Abstract
Human mesenchymal stem cells (hMSCs) possess broad prospects in pre-clinical research. In vitro amplification of hMSCs requires appropriate medium to reach the number of seed cells with clinical significance. However, the uncertainty of the heterologous components of the traditional fetal bovine serum (FBS) culture medium has great safety risks. Moreover, existing commercial hMSCs medium is very expensive, therefore a safer and more optimal hMSCs medium is urgently needed. Accordingly, we developed five components adipose-derived hMSCs (hADMSCs) medium without xenogenic components, named E5 SFM. which is mainly composed of knockout serum replacement (KSR), and additionally four components such as fibroblast growth factor and transferrin. Here, we mainly compared the E5 SFM with traditional FBS-containing medium and a commercial medium by surface markers testing, proliferation assay as well as osteogenic, adipogenic and chondrogenic differentiation assessment. We demonstrated that hADMSCs cultured in the E5 SFM showed similar morphological characteristics and immunophenotypes to those in other media. Notably, cell proliferative capability was similar to that in the commercial medium, but higher than that in the FBS-containing medium and other media. Additionally, their capabilities of adipogenic and osteogenic differentiation were significantly higher than those of other media. Consequently, we concluded that the E5 SFM medium can not only effectively promote cell proliferation of hMSCs, but also has optimal differentiative capacity and clear and simple ingredients.
Keywords: Mesenchymal stem cells, Serum-free, Xeno-free, Cell culture medium
Introduction
Mesenchymal stem cells are adult stem cells with immune regulation function and multi-directional differentiation potential to be differentiated into mesenchymal lineages such as osteogenesis, adipogenesis and chondrogenesis, which can be used as seed cells or gene therapy carriers (Ankrumet al. 2014; Ha et al. 2020). Therefore, they can serve clinically as a potential therapeutic tool and have broad application prospects in cell therapy (Panwar et al. 2021). Generally, human mesenchymal stem cells (hMSCs) can be isolated from the bone marrow, umbilical cord, placenta, fat, and tooth tissue. As observed in our previous study, hMSCs derived from fat are rich in content (Wei et al. 2014), less invasive, and not easy to involve ethical issues, in addition to greater promising benefits brought by adipose tissue (Schneider et al. 2017; Hoang et al. 2022). However, the hMSCs isolated from any kind of tissue are limited to reach the quantity required for clinical application, then culture medium is a key factor for amplification in vitro (Czapla et al. 2019), which plays a decisive role in cell expansion and immunophenotype. In previous studies, culture media containing 10% fetal bovine serum (FBS) was usually used for hMSCs culture. FBS is a mixture of multiple components, contains hormones, growth factors, enzymes, proteins and other undefined constituents, etc., and many of its components are not clearly analyzed. Additionally, xenogenic hallmark has increased its ethical and safety regards (Hemeda et al. 2014; Schepici et al. 2022).
For human cells, FBS is a heterogeneous component with potential risks such as unknown virus infection of animal origin, immune reaction and inter-batch variation (Martin et al. 2022). For example, the extracellular vesicles (EVs) contained in the FBS, including derived secretions, will also pollute the secretions from stem cells, and EV will affect clinical and experimental results due to batch changes (Lee et al. 2022; Urzì et al. 2022). Recently, human platelet lysate (HPL) is used to substitute for FBS to hMSCs culture (Lei et al. 2022). Platelet derivatives can promote in vitro expansion of mesenchymal stem/stromal cells due to platelet-derived growth factors (Burnouf et al. 2016). However, HPL production came under scrutiny due to human welfare concerns with reliability in clinical applications. Additionally, the commercial medium without animal origin and heterologous ingredients can better promote the generation expansion of human hMSCs and maintain the multidirectional differentiation potential, but its high cost limits the need of clinical mass expansion. Therefore, there is a need to develop a hMSCs medium without animal origin or xenogeneic components that supports large scale expansion of hMSCs.
To eliminate the disadvantageous factors of FBS and human origin ingredients, it is significant to develop serum-free medium with definite composition. In the serum-free biochemically defined medium, insulin, epidermal growth factor, transferrin, fibroblast growth factor, heparin and knockout serum replacement (KSR) were added based on DMEM/F-12 medium to constitute a E5 SFM medium. It can maintain cell growth, and its components are simple and clear. In addition, insulin, epidermal growth factor, transferrin and fibroblast growth factor are common components of serum-free medium (Chen and Rabinovitch 1989). Moreover, the addition of heparin will make epidermal growth factor and fibroblast growth factor play the greatest role (Zhang et al. 2021).
Serum replacement is used for the culture of pluripotent stem cells, which was applied to the expansion and culture of hMSCs in vitro. We investigated the ability of KSR to substitute for FBS in adipose-derived hMSCs (hADMSCs) cultures. KSR, which has clear chemical composition, is serum-free, and a source of synthetic protein without undefined growth factor. Additionally, KSR can eliminate many disadvantages of stem cell culture using FBS, including cell differentiation and thermal inactivation. In previous research, we have achieved good results in using KSR to culture embryonic stem cells (Li et al. 2016), so we wonder whether KSR can be used as a substitute for serum to culture hADMSCs. In this study, we extracted hMSCs from human adipose tissue, and compared hADMSCs cultured in the E5 SFM medium with traditional 10% FBS in DMEM, 5% KSR in DMEM/F12, 5% HPL in DMEM/F12 and a commercial StemPro MSC SFM XenoFree medium. In this study, we explored and compared cell proliferative capacity, multilineage differentiations and multipassage assay of hADMSCs cultured in the different media. Significantly, the E5 SFM medium made up for the disadvantage of 10% FBS medium. Therefore, the relatively safe and xeno-free culture medium developed in this study is very suitable for the large-scale expansion of hADMSCs, and is helpful to establish the optimal clinical application conditions for hADMSCs culture.
Materials and methods
Isolation, medium composition and culture of hADMSCs
After obtaining informed consent, adipose tissues were prepared from four female healthy donors, aged between 25 and 45. Procedures were ratified by the Ethics Committee at the Affiliated Huai'an Hospital of Xuzhou Medical University. Fat extraction mixture was first washed using DPBS to take out anesthetic and other substances. Then, the adipose tissue was placed into a new centrifuge tube, which was digested with 0.2% collagenase type I (Gibco, Billings, Montana, USA) for 1 h at 37 °C, and then the undigested tissue was removed through 100 μm strainers, and washed with DPBS and centrifuge. The remaining pellet was suspended and plated in flasks containing different media: (1) StemPro SFM: StemPro MSC SFM XenoFree (Gibco, Billings, Montana, USA); (2) 10% FBS: 10% FBS in DMEM (Gibco, Billings, Montana, USA); (3) E5 SFM: 1 mg/mL recombinant human insulin (Sigma Aldrich, San Francisco, USA), 10 ng/mL recombinant human Epidermal Growth Factor (MCE, Monmouth Junction, USA), 10 ng/mL recombinant human basic Fibroblast Growth Factor (MCE, Monmouth Junction, USA), 1 µg/mL heparin (STEM CELL, Vancouver, BC, Canada) and 5% KSR (Gibco, Billings, Montana, USA) in DMEM/F12 medium (CORNING, Manassas, USA); (4) 5% KSR: 5% KSR (Gibco, Billings, Montana, USA) in DMEM/F12 medium; (5) 5% HPL: 5% HPL (BPM, Tianjin, China) in DMEM. Subsequently, hADMSCs were cultured at 37 °C in a humidified 5% CO2 incubator. When the cells grow to 80–90% confluence, they are passaged and inoculated with 2 × 105/mL density on T75 flask.
The population doubling time
The population doubling time (PDT) was tested from passage 0 to passage 5, and hADMSCs were cultured in a 6-well plate at 1 × 105 cells/well. Cell number was calculated using the formula PDT = t × log2/log (Nf/Ni), where t is the time of cell culture, Ni is the initial number of cells, and Nf is the final number of cells. The cell number was tested by Countess II Automated Cell Counter. Setting 3 technical replicates.
Immunophenotyping
The phenotype of hADMSCs cultured in different media was determined directly by flow cytometry. Cells were gently detached and prepared by single cell suspensions. Cells were washed and adjusted to 1 × 106/mL in stain buffer (BD Biosciences, San Diego, USA), and incubated for a half hour on ice protected from light with 5 μL fluorescent antibodies CD34, CD45, CD73, CD90, CD105, CD11b, CD19 and HLA-DR (562577, 560178, 561254, 555596, 562408, 742638, 560728, 552764, BD Biosciences, San Diego, USA). Resuspended the cell pellet, and stained cells were analyzed by flow cytometry (BD Biosciences, San Jose, USA). Data processing was performed by Flow Jo version 10.
Proliferative capacity
2000 Cells per well were seeded in 96-well plates. 10 μL of CCK8 solution (VC5001L, VICMED, Xuzhou, China) was added to each well of the plate, setting 16 technical replicates per well. Tests were carried out at 6 h, 24 h, 48 h and 72 h. Optical density (OD) was measured at 450 nm by Glomax Discover (Promega, Sunnyvale, USA).
Osteogenic, adipogenic and chondrogenic differentiation
For osteogenesis, hADMSCs were cultured in DMEM medium (Gibco, Billings, Montana, USA) supplemented with 10% FBS (Gibco, Billings, Montana, USA), 10 mM sodium β-glycerophosphate (50020, Sigma Aldrich, San Francisco, USA), 0.2 mM vitamin C (A4403, Sigma Aldrich, San Francisco, USA) and 0.1 μM dexamethasone (D4902, Sigma Aldrich, San Francisco, USA). For adipogenesis, hADMSCs were cultured in DMEM medium (Gibco, Billings, Montana, USA) supplemented with 10% FBS, 5 μM rosiglitazone (R2408, Sigma Aldrich, San Francisco, USA), 10 μg/mL of insulin (91077C, Sigma Aldrich, San Francisco, USA), 0.5 mM IBMX (I7018, Sigma Aldrich, San Francisco, USA), 0.1 mM vitamin C (A4403, Sigma Aldrich, San Francisco, USA), and 1 μM dexamethasone (D4902, Sigma Aldrich, San Francisco, USA). For chondrogenic differentiation, 5 × 105 cells were transferred to a 15 mL centrifuge tube and centrifuged at 300 × g for 5 min. the pellets were cultured in DMEM medium (Gibco, Billings, Montana, USA) supplemented with 20 ng/mL TGFβ1 (P00121, Solarbio, Beijing, China), 10 ng/mL of insulin (91077C, Sigma Aldrich, San Francisco, USA), 0.1 mM vitamin C (A4403, Sigma Aldrich, San Francisco, USA), and 100 nM dexamethasone (D4902, Sigma Aldrich, San Francisco, USA). 3 Technical replicates were set per experiment and repeated 3 times. And the differentiation medium was changed every 3 days.
ALP staining and assay
Cells were stained with ALP for 7 days at osteogenesis induction, and were washed thrice with PBS, 4% paraformaldehyde fixation for 20 min, washed thrice with distilled water, and stained was performed according to BCIP/NBT Alkaline Phosphatase Color Development Kit (C3206, Beyotime, Shanghai, China) protocol for 30 min, and rinsed with distilled water to terminate the reaction. Then, stained plates were photographed by Stereo microscope (OLYMPUS CX21FS1, Tokyo, Japan) and inverted light microscopy (Nikon ECLIPSE Ti, Tokyo, Japan). Furthermore, 4 technical replicates were set and repeated 3 times. Then, 5 images were selected from each group to analyse positive area of ALP staining by ImageJ Version 1.8.0.
Oil red O staining and assay
Oil red O staining were performed at the end of 14th day to detect adipogenic ability, and cells were fixed with 4% paraformaldehyde fixation for 30 min, and distilled water rinsing three times. 60% Oil red O (O0625, Sigma) was used to stain in the dark according to the staining situation, rinsed with distilled water, and stained images were obtained under microscope. Isopropanol extracts the dye from cells and reads the absorbance value at 490 nm by Glomax Discover (Promega, Sunnyvale, USA). 4 Technical replicates were set and repeated 3 times. 5 Images from each group were selected to analyse positive area of Oil red O staining as well as extracting of the dye by isopropanol.
Alcian blue staining
After 3 weeks of chondrogenic differentiation, the chondrogenic pellets have been harvested, and fixed with 4% paraformaldehyde for 30 min. Cryosection and stain with Alcian blue (C0155, Beyotime, Shanghai, China) were performed to examine the presence or production of extracellular matrix of chondrogenic differentiation. The staining effect of chondrogenic differentiation was observed under a microscope, in addition to photograph and induction evaluation. The endogenous acid glycosaminoglycan in the cartilage tissue can be dyed blue green by Alcian blue. 3 Technical replicates were set per experiment and repeated 3 times.
Statistical analysis
The data was analyzed using software GraphPad Prism 8.0 (La Jolla, CA, USA). Results were presented as mean ± standard deviation (SD). For the comparison of cell proliferation data, two-way ANOVA was used, and for other date, a one-way ANOVA was performed to detect statistical differences, and statistically significant was considered if p < 0.05.
Results
hADMSCs showed typical morphological and biological characteristics in the E5 SFM culture medium
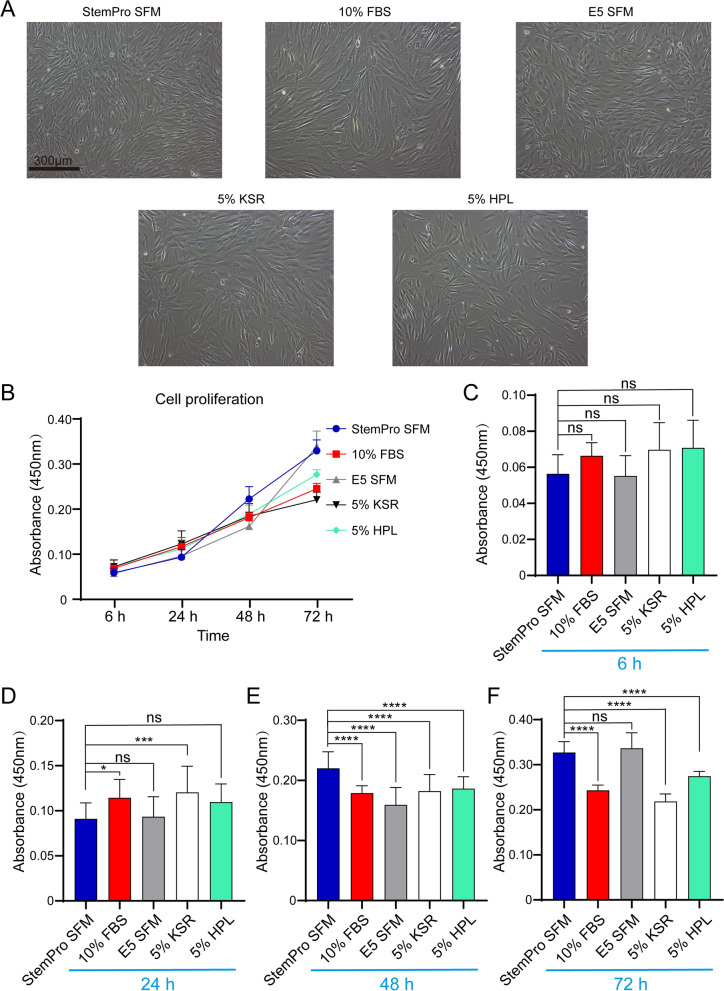
The morphology of hADMSCs, which were cultured to the passage 3 in different media, was compared after cell adherence. hADMSCs were subcultured until they reached about 80% of confluence and passaged at a density of 2 × 104 cells/cm2 in a well of 6-well plate. We mainly compared E5 SFM medium, the commercial medium StemPro MSC SFM XenoFree and traditional 10% FBS medium. At the same time, the effect of culture medium composed of 5% KSR and 5% HPL of other two single components on hADMSCs was also observed. We found that hADMSCs presented typical slender spindle-shaped fibroblasts-like shape in the E5 SFM medium, and had plastic adhesion, and that there was no significant difference when compared with traditional 10% FBS, commercial hADMSCs media and other two media (Fig. 1A).
Fig. 1.
The morphological characteristics and proliferative capacity of hADMSCs in the different culture media. A The representative image shows the microscopic morphological observation after 48 h of cell adhesion. B Growth curve at different time points is obtained by cell counting kit-8 (CCK8) assay in the different culture media. C OD value was measured at 450 nm at 6 h. D OD value was measured at 450 nm at 24 h. E OD value was measured at 450 nm at 48 h. F OD value was measured at 450 nm at 72 h. Data are mean ± SD (n = 16). StemPro SFM StemPro MSC SFM XenoFree, 10% FBS 10% FBS in DMEM, E5 SFM DMEM/F12 medium supplemented with 1 mg/mL insulin, 10 ng/mL epidermal growth factor, 10 ng/mL fibroblast growth factor, 1 µg/mL heparin and 5% KSR, 5% KSR 5% KSR in DMEM/F12 medium, 5% HPL 5% HPL in DMEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001, ns no statistical difference, compared with indicated group
Proliferative capacity of hADMSCs in medium of E5 SFM is nearly equivalent to commercial medium
We tested viable cell number in terms of the proliferation of hADMSCs in different media used cell counting kit-8 (CCK-8) assay. At 0–24 h, the cells were in the incubation adhesion phase, and the proliferation effect was not obvious. The cell cycle of each cell was different, so there were slight differences between the groups, but there was no statistical difference of cell proliferation in different media at 6 h (Fig. 1B, C). At the end of 24 h, they were no significant different in cell proliferation between E5 SFM medium and 5% HPL medium and commercial medium. Only 10% FBS medium and 5% KSR medium had faster cell proliferation than commercial medium (Fig. 1B, D). After entering the logarithmic growth phase, cell proliferation accelerated. At the 48 h time point, the cell counts by CCK-8 assay in commercial medium was significantly higher than that in other media. After 48 h, the cell counts by CCK-8 assay of E5 SFM medium increased (Fig. 1B, E). The growth of hADMSCs was gradually accelerated for up to 72 h after inoculation. At the 72 h time point, the cell counts by CCK-8 assay of E5 SFM medium was similar to that of commercial medium, and there was no statistical difference. And the cell counts by CCK-8 assay of E5 SFM medium was significantly higher than that of other media. At the 72 h time point, the cell counts by CCK-8 assay of hADMSCs in the E5 SFM medium was similar to that of commercial medium, and there was no significant difference compared with the commercial medium, but was significantly higher than that in the 10% FBS medium, 5% HPL medium and 5% KSR medium (Fig. 1B, F). Taken together, these results indicated that E5 SFM medium could be suitable for and able to promote cell growth.
The morphology of primary hADMSCs and population doubling time in the passage 0 until passage 5 before characterization in different media
In order to observe the performance of different culture media on the initial isolated hADMSCs, we observed the growth of cells at different time points in the original culture. After 1 day of adherent growth, fewer cells could be seen, and the morphology of the cells presented a nearly circular or irregular shape (Fig. 2A). Over time, more cells could be seen, and the cell morphology gradually changed to a typical fibroblast morphology. Starting from the fifth day, it could be seen that there are significantly more cells growing in the E5 SFM and StemPro SFM group than in the other groups, and by the nineth day, it was even more pronounced (Fig. 2A). To further analyze the differences in cell growth quantity, we counted the number of cells per unit area. The results further confirmed that the proliferation of hADMSCs with passage 0 in the E5 SFM and StemPro SFM were significantly higher than that of other groups (Fig. 2B). In order to further test the cell proliferation ability of primary cultured hADMSCs, we measured the cell doubling time from passage 0 to passage 5. We found that doubling time of E5 SFM and StemPro SFM were close from passage 0 to passage 5, but the doubling time of these two groups were significantly lower than that of the other groups (Fig. 2C).
Fig. 2.
The morphology of primary hADMSCs and population doubling time in different media. A The representative image shows the morphological observation from the first day to the ninth day after cell adhesion. B The number of cells per unit area were analyzed by ImageJ software. Data are mean ± SD (n = 9). C The population doubling time is obtained by cell count in the passage 0 until passage 5 in different media. Data are mean ± SD (n = 6)
hADMSCs possess classical cell surface makers in the medium of E5 SFM
To test whether hADMSCs cultured in different media meet the requirements of MSC phenotypes stipulated by the International Society of Cell and Gene Therapy (ISCT), we mainly examined the expression of hADMSCs cell surface markers of the traditional 10% FBS medium, commercial medium StemPro SFM and E5 SFM medium using flow cytometry. FACS analysis showed that most hADMSCs population express CD90, CD73 and CD105 (> 95%), and absent expression (< 5%) of CD45, CD11b, CD34, CD14, CD19 and HLA-DR in these five culture media (Fig. 3A–F). The positive expression was defined as fluorescence greater than 95% of the unstained cell control group. The negative expression was defined as a fluorescence below 5% of the unstained cell control group. This result showed that hADMSCs cultured in different media all could meet the requirements of MSCs phenotypes stipulated by the ISCT. And it had no significant difference from 10% FBS medium, commercial medium StemPro SFM and E5 SFM medium, indicating that hADMSCs cultured in the E5 SFM medium containing KSR maintained hMSC unique immune phenotype.
Fig. 3.
Flow cytometric analysis of the expression surface markers of cultured hADMSCs in the different culture media. Fluorescent intensity histogram of control and experimental antibody stains. Unstained control cells (turquoise line), experimental antibody stained cells (red line). A Unstained control cells. B hADMSCs in the commercial culture medium StemPro MSC SFM XenoFree. C hADMSCs in the 10% FBS in DMEM. D hADMSCs in the E5 SFM culture medium. E 5% KSR in DMEM/F12 medium. F 5% HPL in DMEM/F12 medium. (Colour figure online)
hADMSCs grown in E5 SFM medium display better capacity of osteogenesis and adipogenesis differentiation
The osteogenic or adipogenic and chondrogenic lineage differentiation capacity of hADMSCs is a n ecessary requirement for MSCs cultured in vitro. ALP staining assays detected the expression of ALP, an early marker of osteoblasts, at the 7th day of osteogenic induction, which proved that hADMSCs maintained the osteogenic differentiation potential in these media (Fig. 4A, B). The lipid droplets were stained with Oil red O on the 14th day of lipogenic induction. The hADMSCs cultured in these media had lipid droplets formation after adipogenic induction, and had good adipogenic differentiation potential (Fig. 5A, B). Our statistical analysis of the positive area of ALP staining and Oil red O staining showed that the osteogenic and adipogenic capacity of the StemPro MSC SFM XenoFree medium was higher than that of the traditional medium containing 10% FBS and other media, but was lower than that of E5 SFM medium, by means of statistical analysis of the positive area of ALP staining, Oil red O staining and the dye in cells was extracted with isopropanol (Figs. 4C, 5C, D). At 21 days after chondrogenic differentiation induction, we also observed that the cells cultured in these media could be stained with Alcian blue, showing the endogenous acid glycosaminoglycan could be dyed blue green (Fig. 6A, B). Through high-power microscopy, it could be seen that the chondrocyte differentiation effect of the cells cultured in the E5 SFM group was relatively significant, with stronger and more uniform cell differentiation (Fig. 6C). The results demonstrated that the E5 SFM medium was better than the StemPro MSC SFM XenoFree medium and traditional FBS-containing medium in supporting osteogenic or adipogenic and chondrogenic lineage differentiation.
Fig. 4.
ALP staining assays detected the expression of ALP. A Observe the staining condition of each well after ALP staining at 7th day of osteogenesis induction under anatomical microscope. ALP staining was performed to compare the ability of osteogenesis. B The representative image shows ALP staining of cells after induction by the microscope. C Positive area of ALP was normalized to the total area. Data are mean ± SD (n = 5). *p < 0.05; **p < 0.01, ns no statistical difference, compared with indicated group. Control without osteogenic differentiation induction. Yellow box indicates local magnification. (Colour figure online)
Fig. 5.
Oil red O staining indicated the effect of culture medium on adipogenic differentiation of hADMSCs at day 14. A Observe the staining condition of each well after oil red O staining at 14th day of adipogenesis induction under anatomical microscope. Oil red O staining was performed to compare the ability of adipogenesis. B The representative image shows Oil red O staining of cells after induction by the microscope. C Positive area of Oil red O was normalized to the total area. D The dye in cells was extracted with isopropanol and the OD value was measured at a wavelength of 490 nm using a microplate reader for quantitation. Data are mean ± SD (n = 5). Control without adipogenic differentiation induction. ****p < 0.0001, ns no statistical difference, compared with indicated group. Yellow box indicates local magnification. (Colour figure online)
Fig. 6.
Alcian blue staining indicated the effect of culture medium on chondrogenic differentiation of hADMSCs at day 21. A Observe the condition of each chondrogenic pellets without Alcian blue staining. B The representative image shows Alcian blue staining of cells after induction by the microscope. C The representative image shows Alcian blue and Nuclear Fast Red staining of cells after induction by the microscope
hADMSCs grown in E5 medium exhibit typical characteristics after long-term and multiple generations of culture
In order to further observe the effect of E5 SFM on MSCs multi-generation culture, we observed the morphology of cells of passage 1, 5, and 10 (Fig. 7A), tested the ability of osteogenesis, adipogenesis and chondrogenesis at passage 10 (Figs. 7B, C, D), and tested the markers on the cell surface at passage 1, 5, and 10 (Fig. 8A–D). The results showed that hADMSCs could still show typical characteristics after multiple generations of culture in E5 SFM.
Fig. 7.
Multipassage assay in E5 SFM medium. A The representative image shows the morphological observation from the passage1 to 10 in E5 SFM medium. B Observe osteogenic lineage differentiation potential of hADMSCs in E5 SFM medium at day 7 by ALP staining. C Observe adipogenic lineage differentiation potential of hADMSCs in E5 SFM medium at day 14 by Oil red O staining. D Observe chondrogenic lineage differentiation potential of hADMSCs in E5 SFM medium at day 21 by Alcian blue staining. Yellow box indicates local magnification. (Colour figure online)
Fig. 8.
Flow cytometric analysis of the expression surface markers of cultured hADMSCs in E5 SFM medium. Fluorescent intensity histogram of control and experimental antibody stains. Unstained control cells (turquoise line), experimental antibody stained cells (red line). (Colour figure online)
Discussion
One of the major concerns of hMSCs culture is to ensure that it has strong proliferative capacity without compromising its phenotype and characteristics at the same time. In this study, we tested the effects of several different media formulations on hADMSCs, aiming to develop a serum-free and xeno-free, safe and economic medium suitable for clinical large-scale expansion. Although FBS, as a component of traditional culture medium, is beneficial to cell growth, it also contains many unknown components, which has caused serious concerns in clinical use. Additionally, concentrations of FBS components, such as growth factors, vary between batches (van der Valk et al. 2010). Moreover, medium composed of animal serum may lead to immune rejection by host during hMSCs transplantation (Haque et al. 2015). FBS also produces risk of infection by non-human pathogens (Urzì et al. 2022). For safety reasons, the use of animal-derived ingredients in clinical cell therapy is no longer the first choice. As a substitute of FBS, there should be defined composition and low pollution risk.
In order to address these problems, we have developed a new type of culture medium without animal origin and xenobiotic ingredients. According to the ISCT definition of MSCs, MSCs must have plastic adhesion, which express CD90, CD73 and CD105, and lack CD34, CD45, CD14 or CD11b, CD79α Or CD19 and HLA-DR surface makers, and which have capacities of osteogenetic or adipogenetic and chondrogenic differentiation (Le Blanc and Mougiakakos 2012). From these aspects, we analyzed the basic characteristics of hADMSCs growing in different media, including morphology, proliferative ability, multi-lineage differentiation potential, and immunophenotype. Furthermore, we evaluated the impact of the E5 SFM medium on the biological characteristics of hADMSCs, and observed whether it could replace the traditional FBS medium and a commercial medium, so that the cultured hADMSCs could meet the clinical application.
We identified that the hADMSCs cultured in the E5 SFM medium have similar morphology and phenotype with the traditional FBS medium and commercial medium. The cell proliferation ability cultured in E5 SFM medium is similar to that of commercial medium, and its differentiation ability is better than that of commercial medium and FBS medium. At the same time, we also confirmed that the addition of growth factors and other components on the basis of KSR is superior to the simple KSR in terms of cell proliferation, adipogenesis and osteogenesis. Previous studies have shown that KSR or adding insulin on top of KSR could promote cell survival by the PI3K-AKT pathway (Ishii et al. 2015). And we have also found in previous studies that AKT plays an important role in the proliferation of hADMSCs (Li et al. 2016). There are also studies indicating that KSR could upregulate lipid metabolism, providing necessary energy sources (Jin et al. 2018). EGF and bFGF could promote cell proliferation by regulating the proportion of cells that can enter the cell cycle from a resting state or promoting the expression of other factors (Chen and Rabinovitch 1989; Yu et al. 2012). Specifically, heparin could enhance the formation of high affinity complexes between the growth factors and their receptors. In addition, heparin could also prevent the protein hydrolysis of growth factors (Zhang et al. 2021). Moreover, previous studies have confirmed that KSR, EGF, and bFGF can promote the differentiation of MSCs and other cells (Jin et al. 2018; Khan et al. 2020). We mainly refer to the product instructions and literature reports for the usage concentrations of these components (Khan et al. 2020). Compared with the traditional culture medium containing FBS, the E5 SFM medium has no animal origin, and the ingredients are relatively defined, which can reduce the risk of animal-origin pollution and disease transmission. Here, our results show that the new type xeno-free and serum-free medium of hADMSCs can be used as a substitute for traditional FBS medium and commercial hADMSCs medium, and can be used for large-scale expansion of clinical hADMSCs.
Conclusion
In brief, we have developed the medium containing KSR, which is xeno-free and serum-free. hADMSCs cultured in this medium is better than or close to commercial medium in terms of morphology, phenotype, proliferation and differentiation.
Acknowledgements
We would like to thank Professor Weiren Pan and Jian Wang from Xuzhou Medical University for critical reading as well as proof reading of the manuscript during the writing and review. Especially, we thank Jiangsu Key Laboratory of Brain Disease Bioinformation, Research Center for Biochemistry and Molecular Biology, Xuzhou Medical University.
Author contributions
SC carried out data curation, formal analysis, methodology and writing-original draft. Investigation, validation and visualization were performed by LM. SW contributed to investigation and methodology. YX carried out methodology, project administration, supervision. WC was responsible for project administration, resources, supervision. JW was responsible for project administration, resources, supervision, writing-review and editing. All authors read and approved the final manuscript.
Funding
This work was supported by Xuzhou Medical University (to J. W.).
Data availability
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Declarations
Conflict of interest
All authors have no competing conflict of interest to declare.
Ethical approval
The study was approved by Ethics Committee at the Affiliated Huai'an Hospital of Xuzhou Medical University, approval number (Project No. HEYLL202158).
Informed consent
After obtaining written informed consent, adipose tissues that were prepared from healthy donors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenbin Chen, Email: Chenwenbin69@163.com.
Jianfeng Wei, Email: wjf@xzhmu.edu.cn.
References
- Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf T, Strunk D, Koh MB, Schallmoser K. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–387. doi: 10.1016/j.biomaterials.2015.10.065. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rabinovitch PS. Platelet-derived growth factor, epidermal growth factor, and insulin-like growth factor I regulate specific cell-cycle parameters of human diploid fibroblasts in serum-free culture. J Cell Physiol. 1989;140:59–67. doi: 10.1002/jcp.1041400108. [DOI] [PubMed] [Google Scholar]
- Czapla J, Matuszczak S, Kulik K, Wiśniewska E, Pilny E, Jarosz-Biej M, et al. The effect of culture media on large-scale expansion and characteristic of adipose tissue-derived mesenchymal stromal cells. Stem Cell Res Ther. 2019;10:235. doi: 10.1186/s13287-019-1331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020;9:1157. doi: 10.3390/cells9051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque N, Kasim NH, Rahman MT. Optimization of pre-transplantation conditions to enhance the efficacy of mesenchymal stem cells. Int J Biol Sci. 2015;11:324–334. doi: 10.7150/ijbs.10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014;16:170–180. doi: 10.1016/j.jcyt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7:272. doi: 10.1038/s41392-022-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Nhiayi MK, Tse E, Cheng J, Massimino M, Durden DL, et al. Knockout serum replacement promotes cell survival by preventing BIM from inducing mitochondrial cytochrome C release. PLoS ONE. 2015;10:e0140585. doi: 10.1371/journal.pone.0140585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JX, Lee S, Setyawan EMN, Taweechaipaisankul A, Kim GA, Han HJ, et al. A potential role of knockout serum replacement as a porcine follicular fluid substitute for in vitro maturation: lipid metabolism approach. J Cell Physiol. 2018;233:6984–6995. doi: 10.1002/jcp.26489. [DOI] [PubMed] [Google Scholar]
- Khan AA, Huat TJ, Al Mutery A, El-Serafi AT, Kacem HH, Abdallah SH, et al. Significant transcriptomic changes are associated with differentiation of bone marrow-derived mesenchymal stem cells into neural progenitor-like cells in the presence of bFGF and EGF. Cell Biosci. 2020;10:126. doi: 10.1186/s13578-020-00487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kang MH, Jang JE, Lee JE, Yang Y, Choi JY, et al. Comparative analysis of mesenchymal stem cells cultivated in serum free media. Sci Rep. 2022;12:8620. doi: 10.1038/s41598-022-12467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei T, Liu Y, Deng S, Xiao Z, Yang Y, Zhang X, et al. Hydrogel supplemented with human platelet lysate enhances multi-lineage differentiation of mesenchymal stem cells. J Nanobiotechnol. 2022;20:176. doi: 10.1186/s12951-022-01387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Wei JF, Fan LY, Wang SH, Zhu L, Li TP, et al. miR-302 regulates pluripotency, teratoma formation and differentiation in stem cells via an AKT1/OCT4-dependent manner. Cell Death Dis. 2016;7:e2078. doi: 10.1038/cddis.2015.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KE, Kalelkar PP, Coronel MM, Theriault HS, Schneider RS, García AJ. Host type 2 immune response to xenogeneic serum components impairs biomaterial-directed osteo-regenerative therapies. Biomaterials. 2022;286:121601. doi: 10.1016/j.biomaterials.2022.121601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar U, Mishra K, Patel P, Bharadva S, Vaniawala S, Shah A, et al. Assessment of long-term in vitro multiplied human Wharton's jelly-derived mesenchymal stem cells prior to their use in clinical administration. Cells Tissues Organs. 2021;210:239–249. doi: 10.1159/000517423. [DOI] [PubMed] [Google Scholar]
- Schepici G, Gugliandolo A, Mazzon E. Serum-free cultures: could they be a future direction to improve neuronal differentiation of mesenchymal stromal cells? Int J Mol Sci. 2022;23:6391. doi: 10.3390/ijms23126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Unger M, van Griensven M, Balmayor ER. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur J Med Res. 2017;22:17. doi: 10.1186/s40001-017-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzì O, Bagge RO, Crescitelli R. The dark side of foetal bovine serum in extracellular vesicle studies. J Extracell Vesicles. 2022;11:e12271. doi: 10.1002/jev2.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Valk J, Brunner D, De Smet K, Fex Svenningsen A, Honegger P, Knudsen LE, et al. Optimization of chemically defined cell culture media—replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro. 2010;24:1053–1063. doi: 10.1016/j.tiv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Wei J, Li H, Wang S, Li T, Fan J, Liang X, et al. let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev. 2014;23:1452–1463. doi: 10.1089/scd.2013.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Matsuda Y, Takeda A, Uchinuma E, Kuroyanagi Y. Effect of EGF and bFGF on fibroblast proliferation and angiogenic cytokine production from cultured dermal substitutes. J Biomater Sci Polym Ed. 2012;23:1315–1324. doi: 10.1163/092050611X580463. [DOI] [PubMed] [Google Scholar]
- Zhang N, Lin J, Lin VPH, Milbreta U, Chin JS, Chew EGY, et al. A 3D fiber-hydrogel based non-viral gene delivery platform reveals that microRNAs promote axon regeneration and enhance functional recovery following spinal cord injury. Adv Sci (Weinh) 2021;8:e2100805. doi: 10.1002/advs.202100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.