Abstract
Key Clinical Message
Hepatitis E virus (HEV) infection can be manifested with several neurological syndromes including GBS. Therefore, healthcare providers should consider HEV in their differential diagnosis for patients with neurological disorders.
Abstract
We report a case of Guillain‐Barré syndrome associated with hepatitis E virus infection. The current case‐report demonstrates diagnostic challenge to identify GBS case in a limited‐resources country like Sudan. However, HEV infection should be highly suspected in patients with neurological manifestation with high liver enzymes.
Keywords: critical care medicine, infectious disease, neurology, transdisciplinary one health strategy
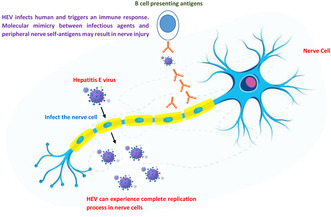
Illustrates the likely pathways through which Hepatitis E virus may trigger the development of Guillain‐Barré Syndrome.
1. INTRODUCTION
Guillain‐Barré Syndrome (GBS) is an acute inflammatory disease that affects the peripheral nervous system. It is caused by an aberrant immune response triggered by viral, bacterial, or parasitic infections, a phenomenon known as molecular mimicry. 1 Several infectious agents have been confirmed to be involved in the development of GBS, including but not limited to COVID‐19, hepatitis E and B viruses, malaria, Influenza A virus, as well as major arboviruses like dengue, West Nile, and Zika viruses. 1 , 2 , 3 These infections can lead to an immune response that mistakenly targets the peripheral nerves, causing the characteristic demyelination and dysfunction of both motor and sensory nerve fibers observed in GBS. Interestingly, it has been observed that approximately two thirds of patients with GBS experience respiratory or gastrointestinal symptoms before the onset of the disease. Surprisingly, a third of the patients remain asymptomatic until the clinical presentation of GBS. 4 This fact emphasizes the challenges faced in the surveillance and early detection of GBS. Consequently, the global, regional, and national burden of GBS is significantly underestimated, especially in resource‐limited settings such as Sudan. 1
Hepatitis E virus (HEV) infection is one of the most common causes of acute viral hepatitis and acute jaundice syndrome worldwide. HEV infection is an important global health issue that contribute significantly in the global morbidity and mortality due to infectious diseases. 5 , 6 The World Health Organization (WHO) estimates that annually over 20 million cases and around 45,000 deaths related to HEV infection reported worldwide. 6 The clinical presentation of HEV infection could be classified into hepatic and extra‐hepatic form of the disease, with the hepatic form further subdivided into acute hepatitis, fibrosis, or cirrhosis. 7 The extra hepatic manifestation of HEV includes neurological, hematological, and renal disorders. Interestingly, 16% of acute hepatitis E infection involves neurological manifestations. 8 High proportion of HEV infection is associated with neuralgic amyotrophy and in GBS. 9 HEV infection was confirmed in 5%–11% of patients presented to outpatient clinics in Netherlands, Japan, Belgium, and Bangladesh. 10 , 11 , 12 , 13 , 14
In Sudan, the prevalence of HEV is rapidly growing during the recent years; this rapid growth is mainly influenced by climate changes. 15 In this communication, we report the first case of Guillain–Barre syndrome associated with HEV infection from Sudan.
2. CASE PRESENTATION
A 33‐year‐old male presented to the Omdurman Teaching Hospital with fatigue, cough, mild jaundice, and he reported having dark color urine for 9 days. The patient was referred to the general hospital Royal Care in Khartoum state where a blood sample was collected. The liver function test showed an elevated level of aspartate aminotranferease (AST) 290 U/L, alanine aminotransferease (ALT) of 466 U/L, and total bilirubin of 3.3 mg/dL, direct bilirubin of 1.3 mg/dL. Based on the clinical presentation, the patient was tested for hepatotrophic viruses including adenovirus, hepatitis A, B, C, D, and E viruses using an enzyme‐linked immunosorbent assay (ELISA); all were negative except for HEV infection that came positive for HEV‐IgM. Additionally, the patient was screened for various related infections that manifest the liver including yellow fever, malaria, and Rift Valley fever and all were negative.
After 8 days, the patient arrived at the emergency room (ER) complaining of distal ascending paraesthesia to the elbow and knee, and he was unable to walk without assistance. The patient reported that 24 h prior to admission, he experienced lower limb weakness, numbness, and tingling sensation in hands and feet. Neurological examinations revealed mild lower limb paralysis (Medical Research Council scale (MRC) 5/5 at upper limbs and 1/5 at lower limbs), mild hypoesthesia (reduced sensation to pinprick distal to the ankle joint), and generalized areflexia with down going plantar reflex. He reported no history of blood transfusions, risky sexual behavior, or sharing needles.
Therefore, the involvement of GBS was suspected, and lumbar puncture was conducted on the second day of admission to the hospital. Cerebrospinal fluid (CSF) examination showed 0/μL monocyte, random blood glucose was 4.6 mmol/L, and protein level was 275.3 mg/dL, which suggested albuminocytologic dissociation. Nerve conduction investigations showed evidence of demyelinating neuropathy with dysfunction of motor and sensory nerve fibers. This confirmed the involvement of GBS due to HEV infection. Figure 1 illustrate the mechanism of HEV‐induced GBS development (Figure 1).
FIGURE 1.
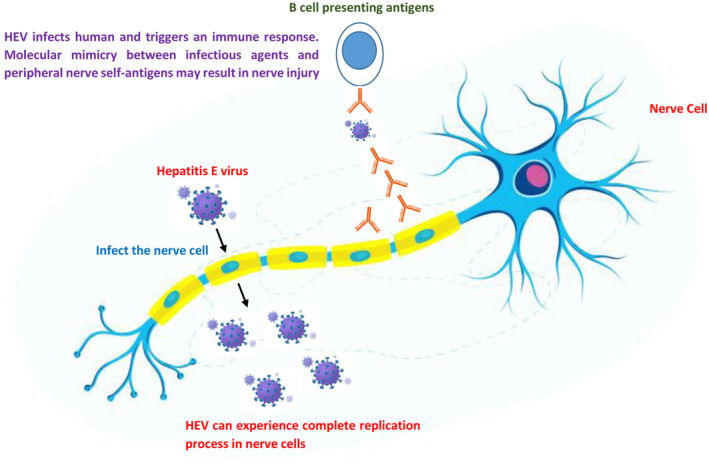
Illustrates the likely pathways through which hepatitis E virus may trigger the development of Guillain‐Barré Syndrome.
The patient was treated with intravenous immunoglobulin at a dose of 0.4 mg/kg per day for 5 days. During the next 2 weeks, his clinical condition and muscle power improved gradually, and the patient had no complaints of respiratory distress or malaise. Repeat CSF examination 2 weeks after admission revealed 10/μL monocyte and 85.7 mg/dL protein level.
3. DISCUSSION
Hepatitis E is a water borne and enterically transmitted infection that is hyper‐endemic in low middle‐income countries and mesoendemic in developed countries. 15 , 16 The virus is well recognized to induce hepatitis; however, neurological syndromes associated with HEV infection cause significant morbidity. 17 Limited data exists regarding the neurological consequences of HEV infection, with most studies originating from India and involving HEV genotype 1. 18 However, several developed countries have recently reported an uptick in HEV infections (primarily genotype 3) resulting in GBS. 18 A study conducted in the Netherlands found that 10 GBS patients had a higher concentration of anti‐HEV immunoglobulin (IgM) antibodies (5.0%) compared to a healthy control (0.5%), resulting in an odds ratio of 10.5, and a 95% confidence interval of 1.3–82.6. HEV RNA was detected in the blood of three of these patients. 11
Neurological complications of HEV infection reported so far includes; GBS and neuralgic amyotrophy considered as the most commonly associated conditions. Interestingly, the virus can also lead to transverse myelitis, encephalitis, cranial nerve palsy, and meningoradiculitis. 11 , 17
GBS is the most common cause of acute flaccid paralysis, however, it is challenging to diagnose in limited capacity and resources settings as it requires a complete medical history, neurological examination, electrophysiological test, and CSF analysis. 19 In addition, healthcare providers need to understand that electromyography (EMG) results are typically normal during the acute phase. Therefore, it is crucial and mandatory to gather a comprehensive medical history and conduct thorough clinical examinations.
Several hypotheses were developed about mechanisms of HEV inducing GBS, however up to date, the exact mechanism is still unclear. 20 The possible scenarios according to the published literature includes either by the direct viral damage due to the replication of the virus within the nervous system or by indirect way that is, immune response or what is called as molecular mimicry. 21 , 22 , 23 In Figure 1, we illustrate mechanisms of developing GBS due to HEV infection (Figure 1).
Our case report, urges for further investigation to estimate the actual burden of hepatitis E and the burden of the associated GBS. Particularly in countries like Sudan, where heavy burden of wide range of infectious diseases with overlapping symptoms and potential involvement in GBS development including Covid‐19, malaria, and several arboviruses are prevalent in the country. 1 , 24 , 25 Major arboviral diseases that are associated with GBS and endemic in the country include Chikungunya, Crimean‐Congo Hemorrhagic fever (CCHF), 26 dengue, Yellow fever, and Rift Valley fever. 24 , 27 Therefore, reducing the burden and prevalence of GBS in Sudan and similar settings would require the implementation of a transdisciplinary One Health strategy with an integrated surveillance and response system for the early preparedness, detection, and response.
In summary, GBS is an extrahepatic manifestation of HEV infection. Furthermore, HEV infection should be suspected in patients with neurological manifestations with increased liver enzymes. In this case, CSF examination demonstrated an elevated level of proteins with pleocytosis, which supported our suspicion of GBS involvement. Healthcare providers should be attention to such association of clinical presentations to improve and facilitating making the accurate diagnosis as early as possible for a better case management.
AUTHOR CONTRIBUTIONS
Ayman Ahmed: Conceptualization; data curation; formal analysis; investigation; methodology; resources; supervision; validation; visualization; writing – original draft; writing – review and editing. Sarah Misbah EL‐Sadig: Conceptualization; data curation; formal analysis; investigation; methodology; writing – review and editing. Emmanuel Edwar Siddig: Conceptualization; data curation; formal analysis; investigation; methodology; resources; supervision; validation; visualization; writing – original draft; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The author reports no conflicts of interest in this work.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Ahmed A, EL‐Sadig SM, Siddig EE. Guillain–Barre syndrome associated with hepatitis E virus infection: A case report. Clin Case Rep. 2023;11:e7863. doi: 10.1002/ccr3.7863
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ahmed A, El‐Amin R, Musa AM, et al. Guillain‐Barre syndrome associated with COVID‐19 infection: a case series. Clin Case Rep. 2023;11(2):e6988. doi: 10.1002/ccr3.6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dalugama C, Shelton J, Ekanayake M, Gawarammana IB. Dengue fever complicated with Guillain‐Barré syndrome: a case report and review of the literature. J Med Case Reports. 2018;12(1):137. doi: 10.1186/s13256-018-1626-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanjalkar M, Karnad DR, Narayana RV, Shah PU. Guillain‐Barre syndrome following malaria. J Infect. 1999;38(1):48‐50. doi: 10.1016/s0163-4453(99)90031-2 [DOI] [PubMed] [Google Scholar]
- 4. Willison HJ, Jacobs BC, van Doorn PA. Guillain‐Barré syndrome. Lancet. 2016;388(10045):717‐727. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed A, Ali Y, Siddig EE, et al. Hepatitis E virus outbreak among Tigray war refugees from Ethiopia Sudan. Emerg Infect Dis. 2022;28(8):1722‐1724. doi: 10.3201/eid2808.220397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang B, Meng XJ. Hepatitis E virus: host tropism and zoonotic infection. Curr Opin Microbiol. 2021;59:8‐15. doi: 10.1016/j.mib.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willauer AN, Sherman KE. Hepatitis E virus: has anything changed? Curr Opin Gastroenterol. 2023;39:169‐174. doi: 10.1097/MOG.0000000000000918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lhomme S, Abravanel F, Cintas P, Izopet J. Hepatitis E virus infection: neurological manifestations and pathophysiology. Pathogens. 2021;10(12):1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abravanel F, Pique J, Couturier E, et al. Acute hepatitis E in French patients and neurological manifestations. J Infect. 2018;77(3):220‐226. [DOI] [PubMed] [Google Scholar]
- 10. Webb GW, Dalton HR. Hepatitis E: an underestimated emerging threat. Ther Adv Infect Dis. 2019;6:204993611983716. doi: 10.1177/2049936119837162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Den Berg B, Van Der Eijk AA, Pas SD, et al. Guillain‐Barré syndrome associated with preceding hepatitis E virus infection. Neurology. 2014;82(6):491‐497. [DOI] [PubMed] [Google Scholar]
- 12. Geurtsvankessel CH, Islam Z, Mohammad QD, Jacobs BC, Endtz HP, Osterhaus ADME. Hepatitis E and Guillain‐Barré syndrome. Clin Infect Dis. 2013;57(9):1369‐1370. [DOI] [PubMed] [Google Scholar]
- 13. Stevens O, Claeys KG, Poesen K, Saegeman V, Van Damme P. Diagnostic challenges and clinical characteristics of hepatitis E virus‐associated guillain‐Barré syndrome. JAMA Neurol. 2017;74(1):26‐33. [DOI] [PubMed] [Google Scholar]
- 14. Fukae J, Tsugawa J, Ouma S, Umezu T, Kusunoki S, Tsuboi Y. Guillain–Barré and miller fisher syndromes in patients with anti‐hepatitis E virus antibody: a hospital‐based survey in Japan. Neurol Sci. 2016;37(11):1849‐1851. [DOI] [PubMed] [Google Scholar]
- 15. Ahmed A, Mohamed NS, Siddig EE, Algaily T, Sulaiman S, Ali Y. The impacts of climate change on displaced populations: a call for action. J Clim Change Heal. 2021;1(3):100057. [Google Scholar]
- 16. Ahmed A, Yousif A, Mohamed NS, Zinsstag J, Siddig EE, Khairy A. Hepatitis E virus outbreak among Tigray War Refugees from Ethiopia, Sudan (response). Emerg Infect Dis. 2023;29(2):460‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ripellino P, Pasi E, Melli G, et al. Neurologic complications of acute hepatitis E virus infection. Neurol Neuroimmunol Neuroinflamm. 2019;7(1):e643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamar N, Bendall RP, Peron JM, et al. Hepatitis E virus and neurologic disorders. Emerg Infect Dis. 2011;17(2):173‐179. doi: 10.3201/eid1702.100856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain‐Barré syndrome in ten steps. Nat Rev Neurol. 2019;15(11):671‐683. doi: 10.1038/s41582-019-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu H, Ma Y. Hepatitis E virus‐associated Guillain–Barre syndrome: revision of the literature. Brain Behav. 2020;10(1):e01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drave SA, Debing Y, Walter S, et al. Extra‐hepatic replication and infection of hepatitis E virus in neuronal‐derived cells. J Viral Hepat. 2016;23(7):512‐521. [DOI] [PubMed] [Google Scholar]
- 22. Zhou X, Huang F, Xu L, et al. Hepatitis E virus infects neurons and brains. J Infect Dis. 2017;215(8):1197‐1206. [DOI] [PubMed] [Google Scholar]
- 23. Shi R, Soomro MH, She R, et al. Evidence of hepatitis E virus breaking through the blood–brain barrier and replicating in the central nervous system. J Viral Hepat. 2016;23(11):930‐939. [DOI] [PubMed] [Google Scholar]
- 24. Ahmed A, Dietrich I, LaBeaud AD, Lindsay SW, Musa A, Weaver SC. Risks and challenges of arboviral diseases in Sudan: the urgent need for actions. Viruses. 2020;12(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohamed NS, Ali Y, Muneer MS, Siddig EE, Sibley CH, Ahmed A. Malaria epidemic in humanitarian crisis settings the case of South Kordofan state, Sudan. J Infect Dev Ctries. 2021;15(1):168‐171. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed A, Ali Y, Salim B, Dietrich I, Zinsstag J. Epidemics of Crimean‐Congo Hemorrhagic Fever (CCHF) in Sudan between 2010 and 2020. Microorganisms. 2022;10(5):928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ali Y, Siddig E, Mohamed N, Ahmed A. Rift valley fever (RVF) and Malaria Co‐infection: a case report. Authorea May. 2023;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


