Abstract
OBJECTIVE
To evaluate the efficacy and safety of Buyang Huanwu decoction (BYHWD) in treating diabetic peripheral neuropathy (DPN).
METHODS
Eight electronic databases, including China National Knowledge Infrastructure Database, Wanfang Database, China Science and Technology Journal Database, Chinese Biomedical Literature Database, Cochrane Library, Embase, Web of Science, and PubMed, were searched for randomized controlled trials (RCTs) of BYHWD to treat DPN. We identified all RCTs related to BYHWD and those on the treatment of DPN with the combination of mecobalamin. RevMan software was used for the statistical analysis.
RESULTS
Twenty-one RCTs with a total of 1945 patients were included. The methodological quality of the literature included was low. Meta-analysis showed that the efficacy of the treatment group was significantly better than that of the control group in the treatment of DPN with BYHWD [risk ratio (RR) = 0.33, 95% CI (0.27, 0.40), Z = 11.25, P < 0.000 01]. The median nerve of median motor nerve conduction velocity (MNCV) [mean difference (MD) = 4.16, 95% CI (1.35, 6.98)] and median sensory NCV (SNCV) [(MD = 3.28, 95% CI (2.35, 4.22)] were improved in the treatment group. The MNCV in the common peroneal nerve [(MD = 1.63, 95% CI (0.39, 2.87)] and SNCV [(MD = 4.56, 95% CI (3.16, 5.97)] were significantly higher than those in the control group (P < 0.01). Plasma viscosity [(MD = -0.15, 95% CI (-0.20, -0.09), Z = 5.17, P < 0.01)], whole blood high shear [(MD = - 0.83, 95% CI (-1.56, -0.11), Z = 2.26, P = 0.02)]and whole blood low shear [(MD = - 1.61, 95% CI (-2.28, -0.94), Z = 4.68, P < 0.01)] decreased significantly after treatment. There was no significant difference in fasting blood glucose [(MD = -0.42, 95% CI (- 0.89, 0.05), Z = 1.76, P = 0.08)] between the treatment and control groups; postprandial blood glucose [(MD = -0.62, 95% CI (- 1.19, 0.05), Z = 2.12, P = 0.03)] decreased significantly. No significant difference was found in the blood lipid levels between the treatment and control groups, including triglycerides [(MD = -0.21, 95% CI (-0.52, 0.10), Z = 1.34, P = 0.18)] and cholesterol [(MD = -0.13, 95% CI (- 0.27, 0.00), Z = 1.92, P = 0.06)]. Of the 21 RCTs, only five reported adverse reactions, and four studies reported the length of follow-up. No serious adverse events were reported. None of the studies reported the quality of life and economic conditions.
CONCLUSIONS
Our study suggests that BYHWD has a significant therapeutic effect on DPN. High-quality, large-scale RCTs are needed to provide more reliable evidence.
Keywords: diabetic peripheral neuropathy, safety, treatment outcome, nerve conduction studies, hemorheology, randomized controlled trial, Meta-analysis , Buyang Huanwu decoction
1. INTRODUCTION
Diabetes is a growing public health concern worldwide, and according to data from the IDF Diabetes Atlas, the number of adults diagnosed with diabetes has tripled over the past 20 years and is predicted to increase to 700 million by 2045. With the increase in the incidence of diabetes, the number of patients suffering from diabetic complications has dramatically grown.1 Diabetic peripheral neuropathy (DPN) is one of the most common complications of diabetes. It is characterized by limb numbness, antselectric shock-like sensation, tingling, burning, drilling pain, tactile hypersensitivity, and a decrease in muscle strength by varying degrees.2 DPN increases the risk of disability due to foot amputation and ulceration. Apart from the negative impacts on the quality of life of patients with DPN, their mortality is also higher than those with DM alone.3 Data from several studies suggests that the risk of amputation in patients with diabetes is 10-20-fold higher than in those without diabetes. Furthermore, the missed diagnosis of DPN affects more than 40-60 million people worldwide and puts them at risk of lower extremity amputation.4
In recent years, there has been an increasing interest in the potential of natural medicine products, such as Traditional Chinese Medicine (TCM), to prevent and treat chronic diseases. Buyang Huanwu decoction (补阳还五汤, BYHWD) was first recorded in a famous Chinese medical book, Yi Lin Gai Cuo,5 by Wang Qingren. The decoction is composed of Huangqi (Radix Astragali Mongolici), Danggui (Radix Angelicae Sinensis), Chuanxiong (Rhizoma Chuanxiong), Honghua (Flos Carthami), Taoren (Semen Persicae), Chishao (Radix Paeoniae Rubra), and Dilong (Pheretima Aspergillum). BYHWD has a therapeutic effect on patients with blood stasis of Qi and Yin deficiency. Previous clinical trials and animal experiments have confirmed that BYHWD can lower blood sugar, significantly improve conduction function, promote the regeneration and repair of neurons, and reduce nerve cell damage.6,7 However, limited systematic reviews have been conducted. BYHWD is a derivative of vitamin B12, which nourishes and repairs nerves, promotes the synthesis of nucleic acids and proteins, improves the metabolism of neurons and Schwann cells, and restores nerve conduction function. Alprostadil can improve nerve microcirculation, and lipoic acid has an antioxidant effect. Therefore, mecobalamin has been used as a control in many studies.
We aimed to collect randomized controlled trials (RCTs) about BYHWD versus mecobalamin for DPN based on evidence-based medicine and conduct a Meta-analysis of its efficacy.
2. MATERIALS AND METHODS
2.1. SEARCH STRATEGY
The following electronic databases were searched for relevant RCTs: China National Knowledge Infrastructure Database, China Science and Technology Journal Database, Chinese Biomedical Literature Database, Wanfang Database, Web of Science, PubMed, Embase, and the Cochrane library. We searched for all trials published before July 9, 2022. We searched the databases in both Chinese and English, as detailed in supplementary Table 1. To include unpublished studies, we also searched the website of the international clinical trial registry provided by the United States National Institutes of Health (available at http://clinicaltrials.gov/) and the Chinese Clinical Trial Registry (available at http://www.chictr.org.cn/index.aspx). The search was conducted independently by two researchers. There were no language, country, or region restrictions.
2.2. Study selection
All studies were RCTs related to BYHWD or modified BYHWD, which were conducted to add or reduce drugs based on BYHWD according to symptoms. Baseline data, if available, were included in the study. No sex, age, or ethnicity restrictions were used.
All included trials met the following selection criteria: (a) Diagnostic criteria of the study subjects: All patients need to be clearly diagnosed with diabetes mellitus. If patients had clinical symptoms such as pain, numbness, and paresthesia and one of the five abnormalities in ankle reflex, acupuncture pain, vibration, pressure, temperature, or no clinical symptoms yet had two out of the five mentioned abnormalities, DPN could be considered to be diagnosed;2 (b) Study type: RCTs; (c) Intervention measures: the control group could be blank, placebo, or mecobalamin. The treatment group should have been treated with BYHWD orally. Routine diabetes education, reasonable diet, exercise therapy, blood glucose control, blood pressure control, and other routine treatment were used in two groups; (d) Intervention time: no less than six weeks; (e) Others: there were no restrictions on the language used. The exclusion criteria were as follows: (a) Allocation methods used in semi-RCTs, such as odd and even number, order of admission or visit, date of birth, number of case records, and different treatment plans; (b) Repeated or identical articles; (c) Articles which did not report relevant outcome indicators or the full text could not be obtained; (d) Interventions such as fumigation, foot bath therapy, acupuncture, acupoint injection, electro-acupuncture, massage, and iontophoresis of TCM were excluded. RCTs that reported patients with neuropathy and severe arteriovenous vascular disease caused by another inducement were also excluded.
The main outcome indicators included the total effective treatment rate and nerve conduction velocity (NCV). The total effective rate was calculated by referring to the guiding principles for clinical research of new Chinese medicines.8 The nerve condition of patients was evaluated by the motor NCV (MNCV) and the sensory NCV (SNCV); the higher the score, the better the improvement effect. The efficacy scores were evaluated based on the nimodipine method: the efficacy index (n) = the point reduction before and after treatment / the points before treatment × 100%. Significant improvement in clinical symptoms and signs and a score reduction of no less than 70% indicates a great effect in treating clinical symptoms and that signs have been improved significantly. A score reduction of no less than 30% indicates a good effect.
If the clinical symptoms and signs are close to or worse than before treatment and the score reduction is less than 30%, it is deemed invalid. Secondary outcome indicators include hemorheology, fasting blood glucose (FPG) or 2 h postprandial blood glucose (2hPG), and blood lipids [total cholesterol, triglycerides (TG)].
2.3. Data extraction
We extracted information from at least three studies using a form to verify its applicability. Two independent reviewers (TANG Yiting and CHEN Yupeng) extracted the following information from the studies: organizational aspects (including reviewer’s name, the first author of the articles, year of publication, publication source), trial characteristics (design of the study, number of participants, number of groups, methods of randomization, methods of allocation concealment, blinding, primary aims of the study), participants (age, ethnicity, sex, diagnosis, concurrent conditions), interventions and controls (name of the intervention, length of treatment, type and name of control, additional treatment), outcome measurements (type of outcome, definition of the outcome, time point for assessment, length of follow-up), results (name of the outcome, mean, standard deviation, events observed after the intervention, total sample size), and other research information. If there was a disagreement between the two reviewers, a consensus was achieved by discussion among all reviewers.
2.4. Quality assessment
Two researchers (HE Puyu and ZHAO Liming) independently assessed the quality of the literature. According to the seven items in the Cochrane Handbook, 5.1 Bias Risk Assessment,9 independent literature quality evaluation includes the random sequence generation method, allocation plan hiding, blinding (subjects, trial personnel), blinding (outcome evaluator), incomplete outcome reports, selective outcome reports, and other sources of bias. We filled in the results according to three levels: low risk, high risk, and unclear. Any dispute was resolved through discussion or by referring to the opinion of a third-party researcher (NI Qing).
2.5. Statistical analysis
The relative risk (RR), MD, and their corresponding 95% confidence intervals (95% CI) were calculated using RevMan software version 5.3 for dichotomous and continuous outcomes, respectively. The I 2 test was used to evaluate the heterogeneity. When P was ≥ 0.1 and I 2 ≤50%, this indicated that there was no obvious heterogeneity between clinical studies, and the fixed effects model was adopted; when P was < 0.1, and I 2>50%, this indicated that there was a significant difference between the clinical studies. Meta-analysis was carried out according to sex, age, doses of BYHWD, and basic medication of the subjects to explore the source of heterogeneity, and subgroup analysis was carried out after clarifying the source of heterogeneity; when there was still obvious heterogeneity, the random effect model was selected. Sensitivity analysis was used to determine the stability of the results. When the number of research articles was greater than 10, a funnel plot was used to assess the publication bias.
3. RESULTS
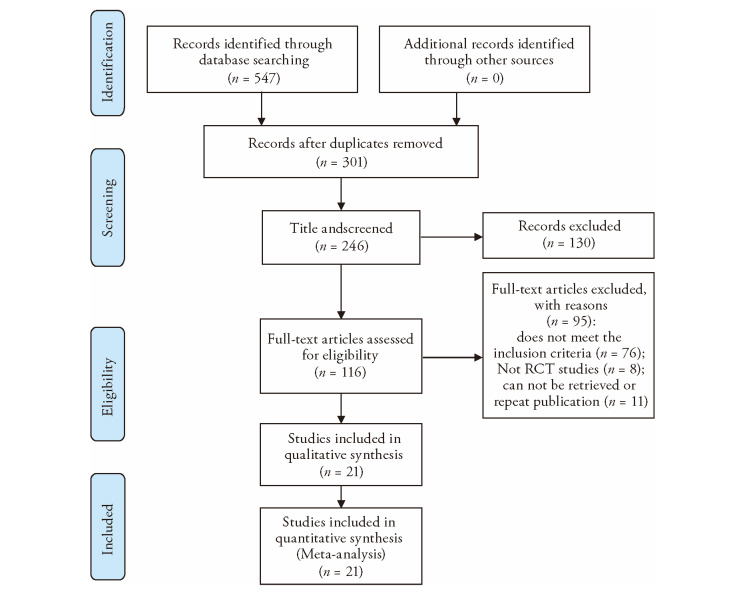
In our initial literature search, 633 articles were found in eight electronic databases, of which 277 were repeated, and 356 were retained. After reading the titles and abstracts, 131 articles out of 356 were excluded because they were retrospective research, experimental research, experience, theoretical discussion, systematic review, and literature review based studies. A total of 146 articles underwent full text screening, among which 125 were excluded for the following reasons: the participants did not meet the inclusion criteria (n = 100), repetition or non-retrieval (n = 12), and non-RCTs (n = 13). Finally, 21 RCTs met our eligibility criteria and were included in the systematic evaluation (Figure 1).
Figure 1. Flow diagram.
3.1. Characteristics of the included trials
We included 21 trials,10,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓,⇓-30 which all were RCTs with two parallel arms. All trials were conducted or published before July 9, 2022, in China. The characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of the trials included in the Meta-analysis
| Source | Sample | Sex | Age (years) | Intervention | Treatment | DN | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| (T/C) | (male | period | duration | measure | |||||
| /female) | Treatment group | Control group | (weeks) | (years) | |||||
| Ning RZ et al 202010 | 30/30 | T:13/17 | T:62.53±3.67 | Modified BYHWD (150 mL, bid)+ Mecobalamine (500 μg tid, po)+BT | Mecobalamine (500 μg tid, po)+BT | 12 | T:1.83 ± 1.78 | CER | |
| C:16/14 | C:61.43±4.29 | C: 2.2 ± 1.17 | |||||||
| Han Y 200518 | 30/20 | Not reported | Not reported | Modified BYHWD (bid)+BT | Mecobalamin (500 μg, tid, po)+BT | 14 | Not reported | NCV+Hemorheology | |
| Gong LZ et al 201719 | 60/60 | Not reported | Not reported | Modified BYHWD (200 mL, bid) | Mecobalamin (1 mg,qd, iv)+BT | 8 | Not reported | NCV | |
| +BT | |||||||||
| Wu Y, Ma D 201520 | 60/60 | T:27/33 | T:45-75 | Modified BYHWD+ BT | Mecobalamin (0.5-1 mg, qd, iv)+BT | 12 | T:0.25-3 | CER+NCV | |
| C: 32/28 | C: 48-74 | Mecobalamin (0.5-1 mg, qd, iv) | C: 0.42-3 | ||||||
| Sun SZ, Wang YZ 200821 | 42/42 | T:23/19 | T:44-73 | BYHWD (250 mL, bid)+Mecobalamin | Mecobalamin (0.5 mL, qd, im)+BT | 12 | T:0.8-13.5 | CER+NCV+ Hemorheology | |
| C: 22/20 | C: 45-72 | (0.5 mL, qd, im)+BT | C: 0.6-13.6 | ||||||
| Zhang T 200822 | 60/60 | T:34/26 | T:54.6±5.9 | Modified BYHWD (bid)+BT | Mecobalamin (500 μg, qd, im)+BT | 12 | T:0.5-3 | CER+NCV | |
| C: 32/28 | C: 54.8±5.8 | C: 0.5-3 | |||||||
| Sun BX, Li LQ 201723 | 47/47 | T:24/23 | T:59.64±10.51 | Modified BYHWD (bid)+BT | Mecobalamin (0.5 mg, qd, im)+BT | 12 | T:6.87±3.41 | CER | |
| C: 22/25 | C: 59.35±10.33 | C: 7.05±3.81 | |||||||
| Yang YQ, Xing DZ 201924 | 38/38 | T:19/19 | T:67.91±2.18 | Modified BYHWD (250 mL, bid) + Mecobalamin (0.5-1 mg, qd,im) | Mecobalamin (0.5-1 mg, qd, im) | 12 | Not reported | CER | |
| C: 18/20 | C: 68.59±2.12 | ||||||||
| Liu B 201811 | 41/41 | T:22/19 | T:59.85±2.82 | Modified BYHWD (200 mL, bid)+ Mecobalamine (0,5 mg, tid, po) + BT | Mecobalamine (0.5 mg, tid, po)+BT | 8 | Not reported | CER+NCV | |
| C: 21/20 | C: 59.35±2.76 | ||||||||
| Pan XJ 201712 | 30/30 | T:13/17 | T:50.74±9.25 | Modified BYHWD (150 mL, bid) + | Mecobalamine (500 μg, tid, po)+BT | 12 | T:8.45±1.26 | CER+NCV+Hemorheology | |
| C: 16/14 | C: 49.62±9.83 | Meco-balamine (500 μg, tid, po) + BT | C: 8.72±1.35 | ||||||
| Li YF 200913 | 36/36 | T:20/16 | T:42-69 | Modified BYHWD (bid) + BT | Mecobalamine (500 μg, tid, po)+BT | 8 | Not reported | CER+NCV+Hemorheology | |
| C: 16/20 | C: 41- | ||||||||
| Peng H 200914 | 30/30 | T:18/12 | T:52.7±11.5 | Modified BYHWD (200 mL, bid) +Mecobalamine (0.5 mg, tid, po)+BT | Mecobalamine (0.5 mg, tid, po)+BT | 12 | T:6.52±1.21 | CER+ NCV+Hemorheology | |
| C: 14/16 | C: 50.8±12.4 | C: 6.46±1.12 | |||||||
| Luo XY 200715 | 40/40 | T:22/18 | T:66.68±9.82 | BYHWD (100 mL, bid)+Mecobalamine ( | Mecobalamine (500 μg, tid, po)+BT | 8 | Not reported | CER +NCV+Hemorheology | |
| C: 21/19 | C: 66.9±10.53 | 500 μg, tid, po)+BT | |||||||
| Wang SX et al 200516 | 130/78 | T:78/52 | T:56.6±6.3 | Modified BYHWD (bid)+BT | Mecobalamine (500 μg, tid, po)+BT | 8 | T:2.3±1.2 | CER+ NCV+Hemorheology | |
| C: 45/33 | C: 55.8±6.5 | C: 2.3±1.5 | |||||||
| Liu CH et al 200317 | 42/26 | T:22/10 | T:55.6±4.5 | Modified BYHWD (100 mL, bid)+BT | Blank+BT | 8 | Not reported | CER+ NCV+Hemorheology | |
| C: 14/12 | C: 57.8± 3.4 | ||||||||
| CuiY, Pan MX 200425 | 100/92 | T:58/42 | T:39-73 | Modified BYHWD (200 mL, bid)+ Meco- | Mecobalamine (500 μg, qod, im) +BT | 8 | T:0.25-10 | CER+ NCV+Hemorheology | |
| C: 52/40 | C: 37-70 | balamine (500 μg, qod, im) +BT | C: 0.25-11 | ||||||
| Luo XY 200715 | 40/40 | T:22/18 | T:66.68±9.82 | BYHWD (100 mL, bid)+Mecobalamine | Mecobalamine (500 μg, tid, po)+BT | 8 | Not reported | CER +NCV+Hemorheology | |
| C: 21/19 | C: 66.9±10.53 | 500 μg, tid, po)+BT | |||||||
| Zhuang HZ 201126 | 30/30 | T:14/16 | T:54.1±3.7 | Modified BYHWD (bid)+Mecobalamine | Mecobalamine (500 μg, qd, im)+BT | 8 | T:4.8±2.2 | CER | |
| C:16/14 | C:55.1±3.9 | (500 μg,qd, im) +BT | C:5.1±3.3 | ||||||
| Liu JG 201527 | 54/54 | T:28/26 | T:62.07±6.93 | Modified BYHWD (200 mL, bid)+Mecob- | Mecobalamine (0.5 mg, tid, po)+BT | 12 | T:5.45±2.81 | CER | |
| C:29/25 | C:61.58±6.52 | alamine (0.5 mg, tid, po) +BT | C:5.16±2.70 | ||||||
| Ji TC 201428 | 32/31 | T:18/14 | T:40-73 | Modified BYHWD (bid)+ Mecobalamine (500 μg, tid)+BT | Mecobalamine (500 μg, tid)+BT | 6 | T:1-15 | CER | |
| C:16/15 | C:41-74 | C:1-14 | |||||||
| Jiao FE et al 201329 | 56/52 | T:30/26 | T:40-72 | Modified BYHWD (bid)+ Mecobalamine (500 μg, tid)+BT | Mecobalamine (500 μg, tid,)+BT | 8 | T:0.33-8.5 | CER | |
| C:31/21 | C:42-70 | C:0.25-8 | |||||||
| Jiang ZS et al 200530 | 30/30 | T:18/12 | T:48.6±9 | Modified BYHWD (200 mL, bid) + Mecobalamine (first four weeks, im; | Mecobalamine (first four weeks, im; last four weeks, po)+BT | 8 | T:13.6± 8.3a | CER+NCV | |
| C:16/14 | C:50±10.8 | last four weeks, po)+BT | C:13± 9.8a | ||||||
Notes: T:treatment group; C:control group; CER: clinical effectiveness; NCV: nerve conduction velocity; BYHWD: Buyang Huanwu decoction; BT:basic treatment; bid: bis in die; qd: quaque die; tid: ter in die; a: month.
In this Meta-analysis, there were 1945 DPN patients: 1018 in the treatment group and 927 in the control group, all of whom came from hospital wards or outpatient clinics. The sample sizes ranged from 20 to 130. Most patients were aged between 37 and 73 years. Six studies12,18,20,⇓-22,30 recruited patients with type 2 diabetes mellitus, while the remaining 15 studies 10-11,13,⇓,⇓,⇓-17,19,23,⇓,⇓,⇓,⇓,⇓-29did not report the type of diabetes.
The World Health Organization diabetes mellitus diagnostic criteria (1999 or 2010 or not indicated) were mentioned in 13 studies;11,13,14,16,18,⇓,⇓,⇓-22,26,28,⇓-30 two studies17,25used the International Diabetes Federation diagnostic criteria (1997); one study12used the Chinese Diabetes Society diagnostic criteria (2013); two other studies10,23 used the WTO or Chinese guidelines for the prevention and treatment of diabetes (2017), and the remaining studies15,24,27included no diagnostic criteria.
In the treatment group, BYHWD alone was used in six trials;13,16,17,19,22,23 modified BYHWD was used in 15 trials;10,⇓-12,14,15,18,20,21,24,⇓,⇓,⇓,⇓,⇓-30 six trials13,16,17,19,22,23 used BYHWD or modified BYHWD in the treatment group and 14 trials used mecobalamin on te premise of BYHWD or modified BYHWD in the control group, except for in one study17 which was blank.
Our study divided the clinical effective rate into effective and ineffective according to whether the symptoms improved. NCV and the total effective rate of treatment were also used as the main outcome indicators, and hemorheology, blood glucose, and blood lipids as the secondary outcome indicators. Only five studies reported the occurrence of adverse events. Withdrawal and loss to follow-up were recorded in four studies, and the effects on quality of life and economic loss were not reported in any of the studies.
3.2. Methodological quality assessment
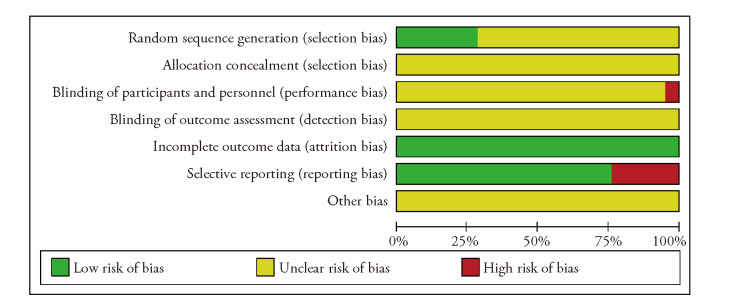
Quality was evaluated according to the Cochrane risk assessment criteria bias. Among the 21 RCTs, six RCTs were randomly grouped using the random number table method, and “random” grouping was only mentioned in the remaining 15 RCTs.
Allocation concealment was not mentioned in any of the studies. None of the 20 RCTs was a double-blind and placebo-controlled trial. One RCT was reported as “single-blind”. All 21 RCTs provided complete baseline data. Five studies were not reported according to the established plan, and sample size estimation methods for other biases were not reported in 21 RCTs. The quality of all the studies was very low, which affected the subsequent confidence intervals. This suggests that the follow-up results should be treated objectively (Figure 2).
Figure 2. Quality assessment of the trials included in the Meta-analysis.
3.3. Primary outcomes
3.3.1. Efficacy
This study showed a significant difference in clinical efficacy between the treatment and control groups. The heterogeneity test showed that the heterogeneity was small between the groups (P = 0.68, I 2 = 0). Therefore, the fixed effect model was selected for analysis. The efficacy in the treatment group was better than that in the control group [(RR = 0.33, 95% CI (0.27, 0.40), Z = 11.25, P < 0.000 01)]. To compare the clinical efficacy of BYHWD and modified BYHWD with that of the control group, we conducted a subgroup analysis. Compared with the control group, there was a significant difference between the BYHWD alone and control groups [(n = 682, RR = 0.34, 95% CI (0.25, 0.45), Z = 7.15, P < 0.00001)], and there was no heterogeneity (χ2 = 5.53, P = 0.35, I 2 = 10%). Modified BYHWD plus conventional Western Medicine had a significant difference compared with the control group [(n = 1213, RR = 0.33, 95% CI (0.25, 0.42), Z = 8.71, P < 0.000 01)], and there was no heterogeneity (χ2 = 10.15, P = 0.68, I 2 = 0%). The analysis also concluded that BYHWD plus mecobalamin could significantly increase the efficacy rate compared to mecobalamin alone (supplementary Figure 1).
3.3.2. NCVs
As a continuous variable, the NCV showed great heterogeneity between the two groups. We performed a subgroup analysis of the BYWHD/modified BYHWD and control groups.
Seven trials,12,13,16,17,20,23,25 containing a total of 742 patients, regarded median MNCV [(MD = 4.16, 95% CI (1.35, 6.98), Z = 2.90, P < 0.01)] as the main outcome. The heterogeneity between the two groups was large (χ2 = 78.31, P < 0.01, I 2 = 94%), so a random effect model was used for the analysis. Four trials were conducted between BYHWD alone and control groups [(n = 370, MD = 3.85, 95% CI (-2.85, 9.86), Z = 1.26, P = 0.21)], with heterogeneity (χ2 = 54.63, P < 0.01, I 2 = 96%). Three trials were conducted between modified BYHWD plus conventional western medicine and control groups [(n = 372, MD = 4.57, 95% CI (1.90, 7.23), Z = 3.36, P < 0.01)], with heterogeneity (χ2 = 16. 48, P < 0. 01, I 2 = 88%).
Nine trials,12,⇓,⇓,⇓,⇓-17,20,23,25 involving a total of 954 patients revealed median SNCV [(MD = 3.28, 95% CI (2.35, 4.22), Z = 6.86, P < 0.01)]as the main outcome. The heterogeneity between the two groups was large (χ2 = 42.65, P < 0.01, I 2 = 81%), so a random-effects model was used for the analysis. Four trials were included in the subgroup analysis to observe the improvement in median SNCV [(n = 442, MD = 4.16, 95% CI (2.02, 6.29), Z = 3.81, P < 0.01)] between BYHWD alone and the control group, with heterogeneity (χ2 = 19.09, P < 0.01, I 2 = 84%). Five trials were conducted between modified BYHWD plus conventional western medicine and the control group [(n = 512, MD = 2.74, 95% CI (1.61, 3.86), Z = 4.76, P < 0.01)], with heterogeneity (χ2 = 21.21, P < 0.01, I 2 = 81%).
Eleven trials,12,⇓,⇓,⇓,⇓-17,20,21,23,25,30 involving a total of 1098 patients revealed peroneal MNCV [(MD = 1.63, 95% CI (0.39, 2.87), Z = 2.58, P = 0.010)]as the main outcome. The heterogeneity between the two groups was large (χ2 = 91.79, P < 0.01, I 2 = 89%), so a random random-effects model was used for the analysis. Four trials were conducted to observe the improvement of peroneal MNCV [(n = 442, MD = 0.96, 95% CI (-2.29, 4.22), Z = 0.58, P = 0.56)] between BYHWD alone and the control group, with heterogeneity (χ2 = 42.57, P < 0.01, I 2 = 93%).
Seven trials were included in a subgroup analysis between modified BYHWD plus conventional western medicine and control groups [(n = 656, MD = 1.97, 95% CI (0.60, 3.33), Z = 2.82, P < 0.01)], with heterogeneity (χ2 = 48.46, P < 0.01, I 2 = 88%).
Nine trials,12,13,16,17,20,21,23,25,30 involving a total of 958 patients revealed peroneal SNCV [(MD = 4.56, 95% CI (3.16, 5.97), Z = 6.36, P < 0.01)] as the main outcome. The heterogeneity between the two groups was large (χ2 = 52.56, P < 0.01, I 2 = 85%), so a random random-effects model was used for analysis. Four trials were included in a subgroup analysis to observe the improvement in peroneal SNCV [(n = 442, MD = 5.18, 95% CI (2.08, 8.29), Z = 3.27, P = 0.0001)] between BYHWD alone and the control group, with heterogeneity (χ2 = 41.05, P < 0.01, I 2 = 93%). Five trials were included in a subgroup analysis between modified BYHWD plus conventional western medicine and control group [(n = 516, MD = 4.15, 95% CI (2.95, 5.35), Z = 6.80, P < 0.01)], with heterogeneity (χ2 = 10.12, P = 0.04, I 2 = 60%).
The analysis also concluded that BYHWD plus mecobalamin could significantly improve NCV compared to mecobalamin alone.
3.4. Secondary outcomes
3.4.1. Hemorheology
Eight trials12,⇓,⇓-15,17,18,21,25assessed the variation in hemorheology. This Meta-analysis mainly evaluated the changes in plasma viscosity, whole blood high shear, and whole blood low shear. The heterogeneity test revealed that the heterogeneity in plasma viscosity was large (χ2 = 20.97, P = 0.004, I 2 = 67%), so a random effect model was used for the analysis. The difference in plasma viscosity was statistically significant between the two groups [(n = 676, MD =-0.15, 95% CI (-0.20, -0.09), Z = 5.17, P < 0.01)].
Four trials12,⇓,⇓-15 assessed the variation in whole blood high shear, and the heterogeneity was large (χ2 = 90.83, P < 0.01, I2 = 97%), so a random-effects model was used for the analysis. The difference in whole blood high shear was statistically significant between the two groups [(n = 272, MD = -0.83, 95% CI (-1.56, -0.11), Z = 2.26, P = 0.02)].
Four trials12,⇓,⇓-15 assessed the variation in whole blood low shear, and the heterogeneity was large (χ2 = 21.95, P < 0.01, I2 = 86%), so a random-effects model was used for the analysis. The difference in whole blood low shear was statistically significant between the two groups [(n = 272, MD = -1.61, 95% CI (-2.28, -0.94), Z = 4.68, P < 0.01)].
3.4.2. Glycemic control
Eight trials12,⇓-14,18,21,23,24,30evaluated the level of fasting blood glucose changes, with high heterogeneity (χ2 = 74.04, P < 0.01, I 2 = 91%). Therefore, a random-effects model was used for the statistical analysis. There was no significant statistical difference between the treatment and control groups [(n = 556, MD = -0.42, 95% CI (-0.89, 0.05), Z = 1.76, P = 0.08)].
Five trials12,⇓-14,23,24 evaluated the changes in 2 h postprandial blood glucose, with great heterogeneity (χ2 = 40.76, P < 0.01, I 2 = 90%). Therefore, a random-effects model was used for the statistical analysis. There was a statistically significant difference between the treatment and control groups [(n = 362, MD = -0.62, 95% CI (-1.19, -0.05), Z = 2.12, P = 0.03)].
3.5. Adverse and other effects
Adverse reactions were reported in five trials.18,19,21,22,24 Three trials18,19,21 indicated that there were no adverse reactions in the two groups. One trial22reported that some patients had stomach discomfort, but this symptom was relieved in the treatment and observation groups. Furthermore, one experiment24 reported that patients had a mild headache, stomach discomfort, and rash in both the treatment and observation groups. Therefore, the safety of Chinese herbal medicines cannot be evaluated. The length of follow-up was reported in two trials.21-22
Publication bias was evaluated using funnel plots. The shape of the clinical efficacy and MNCV funnel plots were not completely symmetrical, indicating that publication bias may exist (Figure 3).
Figure 3. Funnel plot for assessing publication bias.
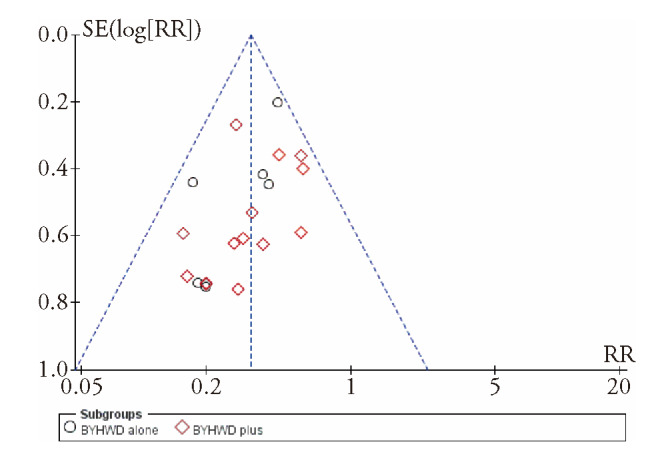
SE: standard error; RR: relative risk; BYHWD: Buyang Huanwu decoction.
3.6. Sensitivity and subgroup analysis
Sensitivity analysis was used to explore the sources of heterogeneity. To investigate specific factors affecting the overall efficacy of RCTs, we performed a subgroup analysis on the treatment duration, sample size, blank or placebo effects, and DPN duration. There was much overlap and difference in confidence intervals and overall risk ratios for MNCV. All I 2 values were more than 50%, indicating a high heterogeneity. There was a significant difference (P < 0.05) in the overall risk ratios for all subgroup analyses. We conducted a sensitivity analysis by removing the trials one by one and found that the I 2 became 0% after removing one trial;16 the data before and after this adjustment were RR = -0.4, 95% CI (-3.3, 2.49), P < 0.01, and RR = 1.67, 95% CI (1.16, 2.18), P = 0.71, respectively (supplementary Table 1).
After reading the full text, we found that heterogeneity may originate from an unclear course of DPN, unclear random methods, and no reports of missing data or visits.
4. DISCUSSION
4.1. Analysis of efficacy
This Meta-analysis showed that the efficacy of BYHWD in the treatment of DPN was significantly better than that in the control group, indicating that BYHWD has a therapeutic effect on DPN. One clinical study showed that the efficacy of BYHWD in the treatment of diabetes was better than that in the control group [Liuwei Dihuang pills (六味地黄丸)] (P < 0.05). There appear to be significant differences in the treatment of patients with diabetes from Qi deficiency and blood stasis in terms of improving symptoms or lowering blood sugar.31
It has been shown that the mechanism of BYHWD in treating DPN is to increase the activity of superoxide dismutase (SOD), improve the function of mitochondria, increase the production of adenosine triphosphate, improve the symptoms of Qi deficiency, resist the damage to blood vessels caused by free radical reactions, indirectly inhibit platelet aggregation, and improve the symptoms of blood stasis. It has a good effect on antioxidant injury and a protective effect on Schwann cell injury induced by H2O2. It can alter the expression of adhesion molecules in vascular endothelial cells and participate in the growth and differentiation of nerve cells.32,⇓-34
NCVs were included as the main outcome indicators. Most studies provided NCVs, but the observation sites were not consistent and included the superficial peroneal, tibial, and posterior tibial nerves. Only about half of the studies observed the conduction velocity of the median and peroneal nerves.
In the included trials, plasma viscosity was only observed in eight trials, and whole blood high shear and low shear were observed in four experiments. We found significant differences in the decrease in plasma viscosity, whole blood high shear degree, and whole blood low shear degree. However, our study was limited, which might have adversely affected the improvement of hemorheology by BYHWD. Modern pharmacological studies have shown that Huangqi (Radix Astragali Mongolici) can reduce the platelet adhesion rate, inhibit platelet aggregation, and prevent thrombosis, thereby reducing whole blood and plasma viscosity. Components of BYHWD, such as sodium ferulate in Danggui (Radix Angelicae Sinensis) and paeonol in Chishao (Radix Paeoniae Rubra), have obvious effects on anti-platelet aggregation and improving microcirculation. Ligustrazine in Chuanxiong (Rhizoma Chuanxiong) can dilate blood vessels and significantly improve microcirculation, reducing platelet surface activity and inhibiting platelet aggregation. Moreover, Taoren (Semen Persicae), Honghua (Flos Carthami), and Dilong (Pheretima Aspergillum) have strong analgesic, anti-inflammatory, and inhibitory effects on platelet aggregation.35,⇓,⇓,⇓-39 Eight trials evaluated the changes in fasting blood glucose, and five trials assessed the changes in 2 h postprandial blood glucose; these trials found that there was a significant improvement in these two factors.
Although our Meta-analysis showed no significant improvement in the fasting blood glucose of DPN in BYHWD treatment between the treatment and control groups, we could not evaluate the efficacy of blood sugar well.
Triglyceride and hypercholesterolemia levels were evaluated in five trials, and there was no significant difference between the treatment and control groups.
All studies did not include the follow-up period and did not evaluate the life quality of patients. Therefore, the long-term efficacy of BYHWD could not be evaluated.
4.2. Quality of the evidence and potential biases
Most of the studies included in this study were small-sample RCTs, and only two trials included more than 100 cases. The samples in 19 RCTs were small, and sample size estimation methods were not reported in 21 RCTs. The methodological quality of all trials was very low. The random number table method for the grouping was mentioned in six RCTs. “Random” was mentioned in only 15 RCTs, but the specific method of randomization was not detailed. Allocation concealment was not mentioned in any of the studies, which may have caused selection bias. Blinding was not reported in any of the studies. However, it is difficult for TCM decoctions to implement a blind method for participants, and BYHWD is not standardized, so it is inevitable to trigger bias to some extent.
Sensitivity and subgroup analyses were performed to test the stability of the results. For the main outcome, MNCV, a random-effects model was used for the Meta-analysis, not a fixed effects model, since I 2 > 50%. The sources of heterogeneity were analyzed regarding the treatment time, sample size, blank or placebo adoption, and disease course. Except for one study, it was found to be consistent with the results of previous studies, indicating that this Meta-analysis was stable.
The funnel plot of clinical efficiency was asymmetrical, so we checked all the studies. However, no negative tests were found. This could be due to all the studies having been published in China or that negative results were not published, indicating the existence of publication bias. Furthermore, the patients and researchers were aware of NCV as the main outcome index, which may have led to bias. Based on the above, we should be cautious regarding the methodological quality evaluation of this study.
4.3. Limitations
There are several potential limitations to this Meta-analysis. First, the duration of the RCTs included did not exceed 12 weeks, and most studies did not mention the follow-up period, which made it impossible to assess the long-term quality of life changes. Second, adverse events were largely unreported in the trials included. Third, there was no sample size calculation in all studies. Fourth, although BYHWD was taken twice a day in all the trials, the period, dose, and composition were inconsistent, which could have affected the reliability of this Meta-analysis. Fifth, the studies included were limited, as all were conducted in China with a limited number of cases and inconsistent controls. Finally, the research protocols were not always published, which may lead to overestimation and underestimation of the results and affect the reliability.
In conclusion, this Meta-analysis of RCTs showed that BYHWD could improve the neurological symptoms of patients with DPN, increase the NCV, reduce the indices of hemorheology, and have a significant therapeutic effect on DPN. Due to the low quality of the included trials, our findings may be biased. Therefore, high-quality and large-scale RCTs are needed to evaluate the efficacy and safety of BYHWD in the treatment of DPN more thoroughly.
5. SUPPORTING INFORMATION
Supporting data to this article can be found online at http://journaltcm.com.
Contributor Information
Bing PANG, Email: 307636788@qq.com.
Qing NI, Email: niqing669@163.com.
REFERENCES
- 1. Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378: 31-40. [DOI] [PubMed] [Google Scholar]
- 2. Chinese Diabetes Society . Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition). Zhong Guo Tang Niao Bing Za Zhi 2018; 10: 4- 67. [Google Scholar]
- 3. Lee CC, Perkins BA, Kayaniyil S, et al. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: The PROMISE Cohort. Diabetes Care 2015; 38: 793-800. [DOI] [PubMed] [Google Scholar]
- 4. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res Clin Pract 2019; 157: 107843. [DOI] [PubMed] [Google Scholar]
- 5. Wang QR (Qing dynasty), Yi Lin Gai Cuo. Beijing: China Medical Science and Technology Press, 2011: 1- 67. [Google Scholar]
- 6. Xu S, Hua WJ. Research progress of Buyang Huanwu decoction in the treatment of diabetic peripheral neuropathy. Zhong Yi Xue 2020; 9: 92-7. [Google Scholar]
- 7. Pan R, Cai J, Zhan L, et al. Buyang Huanwu decoction facilitates neurorehabilitation through an improvement of synaptic plasticity in cerebral ischemic rats. BMC Complement Altern Med 2017; 17: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng XY. Guiding principles for clinical research of new drugs of Traditional Chinese Medicine. Beijing: China Medical Science and Technology Press, 2002: 233- 7. [Google Scholar]
- 9. Higgins JPT, Green S. Corchrane Reviewers’ Handbook 5.2 [updated March 2013], ReviewManager (RevMan) [Computer program]. Version 5.2; 2013. [Google Scholar]
- 10. Ning RZ, Zhang TY, Ma J. Effect of modified Buyang Huanwu decoction on diabetic peripheral neuropathy and insulin-like growth factor. Zhong Yi Yao Xue Bao 2020; 48: 41-4. [Google Scholar]
- 11. Liu B. Therapeutic effect of modified Buyang Huanwu ecoction on diabetic peripheral neuropathy of Qi deficiency and blood stasis type. Tang Niao Bing Xin Shi Jie 2018; 21: 179-80. [Google Scholar]
- 12. Pan XJ. Therapeutic effect of Buyang Huanwu decoction on T2DM peripheral neuropathy of Qi deficiency and blood stasis type. Yunnan: Yunnan university of Chinese medicine, 2017: 1-51. [Google Scholar]
- 13. Li YF. Clinical study of modified Buyang Huanwu decoction in the treatment of diabetic peripheral neuropathy. Guangxi: Guangxi university of Chinese medicine, 2009: 1-55. [Google Scholar]
- 14. Peng H. Clinical observation on the treatment of diabetic peripheral neuropathy with modified Buyang Huanwu decoction. Hubei: Hubei University Of Chinese Medicine, 2009: 1-36. [Google Scholar]
- 15. Luo XY. Clinical study on Buyang Huanwu decoction in the treatment of type 2 diabetic peripheral neuropathy. Hubei: Hubei University Of Chinese Medicine, 2007: 1-42. [Google Scholar]
- 16. Wang SX, Chen L, Ma XJ. 130 cases of diabetic peripheral neuropathy treated with modified Buyang Huanwu decoction. Zhong Guo Zhong Yi Yao Xin Xi Za Zhi 2005; 12: 60-1. [Google Scholar]
- 17. Liu CH, Fan GJ, Tang XY. Clinical observation on 42 cases of diabetic peripheral neuropathy treated with Buyang Huanwu decoction. Sichuan Zhong Yi 2003; 21: 31-2. [Google Scholar]
- 18. Han Y. Treatment of 30 cases of type 2 diabetic peripheral neuropathy with Buyang Huanwu decoction: a control study of 20 cases treated with Mecobalamin tablets. Zhejiang Zhong Yi Za Zhi 2005; 40: 430-1. [Google Scholar]
- 19. Gong LZ, Li YF, Zhang JX, Zhang YP, Qiao XY, Zhang J. Clinical observation on the treatment of diabetic peripheral neuropathy with Buyang Huanwu decoction. Shi Yong Zhong Yi Yao Za Zhi 2017; 33: 1364-5. [Google Scholar]
- 20. Wu Y, Ma D. Clinical observation of Buyang Huanwu decoction combined with mecobalamin injection in the treatment of diabetic peripheral neuropathy. Lin Chuang He Li Yong Yao Za Zhi 2015; 8: 34-5. [Google Scholar]
- 21. Sun SZ, Wang YZ. Clinical observation on 42 cases of diabetic peripheral neuropathy treated by Buyang Huanwu decoction combined with mecobalamin. Jiangsu Zhong Yi Yao 2008; 40: 48-9. [Google Scholar]
- 22. Zhang T. Buyang Huanwu decoction in treating 60 cases of diabetic peripheral neuropathy. Tianjin Zhong Yi Yao 2008; 25: 216. [Google Scholar]
- 23. Sun BX, Li LQ. Clinical effect of Buyang Huanwu decoction on diabetic peripheral neuropathy. Ji Yin Zu Xue Yu Ying Yong Sheng Wu Xue 2017; 36: 3398-402. [Google Scholar]
- 24. Yang YQ, Xing DZ. Clinical study of modified Buyang Huanwu decoction combined with mecobalamin in the treatment of senile diabetic peripheral neuropathy. Xin Zhong Yi 2019; 51: 67-9. [Google Scholar]
- 25. Cui Y, Pan MX. Therapeutic effect of Buyang Huanwu decoction combined with mecobalamin on diabetic peripheral neuropathy. Hebei Zhong Yi 2004; 26: 374-5. [Google Scholar]
- 26. Zhuang HZ. 30 cases of diabetic peripheral neuropathy treated with integrated Traditional Chinese and Western Medicine. Zhong Guo Zhong Yi Yao Xian Dai Yuan Cheng Jiao Yu 2011, 9: 60. [Google Scholar]
- 27. Liu JG. Therapeutic effect of Buyang Huanwu decoction on diabetic peripheral neuropathy. Zhong Yi Lin Chuang Yan Jiu 2015, 7: 113-4. [Google Scholar]
- 28. Ji TC. Clinical observation on the treatment of diabetic peripheral neuropathy with Buyang Huanwu decoction. Zhong Yi Lin Chuang Yan Jiu 2014; 6: 74-5. [Google Scholar]
- 29. Jiao FE, Cong K, Ji XY. Therapeutic effect of Buyang Huanwu decoction on diabetic peripheral neuropathy. Liaoning Zhong Yi Za Zhi 2013; 40: 740. [Google Scholar]
- 30. Jiang ZS, Ren ZX, Zhang SL, Li HY. Clinical study on the treatment of diabetic peripheral neuropathy by supplementing Qi and activating Yang, activating blood and dredging collaterals. Shandong Zhong Yi Yao Da Xue Xue Bao 2005; 29: 200-2. [Google Scholar]
- 31. Zhao L. Clinical observation of modified Buyang Huanwu decoction in the treatment of type Ⅱ diabetes mellitus. Hubei Zhong Yi Za Zhi 2002; 24: 8-9. [Google Scholar]
- 32. Xu XM, Chen XJ, Chen ran, Zhang JY, Zhang YL. Effect of Buyang Huanwu decoction on whole blood specific viscosity, TXB2, PLO and SOD in rats. Shanxi Yi Ke Da Xue Xue Bao 2002; 33: 212-4. [Google Scholar]
- 33. Xing SL, Li ZH, Sun JH, Liu YP, Pu LH. Antioxidant effect of Buyang Huanwu decoction. Jie Pou Xue Za Zhi 2005; 28: 529-32. [Google Scholar]
- 34. Chen LG, Qu Y, GE HY, Hu XQ, Nie YA, He MQ. Effect of Buyang Huanwu decoction on the expression of vascular endothelial cell adhesion molecules in rats with blood stasis syndrome. Zhong Cao Yao 2005; 36: 706-9. [Google Scholar]
- 35. Wang QY. Effect of astragalus injection on hemorheology in rabbits with blood stasis syndrome. Zhong Yao Yao Li Xue Yu Lin Chuang 2004; 20: 19-20. [Google Scholar]
- 36. Jiang DF, Yin CH, Yu NG, et al. Effect of Astragalosides on hemorheology in aged rats. Ji Chu Zhong Yi Za Zhi 2002; 16: 15-6. [Google Scholar]
- 37. Zhuang WQ. Clinical efficacy of Jingui Shenqi pill combined with Buyang Huanwu decoction in the treatment of diabetic peripheral neuropathy. clinical efficacy of diabetic peripheral neuropathy. Tang Niao Bing Xin Shi Jie 2017; 20: 168-9. [Google Scholar]
- 38. Jiang JL, Zhang RL, Zhang RP. Clinical study of Buyang Huanwu decoction and Huangqi Guizhi Wuwu decoction combined with western medicine in the treatment of diabetic peripheral neuropathy. Zhong Xi Yi Jie He Xin Nao Xue Guan Bing Za Zhi 2020; 18: 840-2. [Google Scholar]
- 39. Li S, Liu SR. Treatment of 47 cases of diabetic peripheral neuropathy with Buyang Huanwu decoction. Guang Ming Zhong Yi 2016; 31: 674-5. [Google Scholar]