Abstract
The serotypes of 88 nonreplicate nosocomial Pseudomonas aeruginosa isolates from 11 Greek hospitals were studied in relation to their antibiotic susceptibilities. Rates of resistance to β-lactams, aminoglycosides, and quinolones ranged from 31 to 65%, except for those to ceftazidime (15%) and imipenem (21%). Four serotypes were dominant: O:12 (25% of isolates), O:1 (17%), O:11 (16%), and O:6 (10%). Multidrug resistance rates in the major serogroups O:12 (91%) and O:11 (79%) were higher than those in serogroups O:1 (40%) and O:6 (43%). Further typing with respect to pulsed-field gel electrophoresis patterns following XbaI digestion of genomic DNA discriminated the isolates into 74 types. Pulsed-field gel electrophoresis revealed that the ubiquitous O:12 group was genetically homogeneous, since 95% of strains belonged to two clusters of genotypic similarity, while the O:11 strains, present in 8 of the 11 hospitals, were distributed among five such clusters. Therefore, apart from the already reported O:12 multidrug-resistant European clone, an O:11 population, characterized by a serotype known to be dominant in the environment and the hospital in several parts of the world, but previously not associated with multidrug resistance to antibiotics, has progressed to a multidrug-resistant state.
Pseudomonas aeruginosa is an important nosocomial pathogen, ranking among the three most frequently isolated in intensive care units (20). Nosocomial strains, in sharp contrast to community-acquired strains, exhibit high rates of resistance to antibiotics and are frequently multidrug resistant (2, 16, 24), a fact probably related to the ease with which they can develop resistance in a hospital environment (11, 15). A genetically homogeneous multidrug-resistant P. aeruginosa clone belonging to serogroup O:12 has been reported to be dominant in Greece since 1987 (10) and in the rest of Europe since 1989 (18). By contrast, the most frequently encountered serogroups, both clinically and in the environment, such as O:11, O:6, and O:1 (13), have not been associated with multidrug resistance (9, 17). Strains belonging to O:11 have been connected to environmental sources (1, 13) as well as hospital outbreaks (3).
Apart from serotyping, several other typing systems, assessing either phenotype (12, 19) or genotype (6, 7), have been used to discriminate among P. aeruginosa strains. We have performed a multicenter study to examine the clonal relationships in the population of Greek nosocomial P. aeruginosa strains and the current position of the O:12 clone. We have used the methods of antibiogram typing, serotyping, and DNA fingerprinting by pulsed-field gel electrophoresis (PFGE) of genomic DNA digested with XbaI. We have shown that while the O:12 serogroup is still highly multidrug resistant and composed of two genetic clones, the O:11 serogroup, previously related to low resistance rates (9), is also now strongly represented among multidrug-resistant isolates, although it remains genetically more heterogeneous.
MATERIALS AND METHODS
Bacterial strains.
A total of 88 nonreplicate P. aeruginosa strains, made up of 5 to 9 strains from each of 11 Greek hospitals, were chosen at random from the total P. aeruginosa isolates for 1994 and 1995. They had been identified to the species level by standard microbiological methods (4). Extrapolating from the total of 973 susceptible and resistant P. aeruginosa isolates submitted by 13 hospitals to the Greek Antibiotic Surveillance Network for 1996, the first year of its operation, our sample represented more than 10% of the total P. aeruginosa isolates from the participating hospitals for 1994 and 1995 (23a).
Antibiotic susceptibility testing.
Susceptibilities to the antibiotics listed in Table 1 were determined by the disk diffusion method in accordance with published standards (14).
TABLE 1.
Rates of resistance to selected antipseudomonadal antibiotics
| Antibiotic | % of isolates with resistance statusa of:
|
||
|---|---|---|---|
| R | I | S | |
| Ticarcillin | 65 | 4 | 31 |
| Ceftazidime | 15 | 7 | 78 |
| Aztreonam | 33 | 30 | 37 |
| Imipenem | 21 | 6 | 73 |
| Gentamicin | 47 | 1 | 52 |
| Tobramycin | 37 | 1 | 62 |
| Amikacin | 35 | 7 | 58 |
| Netilmicin | 40 | 4 | 56 |
| Ciprofloxacin | 31 | 9 | 60 |
| Norfloxacin | 31 | 3 | 66 |
R, resistant; I, intermediately resistant; S, susceptible.
Statistical analysis.
A two-tailed χ2 test with Yates’ correction was used.
Serotyping.
Serotyping was performed by agglutination on slides, as previously described (8), with commercially obtained antisera (Difco).
PFGE of macrorestricted genomic DNA.
Agarose-embedded genomic DNA was digested with restriction endonuclease XbaI (New England Biolabs) and electrophoresed in a CHEF DRIII apparatus (Bio-Rad), as described elsewhere (22). The gels were run at 14°C, 6 V cm−1, and a 120° switch angle, for 18 h, with a linear switch time ramp of 0.5 to 20 s. Lambda phage DNA concatamers (New England Biolabs) were used as DNA size markers. After electrophoresis, the gels were stained with a 0.5-μg ml−1 concentration of ethidium bromide and video images were obtained by UV illumination (Vilber Lourmat, Marne-La-Vallée, France).
Analysis of genetic variability.
The images were processed by use of BIO-GENE1 software (Vilber Lourmat), and a dendrogram was computed after comparison of the molecular weights of the DNA fragments by using the Jaccard coefficient and UPGMA (unweighted pair group method using arithmetical averages) clustering. A 60% similarity cutoff was used to group isolates in clusters of related genotypes. Struelens et al. (21) had used an 80% cutoff to assign relatedness to consecutive strains from the same patients, isolated in two centers over a period of 20 months. We decided to use a lower value to allow for the greater variation with respect to time (2 years), place (11 centers), and origin (distinct patients) among our strains and for the differences between the Dice and Jaccard coefficients.
RESULTS
A total of 88 nonreplicate P. aeruginosa strains, made up of five to nine strains from each of 11 Greek hospitals and representing approximately 10% of the total P. aeruginosa isolates for 1994 and 1995, were chosen at random. They had been isolated from urine (30%), bronchial secretions (15%), pus (14%), blood (11%), sputum (9%), wound exudates (9%), the trachea (2%), and cervical secretions (2%), while one strain had originated from a venal catheter swab and three were from unknown sources.
The rates of resistance of isolates to selected antipseudomonadal antibiotics are shown in Table 1. One-third to two-thirds of all isolates were resistant to at least one of the antibiotics tested, with the exception that 15 and 21% of isolates were resistant to ceftazidime and imipenem, respectively. Grouping isolates with intermediate and high resistance together, 32 antibiotic resistance phenotypes could be distinguished, the most frequent ones being full susceptibility (15%), followed by resistance to ticarcillin alone (11%) and resistance to all antibiotics tested (10%). Overall, 52% of isolates were multidrug resistant, i.e., resistant to antibiotics belonging to two or more distinct classes, 40% being resistant to β-lactams, aminoglycosides, and quinolones and 11% being resistant to only the first two compound families. Finally, 32% were resistant to one antibiotic class only, 26% to β-lactams and 6% to aminoglycosides; quinolone resistance was always crossed to resistance to either or both of the other two classes.
Multidrug resistance rates were significantly higher (P = 0.02811) among respiratory tract isolates (sputum and bronchial secretions, 53%) or urine (75%) than among blood (22%) or wound (pus and wound exudates, 22%) isolates.
Serotyping is perhaps the most widespread method of first line typing for P. aeruginosa (7). This method resolved the isolates into 13 serogroups, with one strain being polyagglutinable. Serogroup O:12 was dominant, being represented by 25% of isolates, followed by O:1 (17%), O:11 (16%), O:6 (10%), O:3 (8%), O:5 (6%), O:10 (4%), O:4 (3%), and O:2, O:9, and O:17 (2% each), while one isolate each belonged to O:7 and O:8. As shown in Table 2, serogroups O:12 and O:11 contained the largest proportions of multidrug-resistant isolates, while O:6 contained the largest proportion of susceptible ones.
TABLE 2.
Distribution of resistance phenotypes among different serogroups
| Resistance phenotypea | No. (%) of isolates in serogroup
|
No. of MR isolatesb | Total no. (%) of isolates (n = 88) | ||||
|---|---|---|---|---|---|---|---|
| O:12 (n = 22) | O:11 (n = 14) | O:1 (n = 15) | O:6 (n = 9) | Other (n = 28) | |||
| T | 1 | 3 | 1 | 5 | 8 (11) | ||
| A | 1 | 2 | 1 | 1 | 5 (6) | ||
| M | 1 | 1 | 2 | ||||
| I | 2 | 2 | |||||
| AP | 1 | 1 | 1 | ||||
| TA | 1 | 1 | 5 | 7 (8) | |||
| TG | 1 | 1 | 1 | ||||
| GN | 1 | 1 | |||||
| MN | 1 | 1 | |||||
| TAG | 1 | 1 | 2 | 2 | |||
| TCA | 1 | 1 | |||||
| TGB | 1 | 1 | 1 | ||||
| GMN | 1 | 1 | |||||
| TCAN | 1 | 1 | 1 | ||||
| AGMN | 1 | 1 | 1 | ||||
| TAMP | 1 | 1 | 1 | ||||
| TIPR | 1 | 1 | 1 | ||||
| TAIPR | 1 | 1 | 1 | ||||
| TCAGMN | 1 | 1 | 1 | ||||
| TAGBMN | 1 | 1 | 1 | ||||
| TCAGBMN | 2 | 2 | 2 | ||||
| TCAGMPR | 1 | 1 | 1 | ||||
| TAGBMNP | 1 | 1 | 1 | ||||
| TAGBNPR | 1 | 1 | 1 | ||||
| TAIGBNP | 1 | 1 | 1 | ||||
| TCAIGMNP | 1 | 1 | 1 | ||||
| TAGBMNPR | 4 | 1 | 5 | 5 (6) | |||
| TAIGBNPR | 1 | 1 | 2 | 2 | |||
| TCAGBMNPR | 2 | 1 | 3 | 3 | |||
| TAIGBMNPR | 6 (27) | 1 | 7 | 7 (8) | |||
| TCAIGBMNPR | 6 (27) | 2 | 1 | 9 | 9 (10) | ||
| Fully susceptible | 1 (7) | 1 (7) | 3 (33) | 8 (29) | 13 (15) | ||
| Total MRb | 20 (91) | 11 (79) | 6 (40) | 3 (43) | 6 (25) | 46 (52) | |
| % of total MRb | 43 | 24 | 13 | 7 | 13 | ||
T, ticarcillin; C, ceftazidime; A, aztreonam; I, imipenem; G, gentamicin; B, tobramycin; M, amikacin; N, netilmicin; P, ciprofloxacin; R, norfloxacin.
MR, multidrug resistant (resistant to two or more antibiotic classes). High and intermediate levels of resistance have been grouped together.
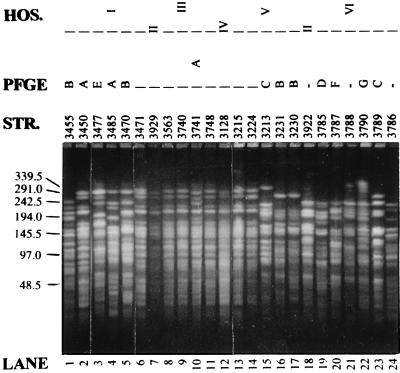
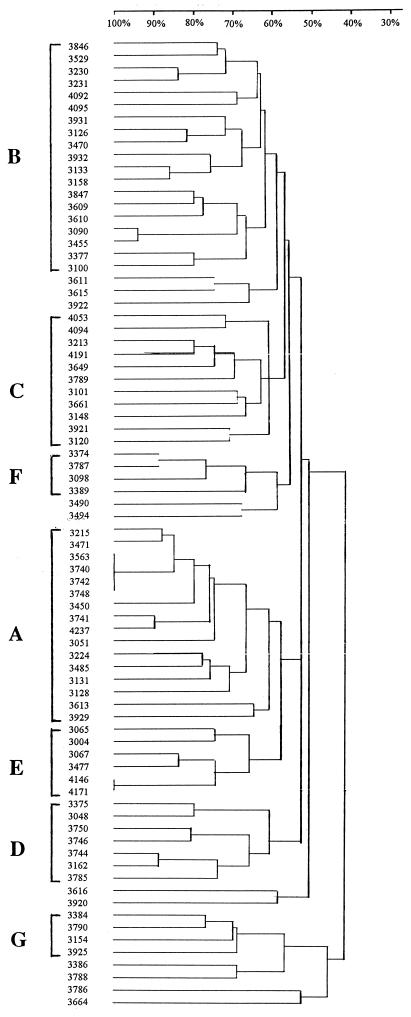
DNA fingerprinting by PFGE of genomic DNA after digestion with an appropriate restriction endonuclease is currently considered the “gold standard” for bacterial typing (23) and has been shown to be the method of preference for P. aeruginosa (6). PFGE after digestion of genomic DNA with XbaI resulted in 74 distinct genomic fingerprints among the 79 examined isolates (Fig. 1). Four O:12 isolates from a single hospital appeared identical, while two other O:12 isolates from a different hospital also displayed indistinguishable PFGE patterns; these presumably were epidemic or endemic strains. When the different fingerprints were compared on a dendrogram, seven groups emerged; these groups consisted of strains with pattern similarity equal or greater than 60% and contained at least four isolates each (Fig. 2). The phenotypic characteristics of strains belonging to these genetic clusters are summarized in Table 3. Most strikingly, group A was composed of closely related isolates (Fig. 1), 81% of which belonged to serogroup O:12 and all of which were multidrug resistant. Group E, on the other hand, was composed primarily of O:11 isolates, while group C contained 45% O:1 and 54% susceptible isolates. Groups A, B, and C were widespread, with representative strains in eight, nine, and seven hospitals, respectively. Finally, 12% of isolates remained outside of these DNA fingerprinting clusters.
FIG. 1.
Representative PFGE of selected strains. All lanes are from the same gel. The positions of migration of the λ DNA concatamers are shown to the left of the gel, and their sizes are indicated in kilobases. HOS., hospital; PFGE, PFGE pattern similarity cluster; STR., strain number; —, strain not belonging to a PFGE pattern similarity cluster.
FIG. 2.
Dendrogram indicating similarity of PFGE patterns of different isolates. Strain numbers are indicated on the left of the figure. A similarity scale ranging from 30 to 100% is at the top. Clusters of PFGE patterns exhibiting similarity of >60% are indicated by capital letters (A to G).
TABLE 3.
Phenotypic characteristics of genotypically related strains
| PFGE group | No. of hospitalsa | No. (%) of strains | % of strains belonging to serogroup
|
% of strains belonging to resistance phenotypeb
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| O:1 | O:6 | O:11 | O:12 | MR | RES | SU | |||
| A | 8 | 16 (20) | 12 | 81 | 100 | ||||
| B | 9 | 19 (24) | 16 | 16 | 32 | 42 | 32 | 26 | |
| C | 7 | 11 (14) | 45 | 9 | 9 | 37 | 54 | ||
| D | 4 | 7 (9) | 14 | 43 | 57 | 29 | 14 | ||
| E | 3 | 6 (8) | 17 | 83 | 67 | 33 | |||
| F | 3 | 4 (5) | 25 | 50 | 50 | 50 | |||
| G | 4 | 4 (5) | 50 | 25 | 50 | 50 | |||
| Other | 7 | 12 (15) | 8 | 33 | 8 | 42 | 42 | 16 | |
Number of hospitals where representative isolates were present.
MR, resistant or intermediately resistant to two or more antibiotic classes; RES, resistant or intermediately resistant to one antibiotic class; SU, susceptible. Calculations are based on the 79 isolates that were typed by PFGE.
DISCUSSION
We studied 88 nosocomial P. aeruginosa isolates from 11 Greek hospitals in an attempt to correlate their genotype with important phenotypic characteristics, such as serotype and antibiotic resistance. Although our sample was relatively not large, it did represent over 10% of the calculated total P. aeruginosa isolates from the participating hospitals for 1994 and 1995 and can therefore be considered as a reflection of the actual situation.
Rates of resistance generally exceeded 33%, apart from those to ceftazidime and imipenem, resulting in an overall multidrug resistance rate of 52%. Compared to those observed in a similar study conducted in 1989 (5), resistance rates to ceftazidime, gentamicin, tobramycin, and netilmicin have remained relatively constant or have even decreased slightly, while increases were noted for aztreonam (+50%), imipenem (+40%), amikacin (+35%), and norfloxacin (+30%).
Of the three dominant serogroups, O:12, O:11, and O:1, the first two showed a high degree of multidrug resistance, in contrast to that of the latter. This signalled a change from a previous Greek study of 1982, where the dominant serogroups were, in decreasing order, O:11, O:6, and O:12 (9) and may be due partly to the expansion of the multidrug resistant O:12 clone, already obvious in 1987 (10). High multidrug resistance rates were observed for strains from urine, bronchial secretions, and sputum; for the first two sources, this correlated with large proportions of strains belonging to serogroups O:11 (21 and 25%, respectively) and O:12 (37 and 33%, respectively), in comparison to those belonging to other serogroups (0 to 12% for urine, and 0 to 17% for bronchial secretions). Conversely, the relatively high susceptibility rates in strains isolated from wound exudates correlated with smaller proportions of O:11 and O:12 (0 and 14%, respectively).
PFGE profiles of different isolates could be grouped according to band pattern similarity of 60% or greater. This was below the 80% similarity observed by Struelens et al. (21) for consecutive strains from the same patients, isolated in two centers for a period of 1 to 20 months. We decided on a lower value to allow for the greater variation with respect to time (2 years), place (11 centers), and origin (distinct patients) among our strains and for the differences between the Dice and Jaccard coefficients used to calculate similarity in the previously mentioned study and the present study, respectively.
It was thus observed that the genetic composition of the O:12 population was relatively homogeneous: 65% belonged to group A of genotypically similar strains, and 30% belonged to group B. The O:12 serogroup contained no susceptible isolates; on the contrary, the vast majority (91%) of O:12 isolates were multidrug resistant. Put another way, O:12 was the largest single serogroup (43%) among multidrug-resistant isolates, as well as among group A strains (81%). Two major multidrug resistance phenotypes, TAIGBMNPR and TCAIGBMNPR (Table 2), each represented 27% of the O:12 isolates. All strains belonging to the first resistance phenotype also belonged to PFGE group A, while those belonging to the second were divided among PFGE groups A (four isolates) and B (two isolates). This picture confirmed the previously reported genetic homogeneity of multidrug-resistant serogroup O:12 in Greece and Europe (10, 18) and demonstrated the persistence of multidrug-resistant O:12 strains among Greek nosocomial P. aeruginosa. The importance of the O:12 serogroup is underlined by the fact that it has overcome, in numbers of isolates, in the past decade, both O:11 and O:6.
However, in the present study, it was noted additionally that 24% of all multidrug resistant strains belonged to serotype O:11, compared with only 7 to 13% that belonged to the remaining serogroups. This was striking because previously, in Greece as elsewhere, O:11 had not been associated with multidrug resistance (9, 17, 25). Both O:12 and O:11 multidrug resistant strains were well dispersed, being found in all and 8, respectively, of the 11 hospitals studied. Although O:11 isolates were genetically more diverse than O:12 isolates, making up substantial proportions of genotypic groups B (16%), C (9%), D (43%), E (83%), and F (50%), none clustered in group A, which contained primarily O:12 isolates. In agreement with their greater genotypic diversity, O:11 isolates were distributed among different resistance phenotypes more evenly than the O:12 group isolates were (Table 2). Finally, serogroup O:6 displayed a greater genotypic heterogeneity (PFGE discriminatory index = 1) than O:12 strains did (0.15), as expected of a largely (33%) susceptible population which has not resulted from selection under antibiotic pressure in a nosocomial environment.
Thus, the present study has demonstrated the progression of an O:11 population to a multidrug-resistant state. The O:11 serogroup has been frequently and ubiquitously observed in the environment as well as in the hospital but was not characterized previously by multidrug resistance to antibiotics. The molecular mechanisms involved in this progression are currently under investigation. It would seem that we are witnessing the inverse of the appearance of multidrug-resistant O:12, which was due to the expansion of a nondominant serogroup in a nosocomial environment, following development of resistance. This observation is cause for concern and once again emphasizes the need for appropriate epidemiological monitoring of strains implicated in nosocomial infections, by using both traditional and molecular methods, to allow the implementation of adequate and prompt preventive measures.
ACKNOWLEDGMENTS
C.F., a student in the Department of Biomedical Laboratory Sciences, Uppsala University College of Health and Caring Sciences, Uppsala, Sweden, was carrying out her final-year project in the Department of Microbiology, Medical School, University of Athens, Athens, Greece, under an exchange scheme supported by the European Union SOCRATES Programme.
We gratefully acknowledge the technical assistance of E. Christou in serotyping. We thank P. Menounos for use of the BIO-GENE1 software in his laboratory (School of Officers Nurses, Athens, Greece) and L. Tzouvelekis (Pasteur Institute, Athens, Greece) for help with statistical analysis.
REFERENCES
- 1.Alcock S R. Acute otitis externa in divers working in the North Sea: a microbiological survey of seven saturation dives. J Hyg Camb. 1977;78:395–409. doi: 10.1017/s0022172400056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald L, Phillips L, Monnet D, McGowan J E, Jr, Tenover F, Graves R. Antimicrobial resistance in isolates from inpatients and outpatients in the United States: increasing importance of the intensive care unit. Clin Infect Dis. 1997;24:211–215. doi: 10.1093/clinids/24.2.211. [DOI] [PubMed] [Google Scholar]
- 3.Farmer J J, III, Weinstein R A, Zierdt C H, Brokopp C D. Hospital outbreaks caused by Pseudomonas aeruginosa: importance of serogroup O11. J Clin Microbiol. 1982;16:266–270. doi: 10.1128/jcm.16.2.266-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilardi G L. Pseudomonas and related genera. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 429–441. [Google Scholar]
- 5.The Greek Society for Microbiology. Antibiotic resistance among gram-negative bacilli in 19 Greek hospitals. J Hosp Infect. 1989;14:177–180. doi: 10.1016/0195-6701(89)90124-2. [DOI] [PubMed] [Google Scholar]
- 6.Grundmann H, Schneider C, Hartung D, Daschner F D, Pitt T L. Discriminatory power of three DNA-based typing techniques for Pseudomonas aeruginosa. J Clin Microbiol. 1995;33:528–534. doi: 10.1128/jcm.33.3.528-534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The International Pseudomonas aeruginosa Typing Study Group. A multicenter comparison of methods for typing strains of Pseudomonas aeruginosa predominantly from patients with cystic fibrosis. J Infect Dis. 1994;169:134–142. doi: 10.1093/infdis/169.1.134. [DOI] [PubMed] [Google Scholar]
- 8.Legakis N J, Tzouvelekis L S, Tsakris A, Legakis J N, Vatopoulos A C. On the incidence of antibiotic resistance among aerobic Gram-negative rods isolated in Greek hospitals. J Hosp Infect. 1993;24:233–237. doi: 10.1016/0195-6701(93)90052-2. [DOI] [PubMed] [Google Scholar]
- 9.Legakis N J, Aliferopoulou M, Papavassiliou J, Papapetropoulou M. Serotypes of Pseudomonas aeruginosa in clinical specimens in relation to antibiotic susceptibility. J Clin Microbiol. 1982;16:458–463. doi: 10.1128/jcm.16.3.458-463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legakis N J, Koukoubanis N, Malliara K, Michalitsianos D, Papavassiliou J. Importance of carbenicillin and gentamicin cross-resistant serotype O:12 Pseudomonas aeruginosa in six Athens hospitals. Eur J Clin Microbiol. 1987;6:300–303. doi: 10.1007/BF02017618. [DOI] [PubMed] [Google Scholar]
- 11.Manian F A, Meyer L, Jenne J, Owen A, Taff T. Loss of antimicrobial susceptibility in aerobic Gram-negative bacilli repeatedly isolated from patients in intensive-care units. Infect Control Hosp Epidemiol. 1996;17:222–226. doi: 10.1086/647284. [DOI] [PubMed] [Google Scholar]
- 12.Maniatis A N, Karkavitsas C, Maniatis N A, Tsiftsakis E, Genimata V, Legakis N J. Pseudomonas aeruginosa folliculitis due to non-O:11 serogroups: acquisition through use of contaminated synthetic sponges. Clin Infect Dis. 1995;21:437–439. doi: 10.1093/clinids/21.2.437. [DOI] [PubMed] [Google Scholar]
- 13.Mayo M S, Cook W L, Schlitzer R L, Ward M A, Wilson L A, Ahearn D G. Antibiograms, serotypes, and plasmid profiles of Pseudomonas aeruginosa associated with corneal ulcers and contact lens wear. J Clin Microbiol. 1986;24:372–376. doi: 10.1128/jcm.24.3.372-376.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disc susceptibility tests, vol. 13, no. 24. Publication no. M2-A5. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 15.Ogle J W, Reller L B, Vasil M L. Development of resistance in Pseudomonas aeruginosa to imipenem, norfloxacin, and ciprofloxacin during therapy: proof provided by typing with a DNA probe. J Infect Dis. 1988;157:743–748. doi: 10.1093/infdis/157.4.743. [DOI] [PubMed] [Google Scholar]
- 16.Paraskaki I, Lebessi E, Legakis N J. Epidemiology of community-acquired Pseudomonas aeruginosa infections in children. Eur J Clin Microbiol Infect Dis. 1996;15:782–786. doi: 10.1007/BF01701519. [DOI] [PubMed] [Google Scholar]
- 17.Patzer J, Dzierzanowska D. The resistance patterns and serotypes of Pseudomonas aeruginosa strains isolated from children. J Antimicrob Chemother. 1991;28:869–875. doi: 10.1093/jac/28.6.869. [DOI] [PubMed] [Google Scholar]
- 18.Pitt T L, Livermore D M, Pitcher D, Vatopoulos A C, Legakis N J. Multiresistant serotype O 12 Pseudomonas aeruginosa: evidence for a common strain in Europe. Epidemiol Infect. 1989;103:565–576. doi: 10.1017/s095026880003096x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poh C L, Yeo C C. Recent advances in typing of Pseudomonas aeruginosa. J Hosp Infect. 1993;24:175–181. doi: 10.1016/0195-6701(93)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Spencer R C. Predominant pathogens found in the European prevalence of infection in intensive care study. Eur J Clin Microbiol Infect Dis. 1996;15:281–285. doi: 10.1007/BF01695658. [DOI] [PubMed] [Google Scholar]
- 21.Struelens M J, Schwam V, Deplano A, Baran D. Genome macrorestriction analysis of diversity and variability of Pseudomonas aeruginosa strains infecting cystic fibrosis patients. J Clin Microbiol. 1993;31:2320–2326. doi: 10.1128/jcm.31.9.2320-2326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tassios P T, Gennimata V, Spaliara-Kalogeropoulou L, Kairis D, Koutsia C, Vatopoulos A C, Legakis N J. Multi-resistant Pseudomonas aeruginosa serogroup O:11 outbreak in an intensive care unit. Clin Microbiol Infect. 1997;3:621–628. doi: 10.1111/j.1469-0691.1997.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 23.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Vatopoulous, A. C. Personal communication.
- 24.Vatopoulos A C, Tzouvelekis L S, Tzelepi E, Legakis N J. Cross resistance to norfloxacin and other antimicrobial agents among clinical isolates of gram-negative bacteria. Int J Exp Clin Chemother. 1990;3:201–205. [Google Scholar]
- 25.Visca P, Chiarini F, Vetriani C, Mansi A, Serino L, Orsi N. Epidemiological typing of uropathogenic Pseudomonas aeruginosa strains from hospitalized patients. J Hosp Infect. 1991;19:153–165. doi: 10.1016/0195-6701(91)90219-x. [DOI] [PubMed] [Google Scholar]