Abstract
OBJECTIVE
To investigate the outcome of Chinese water-soluble propolis (WSP) on the inflammatory response and oxidative stress (OS) of colonic mucosa in rats with ulcerative colitis.
METHODS
Dextran sulfate sodium (DSS) was employed to establish the ucerative colitis (UC) rat model. Forty-eight male rats were arbitrarily separated into six groups, namely control, UC, low-dose water-soluble propolis (L-WSP), medium-dose water-soluble propolis (M-WSP), high-dose water-soluble propolis (H-WSP), and sulfasalazine (Sulfa). In this study, we adopted a method of pre-administration and reconstruction of the model that assessed the water-soluble propolis mediated protection against DSS-induced UC rats. Moreover, we examined the body weight (BW), disease activity index (DAI), bloody stool, colon length, and intestinal mucosal injury index of rats. In addition, using enzyme linked immunosorbent assays, we assessed indicators, such as, colonic myeloperoxidase (MPO), interleukin-6 (IL-6), interleukin-9 (IL-9), tumor necrosis factor-ɑ (TNF-ɑ), superoxide dismutase (SOD), malondialdehyde, and glutathione peroxidase (GSH-Px) levels.
RESULTS
The pro-inflammatory cytokine expression, as well as OS, was increased in the model rats. However, upon WSP intervention, both pro-inflammatory cytokine levels and OS reduced dramatically, and the therapeutic effect was dose-dependent.
CONCLUSION
WSP downregulates OS by enhancing the function of endogenous antioxidant enzymes like SOD and GSH-Px, that inhibit neutrophil activity, as well as diminish pro-inflammatory cytokines like TNF-ɑ, IL-6, and IL-9, along with mechanisms that attenuate intestinal inflammation in UC rat model.
Keywords: ulcerative colitis, natural products, oxidative stress, water-soluble propolis, pro-inflammatory cytokine
1. INTRODUCTION
The gut immune system provides a swift and successful immune response to pathogenic bacteria while remaining tolerant to food and microbiota antigens. Dysregulation between the immune system and microbiota can lead to abnormal inflammatory responses like inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), which is manifested by a persistent intermittent inflammation, with active disease alternation with varying degrees of remission.1 Among them, UC is an inflammation-based illness involving the colonic mucosa, and it presents as abdominal pain, cramping, bloody diarrhea, rectal bleeding, weight loss, fever, and fatigue, and symptom initiation may occur sequentially or simultaneously. UC pathogenesis is strongly correlated with factors, such as heredity, immunity, and environment. However, no clear evidence suggests a direct link between these factors and UC pathogenesis. In fact, UC, etiology is currently unknown. As a result, curing UC is tremendous challenge. Unfortunately, delayed healing increases the likelihood of developing colorectal cancer.2
Traditional UC therapies include 5-aminosalicylic acid, thiopurines, and corticosteroids.3 However, these drugs are accompanied with severe side effects and unsatisfactory therapeutic effects. Therefore, it is necessary to develop new treatment strategies that are highly efficacious and produce minimal side effects. Natural ingredients are an interesting source of new therapies. In fact, natural ingredients and their compounds make excellent candidates for developing novel drugs, owing to their wide-ranging therapeutic spectrum, reduced toxicity, fewer side effects, and diminished cost. Being an inflammation-based disease, UC is generally treated with compounds having anti-inflammatory properties, and there is an urgent need to examine the anti-inflammatory aspects of natural ingredients on UC.
Propolis is a resinous substance collected by foraging bees, from buds and exudates of different plant species. It mixes with β-glycosidase in bee saliva, and possesses a myriad of biological activities like anti-inflammation, antioxidant, antitumor, antiviral, immune regulation, and so on.4 Propolis was used in folk medicine since ancient times, and the biological activity of propolis is mainly attributed to its polyphenolic compounds, like flavonoids. Being a natural product, propolis is widely used in food, medicine, health care products, and cosmetics. Emerging evidences reported that propolis has anti-ulcer properties and promotes mucosal healing and granuloma formation.5 These anti-inflammatory properties make propolis excellent natural candidate for improving UC. Propolis composition varies slightly depending on the geographical location of its origin, and its chemical composition strongly associates with the plant from which it is derived.6 Typical propolis has various plant origins. Chinese propolis mostly originates from poplar.7 Multiple reports suggested that both Chinese and Brazilian propolis strongly suppresses the expression and release of bacterial lipopolysaccharide-induced inflammatory factors in macrophages, thereby alleviating reactive oxygen species (ROS) accumulation in cells, despite their differences in chemical composition.8 Therefore, propolis plays a strong anti-inflammatory action during inflammatory diseases.
The goal of our research was to assess the antioxidant and anti-inflammatory properties of Chinese water-soluble propolis (WSP) in a dextran sulfate sodium (DSS)-triggered UC rat model, and to detect remission of WSP. It is our belief that a better understanding of the mechanism of action behind intestinal inflammation in UC rats can effectively promote the application and industrial development of propolis, and provide important reference information for the generation of novel drugs that treat UC.
2. MATERIALS
2.1. Experimental animals
In all, we employed 48 specific pathogen free (SPF) grade male Sprageu-Dawley (SD) rats (license number: SCXK (Lu) 20190003), six-month-old, weighing (180 ± 20) g. The quality inspection was done at the Shandong Laboratory Animal Center. The study was approved by the Biomedical Ethics Committee of Anhui Medical College [approval number (2021) 160].
2.2. Drugs and reagents
DSS (MW: 36 000-50 000; MP Biomedicals, Santa Ana, Canada); superoxide dismutase (SOD) enzyme linked immunosorbent assays (ELISA) kit, and glutathione peroxidase (GSH-Px) ELISA kit, were purchased from CUSABIO Company (Wuhan, China). ROS detection ELISA kit, malondialdehyde (MDA), and myeloperoxidase (MPO) detection kit were acquired from Nanjing Jiancheng Biomedical Engineering Institute (Nanjing, China). Interleukin-6 (IL-6), interleukin-9 (IL-9), tumor necrosis factor-α (TNF-α) kits were obtained from Hefei Zhihongtek Biotechnology Co., Ltd. (Hefei, China). Paraformaldehyde, as well as hematoxylin and eosin, were purchased from Biosharp Company (Guangzhou, China). Water-soluble propolis was obtained from Guangzhou Jiehe Bee Industry Co., Ltd. (Guangzhou, China) (batch No. 20210325-B).
3. METHODS
3.1. Animal grouping
Forty-eight male SD rats were conditionally fed for 1 week prior to the experiment. They were housed in cages, with sawdust in the room [(23 ± 2) ℃] ambient temperature, and 12-hour light-dark cycle. The rats were randomly divided into six groups, namely, control (CON), ulcerative colitis (UC), low-dose propolis group (L-WSP), medium-dose propolis group (M-WSP), high-dose propolis group (H-WSP), and sulfasalazine groups (Sulfa), 8 per group.
3.2. Method of administration
This experiment adopted the method of pre-administration and reconstruction of the model. During the entire experiment, the CON and UC rats were orally gavaged with distilled water, and the L-WSP, M-WSP, and H-WSP rats were orally gavaged with WSP, in dosages 50, 100 and 200 mg/kg, respectively. In the Sulfa group, sulfasalazine 100 mg/kg was orally administered. After performing the above steps for 7 d, the CON rats were allowed to freely drink distilled water for 7 d; whereas the UC, L-WSP, M-WSP, H-WSP, and Sulfa rats were made to freely drink 5% DSS solution for 7 d. Finally, the free DSS drinking was terminated, and all groups were provided with distilled water for 2 d. Rats were sacrificed via cervical dislocation.
3.3. Assessment of body weight (BW) changes and blood in the stool
Starting from the first day (day 1) of DSS consumption till day 9, the BW, blood in the stool, and fecal characteristics of rats were monitored at the same time daily, and the data was recorded in detail. The rats were sacrificed on day 10. The day 1 BW was marked as the starting BW prior to the experiment.9
Weight change (%) = [(Day X BW-Day 1 BW) / Day 1 BW] × 100%
The stool blood detection was done with the occult blood detection kit, according to the kit directions and references.10
3.4. Disease activity index (DAI) score
From day 2 of DSS consumption, we evaluated DAI, according to the following criteria based on BW changes, fecal characteristics, and blood in the stool, DAI = weight loss score + fecal characteristics score + blood in stool score. The scoring criteria are presented in Table 1.10
Table 1.
Disease activity index scoring criteria
| Score | Weight loss (%) | Stool traits | Occult blood |
|---|---|---|---|
| 0 | <1 | normal | (-) |
| 1 | ≥1 - ≤5 | Between normal and loose stools | (+) |
| 2 | >5 - ≤10 | Loose stools | (++) |
| 3 | >10 - ≤15 | between loose stools and diarrhea | (+++) |
| 4 | >15 | diarrhea | gross bloody stool |
3.5. Sample collection and processing
Upon sacrifice, the colonic intestinal segment more than 2 cm from the rat anus was dissected and completely removed from the abdominal cavity. The colon was flattened to measure its length. Subsequently, the colon was dissected along the longitudinal axis, washed with chilled normal saline, cleaned of its contents, and placed on a filter paper to blot dry. The colon weight/length (W/L) ratio was computed as follows11:
Colon W/L ratio = colon weight (g) / colon length (cm)
The colonic mucosal damage index (CMDI) was also calculated, and the scoring index is presented in Table 2.11
Table 2.
Colonic mucosal damage index scoring standard
| Score | Macroscopic observation of colonic mucosa |
|---|---|
| 0 | Normal colon (no damage) |
| 1 | Mild congestion, no ulcers |
| 2 | Ulcers or small areas of inflammation with flashpoints, congestion and edema |
| 3 | Large areas of inflammation and ulcers < 1 cm |
| 4 | Extensive tissue damage and > 1cm extension |
The lesion tissue was collected from the same part of the colon in each group, and some tissues were fixed in EP tubes with 4% paraformaldehyde. The remaining colon was placed in EP tubes and chopped, and homogenized with 5 times the volume of normal saline, followed by centrifugation at 10 000 r/min for 10 min. The supernatant was then collected, and stored at -80 ℃.
3.6. Histopathological observation of the colon
We performed an overnight wash of the fixed colon tissue using running water, prior to dehydration with graded alcohol, clearance with xylene, embedding in paraffin, slicing into 5 μm thick sections, staining with hematoxylin-eosin (HE), and, finally, observation under a light microscope. The colon histological (histological score, HS) scoring criteria for detection of colonic damage are presented in Table 3.11
Table 3.
Histological colitis scoring system
| Score | Histological changes |
|---|---|
| 0 | Normal histology |
| 1 | Histological damage limited to endothelial cells with mild inflammatory cell infiltration |
| 2 | Focal ulceration, histological destruction limited to the mucosal layer, abnormal glandular structure of the intestinal wall, mild inflammatory cell infiltration |
| 3 | Focal, transmural ulceration and inflammation, with mild to moderate inflammatory cell infiltration |
| 4 | Large transmural ulceration and inflammation, moderate inflammatory cell infiltration |
| 5 | Large ulcers and inflammation, lesions extending from mucosa to serosa, severe inflammatory cell infiltration |
3.7. Detection of pro-inflammatory cytokines in the colon tissue homogenate
We swiftly recovered the cryopreserved colon tissue homogenate supernatant, and measured IL-6, IL-9 and, TNF-α levels in the colon tissue homogenate of each group, following kit directions.
3.8. Determination of the OS index in rat colon tissue
The colonic tissues of each group were retrieved from the -80 ℃ refrigerator. Approximately, 100 mg of colonic tissues were weighed and placed in an ice box, and homogenized into a 10% tissue homogenate with iced saline. Next, we diluted 1/4 of the homogenate to a volume fraction of 1%. This dilution then underwent centrifugation at 14 000 r/min for 15 min at 4 ℃, and the supernatant was used to detect ROS, MDA, MPO, SOD, and GSH-Px activities, following kit directions.
3.9. Statistical analysis
All data were expressed as mean ± standard deviation (χ ± s) and were anlayzed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). Comparison among multiple groups was done with one-way analysis of variance, whereas pairwise comparisons between multiple groups, was done with the independent sample t test, in presence of normally distributed, and using the nonparametric rank sum test, in presence of normally distributed. P < 0.05 was considered statistically significant.
4. RESULTS
4.1. WSP improves DSS-induced colonic inflammation in UC rats
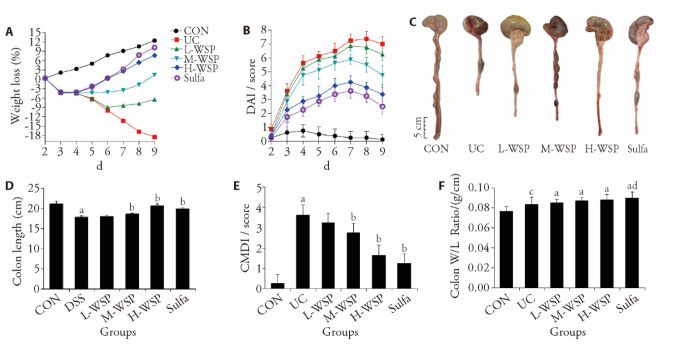
In this study, rats were treated with WSP and Sulfa for 16 d, and the UC model was established on the 8th day by drinking 5% DSS freely. Based on our results, UC rat BW reduced gradually and significantly, whereas, the DAI was markedly upregulated, compared to CON rats. Upon intervention with various WSP dosages, the M-WSP and H-WSP doses markedly suppressed DSS-induced weight loss (Figure 1A). Moreover, the DAI score was markedly reduced as well (Figure 1B).
Figure 1. Effects of WSP on weight loss, DAI score, colon length, CMDI socre and colon W/L ratio over the whole experimental period.
A: weight loss; B: DAI score; C-D: colon length; E: CMDI socre; F: colon W/L ratio. CON: control group; UC: ulcerative colitis group; L-WSP: low-dose water-soluble propolis group (50 mg·kg-1·d-1); M-WSP: medium-dose water-soluble propolis group (100 mg·kg-1·d-1); H-WSP: high-dose water-soluble propolis group (200 mg·kg-1·d-1); Sulfa: sulfasalazine group (100 mg·kg-1·d-1). The drug was administered by gavage once a day for 16 d. DAI: disease activity index; CMDI: colonic mucosal damage index; colon W/L: colon weigh (g) / colon length (cm). Data are expressed as mean ± standard deviation (n = 8). Compared to CON group, aP < 0.01, cP < 0.05; compared to UC group, bP < 0.01, dP < 0.05.
Relative to the CON rats, the UC rat colonic length was remarkably shortened (Figure 1C, 1D); the colonic mucosa exhibited hyperemia, edema, local erythema, as well as erosion; and the CMDI score was increased (Figure 1E). Relative to the UC, the M-WSP and H-WSP rats exhibited markedly longer colon length; the colonic mucosal congestion and edema were significantly reduced; the erosion was repaired; and the CMDI score was reduced, and the differences were statistically significant (P < 0.01). Relative to the CON rats, the colon W/L ratio increased in each group (Figure 1F). Based on these evidences, WSP can effectively relieve DSS triggered UC in rats.
4.2. WSP reduces the degree of colon tissue lesions in UC rats
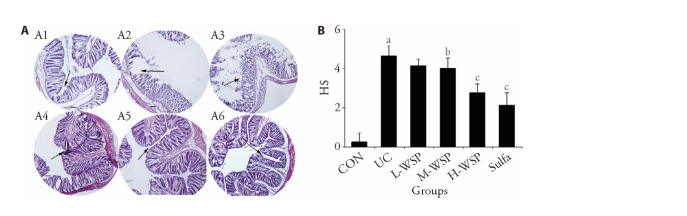
We observed the HE-stained pathological sections, and as illustrated in Figure 2A, the colonic mucosal epithelium in CON rats exhibited a complete structure, and the epithelial cells were normal in morphology and structure, tightly arranged, and without inflammatory cell infiltration. In UC rats, however, there was a large presence of inflammatory and goblet cells, whereas, the glandular crypts were largely absent. Relative to the CON rats, the HS score of UC rats was markedly elevated (P < 0.01). Upon drug intervention, the intestinal epithelial structure was relatively intact in the H-WSP and Sulfa rats, and the level of damage was markedly relieved. Relative to the CON rats, the HS scores in the H-WSP and Sulfa rats were both significantly decreased (Figure 2B) (P < 0.01). Moreover, we observed a relatively low quantity of inflammatory cell infiltration, along with a reduced number of goblet, as well as crypt loss in the M-WSP rats. In addition, the HS score was decreased, relative to the CON rats (P < 0.05). In contrast, there was some inflammatory cell infiltration in the L-WSP rats. Given these evidences, we speculate that a certain concentration of WSP significantly protects the colonic epithelial cell structure, reduces inflammatory cell infiltration, and lowers histological inflammation, thereby protecting the colonic mucosa from damage.
Figure 2. Effects of WSP on microscopic changes in colon tissue of rats in each group.
A: representative images of microscopic appearance colon slices with HE staining of all groups, magnification × 40; A1: CON; A2: UC; A3: L-WSP; A4: M-WSP; A5: H-WSP; A6: Sulfa; B: Score of histological changes. CON: control group; UC: ulcerative coloits group; L-WSP: low-dose water-soluble propolis group (50 mg·kg-1·d-1); M-WSP: medium-dose water-soluble propolis group (100 mg·kg-1·d-1); H-WSP: high-dose water-soluble propolis group (200 mg·kg-1·d-1); Sulfa: sulfasalazine group (100 mg·kg-1·d-1). The drug was administered by gavage once a day for 16 d. HS: histological score. Data are expressed as mean ± standard deviation (n = 8). Compared to CON group, aP < 0.01; compared to UC group, bP < 0.05, cP < 0.01.
4.3. WSP reduces inflammatory response of colon tissue in UC rats
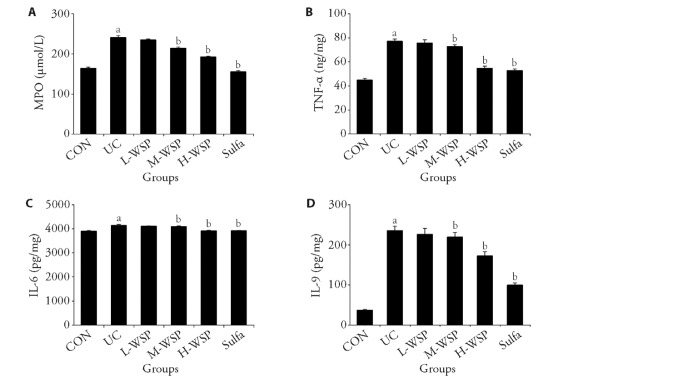
MPO, an enzyme present almost exclusively in neutrophils, is a marker of acute inflammatory responses. As shown in Figure 3A, MPO activity was significantly up-regulated in the colon tissue homogenate of UC rats, suggesting the presence of excess neutrophils in the inflamed colonic mucosa during UC. Subsequently, neutrophil infiltration and activation leads to enhanced levels of pro-inflammatory cytokines. As illustrated in Figure 3B-3D, relative to CON rats, the expressions of IL-9, IL-6, and TNF-α in DSS rats were markedly enhanced (P < 0.01). Upon WSP intervention, MPO, IL-9, IL-6, and TNF-α levels in the M-WSP and H-WSP rats were markedly decreased (P < 0.01). Based on these data, medium and high dosages of WSP inhibit neutrophil, downregulate pro-inflammatory cytokine levels, and relieve colonic inflammation.
Figure 3. Effects of WSP on the colonic inflammatory response in UC rats.
A: MPO levels in colon tissue homogenate; B: TNF-α levels in colon tissue homogenate; C: IL-6 levels in colon tissue homogenate; D: IL-9 levels in colon tissue homogenate. CON: control group; UC: ulcerative colitis group; L-WSP: low-dose water-soluble propolis group (50 mg·kg-1·d-1); M-WSP: medium-dose water-soluble propolis group (100 mg·kg-1·d-1); H-WSP: high-dose water-soluble propolis group (200 mg·kg-1·d-1); Sulfa: sulfasalazine group (100 mg·kg-1·d-1). The drug was administered by gavage once a day for 16 d. MPO: myeloperoxidase; TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; IL-9: interleukin-9. Data are expressed as mean ± standard deviation (n = 8). Compared to CON group, aP < 0.01; compared to UC group, bP < 0.01.
4.4. WSP alleviates OS injury in colon tissue of UC rats
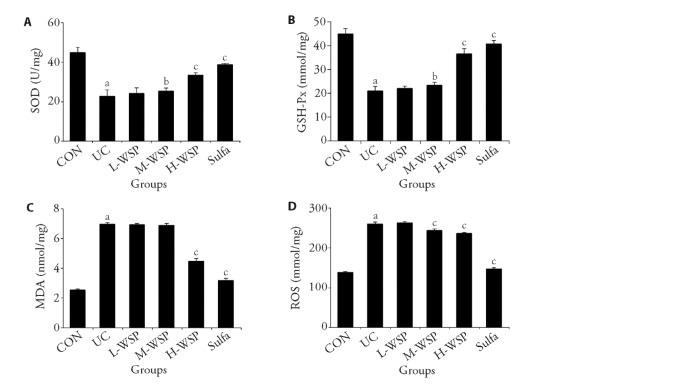
As depicted in Figure 4, the SOD and GSH-Px levels in the colon tissue homogenate of UC rats were drastically diminished, compared to the CON rats. In contrast, the ROS and MDA levels were remarkably enhanced, suggesting that UC rats had relatively high levels of OS. Upon WSP intervention, both medium and high dosages of WSP significantly increased SOD and GSH-Px levels, and markedly reduced ROS levels. However, high dosages of WSP significantly reduced MDA levels, thus relieving OS. Hence, WSP protected colon tissue from the detrimental effects of enhanced OS during UC.
Figure 4. Effects of WSP on the SOD, GHS-Px, MDA and ROS contents in the colon tissue of rats in each group.
A: SOD contents in colon tissue homogenate; B: GSH-Px contents in colon tissue homogenate; C: MDA contents in colon tissue homogenate; D: ROS contents in colon tissue homogenate. CON: control group; UC: ulcerative colitis group; L-WSP: low-dose water-soluble propolis group (50 mg·kg-1·d-1); M-WSP: medium-dose water-soluble propolis group (100 mg·kg-1·d-1); H-WSP: high-dose water-soluble propolis group (200 mg·kg-1·d-1); Sulfa: sulfasalazine group (100 mg·kg-1·d-1). The drug was administered by gavage once a day for 16 d. SOD: superoxide dismutase; GSH-Px: glutathione peroxidase; MDA: malondialdehyde; ROS: reactive oxygen species. Data are expressed as mean ± standard deviation (n = 8). Compared to CON group, aP < 0.01; compared to UC group, bP < 0.05, cP < 0.01.
5. DISCUSSION
5.1. WSP alleviates intestinal inflammation in DSS-induced UC rats
UC is a long-term recurring inflammatory illness involving the digestive tract. It is highly damaging to both patient physical and mental fitness, and it may enhance risk colon and bowel cancer. Thus far, there is no specific drug that completely cures UC. The long-term goal is to prevent disability, colectomy, or colorectal cancer. Given these circumstances, UC treatment still remains an area of significant medical need. As such, it is crucial to identify alternative and more tolerable treatments for this disease, and this area of study is hot topic of today.
Since safety, reduced toxicity, and efficacy are the primary concerns, natural products and their derivatives are the main focus of UC research.12 Growing evidences confirm that phenols, particularly flavonoids, possess strong anti-inflammatory and antioxidant activities both in vitro and in vivo, and compounds with these two properties seem promising for UC treatment. Multiple reports demonstrated that dietary polyphenol-rich foods, such as green tea, coffee, berries, grapes, and other fruits/vegetables, play an essential role in maintaining an intact intestinal mucosal barrier while increasing the expression of tight junction-related proteins, which help relieve intestinal inflammation.13 In fact, UC patients, who received a standard anthocyanin-rich bilberry preparation daily for 6 weeks, exhibited significantly reduced intestinal inflammation.14 Polyphenol-rich cranberry extract can abrogate dietlinked obesity, insulin resistance, and intestinal inflammation.15 Propolis is a natural antibiotic that is rich in flavonoids, terpenes, and other substances.16 It has a good antioxidant effect, whereby it removes free radicals from the body and reduces lipid peroxides and lipids, protection of cell membranes, enhancement of cell viability, and regulation of tissue and organ functions, propolis is expected to reduce inflammatory damage and/or improve colonic mucosal healing in UC patients.
UC symptoms, namely, weight loss, abdominal pain, diarrhea, blood in stool, loss of appetite, and so on, form an important basis for the diagnosis of UC. DSS-induced rodent UC model is currently considered to be the best model representing human UC.17 It has the typical characteristics of UC, and can be used as a tool to study the cellular and molecular mechanisms underlying UC. It is also increasingly used in anti-inflammatory drugs development. In this study, a 5% DSS-induced rat UC model was employed. During establishment of the disease, UC rats developed clinical symptoms similar to human UC patients, namely, significant weight loss, increased DAI score, diarrhea, bloody stools, and so on. Prior investigations revealed that the colon length of UC models is inversely proportional to the severity of experimental UC. Moreover, UC is manifested by persistent inflammation of the colonic mucosa and submucosa. Another important indicator of UC is histopathological evaluation, and our study also demonstrated that the UC rat colon length was remarkably shortened, and erythema and, as well as erosion of the colonic mucosa, was apparent with the naked eye. Colon HE staining revealed massive inflammatory cell infiltration, and a large number of goblet cells and crypts were missing, indicating that the modeling was successful. Sulfa is a first-line drug used in the clinical treatment of UC. In this experiment, we selected sulfa as the positive control in order to better evaluate the efficacy of WSP on UC. Based on our analysis, relative to the CON rats, UC rats, who received medium and high concentrations of WSP via gavage, exhibited enhanced BW and colon length, and diminished DAI, CMDI, and HS scores, and the differences were statistically significant. This suggests that medium and high concentrations of WSP can effectively reduce intestinal mucosal inflammation, and successfully alleviate colon tissue damage in UC rats. Relative to CON rats, the colon W/L ratio increased significantly in each group, indicating that, during colon tissue regeneration, new blood vessels formed and fibroblasts grew, resulting in a thicker colon. In addition, the W/L ratio of the colon reflected the degree of tissue repair. The primary roles of the colon are to extract water and electrolytes, form stool, and utilize peristalsis to induce defecation. When the colon is unable to absorb nutrients, diarrhea ensues. Taken together, these evidences suggest that medium and high dosages of WSP promotes colonic mucosal ulcer repair, and, simultaneously, restores colonic absorptive function.
5.2. Analysis of the WSP-based mechanism that downregulates intestinal inflammation in DSS-induced UC rats
It is well known that UC severity correlates with MPO activity, a neutophil-specific enzyme. Moreover, MPO activity directly reflects neutrophil presence in the inflamed colon. Upon neutrophil activation, MPO is released into the extracellular space or phagosome, leading to UC tissue damage and intestinal mucosal dysfunction. This accelerates the development of local intestinal inflammation.18 Thus, MPO is a sign of acute inflammatory response. Our observation of MPO activity in the colon tissue homogenate samples revealed that relative to the CON rats, the MPO activity in UC rats was markedly increased. However, in UC rats, that received medium and high concentrations of WSP via gavage, the MPO activity was significantly reduced, thus, indicating that medium and high dosages of WSP inhibits neutrophils and alleviates intestinal inflammation.
Neutrophils migration to the inflamed colonic mucosa results in enhanced accumulation of pro-inflammatory cytokines, and the subsequent imbalance between pro- and anti-inflammatory cytokines contributes to UC pathogenesis. It was earlier demonstrated that UC severity is intricately correlated with pro-inflammatory cytokines synthesis.19
TNF-α, IL-6 and IL-9 are typical pro-inflammatory cytokines found in UC. As such, activity suppression of these cytokines is critical target for UC therapy. It was previously reported that limiting the action of these cytokines restores the function of the intestinal mucosal barrier, and subsequently alleviates UC inflammatory response. It was reported that the TNF-α, a major contributor to UC pathogenesis, enhances endothelial permeability, and induces intestinal recruitment of neutrophils by upregulating intracellular adhesion molecule expression.20 Hence, anti-TNF-α constitutes the main direction of UC treatment. Chaudhary and other studies reported that Bawei Xile San (八味喜乐散) inhibits DSS-induced TNF-α expression in UC mice, and, subsequently, improves their mucosal barrier function.21 Likewise, in clinical practice, TNF-α blockers, like infliximab and adalimumab were successfully used to treat UC patients.
Furthermore, macrophages and CD4+ T cells in the intestinal mucosal lamina propria of UC patients produce an excess of pro-inflammatory cytokine IL-6. The association of IL-6 with its soluble receptor (sIL-6R) induces cells to produce the membranal gp130 protein. Next, IL-6 transmits signals via gp130 to activate the signal transducer and activator of transcription 3 (STAT3) protein, which, in turn, accelerates production of the anti-apoptotic factors B-lymphoma-2 (Bcl-2) and Bcl-xL, which results in apoptosis-resistant T cells. The subsequent T cell accumulation leads to chronic inflammation.22 Thus, IL-6 is a potential therapeutic target for UC. Wang et al 23 demonstrated that salidroside suppresses the release of pro-inflammatory cytokine IL-6 by attenuating nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and JAK STAT3 networks, thereby alleviating inflammation associated with UC.
IL-9 is a new subtype of T helper (Th) cells.24 Following the production of Th9 cells, IL-9 and its receptors promote UC development. Excess IL-9 expression was demonstrated in the inflamed mucosal biopsies from UC patients, IL-9 regulates the intestinal mucosal ulcer healing by altering the intestinal mucosal barrier function. As such, inhibiting IL-9 action was shown to lower intestinal inflammation in DSS-stimulated colitis mice.25 These evidences suggest that IL-9 may be another key regulator of UC development. In our study, we demonstrated that the TNF-α, IL-6 and IL-9 levels in the colon tissue homogenate of DSS-stimulated UC rats were markedly elevated, compared to CON rats. In contrast, in the M-WSP and H-WSP rats, the TNF-α, IL-6 and IL-9 levels were markedly reduced, relative to the UC rats. Thus, a certain dose of WSP intake can effectively reduce pro-inflammatory cytokines TNF-α, IL-6 and IL-9 in the colon tissue, and relieve the intestinal inflammatory response associated with UC.
Dysregulation of the intestinal mucosal barrier is one of the pathogenesis of UC.26 Under normal circumstances, the intestinal mucosal barrier only allows a small amount of antigens and bacteria to pass through. Upon disruption of the barrier function, the passage of antigens and bacteria are enhanced, which, in turn, damages the integrity of the intestinal mucosa, and results in enhanced in ROS synthesis, which promotes pathological conditions like UC.27 The physiological protective mechanisms of the intestinal mucosal integrity include endogenous antioxidant defense systems (eg, SOD, GSH-Px) that prevent ROS production. Several reports suggested that UC is correlated with excess ROS production, which mediates lipid peroxidation and downstream inflammatory responses.28 MDA is the end product of lipid peroxidation, which is an indicator of OS that leads to cellular damage.29 The membranal lipids are attacked by free radicals, which activates the lipid peroxidation process, which ultimately leads to cell damage. Therefore, MDA levels reflect cellular damage, and it is a common indicator of lipid peroxidation and OS. In this study, we demonstrated that the ROS and MDA contents in the UC rat colon tissue were markedly enhanced, suggesting that the colon tissue was exposed to DSS, which resulted in an excess of free radicals, thus, leading to OS, lipid peroxidation, and augmented MDA content. We also demonstrated that M-WSP and H-WSP interventions effectively suppressed ROS and MDA contents in the colon tissue of UC rats, as opposed to no intervention.
OS, caused by excess ROS synthesis or diminished antioxidant activity, is another known factor that triggers inflammatory response in UC.30 Excess ROS can promote direct oxidative damage to cellular proteins, membrane lipids, and DNA, leading to cell death. Continued production of ROS (including superoxide, hydrogen peroxide, and peroxysulfite) reduces endogenous antioxidant enzymes. Hence, anti-oxidative stress may be a good option for UC treatment. UC is inseparable from the imbalance of OS in the body. Excessive ROS may have deleterious effects on target cellular components like DNA, proteins, and lipids. During UC, ROS can cause DNA damage, which may lead to cancer development. Endogenous antioxidant enzymes SOC and GSH-Px are known to resist free radical attacks within biological systems, while exerting antioxidant protection ability.31 Their function as first-line defense antioxidants is associated with the rapid inhibition or prevention of free radicals or ROS formation. With the increase in oxygen free radicals within the body, the free radical scavenger SOD is produced, which scavenges oxygen free radicals. Therefore, the degree of SOD activity can indirectly reflect damage to the body tissue. Additionally, GSH-Px protects membrane structural and functional integrity by removing MDA and OS lipid peroxides. In this study, we demonstrated that the SOD and GSH-Px content in the UC rat colon tissue drastically low. Upon M-WSP and H-WSP intervention, however, the SOD and GSH-Px levels rose significantly. Based on these results, a certain dose of WSP alleviates DSS-induced UC in rats by enhancing antioxidant activity, while inhibiting lipid peroxidation.
In conclusion, Flavonoid-rich WSP enhances the scavenging abilities of oxygen free radicals and reduces oxidative stress products by augmenting activities of endogenous antioxidant enzymes like SOD and GSH-Px in colon tissue. It, simultaneously, suppresses neutrophil activity, and down-regulates pro-inflammatory cytokines TNF-α, IL-6, and IL-9 to alleviate intestinal inflammation in a UC rat model in a concentration-dependent manner. Given these evidences, WSP is an excellent drug of choice for treating UC.
6. ACKNOWLEDGEMENTS
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor. com) for its linguistic assistance during the preparation of this manuscript.
Contributor Information
Hua ZHOU, Email: zhouhua@ahyz.edu.cn.
Haihua WANG, Email: 840099827@qq.com.
REFERENCES
- 1. Sood A, Mahajan R, Singh A, Midha V, Mehta V, Naranet V. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J Crohns Colitis 2019; 13: 1311-7. [DOI] [PubMed] [Google Scholar]
- 2. Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and Meta-analysis. Lancet Gastroenterol Hepatol 2017; 2: 269-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang C, Merlin D. Nanoparticle-mediated drug delivery systems for the treatment Of IBD: current perspectives. Int J Nanomedicine 2019; 14: 8875-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou H, Wang H, Shi N, Wu F. Potential protective effects of the water-soluble Chinese propolis on hypertension induced by high-salt intake. Clin Transl Sci 2020; 13: 907-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mendonça MAA, Ribeiro ARS, Lima AK, et al. Red Propolis and its dyslipidemic regulator formononetin: evaluation of antioxidant activity and gastroprotective effects in rat model of gastric ulcer. Nutrients 2020; 12: 2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol 2011; 133: 253-60. [DOI] [PubMed] [Google Scholar]
- 7. Wei P, Ding Y, Lu Q, Tan J, Zhang JL, Liu R. Flavonoids in propolis and poplar resin and their inhibition of α-glucosidase activity. Hua Zhong Nong Ye Da Xue Xue Bao 2018; 37: 92-9. [Google Scholar]
- 8. Cornara L, Biagi M, Xiao J, Burlando B. Therapeutic properties of bioactive compounds from different honeybee products. Front Pharmacol 2017; 8: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma Q, Weng YJ, Li J, Xu SY, He YF, Ma XD. Therapeutic effect of Shenling Baizhu powder on ulcerative colitis in mice. Zhong Guo Shi Yan Dong Wu Xue Bao 2021; 29: 785-92. [Google Scholar]
- 10. Dong J, Wang SK, Wu P, et al. Protective effect of polyphenol of houttuynia cordata on ulcerative colitis induced by sodium dextran sulfate (DSS) in mice. Xian Dai Shi Pin Ke Ji 2021; 37: 7-13. [Google Scholar]
- 11. Yang XJ, Fu Y, Wang JJ, et al. Effect of coptidis Rhizoma-Magnoliae officinalis cortes on TNBS-induced ulcerative colitis in rats by inhibiting PI3K/Akt signaling pathway. Zhong Cao Yao 2021; 52: 4587-97. [Google Scholar]
- 12. Nascimento RPD, Machado APDF, Galvez J, Cazarin CBB, Maróstica Junior MR. Ulcerative colitis: gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci 2020; 258: 118129. [DOI] [PubMed] [Google Scholar]
- 13. Chen Z, Chen H, Li X, et al. Fumonisin B1 damages the barrier functions of porcine intestinal epithelial cells in vitro. J Biochem Mol Toxicol 2019; 33: e22397. [DOI] [PubMed] [Google Scholar]
- 14. Roth S, Spalinger MR, Gottier C, et al. Bilberry-derived anthocyanins modulate cytokine expression in the intestine of patients with ulcerative colitis. PLoS One 2016; 11: e0154817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santana DG, Oliveira AS, Souza MTS, et al. Vaccinium macrocarpon Aiton extract ameliorates inflammation and hyperalgesia through oxidative stress inhibition in experimental acute pancreatitis. Evid Based Complement Alternat Med 2018; 2018: 9646937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. da Silva LM, de Souza P, Jaouni SKA, Harakeh S, Golbabapour S, de Andrade SF. Propolis and its potential to treat gastrointestinal disorders. Evid Based Complement Alternat Med 2018; 2018: 2035820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu X, Guo Y, Min X, Pei L, Chen X. Neferine, a bisbenzylisoquinoline alkaloid, ameliorates dextran sulfate sodium-induced ulcerative colitis. Am J Chin Med 2018; 46: 1263-79. [DOI] [PubMed] [Google Scholar]
- 18. Wang YJ, Lu Y, Zhou YF, et al. Therapeutic effect and mechanism of limonin on dextran sulfate sodium salt-induced ulcerative colitis in mice. Sheng Wu Jia Gong Guo Cheng 2018; 16: 57-62. [Google Scholar]
- 19. Bao CH, Wang CY, Li GN, et al. Effect of mild moxibustion on intestinal microbiota and NLRP6 inflammasome signaling in rats with post-inflammatory irritable bowel syndrome. World J Gastroenterol 2019; 25: 4696-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol 2018; 30: 1-10. [DOI] [PubMed] [Google Scholar]
- 21. Pouillon L, Bossuyt P, Peyrin-Biroulet L. Considerations, challenges and future of anti-TNF therapy in treating inflammatory bowel disease. Expert Opin Biol Ther 2016; 16: 1277-90. [DOI] [PubMed] [Google Scholar]
- 22. Chaudhary G, Mahajan UB, Goyal SN, Ojha S, Patil CR, Subramanya SB. Protective effect of Lagerstroemia speciosa against dextran sulfate sodium induced ulcerative colitis in C57BL/6 mice. Am J Transl Res 2017; 9: 1792-800. [PMC free article] [PubMed] [Google Scholar]
- 23. Kasembeli MM, Bharadwaj U, Robinson P, Tweardy DJ. Contribution of STAT3 to inflammatory and fibrotic diseases and prospects for its targeting for treatment. Int J Mol Sci 2018; 19: 2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Pan Y, Cao Y, Zhou W, Lu J. Salidroside regulates the expressions of IL-6 and defensins in LPS-activated intestinal epithelial cells through NF-κB/MAPK and STAT3 pathways. Iran J Basic Med Sci 2019; 22: 31-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D, Li H, Duan YY, et al. TL1A modulates the severity of colitis by promoting Th 9 differentiation and IL-9 secretion. Life Sci 2019; 231: 116536 [DOI] [PubMed] [Google Scholar]
- 26. Tian L, Li Y, Zhang J, Chang R, Li J, Huo L. IL-9 promotes the pathogenesis of ulcerative colitis through STAT3/SOCS3 signaling. Biosci Rep 2018; 38: BSR20181521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomas S, Hoxha K, Alexander W, et al. Intestinal barrier tightening by a cell-penetrating antibody to Bin1, a candidate target for immunotherapy of ulcerative colitis. J Cell Biochem 2019; 120: 4225-37. [DOI] [PubMed] [Google Scholar]
- 28. Wan Y, Yang L, Jiang S, Qian D, Duan J. Excessive apoptosis in ulcerative colitis: crosstalk between apoptosis, ROS, ER stress, and intestinal homeostasis. Inflamm Bowel Dis 2022; 28: 639-48. [DOI] [PubMed] [Google Scholar]
- 29. Sonninen TM, Goldsteins G, Laham-Karam N, Koistinaho J, Lehtonen Š. Proteostasis disturbances and inflammation in neurodegenerative diseases. Cells 2020; 9: 2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Debbarh H, Louanjli N, Aboulmaouahib S, et al. Antioxidant activities and lipid peroxidation status in human follicular fluid: age-dependent change. Zygote 2021; 29: 490-4. [DOI] [PubMed] [Google Scholar]
- 31. Zhao XX, Ma SB, Wen JP, et al. Reactive oxygen species-responsive polyether micelle nanomaterials for targeted treatment of ulcerative colitis. J Biomed Nanotechnol 2022; 18: 120-31. [DOI] [PubMed] [Google Scholar]