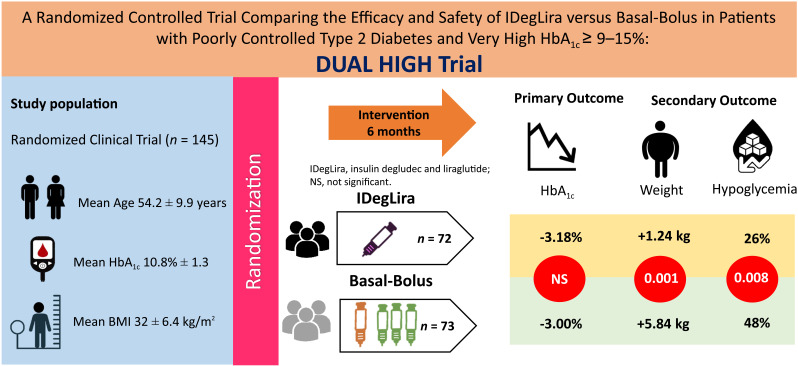
Abstract
OBJECTIVE
In participants with type 2 diabetes (T2D) and HbA1c >9.0–10.0%, guidelines recommend treatment with basal-bolus insulin.
RESEARCH DESIGN AND METHODS
This randomized trial compared the efficacy and safety of insulin degludec and liraglutide (IDegLira) and basal-bolus among participants with high HbA1c ≥9.0–15.0%, previously treated with 2 or 3 oral agents and/or basal insulin, allocated (1:1) to basal-bolus (n = 73) or IDegLira (n = 72). The primary end point was noninferiority (0.4%) in HbA1c reduction between groups.
RESULTS
Among 145 participants (HbA1c 10.8% ± 1.3), there was no statistically significant difference in HbA1c reduction (3.18% ± 2.29 vs. 3.00% ± 1.79, P = 0.65; estimated treatment difference (ETD) 0.18%, 95% CI −0.59, 0.94) between the IDegLira and basal-bolus groups. IDegLira resulted in significantly lower rates of hypoglycemia <70 mg/dL (26% vs. 48%, P = 0.008; odds ratio 0.39, 95% CI 0.19, 0.78), and less weight gain (1.24 ± 8.33 vs. 5.84 ± 6.18 kg, P = 0.001; ETD −4.60, 95% CI −7.33, −1.87).
CONCLUSIONS
In participants with T2D and HbA1c ≥9.0–15.0%, IDegLira resulted in similar HbA1c reduction, less hypoglycemia, and less weight gain compared with the basal-bolus regimen.
Graphical Abstract
Introduction
Landmark studies have shown that persistent hyperglycemia in participants with type 2 diabetes (T2D) is associated with short- and long-term complications (1–4). Participants with severe hyperglycemia usually respond poorly to oral antidiabetes agents and frequently require insulin therapy (4,5). Guidelines recommend initiating basal insulin and progressively step-up to basal-bolus insulin in participants with high HbA1c >10% (86 mmol/mol), particularly if symptomatic or with catabolic symptoms (6). The basal-bolus insulin regimen increases hypoglycemia risk and weight gain (7,8), is labor intensive, and requires multiple daily injections (MDI), which may decrease treatment adherence (9–12). However, simplified regimens may improve adherence and glycemic control (10,11,13).
Prior studies demonstrated the efficacy and safety of fixed-ratio combination (FRC) of basal insulin and glucagon-like peptide 1 receptor agonists (GLP1-RA) (14–18) but excluded participants with very high HbA1c (≥9.0–15.0%, 75–140.4 mmol/mol). Accordingly, this randomized controlled trial (RCT) compared the efficacy and safety of insulin degludec and liraglutide (IDegLira) and a basal-bolus insulin regimen in glycemic control (efficacy end point), hypoglycemia, and weight gain (safety end points) in participants with T2D and HbA1c ≥9.0–15.0% (75–140.4 mmol/mol).
Research Design and Methods
This prospective RCT was conducted at two academic clinics in Atlanta, GA. This trial was approved by Emory University’s Institutional Review Board and was registered with clinicaltrials.gov (NCT03737240). We screened male and female participants between 18 and 80 years of age, HbA1c between 9.0 and 15.0% (75–140.4 mmol/mol), history of T2D for at least 6 months, treated with two or more oral antidiabetic agents and/or with basal insulin (total daily dose ≤50 units/day). Exclusion criteria are listed in the Supplementary Materials (Supplementary Tables 1 and 3 and pages 3–10). After randomization, the insulin regimen was adjusted by the study team following a validated titration algorithm (14,15) (Supplementary Table 2).
Statistical Analysis
Our hypothesis was that participants randomized to IDegLira would experience similar HbA1c reduction from baseline to end-of-study compared with the basal-bolus group. To test the noninferiority of IDegLira, we assumed a noninferiority margin of 0.4% for HbA1c reduction, and the corresponding SD bounded above by 0.85%. Based on a one-sided, two-sample t test, we assumed that 57 participants per group (total 114), with an attrition rate of 15% and total of 134 (67 per group) randomized, would provide a power of 80%, with α (type 1 error) set as 0.05 to detect noninferiority (Supplementary Material, Statistical Considerations).
Results
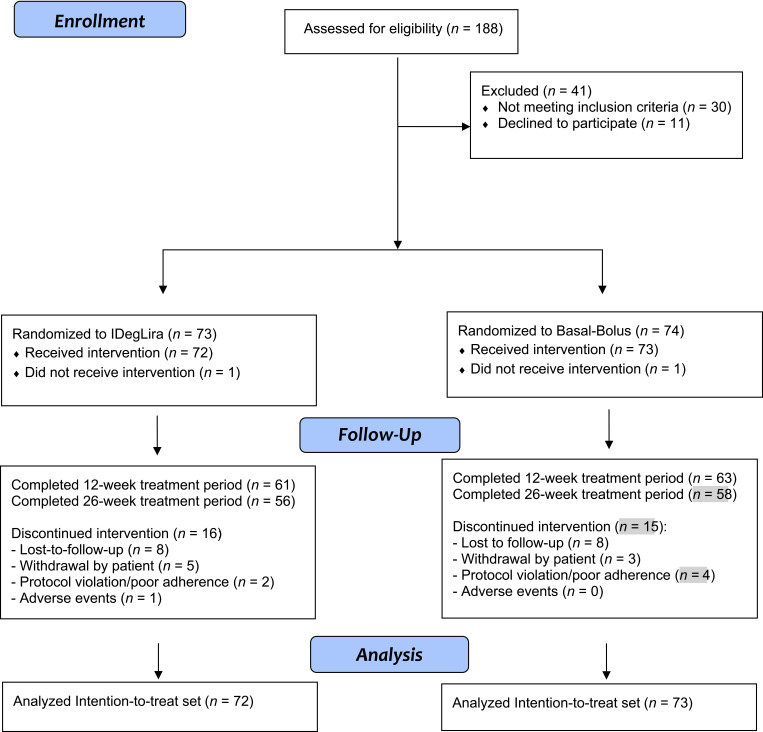
The intention-to-treat analysis included 145 participants, with 72 participants allocated to IDegLira and 73 to the basal-bolus group, of which 56 (78%) and 58 (79%) completed the intervention up to 26 weeks, respectively (Fig. 1). Baseline demographic and clinical characteristics are shown in Table 1.
Figure 1.
Participant flow diagram.
Table 1.
Baseline demographic and clinical characteristics of study participants
| IDegLira | Basal-bolus | |
|---|---|---|
| n = 72 | n = 73 | |
| Age, years | 54.5 ± 10.1 | 53.8 ± 9.7 |
| Sex | ||
| Male | 28 (39) | 35 (48) |
| Female | 44 (61) | 38 (52) |
| Race/ethnicity | ||
| Black | 63 (88) | 61 (84) |
| White | 3 (4.2) | 7 (9.6) |
| Hispanic | 6 (8.3) | 5 (6.8) |
| Weight, kg | 93.1 ± 20.5 | 91.9 ± 19.5 |
| BMI, kg/m2 | 32.4 ± 6.5 | 31.5 ± 6.2 |
| Waist circumference, cm | 108.7 ± 14.3 | 106.8 ± 14.9 |
| Blood pressure, mmHg | ||
| Systolic | 134.2 ± 18.5 | 134.3 ± 17.4 |
| Diastolic | 75.4 ± 11.2 | 78.6 ± 10.8 |
| Diabetes duration | ||
| <20 years | 62 (86.2) | 64 (87.7) |
| >20 years | 10 (13.8) | 9 (12.3) |
| Oral antidiabetes therapy | 22 (30.6) | 25 (34.3) |
| Insulin only | 10 (13.8) | 12 (16.4) |
| Oral and insulin therapy | 40 (55.6) | 36 (49.3) |
| Insulin dose, units/kg/day | 0.31 ± 0.17 | 0.31 ± 0.13 |
| Laboratory testing | ||
| HbA1c, % | 10.8 ± 1.4 | 10.7 ± 1.3 |
| HbA1c, mmol/mol | 95.0 ± 15.3 | 93.0 ± 14.2 |
| Fasting blood glucose, mg/dL | 234.3 ± 82.3 | 238.1 ± 90.6 |
| Fasting blood glucose, mmol/L | 13.0 ± 4.6 | 13.2 ± 5.0 |
| Creatinine, mg/dL | 0.9 ± 0.4 | 0.9 ± 0.3 |
| GFR >30–60, mL/min/1.73 m2 | 23 (32) | 26 (36) |
| GFR >60, mL/min/1.73 m2 | 49 (68) | 47 (64) |
| Total cholesterol, mg/dL | 176.0 ± 52.6 | 172.9 ± 47.4 |
| Triglycerides, mg/dL | 150.2 ± 85.5 | 150.2 ± 85.5 |
| LDL, mg/dL | 102.1 ± 43.9 | 94.9 ± 41.7 |
| HDL, mg/dL | 44.4 ± 9.2 | 46.1 ± 17.5 |
Data are reported as means ± SD or n (%).
GFR, glomerular filtration rate.
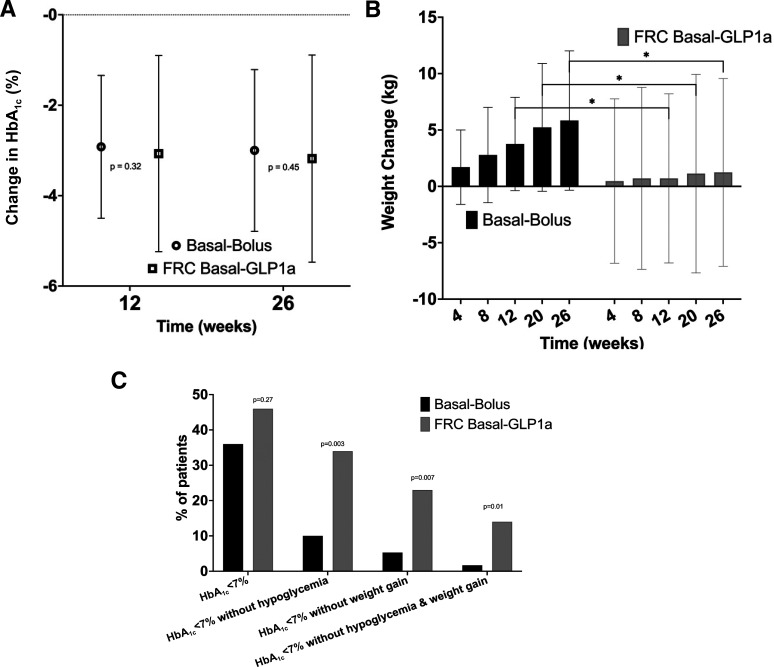
There was no statistically significant difference on the primary end point of HbA1c reduction between the IDegLira and basal-bolus groups (3.18% ± 2.29 vs. 3.00% ± 1.79, P = 0.65; estimated treatment difference [ETD] 0.18%, 95% CI −0.59, 0.94) after 26 weeks (Fig. 2A).
Figure 2.
Efficacy and composite outcomes. A: HbA1c change from baseline (week 12, and week 26). B: Change in body weight from baseline to week 26. C: Participants achieving composite outcomes: efficacy (HbA1c) targets and safety (hypoglycemia and/or weight) targets, from baseline to week 26. *P < 0.05. Data are presented as mean ± SD, except where otherwise noted.
The IDegLira group met the prespecified safety outcome of resulting in a significantly lower hypoglycemia rate <70 mg/dL (26% vs. 48%, P = 0.008; odds ratio 0.39, 95% CI 0.19, 0.78) and decreased number of episodes (0.9 vs. 2.9 episodes, P = 0.002). Clinically significant hypoglycemia rate (<54 mg/dL) was numerically lower in IDegLira group compared with basal-bolus groups (9.7% vs. 19%, P = 0.2; odds ratio 0.45, 95% CI 0.17, 1.20) (Table 2).
Table 2.
Primary and secondary efficacy and safety outcomes
| IDegLira | Basal-bolus | ||
|---|---|---|---|
| Variables | n = 72 | n = 73 | P value |
| Efficacy outcomes | |||
| HbA1c change from baseline | |||
| HbA1c change at 12 weeks, % | −3.07 ± 2.17 | −2.92 ± 1.58 | 0.66 |
| HbA1c change at 12 weeks, mmol/mol | −33.55 ± 23.71 | −31.91 ± 17.26 | |
| HbA1c change at 26 weeks, % | −3.18 ± 2.29 | −3.00 ± 1.79 | 0.65 |
| HbA1c change at 12 weeks, mmol/mol | −34.75 ± 25.02 | −32.79 ± 19.56 | |
| Weight change from baseline, kg | |||
| Body weight change at 4 weeks | 0.47 ± 7.29 | 1.70 ± 3.30 | 0.23 |
| Body weight change at 8 weeks | 0.71 ± 8.07 | 2.78 ± 4.23 | 0.10 |
| Body weight change at 12 weeks | 0.71 ± 7.50 | 3.76 ± 4.14 | 0.007 |
| Body weight change at 20 weeks | 1.13 ± 8.80 | 5.23 ± 5.67 | 0.008 |
| Body weight change at 26 weeks | 1.24 ± 8.33 | 5.84 ± 6.18 | 0.001 |
| Safety outcomes | |||
| Hypoglycemia | |||
| Blood glucose <70 mg/dL (3.9 mmol/mol) | 19 (26) | 35 (48) | 0.008 |
| Blood glucose <54 mg/dL (3.0 mmol/mol) | 7 (9.7) | 14 (19) | 0.16 |
| Composite outcomes | |||
| HbA1c <7% without hypoglycemia at | |||
| 12 weeks | 21 (34) | 8 (13) | 0.005 |
| 26 weeks | 19 (34) | 6 (10) | 0.003 |
| HbA1c <7% without weight gain at | 12 (20) | 2 (3.2) | 0.004 |
| 12 weeks | |||
| 26 weeks | 13 (23) | 3 (5.3) | 0.007 |
| HbA1c <7% without hypoglycemia or weight gain at | 10 (17) | 2 (3.2) | 0.015 |
| 12 weeks | |||
| 26 weeks | 8 (14) | 1 (1.7) | 0.016 |
| Common adverse events | |||
| Nausea, weeks 1–12 | 26 (36) | 3 (4.1) | <0.001 |
| Nausea, weeks 1–26 | 27 (38) | 3 (4.1) | <0.001 |
| Vomiting, weeks 1–12 | 8 (11) | 1 (1.4) | 0.017 |
| Vomiting, weeks 1–26 | 9 (13) | 1 (1.4) | 0.009 |
| Abdominal pain, weeks 1–12 | 11 (15) | 4 (5.5) | 0.06 |
| Abdominal pain, weeks 1–26 | 13 (18) | 6 (8.2) | 0.09 |
| Diarrhea, weeks 1–12 | 6 (8.3) | 4 (5.5) | 0.53 |
| Diarrhea, weeks 1–26 | 8 (11) | 6 (8.2) | 0.59 |
Data are presented as mean ± SD or cumulative n (%) of participants with one or more events during the study duration. In the basal-bolus group, the sample size for HbA1c at 12 weeks was n = 63 and at 26 weeks was n = 58. In the IDegLira group, the sample size for HbA1c at 12 weeks was n = 61 and at 26 weeks was n = 56. P values were obtained from the Wilcoxon test, χ2 test, or Fisher exact test, when appropriate.
After 26 weeks of treatment, the basal-bolus intervention resulted in greater weight gain (5.84 ± 6.18 kg vs. 1.24 ± 8.33 kg; ETD +4.60 kg, 95% CI 1.87, 7.33, P = 0.001) compared with IDegLira. Body weight increased progressively in the basal-bolus group, while IDegLira resulted in less weight gain over the study period (Fig. 2B).
Compared with basal-bolus, IDegLira treatment was associated with greater proportion of participants achieving the prespecified composite outcome of a target HbA1c of <7.0% (53 mmol/mol) with no hypoglycemia <70 mg/dL after 12 weeks (34% vs. 13%, P = 0.005) and after 26 weeks (34% vs. 10%, P = 0.003). IDegLira treatment was associated with greater proportion of participants achieving a target HbA1c of <7.0% (53 mmol/mol) with no weight gain after 12 weeks (20% vs. 3.2%, P = 0.004) and after 26 weeks (23% vs. 5.3%, P = 0.007). In addition, IDegLira treatment was associated with greater proportion of participants achieving a target HbA1c of <7.0% (53 mmol/mol) without hypoglycemia <70 mg/dL and without weight gain after 12 weeks (17% vs. 3.2%, P = 0.015) and after 26 weeks (14% vs. 1.7%, P = 0.016) (Fig. 2C and Table 2). Nine participants (12.5%) in the IDegLira group met the prespecified “treatment failure” definition, requiring an additional injection of basal insulin.
A higher proportion of participants in the IDegLira group reported nausea (38.0% vs. 4.1%, P < 0.001), vomiting (13% vs. 1.4%, P = 0.009), and abdominal pain (18% vs. 8.2%, P = 0.009) over the study duration (Table 2). Most of the gastrointestinal adverse effects were reported early in the titration period (Supplementary Fig. 3). One participant had nonproliferative retinopathy before randomization to the IDegLira group that progressed and was withdrawn from the study. Additional secondary outcomes are reported in the Supplementary Material (Supplementary Figs. 2 and 3).
Conclusions
This prospective RCT demonstrated the similar efficacy in HbA1c reduction of IDegLira and resulted in significantly lower hypoglycemia and less weight gain compared with basal-bolus insulin therapy in patients with T2D and high HbA1c ≥9.0–15.0% (75–140.4 mmol/mol).
Despite recent advances in pharmacology, only 60–70% of participants with T2D met personalized HbA1c targets from 1999 to 2018 in the U.S. (16), with 13.2% having HbA1c values >9.0% (75 mmol/mol) (17). Participants with severe hyperglycemia frequently respond poorly to oral antidiabetes agents and require insulin therapy for at least a period of time (5). As expected, we observed that insulin requirements were high during the titration phase (first 3 of months of the study) given the elevated baseline glucose value. However, IDegLira treatment resulted in a significantly higher proportion of participants achieving a target HbA1c of <7.0% (53 mmol/mol) during the 12-week titration period. There was significantly higher weight gain with basal-bolus treatment compared with IDegLira, particularly during the first 12 weeks of insulin titration, and less weight gain with IDegLira at the end of the study (26 weeks). These findings are of contemporary interest given a recent paradigm change in the management of participants with T2D to a more patient-centered approach, favoring therapeutic options associated with weight neutral or weight loss effect (18).
Our study challenges the current widespread practice of basal-bolus insulin therapy as the most effective option for glycemic control in participants with T2D and severe hyperglycemia (i.e., HbA1c >10%, 86 mmol/mol), who are usually excluded from clinical trials, and suggests that combination therapy with basal insulin and GLP1-RA, in an FRC daily injection, results in better patient-centered outcomes. Previous studies have demonstrated that a combination of basal insulin and GLP1-RA have similar efficacy in lowering HbA1c, with benefits on hypoglycemia and weight gain (15), but excluded this population with severe hyperglycemia and HbA1c ≥9.0–15.0% (75–140.4 mmol/mol) (19,20). Ongoing st-udies assessing the efficacy and safety of the combination of weekly insulin and GLP1-RA, in FRC or alone, or more potent weekly GLP1-RA or dual/triple agonists alone, may provide another alternative for patients with poorly controlled T2D. However, these studies usually do not include patients with very high HbA1c and will take years to be completed. Our study was designed to clarify the clinical need for better therapeutic strategies that will allow glycemic targets to improve and also patient-centered outcomes in participants with severe hyperglycemia and HbA1c >9.0–15.0% (75–140.4 mmol/mol), despite the failure of oral antidiabetes agents and/or basal insulin. Our approach in a real-word diabetes clinic and serving underserved populations demonstrated the benefits of a simpler treatment (daily FRC injection) and underscores the complexity and burden of the basal-bolus regimen. Notably, our participants are highly representative of racial minorities who are usually disproportionally affected by higher rates of complications.
Despite being efficacious and widely implemented, basal-bolus insulin therapy is a complex and burdensome regimen for participants, requiring insulin multiple daily injections, multiple glucose checks per day, and ongoing diabetes education. Furthermore, it is associated with increased risk of hypoglycemia, poor adherence, and weight gain (15). Our study demonstrated similar efficacy on glycemic improvement, but lower hypoglycemia rates and less weight gain, with more participants achieving glycemic targets (e.g., HbA1c <7%, 53 mmol/mol) without hypoglycemia and without weight gain. In accordance with prior studies (15,19), a higher proportion of participants in the IDegLira group reported gastrointestinal adverse events. We acknowledge that ∼20% of participants did not finish all visits, mostly occurring during the coronavirus disease 2019 pandemic and in concordance with prior studies (19).
In conclusion, we demonstrated that a simpler and more physiologic treatment approach, with a single daily injection of basal insulin and liraglutide, compared with the traditional approach of basal-bolus insulin, was not inferior in achieving glycemic control in participants with T2D and severe hyperglycemia (HbA1c >9.0–15.0%, 75–140.4 mmol/mol). This simpler regimen resulted in less hypoglycemia and less weight gain. Our study supports a paradigm change from the widely use basal-bolus approach to a simpler regimen with basal insulin and GLP1-RA as a more patient-centered option for participants with severe hyperglycemia and HbA1c >9.0–10.0% (75–86 mmol/mol).
Article Information
Acknowledgments. The authors extend personal thanks to Professor Clare Bradley, Health Psychology Research, Royal Holloway University of London, Surrey, U.K., for granting permission to use the Diabetes Treatment Satisfaction Questionnaire (DTSQ) questionnaire.
Funding. R.J.G. is supported in part by grants from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under award numbers P30DK111024-04S2 and 1K23DK123384-03. P.V. is supported in part by NIH/NIDDK grant 1K23DK113241. F.J.P. is supported in part by NIH National Institute of General Medical Sciences under award number K23GM12822103 and NIH/NIDDK P30DK111024 and P30DK11102405S1. G.E.U. is partly supported by NIH National Center for Advancing Translational Sciences Award program research grant 3UL1TR002378-05S2, and from NIH/NIDDK (2P30DK111024-06) and National Center for Research Resources.
Duality of Interest. This study was an investigator-initiated clinical trial funded by a grant from Novo Nordisk (IIS to Emory University). A contract was developed between Novo Nordisk and Emory University (primary investigator: R.J.G.), which became the academic sponsor of the study. R.J.G. received unrestricted research support (to Emory University) from Novo Nordisk, Eli Lilly and Dexcom Inc., and consulting fees from Sanofi, Novo Nordisk, Eli Lilly, Pfizer, Boehringer, Bayer, and Weight Watchers. P.V. has received consulting fees from Merck and Boehringer-Ingelheim. F.J.P. has received consulting fees from Merck, Boehringer-Ingelheim, Eli Lilly, and Medscape, and research support from Merck, Ideal Medical Technologies, Insulet, and Dexcom Inc. G.M.D. and has received research support from Insulet and consulting fees from Medscape. M.F. receives research support from Dexcom Inc. G.E.U. has received research support (to Emory University) from Bayer, Abbott, and Dexcom.
The funder of the study had no role in study design, data analysis, and interpretation, or writing of the report.
Author Contributions. R.J.G. wrote the initial research proposal and critically reviewed research data and wrote the initial manuscript. B.M., M.F.S., C.Z., B.S.A., and J.S. screened, consented, and followed participants in the study, reviewed the data and analysis, and edited the manuscript. P.V., F.J.P., G.M.D., M.F., and G.E.U. reviewed the initial research proposal, conducted the study, reviewed the data and analysis, and edited the manuscript. L.P. generated the random allocation sequence and performed the statistical analysis. R.J.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as oral presentation at the 82nd Scientific Sessions of the American Diabetes Association, virtual and at New Orleans, LA, 3–7 June 2022.
Footnotes
Clinical trial reg. no. NCT03737240, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.23556180.
This article is featured in a podcast available at diabetesjournals.org/journals/pages/diabetes-core-update-podcasts.
References
- 1. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 3. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 4. DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 2013;36(Suppl. 2):S127–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrannini E. The stunned beta cell: a brief history. Cell Metab 2010;11:349–352 [DOI] [PubMed] [Google Scholar]
- 6. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S125–S143 [DOI] [PubMed] [Google Scholar]
- 7. Garber AJ. Insulin intensification strategies in type 2 diabetes: when one injection is no longer sufficient. Diabetes Obes Metab 2009;11(Suppl. 5):14–18 [DOI] [PubMed] [Google Scholar]
- 8. Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: developing a diabetes mellitus comprehensive care plan—2022 update. Endocr Pract 2022;28:923–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012;29:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peyrot M, Rubin RR, Khunti K. Addressing barriers to initiation of insulin in patients with type 2 diabetes. Prim Care Diabetes 2010;4(Suppl. 1):S11–S18 [DOI] [PubMed] [Google Scholar]
- 11. Rubin RR, Peyrot M, Kruger DF, Travis LB. Barriers to insulin injection therapy: patient and health care provider perspectives. Diabetes Educ 2009;35:1014–1022 [DOI] [PubMed] [Google Scholar]
- 12. Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. J Gen Intern Med 2005;20:479–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Josse RG, Woo V. Flexibly timed once-daily dosing with degludec: a new ultra-long-acting basal insulin. Diabetes Obes Metab 2013;15:1077–1084 [DOI] [PubMed] [Google Scholar]
- 14. Lingvay I, Pérez Manghi F, García-Hernández P, et al.; DUAL V Investigators . Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial [published correction appears in JAMA 2016;315:2125]. JAMA 2016;315:898–907 [DOI] [PubMed] [Google Scholar]
- 15. Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care 2018;41:1009–1016 [DOI] [PubMed] [Google Scholar]
- 16. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. Adults, 1999–2018. N Engl J Med 2021;384:2219–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Accessed 18 January 2022. Available from https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 18. Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet 2022;399:394–405 [DOI] [PubMed] [Google Scholar]
- 19. Abreu M, Tumyan A, Elhassan A, et al. A randomized trial comparing the efficacy and safety of treating patients with type 2 diabetes and highly elevated HbA1c levels with basal-bolus insulin or a glucagon-like peptide-1 receptor agonist plus basal insulin: the SIMPLE study. Diabetes Obes Metab 2019;21:2133–2141 [DOI] [PubMed] [Google Scholar]
- 20. Abdul-Ghani M, Migahid O, Megahed A, DeFronzo RA, Zirie M, Jayyousi A. Efficacy of exenatide plus pioglitazone vs basal/bolus insulin in T2DM patients with very high HbA1c. J Clin Endocrinol Metab 2017;102:2162–2170 [DOI] [PubMed] [Google Scholar]