Abstract
Gray mold is a destructive plant disease caused by a fungal pathogen Botrytis cinerea. The use of plant growth promoting rhizobacteria (PGPR) has proven to be a promising method to control this disease. Bacillus velezensis K01 was isolated from the rhizosphere of planting tomatoes. Strain K01 has a range of roles, including the ability to solubilize phytate phosphorus, stimulate resistant response, and produce indoleacetic acid (IAA), protease, cellulase, and antimicrobial substances. Strain K01 was found to inhibit 12 phytopathogenic fungi and 5 phytopathogenic bacteria. Specially, strain K01 demonstrated a biocontrol efficiency of over 78% against gray mold caused by B. cinerea on the leaves and fruits of tomato and pepper. Additionally, K01 was found to promote the growth of maize seedlings. Further genomic analysis revealed that K01 belongs to B. velezensis, which is consistent with phylogenetic analysis, average nucleotide polymorphism (ANI), and digital DNA–DNA hybridization (dDDH). The genome of strain K01 had a size of 3,927,799 bp and deduced 3866 predicted genes, with an average guanine-cytosine (GC) content of 46.5%. Based on the analyses of genomic secondary metabolites, over 18.4% of the genome was annotated to 12 gene clusters related to antimicrobial metabolite synthesis. Additionally, genome annotation and comparative genomics identified several genes associated with plant growth promotion and environmental adaption. These findings suggest that B. velezensis K01 has the potential to serve as a new biocontrol agent for management of gray mold on tomato and pepper.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13568-023-01596-x.
Keywords: Bacillus velezensis, Biocontrol efficiency, Botrytis cinerea, Antagonistic activities, Secondary metabolite
Introduction
Plant diseases are one of the important reasons leading to the decrease of crop yield. According to the Food and Agriculture Organization (FAO), 14% of global crop production losses are caused by plant diseases each year, with fungal diseases accounting for 42% and bacteria for 27% (Roca-Couso et al. 2021). Botrytis cinerea is a necrotrophic fungus that has been reported to infect more than 1,400 plant species worldwide and causes significant economic losses of USD 10 to 100 billion over the whole growth period of the plant (Boyno et al. 2022; Nifakos et al. 2021). Control of gray mold disease caused by this fungus primarily relies on the use of chemical fungicides. However, due to the public concern for the adverse effects of fungicides on the environment and human health, applied biological control agents have emerged as a more sustainable alternative to control the plant disease in farming system, especially in organic farming system (Karthika et al. 2020; Nifakos et al. 2021). In 2021, over 76.4 million hectares of organic agricultural land were recorded in 191 countries, and the area of organic agricultural land is increased for 108.4% in past 10 years (Willer et al. 2023). The development of an effective microbial strategy comprising fungicidal properties could be a viable option for plant preharvest and postharvest health management practices in organic farming (Panneerselvam et al. 2019).
Bacillus is one of the most studied plant growth promoting rhizobacteria (PGPR) and has been shown to exhibit excellent capacities to promote plant growth, improve plant resistance systems, and inhibit the growth of plant pathogens (Lastochkina et al. 2019; Radhakrishnan et al. 2017; Saxena et al. 2020; Shafi et al. 2017). Species of Bacillus including B. subtilis, B. amyloliquefaciens, B. methylotrophicus, B. polymyxa, B. cereus, B. coagulans, B. subterraneous, B. licheniformis, B. pumilus, B. circulans, B. velezensis, B. megaterium, B. firmus, B. aquimaris, B. vietnamensis, and B. aerophilus have been reported to be suitable for biological control of plant disease and plant growth promoting (Dunlap et al. 2016; Fan et al. 2017; Saxena et al. 2020).
B. velezensis is a species within the genus Bacillus, which is widely found in soil, food, gut, and marine environments (Vairagkar et al. 2021). It has attracted significant attention due to its fast growth, harmlessness to humans and animals, and environmentally-friendly characteristics (Rabbee et al. 2019; Ye et al. 2018). B. velezensis was first discovered in 1998 and subsequently identified as a new species in 2016 based on comparative analysis of B. velezensis, B. methylotrophicus, and Bacillus amylolyticus genomes (Fan et al. 2017; Krebs et al. 1998; Ruiz-Garcia et al. 2005). Currently, B. velezensis, B. amyloliquefaciens, Bacillus siamensis, and Bacillus nakamurai are classified in the operational group of B. amyloliquefaciens within the Subtilis Clade of the Bacillus genus (Fan et al. 2017; Ngalimat et al. 2021). In 2007, the genome of the B. velezensis strain FZB42 was sequenced, and since then, 662 B. velezensis strains were whole-genome sequenced and the sequences were made available in the National Center for Biotechnology Information (NCBI) database. These reports mainly focus on analyzing and comparing the genes involved in the biosynthesis of secondary metabolites, as well as genes involved in beneficial plant-bacterial interactions in B. velezensis (Ngalimat et al. 2021).
Due to its abundant secondary metabolites and effects on plant growth promotion and disease inhibition, B. velezensis has received considerable attention in agriculture application (Saxena et al. 2020). For example, strains such as WZ-37 and BY6 could increase plant height, stem diameter, growth of tomato and poplar seedlings by produce indoleacetic acid (IAA), NH3, and 1-aminocyclopropane-1-carboxylate deaminase (ACC-deaminase), and strain CE 100 could decrease the incidence of Phytophthora root rot diseases and increase the survival rate of Chamaecyparis obtusa seedlings (Chen et al. 2021a; Moon et al. 2021; Zhang et al. 2021). B. velezensis produces a variety of secondary metabolites, such as surfactant, phonomycin, bacteriocin D, polylactic acid protein, and bacteriocin that can inhibit the development of various plant diseases caused by Fusarium graminearum, B. cinerea, Corynespora cassiicola, Alternaria alternata, and Phytophthora capsici on crops such as maize, grapes, cucumber, rice, chili, wheat, and watermelon (Cao et al. 2021; Chen et al. 2021b; Jiang et al. 2019; Wang et al. 2018; Xu et al. 2020). Currently, the utilization of B. velezensis mainly focus on promoting plant growth and biocontrol of preharvest and postharvest plant diseases. Studies on the biocontrol of postharvest diseases of plants by B. velezensis has been scarcely studied.
The objective of this study was to investigate the potential probiotic capabilities of the B. velezensis strain K01 through mining genomic sequencing information, and to determine if K01 has the potential for use as a PGPR in agriculture by conducting experiments on plant growth promotion and pathogen inhibition. Specifically, the main aims of our research were to investigate the function of K01 on (a) promoting the growth of maize, (b) antagonizing plant pathogenic fungi and controlling pepper and tomato leaf and fruit gray mold caused by B. cinerea, and (c) comparing and analyzing the genes associated with plant growth-promoting functions in the genome.
Materials and methods
Strains and culture conditions
The B. velezensis strain K01 was isolated from tomato rhizosphere in Weixian county, Hebei province of China, and identified as B. velezensis (deposited in China Center for Type Culture Collection with the deposition number of CCTCC No. M2020871). Eight plant pathogenic bacteria were incubated in Luria-Bertani (LB) medium at 28 °C. Pseudomonas syringae, Pseudomonas tolaasii, Pectobacterium carotovorum and Ralstonia solanacearum were kindly provided by Dr. Jie Feng (Plant Protection Institute, The Chinese Academy of Agricultural Sciences, Beijing, China) (Guo et al. 2007). Agrobacterium vitis K308 was kindly provided by Dr. Allen Kerr (Department of Plant Pathology, Waite Agricultural Research Institute, University of Adelaide, South Australia) (Kerr et al. 1980), and Xanthomonas citri, Xanthomonas campestris pv. campestris, and X. campestris pv. vesicatoria, were collected by our laboratory (Guo et al. 2007). Twelve pathogenic fungi were incubated in potato dextrose agar (PDA) medium at 22 °C. B. cinerea B02 (deposited in Agricultural Culture Collection of China with the deposition number of ACCC 35,467), Sclerotinia sclerotiorum, and Colletotrichum destructivum were kindly provided by Dr. Yue Liang (College of Plant Protection, Shenyang Agricultural University, Shenyang, China) (An et al. 2019). Alternaria solani, Ceratocystis fimbriata, Fusarium moniliforme, Fusarium oxysporum, F. oxysporum f. sp. lilii, F. oxysporum f. sp. niveum, Fusarium verticillioides, Rhizoctonia cerealis, and Rhizoctonia solani were kindly provided by Dr. Xili Liu (Department of Plant Pathology, China Agricultural University, Beijing, China) (Guo et al. 2007).
Determination of antimicrobial activity in vitro
The antibacterial capacity of K01 on inhibiting the growth of the phytopathogenic bacteria was tested on LB medium according to the method of Jiao et al. (2013). The antifungal capacity of K01 on inhibiting the growth of the phytopathogenic fungi was tested on PDA medium according to the method of Liu et al. (2021).
Biocontrol assay
The inhibition activity of K01 against grey mold caused by B. cinerea in tomato and pepper leaves was examined using method of Guo et al. (2007) and Chen et al. (2022) with some modifications. Five layers of sterile filter paper were spread in a petri dish (130 × 130 mm), and soaked with 10 mL sterile water. The leaves were washed three times with sterile water and placed on the moist filter paper. Bacterial cultural suspensions of K01 with 108 colony-forming units (cfu mL− 1) was sprayed on the leaf surface. After drying the leaves, a mycelial plug of B. cinerea at 5 mm in diameter was placed upside down on the surface of the respective leaf. Sterile water served as a negative control and 20 mg L− 1 carbendazim solution was used as a positive control. At 72 h post inoculation of B. cinerea, the leaves were photographed with visible light and an ultraviolet lamp (365 nm; Analytik Jena US, Upland, CA, USA), and the size and area of lesions area on leaves were analyzed using the software APS Assess (APS Press, Canada). The grey mold inhibition activity was calculated as follows:
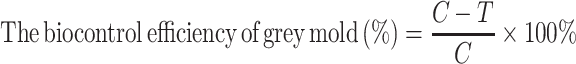 |
Where C is the average area of the lesion of the negative control group, and T is the average area of the lesion of leaves treated with K01.
The biocontrol activity of K01 against grey mold caused by B. cinerea on fruits of tomatoes, peppers, and bell peppers was analyzed according to the methods of Liu et al. (2020a) with some modifications. A 3 or 5 mm2 area of wound was created with a sterile needle on the surface-disinfected of tomatoes (3 mm2), peppers (5 mm2), and bell peppers (5 mm2) fruit. Ten microliters of K01 cultural solution with 108 cfu mL− 1 were dropped on the wound of each fruit. A mycelial plug of B. cinerea with 3 or 5 mm in diameter was placed upside down on the wound of the fruit, and the fruits were cultivated at 22 °C for three days, Then, the fruits were photographed and the size of lesion area on fruits was analyzed by the method descripted above. Carbendazim solution (20 mg L− 1) was used as a positive control, and sterile deionized water was used as a negative control. Five replicates were set for each treatment, and the experiment was repeated three times.
Plant growth promoting assay
The test tube maize seedling experiments according to the methods of Li et al. (2014) with some modifications. Maize caryopses were surface sterilized with 75% ethanol and germinated at 28 °C overnight. The sprouting maize caryopses were soaked in K01 bacterial suspension with at 106 cfu mL− 1 or 105 cfu mL− 1 for 4 h respectively. The caryopses rolled in Whatman columnar rolled filter paper, and the columnar filter paper was inserted into a tube with 25 mm of diameter and 200 mm of length. Fifteen mL Hoagland solution was added in to tubes. The tubes were incubated in a constant temperature incubator at 16 h light and 8 h dark at 22 ℃ and 70% relative humidity. There were three seedlings per test tube and five replicates per treatment. Maize caryopses were treated by sterile deionized water as the negative control. The maize samples were collected after culturing for 15 days, stem weight, and root weight were determined.
Determination of hydrolytic activity and IAA production
Production of proteases by K01 was determined by using skim milk agar plates (Kumar et al. 2005). Cellulase was measured according to methods of Teather and Wood (1982). According to Nautiyal (1999) and Sethi and Subba-Rao (1968), the phosphate growth medium (NBRIP: glucose, 10 g; Ca3(PO4)2, 5 g; MgCl2•6H2O, 5 g; MgSO4•7H2O, 0.25 g; KCl, 0.2 g and (NH4)2SO4, 0.1 g.) of the National Institute of Botany was used to test the solubilization of tricalcium phosphate and calcium phytate activity of K01. Quantitative analysis of soluble phosphate, protease and cellulase activity were conducted according to the method of King (1932), Fischer et al. (2006), and Lynd and Zhang (2002), respectively. The production of IAA was determined using the method of Patten et al. (2002) in nutrient broth with and without tryptophan (0.5 g L− 1).
Genome DNA isolation, sequencing, and the annotation
Genomic DNA of K01 was extracted using a bacterial genome isolation kit (DP302-02, Tiangen, Biotech (Beijing) Co., Ltd. Beijing, China). The whole genome of K01 was sequenced using an Illumina HiSeq 2500 system and PacBio SMRT sequencing technique (Wang et al. 2021). Subsequently, a PCR-free SMRT bell library was constructed based on the PacBio RSII/Sequel sequencing system, and the sequencing was carried out using the PacBio SMRT Technology. The hierarchical genome-assembly process method (HGAP) provided by PacBio was utilized to obtain the denovo assembly results of K01 with high integrity. The Kyoto Encyclopedia of Genes and Genomes database (KEGG, https://www.genome.jp/kegg/) and Clusters of Orthologous Groups (COG, ftp://ftp.ncbi.nlm.nih.gov/pub/COG) were employed for predicting the functional proteins (Wu et al. 2020). A circular genome map was generated using the CGview Server (http://stothard.afns.ualberta.ca/cgview_server/) to visualize gene annotation results (Grant and Stothard 2008).
Taxonomic classification of the stain K01
A phylogenetic tree was constructed based on 16 S rRNA gene sequences given in the Online Resource (Table S1). The phylogenetic tree was generated using the maximum-likelihood method by Molecular Evolutionary Genetics Analysis version 11 (MEGA 11, Tamura et al. 2021). The average nucleotide polymorphism (ANI) values were analyzed using Jspecies WS (http://jspecies.ribohost.com/jspeciesws/), while the digital DNA–DNA hybridization (dDDH) analysis was conducted utilizing the Genome-to-Genome Distance Calculator (GGDC v3.0) (https://ggdc.dsmz.de/).
Genes related to plant growth promotion and environmental adaption
AntiSMASH (https://antismash.secondarymetabolites.org/) was used to predict gene clusters in K01 genome related to secondary metabolism (Blin et al. 2023). Annotated gene sequences from the genome of K01 were blasted using the carbohydrate active enzyme (CAZy) database (https://bcb.unl.edu/dbCAN2/blast.php) (Zhang et al. 2022). Genes related to plant growth promoting including IAA production, phosphate solubilization, and antibiotic substance synthesis were analyzed in the K01 genome using the Basic Local Alignment Search Tool (BLAST) of NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical analysis
All data processed in the experiment were expressed as mean ± standard deviation (SD). All treatments for each experiment were compared by the t-test or one-way analysis of variance (ANOVA), and the post-hoc multiple comparison analysis using the Duncan multiple comparison test (p < 0.05) with IBM SPSS Statistics 23.0 (IBM Corp., Armonk, NY, USA). All figures were completed with Origin Pro 2021 (Origin Lab Inc., Northampton, MA, USA).
Data availability
The complete genome sequence of B. velezensis K01 have been deposited in the NCBI GenBank with the accession number CP059344.1.
Result
The antagonistic ability of K01 strain in vitro
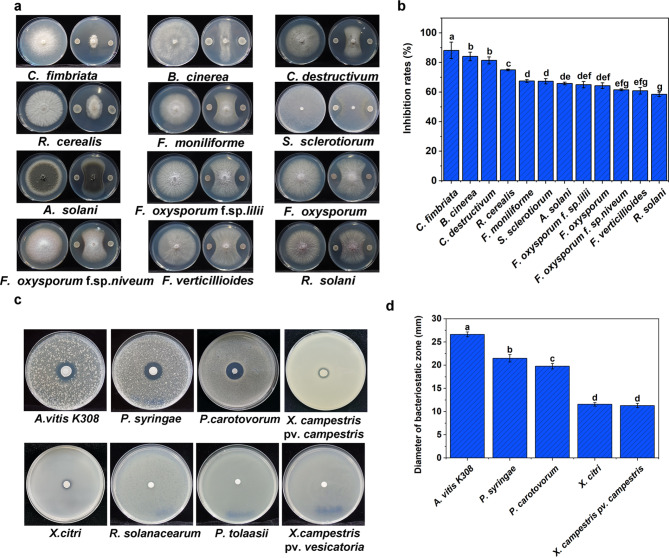
In this study, K01 strain was observed to display varying abilities in suppressing the growth of plant pathogenic fungi and bacteria in vitro (Fig. 1). The inhibition rate of K01 strain against 14 pathogenic fungi was more than 60%, except for R. solani with 58.4%. The highest inhibition to pathogenic fungi was found in C. fimbriata (88.1%), followed by B. cinerea (84.1%) and C. destructivum (81.4%) (Fig. 1a and b). The diameter of the inhibitory zones of K01 to pathogenic bacteria of A. vitis, P. syringae, P. carotovorum, X. citri, and X. campestris pv. campestris were 26.7, 21.5, 19.8, 11.6 and 11.3 mm, respectively (Fig. 1c and d). No antagonistic activity of K01 against P. tolaasii, X. campestris pv. vesicatoria and R. solanacearum were observed.
Fig. 1.
Antagonistic activity of K01 to phytopathogenic fungi (a, b) and phytopathogenic bacteria (c, d) The error bar represents the SD of the mean value of three biological repeats. Different letters above the bars indicate significant differences between treatments (one-way ANOVA, P < 0.05)
Biocontrol gray mold on tomato and pepper by K01
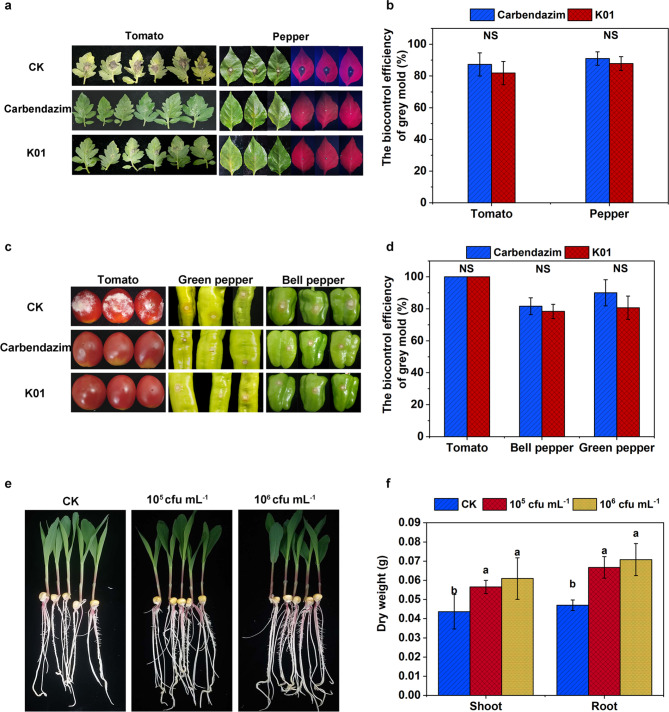
The effects of strain K01 on preharvest and postharvest gray mold in tomato and pepper are shown in Fig. 2a-d. Treatment with K01 significantly reduced the lesion area caused by B. cinerea infection on tomato and pepper leaves (Fig. 2a). The biocontrol efficiency of K01 against gray mold on tomato and pepper leaves reached 81.9% and 87.8%, respectively (Fig. 2b). Moreover, K01 was found to be highly effective in controlling the postharvest diseases of tomato and pepper caused by B. cinerea (Fig. 2c). When fruits of tomato, green pepper, and bell pepper were treated with K01, the biocontrol efficiency of K01 against gray mold on the fruits reached 100%, 80.7%, and 78.4%, respectively (Fig. 2d). The biological control ability of K01 on gray mold of leaves and fruits of tomato, green pepper, and bell pepper was found to be not significantly different from that of carbendazim (Fig. 2a-d). These results indicate that K01 has the potential to be used as a biocontrol agent for effectively controlling gray mold on leaves and fruits of tomato and pepper.
Fig. 2.
Biocontrol capability of K01 strain (a) K01 reduced the occurrence of gray mold in leaves, the leaves of pepper were photographed with visible light (the left three leaves) and ultraviolet lamp (the right three leaves), (b) The control effect of K01 on leaf gray mold in vitro, (c) K01 reduced the occurrence of postharvest gray mold, (d) Control effect of K01 on postharvest gray mold, (e) Growth promoting effect of K01 on maize seedlings, (f) Effects of K01 on stem and root dry weight of maize seedlings. The error bar represents the SD of the mean value of three biological repeats. The “NS” above the bars indicate no significant differences between treatments (t-test, P < 0.05), and different letters above the bars indicate significant differences between treatments (one-way ANOVA, P < 0.05)
Growth promoting ability of the K01 strain
Upon soaking maize caryopses in K01 fermentation solution, the biomass of maize seedlings increased as compared to the control group, indicating that K01 had the capacity to promote the growth of maize seedlings (Fig. 2e-f). Treatment of maize caryopses with K01 at concentrations of 105 cfu mL− 1 and 106 cfu mL− 1 resulted in an increase of 29.7%, 42.0% and 39.7%, 50.6% in the dry weight of shoot and root of maize seedlings, respectively (Fig. 2f). Strain K01 has demonstrated the ability to dissolve phytate phosphorus as well as produce cellulase and protease (Fig. 3a, c, d). The content of soluble phosphorus in the media’s supernatant reached 75.3 mg/L when phytate phosphorus was supplied. The protease and cellulase activity of K01 was found to be 2683.2 and 7.6 U mL− 1, respectively. In addition, strain K01 produced 5.7 mg L− 1 IAA in DF medium supplemented with tryptophan.
Fig. 3.
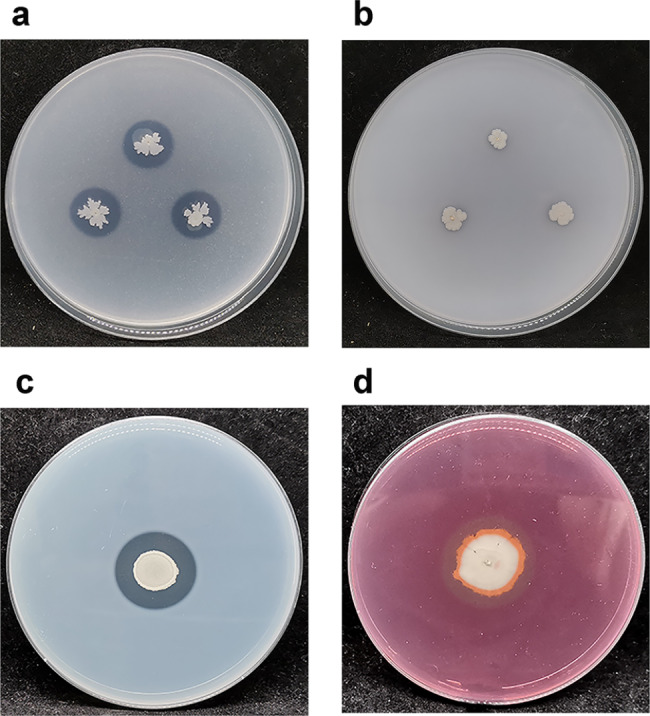
Assays for characteristics related to biocontrol (a) Three transparent halos were found on the plates containing phytate phosphorus, indicating that strain K01 has excellent potential for phytate phosphorus solubilization, (b) No transparent halo were found on the plates containing tricalcium phosphate, indicating that strain K01 may not be able to dissolve inorganic phosphorus on media containing tricalcium phosphate, (c) Protease production by K01 on skimmed milk agar plates, (d) Cellulase production by K01 on congo red cellulose plates
Identification of strain K01
To determine the genetic relationships of K01 with other strains in Bacillus, a phylogenetic tree was established based on the 16S rRNA gene sequence, and ANI and dDDH calculations among Bacillus strains were performed. As expected, fourteen Bacillus strains were clustered into two major clades. Among of them, K01 and four strains of B. velezensis (QST713, KCTC13012T, FZB42 and QST713), B. subtilis subsp. subtilis str. 168T, Bacillus amyloliquefaciens DSM7T, Bacillus atrophaeus NRRLNRS213T, Bacillus licheniformis DSM13T and Bacillus salacetis SKP7-4T were clustered into one major clade. Additional Bacillus aerolatus CX253T, Bacillus acidicola FJAT-2406T, Bacillus cereus ATCC14579T and Bacillus alkalicellulosilyticus FJAT-44,921T were clustered into another clade. One Rouxiella strain, one Brucella strain, one Citrobacter strain, one Escherichia strain and one Pseudomonas strain were in other clades (Fig. 4a). Based on the observed genetic distance relationships, strain K01 was closely clustered together with B. velezensis QST713, the type strain B. velezensis KCTC13012T and B. velezensis FZB42, successively followed by the type strain B. amyloliquefaciens DSM7T, the type strain B. subtilis subsp. subtilis str. 168T and strain B. velezensis SQR9 (Fig. 4a).
Fig. 4.
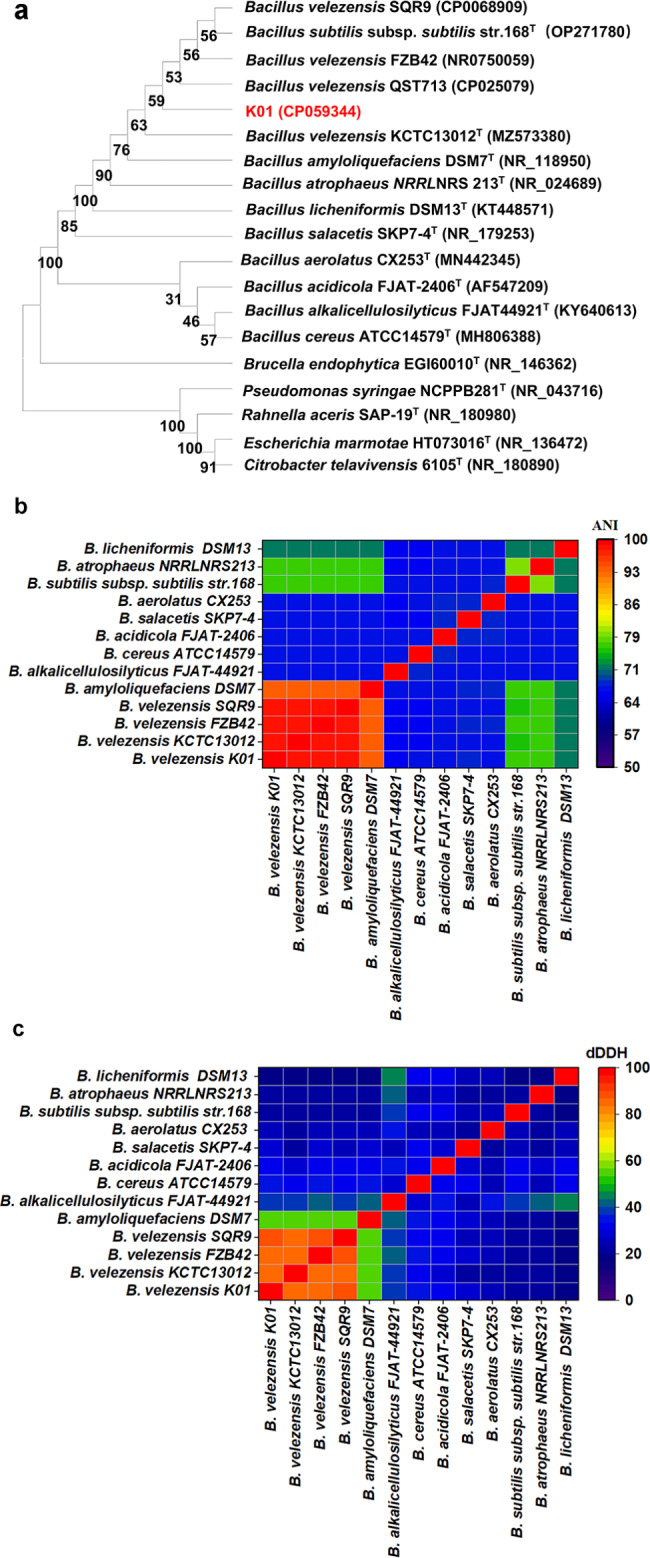
Phylogenetic classification of the strain K01 (a) Phylogenetic neighbor-joining tree reconstructed based on 16S rRNA genes of the selected strains of bacteria. Bootstrap values (1,000 replicates) were shown at the branch points. (b) ANI values were computed for a pairwise genome comparison using the Jspecies WS, and the heatmap was expressed as the percentage of ANI among the selected Bacillus strains. (c) dDDH values were calculated by using the Genome-to-Genome Distance Calculator (GGDC), and the heatmap was expressed as the percentage of dDDH among the selected Bacillus strains
In the study, ANI and dDDH calculations among Bacillus strains were performed. The ANI values between K01 and KCTC13012T, FZB42 and SQR9 were 97.8%, 98.2% and 98.4%, respectively (Fig. 4b), and the dDDH values between K01 and these three strains were 84.5%, 85.4% and 89.7%, respectively (Fig. 4c). The ANI values between K01 and B. amyloliquefaciens DSM 7, B. alkalicellulosilyticus FJAT-44,921T, B. cereus ATCC14579 T, B. acidicola FJAT-2406T, B. salacetis SKP7-4T, B. aerolatus CX253T, B. subtilis subsp. subtilis str.168T, B. atrophaeus NRRLNRS213T, and B. licheniformis DSM13T were 93.4%, 66.7%, 66.7%,67.7%, 67.9%, 67.8%, 76.3%, 76.8%, and 71.9%, respectively (Fig. 4b), and the dDDH values between K01 and these strains were 55.5%, 38.1%, 33.7%, 30.9%, 26.8%, 25.7%, 20.9%, 20.8%, and 19.4%, respectively (Fig. 4c). Based on the results of phylogenetic tree, ANI and dDDH, strain K01 has been identified as B. velezensis.
Comparative genome analysis of strain K01 with other B. velezensis strains
The genome assembly revealed that the K01 genome consisted of a single circular chromosome of 3,927,799 bp with an average GC content of 46.5%, and did not possess any plasmids (Table 1). The K01 genome was predicted to have 3,866 protein-coding genes, 86 tRNA genes, and 27 rRNA genes. The general genome structure and functions of strain K01 were illustrated in a graphical circular genome map (Fig. 5a). When comparing the genomes of B. velezensis K01, FZB42, and QST713, it was observed that the genome size of strain K01 was similar to FZB42 (3,918,589 bp), but smaller than that of QST713 (4,233,757 bp) (Table 1). Additionally, the average of G + C content of K01 was similar to FZB42 (46.5%) but higher than that of QST713 (45.9%) (Table 1). Moreover, the genome of K01 contained 91 pseudo genes, which was higher than the number found in FZB42 (59) and QST713 (68).
Table 1.
The information of K01 genome and its comparison with B. velezensis FZB42 and QST713 strains
| Properties | K01 | FZB42 | QST713 |
|---|---|---|---|
| GenBank accession No. | CP059344.1 | CP000560.2 | CP025079.1 |
| RefSeq | NZ_CP059344.1 | NC_009725.2 | NZ_CP025079.1 |
| Size(bp) | 3,927,799 | 3,918,589 | 4,233,757 |
| G + C content/% | 46.5 | 46.5 | 45.9 |
| Number of genes | 3,866 | 3,855 | 4,212 |
| Protein-coding sequences (CDS) | 3657 | 3,675 | 4,037 |
| Percent of coding region (%) | 94.6 | 95.3 | 95.8 |
| Ribosomal RNA operons | 27 | 29 | 25 |
| Number of tRNAs | 86 | 88 | 77 |
| Other RNA | 5 | 4 | 5 |
| Pseudogenes | 91 | 59 | 68 |
| Bacillibactin (NRPSa) | 50,501 | 98.4% | 98.3% |
| Bacilysin (NRPS) | 41,418 | 98.8% | 98.8% |
| Fengycin (NRPS) | 134,310 | 97.6% | 97.5% |
| Bacillaene (NRPS) | 100,565 | 98.2% | 98.0% |
| Surfactin (NRPS) | 65,407 | 98.2% | 98.1% |
| Difficidin (transAT-PKSb) | 92,359 | 98.0% | 98.0% |
| Macrolactin H (transAT-PKS) | 88,232 | 98.1% | 98.0% |
| Butirosin A / Butirosin B (PKS-like) | 41,244 | 98.5% | 98.4% |
| Unknow (lanthipeptide-class-ii) | 28,888 | / | / |
| Unknow (T3PKS) | 40,726 | / | / |
| Unknow (terpene) | 20,126 | / | / |
| Unknow (terpene) | 20,740 | / | / |
a NRPS: non-ribosomal peptide synthetases, and b PKS: polyketide synthases
Fig. 5.
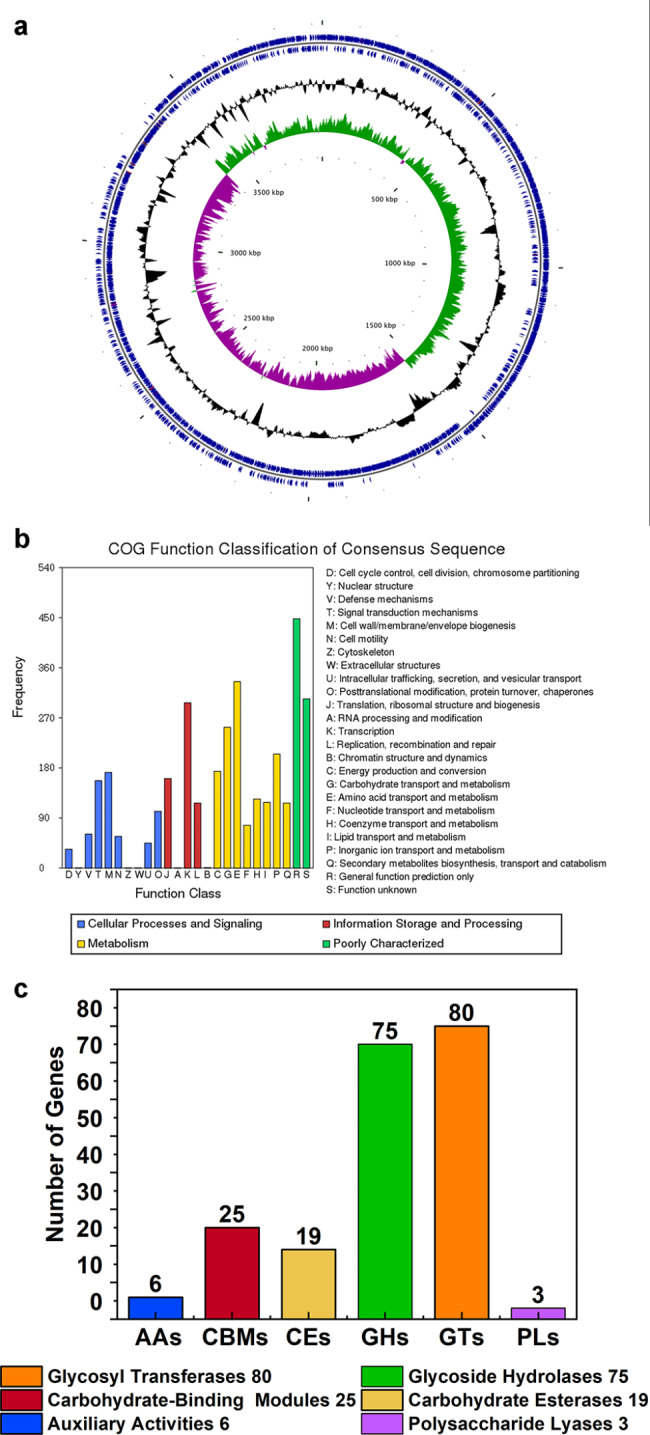
Annotation results of K01 genome and classification of CAZy gene (a) Circle graph: from inside to outside, the first circle is the length coordinate of genome, the second circle represents GC skew (GC SKEw = (G-C)/(G + C)), the third circle represents the average GC% content, and the outer bulge represents higher than the average and the inner concave represents lower than the average, the fourth circle was COG annotation result of negative strand gene, the fifth circle is the prediction results of negative chain genes, rRNA and tRNA, the sixth circle is the prediction results of plus-strand genes, rRNA and tRNA, the seventh circle is COG annotation result of plus-strand gene, (b) COG functional classification results of K01 genome, (c) CAZy gene classification in the K01 genome, the number on the column indicates the number of genes in that class
Of the 3,866 protein-coding genes in the K01 genome, 3,749 (97.0%) were annotated using the COG database. Among these annotated genes, 3131 were classified and annotated as being involved in amino acid transport and metabolism, transcription, carbohydrate transport and metabolism, inorganic ion transport and metabolism, energy production and conversion, and cell wall/membrane biogenesis (Fig. 5b). Within the genome, 31.3% of genes were classified under metabolism, with 219, 172, 140, 149, 69, 98, 73 and 61 genes been annotated for amino acid transport and metabolism, inorganic ion transport and metabolism, energy production and conversion, nucleotide transport and metabolism, coenzyme transport and metabolism, lipid transport and metabolism, and secondary metabolites biosynthesis transport and metabolism, respectively.
Gene clusters involved in the synthesis of secondary metabolites
Using the AntiSMASH database, 12 putative secondary metabolite biosynthetic gene clusters (BGCs) were identified in the genome of K01, covering 18.4% (724,516 bp) of the entire genome. Of these BGCs, 5 corresponded to non-ribosomal peptide synthetases (NRPS), 2 to transAT-polyketide synthases (PKS), 2 to terpenes, 1 to PKS-like, 1 to lanthipeptide-class-ii, and 1 to T3PKS (Table 1). Most of these compounds were associated with the production of NRPS and PKS systems. Among the 12 BGCs, 8 clusters were identified to play a key role in the synthesis of bacillibactin, bacilysin, fengycin, bacillaene, surfactin, difficidin, macrolactin H and butirosin A / butirosin B (Table 1). To determine the variation of BGCs among B. velezensis strains and K01, their relatedness was investigated by searching for homologues all of the 8 gene clusters with B. velezensis FZB42 and QST713 (Table 1 and Online Resource Table S2). We identified the gene clusters responsible for synthesizing bacillibactin, bacilysin, fengycin, bacillaene, surfactin, difficidin, macrolactin H and butirosin A / butirosin B in the genome of K01. These gene clusters contain the dhbABCEF, bacABCDE, fenABCDE, bmyABCD, srfAAABACAD, dfnAYXBCDEGFHIJ, mlnABCDEFG and btrXW genes, which are 50,501 bp, 41,418 bp, 134,310 bp, 100,565 bp, 65,407 bp, 92,359 bp, 88,232 and 41,244 bp long, respectively. The amino acid sequence encoded by the gene clusters shared 98.4%, 98.8%, 97.6%, 98.2%, 98.2%, 98.0%, 98.1%, 98.5% and 98.3%, 98.8%, 97.5%, 98.0%, 98.1%, 98.0%, 98.0%, 98.4% similar with the gene cluster in B. velezensis FZB42 and QST713, respectively (Table 1 and Online Resource Table S2). Additionally, we identified 4 gene clusters in the genome of that encode PKS-like, lanthipeptide-class-ii, T3PKS (unknown), and terpene (unknown). These gene clusters were not found in the genomes of FZB42 and QST713.
Analysis of CAZyme Genes in the B. velezensis K01 genome
The genome of B. velezensis K01 contained 208 CAZyme genes, including 6 auxiliary activity (AA) genes, 25 carbohydrate-binding module (CBM) genes, 19 carbohydrate esterase (CE) genes, 75 glycoside hydrolase (GH) genes, 80 glycosyl transferase (GT) genes, and 3 polysaccharide lyase (PL) genes (Fig. 5c and Online Resource Table S3). Lignocellulosic genes related to cellulose degradation were presented, including 15 GH13 (α-glucosidase), 2 GH5 (endo-1,4-β-glucanase), 2 GH30 (glucan endo-1,6-β-glucosidase), 5 GH4 (6-phospho-β-glucosidase, 6-phospho-α-glucosidase), 5 GH1 (6-phospho-β-galactosidase), 1 GH16 (β-glucanase), and 3 GH32 (two sucrose-6-phosphate hydrolase and levanase) (Online Resource Table S3). Furthermore, two cellodisaccharide hydrolase genes (GH9 and GH51) were also identified in the genome of K01. In addition, genes related to the degradation of hemicellulose (GH11, GH26, GH43, GH51, GH53, CE7), pectin (PL1and PL9), amylase (GH13, GH126, CBM34), and chitin (GH18, GH23, GT2, CE4, CBM50 and AA10) were detected in the entire genome of K01 (Online Resource Table S3).
Genes related to plant growth promotion and environmental adaption
The draft genome of B. velezensis K01 was annotated to identify genes associated with plant-beneficial traits. Specifically, genes coding for plant bacteria interactions traits related to IAA synthesis were identified and annotated in genome of B. velezensis K01 (IAA synthesis, Online Resource Table S2). The genome was found to contain a total of 6 key genes which are involved in tryptophan synthesis (trpABCDE and TRP1), as well as a key enzyme, aldehyde dehydrogenase (NAD+), in the pathway that produced IAA using tryptophan as a precursor. A metabolic pathway analysis using the KEGG database revealed that the genome of B. velezensis K01 contains 47 key genes for organic acid synthesis in glycolysis (EMP) pathway, the tricarboxylic acid cycle (TCA) pathway, the pentose phosphate pathway, and the oxidative phosphorylation pathway (Phosphorus dissolution, Online Resource Table S2). Furthermore, alkaline phosphatase coding genes, including phoA, phoD, two-component system phoR-phoP regulating phosphate synthesis and metabolism, and histidine phosphatase family protein phoE were also identified in the genome of K01 (Phosphorus dissolution, Online Resource Table S2). These identified genes likely play a significant role in the process of phosphate dissolution of strain K01 for promoting plant growth. Through screening and statistical analysis of protease-related genes in the whole genome of the strain, a total of 36 protease-related genes (Protease, Online Resource Table S2) were identified. These include 2 extracellular proteases epr and vpr, 6 metalloproteases ymfH, ymfF, ymcG, yhfN, ftsH, ywhC, 4 serine proteases aprX, htrB, htrA, yyxA and a major intracellular serine protease, 2 cysteine proteases and 21 other proteases.
The genome of K01 was found to contain 26 genes encoding flagellar synthesis, covering the synthesis genes of the flagellar matrix, flagellar hook, and flagellar filament (Flagella synthesis, Online Resource Table S2). The flagellar collective includes fliF, which encodes the MS ring, fliE, flgB and flgC, which encode the proximal end of the matrix rod, flgG, which encodes the distal end of the matrix rod, flgH, which encodes the L ring, and flgI, which encodes the P ring. Additionally, the genes for the flagellar base includes flhA, flhB, fliH, fliI, fliK, fliO, fliQ, fliP and fliR. The genes for the flagellate hook part includes flgE, which encodes the flagellate hook, flgD, flgK and flgL, which encode proteins at the junction of the flagellate hook and the flagellate filament. The genes for the flagellar filament part includes fliC, which encodes the flagellar filament, as well as the flagellar cap gene fliD, the flagellar secretion chaperone genes fliS and fliT. The whole genome of strain K01 was found to contain genes related to chemotaxis (Chemotaxis, Online Resource Table S2), which include those for methyl-accepting chemotaxis protein (MCP), methyl transferase chemotaxis protein CheR, protein-glutamate methylesterase/glutaminase TheB (EC: 3.1.1.61 and EC: 3.5.1.44), sensor kinase TheA (EC: 2.7.13.3), purine-bond chemotactic protein TheW, chemotactic proteins TheV, TheY, TheC, TotA, TotB, flagellum motoswitch proteins FliG, FliM, and FliN, and ribose transport system substrate binding protein TbsB).
Discussion
In recent years, the application of PGPR such as Bacillus has become a significant practice in agricultural production for controlling plant diseases and promoting plant growth (Shafi et al. 2017; Saxena et al. 2020). However, there are still some gaps in the specific mechanisms by which they promote plant growth and adaptation at the molecular level especially for B. velezensis. In this study, B. velezensis K01 which was isolated from tomato rhizosphere. Strain K01 produced IAA and phosphatases, which were key factors to promote plant growth, and it presented broad spectrum antagonistic activities against various plant pathogens and exhibited a significant effect in controlling B. cinerea on tomato and pepper. All of these characteristics indicated that K01 will be a PGPR. The complete genome of B. velezensis K01 was sequenced and compared with other Bacillus strains to better understand the mechanisms for plant-growth promotion and environmental adaption.
The phylogenetic trees showed that K01 clustered together with QST713, FZB42, KCTC13012Tand the three strains both belonged to the B. velezensis species, forming a tight cluster with B. amyloliquefaciens DSM7T and B. subtilis subsp. subtilis str. 168T, which was consistent with a previous study (Dunlap et al. 2016; Fan et al. 2017). Nevertheless, constructing the phylogenetic tree solely based on 16S rRNA sequences is less likely to differentiate between closely Bacillus species (Fan et al. 2017). Strains revealing ANI values ≥ 96% and dDDH values ≥ 70% were typically considered as the same species (Zhang et al. 2016). To more accurately determine the phylogenetic and taxonomic relationships between K01 and other Bacillus species, genomes must also be used to assess the similarity of closely related strains utilizing ANI and dDDH determination (Dunlap et al. 2016; Fan et al. 2017). However, our study showed that ANI and dDDH values (< 96% and < 70%, respectively) were calculated between B. velezensis K01 and B. amyloliquefaciens DSM7T (93.4% and 55.5%), B. subtilis subsp. Subtilis str. 168T (76.3% and 20.9%) strains (Fig. 4b-c).
In this study, strain K01 exhibited significant antagonistic effects against 12 pathogenic fungi (with inhibition rates of 58.4-88.1%) and 5 bacteria (Fig. 1). B. velezensis strains that have been reported to have the same ability include but not limited to FZB42, GS-1, BA-26, BS1, ZF2, BY6 and so on (Chen et al. 2007; Zhang et al. 2021, 2022; Wang et al. 2021; Shin et al. 2021; Xu et al. 2020). B. velezensis produces secondary metabolites against various phytopathogenic fungi and bacteria (Berezhnaya et al. 2019; Xu et al. 2020; Zhang et al. 2021). In K01 genome, 12 gene clusters related to antimicrobial compound synthesis were identified, and 8 gene clusters coding bacillibactin, bacilysin, fengycin, bacillaene, surfactin, difficidin, macrolactin H, and butirosin A / butirosin production shared 97.5–98.8% similarity to that in B. velezensis FZB42 and QST713. However, there were 2 gene clusters coding terpenes, and 1 gene cluster coding lanthipeptide, and 1 gene cluster coding an unknow T3PKS substance were unique identified in K01 genome compared to FZB42 and QST713. Lanthipeptides are ribosomally synthesized and post-translationally modified peptides (RiPPs) which was detected and isolated in few of bacteria with the antifungal, antibacterial, antiviral, and other activities (Repka et al. 2017). Lanthipeptide biosynthetic gene clusters were predicted in genome of Bacillus clausii, B. cereus, Bacillus thuringiensis, Bacillus mycoides, except in genome of B. velezensis genomes (Marsh et al. 2010, Rabbee et al. 2020). Terpenes are generally volatile, incuding α-Pinene and oxigenated terpene, which had been reported volatile antibacterial compound (De la Cruz-López et al. 2022; Stamenković et al. 2022). These metabolite synthesis gene clusters were also found in the whole genome sequence of B. velezensis ZF2, BIM B-439D and BY6 strains (Rabbee and Baek 2020; Xu et al. 2020; Berezhnaya et al. 2019; Zhang et al. 2021). Difficidin, macrolactin and bacilysin are primarily responsible for inhibiting pathogenic fungi, while bacillaene, fengycin and surfactin are effective against pathogenic bacteria, and bacillibactin is known to inhibit microbial competitors (Rabbee and Baek 2020). The antagonistic activity of K01 to phytopathogens was involved likely with the antimicrobial compounds predicted BGCs by antiSMASH in K01 genome.
Furthermore, we discovered that B. velezensis K01 has the ability to synthesize protease (Fig. 3c) and cellulase (Fig. 3d). Such activities have been reported to contribute to antagonism against phytopathogens and biocontrol of plant diseases (Xu et al. 2020). PGPRs may secrete proteases, cellulases, glucanases and chitinases to degrade components of the fungal cell wall, such as chitin, glucan, and protein, and thereby disrupt the integrity of the cell wall, ultimately reducing the pathogenicity of the phytopathogen (Huang et al. 2017). K01 strain was found to carry 5 genes related to serine proteases (Protease, Online Resource Table S2). Serine proteases are ubiquitous in the genomes of cells and viruses and employ a nucleophilic serine residue in their active sites to cleave peptides (Liu et al. 2020b). Furthermore, genes related to degradation of cellulose, hemicellulose, pectin, amylase and chitin were also detected in the genome of K01 (Online Resource Table S3). Various hydrolytic enzymes (glucanases and proteases), in turn, exhibit antifungal properties by affecting the structural elements of fungal cell walls and membranes (Lyagin et al. 2023). The endoglucanases (GH16), exo-glucanases (GH1), and β-cellobiose hydrolases (GH5) found in the genome of K01 have been identified. We therefore hypothesized that the antifungal activity of K01 might be expected to be related to the ability to decompose hydrolase activity.
Gray mold disease, caused by B. cinerea, can produce symptoms on all parts of vegetables, such as leaves, stem and fruits (Jiang et al. 2018; Yan et al. 2022). B. velezensis K01 demonstrated significant effectiveness (with biocontrol efficiency of more than 78%) in preventing and controlling preharvest and postharvest gray mold diseases in tomato and pepper (Fig. 2). The biocontrol efficiency of K01 against gray mold diseases was higher than that of B. velezensis A4 in tomato, strawberry, apple and kiwifruit fruits (30–70%), B. velezensis Bvel1 in grape fruits (14–75%), B. velezensis S3 and S6 in grape leaves (about 50%), and B. velezensis 5YN8 and DSN012 in pepper leaves (about 50%) (Calvo et al. 2020; Jiang et al. 2018; Nifakos et al. 2021; Zhao et al. 2022). B. velezensis has proven to be a promising and safe biocontrol agent for effectively managing gray mold disease.
Genome mining revealed that the strain K01 possesses the genetic potential to synthesize secondary metabolites, and previous studies have confirmed that several secondary metabolites can effectively control gray mold. For example, fengycin A, isolated from B. velezensis G341, showed in vivo antifungal activity against tomato gray mold, while iturin and different fengycin derived from B. velezensis BA-26 and surfactins, iturins and fengycins of strains B. velezensis BBC023 and BBC047 had significant inhibitory effects on B. cinerea (Lim et al. 2017; Salvatierra-Martinez et al. 2018; Wang et al. 2021). Indeed, several studies have highlighted that the successful use of Bacillus strains as biocontrol agents is not only dependent on the synthesis and secretion of various secondary metabolites with growth-promoting and antibacterial effects, but also on their ability to colonization of plants (Nifakos et al. 2021). The genome of K01 contains numerous genes responsible for flagella biosynthesis, chemotaxis, and environmental sensing receptor proteins (Online Resource Table S2), which are essential for bacterial colonization, flagella biosynthesis, and plant defense induction (Chen et al. 2007; Rabbee et al. 2019; Xu et al. 2020). These results may highlight the important potential application of K01 in agriculture.
In addition, B. velezensis K01 demonstrated a significant growth-promoting effect on maize. The growth-promoting effect of K01 was higher than that of strain B. velezensis BY6 on Populus davidiana seedlings (by approximately 30%) but less than that of strain B. velezensis BS1 on green pepper seedlings (74%) (Shin et al. 2021; Zhang et al. 2021). The reason for this phenomenon may be related to the efficiency of PGPR in synthesizing IAA. IAA production was identified in K01 cultural medium, and the IAA yield of strain K01 being less than that of B. velezensis T12r (48.3 mg ml–1) (Abdel-Hamid et al. 2021). IAA production is predominantly linked to the enzyme responsible for converting tryptophan (Abu-Zaitoon et al. 2012). Through a comparative genome analysis, we discovered that strain K01 contains the tryptophan operon trpABCDE and TRP1, as well as the gene for an aldehyde dehydrogenase (NAD+) (IAA synthesis, Online Resource Table S2). Aldehyde dehydrogenase is involved in two IAA synthesis pathways: the tryptamine side chain oxidase pathway (TSO) and the tryptamine pathway (TAM), which oxidize indole acetaldehyde to indole acetic acid (Li et al. 2018). The synthesis pathways of IAA encoded in the strain K01 genome likely include both the TSO and the TAM pathway. Phosphorus is an essential nutrient required for plants, and its bioavailability is associated with increases in plant growth (Mpanga et al. 2020). In the natural environment, the phosphorus in soil is predominantly composed of insoluble phosphorus (Abdel-Hamid et al. 2021). Microbes can enhance phosphorus solubilization by producing organic acids, as well as by synthesizing various phosphate-solubilizing enzymes such as phytase, phosphatase, and C-P lyase (Abdel-Hamid et al. 2021; Gouda et al. 2018). Our study revealed the formation of a transparent circle on the phosphate-soluble plate (calcium phytate), indicating that strain K01 has excellent potential for phytate phosphorus solubilization (Fig. 3a). The phosphate solubilizing activity of K01 was higher than that of B. velezensis H11 (10.5 mg L–1) but lower than that of strain B. velezensis T12r and T13r (149.5 and 159.5 mg L–1) (Abdel-Hamid et al. 2021; Gutierrez-Santa Ana et al. 2020). A significant number of genes related to organic acid synthesis and alkaline phosphatase were identified in the genome of K01 strain, which may contribute to the ability of K01 to convert insoluble organic and inorganic phosphorus to soluble phosphorus (Phosphorus dissolution, Online Resource Table S2). The presence of genes related to phosphorus solubilization in the genome of K01 suggested that this strain is capable of converting both inorganic and organic phosphorus. These findings suggest that the B. velezensis K01 strain is endowed with PGPR traits, such as IAA production and phosphate solubilization. In the meantime, these characteristics of strain K01 may account for the growth promotion observed here in maize following inoculation.
In summary, we have shown that B. velezensis K01 has a broad-spectrum antagonistic activity. Additionally, it has the potential to effectively control preharvest and postharvest gray mold diseases in tomato and pepper. Based on genome sequencing information, we found 12 antimicrobial compounds synthesis gene clusters in the K01 genome, and several genes related to the synthesis of hydrolytic enzymes such as phosphatase, cellulase, and protease, which potentially are involved in the antimicrobial and plant growth promoting traits. This paper confirms evidences for B. velezensis K01 to serve as a PGPR and biological control agent applying in tomato, pepper, and maize production.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Dr. Xili Liu (Department of Plant Pathology, China Agricultural University, Beijing, China), Jie Feng (Plant Protection Institute, The Chinese Academy of Agricultural Sciences, Beijing, China), Yue Liang (Plant Protection College, Shenyang Agricultural University, Shenyang, China) and Allen Kerr (Department of Plant Pathology, Waite Agricultural Research Institute, University of Adelaide) for providing the plant phytopathogenic fungi and bacteria.
Author contributions
Conceived and designed the experiments: YG. Performed the experiments: YX, YZ, and XW. Analyzed the data: YG, YX, QX, KH, and MX. Contributed materials/analysis tools: KH and MX. Wrote the paper: YG, YX, and QX. All authors have read and agreed to the published version of the manuscript.
Funding information
The study was funded by Special Fund for Key Science & Technology Program in Xinjiang Province of China (No. 2022B02053-1 & 2022B02021) and Science and Technology Project of Yunnan Branch of China National Tobacco Corporation (2023530000241018).
Data Availability
Available upon the request.
Declarations
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by affiliated organizations.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Hamid MS, Fouda A, El-Ela HKA, El-Ghamry AA, Hassan SE-D. Plant growth-promoting properties of bacterial endophytes isolated from roots of Thymus vulgaris L. and investigate their role as biofertilizers to enhance the essential oil contents. Biom conc. 2021;12(1):175–196. doi: 10.1515/bmc-2021-0019. [DOI] [PubMed] [Google Scholar]
- Abu-Zaitoon YM, Bennett K, Normanly J, Nonhebel HM. A large increase in IAA during development of rice grains correlates with the expression of tryptophan aminotransferase OsTAR1 and a grain-specific YUCCA. Physiol Plant. 2012;146(4):487–499. doi: 10.1111/j.1399-3054.2012.01649.x. [DOI] [PubMed] [Google Scholar]
- An MN, Tong ZP, Ding CS, Wang ZH, Sun HY, Xia ZH, Qi MF, Wu YH, Liang Y. Molecular characterization of a thaumatin-like protein PR-NP24 in tomato fruits. J Agric Food Chem. 2019;67:13001–13009. doi: 10.1021/acs.jafc.9b05256. [DOI] [PubMed] [Google Scholar]
- Berezhnaya AV, Evdokimova OV, Valentovich LN, Sverchkova NV, Titok MA, Kolomiyets EI (2019) Molecular genetic and functional analysis of the genome of bacteria bacillus velezensis BIM B-439D. Appl Biochem Micro + 55. 4386–396. 10.1134/S0003683819040033
- Blin K, Shaw S, Augustijn HE, Reitz ZL, Biermann F, Alanjary M, Fetter A, Terlouw BR, Metcalf WW, Helfrich EJN, van Wezel GP, Medema MH, Weber T. antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualization. Nucleic Acids Res. 2023 doi: 10.1093/nar/gkad344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyno G, Demir S, Danesh YR. Effects of some biological agents on the growth and biochemical parameters of tomato plants infected with Alternaria solani (Ellis & Martin) Sorauer. Eur J Plant Pathol. 2022;162(1):19–29. doi: 10.1007/s10658-021-02398-2. [DOI] [Google Scholar]
- Calvo H, Mendiara I, Arias E, Gracia AP, Blanco D, Venturini ME. Antifungal activity of the volatile organic compounds produced by Bacillus velezensis strains against postharvest fungal pathogens. Postharvest Biol Tec. 2020;166:111208. doi: 10.1016/j.postharvbio.2020.111208. [DOI] [Google Scholar]
- Cao Y, Ding W, Liu C. Unraveling the metabolite signature of endophytic Bacillus velezensis strain showing defense response towards Fusarium oxysporum. Agronomy. 2021;11(4):683. doi: 10.3390/agronomy11040683. [DOI] [Google Scholar]
- Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Sussmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol. 2007;25(9):1007–1014. doi: 10.1038/nbt1325. [DOI] [PubMed] [Google Scholar]
- Chen XL, Huang HF, Zhang SM, Zhang Y, Jiang JB, Qiu YW, Liu JY, Wang AX. Bacillus velezensis WZ-37, a new broad-spectrum biocontrol strain, promotes the growth of tomato seedlings. Agriculture. 2021;11(7):581. doi: 10.3390/agriculture11070581. [DOI] [Google Scholar]
- Chen Z, Zhao L, Dong YL, Chen WQ, Li CL, Gao XL, Chen RJ, Li LH, Xu ZJ (2021b) The antagonistic mechanism of Bacillus velezensis ZW10 against rice blast disease: evaluation of ZW10 as a potential biopesticide. PLoS ONE 16(8). 10.1371/journal.pone.0256807 [DOI] [PMC free article] [PubMed]
- Chen ZY, Sun HY, Hu T, Wang ZH, Wu WL, Liang Y, Guo YB. Sunflower resistance against Sclerotinia sclerotiorum is potentiated by selenium through regulation of redox homeostasis and hormones signaling pathways. Environ Sci Pollut R. 2022;29:38097–38109. doi: 10.1007/s11356-021-18125-7. [DOI] [PubMed] [Google Scholar]
- De la Cruz-López N, Cruz-López L, Holguín-Meléndez F, Guillén-Navarro GK, Huerta-Palacios G. Volatile organic compounds produced by cacao endophytic bacteria and their inhibitory activity on Moniliophthora roreri. Curr microbiol. 2022;79(2):35. doi: 10.1007/s00284-021-02696-2. [DOI] [PubMed] [Google Scholar]
- Dunlap CA, Kim SJ, Kwon SW, Rooney AP. Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. plantarum and ‘Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int J Syst Evol Micr. 2016;66(3):1212–1217. doi: 10.1099/ijsem.0.000858. [DOI] [PubMed] [Google Scholar]
- Fan B, Blom J, Klenk HP, Borriss R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an operational Group B. amyloliquefaciens within the B. subtilis species complex. Front Microbiol. 2017;8:22. doi: 10.3389/fmicb.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Bachle D, Fotin-Mleczek M, Jung G, Kalbacher H, Brock R. A targeted protease substrate for a quantitative determination of protease activities in the endolysosomal pathway. ChemBioChem. 2006;7(9):1428–1434. doi: 10.1002/cbic.200600209. [DOI] [PubMed] [Google Scholar]
- Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res. 2018;206:131–140. doi: 10.1016/j.micres.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Guo YB, Zheng H, Yang YL, Wang HM. Characterization of Pseudomonas corrugata strain P94 isolated from soil in Beijing as a potential biocontrol agent. Curr Microbiol. 2007;55(3):247–253. doi: 10.1007/s00284-007-0120-3. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Santa Ana A, Carrillo-Cerda HA, Rodriguez-Campos J, Kirchmayr MR, Contreras-Ramos SM, Velazquez-Fernandez JB. Volatile emission compounds from plant growth-promoting bacteria are responsible for the antifungal activity against F. solani. 3 Biotech. 2020;10(7):292. doi: 10.1007/s13205-020-02290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Li QC, Hou Y, Li GQ, Yang JY, Li DW, Ye JR. Bacillus velezensis strain HYEB5-6 as a potential biocontrol agent against anthracnose on Euonymus japonicus. Biocontrol Sci Techn. 2017;27(5):636–653. doi: 10.1080/09583157.2017.1319910. [DOI] [Google Scholar]
- Jiang CH, Liao MJ, Wang HK, Zheng MZ, Xu JJ, Guo JH. Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol Control. 2018;126:147–157. doi: 10.1016/j.biocontrol.2018.07.017. [DOI] [Google Scholar]
- Jiang CH, Yao XF, Mi DD, Li ZJ, Yang BY, Zheng Y, Qi YJ, Guo JH. Comparative transcriptome analysis reveals the biocontrol mechanism of Bacillus velezensis F21 against fusarium wilt on watermelon. Front Microbiol. 2019;10:652. doi: 10.3389/fmicb.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao ZW, Wu N, Hale L, Wu W, Wu D, Guo YB. Characterisation of Pseudomonas chlororaphis subsp. aurantiaca strain Pa40 with the ability to control wheat sharp eyespot disease. Ann Appl Biol. 2013;163(3):444–453. doi: 10.1111/aab.12068. [DOI] [Google Scholar]
- Karthika S, Varghese S, Jisha MS (2020) Exploring the efficacy of antagonistic rhizobacteria as native biocontrol agents against tomato plant diseases. 3 Biotech 10(7). 10.1007/s13205-020-02306-1 [DOI] [PMC free article] [PubMed]
- Kerr A. Biological control of crown gall through production of agrocin 84. Plant Dis. 1980;64(1):28–30. [Google Scholar]
- King EJ. The colorimetric determination of phosphorus. Biochem J. 1932;26:292–297. doi: 10.1039/AN9588300406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs B, Höding B, Kübar S, Workie MA, Junge H, Schmiede-knecht G, Grosch R, Bochow H, Hevesi M. Use of Bacillus subtilis as biocontrol agent. I. Activities and characterization of Bacillus subtilis strains. J Plant Dis Protect. 1998;105(2):181–197. [Google Scholar]
- Kumar RS, Ayyadurai N, Pandiaraja P, Reddy AV, Venkateswarlu Y, Prakash O, Sakthivel N. Characterization of antifungal metabolite produced by a new strain Pseudomonas aeruginosa PUPa3 that exhibits broad-spectrum antifungal activity and biofertilizing traits. J Appl Microbiol. 2005;98(1):145–154. doi: 10.1111/j.1365-2672.2004.02435.x. [DOI] [PubMed] [Google Scholar]
- Lastochkina O, Seifikalhor M, Aliniaeifard S, Baymiev A, Pusenkova L, Garipova S, Kulabuhova D, Maksimov I. Bacillus spp.: efficient biotic strategy to control postharvest diseases of fruits and vegetables. Plants. 2019;8(4):97. doi: 10.3390/plants8040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jiao ZW, Hale L, Wu WL, Guo YB. Disruption of gene pqqA or pqqB reduces plant growth promotion activity and biocontrol of crown gall disease by Rahnella aquatilis HX2. PLoS ONE. 2014;9(12):e115010. doi: 10.1371/journal.pone.0115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MS, Guo R, Yu F, Chen X, Zhao HY, Li HX, Wu J. Indole-3-acetic acid biosynthesis pathways in the plant-beneficial bacterium Arthrobacter pascens ZZ21. Int J Mol Sci. 2018;19(2):443. doi: 10.3390/ijms19020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM, Yoon MY, Choi GJ, Choi YH, Jang KS, Shin TS, Park HW, Yu NH, Kim YH, Kim JC. Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus velezensis G341 against various phytopathogenic fungi. Plant Pathol J. 2017;33(5):488–498. doi: 10.5423/ppj.oa.04.2017.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Zhang Z, Xu ZX, Wang LN, Chen CH, Ren ZH. Over expression of SlMYB75 enhances resistance to Botrytis cinerea and prolongs fruit storage life in tomato. Plant Cell Rep. 2020;40:43–58. doi: 10.1007/s00299-020-02609-w. [DOI] [PubMed] [Google Scholar]
- Liu XQ, Xie JT, Fu YP, Jiang DH, Chen T, Cheng JS. The subtilisin-like protease bcser2 affects the sclerotial formation, conidiation and virulence of Botrytis cinerea. Int J Mol Sci. 2020;21(603):2–15. doi: 10.3390/ijms21020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Zeng QC, Yalimaimaiti NEZY, Wang W, Zhang RF, Yao J. Comprehensive genomic analysis of Bacillus velezensis AL7 reveals its biocontrol potential against Verticillium wilt of cotton. Mol Genet Genom. 2021;296:1287–1298. doi: 10.1007/s00438-021-01816-8. [DOI] [PubMed] [Google Scholar]
- Lyagin I, Aslanli A, Domnin M, Stepanov N, Senko O, Maslova O, Efremenko E (2023) Metal nanomaterials and hydrolytic enzyme-based formulations for improved antifungal activity. Int J Mol Sci 24(14). 10.3390/ijms241411359 [DOI] [PMC free article] [PubMed]
- Lynd LR, Zhang YH. Quantitative determination of cellulase concentration as distinct from cell concentration in studies of microbial cellulose utilization: analytical framework and methodological approach. Biotechnol Bioeng. 2002;77(4):467–475. doi: 10.1002/bit.10142. [DOI] [PubMed] [Google Scholar]
- Marsh AJ, O’Sullivan O, Ross RP, Cotter PD, Hill C. In silico analysis highlights the frequency and diversity of type 1 lantibiotic gene clusters in genome sequenced bacteria. BMC Genomics. 2010;11:679. doi: 10.1186/1471-2164-11-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JH, Won SJ, Maung CEH, Choi JH, Choi SI, Ajuna HB, Ahn YS. Bacillus velezensis CE 100 inhibits root rot diseases (Phytophthora spp.) and promotes growth of japanese cypress (Chamaecyparis obtusa Endlicher) seedlings. Microorganisms. 2021;9(4):821. doi: 10.3390/microorganisms9040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpanga IK, Ludewig U, Dapaah HK, Neumann G. Acquisition of rock phosphate by combined application of ammonium fertilizers and Bacillus amyloliquefaciens FZB42 in maize as affected by soil pH. J Appl Microbiol. 2020;129(4):947–957. doi: 10.1111/jam.14654. [DOI] [PubMed] [Google Scholar]
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- Ngalimat MS, Yahaya RSR, Baharudin MMA-a, Yaminudin SM, Karim M, Ahmad SA, Sabri S. A review on the biotechnological applications of the operational group Bacillus amyloliquefaciens. Microorganisms. 2021;9(3):614. doi: 10.3390/microorganisms9030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nifakos K, Tsalgatidou PC, Thomloudi EE, Skagia A, Kotopoulis D, Baira E, Delis C, Papadimitriou K, Markellou E, Venieraki A, Katinakis P. Genomic analysis and secondary metabolites production of the endophytic Bacillus velezensis Bvel1: a biocontrol agent against Botrytis cinerea causing bunch rot in post-harvest table grapes. Plants. 2021;10(8):1716. doi: 10.3390/plants10081716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam P, Senapati A, Kumar U, Sharma L, Lepcha P, Prabhukarthikeyan SR, Jahan A, Parameshwaran C, Govindharaj GPP, Lenka S, Nayak PK, Mitra D, Sagarika MS, Thangappan S, Sivakumar U (2019) Antagonistic and plant-growth promoting novel Bacillus species from long-term organic farming soils from Sikkim, India. 3 Biotech 9(11). 10.1007/s13205-019-1938-7 [DOI] [PMC free article] [PubMed]
- Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol. 2002;68(8):3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbee MF, Baek KH. Antimicrobial activities of lipopeptides and polyketides of Bacillus velezensis for agricultural applications. Molecules. 2020;25(21):4973. doi: 10.3390/molecules25214973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbee MF, Ali MS, Choi J, Hwang BS, Jeong SC, Baek KH. Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules. 2019;24(6):1046. doi: 10.3390/molecules24061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R, Hashem A, Abd-Allah EF. Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Front Physiol. 2017;8:667. doi: 10.3389/fphys.2017.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka LM, Chekan JR, Nair SK, van der Donk WA. Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chem Rev. 2017;117(8):5457–5520. doi: 10.1021/acs.chemrev.6b00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Couso R, Flores-Felix JD, Rivas R. Mechanisms of action of microbial biocontrol agents against Botrytis cinerea. J Fungi. 2021;7(12):1045. doi: 10.3390/jof7121045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Garcia C, Bejar V, Martinez-Checa F, Llamas I, Quesada E. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Velez in Malaga, southern Spain. Int J Syst Evol Micr. 2005;55(1):191–195. doi: 10.1099/ijs.0.63310-0. [DOI] [PubMed] [Google Scholar]
- Salvatierra-Martinez R, Arancibia W, Araya M, Aguilera S, Olalde V, Bravo J, Stoll A. Colonization ability as an indicator of enhanced biocontrol capacity-An example using two Bacillus amyloliquefaciens strains and Botrytis cinerea infection of tomatoes. J Phytopathol. 2018;166(9):601–612. doi: 10.1111/jph.12718. [DOI] [Google Scholar]
- Saxena AK, Kumar M, Chakdar H, Anuroopa N, Bagyaraj DJ. Bacillus species in soil as a natural resource for plant health and nutrition. J Appl Microbiol. 2020;128(6):1583–1594. doi: 10.1111/jam.14506. [DOI] [PubMed] [Google Scholar]
- Sethi RP, Subba-Rao NS. Solubilization of tricalcium phosphate and calcium phytate by soil fungi. J Gen Appl Microbiol. 1968;14(3):329–331. doi: 10.2323/jgam.14.329. [DOI] [Google Scholar]
- Shafi J, Tian H, Ji MS. Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotec Eq. 2017;31(3):446–459. doi: 10.1080/13102818.2017.1286950. [DOI] [Google Scholar]
- Shin JH, Park BS, Kim HY, Lee KH, Kim KS. Antagonistic and plant growth-promoting effects of Bacillus velezensis BS1 isolated from rhizosphere soil in a pepper field. Plant Pathol J. 2021;37(3):307–314. doi: 10.5423/ppj.nt.03.2021.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenković JG, Petrović GM, Đorđević AS. Phytochemical analysis and antibacterial activity of Achillea coarctata poir. Essential oils. Chem Biodivers. 2022;19(10):e202200578. doi: 10.1002/cbdv.202200578. [DOI] [PubMed] [Google Scholar]
- Teather RM, Wood PJ. Use of congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microb. 1982;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vairagkar U, Nipanikar-Gokhale P, Mirza Y. Genomic insights into biocontrol potential of edible seaweed-associated Bacillus velezensis MTCC 10456 from Gulf of Mannar. Arch Microbiol. 2021;203(6):2941–2952. doi: 10.1007/s00203-021-02244-3. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Guo RJ, Wang WC, Ma GZ, Li SD. Insight into the surfactin production of Bacillus velezensis B006 through metabolomics analysis. J Ind Microbiol Biot. 2018;45(12):1033–1044. doi: 10.1007/s10295-018-2076-7. [DOI] [PubMed] [Google Scholar]
- Wang BQ, Liu C, Yang XM, Wang YN, Zhang FY, Cheng HC, Zhang LP, Liu HW. Genomics-guided isolation and identification of active secondary metabolites of Bacillus velezensis BA-26. Biotechnol Biotec Eq. 2021;35(1):895–904. doi: 10.1080/13102818.2021.1934540. [DOI] [Google Scholar]
- Willer H, Schlatter B, Trávníček J. The world of organic agriculture. statistics and emerging trends 2023. In:, Schlatter B, Trávníček J, Willer H (eds) (2023) Current statistics on organic agriculture worldwide: area, operators, retail sales and international trade, Online Version 2 of February 23, 2023. Frick and Bonn
- Wu RY, Qin YX, Shen Q, Li PL. The complete genome sequence of Bacillus velezensis LPL061, an exopolysaccharide-producing bacterium. 3 Biotech. 2020;10:1–10. doi: 10.1007/s13205-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Xie XW, Zhao YR, Shi YX, Chai AL, Li L, Li BJ (2020) Whole-genome analysis of Bacillus velezensis ZF2, a biocontrol agent that protects Cucumis sativus against Corynespora leaf spot diseases. 3 Biotech 10(4):1–10. 10.1007/s13205-020-2165-y [DOI] [PMC free article] [PubMed]
- Yan YC, Xu WH, Chen WJ, Hu YL, Wang ZG. Complete genome sequence of Bacillus velezensis YYC, a bacterium isolated from the tomato rhizosphere. Arch Microbiol. 2022;204:1–5. doi: 10.1007/s00203-021-02709-5. [DOI] [PubMed] [Google Scholar]
- Ye M, Tang XF, Yang R, Zhang HF, Li FS, Tao FZ, Li F, Wang ZG. Characteristics and application of a novel species of Bacillus: Bacillus velezensis. ACS Chem Biol. 2018;13(3):500–505. doi: 10.1021/acschembio.7b00874. [DOI] [PubMed] [Google Scholar]
- Zhang YC, Fan QR, Loria R. A re-evaluation of the taxonomy of phytopathogenic genera Dickeya and Pectobacterium using whole-genome sequencing data. Syst Appl Microbiol. 2016;39:252–259. doi: 10.1016/j.syapm.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Zhang P, Diao J, Xie GQ, Ma L, Wang LH (2021) A complete genome sequence of the wood stem endophyte Bacillus velezensis BY6 strain possessing plant growth-promoting and antifungal activities. Biomed Res Int 2021. 10.1155/2021/3904120 [DOI] [PMC free article] [PubMed]
- Zhang YH, Zhao MX, Chen W, Yu HL, Jia WT, Pan HY, Zhang XH. Multi-omics techniques for analysis antifungal mechanisms of lipopeptides produced by Bacillus velezensis GS-1 against Magnaporthe oryzae in vitro. Int J Mol Sci. 2022;23(7):3762. doi: 10.3390/ijms23073762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HL, Liu K, Fan YZ, Cao JC, Li HH, Song W, Liu YS, Miao M (2022) Cell-free supernatant of Bacillus velezensis suppresses mycelial growth and reduces virulence of Botrytis cinerea by inducing oxidative stress. Front Microbiol 13. 10.3389/fmicb.2022.980022 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete genome sequence of B. velezensis K01 have been deposited in the NCBI GenBank with the accession number CP059344.1.
Available upon the request.