Abstract
Key Clinical Message
In this case report, the utility of MDCT in elucidating the pathophysiology and etiology of prosthetic aortic valve dysfunction allowed us to distinguish thrombosis from pannus as an etiology of prosthetic valve dysfunction. MDCT also guided the success of therapy.
Abstract
The diagnosis and management of prosthetic aortic valve thrombosis (PAVT) is challenging. The accurate diagnosis of this entity and its prompt management is vital to improving the prognosis of PAVT patients. Multidetector CT plays a central role in this effort. We present a case of PAVT in which the use of MDCT was useful in guiding management.
Keywords: aortic valve, fibrinolysis, prosthesis
MDCT showing bileaflet mechanical aortic valve, with thrombus interfering with function of the posterior leaflet (shown with arrow).

1. HISTORY OF PRESENTATION
A 61‐year‐old male presented to hospital with dizziness and dyspnea at rest and on exertion of 3 weeks duration. He admitted to running out of his medications about a month prior to presentation to the emergency department. At presentation, the patient was afebrile, New York Heart Association (NYHA) functional class III, with an average pulse of 77, respiratory rate 22, and blood pressure 100/82. An electrocardiograph revealed atrial flutter at a rate of 79 with right bundle branch block. Results of initial biochemical laboratory investigations including high‐sensitivity troponin 1, hemogram, were normal. Significant results of biochemical tests included NT proBNP >3000, and subtherapeutic INR 1.2. Chest x‐ray showed mild pulmonary vascular congestion.
2. MEDICAL HISTORY
His past medical history was significant for aortic root replacement using modified Bentall procedure secondary to acute type 1 aortic dissection, and concomitant aortic valve replacement using 23 mm Carbomedic bileaflet mechanical valve conduit 3 years ago. He also had a ventricular septal defect repaired at the same time. The rest of his history included heart failure with preserved ejection fraction, coronary artery disease status post coronary artery bypass graft, paroxysmal atrial fibrillation on Coumadin, peripheral artery disease, Type II diabetes mellitus, and hyperlipidemia.
3. DIFFERENTIAL DIAGNOSIS
The differential diagnosis included valvular thrombus, or valve pannus, in the setting of acute decompensated heart failure.
4. INVESTIGATIONS
A transthoracic echocardiogram (TTE) revealed a moderately to severely reduced left ventricular systolic function with an estimated ejection fraction of 30%–35%, moderate transvalvular aortic regurgitation, with increased transprothetic pressure gradients. Prosthetic transvalvular velocities (Vmax 3.55 m/s) were increased with peak gradient of 51 mmHg and mean gradient of 32 mmHg (Figure 1). A transesophageal echocardiogram (TEE) revealed an abnormally functioning mechanical valve with transvalvular peak gradient of 49 mmHg, mean gradient of 29 mmHg, Doppler velocity index of 0.21, and acceleration time of 114 msec, consistent with prosthetic aortic valve stenosis (Video S1, Figure 2). Leaflet mobility and sub valvular structures could not be evaluated because of acoustic shadowing on esophageal views. Valve fluoroscopy revealed an immobile disc without any obvious obstructive lesions (Video S2). A Multidetector computed tomography (MDCT) was performed to help in assessing the etiology of valve dysfunction. It revealed a low‐density lesion (40HU), consistent with thrombus, measuring 8 × 5 mm interfering with the mobility of the posterior disc of the prosthetic aortic valve (Figure 3).
FIGURE 1.
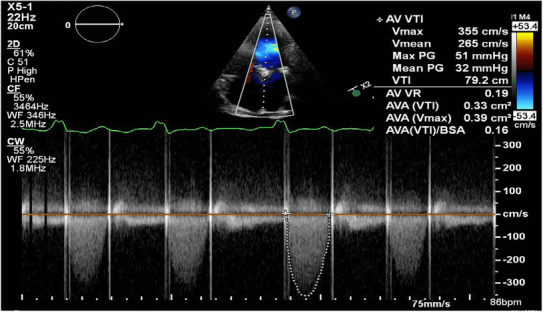
Transprosthetic pressure gradient on transthoracic echocardiography. Transthoracic echocardiography showing increased transprosthetic pressure gradient (PG; maximum PG 51 mmHg; mean PG 32 mmHg). VTI, velocity time integral.
FIGURE 2.
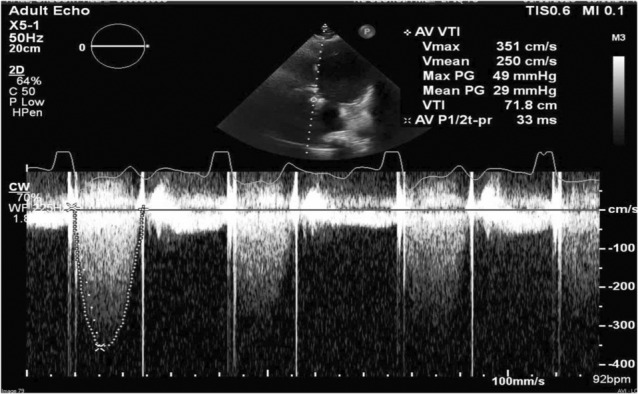
Transesophageal echocardiography showing increased transprosthetic velocity of 3.51 m/s with mean gradient 29 mmHg.
FIGURE 3.

MDCT showing bileaflet mechanical aortic valve, with thrombus interfering with function of the posterior leaflet (shown with arrow).
5. MANAGEMENT
The therapeutic options available in the management of our patient were thrombolytic therapy and emergency cardiac surgery. The heart team evaluated and reviewed the images obtained via MDCT, objectively elected to pursue thrombolytic therapy, tissue plasminogen activator (tPA) infusion was started. The patient received 10 mg IV bolus followed by 90 mg over approximately 2 h. A repeat aortic valve fluoroscopy demonstrated restricted valve motion (Video S3). A second round of tPA was administered at the same dosage. A limited echocardiogram showed improved gradient across the aortic valve with peak gradient of 16 mmHg and mean gradient of 10 mmHg and no regurgitation. The heart team decision was made to repeat tPA infusion at a lower dose. The patient received 1 mg/h of tPA infusion for 25 h. A repeat TTE revealed more improvement of transaortic velocity/gradient with peak gradient 15 mmHg and mean gradient of 8 mmHg but with a small thrombus still present. Patient symptoms had resolved. A valve fluoroscopy obtained a couple of days later still showed no significant movement of one of the mechanical aortic valve leaflets (Video S4). The patient received a fourth dose of low‐dose tPA infusion as the previous, described above. Follow‐up MDCT obtained 3 days after the fourth tPA infusion, revealed resolution of the thrombus with normal leaflets excursion (Video S5). There were no hemorrhagic complications. IV heparin used between tPA treatment was resumed after the fourth dose as a bridge to warfarin until achievement of therapeutic INR.
6. DISCUSSION
Prosthetic valve thrombosis (PVT) is one of the major causes of primary valve failure with an incidence of 0.5%–8% in mechanical valves in the aortic position. 1 Current studies have shown that there is a high risk of thromboembolic episodes, including strokes, especially during the first 10 postoperative days and up until 90 days. However, anticoagulation has reduced the risk of thromboembolic events. Anticoagulation been indicated in all patients with mechanical valve replacement for 3 months and or more according to other coexisting risk factors. 2
The clinical presentation of prosthetic aortic valve thrombosis (PAVT) is variable, with symptoms including dyspnea, decreased exercise capacity, palpitation, chest pain, vertigo, and cerebrovascular accident. On physical examination, stenotic or regurgitant murmurs may be revealed. Hemodynamic stability may depend on the number of leaflets involved with better hemodynamic conditions seeing if a single leaflet is involved as opposed to two leaflets. 3 In patients with prosthetic valve presenting with symptoms and signs suggestive of valve dysfunction, echocardiography examination should be urgently performed especially if there is suspicion for PVT.
Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) are the standard techniques for the evaluation of prosthetic valve function. 4 Cinefluoroscopic is, however, considered the gold standard as TTE and TEE do not always allow for quantitative evaluation of leaflet motion. Cinefluoroscopic has the advantages of being low cost, fast, and easily repeatable so it can be used for evaluation of valve motion during thrombolytic treatment. 5 It is however not useful in assessing the etiology of prosthetic valve failure. 5 MDCT diagnostic yield is in the identification of the etiology of prosthetic valve failure. 6 Determining the cause of valvular dysfunction is important to guide therapy as surgery is the only option for pannus, PVT may require nonsurgical approaches.
The optimal management of PAVT is controversial in part due to the lack of clinical trials. Treatment options include surgery, thrombolytic therapy, and anticoagulation therapy, the latest being inferior to the first two. 7 Surgery is indicated in patients with contraindications for fibrinolytic, large emboli burden (>0.8 cm 2), presence of a possible pannus formation, and sometimes, patient's preference. 8 Although the success rates of both thrombolytic therapy and surgery are less than 10%, the former is much less with 5% at 30 days without rebleeding rates. 8
In a systematic review and meta‐analysis of observational studies, urgent surgery was found not superior to thrombolytic therapy at restoring valve function, but substantially reduced the occurrence of thromboembolic events, major bleeding, and recurrent PVT. 9 The authors recommended that in experienced centers, urgent surgery be preferred over thrombolytic therapy for treating left‐sided PVT, pending the results of randomized controlled trials. 10
In a multicenter observational prospective study of thrombolytic therapy involving 158 patients with PVT, the authors recommended a low‐dose and slow/ultraslow infusion of tPA as a viable treatment in patients with obstructive PVT. The patients in the study received slow (6 h) and/or ultraslow (25 h) infusion of low‐dose tPA (25 mg) mostly in repeated sessions. 9 This study found that a low‐dose and slow/ultraslow infusion of tPA were associated with low complications and mortality and high success rates. 9 Furthermore, the TROIA trial also showed that slow infusion of 25 mg tPA without a bolus appears to be the safest thrombolytic regimen with lower complication and mortality rates for PVT compared with higher doses or rapid infusions. 10 Recently, Özkan and his colleague also demonstrated that ultraslow (25 h) infusion of low‐dose (25 mg) tPA without bolus appears to be associated with quite low nonfatal complications and mortality for PVT patients without loss of effectiveness, when compared with higher doses or faster infusions of tPA. 11 This ultraslow thrombolytic infusion approach could be used with repetition without increasing adverse events including major bleeding and intracranial bleeding. 11 , 12
In the era of cardiac multimodality imaging, evaluation and management of prosthetic valve thrombosis should not be done only based on clinical findings, or indirect imaging modalities. Rather, MDCT should be incorporated into the decision‐making process, as its diagnostic yielding in identifying the etiology of PVT is invaluable.
7. FOLLOW‐UP
At his 1‐week follow‐up, he was still asymptomatic. He reported medication compliance although his INR had dropped from 2.6 to 1.6. The dose of warfarin was adjusted with lovenox bridging on outpatient basis.
8. CONCLUSION
We present a case of PAVT treated with repeated infusions of tPA using a combination of high dose with rapid infusion and low dose with slow infusion. The treatment was successful and well tolerated. Prompt detection and treatment of PVT is importance to avoid catastrophic outcomes. Multimodality techniques, including MDCT helped to reveal etiology of the prosthetic aortic valve dysfunction, including thrombosis.
AUTHOR CONTRIBUTIONS
Sheriff Dodoo: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; validation; writing – original draft; writing – review and editing. Christelle Yakana Moyine: Data curation; writing – original draft; writing – review and editing. Alicia Agyemang‐Sarpong: Data curation; formal analysis; validation; writing – original draft; writing – review and editing. Abdullah Ismail: Writing – review and editing. Nina Le: Writing – review and editing. Falgun Patel: Supervision. Nima Ghasemzadeh: Supervision. Ronnie Ramadan: Supervision. Khaja Mohammed: Supervision. Glen Henry: Supervision; writing – review and editing. Ioannis Parastatidis: Supervision.
CONFLICT OF INTEREST STATEMENT
The authors have no potential or actual conflicts of interest to disclose.
FUNDING INFORMATION
There is no funding source for this work.
CONSENT
Written Informed consent was obtained to publish this report in accordance with the journal's patient's consent policy. Additionally, written informed consent and authorization were obtained prior to preparation of this manuscript. This would be made available to editor on request.
Supporting information
Video S1.
Video S2.
Video S3.
Video S4.
Video S5.
Dodoo SN, Moyine CY, Agyemang‐Sarpong A, et al. The role of multidetector CT scan in the management of prosthetic aortic valve thrombosis: A case report. Clin Case Rep. 2023;11:e7824. doi: 10.1002/ccr3.7824
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions
REFERENCES
- 1. Gürsoy MO, Kalçık M, Yesin M, et al. A global perspective on mechanical prosthetic heart valve thrombosis: diagnostic and therapeutic challenges. Anatol J Cardiol. 2016;16(12):980‐989. doi: 10.14744/AnatolJCardiol.2016.7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heras M, Chesebro JH, Fuster V, et al. High risk of thromboemboli early after bioprosthetic cardiac valve replacement. J Am Coll Cardiol. 1995;25(5):1111‐1119. doi: 10.1016/0735-1097(94)00563-6 [DOI] [PubMed] [Google Scholar]
- 3. Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long‐term management. Circulation. 2009;119(7):1034‐1048. doi: 10.1161/CIRCULATIONAHA.108.778886 [DOI] [PubMed] [Google Scholar]
- 4. Muratori M, Montorsi P, Teruzzi G, et al. Feasibility and diagnostic accuracy of quantitative assessment of mechanical prostheses leaflet motion by transthoracic and transesophageal echocardiography in suspected prosthetic valve dysfunction. Am J Cardiol. 2006;97(1):94‐100. doi: 10.1016/j.amjcard.2005.07.112 [DOI] [PubMed] [Google Scholar]
- 5. Montorsi P, De Bernardi F, Muratori M, et al. Role of cine‐fluoroscopy, transthoracic, and transesophageal echocardiography in patients with suspected prosthetic heart valve thrombosis. Am J Cardiol. 2000;85(1):58‐64. doi: 10.1016/s0002-9149(99)00607-4 [DOI] [PubMed] [Google Scholar]
- 6. Suchá D, Symersky P, Tanis W, et al. Multimodality imaging assessment of prosthetic heart valves. Circ Cardiovasc Imaging. 2015;8(9):e003703. doi: 10.1161/CIRCIMAGING.115.003703 [DOI] [PubMed] [Google Scholar]
- 7. Biteker M, Altun I, Basaran O, Dogan V, Yildirim B, Ergun G. Treatment of prosthetic valve thrombosis: current evidence and future directions. J Clin Med Res. 2015;7(12):932‐936. doi: 10.14740/jocmr2392w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the Management of Patients with Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143(5):e35‐e71. doi: 10.1161/CIR.0000000000000932. Erratum in: Circulation. 2021 Feb 2;143(5):e228. Erratum in: Circulation. 2021 Mar 9;143(10):e784. [DOI] [PubMed] [Google Scholar]
- 9. Karthikeyan G, Senguttuvan NB, Joseph J, Devasenapathy N, Bahl VK, Airan B. Urgent surgery compared with fibrinolytic therapy for the treatment of left‐sided prosthetic heart valve thrombosis: a systematic review and meta‐analysis of observational studies. Eur Heart J. 2013;34(21):1557‐1566. doi: 10.1093/eurheartj/ehs486 [DOI] [PubMed] [Google Scholar]
- 10. Özkan M, Gündüz S, Biteker M, et al. Comparison of different TEE‐guided thrombolytic regimens for prosthetic valve thrombosis: the TROIA trial. JACC Cardiovasc Imaging. 2013;6(2):206‐216. doi: 10.1016/j.jcmg.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 11. Özkan M, Gündüz S, Gürsoy OM, et al. Ultraslow thrombolytic therapy: a novel strategy in the management of PROsthetic MEchanical valve thrombosis and the prEdictors of outcomE: the ultra‐slow PROMETEE trial. Am Heart J. 2015;170(2):409‐418. doi: 10.1016/j.ahj.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 12. Gündüz S, Özkan M, Yesin M, et al. Prolonged infusions of low‐dose Thrombolytics in elderly patients with prosthetic heart valve thrombosis. Clin Appl Thromb Hemost. 2017;23(3):241‐247. doi: 10.1177/1076029615609698 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1.
Video S2.
Video S3.
Video S4.
Video S5.
Data Availability Statement
Data available on request due to privacy/ethical restrictions


