Abstract
Recent studies have identified inequality in the distribution of air pollution attributable health impacts, but to our knowledge this has not been examined in Canadian cities. We evaluated the extent and sources of inequality in air pollution attributable mortality at the census tract (CT) level in seven of Canada's largest cities. We first regressed fine particulate matter (PM2.5) and nitrogen dioxide (NO2) attributable mortality against the neighborhood (CT) level prevalence of age 65 and older, low income, low educational attainment, and identification as an Indigenous (First Nations, Métis, Inuit) or Black person, accounting for spatial autocorrelation. We next examined the distribution of baseline mortality rates, PM2.5 and NO2 concentrations, and attributable mortality by neighborhood (CT) level prevalence of these characteristics, calculating the concentration index, Atkinson index, and Gini coefficient. Finally, we conducted a counterfactual analysis of the impact of reducing baseline mortality rates and air pollution concentrations on inequality in air pollution attributable mortality. Regression results indicated that CTs with a higher prevalence of low income and Indigenous identity had significantly higher air pollution attributable mortality. Concentration index, Atkinson index, and Gini coefficient values revealed different degrees of inequality among the cities. Counterfactual analysis indicated that inequality in air pollution attributable mortality tended to be driven more by baseline mortality inequalities than exposure inequalities. Reducing inequality in air pollution attributable mortality requires reducing disparities in both baseline mortality and air pollution exposure.
Keywords: air pollution, environmental injustice, environmental racism
Key Points
Census tracts with a higher prevalence of low income and Indigenous identity had significantly higher air pollution attributable mortality
The magnitude of inequality differed among seven Canadian cities
Inequality in air pollution attributable mortality tended to be driven more by baseline mortality inequalities than exposure inequalities
1. Introduction
Numerous studies have quantified the public health impacts of air pollution globally and nationally. Globally, air pollution is the leading environmental risk factor for mortality (GBD, 2019 Risk Factor Collaborators, 2020). In Canada, it is estimated that approximately 15,000 deaths annually are attributable to air pollution (Health Canada, 2021). Increasingly, attention is turning toward the distribution of these impacts from the perspectives of environmental racism and environmental injustice. Studies have identified inequality in the distribution of air pollution exposure and attributable public health impacts by racialized group membership and socioeconomic status (SES) between and within cities and counties (Buzzelli et al., 2003; Castillo et al., 2021; Clark et al., 2014; Crouse et al., 2009; Doiron et al., 2020; Fann et al., 2018; Giang & Castellani, 2020; Jerrett et al., 2001; Martenies et al., 2017; Pinault, 2016; Pinault et al., 2016; Rosofsky et al., 2018; Sohrabi et al., 2020; Southerland et al., 2021; Spiller et al., 2021). While the distribution of wealth in Canada is more equitable than in the US, health disparities are nonetheless evident, and there is evidence that disparities are widening (Shahidi et al., 2020). Moreover, it is well established that there are substantial disparities in health status between Indigenous and non‐Indigenous populations in Canada (Smylie & Firestone, 2015), including reduction of life expectancy of over 10 years (Tjepkema et al., 2019), and up to three‐fold higher age‐standardized rates of acute care hospitalization (Bougie, 2021) in some Indigenous groups compared to the non‐Indigenous population. To our knowledge, the distribution of air pollution health impacts within Canadian cities has not been examined, and in particular, disparities by Indigenous identity have not been evaluated.
In addition to examining the overall public health impacts of air pollution, addressing environmental injustice and environmental racism requires evaluation of the distribution of the benefits or damages resulting from policies and programs that aim to reduce environmental exposures. In both Canada and the US, regulatory impact assessments require analysis of the distribution of costs and benefits of proposed regulatory or other initiatives (Carey, 2022; Treasury Board of Canada Secretariat, 2022). In practice, this is often done only qualitatively. Thus, development of capacity and methods to conduct quantitative analysis of distributional impacts is required. This need is highlighted by legislation currently being considered in both countries to specifically address environmental injustice and environmental racism (Grijalva, 2021; May, 2021).
In this study, we examine the distribution of baseline mortality rates, air pollution exposure and air pollution attributable mortality, and quantify the extent and sources of inequality in air pollution attributable mortality within seven of Canada's largest cities. Comparing results across multiple cities allows us to determine whether patterns of inequality differ and hypothesize why this might be the case. We assess inequality in air pollution attributable mortality for fine particulate matter (PM2.5) and nitrogen dioxide (NO2), which have different patterns of spatial variability. Finally, we report alternative metrics of inequality, with a view to evaluating their utility in future assessments of environmental injustice and environmental racism.
2. Materials and Methods
2.1. Mortality, Population and Demographic Data
The analysis was conducted by census tract (CT) in seven of Canada's largest cities—Montreal, Ottawa, Toronto, Winnipeg, Calgary, Edmonton, and Vancouver (Figure 1). Our choice of seven cities was motivated by a desire to expand the analysis beyond the three largest cities (Toronto, Montreal, and Vancouver) that have typically been examined in these types of analyses in Canada, including cities from additional provinces/regions and with different densities and physical geography. The present analysis serves as a proof of concept for expanding the geographic scope to additional locations in future analyses. CTs are small, generally temporally stable geographic units with populations usually less than 10,000, that are intended to represent neighborhoods (Government of Canada, 2021c). For Montreal and Toronto, we used Census Division (Government of Canada, 2021a) boundaries to define the city perimeter. For Winnipeg, Calgary and Edmonton, we used Census Subdivision (CSD) (Government of Canada, 2021b) boundaries. For Vancouver we used the combined CSD boundaries of Vancouver, Burnaby, West Vancouver, and North Vancouver (City and District), which includes traditional territories of the Musqueam Indian Band, Squamish Nation, and Tsleil‐Waututh Nation, some of whose members live on‐reserve on the Burrard Inlet 3, Capilano 5, Kitsilano 6, Mission 1, Musqueam 2, and Seymour Creek 2 reserves, with boundaries contiguous with these CSDs. For Ottawa, we used the CSD boundary, excluding 22 primarily rural CTs outside the principal population center.
Figure 1.
Locations of included cities.
Mortality count data by CT for all ages and for those age 25 years and older were obtained from Statistics Canada for 2013–2015 (the most recent years for which data were available) for all cities except Montreal, for which data were only available for 2014 and 2015 (in other cities, mortality rates based on 2013–2015 exhibited a correlation of 0.99 with rates based on 2014–2015). We employed multiyear averages of mortality data in order to reduce the probability of potentially unrepresentative outlying values from a single year. Because population counts at the CT level are only available for census years (in this case, the closest year being 2016), we used inter‐censal estimates at the province level to adjust 2016 CT level population counts to 2013–2015 in order to compute denominators for the calculation of mortality rates (mortality count/population). Mortality rates were then averaged over 2013–2015.
Population counts and sociodemographic data by CT on percent of the population with income less than the low income cutoff (LICO), with less than a high school education (i.e., did not complete high school), or identifying as an Indigenous (Aboriginal) or Black person, were obtained from the 2016 census (Government of Canada, 2017; Statistics Canada, 2018). LICOs are defined as income levels below which families spend a disproportionate share of their income on necessities, and are family size and community‐size specific (Statistics Canada, 2017a). Indigenous (Aboriginal) identity refers to individuals who identify as First Nations, Métis or Inuit, as defined under the Constitution Act, 1982 (Statistics Canada, 2017b). We employ the term “Indigenous” rather than “Aboriginal” in keeping with current scholarship (Smylie & Firestone, 2015). Percent of the population 65 years and older by CT was also obtained to account for the strong dependence of mortality risk on age.
2.2. Exposure Data
As with mortality data, we employed multiyear averages of air pollution concentration data in order to reduce the probability of potentially unrepresentative outlying values from a single year. Three‐year average PM2.5 concentrations for 2013–2015 were calculated for each CT based on a 0.01° × 0.01° (approximately 1 km × 1 km) resolution surface that combines Aerosol Optical Depth retrievals from the National Aeronautics and Space Administration Moderate Resolution Imaging Spectroradiometer, Multi‐angle Imaging SpectroRadiometer, and Sea‐Viewing Wide Field‐of‐View Sensor instruments, with the GEOS‐Chem chemical transport model and ground‐level observations [edit ref V4.NA.02] (van Donkelaar et al., 2019). NO2 data for 2013–2015 were derived from a national land use regression (LUR) model incorporating ground monitoring data, remote sensing and land use patterns, with a resolution of 30 m × 30 m (Hystad et al., 2011). The LUR model was based on a single year (2006), then temporally scaled to other years based on ground monitoring data (CANUE—The Canadian Urban Environmental Health Research Consortium, 2018). PM2.5 and NO2 data were assigned to CTs by averaging values mapped to six character postal codes as points (CANUE—The Canadian Urban Environmental Health Research Consortium, 2023; DMTI Spatial, 2015) falling within each CT. This approach serves as an approximation of population weighting since density of postal codes mirrors population density (Giang & Castellani, 2020). These PM2.5 mass and NO2 data have been used extensively in air pollution epidemiology studies in Canada (Pappin et al., 2019). The temporal and spatial specification of data sources employed in the analysis is summarized in Table 1.
Table 1.
Temporal and Spatial Specification of Data Sources
| Data | Year(s) | Geographic resolution |
|---|---|---|
| Mortality counts | 2013–2015 a | Census tract |
| Population counts | 2016 | Census tract |
| 2013–2016 | Province | |
| Demographic characteristics | 2016 | Census tract |
| PM2.5 | 2013–2015 | Census tract (from 1 km × 1 km surface) |
| NO2 | 2013–2015 | Census tract (from 30 m × 30 m surface) |
2014–2015 for Montreal.
2.3. Statistical Analysis
All statistical analyses were conducted in R (R Core Team, 2019) using the DescTools (Signorell, 2021), CARBayes (Lee, 2013), coda (Plummer et al., 2006), and rineq (Devleesschauwer et al., 2017) packages. Results from regression models by city were pooled using a random effects model employing the metafor package (Viechtbauer, 2010). Maps were generated using the rgdal (Bivand et al., 2019) and tmap (Tennekes, 2018) packages. Interactive maps were created using the R shiny package (Chang et al., 2022).
2.3.1. Air Pollution Attributable Mortality
Air pollution attributable mortality per 100,000 population (M AP) was calculated as the attributable fraction multiplied by the baseline mortality rate from internal causes per 100,000 population,
where β is the log hazard ratio (HR) or log relative risk (RR), x i is the CT average pollutant concentration, x 0 is the counterfactual pollutant concentration, and M 0 is the CT baseline mortality rate. β values for the association of long term exposure to PM2.5 and NO2 with mortality were derived from a large nationally representative population‐based Canadian cohort study employing the same PM2.5 and NO2 exposure surfaces that we employed, mapped to residential postal codes (HRs 1.072, 95% confidence interval (CI) 1.060–1.084 per 10 μg/m3 PM2.5 and 1.065, 95% CI 1.056–1.074 per 10 ppb NO2) (Crouse et al., 2015). We consider long‐term exposure to be duration over a period of years, associations with which are examined using cohort studies, in contrast to short‐term exposure, which refers to duration over days to weeks, associations with which are examined using time series or case crossover studies. Although numerous studies have examined the association between long term exposure to NO2 and mortality, the evidence is considered weaker than that for PM2.5 (Huangfu & Atkinson, 2020; Stieb et al., 2021). As a sensitivity analysis, we therefore considered an alternative β value for NO2 based on an international study of short term exposure including 25 Canadian cities (Meng et al., 2021). The value is based on results provided to us by the author for the 25 Canadian cities from a model including PM2.5 (RR 1.0042, 95% CI 0.9977–1.0108 per 10 ppb). Following Castillo et al. (2021), we consider the HR and RR constant in time and space, thus we do not propagate the 95% CIs through the analysis. Consistent with the respective sources of concentration response functions, baseline mortality rates (M 0) for mortality attributable to long‐term exposure to PM2.5 and NO2 were based on the population 25 years of age or older, while baseline mortality rates for mortality attributable to short‐term exposure to NO2 were based on all ages. As an additional sensitivity analysis, we followed the example of a recent paper (Spiller et al., 2021) that employed racialized‐group specific β values derived from a recent analysis of the US Medicare cohort (Di et al., 2017), that found that the HR for the association between PM2.5 and mortality was significantly larger in the Black population compared to the white population, but not in the Native American population compared to the white population, nor in those eligible for Medicaid versus not eligible (as a measure of SES). We employed population‐wide versus SES specific HRs for PM2.5 and NO2 from the same source as the base case (Crouse et al., 2015). To our knowledge, HRs specific to Indigenous populations in Canada are not available. Details of the derivation and application of SES specific HRs are provided in Supporting Information S1 (Text S1).
In keeping with previous papers that have examined inequality in the distribution of air pollution attributable health impacts between counties and neighborhoods, we employed a counterfactual pollutant concentration (x 0) of 0. As a sensitivity analysis, we employed natural background concentrations (1.8 μg/m3 for PM2.5 and 0.15 ppb for NO2, applied nationally) estimated by Environment and Climate Change Canada based on a review of monitoring data from rural and remote monitoring sites during time periods classified as being influenced primarily by background air mass types (Health Canada, 2021). These concentrations are comparable in concept to the US Environmental Protection Agency “policy relevant background,” defined as concentrations that would occur in the absence of anthropogenic emissions in continental North America (National Center for Environmental Assessment‐RTP Division Office of Research and Development, 2009). The value for PM2.5 is similar to background concentrations employed in a US analysis, which ranged from 0.74 to 1.72 μg/m3 by region (Fann et al., 2012).
Estimates of attributable mortality were calculated only for census tracts with a population of at least 1,000. Of 57 census tracts with population < 1,000, 84% were missing data for baseline mortality and 42%–53% were missing data on percent of the population 65 years or older, identifying as a Black or Indigenous person, with income less than the LICO and less than a high school education. Mean PM2.5 concentrations were slightly higher (7.9 vs. 7.5 μg/m3), NO2 concentrations higher (16.6 vs. 13.9 ppb), and land surface area larger (3.7 vs. 2.0 km2) in census tracts with population < 1,000 versus 1,000 or greater.
2.3.2. Analysis of Inequality
We used multiple methods to quantify the extent and sources of inequality in the distribution of air pollution attributable mortality in order to compare our results to earlier studies that have employed differing approaches, triangulate our findings and evaluate the utility of alternative methods in future analyses of environmental injustice and environmental racism.
First, similar to earlier analyses of inequality in air pollution exposure (Buzzelli et al., 2003; Jerrett et al., 2001; Pinault, 2016; Pinault et al., 2016), we regressed air pollution attributable mortality against the prevalence of population characteristics (percent of the population age 65 years and older, with income less than the LICO, with less than a high school education, and identifying as an Indigenous and/or Black person) by CT in each city. Each characteristic was first included separately in a general linear model, then in a multivariate general linear model, and finally those displaying consistent associations with attributable mortality were included together in a multivariate conditional autoregressive model accounting for spatial autocorrelation (Lee, 2013; Leroux et al., 2000). City‐specific results were then pooled using a random effects meta‐analysis.
Second, we calculated the concentration index of the distribution of baseline mortality rate, air pollution exposure and air pollution attributable mortality by rank order of CTs based on the prevalence of the same population characteristics as considered in regression models, as well as the Atkinson index and Gini coefficient by quantile of the prevalence of these CT characteristics. The concentration index, which we refer to henceforth as the “inequality index” to avoid confusion with pollutant concentrations (Giang & Castellani, 2020), is based on the concentration curve, which as applied here depicts the proportion of the total burden of an adverse health outcome experienced by the population ranked in order from highest to lowest prevalence of disadvantage. The index varies from −1 to 1, with negative values indicating that disadvantaged populations experience a disproportionate share of the burden, positive values indicating the converse, and 0 indicating equality (Giang & Castellani, 2020). Atkinson index and Gini coefficient values range from 0 to 1, with higher values indicating greater inequality (Fann et al., 2018; Rosofsky et al., 2018). The Atkinson Index permits decomposition of inequalities within and between population subgroups, while the Gini coefficient does not (Fann et al., 2018). An epsilon value of 0.75 was used in calculating the Atkinson index, for comparability with Fann et al. (2018), Rosofsky et al. (2018), Martenies et al. (2017), and Clark et al. (2014). The Atkinson Index and Gini coefficient were calculated by tertile of CT characteristics, with a sensitivity analysis by quintile. Computation of the inequality index, Atkinson index and Gini coefficient are detailed in Supporting Information S1 (Text S2).
Finally, we conducted a counterfactual analysis to determine the extent to which observed disparities in air pollution attributable mortality, measured using the inequality index, would be reduced if CTs with a prevalence of disadvantage related to racialized group membership or SES above the median prevalence among all CTs in each city, experienced the same baseline mortality rate or air pollution exposures as other CTs. The statistical significance of differences between inequality index values for the base case versus counterfactual scenarios was assessed using Cochran's Q (Viechtbauer, 2010).
Research ethics board approval was not required as all data were aggregate in nature.
3. Results
3.1. Descriptive Findings
The seven cities included in our analysis comprised 2,070 CTs, with a total population of approximately 9.5 million. Of these, 59 CTs (2.9%) were excluded because the population was less than 1,000 or missing, and an additional 21 were excluded due to missing data for mortality rate (n = 18, 0.9%), demographic characteristics (n = 2, 0.1%), or air pollution (n = 1, 0.04%), leaving 1,990 (96.1%) included in our analysis. The principal reason for missing mortality or demographic data is suppression of small counts for privacy protection. The mean and 95th percentile of surface area of CTs included in our analysis were 2.0 and 5.3 km2 respectively.
The distributions of demographic characteristics, mortality rates, air pollution exposure, and air pollution attributable mortality rates among CTs are summarized in Table 2. The mean percentage of the population aged 65 years or older, with income below the LICO and with less than a high school education was relatively consistent among the seven cities. The mean percentages identifying as a Black and/or Indigenous person were more variable. Mean baseline mortality rates varied considerably and were lowest in Calgary and highest in Winnipeg. Mean PM2.5 values exhibited little variability between cities, while mean NO2 values were lowest in Ottawa and Winnipeg, with similar values in the other cities. Mean PM2.5 attributable mortality rate was lowest in Calgary and highest in Montreal, while mean NO2 attributable mortality rate was lowest in Ottawa and highest in Montreal. Table 2 also summarizes the range of values observed within cities. Baseline mortality rate ranged roughly 20–40 fold, with the exception of Montreal where the range was nearly 90 fold, PM2.5 varied less than two fold, NO2 varied three to five fold, and both PM2.5 and NO2 attributable mortality rate varied approximately 20–90 fold, with the greatest variability in Montreal and least in Vancouver.
Table 2.
Descriptive Statistics—Population Characteristics, Baseline Mortality Rates, Air Pollution Concentrations, and Air Pollution Attributable Mortality Rates by Census Tract
| Variable | Montreal | Ottawa | Toronto | Winnipeg | Calgary | Edmonton | Vancouver |
|---|---|---|---|---|---|---|---|
| Mean, all census tracts | |||||||
| Percent age 65+ | 15.9 | 16.9 | 16.1 | 15.9 | 12.2 | 13.6 | 16.5 |
| Percent Black population | 8.7 | 6.8 | 8.7 | 3.8 | 3.9 | 5.7 | 1.0 |
| Percent Indigenous population | 0.7 | 2.5 | 0.9 | 13.6 | 3.0 | 6.1 | 3.4 |
| Percent < LICO a | 19.0 | 12.1 | 17 | 13.9 | 9.0 | 10.6 | 16.5 |
| Percent < High school | 16.2 | 12.2 | 16.9 | 17.9 | 13.6 | 16.2 | 12.5 |
| Mortality/100,000 (age 25+) | 979.0 | 914.3 | 814.2 | 1,125.7 | 638.3 | 857.2 | 809.7 |
| PM2.5 (μg/m3) | 8.3 | 6.3 | 8.5 | 5.6 | 6.0 | 8.0 | 6.1 |
| NO2 (ppb) | 14.8 | 6.3 | 15.9 | 9.6 | 13.3 | 15.8 | 15.2 |
| PM2.5 attributable mortality/100,000 | 55.4 | 40.0 | 46.5 | 43.0 | 27.3 | 47.7 | 33.7 |
| NO2 attributable mortality/100,000 | 85.0 | 37.7 | 77.2 | 66.5 | 53.5 | 81.2 | 72.8 |
| Ratio of maximum to minimum over all census tracts | |||||||
| Mortality per 100k (age 25+) | 89.8 | 35.2 | 38.3 | 26.3 | 42.3 | 37.4 | 17.0 |
| PM2.5 (μg/m3) | 1.3 | 1.4 | 1.2 | 1.2 | 1.8 | 1.5 | 1.3 |
| NO2 (ppb) | 4.7 | 3.1 | 3.0 | 3.0 | 2.6 | 2.6 | 4.9 |
| PM2.5 attributable mortality per 100k | 93.6 | 45.8 | 35.3 | 27.7 | 59.4 | 47.5 | 18.9 |
| NO2 attributable mortality per 100k | 84.5 | 54.3 | 42.3 | 51.2 | 61.0 | 48.2 | 20.3 |
Low Income Cut‐Off.
Descriptive results from sensitivity analyses are shown in Supporting Information S1 (Table S1). Not surprisingly, average air pollution attributable mortality rates were lower when estimated natural background concentrations were employed as counterfactuals rather than zero, to a greater degree for PM2.5 than for NO2, consistent with the estimated natural background concentration for NO2 being closer to zero. Average air pollution attributable mortality rates were generally not sensitive to employing SES‐specific HRs. NO2 attributable mortality rates based on short‐term exposure were considerably lower than those based on long term exposure, in keeping with the relative magnitudes of the respective HR and RR. The ratio of maximum to minimum air pollution attributable mortality rates generally increased with the application of SES‐specific HRs for NO2 but not for PM2.5. These ratios were generally insensitive to other sensitivity analyses.
We found that the 10 CTs with the highest PM2.5 or NO2 attributable mortality all pertained to NO2 exposure and comprised eight CTs primarily in north and east Montreal, one in Winnipeg's north end and one in the downtown east side of Vancouver (Table 3). These CTs were in the highest quintile of multiple factors including prevalence of age ≥ 65 (8 of 10), income < LICO (5 of 10), education < HS (4 of 10), identification as an Indigenous (2 of 10) or Black person (2 of 10), baseline mortality (9 of 10) and NO2 concentration (3 of 10).
Table 3.
Characteristics of Census Tracts With 10 Highest Air Pollution Attributable Mortality Rates a
| City | CTUID b | Pollutant | Attributable mortality 100k | Percent ≥65 | Percent <LICO c | Percent <HS d | Percent identifying as Black person | Percent identifying as Indigenous person | Baseline mortality /100k | NO2 (ppb) |
|---|---|---|---|---|---|---|---|---|---|---|
| Montreal | 4620148.00 | NO2 | 747.3 | 23.0 | 19.0 | 5.8 | 4.0 | 0.0 | 6,987.0 | 16.2 |
| Montreal | 4620251.01 | NO2 | 681.9 | 28.0 | 21.0 | 30.4 | 10.6 | 0.3 | 5,273.7 | 18.1 |
| Winnipeg | 6020035.00 | NO2 | 641.5 | 25.0 | 36.0 | 30.9 | 3.3 | 41.5 | 6,656.2 | 10.3 |
| Montreal | 4620057.00 | NO2 | 621.7 | 28.0 | 37.0 | 15.9 | 5.4 | 0.0 | 4,262.9 | 17.9 |
| Montreal | 4620277.00 | NO2 | 545.7 | 27.0 | 22.0 | 18.5 | 20.9 | 0.5 | 5,236.9 | 14.6 |
| Vancouver | 9330058.00 | NO2 | 511.8 | 21.3 | 65.0 | 27.0 | 2.5 | 15.6 | 2,258.8 | 22.5 |
| Montreal | 4620192.00 | NO2 | 496.2 | 35.0 | 11.0 | 13.1 | 8.3 | 2.0 | 6,245.7 | 13.5 |
| Montreal | 4620016.00 | NO2 | 476.5 | 16.0 | 31.0 | 22.2 | 6.1 | 1.6 | 4,035.1 | 15.9 |
| Montreal | 4620383.01 | NO2 | 442.8 | 31.0 | 11.0 | 13.0 | 3.8 | 0.0 | 5,484.9 | 13.5 |
| Montreal | 4620132.00 | NO2 | 415.6 | 20.0 | 41.0 | 5.1 | 4.7 | 0.9 | 3,046.6 | 16.2 |
Note. Bold indicates >80th percentile of all CTs, all cities.
Based on income‐specific HR.
Census Tract Unique Identifier.
Low Income Cut‐Off.
High School.
Interactive maps of demographic characteristics, baseline mortality rates, air pollution exposures and air pollution attributable mortality rates by city are available at https://apinequality.shinyapps.io/inequality/ (in keeping with Statistics Canada data disclosure policy, mortality rates shown on interactive maps are based on mortality counts randomly rounded to base 5; rates based on randomly rounded vs. raw counts had a correlation of 1). Sample static maps are shown for Calgary in Figure 2. Spatial patterns were most consistently evident in all cities for PM2.5 and NO2 concentrations (i.e., higher concentrations in the downtown core and/or along major roadways), and in some cities for percent of population with income below the LICO or identifying as an Indigenous person (i.e., concentration in certain areas of the city as opposed to more random scatter). Spatial patterns of air pollution attributable mortality generally more closely paralleled those of baseline mortality than air pollution concentrations. No clear spatial patterns were evident for the other variables.
Figure 2.
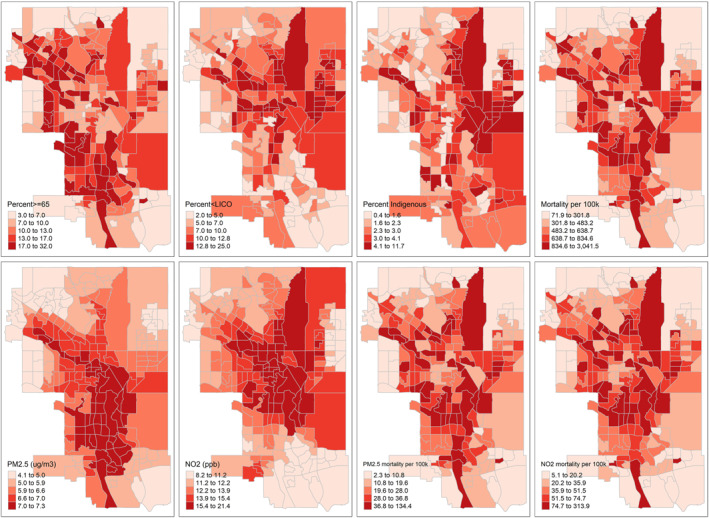
Distribution of demographic characteristics, baseline mortality rates, air pollution concentrations and air pollution attributable mortality rates by census tract in Calgary; quintiles as cutpoints. LICO = Low Income Cut‐Off. Created with R tmap package.
3.2. Analysis of Inequality
In multivariate general linear models, only percent of the population 65 years and older, percent with household income less than the LICO, and percent identifying as an Indigenous person were consistently significantly positively associated with air pollution attributable mortality (Table S2 in Supporting Information S1). Regression results by city from multivariate conditional autoregressive models accounting for spatial autocorrelation are presented in Figure 3. Inclusion of percent age 65 years and older both adjusts for the strong dependency of mortality risk on age, and provides a comparator for the magnitude of the associations with other CT characteristics. Pooled estimates of the association of percent 65 or older, low income and identifying as an Indigenous person with attributable mortality were positive, significant and similar in magnitude for PM2.5 and NO2. Significant heterogeneity was observed between cities. Findings were not sensitive to excluding individual cities (Table S3 in Supporting Information S1).
Figure 3.
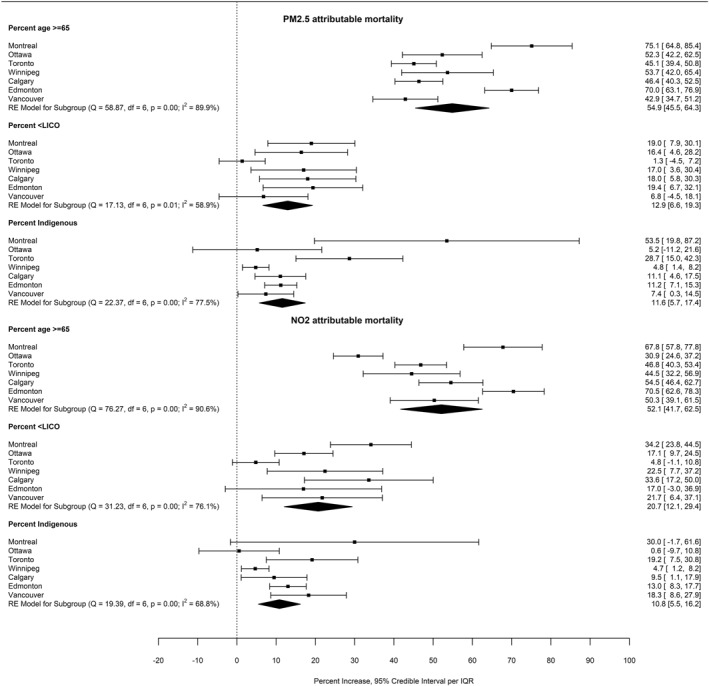
Percent increase in PM2.5 and NO2 attributable mortality per interquartile range increase in census tract prevalence of age ≥ 65, income < Low Income Cut‐Off and identifying as an Indigenous person, by city and pooled across cities. Based on conditional autoregressive multivariate models including the three variables and accounting for spatial autocorrelation. Figure created with R metafor package using results generated by the CARBayes package.
Inequality index values by city are shown in Figure 4. They were most strongly negative for percent of the population greater than 65 years of age, and in relation to this characteristic, values were most strongly negative for baseline mortality rates and air pollution attributable mortality. Consistent with our results from regression models, inequality index values were more strongly negative in relation to percent of the population with income less than the LICO and identifying as an Indigenous person, than in relation to percent of the population with less than a high school education or identifying as a Black person. Inequality index values in relation to income, education and identifying as a Black and/or Indigenous person tended to be greatest for NO2 attributable mortality followed by PM2.5 attributable mortality, and were least strongly negative for PM2.5 pollutant concentrations. Values were generally closer to zero or positive in Montreal, Toronto and Vancouver compared to the other cities in relation to all variables other than age 65 or older. Results were not sensitive to the application of estimated natural background as the counterfactual pollutant concentration, or substitution of a β value for NO2 based on short term exposure (Figure S1 in Supporting Information S1). Substitution of income specific β values had relatively little impact on inequality index values for PM2.5 attributable mortality (Figure S1 in Supporting Information S1). In contrast, inequality index values became more strongly negative for NO2 attributable mortality in relation to percent of the population with income less than the LICO, less than a high school education, and identifying as a Black and/or Indigenous person (Figure S1 in Supporting Information S1).
Figure 4.
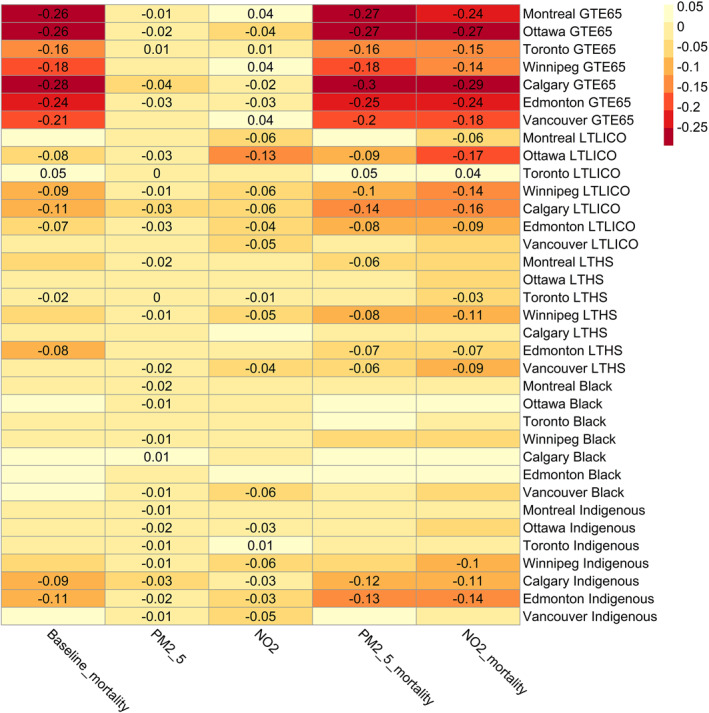
Heatmap of inequality index values for baseline mortality rate/100,000 population, PM2.5 concentration, NO2 concentration, PM2.5 attributable mortality/100,000 population and NO2 attributable mortality/100,000 population by city and population characteristic. GTE = greater than or equal to, LTLICO = less than Low Income Cut‐Off, LTHS = less than High School. Only values significantly different from zero are shown. Figure created with R pheatmap package using inequality index values generated by the rineq package.
Atkinson index values and Gini coefficients pertaining to within city inequality based on tertiles of population characteristics paralleled findings for the inequality index (Figures S2 and S3 in Supporting Information S1). There tended to be less variability in Atkinson index values than Gini coefficients. Atkinson index values tended to be slightly larger based on quintiles versus tertiles of population characteristics, whereas Gini coefficients were not consistently larger based on quintiles versus tertiles (Figures S4 and S5 in Supporting Information S1).
Results of the counterfactual analysis comparing inequality index values in the base case to pollutant and mortality counterfactuals are shown in Figure 5. Only values significantly different from zero are shown. Inequality index values were reduced in magnitude compared to the base case (indicating reduced inequality) in all counterfactual scenarios in Ottawa, Winnipeg, Calgary and Edmonton. In these cities, the reduction in inequality tended to be greater for the mortality counterfactual compared to the air pollution counterfactual. Inequality index values differed significantly from the base case for all mortality counterfactual scenarios in Calgary, and for mortality counterfactual scenarios related to prevalence of identifying as an Indigenous person in Edmonton (p < 0.05). Results were less consistent in Montreal, Toronto and Vancouver. The change in spatial distribution of PM2.5 and NO2 attributable mortality in Calgary according to air pollution and baseline mortality counterfactuals is shown in Figure 6. Interactive maps of counterfactual analyses for all cities are available at https://apinequality.shinyapps.io/inequality/.
Figure 5.
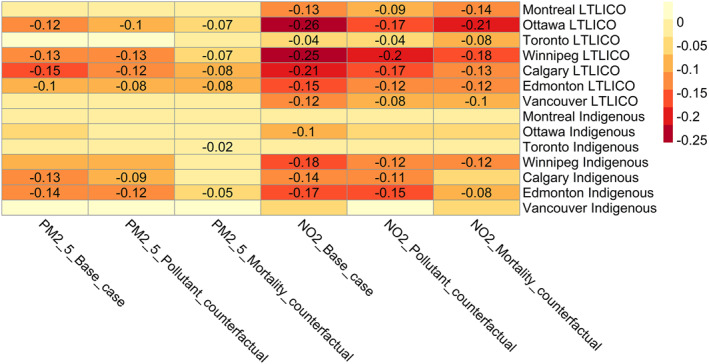
Heatmap of inequality index values for PM2.5 and NO2 attributable mortality/100,000, comparing base case, pollutant counterfactual and mortality counterfactual. All analyses including the base case employed income quintile specific hazard ratios for the association between air pollution and mortality. LTLICO = less than Low Income Cut‐Off. Only values significantly different from zero are shown. Figure created with R pheatmap package using inequality index values generated by the rineq package.
Figure 6.
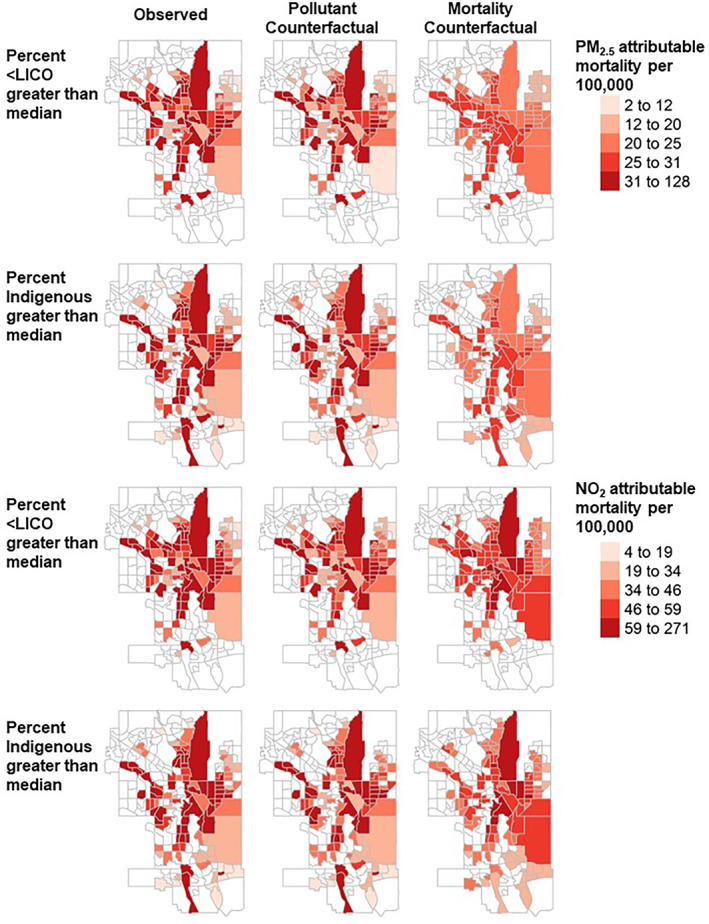
Observed and counterfactual spatial distribution of air pollution attributable mortality per 100,000 population in Calgary. Only census tracts above the median prevalence of income less than the low income cut‐off (LICO) and identifying as an Indigenous person are shaded. Counterfactual scenarios set air pollution concentrations or baseline mortality rates to the mean observed in census tracts below the median prevalence of income less than the low income cut‐off (LICO) and Indigenous identity. Created with R tmap package.
4. Discussion
In our analysis of the distribution of air pollution attributable mortality rates within Canadian cities, we found that CTs with a higher prevalence of low income and Indigenous identity had significantly higher air pollution attributable mortality. Inequality index, Atkinson index, and Gini coefficient values were consistent in revealing different degrees of inequality among the cities. Counterfactual analysis indicated that reducing inequality in baseline mortality rates tended to have a greater impact on reducing inequality in air pollution attributable mortality than reducing inequality in air pollution concentrations. To our knowledge, our study is the first to report inequality in the distribution of air pollution attributable mortality by prevalence of both low income and identification as an Indigenous person. Our results were generally consistent among multiple methods of assessing the extent and sources of inequality in air pollution attributable mortality. In particular, inequality tended to be least for PM2.5 concentrations, somewhat greater for NO2 concentrations (NO2 is well known to be more spatially heterogeneous than PM2.5 (Wang et al., 2020)), greater for baseline mortality rates, and greatest for PM2.5 and NO2 attributable mortality. Not surprisingly, the magnitude of inequality in air pollution attributable mortality reflects compounding of the magnitudes of inequality in baseline mortality rates and air pollution concentrations.
We found that the degree of inequality varied between cities. Inequality tended to be least in Montreal, Toronto and Vancouver compared to the other cities. Each city has a unique physical and human geography which could explain the observed differences. Montreal is an island bounded on all sides by the St. Lawrence River, Toronto is bounded on one side by Lake Ontario, and Vancouver is bounded by the Pacific Ocean, Fraser River and North Shore mountains. Montreal, Toronto and Vancouver are also the most populous and are characterized by greater average population density (respectively 3,902, 4,332, and 2,235 persons per km2) than the other cities included in our analysis (1,146–1,703 per km2). We hypothesize that greater density may be associated with greater mixing of population subgroups relative to the distribution of baseline mortality rates and air pollution exposure, reducing the degree of inequality in air pollution attributable health impacts. Alternatively, these findings could be influenced by the sensitivity of results to the level of spatial aggregation, that is, the modifiable areal unit problem (Tuson et al., 2019). Particularly in denser cities and denser areas within cities, the variables of interest may vary at a smaller scale or according to different boundaries than CTs. Nonetheless, we found that the 10 highest air pollution attributable mortality rates were observed in relation to NO2 in eight CTs in Montreal, one in Winnipeg and one in Vancouver. Several of the Montreal CTs and the Winnipeg and Vancouver CTs in particular corresponded with neighborhoods (Point Douglas and the Downtown East Side respectively) that experience a high prevalence of severe poverty and homelessness (Manitoba Collaborative Data Portal, 2019; Mauboules, 2020), potentially further increasing residents' vulnerability and exposure to air pollution.
Counterfactual analysis revealed that reducing inequality in baseline mortality rates tended to result in greater reductions in disparities in air pollution attributable mortality than reducing inequality in air pollution exposure. These findings are consistent with our observation that inequality indices generally indicated greater inequality in baseline mortality rates than air pollution concentrations. To the extent that variability in baseline mortality rates is partially determined by variability in air pollution exposure, baseline mortality counterfactuals do not entirely exclude the contribution of inequality in air pollution exposure. Nonetheless, since the air pollution attributable fraction is small, the findings highlight the importance of accounting for neighborhood level variability in both baseline mortality and air pollution exposure in analyses of the distribution of air pollution attributable health impacts. These findings also suggest that reducing inequality in baseline mortality rates by improving overall health status in disadvantaged communities merits greater consideration as an important strategy for reducing inequality in the distribution of air pollution attributable mortality.
While previous studies have documented inequality in the distribution of air pollution exposure within Canadian cities, most have focused on single cities, or only the three largest cities. In an analysis of Canada's three largest cities (Montreal, Toronto, Vancouver), Pinault et al. (2016) found that dissemination areas (DAs) with a larger proportion of the population that did not speak English or French had significantly greater exposure to NO2. Measures of social deprivation were also significantly associated with NO2 exposure, but associations differed by city (Pinault et al., 2016). In a similar analysis focused on children, those in lower income DAs had significantly greater NO2 exposure, and in some cities, children living in DAs with larger proportions of lone parent families and people of color (“visible minority”) also had greater exposures (Pinault, 2016). Earlier analyses employing similar methods in the industrial city of Hamilton, Canada found that total suspended particulate matter concentrations were significantly negatively associated with dwelling values, and significantly positively associated with prevalence of low income and unemployment (Jerrett et al., 2001), but that inequalities narrowed over time (Buzzelli et al., 2003). Doiron et al. (2020) conducted an analysis of multiple built environment features by postal code in Montreal, Toronto and Vancouver, and reported that postal codes characterized by greater deprivation were less walkable, and had higher NO2 concentrations and lower vegetation indices reflecting green space. Crouse et al. (2009) reported that while some Montreal neighborhoods were characterized by a high prevalence of deprivation together with high NO2 concentrations, this was not true of all deprived neighborhoods, and some wealthy neighborhoods also had high NO2 concentrations. This finding is consistent with our hypothesis that in some cities, there may be greater mixing of population subgroups, influencing the distribution of baseline mortality and air pollution exposure. Giang and Castellani computed concentration index values for PM2.5, NO2, carbon monoxide, ozone and sulfur dioxide individually and as a joint hazard index in Montreal, Toronto and Vancouver and reported values that were generally of comparable direction and magnitude relative to our findings (Giang & Castellani, 2020). They also found greater inequality in NO2 compared to PM2.5 concentrations.
In a study similar to ours in the Bay area of California, using census block group (CBG) level mortality rates and high resolution exposure estimates, Southerland et al. (2021) found that air pollution attributable mortality rates varied by 38 and 5 fold respectively for NO2 and PM2.5 among CBGs. We observed somewhat greater variability in both PM2.5 and NO2 attributable mortality (up to 80–90 fold in Montreal). Attributable mortality rates varied substantially depending on the source of the exposure data in Southerland et al. (2021) analysis, and aggregated to the county level were approximately 15% higher based on CBG level versus county level mortality rates. In addition, applying CBG versus county level baseline mortality rates resulted in 3–5 times more variability in air pollution attributable mortality (Southerland et al., 2021). Finally, CBGs where greater than 50% of the population was minority accounted for 75% of the air pollution attributable mortality burden (Southerland et al., 2021).
Another similar study examined the distribution of PM2.5 attributable mortality and morbidity among neighborhoods, zip codes and wards in Washington DC, employing neighborhood and zip code level baseline mortality and morbidity rates (Castillo et al., 2021). Castillo et al. (2021) found that PM2.5 attributable mortality and morbidity were negatively associated with income and educational attainment, and positively associated with prevalence of Black residents, poverty and unemployment. Similar to our findings, Castillo et al. (2021) noted that spatial patterns of air pollution attributable mortality paralleled those of baseline mortality rates. An earlier study in Houston, employing county level baseline mortality rates also found that census tracts with lower average incomes experienced greater PM2.5 and NO2 exposure and higher air pollution attributable mortality (Sohrabi et al., 2020). A study in Detroit, employing air pollution concentrations mapped to CBG level and zip code level baseline mortality and morbidity rates, evaluated inequalities in air pollution attributable mortality and morbidity using the Atkinson index and inequality index (Martenies et al., 2017). They reported Atkinson index values of 0.003 and 0.009 for PM2.5 and NO2 exposure respectively, and 0.045 and 0.137 for PM2.5 and NO2 attributable health burden respectively. These values are somewhat larger than what we observed, but are consistent in that we also observed larger values for NO2 than for PM2.5, and for attributable burden than for exposure. Inequality index values were of comparable magnitude to what we observed, and were particularly strongly negative in relation to point source PM2.5 and percent Latino (−0.117), and mobile source NO2 and percent of households in poverty (−0.084).
Rosofsky et al. (2018) examined trends in inequality in PM2.5 and NO2 exposure within Massachusetts at the CBG level by racialized group membership, income and educational attainment. They reported Atkinson index values for PM2.5 and NO2 respectively of approximately 0.0001 and 0.003 based on racialized group membership and <0.0001 and 0.001 based on income and education (Rosofsky et al., 2018). An upward trend in inequality was observed for NO2, while there was more year to year variability for PM2.5, despite decreasing trends in concentrations of both pollutants (Rosofsky et al., 2018). Within city Atkinson index values by Indigenous identity in our analysis were comparable to their results for racialized group membership, while for low income, we observed larger values in some cities, particularly for NO2. Clark et al. (2014) examined inequality in NO2 concentrations by income at the CBG level nationally in the U.S. and reported Atkinson index values of 0.009 in small urban areas, 0.015 in medium urban areas and 0.018 in large urban areas, somewhat larger than the values we observed by income in most cities except Ottawa.
To our knowledge, only one previous study applied population subgroup specific HRs and baseline mortality rates in examining the distribution of air pollution attributable mortality. Spiller et al. (2021) estimated PM2.5 attributable mortality by US county using mortality rates and HRs specific to Black populations. They reported that employing population‐wide rather than subgroup specific parameters dramatically underestimated impacts in Black populations as well as disparities between Black and white populations, although it did not affect the total number of attributable deaths (Spiller et al., 2021). We found that employing income specific HRs had less impact on inequality in PM2.5 attributable mortality than NO2 attributable mortality.
Finally, Fann et al. (2018) conducted a county level analysis in the US and found that inequality in the distribution of PM2.5 attributable mortality, quantified using the Atkinson index and Gini coefficient declined between 2005 and 2014. 2014 values of the Atkinson index and Gini coefficient by educational attainment were approximately 0.02 and 0.1 respectively for PM2.5 exposure and attributable fraction. In contrast, based on groupings by income and Indigenous identity, we observed Atkinson index values of 0.0–0.001 for PM2.5 exposure, 0.001–0.033 for PM2.5 attributable mortality, 0.0–0.018 for NO2 exposure and 0.00–0.045 for NO2 attributable mortality. We observed Gini coefficient values of 0.00–0.05 for PM2.5 exposure, 0.04–0.22 for PM2.5 attributable mortality, 0.01–0.18 for NO2 exposure and 0.03–0.27 for NO2 attributable mortality. These results indicate that inequality in PM2.5 and NO2 attributable mortality within some Canadian cities exceeds inequality in exposure and attributable fraction between US counties.
Strengths of our analysis compared to previous literature include analysis of multiple cities, which allowed us to discern differences in the magnitude and drivers of inequality between cities, and application of multiple methods for examining the magnitude and sources of disparities among population groups. Applying multiple methods permitted us to compare our findings to previous literature employing differing methods, triangulate our results and evaluate their utility in future analyses of environmental injustice and environmental racism. Multivariate regression models accounting for spatial autocorrelation allowed us to attempt to isolate the independent effects of individual variables reflecting potential sources of inequality. Of the inequality metrics, the inequality index demonstrated greater variability than the Atkinson index or Gini coefficient, potentially reflecting greater sensitivity. In the context of regulatory impact assessments, the estimated change in the inequality index value in relation to a proposed policy could be used to quantify distributional impacts. Analysis of counterfactual scenarios permitted us to evaluate the relative contributions of inequality in exposure and baseline mortality rates reflecting underlying health status to disparities in air pollution attributable mortality, highlighting the possibly underappreciated role of inequality in underlying health status.
Limitations of our study include the incomplete nature of the low income variable, which does not capture all aspects of economic security, such as wealth, the potential for air pollution gradients to exist that might not be fully resolved by the exposure data, and possible numerator‐denominator bias with respect to under‐counting of urban Indigenous populations, but not deaths (Smylie & Firestone, 2015). Our finding that inequality in air pollution attributable mortality was driven to a greater extent by inequality in baseline mortality rates than in air pollution exposure could be sensitive to the relative spatial resolution of baseline mortality rate and air pollution exposure data. If exposure data were more highly spatially resolved, our findings in this regard may have differed. However, NO2 data were already highly resolved (30 m × 30 m), and there was little spatial variability in PM2.5. Since the spatial distribution of NO2 concentrations was based on a LUR for a single year, estimated NO2 concentrations may not reflect changes in the spatial distribution of sources of NO2 over time. However, there is evidence that spatial gradients in traffic‐related air pollutants such as NO2 tend to be stable over time (Cesaroni et al., 2012; de Hoogh et al., 2018; Eeftens et al., 2011). Estimates of air pollution attributable mortality were missing for 3.8% of CTs (0.9% due to missing baseline mortality rate and 2.9% which we excluded due to small (<1,000) or missing population), varying from 0% in Calgary to 6.0% in Edmonton. If the probability of being missing is not random, our results could be biased. However, our results were not sensitive to exclusion of individual cities from the analysis. A sensitivity analysis revealed that, not surprisingly, employing a concentration response function specific to low income populations increased the degree of disparity in air pollution attributable mortality, particularly for NO2. Additional research is needed to quantify differences in air pollution related mortality risks in Canada, particularly by racialized group membership, and accounting for the intersection of lower SES and racialized group membership.
5. Conclusions
We observed significant disparities in air pollution attributable mortality within Canadian cities, in relation to the prevalence of low income and Indigenous identity. The magnitude of disparities differed between cities, suggesting that factors unique to individual cities may play an important role in driving observed inequality. The inequality (concentration) index provided a sensitive measure of inequality that could be readily applied in regulatory impact assessments to examine distributional impacts. In counterfactual analyses, disparities in air pollution attributable mortality tended to be reduced to a greater degree by reducing disparities in baseline mortality rates than reducing disparities air pollution exposure. This highlights the importance of accounting for neighborhood level variability in both baseline mortality and air pollution exposure in analyses of the distribution of air pollution attributable health impacts, and suggests that reducing inequality requires reducing disparities in both baseline mortality risk and air pollution exposure.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Acknowledgments
The authors thank Mr. Phil Blagden and Dr. Li Chen, Health Canada, for helpful comments. NO2 and PM2.5 metrics, indexed to DMTI Spatial Inc. postal codes, were provided by CANUE (Canadian Urban Environmental Health Research Consortium). This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Stieb, D. M. , Smith‐Doiron, M. , Quick, M. , Christidis, T. , Xi, G. , Miles, R. M. , et al. (2023). Inequality in the distribution of air pollution attributable mortality within Canadian cities. GeoHealth, 7, e2023GH000816. 10.1029/2023GH000816
Data Availability Statement
Mortality baseline rate data at the census tract (neighborhood) level used in this study are not publicly available due to confidentiality of personal information. These data were made available to Health Canada through Statistics Canada. Surface PM2.5 and NO2 data sets for this study are available at https://www.canuedata.ca/metadata.php. Demographic data by census tract are available at: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/download-telecharger/comp/page_dl-tc.cfm?Lang=E.
References
- Bivand, R. , Kitt, T. , & Rowlington, B. (2019). R package “rgdal”: Bindings for the ‘geospatial’ data abstraction library. (version 1.4‐8). Retrieved from https://CRAN.R-project.org/package=rgdal
- Bougie, E. (2021). Acute‐care hospitalizations among First Nations people, Inuit and Métis: Results from the 2006 and 2011 Canadian Census Health and Environment Cohorts. Health Reports, 32(82), 18. [DOI] [PubMed] [Google Scholar]
- Buzzelli, M. , Jerrett, M. , Burnett, R. , & Finklestein, N. (2003). Spatiotemporal perspectives on air pollution and environmental justice in Hamilton, Canada, 1985‐1996. Annals of the Association of American Geographers, 93(3), 557–573. 10.1111/1467-8306.9303003 [DOI] [Google Scholar]
- CANUE—The Canadian Urban Environmental Health Research Consortium . (2018). NO2‐Supplementary‐Methods‐Documentation‐2013‐2016.pdf (pp. 1–2). Retrieved from https://canue.ca/wp-content/uploads/2020/05/NO2-Supplementary-Methods-Documentation-2013-2016.pdf
- CANUE—The Canadian Urban Environmental Health Research Consortium . (2023). Data portal. Retrieved from https://www.canuedata.ca/metadata.php
- Carey, M. (2022). Cost‐benefit analysis in federal agency rulemaking. Congressional Research Service. Retrieved from https://crsreports.congress.gov/ [Google Scholar]
- Castillo, M. D. , Kinney, P. L. , Southerland, V. , Arno, C. A. , Crawford, K. , van Donkelaar, A. , et al. (2021). Estimating intra‐urban inequities in PM2.5 ‐attributable health impacts: A case study for Washington, DC. GeoHealth, 5(11). 10.1029/2021GH000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni, G. , Porta, D. , Badaloni, C. , Stafoggia, M. , Eeftens, M. , Meliefste, K. , & Forastiere, F. (2012). Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environmental Health, 11(1), 48. 10.1186/1476-069X-11-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, W. , Cheng, J. , Allaire, J. , Sievert, C. , Schloerke, B. , Xie, Y. , et al. (2022). R package “Shiny”: Web application framework for R. (version 1.7.3). Retrieved from https://shiny.rstudio.com/
- Clark, L. P. , Millet, D. B. , & Marshall, J. D. (2014). National patterns in environmental injustice and inequality: Outdoor NO2 air pollution in the United States. PLoS One, 9(4), e94431. 10.1371/journal.pone.0094431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse, D. L. , Peters, P. A. , Hystad, P. , Brook, J. R. , van Donkelaar, A. , Martin, R. V. , et al. (2015). Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow‐up in the Canadian census health and environment cohort (CanCHEC). Environmental Health Perspectives, 123(11), 1180–1186. 10.1289/ehp.1409276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse, D. L. , Ross, N. A. , & Goldberg, M. S. (2009). Double burden of deprivation and high concentrations of ambient air pollution at the neighborhood scale in Montreal, Canada. Social Science & Medicine, 69(6), 971–981. 10.1016/j.socscimed.2009.07.010 [DOI] [PubMed] [Google Scholar]
- de Hoogh, K. , Chen, J. , Gulliver, J. , Hoffmann, B. , Hertel, O. , Ketzel, M. , et al. (2018). Spatial PM2.5, NO2, O3 and BC models for Western Europe—Evaluation of spatiotemporal stability. Environment International, 120, 81–92. 10.1016/j.envint.2018.07.036 [DOI] [PubMed] [Google Scholar]
- Devleesschauwer, B. , Willimes, S. , Van Malderen, C. , Konings, P. , & Speybroeck, N. (2017). rineq‐package: Statistical analysis of health inequalities (version 0.0.1). Retrieved from https://rdrr.io/github/brechtdv/rineq/man/rineq-package.html
- Di, Q. , Wang, Y. , Zanobetti, A. , Wang, Y. , Koutrakis, P. , Choirat, C. , et al. (2017). Air pollution and mortality in the Medicare population. New England Journal of Medicine, 376(26), 2513–2522. 10.1056/NEJMoa1702747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DMTI Spatial . (2015). CanMap postal code suite v2015.3 [Computer file]. DMTI Spatial. [Google Scholar]
- Doiron, D. , Setton, E. M. , Shairsingh, K. , Brauer, M. , Hystad, P. , Ross, N. A. , & Brook, J. R. (2020). Healthy built environment: Spatial patterns and relationships of multiple exposures and deprivation in Toronto, Montreal and Vancouver. Environment International, 143, 106003. 10.1016/j.envint.2020.106003 [DOI] [PubMed] [Google Scholar]
- Eeftens, M. , Beelen, R. , Fischer, P. , Brunekreef, B. , Meliefste, K. , & Hoek, G. (2011). Stability of measured and modelled spatial contrasts in NO2 over time. Occupational and Environmental Medicine, 68(10), 765–770. 10.1136/oem.2010.061135 [DOI] [PubMed] [Google Scholar]
- Fann, N. , Coffman, E. , Timin, B. , & Kelly, J. T. (2018). The estimated change in the level and distribution of PM2.5‐attributable health impacts in the United States: 2005–2014. Environmental Research, 167, 506–514. 10.1016/j.envres.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann, N. , Lamson, A. D. , Anenberg, S. C. , Wesson, K. , Risley, D. , & Hubbell, B. J. (2012). Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Analysis: An International Journal, 32, 81–95. 10.1111/j.1539-6924.2011.01630.x [DOI] [PubMed] [Google Scholar]
- Giang, A. , & Castellani, K. (2020). Cumulative air pollution indicators highlight unique patterns of injustice in urban Canada. Environmental Research Letters, 15(12), 124063. 10.1088/1748-9326/abcac5 [DOI] [Google Scholar]
- Government of Canada, S. C . (2017). Download, census profile, 2016 census. Retrieved from https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/download-telecharger/comp/page_dl-tc.cfm?Lang=E
- Government of Canada, S. C . (2021a). Dictionary, census of population, 2021—Census division (CD). Retrieved from https://www12.statcan.gc.ca/census-recensement/2021/ref/dict/az/Definition-eng.cfm?ID=geo008
- Government of Canada, S. C . (2021b). Dictionary, census of population, 2021—census subdivision (CSD). Retrieved from https://www12.statcan.gc.ca/census-recensement/2021/ref/dict/az/Definition-eng.cfm?ID=geo012
- Government of Canada, S. C . (2021c). Dictionary, census of population, 2021—Census tract (CT). Retrieved from https://www12.statcan.gc.ca/census-recensement/2021/ref/dict/az/Definition-eng.cfm?ID=geo013
- Grijalva, R. H. R. (2021). Environmental justice for all Act. Retrieved from https://www.congress.gov/bill/117th-congress/house-bill/2021/text
- Health Canada . (2021). Health impacts of air pollution in Canada: Estimates of premature deaths and nonfatal outcomes—2021 report. Retrieved from https://epe.lac-bac.gc.ca/100/201/301/weekly_acquisitions_list-ef/2021/21-21/publications.gc.ca/collections/collection_2021/sc-hc/H144-51-2021-eng.pdf
- Huangfu, P. , & Atkinson, R. (2020). Long‐term exposure to NO2 and O3 and all‐cause and respiratory mortality: A systematic review and meta‐analysis. Environment International, 144, 105998. 10.1016/j.envint.2020.105998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hystad, P. , Setton, E. , Cervantes, A. , Poplawski, K. , Deschenes, S. , Brauer, M. , et al. (2011). Creating national air pollution models for population exposure assessment in Canada. Environmental Health Perspectives, 119(8), 1123–1129. 10.1289/ehp.1002976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett, M. , Burnett, R. T. , Kanaroglou, P. , Eyles, J. , Finkelstein, N. , Giovis, C. , & Brook, J. R. (2001). A GIS–environmental justice analysis of particulate air pollution in Hamilton, Canada. Environment and Planning A: Economy and Space, 33(6), 955–973. 10.1068/a33137 [DOI] [Google Scholar]
- Lee, D. (2013). CARBayes: An R package for Bayesian spatial modeling with conditional autoregressive priors. Journal of Statistical Software, 55(13). 10.18637/jss.v055.i13 [DOI] [Google Scholar]
- Leroux, B. , Lei, X. , & Breslow, N. (2000). Estimation of disease rates in small areas: A new mixed model for spatial dependence. In Statistical models in epidemiology, the environment, and clinical trials (pp. 179–191). Springer. [Google Scholar]
- Manitoba Collaborative Data Portal . (2019). Community data map, Winnipeg health region 2019—Interactive web map. Retrieved from https://mangomap.com/cgreenwpg/maps/61783/community-data-map-winnipeg-health-region-2019?preview=true#
- Martenies, S. , Milando, C. , Williams, G. , & Batterman, S. (2017). Disease and health inequalities attributable to air pollutant exposure in Detroit, Michigan. International Journal of Environmental Research and Public Health, 14(10), 1243. 10.3390/ijerph14101243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauboules, C. (2020). Homelessness & supportive housing strategy. Presented at the City of Vancouver, Vancouver. Retrieved from https://council.vancouver.ca/20201007/documents/pspc1presentation.pdf
- May, E. (2021). C‐226 an Act respecting the development of a national strategy to assess, prevent and address environmental racism and to advance environmental justice. Retrieved from https://www.parl.ca/LegisInfo/en/bill/44-1/c-226
- Meng, X. , Liu, C. , Chen, R. , Sera, F. , Vicedo‐Cabrera, A. M. , Milojevic, A. , et al. (2021). Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: Multilocation analysis in 398 cities. BMJ, n534. 10.1136/bmj.n534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, C. J. L. , Aravkin, A. Y. , Zheng, P. , Abbafati, C. , Abbas, K. M. , Abbasi‐Kangevari, M. , et al. (2020). Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. The Lancet, 396(10258), 1223–1249. 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Environmental Assessment‐RTP Division Office of Research and Development . (2009). Integrated science assessment for particulate matter. US Environmental Protection Agency. [Google Scholar]
- Pappin, A. J. , Christidis, T. , Pinault, L. L. , Crouse, D. L. , Brook, J. R. , Erickson, A. , et al. (2019). Examining the shape of the association between low levels of fine particulate matter and mortality across three cycles of the Canadian census health and environment cohort. Environmental Health Perspectives, 127(10), 107008. 10.1289/EHP5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault, L. (2016). Socioeconomic differences in nitrogen dioxide ambient air pollution exposure among children in the three largest Canadian cities. Health Reports, 27(82), 9. [PubMed] [Google Scholar]
- Pinault, L. , Crouse, D. , Jerrett, M. , Brauer, M. , & Tjepkema, M. (2016). Spatial associations between socioeconomic groups and NO2 air pollution exposure within three large Canadian cities. Environmental Research, 147, 373–382. 10.1016/j.envres.2016.02.033 [DOI] [PubMed] [Google Scholar]
- Plummer, M. , Best, N. , Cowles, K. , & Vines, K. (2006). CODA: Convergence diagnosis and output analysis for MCMC. R News, 6(1), 7–11. [Google Scholar]
- R Core Team . (2019). R: A language and environment for statistical computing. Retrieved from https://www.R-project.org/
- Rosofsky, A. , Levy, J. I. , Zanobetti, A. , Janulewicz, P. , & Fabian, M. P. (2018). Temporal trends in air pollution exposure inequality in Massachusetts. Environmental Research, 161, 76–86. 10.1016/j.envres.2017.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi, F. V. , Parnia, A. , & Siddiqi, A. (2020). Trends in socioeconomic inequalities in premature and avoidable mortality in Canada, 1991–2016. Canadian Medical Association Journal, 192(39), E1114–E1128. 10.1503/cmaj.191723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorell, A. (2021). R package “DescTools”: Tools for descriptive statistics (version 0.99.41). Retrieved from https://cran.r-project.org/package=DescTools
- Smylie, J. , & Firestone, M. (2015). Back to the basics: Identifying and addressing underlying challenges in achieving high quality and relevant health statistics for Indigenous populations in Canada. Statistical Journal of the IAOS, 31(1), 67–87. 10.3233/SJI-150864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi, S. , Zietsman, J. , & Khreis, H. (2020). Burden of disease assessment of ambient air pollution and premature mortality in urban areas: The role of socioeconomic status and transportation. International Journal of Environmental Research and Public Health, 17(4), 1166. 10.3390/ijerph17041166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southerland, V. A. , Anenberg, S. C. , Harris, M. , Apte, J. , Hystad, P. , van Donkelaar, A. , et al. (2021). Assessing the distribution of air pollution health risks within cities: A neighborhood‐scale analysis leveraging high‐resolution data sets in the Bay Area, California. Environmental Health Perspectives, 129(3), EHP7679. 10.1289/EHP7679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller, E. , Proville, J. , Roy, A. , & Muller, N. Z. (2021). Mortality risk from PM2.5: A comparison of modeling approaches to identify disparities across racial/ethnic groups in policy outcomes. Environmental Health Perspectives, 129(12), 127004. 10.1289/EHP9001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada . (2017a). Low‐income cut‐offs, after tax (LICO‐AT). Retrieved from https://www12.statcan.gc.ca/census-recensement/2016/ref/dict/fam019-eng.cfm
- Statistics Canada . (2017b). Dictionary, census of population, 2016 aboriginal identity. Retrieved from https://www12.statcan.gc.ca/census-recensement/2016/ref/dict/pop001-eng.cfm
- Statistics Canada . (2018). Age, sex, type of dwelling, families, households, marital status, language, income, immigration and ethnocultural diversity, housing, aboriginal peoples, education, labor, journey to work, mobility and migration, and language of work for Canada, provinces and territories, and health regions, 2016 census catalogue number: 98‐401‐X2016058. Retrieved from https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/download-telecharger/comp/page_dl-tc.cfm?Lang=E
- Stieb, D. M. , Berjawi, R. , Emode, M. , Zheng, C. , Salama, D. , Hocking, R. , et al. (2021). Systematic review and meta‐analysis of cohort studies of long term outdoor nitrogen dioxide exposure and mortality. PLoS One, 16(2), e0246451. 10.1371/journal.pone.0246451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennekes, M. (2018). tmap: Thematic maps in R. Journal of Statistical Software, 84(6), 1–39. 10.18637/jss.v084.i06 30450020 [DOI] [Google Scholar]
- Tjepkema, M. , Bushnik, T. , & Bougie, E. (2019). Life expectancy of First Nations, Métis and Inuit household populations in Canada. Health Reports, 30(12), 3–10. 10.25318/82-003-X201901200001-ENG [DOI] [PubMed] [Google Scholar]
- Treasury Board of Canada Secretariat . (2022). Policy on cost‐benefit analysis. Retrieved from https://www.canada.ca/en/government/system/laws/developing-improving-federal-regulations/requirements-developing-managing-reviewing-regulations/guidelines-tools/policy-cost-benefit-analysis.html
- Tuson, M. , Yap, M. , Kok, M. R. , Murray, K. , Turlach, B. , & Whyatt, D. (2019). Incorporating geography into a new generalized theoretical and statistical framework addressing the modifiable areal unit problem. International Journal of Health Geographics, 18(1), 6. 10.1186/s12942-019-0170-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar, A. , Martin, R. V. , Li, C. , & Burnett, R. T. (2019). Regional estimates of chemical composition of fine particulate matter using a combined geoscience‐statistical method with information from satellites, models, and monitors. Environmental Science & Technology, 53(5), 2595–2611. 10.1021/acs.est.8b06392 [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Wang, Y. , Bechle, M. J. , Kim, S.‐Y. , Adams, P. J. , Pandis, S. N. , Pope, C. A. , et al. (2020). Spatial decomposition analysis of NO2 and PM2.5 air pollution in the United States. Atmospheric Environment, 241, 117470. 10.1016/j.atmosenv.2020.117470 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Mortality baseline rate data at the census tract (neighborhood) level used in this study are not publicly available due to confidentiality of personal information. These data were made available to Health Canada through Statistics Canada. Surface PM2.5 and NO2 data sets for this study are available at https://www.canuedata.ca/metadata.php. Demographic data by census tract are available at: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/download-telecharger/comp/page_dl-tc.cfm?Lang=E.


