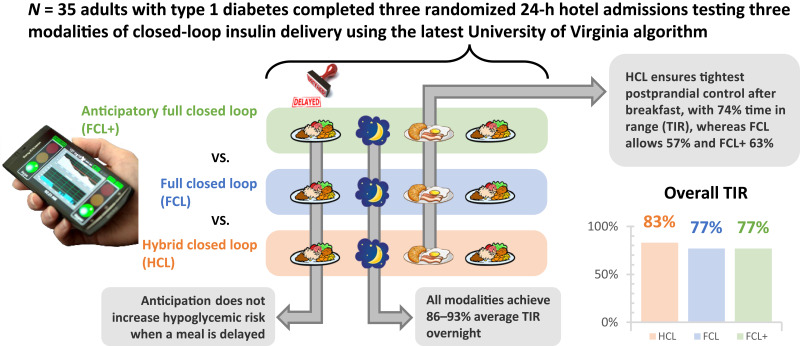
Abstract
OBJECTIVE
Meals are a consistent challenge to glycemic control in type 1 diabetes (T1D). Our objective was to assess the glycemic impact of meal anticipation within a fully automated insulin delivery (AID) system among adults with T1D.
RESEARCH DESIGN AND METHODS
We report the results of a randomized crossover clinical trial comparing three modalities of AID systems: hybrid closed loop (HCL), full closed loop (FCL), and full closed loop with meal anticipation (FCL+). Modalities were tested during three supervised 24-h admissions, where breakfast, lunch, and dinner were consumed per participant’s home schedule, at a fixed time, and with a 1.5-h delay, respectively. Primary outcome was the percent time in range 70–180 mg/dL (TIR) during the breakfast postprandial period for FCL+ versus FCL.
RESULTS
Thirty-five adults with T1D (age 44.5 ± 15.4 years; HbA1c 6.7 ± 0.9%; n = 23 women and n = 12 men) were randomly assigned. TIR for the 5-h period after breakfast was 75 ± 23%, 58 ± 21%, and 63 ± 19% for HCL, FCL, and FCL+, respectively, with no significant difference between FCL+ and FCL. For the 2 h before dinner, time below range (TBR) was similar for FCL and FCL+. For the 5-h period after dinner, TIR was similar for FCL+ and FCL (71 ± 34% vs. 72 ± 29%; P = 1.0), whereas TBR was reduced in FCL+ (median 0% [0–0%] vs. 0% [0–0.8%]; P = 0.03). Overall, 24-h control for HCL, FCL, and FCL+ was 86 ± 10%, 77 ± 11%, and 77 ± 12%, respectively.
CONCLUSIONS
Although postprandial control remained optimal with hybrid AID, both fully AID solutions offered overall TIR >70% with similar or lower exposure to hypoglycemia. Anticipation did not significantly improve postprandial control in AID systems but also did not increase hypoglycemic risk when meals were delayed.
Graphical Abstract
Introduction
In type 1 diabetes (T1D), sustained hyperglycemia is associated with long-term sequelae, requiring intensive insulin management. Compared with traditional basal-bolus approaches, hybrid closed-loop (HCL) systems have resulted in increased time in target range 70–180 mg/dL (TIR). These automated insulin delivery (AID) systems use continuous glucose monitors (CGMs), insulin pumps, and control algorithms to modulate insulin infusion based on rising or falling glucose levels, consistently increasing TIR without added risk of hypoglycemia. An increasing number of these systems are available commercially for individuals with diabetes (1–5).
A key limitation of HCL systems, however, is that they continue to rely on user input of ingested carbohydrates before meals and snacks, a continued obstacle for those with diabetes who desire a more spontaneous lifestyle without the burden of meal planning or estimation of carbohydrates (6,7). The next generation of AID systems aim at a fully closed-loop (FCL) approach that does not rely on meal announcements to reach glycemic control targets. Given the rapid increase in glycemia after carbohydrate ingestion, these FCL systems require new approaches to manage meals, including automated prandial insulin priming (fixed bolus at or shortly after mealtime) and/or anticipation (increased insulin in the hours leading up to a potential meal).
We recently assessed prandial insulin priming for glycemic management in an FCL system (RocketAP) that monitors changes in CGM patterns to determine the probability that a meal-like disturbance has occurred and delivers a small fixed dose of insulin if said probability increases beyond predetermined thresholds (8). Over the course of an unannounced dinner and the following 6 h, this system resulted in 83% TIR. We also previously evaluated a similar closed loop algorithm enhanced with the ability to anticipate mild to moderate physical activity based on patient-specific historical patterns; the system resulted in a reduction in hypoglycemia during exercise without a decrease in TIR (9). However, the efficacy and safety of using a similar anticipation approach for meal management (i.e., increasing insulin delivery before an expected meal to increase insulin action at the time of carbohydrate ingestion) have not been determined, with a particular concern being whether this increased insulin delivery will heighten the risk for hypoglycemia if the anticipated meal does not occur.
In the current study, we sought to assess the safety and efficacy of automatic prandial insulin priming and anticipation, comparing TIR during three 24-h periods with glycemic control using the RocketAP system in three different modalities (in random order): 1) HCL (all carbohydrate intake entered into system), 2) FCL (no carbohydrate announcement), or 3) FCL+ (no carbohydrate announcement enhanced with meal anticipation). We hypothesized that the FCL modality would be safe (similar time below range [TBR] as HCL) and provide adequate glycemic control (TIR ≥70%) and that the addition of prandial insulin anticipation would be safe and further increase TIR.
Research Design and Methods
The University of Virginia (UVA) Institutional Review Board for Health Sciences Research (210035; Charlottesville, VA) and the US Food and Drug Administration (investigational device exemption G210051) approved this randomized controlled clinical trial. Each participant provided written informed consent. Inclusion criteria included age 18–70 years, documented diagnosis of T1D, and insulin pump therapy for ≥3 months. Exclusion criteria included diabetic ketoacidosis or a severe hypoglycemic event (defined as seizure or loss of consciousness) in the past 12 months; use of an oral glucose-lowering agent, including metformin; pregnancy; and any medical condition deemed high risk by the clinical investigators.
The study design is outlined in Supplementary Fig. 1. Enrollment and screening visits were performed via phone or secure Internet video connection, during which medical history and insulin use parameters were obtained and documentation of a physical examination within the prior year was reviewed. Female participants of childbearing potential were provided a urine pregnancy test. Once enrolled and screened, participants were trained on the use of a Dexcom G6 CGM system (Dexcom, Inc., San Diego, CA) and collected at least 14 days of sensor data and insulin records from home use. Upon completion of the baseline data collection (see below), participants were randomly assigned to the order of the three modalities to be tested: HCL, FCL, or FCL+. Participants then traveled to UVA for three sequential 24-h assessments of the closed-loop control modalities in a supervised hotel environment.
Baseline Data
A minimum of 14 days of CGM and pump information was required for analysis of baseline glycemic control, meal patterns, and insulin use. For participants already using a Dexcom G6, retrospective data before enrollment could be used. Participants not using Dexcom G6 CGM were trained and sent equipment for a minimum of 30 days of data before study admission. Baseline data were used to construct the participant-specific meal patterns anticipated by FCL+, as described in Corbett et al. (10) For each participant, we also used these daily meal records to estimate the expected timings of breakfast and dinner and plan the admission-day schedule (see section below).
Supervised Admissions
As mentioned above, participants used the three control modalities in randomized order during a supervised (on-site 24 h per day clinical team with remote monitoring capacity) hotel stay. Each modality was analyzed over a 24-h time period beginning at 4:00 p.m. daily. Participants were connected to the study equipment in the morning before the first study period. Between system setup and study start, participants were managed in HCL (i.e., meals are announced, and there is no meal anticipation). At the end of each 24-h period (i.e., approximately 4:00 p.m. the next day), participants were transferred to the next controller modality to which they had been randomly assigned.
The three 24-h study periods were designed to be as similar as possible with respect to the timing and content of food and activity; meals were selected beforehand by participants and repeated for each 24-h period. The timings of breakfast and dinner were designed to test the efficacy and safety of meal anticipation in glycemia. Participants ate breakfast each day at the expected time (from their home schedule, with times ranging from 7:00 to 9:30 a.m.). This facilitated assessment of the meal anticipation system under optimal conditions, when the system adjusted insulin administration starting 2 h before when the meal occurred, allowing for onset of increased insulin action as the carbohydrate content began to be absorbed. For dinner each day, participants ate 1.5 h after the expected time from the baseline analysis (times ranging from 6:30 to 9:30 p.m.). This facilitated assessment of the meal anticipation system during a potentially dangerous period, where the system adjusted insulin administration (potentially increasing) starting 2 h before the expected meal, but there was no ingested carbohydrate for this insulin to act on until 90 min after the expected time, raising potential for hypoglycemia (if the controller did not react adequately to compensate for dropping blood glucose by then reducing insulin). Lunch was at 1:00 p.m., without anticipation. On the HCL day, participants informed the control system before the meal of the carbohydrates they intended to eat. On FCL and FCL+ days, no manual bolus was given at mealtimes. A midafternoon snack of carbohydrate-free food (deli meat and/or cheese) was provided at 4:00 p.m. if desired (and repeated every day if taken).
At 10:30 a.m. for each study period, participants together went on a light walk for 25 min, approximately 1.5 miles. Participants were otherwise asked not to participate in strenuous exercise and, if they performed any physical activity, to repeat the same routine at the same time daily.
Study Devices
After arrival at the study hotel, participants were started on a Tandem t:AP insulin pump set (Tandem Diabetes Care, San Diego, CA) with their home insulin parameters. Study participants also started a Dexcom G6 CGM (Dexcom, Inc.) sensor session within 24–48 h before study start. Both devices were connected to the DiAs system (11) (UVA) using a study-provided Android cellphone that allowed for remote monitoring (DiAs web-based monitoring system) (12) (described further below). A study blood glucose meter (ContourNext Link; Ascencia Diabetes Care, Parsippany, NJ) and study blood ketone meter (Precision Xtra; Abbott, Alameda, CA) were provided to all participants for use as necessary in adherence to the glycemic guidelines (Supplementary Material). Participants were also fitted with a physical activity tracker (Fitbit Charge 3; Fitbit, San Francisco, CA; data not used by the AID system).
Study AID System
This study compared three modalities of the latest UVA AID system: HCL (nonpowered comparator), FCL (control condition), and FCL+ (experimental). The control algorithms have been described previously (8). Briefly, this is a model-predictive control (MPC) system that continually predicts future glycemia and calculates optimal insulin doses to maintain a desired glucose target for the user in combination with an automated bolus priming system (BPS) module, a system designed to provide fixed priming doses in response to a meal-like disturbance. The, novel FCL+ modality enables k parallel identical MPC systems (i.e., cloning the FCL modality), except for the disturbance realization each one is accounting for in its prediction horizon (what each anticipates). In this case, the disturbance refers to the k meal patterns constructed from the baseline data (10). The final insulin dose is the result of the consensus of the k MPCs, where each anticipated meal pattern is weighted based on its likelihood from recent measurements. FCL+ also inherits the BPS module from FCL and HCL. To avoid an excessive computational burden, the number of parallel FCLs composing FCL+ was fixed at five during the disturbance signal clustering step. Additional details of the design have been published previously (10).
This study was conducted in two phases, with n = 18 and n = 17 participants completing each phase, respectively. In the time between these phases, minor adjustments were made to the algorithms: 1) the BPS active timeframe was changed from 24 h per day in the first phase to being disabled from 11:00 p.m. to 7:00 a.m. during the second phase, to remove the chance of priming boluses being delivered during sleep; and 2) in the initial design, the MPC controller used the total insulin-on-board (IOB) history (MPC plus BPS) to modulate the infusion, whereas for the second phase, the controller considered IOB without BPS, therefore becoming more responsive to glycemic fluctuations. The inactivation of the BPS overnight was instituted in response to situations where the BPS delivered insulin doses based on spurious fluctuations in glycemia (e.g., immediately after compression artifacts when the glucose artificially appeared to rise rapidly).
Remote Monitoring and Glycemic Treatment Guidelines
Members of the study team remotely monitored participants’ real-time CGM data through the UVA DiAs web-based monitoring system for the entirety of the hotel portion of the study and alerted study nurses, physicians, and technicians regarding glycemic concerns and device connection issues. In addition, at least one member of the study team was present during outdoor activities and maintained participant monitoring via a mobile device. A majority of treatment decisions were based on CGM data, with self-monitoring of blood glucose performed at the discretion of the medical team. Glycemic guidelines used for treatment decisions are reported in the Supplementary Material. According to the protocol, hypoglycemic treatments were administered with at least one CGM value <60 mg/dL or as requested by the participant because of hypoglycemia-related symptoms.
Outcomes and Statistical Analysis
All glycemic outcomes were computed based on CGM records. CGM data associated with device or protocol issues unrelated to the studied system (e.g., pump occlusion, site failure, CGM disconnection, or prolonged DiAs-pump disconnection) were excluded from the analysis. The primary outcome was percent TIR 70–180 mg/dL during the 5-h period after breakfast. Secondary glycemic outcomes included percent TIR overall and during the 5-h periods after lunch and dinner; percent TBR (<54 and <70 mg/dL) overall, in the 5-h period after every meal, and during the 2 h preceding dinner; percent time above range (TAR; >180, >250, and >300 mg/dL) overall and in the 5-h period after every meal; number of hypoglycemia events (defined as the presence of a hypoglycemia treatment or one CGM measurement <60 mg/dL) overall, in the 5-h period after every meal, and during the 2 h before dinner; and units of insulin injected overall and before and after every meal (5-h postprandial period). Additional outcomes were presented according to standard guidelines (13). A linear mixed-effects regression model was used for both primary and secondary outcomes (if normally distributed), with the respective prerandomization variable (e.g., participant characteristic and study phase) and sex as covariates. Nonparametric Wilcoxon signed rank tests were used in case of nonnormally distributed samples. The significance level was defined as a P value <0.05 (all P values are two tailed). Data are reported as mean ± SD if normally distributed and median (interquartile range) if nonnormally distributed. Statistical analyses were conducted using SPSS version 28 software. No power analysis was calculated for the design of the clinical trial, given the safety and feasibility nature of the pilot study.
Results
Between June 2021 and March 2022, 50 individuals provided signed informed consent, four did not pass screening, and 10 withdrew before random assignment. Thirty-six participants were randomly assigned, and the trial was completed by 35 (97%); one participant was excluded after random assignment because of prevailing hyperglycemia and ketonemia deemed unrelated to the investigational system or algorithms. Demographic characteristics are listed in Table 1 (Supplementary Material). The carbohydrate content for meals, as selected by the participants according to their preference, varied from 52 ± 15 g (range 33–89 g) at dinner, 47 ± 12 g (range 34–81 g) at breakfast, and 58 ± 17 g (range 30–81 g) at lunch. Statistical comparisons throughout are between FCL+ and FCL unless stated otherwise.
Table 1.
Demographics
| Mean ± SD or n (%) | Range | |
|---|---|---|
| Age, years | 44.5 ± 15.4 | 18–68 |
| Diabetes duration, years | 26 ± 14 | 5–55 |
| BMI, kg/m2 | 28.6 ± 5.3 | 21–39 |
| Female sex | 23 (65.7) | NA |
| Previous CGM user | 34 (97.1) | NA |
| Race/ethnicity | ||
| White | 30 (85.7) | NA |
| Hispanic or Latino | 2 (5.7) | NA |
| African American | 2 (5.7) | NA |
| Other | 1 (2.9) | NA |
| HbA1c | ||
| % | 6.7 ± 0.9 | 5.1–9.1 |
| mmol/mol | 50 ± 10 | 32–76 |
NA, not applicable.
Glycemic Control for an Expected Meal: Breakfast
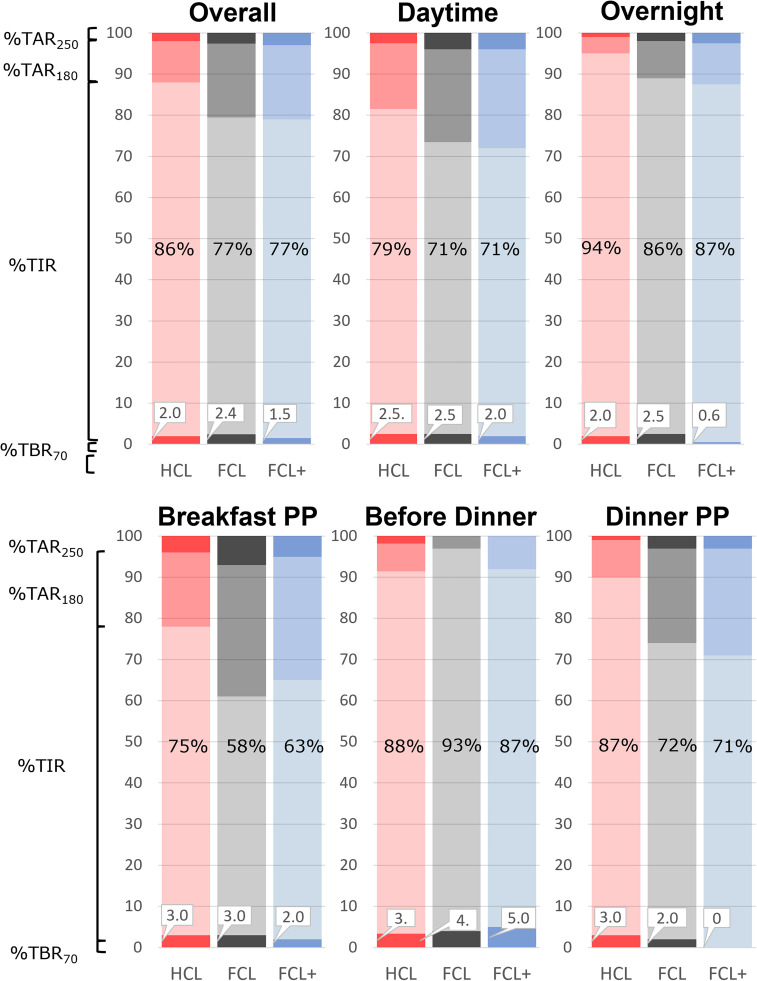
The primary outcome of TIR 70–180 mg/dL for the 5-h period after breakfast was numerically higher for FCL+, although the difference was not statistically significant, with respect to FCL (63 ± 19% vs. 58 ± 21%; P = 0.62) (Fig. 1, Supplementary Table 3, and Supplementary Material). Percent TBR (<54 and <70 mg/dL) and TAR (>180, >250, and >300 mg/dL) were not different between FCL+ and FCL (Supplementary Table 3 and Supplementary Material). No difference in insulin infusion was observed during this postprandial period (9.4 ± 3.3 vs. 9.8 ± 4.0 IU). However, in the 2 h before commencement of the meal, FCL+ delivered more insulin than FCL (4.3 ± 3.8 vs. 3.4 ± 3.7 IU; P = 0.02).
Figure 1.
Percent time <70 (TBR70), TIR, time >180 (TAR180), and time >250 mg/dL (TAR250) for different time windows: overall, daytime (7:00 a.m.–11:00 p.m.), overnight (11:00 p.m.–7:00 a.m.), breakfast 5-h postprandial (PP), dinner 2-h preprandial, and dinner 5-h PP.
Glycemic Control for a Delayed Meal: Dinner
Dinner was delayed 1.5 h from the usual time to observe the glycemic impact of anticipation if the expected meal disturbance did not occur as planned.
Percent TBRs (<54 and <70 mg/dL) were not different between FCL+ and FCL in the 2 h before dinner (0.0% [0.0–0.0%] vs. 0.0% [0.0–0.0%]; P = 1.0 and 0.0% [0.0–0.0%] vs. 0.0% [0.0–0.0%]; P = 0.51, respectively).
Prandial control did not differ between FCL+ and FCL; TIR 70–180 mg/dL for the 5-h period after dinner was 71 ± 34% versus 72 ± 29% (P = 1.0) (Fig. 1). TAR was also not different (Supplementary Table 4 and Supplementary Material). However, we found FCL+ resulted in less time <70 mg/dL than FCL (0.0% [0.0–0.0%] vs. 0.0% [0.0–0.8%]; P = 0.03), a finding confirmed using the low glycemic blood glucose index (0.2 ± 0.3 vs. 0.6 ± 0.7; P = 0.004) (14).
24-H and Overnight Glycemic Outcomes
Both FCL+ and FCL had similar glycemic outcomes over the 24-h testing period, with TIR reaching 77 ± 12% and 77 ± 11%, respectively (Fig. 1, Supplementary Table 1, and Supplementary Material). FCL+ was superior to FCL in the percent time <70 mg/dL (0.3% [0–1.9%] vs. 1.4% [0–4.5%]; P = 0.03). Overnight, FCL+ had less percent time <70 mg/dL (0.0% [0.0–0.0%] vs. 0.0% [0.0–4.0%]; P = 0.02), confirmed by a lower low glycemic blood glucose index in FCL+ (0.4 ± 0.6 vs. 0.9 ± 1; P = 0.02).
HCL Versus FCL
HCL was superior to FCL over the 24-h testing period (Fig. 1, Supplementary Table 5, and Supplementary Material) in mean glucose (126 ± 14 vs. 138 ± 14 mg/dL; P < 0.001), time in the tight range (140–180 mg/dL; 71 ± 13% vs. 60 ± 13%; P < 0.001), TIR (86 ± 10% vs. 77 ± 11%; P < 0.001), and time >180 mg/dL (12 ± 10% vs. 20 ± 11%; P < 0.001). TBR metrics (time <54 and <70 mg/dL) and number of hypoglycemic treatments were similar. HCL also exhibited lower variability in CGM SD (39 ± 12 vs. 48 ± 11 mg/dL; P < 0.001) and coefficient of variation (30 ± 7 vs. 34 ± 6%; P = 0.009). It was also observed that HCL commanded more insulin than FCL (0.6 ± 0.5 vs. 0.5 ± 0.2 IU/kg; P = 0.009). Similarly, HCL was superior to FCL over the 5 h after breakfast (Fig. 1, Supplementary Table 6, and Supplementary Material) in mean glucose (143 ± 32 vs. 160 ± 30 mg/dL; P = 0.02), time in the tight range (52 ± 24% vs. 42 ± 19%; P = 0.05), TIR (75 ± 23% vs. 58 ± 21%; P < 0.001), and time >180 mg/dL (22 ± 24% vs. 39 ± 21%; P < 0.001). TBR metrics (time <54 and <70 mg/dL) and number of hypoglycemic treatments were similar in both interventions. HCL also exhibited lower variability in CGM SD (38 ± 15 vs. 51 ± 14 mg/dL; P < 0.001) and coefficient of variation (26 ± 8% vs. 33 ± 9%; P = 0.001). In this period, HCL commanded more insulin than FCL (0.1 ± 0.09 vs. 0.1 ± 0.04 IU/kg; P = 0.02).
Safety and Adverse Events
FCL+ and FCL were similar in terms of the number of administered hypoglycemic treatments overall (0.0 [0.0–1.0] vs. 0.0 [0.0–1.5]; P = 0.17); during the 5 h after breakfast (0.0 [0.0–0.0] vs. 0.0 [0.0–0.0]; P = 0.28), lunch (0.0 [0.0–0.0] vs. 0.0 [0.0–0.0]; P = 1), and dinner (0.0 [0.0–0.0] vs. 0.0 [0.0–0.0]; P = 0.18); and overnight (0.0 [0.0–0.0] vs. 0.0 [0.0–0.5]; P = 0.07).
Regarding delivery of a BPS bolus not associated with food intake, such misfires occurred 65 times (27 during daytime and 38 overnight) during phase one but only eight times (daytime only) during phase two (after BPS redesign). These 65 events in phase one led to eight occurrences of hypoglycemia (there was no hypoglycemia after BPS misfire in phase two; i.e., 9% of all hypoglycemic events or approximately 12% of undesired BPS actions). Nevertheless, when included as a variable in the analysis, there was no significance regarding study phase in glycemic outcomes, either overnight or otherwise.
Two adverse events not deemed related to the device/algorithm were reported. One was a case of persistent hyperglycemia and ketonemia that required multiple site changes and manual insulin dosing by the study team within 12 h of the beginning of data collection. The event led to >80% of data missing from the first study day (this participant was discharged from the study, and data were not used). The second adverse event was a case of emesis followed by ketonemia of unclear etiology but suspected gastroenteritis that occurred just before study completion (these final 2 h of data were excluded from the analysis).
Two participants had glycemic excursions after a hypoglycemic treatment that activated the BPS and required interruption of automated insulin injection by the study team to prevent further hypoglycemia. In both cases, CGM measurements were considerably higher than fingerstick values, which prevented the CGM-based safety system from constraining BPS injections. The system was adjusted to allow entry of fingerstick values to prevent subsequent events as a temporary solution. One participant had a prolonged cessation of insulin after disconnection of the pump and DiAs platform, which was eventually resolved by replacing the study pump, with subsequent resolution of hyperglycemia.
Conclusions
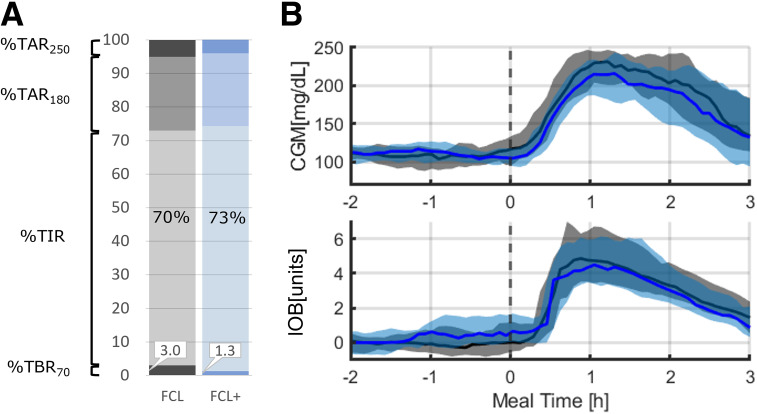
This study demonstrated that an FCL system (with and without anticipation) can provide adequate (per consensus guidelines) glycemic control in adults with T1D in a supervised setting. Meal anticipation (FCL+) did not improve over FCL control for expected glycemic disturbance. This is in contrast to a prior report of anticipation in the setting of exercise, which did improve glycemia after insulin reduction before anticipated physical activity. The lack of a significant effect on glycemia in the current study may be due in part to the presence of high variance in glycemic outcomes around meals and overall tight blood glucose levels before breakfast, limiting opportunities for the algorithm to deliver additional insulin in anticipation (Fig. 2). To investigate this further, we assessed the amount of injected insulin for both systems in the 2 h (the controller’s prediction horizon) before breakfast, showing the anticipatory effect in terms of injected insulin (0.03 ± 0.02 vs. 0.02 ± 0.03 IU/kg; P = 0.02), which could explain the numeric difference in TIR. Of note, we observed no added hypoglycemic risk associated with this increase in insulin delivery. Considering the relatively minor increase in insulin delivery with anticipation, more aggressive anticipation may provide a clearer postprandial control advantage without additional hypoglycemic risk.
Figure 2.
Glucose levels over time by AID system, FCL+ (blue) and FCL (gray), for the time window from 2 h before breakfast to 3 h after breakfast. A: Percent time <70 (TBR70), TIR, time >180 (TAR180), and time >250 mg/dL (TAR250). B: CGM and IOB data shown centered over the meal. Black vertical dotted line represents commencement of the meal. Solid blue and black lines and shaded areas represent mean glucose and 25th–75th percentiles, respectively.
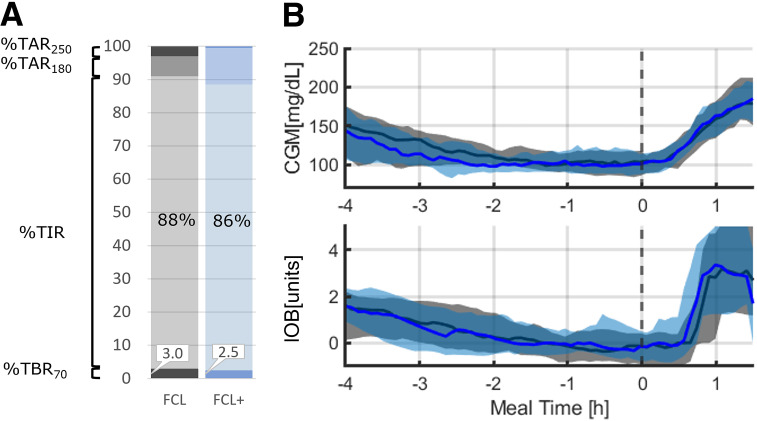
In addition, FCL+ showed no added hypoglycemic risk when an expected meal did not occur (delayed dinner), as depicted in Fig. 3; similar glycemic metrics were observed before and after the meal (Fig. 1).
Figure 3.
Glucose levels over time by AID system, FCL+ (blue) and FCL (gray), for the time window from 4 h before dinner to 1.5 h after dinner. A: Percent time<70 (TBR70), time-in-range (TIR), time>180 (TAR180), and time>250 (TAR250). B: CGM and IOB data shown centered over the meal. Black vertical dotted line represents commencement of the meal. Solid blue and black lines and shaded areas represent mean glucose and 25th–75th percentiles, respectively.
FCL and FCL+ provided TIR above the ADA-recommended threshold of 70% overall (15) without any carbohydrate announcement over a 24-h period and using a single-hormone system in a structured hotel-based study. As expected, using the RocketAP system in HCL did provide higher TIR, and it should also be noted that a prior study demonstrated that the RocketAP controller used in HCL provided superior TIR compared with a currently available AID system (8). In the current study, we noticed a reduction in TIR of 9% when comparing RocketAP in HCL versus FCL. This is not surprising, given that during HCL the quantity of insulin delivered matches the carbohydrates consumed and insulin is given before eating, allowing a greater amount of insulin action as the meal carbohydrate content is absorbed. However, the goal of this line of research is to develop AID systems that substantially reduce user burden while still providing acceptable glycemic control to avoid comorbidities of T1D. In this sense, HCL provides an upper bound of system performance. In clinical practice, FCL systems are most likely to benefit individuals seeking adequate T1D care without the effort of meal announcement. In the current study, the TIR of 77% is in the range recommended by the ADA and in the upper range of previously reported insulin-only FCL performance, that is, 58–78% for insulin-only systems (15–17), although it should be noted that it is difficult to compare individual studies because of differences in activity levels and carbohydrate intake. It is also notable that current FCL assessments were conducted in a highly controlled environment and still require testing in real-world settings.
The RocketAP system uses a meal probability system (BPS) that commands small priming bolus based on the patient’s total daily insulin requirements, with the exact dose depending on how certain the system is that a meal-like disturbance has occurred. This design allows for the possibility of priming insulin being delivered after a CGM glucose artifact (e.g., where a BPS dose was delivered that was not within 3 h after a meal, such as after resolution of a CGM compression event). In the current study, such events occurred 65 times during phase one (leading to eight episodes of hypoglycemia) but only eight times during phase two (after BPS redesign). Although we chose to silence the BPS overnight to prevent doses in the absence of carbohydrate ingestion, a CGM fault detection system may also suffice to prevent unnecessary boluses.
This study benefited from a randomized crossover approach with standardized study days to compare between controller approaches. However, conclusions that can be derived from such a controlled environment must be confirmed in free-living conditions. This structured hotel stay did not include rigorous exercise; however, the 25-min daily walk midmorning, in many cases during a time of peak insulin action from breakfast, was associated with an increase in cases of hypoglycemia, which did not differ between HCL and FCL phases. Further assessment of FCL systems will need to include more strenuous bouts of exercise, including high-intensity interval training, which can result in increases in blood glucose (18). The system was modified slightly between the two phases, inactivating the BPS overnight and altering how IOB was calculated; however, this did not result in marked changes in glycemia, and we combined the data from both phases. Limitations also include a lack of comparison with existing commercial AID systems in this same setting and a participant population that imperfectly reflects national prevalence estimates for race and ethnicity (86% White or Caucasian non-Hispanic or Latino) and for degree of T1D control (mean HbA1c 6.7%). Finally, as a pilot study, we did not perform a priori power calculation to determine sample size, and therefore, the study may not have been powered to detect a difference between groups.
In conclusion, we noted a high percent TIR using FCL over a 24-h period that included high carbohydrate intake. The addition of anticipation, with the controller potentially increasing insulin leading up to an expected meal, did not increase hypoglycemia but also did not significantly increase TIR compared with FCL without anticipation, suggesting that other factors may need to be considered in the algorithm to enable more prominent anticipation. Overall, this study provides support that FCL may be a safe and effective treatment option for adults with T1D, although further rigorous testing with additional challenges in supervised and unsupervised environments is warranted.
Article Information
Acknowledgments. The authors thank the study volunteers and the research staff at the UVA Center for Diabetes Technology.
Funding. This project was funded by a grant from the National Institutes of Health (R01DK129553).
The funding agency had no role in study design, data collection, analyses, or interpretation or in article preparation.
Duality of Interest. J.G.-T. and P.C. report receiving industry research support and royalties from Dexcom, Inc. M.D.D. reports receiving grant or material support from Tandem Diabetes Care, Medtronic, and Dexcom, Inc. M.D.B. has received honoraria and travel reimbursement from Dexcom, Inc., and Tandem Diabetes Care and research support from Dexcom, Inc., Novo Nordisk, and Tandem Diabetes Care. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.G.-T. was involved in the design of the closed-loop control (CLC) system and in the study design, provided technical support remotely and on site for all admissions, analyzed data, performed statistical analyses, and was involved in writing and editing the manuscript. P.C. and J.L.D. were involved in the design of the CLC system, provided technical support remotely and on site for all admissions, and were involved in writing and editing the manuscript. O.V. and R.E.-Z. provided on-site support during screenings and admissions and were involved in writing and editing the manuscript. C.L.K.K. maintained and checked the functionality of the DiAs system and the functionality of the closed-loop systems on the smartphones, conducted the testing of the systems before the study, provided technical support remotely and on site for all admissions, and reviewed and edited the manuscript. C.L.B. coordinated the study monitoring, processed data, and was involved in writing and editing the manuscript. M.C.O. assisted with the development of the protocol, coordinated institutional review board submission and investigational device exemption submission to the US Food and Drug Administration (FDA), contributed to study planning and preparation, and reviewed and edited the manuscript. M.F. was the research coordinator for the study, was responsible for all participant interaction and operations during the trial, and reviewed and edited the manuscript. S.A.B. was the study physician responsible for all participant activities, contributed to the study design, answered queries from the regulatory boards, including the FDA, and was involved in writing and editing the manuscript. M.D.D. was a study physician, contributed to the study design, and was involved in writing and editing the manuscript. M.D.B. contributed to both algorithm and study design, supervised their implementation, and was involved in writing and editing the manuscript. M.D.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT04877730, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.23519412.
J.G.-T., S.A.B., M.D.D., and M.D.B. contributed equally to this work.
References
- 1. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 2. Tauschmann M, Thabit H, Bally L, et al.; APCam11 Consortium . Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown SA, Kovatchev BP, Raghinaru D, et al.; iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breton MD, Kanapka LG, Beck RW, et al.; iDCL Trial Research Group . A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown SA, Forlenza GP, Bode BW, et al.; Omnipod 5 Research Group . Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021;44:1630–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cherñavvsky DR, DeBoer MD, Keith-Hynes P, et al. Use of an artificial pancreas among adolescents for a missed snack bolus and an underestimated meal bolus. Pediatr Diabetes 2016;17:28–35 [DOI] [PubMed] [Google Scholar]
- 7. Lee MH, Paldus B, Vogrin S, et al. Fast-acting insulin aspart versus insulin aspart using a second-generation hybrid closed-loop system in adults with type 1 diabetes: a randomized, open-label, crossover trial. Diabetes Care 2021;44:2371–2378 [DOI] [PubMed] [Google Scholar]
- 8. Garcia-Tirado J, Diaz JL, Esquivel-Zuniga R, et al. Advanced closed-loop control system improves postprandial glycemic control compared with a hybrid closed-loop system following unannounced meal. Diabetes Care 2021;44:2379–2387 [DOI] [PubMed] [Google Scholar]
- 9. Garcia-Tirado J, Brown SA, Laichuthai N, et al. Anticipation of historical exercise patterns by a novel artificial pancreas system reduces hypoglycemia during and after moderate-intensity physical activity in people with type 1 diabetes. Diabetes Technol Ther 2021;23:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corbett JP, Garcia-Tirado J, Colmegna P, Diaz Castaneda JL, Breton MD. Using an online disturbance rejection and anticipation system to reduce hyperglycemia in a fully closed-loop artificial pancreas system. J Diabetes Sci Technol 2022;16:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keith-Hynes P, Mize B, Robert A, Place J. The Diabetes Assistant: a smartphone-based system for real-time control of blood glucose. Electronics 2014;3:609–623 [Google Scholar]
- 12. Place J, Robert A, Ben Brahim N, et al. DiAs web monitoring: a real-time remote monitoring system designed for artificial pancreas outpatient trials. J Diabetes Sci Technol 2013;7:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maahs DM, Buckingham BA, Castle JR, et al. Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care 2016;39:1175–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kovatchev BP. Metrics for glycaemic control—from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol 2017;13:425–436 [DOI] [PubMed] [Google Scholar]
- 15. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forlenza GP, Cameron FM, Ly TT, et al. Fully closed-loop multiple model probabilistic predictive controller artificial pancreas performance in adolescents and adults in a supervised hotel setting. Diabetes Technol Ther 2018;20:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cameron FM, Ly TT, Buckingham BA, et al. Closed-loop control without meal announcement in type 1 diabetes. Diabetes Technol Ther 2017;19:527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riddell MC, Pooni R, Fontana FY, Scott SN. Diabetes technology and exercise. Endocrinol Metab Clin North Am 2020;49:109–125 [DOI] [PubMed] [Google Scholar]