the dramatically increased level of the pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in Aβ1−42-challenged cells (Fig. 4A). Besides, FSTL1 depletion attenuated the phosphorylation (p) of IκBα in Aβ1–42-challenged SH-SY5Y cells (Fig. 4B). In the meantime, the expression of NF-κB p65 subunit was suppressed in the nucleus but was promoted in the cytoplasm by shFSTL1 transfection (Fig. 4C). These data suggested the inhibitory effect of FSTL1 knockdown on Aβ1−42-induced inflammatory responses and activation of NF-κB signaling.
Fig. 4.
FSTL1 activated the NF-κB signaling to promote the secretion of pro-inflammatory cytokines. SH-SY5Y cells divided into three groups: untreated control, Aβ1−42, and Aβ1−42 + shFSTL1. A ELISA assay detected the expression of TNF-α, IL-1β, and IL-6 in the cell supernatants B Western blot detected the expression of IκBα and p-IκBα with GAPDH used as control. C Cytoplasmic and nuclear NF-κB p65 were analyzed by western blot. (One-way ANOVA; *P < 0.05, **P < 0.01)
p300 modulated the H3K27 acetylation to facilitate the transcription of FSTL1 in AD
Subsequently, the factor that caused the upregulation of FSTL1 in AD was explored. UCSC database indicated that H3K27ac was greatly enriched in the promoter region of FSTL1 (Fig. 5A). To confirm whether histone acetylation regulated the transcription of FSTL1, sodium butyrate (NaBu), an inhibitor of histone deacetylase, was employed to treat Aβ1−42-challenged SH-SY5Y cells. NaBu treatment caused a significant elevation in the protein level of FSTL1 and enrichment level of H3K27ac (Fig. 5B, C). Besides, it was observed that H3K27ac enrichment at FSTL1 promoter region was markedly increased in Aβ1−42-treated cells (Fig. 5D), suggesting that histone acetylation might be the epigenetic mechanism regulating the transcription of FSTL1 in AD. Afterward, it was verified whether the histone acetyltransferase p300 is responsible for the activation of FSTL1 transcription in AD. Figure 5E illustrated that treatment with p300 inhibitor A485 remarkably suppressed the mRNA and protein expression of FSTL1 in Aβ1−42-challenged SH-SY5Y cells. Oppositely, p300 overexpression significantly elevated FSTL1 expression (Fig. 5F). More importantly, the enrichment of H3K27ac at FSTL1 promoter region was reduced by A485 and augmented by p300 abundance (Fig. 5G, H), implicating that p300 activated the acetylation of FSTL1 in AD.
Fig. 5.
p300 induces an upregulation of FSTL1 by modulating H3K27 acetylation. A UCSC database predicted the H3K27ac enrichment in the promoter region of FSTL1. B Western blot detected the protein level of FSTL1 in Aβ1−42-challenged SH-SY5Y cells treated or untreated with Nabu (Student’s t-test; **P < 0.01). C ChIP assay assessed the H3K27ac enrichment in Aβ1−42-challenged SH-SY5Y cells treated or untreated with Nabu (One-way ANOVA; ***P < 0.001). D H3K27ac enrichment in SH-SY5Y cells treated or untreated with Aβ1−42 (One-way ANOVA; ***P < 0.001). E The mRNA and protein expression of FSTL1 in Aβ1−42-challenged SH-SY5Y cells treated or untreated with A485 (Student’s t-test; **P < 0.01, ***P < 0.001). F The mRNA and protein expression of FSTL1 in Aβ1−42-challenged SH-SY5Y cells treated or untreated with p300 overexpression plasmid (Student’s t-test; **P < 0.01). G, H Aβ1−42-challenged SH-SY5Y cells were grouped into control and A485 (G) or control and pcDNA3.1/p300 (H) to detect H3K27ac enrichment in the promoter region of FSTL1 (Student’s t-test; **P < 0.01)
RUNX1 acted as a co-activator for p300 in the acetylation of FSTL1 in AD
Based on the above findings, we further investigated the transcriptional factor recruiting p300 to the target promoter region of FSTL1. JASPER website predicted that FSTL1 might be transcriptionally activated by RUNX1 which was previously reported to recruit p300 to the promoter region of the target gene (Chen et al. 2019). Western blot and Luciferase reporter assay proved that RUNX1 overexpression increased the expression and transcription activity of FSTL1 in Aβ1−42-challenged SH-SY5Y cells (Fig. 6A, B). Furthermore, cells co-transfected with RUNX1 and p300 overexpression plasmids exhibited the most significant increase in FSTL1 protein level compared with cells transfected with either oe-RUNX1 or oe-p300 (Fig. 6C). To summarize, RUNX1 recruited p300 to the promoter region of FSTL1, resulting in the stimulated acetylation of FSTL1 in AD.
Fig. 6.
RUNX1 recruited p300 to co-activate the transcription of FSTL1 in AD. A, B The protein level and transcription activation of FSTL1 were determined in Aβ1−42-challenged SH-SY5Y cells transfected with pcDNA3.1/RUNX1 using western blot and luciferase reporter assay (Student’s t-test; **P < 0.01, ***P < 0.001). C Aβ1−42-challenged SH-SY5Y cells were divided into four groups: control, oe-RUNX1, oe-p300, and oe-RUNX1 + oe-p300. Western blot detected the protein level of FSTL1 (One-way ANOVA; ***P < 0.001)
Discussion
FSTL1 was originally identified to be upregulated in osteoblast cells under the stimulation of transforming growth factor-beta 1 (TGF-beta1) (Gu et al. 2018). Studies have suggested that FSTL1 regulates the development of human organs, including the ureter, lung, and central nervous system (Zhang and Wang 2020). Besides, FSTL1 has been manifested to exert contrasting effects on the progression of different types of cancers (Loh et al. 2021; Liu et al. 2021a, b; Li et al. 2020). More importantly, FSTL1 is associated with inflammatory responses in human diseases. For instance, FSTL1 induces M1 polarization and inflammation during liver fibrosis by modulating PKM2 (Rao et al. 2022). FSTL1 depletion suppressed inflammatory responses through the TLR4/MyD88/NF-κB and MAPK pathway in atherosclerosis (Guo et al. 2016). FSTL1 activates the NLRP3/IL-1β signaling and stimulates inflammation in asthmatic mice (Wang et al. 2021). The current investigation first evaluated the expression pattern of FSTL1 in AD and showed that FSTL1 was significantly upregulated in the serum samples of AD patients as well as in the SH-SY5Y cells following Aβ1−42 treatment. We further investigated the effect of FSTL1 on Aβ1−42-induced injuries in SH-SY5Y cells. Experimental data indicated that FSTL1 knockdown substantially enhanced the proliferation, suppressed apoptosis, reduced LDH release, and inhibited ROS production of SH-SY5Y cells after Aβ1−42 stimulation. These data proved that FSTL1 silence had ameliorative effects on Aβ1−42-induced injuries in SH-SY5Y cells.
Considering that autophagy has been reported to participate in FSTL1-mediated pathogenesis, we also evaluated autophagy activity in Aβ1−42-treated SH-SY5Y cells. Western blot revealed that the LC3-II/LC3-I ratio was elevated while the P62 protein level was reduced by Aβ1−42-treatment, which could all be reversed by the introduction of the autophagy inhibitor, Chloroquine, indicating the abnormal activation of autophagy in Aβ1−42-challenged SH-SY5Y cells. Excessive autophagy has been identified to be involved in the progression of neurodegenerative diseases including AD. For instance, Huang et al. proved that oxygen and glucose deprivation/reperfusion (OGD/R) augmented autophagy activity in neurons and compound K suppresses autophagy-mediated apoptosis via the regulation of AMPK-mTOR pathway (Huang et al. 2020). Tang et al. demonstrated that lncRNA RMRP silence suppressed Aβ1−42-stimulated autophagy and neuronal apoptosis through miR-3142/TRIB3 axis in AD (Tang et al. 2022). Wang et al. revealed that Gastrodin protected astrocytes from LPS-triggered excessive autophagy and apoptosis (Wang et al. 2016). To confirm that autophagy activation was involved in FSTL1-mediated injuries in Aβ1−42-induced SH-SY5Y cells, the autophagy activator, rapamycin, was employed to treat Aβ1−42-induced SH-SY5Y cells after FSTL1 silence. It was discovered that the protective effect of FSTL1 silence on SH-SY5Y cells from Aβ1−42-induced injuries was remarkably abrogated by the employment of rapamycin. Taken together, FSTL1 may regulated AD progression through the mediation of autophagy activity.
The therapeutic significance of NF-κB signaling in neurodegenerative diseases has been extensively investigated (Camandola and Mattson 2007; Ali et al. 2020; Li et al. 2018). Particularly, NF-κB-mediated inflammatory responses are closely correlated with the pathogenesis of AD (Nan et al. 2019; Chen et al. 2022; Dong et al. 2019). Interestingly, FSTL1 has been discovered to activate the NF-κB signaling in many cases. Zhang et al. revealed that FSTL1 induced the activation of NF-κB pathway in osteosarcoma cells that the phosphorylated level of NF-κB was reduced by FSTL1 knockdown and elevated by FSTL1 overexpression (Zhang et al. 2020). In rat chondrocytes, FSTL1 promote the expression of matrix metalloproteinase, secretion of inflammatory factors, and the activation of NF-κB pathway (Hu et al. 2019). Another study reported that FSTL1 treatment caused dramatic production of p-IκBα in the cytoplasm and NFκBp65 in the nucleus whereas inhibition of the NF-κB pathway reduced the inflammation and catabolism stimulated by FSTL1 in human primary chondrocytes (Qu et al. 2019). In this study, we investigated the FSTL1 depletion on the inflammatory responses and the status of NF-κB signaling in Aβ1−42-induced SH-SY5Y cells. The knockdown of FSTL1 substantially suppressed the secretion of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6). For the NF-κB signaling, the phosphorylation of IκBα was suppressed and the NF-κB p65 subunit was transferred from nucleus to the cytoplasm in shFSTL1-treated cells. In sum, we revealed that FSTL1 deficiency inhibited Aβ1−42-induced inflammation and activation of NF-κB pathway in AD model cell.
Existing studies have indicated that FSTL1 is aberrantly expressed in multiple diseases and cancers (Kudo-Saito et al. 2018; Mattiotti et al. 2018). However, the mechanism causing the dysregulation of FSTL1 is poorly understood. Histone acetylation and deacetylation are epigenetic mechanisms elaborately regulating the transcription of genes (Zhao et al. 2019). The present investigation proved that H3K27ac was enriched at the FSTL1 promoter region and inhibition of histone deacetylase by NaBu caused increase in both FSTL1 protein expression and H3K27ac enrichment in Aβ1−42-induced SH-SY5Y cells. It was hypothesized that the transcription of FSTL1 may be regulated by histone acetyltransferase p300. The hypothesis was verified as p300 inhibitor suppressed FSTL1 expression and H3K27ac enrichment at FSTL1 promoter region; oppositely, p300 overexpression promoted the expression of FSTL1 and the enrichment of H3K27ac. Furthermore, histone acetyltransferases p300 is a global transcriptional coactivator that participates in the regulation of various transcriptional factors (Ogryzko et al. 1996). For instance, DUX4 recruits p300/CBP to induce global H3K27 acetylation changes in human myogenic cells (Choi et al. 2016). p300 recruited by c-Jun facilitated H3K27 acetylation to activate the transcription of COL6A1 in osteosarcoma (Zhang et al. 2021). Herein, we demonstrated that RUNX1 transcriptionally activated FSTL1 and combined treatment with RUNX1 and p300 overexpression plasmids elevated FSTL1 expression more significantly than overexpressing RUNX1 or p300 alone in AD cell model, suggesting that p300 was recruited to the promoter region of FSTL1 to acetylate FSTL1 in AD.
In conclusion, RUNX1 and p300 co-activated the transcription of FSTL1 through the modulation of H3K27 acetylation, which exacerbated autophagy-mediated injuries and activated NF-κB to stimulate inflammatory responses in Aβ1−42-challenged SH-SY5Y cells.
Author contributions
DD and JX designed this study. YZ and FC performed all the experiments. YZ and DD analyzed the data and prepared the figures. FC, BZ and LM drafted the initial manuscript. YZ reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Protocol for this investigation was approved by the Ethics Committee of 904th Hospital of Joint Logistic Support Force of PLA.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dongmei Dai and Junzheng Xie contribute equally to this research and should be considered as co-first author.
References
- Ali A, et al. NF-κB inhibitors attenuate MCAO induced neurodegeneration and oxidative stress—a reprofiling approach. Front Mol Neurosci. 2020;13:33. doi: 10.3389/fnmol.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Camandola S, Mattson MP. NF-κB as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets. 2007;11:123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- Cao J, et al. Advances in developing novel therapeutic strategies for Alzheimer’s disease. Mol Neurodegeneration. 2018;13:64. doi: 10.1186/s13024-018-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, et al. The RUNX1-ETO fusion protein trans-activates c-KIT expression by recruiting histone acetyltransferase P300 on its promoter. FEBS J. 2019;286:901–912. doi: 10.1111/febs.14751. [DOI] [PubMed] [Google Scholar]
- Chen S, et al. The Neuroprotection of Verbascoside in Alzheimer’s Disease Mediated through Mitigation of Neuroinflammation via Blocking NF-κB-p65 Signaling. Nutrients. 2022 doi: 10.3390/nu14071417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, et al. DUX4 recruits p300/CBP through its C-terminus and induces global H3K27 acetylation changes. Nucleic Acids Res. 2016;44:5161–5173. doi: 10.1093/nar/gkw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucos CA. Sulfiredoxin-1 blood mRNA expression levels negatively correlate with hippocampal atrophy and cognitive decline. F1000Res. 2022;11:114. doi: 10.12688/f1000research.76191.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong P, et al. Oxymatrine exhibits anti-neuroinflammatory effects on Aβ(1–42)-induced primary microglia cells by inhibiting NF-κB and MAPK signaling pathways. Int Immunopharmacol. 2019;74:105686. doi: 10.1016/j.intimp.2019.105686. [DOI] [PubMed] [Google Scholar]
- Feng H, et al. Apatinib-induced protective autophagy and apoptosis through the AKT-mTOR pathway in anaplastic thyroid cancer. Cell Death Dis. 2018;9:1030. doi: 10.1038/s41419-018-1054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, et al. FSTL1 interacts with VIM and promotes colorectal cancer metastasis via activating the focal adhesion signalling pathway. Cell Death Dis. 2018;9:654. doi: 10.1038/s41419-018-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, et al. Knockdown of FSTL1 inhibits oxLDL-induced inflammation responses through the TLR4/MyD88/NF-κB and MAPK pathway. Biochem Biophys Res Commun. 2016;478:1528–1533. doi: 10.1016/j.bbrc.2016.08.138. [DOI] [PubMed] [Google Scholar]
- Hu PF, et al. Follistatin-like protein 1 (FSTL1) promotes chondrocyte expression of matrix metalloproteinase and inflammatory factors via the NF-κB pathway. J Cell Mol Med. 2019;23:2230–2237. doi: 10.1111/jcmm.14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CJ, et al. Assessment of high risk for Alzheimer’s disease using plasma biomarkers in subjects with normal cognition in Taiwan: a preliminary study. J Alzheimers Dis Rep. 2021;5:761–770. doi: 10.3233/ADR-210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XF, et al. Geniposide attenuates Aβ(25–35)-induced neurotoxicity via the TLR4/NF-κB pathway in HT22 cells. RSC Adv. 2018;8:18926–18937. doi: 10.1039/C8RA01038B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, et al. Compound K inhibits autophagy-mediated apoptosis induced by oxygen and glucose deprivation/reperfusion via regulating AMPK-mTOR pathway in neurons. Life Sci. 2020;254:117793. doi: 10.1016/j.lfs.2020.117793. [DOI] [PubMed] [Google Scholar]
- Kudo-Saito C, et al. Blocking the FSTL1-DIP2A Axis improves anti-tumor immunity. Cell Rep. 2018;24:1790–1801. doi: 10.1016/j.celrep.2018.07.043. [DOI] [PubMed] [Google Scholar]
- Kumari E, et al. FSTL1-knockdown improves neural oscillation via decreasing neuronal-inflammation regulating apoptosis in Aβ(1–42) induced AD model mice. Exp Neurol. 2023;359:114231. doi: 10.1016/j.expneurol.2022.114231. [DOI] [PubMed] [Google Scholar]
- Li Q, Liu Y, Sun M. Autophagy and Alzheimer’s Disease. Cell Mol Neurobiol. 2017;37:377–388. doi: 10.1007/s10571-016-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Sibon OCM, Dijkers PF. Inhibition of NF-κB in astrocytes is sufficient to delay neurodegeneration induced by proteotoxicity in neurons. J Neuroinflammation. 2018;15:261. doi: 10.1186/s12974-018-1278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. Follistatin-like 1 (FSTL1) is a prognostic biomarker and correlated with immune cell infiltration in gastric cancer. World J Surg Oncol. 2020;18:324. doi: 10.1186/s12957-020-02070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, et al. Follistatin-like 1 attenuates apoptosis via disco-interacting protein 2 homolog A/Akt pathway after middle cerebral artery occlusion in rats. Stroke. 2014;45:3048–3054. doi: 10.1161/STROKEAHA.114.006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, et al. Autophagy plays a role in FSTL1-induced epithelial mesenchymal transition and airway remodeling in asthma. Am J Physiol Lung Cell Mol Physiol. 2017;313:L27–l40. doi: 10.1152/ajplung.00510.2016. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. FSTL1 aggravates cigarette smoke-induced airway inflammation and airway remodeling by regulating autophagy. BMC Pulm Med. 2021;21:45. doi: 10.1186/s12890-021-01409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YK, et al. FSTL1 increases cisplatin sensitivity in epithelial ovarian cancer cells by inhibition of NF-κB pathway. Cancer Chemother Pharmacol. 2021;87:405–414. doi: 10.1007/s00280-020-04215-9. [DOI] [PubMed] [Google Scholar]
- Loh JJ, et al. FSTL1 secreted by activated fibroblasts promotes Hepatocellular Carcinoma Metastasis and Stemness. Cancer Res. 2021;81:5692–5705. doi: 10.1158/0008-5472.CAN-20-4226. [DOI] [PubMed] [Google Scholar]
- Mattiotti A, et al. Follistatin-like 1 in development and human diseases. Cell Mol Life Sci. 2018;75:2339–2354. doi: 10.1007/s00018-018-2805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BD, Martin J. Short Aβ peptides attenuate Aβ42 toxicity in vivo. J Exp Med. 2018;215:283–301. doi: 10.1084/jem.20170600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan K, et al. HMGB1 gene silencing inhibits neuroinflammation via down-regulation of NF-κB signaling in primary hippocampal neurons induced by Aβ(25–35) Int Immunopharmacol. 2019;67:294–301. doi: 10.1016/j.intimp.2018.12.027. [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, et al. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/S0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Qi LF, et al. Ganoderic acid A promotes Amyloid-β Clearance (In Vitro) and Ameliorates cognitive deficiency in Alzheimer’s disease (mouse model) through autophagy induced by activating Axl. Int J Mol Sci. 2021;22:5559. doi: 10.3390/ijms22115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Liu Y, Li R. FSTL1 promotes inflammatory reaction and cartilage catabolism through interplay with NFκB Signaling Pathways in an in vitro ONFH model. Inflammation. 2019;42:1491–1503. doi: 10.1007/s10753-019-01012-2. [DOI] [PubMed] [Google Scholar]
- Rao J, et al. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut. 2022;71:2539. doi: 10.1136/gutjnl-2021-325150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao T, et al. Autophagy regulates differentiation of ovarian granulosa cells through degradation of WT1. Autophagy. 2022;18:1864–1878. doi: 10.1080/15548627.2021.2005415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó MR, et al. Modulatory effect of myokines on reactive oxygen species in ischemia/reperfusion. Int J Mol Sci. 2020;21:9382. doi: 10.3390/ijms21249382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, et al. Mitochondrial ubiquitin ligase alleviates Alzheimer’s disease pathology via blocking the toxic amyloid-β oligomer generation. Commun Biol. 2021;4:192. doi: 10.1038/s42003-021-01720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z-B, et al. LncRNA RMRP accelerates autophagy-mediated neurons apoptosis through miR-3142/TRIB3 signaling axis in alzheimer’s disease. Brain Res. 2022;1785:147884. doi: 10.1016/j.brainres.2022.147884. [DOI] [PubMed] [Google Scholar]
- Wang XS, et al. Protective Effects of Gastrodin against autophagy-mediated astrocyte death. Phytother Res. 2016;30:386–396. doi: 10.1002/ptr.5538. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. FSTL1 aggravates OVA-induced inflammatory responses by activating the NLRP3/IL-1β signaling pathway in mice and macrophages. Inflamm Res. 2021;70:777–787. doi: 10.1007/s00011-021-01475-w. [DOI] [PubMed] [Google Scholar]
- Xiang S, et al. Knockdown of Follistatin-like 1 disrupts synaptic transmission in hippocampus and leads to cognitive impairments. Exp Neurol. 2020;333:113412. doi: 10.1016/j.expneurol.2020.113412. [DOI] [PubMed] [Google Scholar]
- Xiao X, et al. Knockdown of FSTL1 inhibits microglia activation and alleviates depressive-like symptoms through modulating TLR4/MyD88/NF-κB pathway in CUMS mice. Exp Neurol. 2022;353:114060. doi: 10.1016/j.expneurol.2022.114060. [DOI] [PubMed] [Google Scholar]
- Yang W, et al. Follistatin-like 1 attenuates ischemia/reperfusion injury in cardiomyocytes via regulation of autophagy. BioMed Res. 2019;2019: 9537382. doi: 10.1155/2019/9537382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang Z. Circular RNA hsa_circ_0004812 impairs IFN-induced immune response by sponging mir-1287-5p to regulate FSTL1 in chronic hepatitis B. Virol J. 2020;17:40. doi: 10.1186/s12985-020-01314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, et al. Sulforaphene inhibits the progression of osteosarcoma via regulating FSTL1/NF-κB pathway. Life Sci. 2020;263:118485. doi: 10.1016/j.lfs.2020.118485. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics. 2021;11:1473–1492. doi: 10.7150/thno.51245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, et al. HDAC3 inhibition prevents blood-brain barrier permeability through Nrf2 activation in type 2 diabetes male mice. J Neuroinflammation. 2019;16:103. doi: 10.1186/s12974-019-1495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.