Abstract
Introduction
Fear of progression (FoP) is associated with the quality of life and behavioral change in acute pancreatitis (AP) patients, but lack of assessment tools.
Aim
This study aimed to develop and evaluate the psychometric properties of the Fear of Progression Questionnaire-Short Form in AP patients (AP-FoP-Q-SF).
Methods
Internal consistency, factorial structure, convergent validity, and criterion validity of AP-FoP-Q-SF were assessed. A receiver operating characteristic (ROC) curve analysis was performed to identify the cutoff value for high FoP. Associations between patient variables and FoP were evaluated using multiple logistic regression. Wilcox rank sum test was used to analyses the costs and length of hospital stay of the patients with high FoP.
Results
The two-factor structure showed a good fit. Internal consistency was acceptable (Cronbach's α = 0.771). The cutoff of 26 identified 35.3% of patients with high FoP. High FoP scores were associated with age (OR = 0.96, 95%CI: 0.94–0.98), recurrence times (OR = 1.22, 95%CI: 1.02–1.45) and anxiety (OR = 1.27, 95%CI: 1.16–1.40). Patients with high FoP spent more cost and time in the hospital.
Conclusions
The AP-FoP-Q-SF is a good FoP tool for AP patients in China.
Implications for practice
Clinicians can use the AP-FoP-Q-SF to assess FoP and take promotion programs to avoid worse effects.
Keywords: Acute pancreatitis, Fear of progression, Validation study
Accessible Summary.
What is known on the subject?
-
•
Acute pancreatitis is a recurrent disease associated with diet, smoking and alcohol consumption, and patients with acute pancreatitis are fear of disease recurrence and aggravation.
-
•
To our knowledge, there is no tool for assessing the fear of progression in acute pancreatitis patients.
What this paper adds to existing knowledge?
-
•
This is the first study to develop and validate the tool to assess fear of progression for acute pancreatitis patients.
-
•
There are 35.3% of patients with acute pancreatitis having the high level of fear of progression.
-
•
More medical costs and longer hospital stays were incurred in patients with high fear of disease progression.
What are the implications for practice?
-
•
Clinicians can use the tool to assess fear of progression in patients with acute pancreatitis.
-
•
In clinical work, medical staffs should pay attention to the fear of progression in the patients with acute pancreatitis.
1. Introduction
Acute pancreatitis (AP) is a condition in which the pancreas becomes inflamed over a short period, with favorable outcome in most cases (80%) [1]. However, the etiology of acute pancreatitis is complex; the course of disease is unpredictable and progressive, and the relapses are common [2]. In addition, recurrent episodes of acute pancreatitis can progress to chronic pancreatitis, which can significantly reduce patients' quality of life and is associated with pancreatic cancer [3]. These increase fear in AP patients. Fear is a negative emotion, which reduces the patient's pain threshold, sleep quality, increases the adverse reaction of the digestive system, and takes actions that are not conducive to health [4]. Negative emotion is associated with longer hospital stays, higher hospital costs, and readmission [5,6]. Meanwhile, the lack of ability to self-manage the disease and decreased quality of life of patients with AP are associated with fear of future health [7].
Fear of future health has been found in patients with AP, acute recurrent pancreatitis, and chronic pancreatitis [3,7,8]. Fear in patients with AP is specific and important. However, the psychological problem of fear of disease progression is often underestimated or unrecognized. One reason is the lack of assessment tools, which makes fear of disease progression difficult to assess comprehensively.
In 2003, Professor Dankert proposed the concept of fear of progression (FoP), which is defined as fear of all the biopsychosocial consequences of disease progression or fear of illness recurrence [9]. The most common tools used to assess FoP are the Fear of Progression Questionnaire [10], Fear of Cancer Recurrence Inventory [11] and Fear of Progression Questionnaire-Short Form (FoP-Q-SF) [12]. However, they are not used in acute diseases, and none of them, to our knowledge, specifically assessed FoP in AP patients. The FoP-Q-SF has been validated in a variety of diseases, and our qualitative studies reported that the fear content of patients with AP was similar to the items in the FoP-Q-SF [13]. In order to effectively observe the psychological effect of FoP in AP patients, we need a suitable assessment tool.
Therefore, the purpose of this study was to further develop and improve the content of the questionnaire according to the fear of disease progression in patients with AP and verify its reliability and validity.
2. Methods
The development, validation, and reliability testing of the AP-FoP-Q-SF were performed in a prospective cross-sectional study.
2.1. Participants
A convenience sample of 417 participants was recruited from August 2020 to February 2021 in the gastroenterology departments of three tertiary hospitals in the eastern region of China. The inclusion criteria for inpatients were as follows: (a) diagnosis of AP were based on the 2012 revised Atlanta criteria [14]; (b) Patients aged ≥18 years and <80 years; and (c) agreement to participate in the study. The exclusion criteria were as follows: (a) diagnostic suspicion or a definitive diagnosis of severe illness, such as cancer or chronic failure of the heart, liver or other major organs; (b) pregnancy or breastfeeding; or (c) cognitive impairment or a history of psychiatric illness. The severity and systemic inflammatory response syndrome for AP patients are in accordance with the revised Atlanta consensus [14]. Computed tomography severity index (CTSI) was made according to Balthazar EJ et al. [15].
Patients self-administrated filled out the paper–based questionnaire after informed consent was obtained, and the completeness of the questionnaire was checked by the researcher after the patient finished. Patients with incomplete questionnaires were asked to complete the questionnaire on site. For sentences that were difficult to understand, standard guidelines were used to assist the patient.
2.2. Procedures
2.2.1. Preliminary adjustment of questionnaire items
In qualitative studies, Fear of pain and special treatments, worry about work and the burden on family members were often mentioned by patients with AP [8,16]. Besides, a qualitative study was conducted in 28 AP patients, they also expressed the fear of disease recurrence and further deteriorate, and they had to change their living habits to prevent a recurrence of the disease [13]. The contents of fear in AP patients were basically consistent with the items of the FoP-Q-SF. The FoP-Q-SF is a multidimensional self-report questionnaire comprising 12 items belonging to four categories (affective reactions, partnership/family issues, occupation, and loss of autonomy). Five Likert-style items were used to assess the degree of FoP (ranging from 1 (“never”) to 5 (“very often”)). In addition, Chinese version of FoP-Q-SF was verified (Wu, Ye, Li, & Liu, 2015). The items and expressions of the questionnaire were discussed and reached consensus by an internal expert group, consisting of 3 pancreatic disease experts, 1 psychological expert and 1 scale expert, according to the characteristics of the disease, qualitative study results and the cultural background of AP patients.
2.2.2. Determination of questionnaire items
To develop the AP-FoP-Q-SF, an item analysis comprising the following six guidelines was conducted:
-
1.
If the distribution of answers revealed floor or ceiling effects, i.e., a value of 1 or 5 accounted for more than 50% of the responses, respectively, questions were excluded because of insufficient measurement precision [17].
-
2.
If the answer distribution showed extreme skewness, the items with skewness coefficients >2 were deleted [18].
-
3.
If the commonalities between the items indicated that the item could explain the variability of the same psychological trait, principal component analysis was used to extract one common factor to test the commonness among the items. If the factor loadings were less than 0.5, they were deleted [19].
-
4.
If the Pearson's correlation coefficient between item scores and total scale scores was ≤0.50, the item was deleted [20].
-
5.
The t-value was calculated between the items of the upper 27% and lower 27% of cases to test the discriminability of the questionnaire. If the items showed poor discriminability (t < 3), they were deleted.
-
6.
Cronbach's alpha method was adopted by analyzing whether the Cronbach's alpha of the instrument increased after deleting the current item.
2.2.3. Reliability and validity testing of the questionnaire
2.2.3.1. Validation
Three types of validation were investigated.
-
1.
Content validity: The FOP-Q-SF is a simplified version of the FOP-Q, which has been widely used and verified in a variety of diseases, indicating that relevant experts affirm its content. In addition, the internal expert panel of this study also discussed the content of the questionnaire and ensured that all important aspects were covered in it through consensus.
-
2.
Construct validity: Construct validity refers to the degree to which the questionnaire can measure a certain psychological trait. We used the first half of the data to conduct exploratory factor analysis (EFA) to explore the structure of the questionnaire. We then conducted confirmatory factor analysis (CFA) using the second half of the data to verify the factor structure and assess factor loadings and model fit.
-
3.
Criterion validity: Based on previous studies [21], the Hospital Anxiety and Depression Scale (HADS) and the shortened version of the SF-36 Health Survey (SF-12) were used to verify the criterion validity of the AP-FOP-Q-SF.
2.2.3.2. Reliability
To verify the stability and heterogeneity of the questionnaire results, the Cronbach's alpha and split-half reliability methods were adopted in this study.
2.3. Statistical analysis
The number of participants with no missing demographic or clinical characteristics, a completed AP-FoP-Q-SF and available HADS and SF-12 results was small (N = 416). This was managed using pairwise deletion. Descriptive statistics are used to describe the demographic and clinical characteristics. Frequency and percentages are used to summarize categorical variables. The mean ± standard deviation for normal distributions or the median (interquartile range, IQR) in case of non-normal distributions are used to summarize quantitative measures.
2.3.1. Psychometric validation of AP-FoP-Q-SF
To evaluate the construct validity of the AP-FoP-Q-SF, we randomly divided the sample into two datasets. The Kaiser-Meyer-Olkin test and Bartlett's test of sphericity were used to confirm the sample and item adequacy in one subsample of the two datasets. If the data were suitable for exploratory factor analysis (EFA), a principal component analysis with varimax rotation was used to assess the main factors. An eigenvalue >1 was used to determine the underlying factor structure. The other subsample was used for confirmatory factor analysis (CFA). The maximum-likelihood-robust estimation method was applied to estimate the fit of the underlying structure. The goodness-of-fit was verified by using the following indexes: (1) the relative chi-square (χ2/df < 3), (2) the root mean square error of approximation (RMSEA<0.08), (3) the normed fit index (NFI>0.90), (4) the incremental fit index (IFI>0.90), (5) the Tucker-Lewis index (TLI>0.90) and (6) the comparative fit index (CFI>0.90) [22]. Convergent validity was then confirmed by comparing the Pearson's correlation coefficient of each item with the total score of the AP-FoP-Q-SF, average variance extracted (AVE), and construct reliability (CR). Spearman's correlation coefficients were calculated to test correlations between the FoP-Q-SF scores, the SF-12, and the HADS, and values higher than 0.50 indicated a strong correlation. Finally, measurement invariance was performed to assessthe psychometric equivalence of a construct across groups, using a series of nested models (configural model; metric invariance model; and scalar invariance model). To evaluate these nested models, the variation (Δ) of goodness-of-fit indicators (CFI and RMSEA) of the restricted models was taken into account. The criterion of group invariance was the variation of CFI is less than or equal to 0.010 and RMSEA is less than or equal to 0.015 [23,24].
Internal consistency was examined using Cronbach's alpha and split-half correlations with Spearman-Brown's correction, where values above 0.7 were considered acceptable.
2.3.2. Identification of patient clusters and characteristics associated with fear of progression
A receiver operating characteristic (ROC) curve analysis was performed to identify the most appropriate cutoff value for high FoP, using the HADS anxiety subscale cutoff (≥11 points) as the gold standard to detect moderate or severe FoP [21]. The sensitivity and specificity were calculated. Frequencies and percentages are used to describe the degree to which moderate to severe FoP was present in AP patients.
Univariate and multivariate logistic regression analyses were used to identify patient variables (including demographic, social and economic characteristics, disease status, anxiety/depression and quality of life level) independently associated with high FoP.
2.3.3. The negative effects of high FoP
Wilcox rank sum test was used to analyses the costs and length of hospital stay of the patients with high FoP.
All statistical analyses were performed using SPSS (version 26), AMOS (version 22) and Graph Prism 8. p values < 0.05 were considered significant.
2.4. Ethics
The study was reviewed by the Ethics Committee of Clinical Medical College, Yangzhou University (Ethics Number: 2021ky188).
3. Results
The development study included 417 patients. They all provided informed consent, but one patient only completed part of the study (due to disease). Patient characteristics are presented in Table 1.
Table 1.
Demographic characteristics of the included patients (N = 417).
| Characteristics | N (%) | Mean ± SD |
|---|---|---|
| Age (years) | 51.56 ± 14.30 | |
| Sex | ||
| Female | 179(42.9%) | |
| Male | 238(57.1%) | |
| Education level | ||
| Primary and below | 145(34.8%) | |
| Junior high | 127(30.5%) | |
| Senior high | 105(25.2%) | |
| College and above | 40(9.6%) | |
| Marital status | ||
| Married | 391(93.8%) | |
| Unmarried | 26(6.2%) | |
| Profession | ||
| Farmer or worker | 185(44.4%) | |
| Government or public institution employee | 51(12.2%) | |
| Other | 181(43.3%) | |
| Annual household income | ||
| <40,000¥ | 32(7.7%) | |
| 40,000–100,000¥ | 147(35.3%) | |
| 100,000–200,000¥ | 157(37.6%) | |
| >200,000¥ | 81(19.4%) | |
| Homestyle | ||
| Lives alone | 31(7.4%) | |
| With family | 371(89.0%) | |
| Other | 15(3.6%) | |
| Recurrence Times | 0.70 ± 1.33 | |
| Etiology | ||
| Biliary | 223(53.5%) | |
| Hyperlipidemia | 133(31.9%) | |
| Alcoholism | 12(2.9%) | |
| Other | 49(11.8%) | |
| Severity classification | ||
| SAP | 32(7.7%) | |
| MSAP | 330(79.1%) | |
| MAP | 55(13.2%) | |
| CTSI score | 5.10 ± 1.76 | |
| SIRS score | 0.86 ± 0.92 | |
| FoP score≥26 | 147 (35.3%) | |
Note: CTSI=Computed Tomography Severity Index; FoP=Fear of progression; MAP = Mild Acute Pancreatitis; MSAP=Moderately Severe Acute Pancreatitis; SAP=Severe Acute Pancreatitis; SD= Standard Deviation; SIRS= Systemic Inflammatory Response Syndrome.
3.1. Development of the FoP-Q-SF
The FoP-Q-SF questionnaire initially included 12 items. Experts adjusted the language of the items according to the disease characteristics of AP patients and the Chinese cultural background. We further interpreted “progress” in item 1 as “further deteriorate or recur”. We changed “contracting” and “stranger” in items 6 and 7 to “inheriting” and “others”, respectively. According to the qualitative results, the content of item 11 was changed to “I worry about being a burden to my family”. Three questions (Q6, Q7, Q10) in the FoP-Q-SF were excluded according to the item analysis (Table 2).
Table 2.
Descriptive statistics and item analysis for the AP-FoP-Q-SF (N = 417) Note: SD=Standard deviation.
| Item No. | Mean (SD) | Floor effect (%) | Ceiling effect (%) | Skewness | Factor loading | Item-total correlation | Upper |
Lower |
T | p | Cronbach's α if item deleted | Item exclusion or retention |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||||||||||
| 1 | 3.14 (1.23) | 10.8 | 17.7 | −0.05 | 0.59 | 0.58 | 4.07 (1.02) | 2.36 (1.12) | 12.28 | 0.000 | 0.776 | Retained |
| 2 | 2.33 (1.20) | 32.1 | 7.2 | 0.59 | 0.58 | 0.57 | 3.19 (1.28) | 1.46 (0.80) | 12.36 | 0.000 | 0.777 | Retained |
| 3 | 3.22 (1.20) | 8.4 | 20.4 | −0.01 | 0.51 | 0.51 | 4.08 (0.98) | 2.43 (1.11) | 12.10 | 0.000 | 0.784 | Retained |
| 4 | 2.40 (1.37) | 38.8 | 9.1 | 0.48 | 0.51 | 0.55 | 3.32 (1.42) | 1.56 (0.87) | 11.40 | 0.000 | 0.782 | Retained |
| 5 | 2.23 (1.18) | 31.9 | 8.2 | 0.90 | 0.59 | 0.57 | 3.17 (1.31) | 1.45 (0.77) | 12.19 | 0.000 | 0.777 | Retained |
| 6 | 1.39 (0.87) | 76.5 | 2.9 | 2.76 | 0.43 | 0.43 | 1.84 (1.30) | 1.09 (0.34) | 6.01 | 0.000 | 0.788 | Excluded |
| 7 | 1.79 (1.07) | 52.5 | 4.3 | 1.46 | 0.55 | 0.54 | 2.51 (1.42) | 1.31 (0.59) | 8.41 | 0.000 | 0.780 | Excluded |
| 8 | 2.17 (1.11) | 34.3 | 5.0 | 0.79 | 0.50 | 0.51 | 2.80 (1.26) | 1.55 (0.88) | 8.79 | 0.000 | 0.783 | Retained |
| 9 | 2.88 (1.22) | 16.5 | 12.9 | 0.11 | 0.66 | 0.64 | 3.87 (1.04) | 1.91 (0.93) | 15.28 | 0.000 | 0.769 | Retained |
| 10 | 2.03 (1.05) | 40.3 | 2.4 | 0.77 | 0.44 | 0.46 | 2.47 (1.22) | 1.48 (0.84) | 7.22 | 0.000 | 0.787 | Excluded |
| 11 | 2.87 (1.27) | 16.1 | 13.2 | 0.16 | 0.69 | 0.68 | 3.90 (1.07) | 1.88 (0.94) | 15.44 | 0.000 | 0.764 | Retained |
| 12 | 2.27 (1.29) | 40.0 | 6.7 | 0.61 | 0.56 | 0.60 | 3.23 (1.36) | 1.50 (0.85) | 11.63 | 0.000 | 0.775 | Retained |
3.2. Validation
3.2.1. Content validity
The experts agreed that the AP-FoP-Q-SF covers all the important areas.
3.2.2. Construct validity
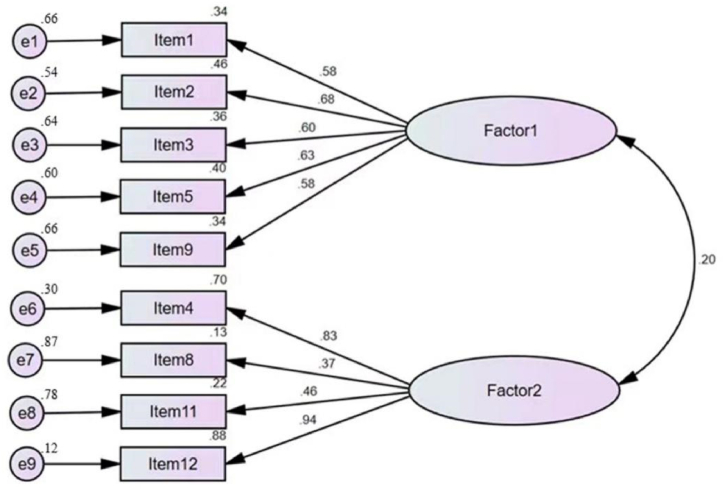
Principal component analysis identified two dimensions, which accounted for 57.4% of the variance in item scores. The factor analysis showed that only one item (item 11) had a factor loading greater than 0.45 in both dimensions, while the other items were significant in a single dimension. The results of the EFA are summarized in Table 3. After discussion among experts in the group and review of the published literature [25], item 11 was classified as factor 1. CFA matched the data to two factorial structures. The patch models are shown in Fig. 1. The goodness-of-fit indices of the 2-factor structural model are shown in Table 4. Factors 1 and 2 were named physical health and social and family, respectively. The results of convergent validity showed that item-total corrections ranging from 0.49–0.67, which were greater than 0.30. The AVE were 0.38 and 0.48, which were below 0.5 in the current study. However, a high level of CR (0.75 and 0.77, respectively) made the AVE value acceptable [26], as shown in Table 5.
Table 3.
Results of exploratory factor analysis (N = 122).
| NO. | Item description | Factor 1 | Factor 2 |
|---|---|---|---|
| 5 | When I am anxious, I have physical symptoms, e.g., rapid heartbeat, stomach ache, or nervousness. | 0.79 | 0.15 |
| 9 | I am afraid of severe medical treatments in the course of my illness. | 0.73 | 0.25 |
| 3 | I am afraid of pain. | 0.70 | 0.04 |
| 2 | I am nervous prior to doctors' appointments or examinations. | 0.70 | −0.00 |
| 1 | I become anxious if I think my disease may further deteriorate or recur. | 0.65 | 0.17 |
| 11 | I worry about being a burden to my family. | 0.56 | 0.46 |
| 4 | The thought that I might become less productive at my job upsets me. | −0.00 | 0.91 |
| 12 | The thought that I might not be able to work due to my illness upsets me. | 0.09 | 0.91 |
| 8 | I am worried I will no longer be able to pursue my hobbies because of my illness. | 0.25 | 0.51 |
Fig. 1.
Confirmatory factor analysis model.
Table 4.
Results of confirmatory factor analysis (N = 295).
| Fit indices | χ2/df | RMSEA | NFI | IFI | TLI | CFI |
|---|---|---|---|---|---|---|
| Value | 2.803 | 0.078 | 0.903 | 0.935 | 0.909 | 0.934 |
Note: χ2/df = chi squared/degrees of freedom, NFI = normed fit index, IFI = incremental fit index, TLI = Tucker-Lewis index, CFI = comparative fit index, and RMSEA = root mean square error of approximation.
Table 5.
Results of Convergent validity.
| Factor & Item. | Item-total correlation | AVE | CR |
|---|---|---|---|
| 1–1 | 0.60 | 0.38 | 0.75 |
| 1–2 | 0.60 | ||
| 1–3 | 0.55 | ||
| 1–5 | 0.60 | ||
| 1–9 | 0.64 | ||
| 2–4 | 0.58 | 0.48 | 0.77 |
| 2–8 | 0.49 | ||
| 2–11 | 0.67 | ||
| 2–12 | 0.61 |
Note: AVE = average variance extracted, CR = construct reliability.
3.2.3. Criterion validity
AP-FoP-Q-SF total scores and the health physical subscores were significantly correlated with HADS depression, HADS anxiety, and SF-12 quality of life (physical and mental component summaries). Social and family subscores were positively correlated with HADS anxiety scores and negatively correlated with mental quality scores. The results of the correlation analysis are shown in Table 6.
Table 6.
Association between AP-FoP-Q-SF scores and validation scale scores (N = 416).
| AP-FoP-Q-SF Scales | HADS anxiety | HADS depression | SF-12 physical score | SF-12 mental score |
|---|---|---|---|---|
| Physical health | 0.6348** | 0.494** | −0.219** | −0.487** |
| Social and family | 0.203** | 0.094 | 0.066 | −0.133** |
| Total score | 0.530** | 0.372** | −0.096* | −0.395** |
Note: * = p < 0.05, ** = p < 0.001.
3.3. Reliability
Cronbach's alpha coefficient for the AP-FoP-Q-SF was 0.771. The Guttman split-half correlation coefficient was calculated at 0.748.
3.4. Measurement invariance
We evaluated measurement invariance across gender and age group (Table 7). All models indicated a relatively good fit (CFI value is close to 0.9, RMSEA<0.10) [27,28]. The result showed the measurement invariance was achieved, allowing group comparisons.
Table 7.
Measurement invariance test according to gender(male vs female) and age group(≤50 vs>50) (N = 417).
| Model | x2 | df | CFI | TLI | RMSEA[90%CI] | SRMR | ΔCFI | ΔRMSEA |
|---|---|---|---|---|---|---|---|---|
| gender (Male,n = 238; Female, n = 179) | ||||||||
| M1: Configural | 152.864 | 52 | 0.906 | 0.869 | 0.096[0.079,0.114] | 0.087 | ||
| M2: Metric | 165.964 | 59 | 0.900 | 0.878 | 0.093[0.077,0.110] | 0.093 | 0.006 | 0.003 |
| M3: Scalar | 183.407 | 66 | 0.890 | 0.880 | 0.092[0.077,0.108] | 0.097 | 0.001 | 0.001 |
| age group(≤50years,n = 193; >50years,n = 224) | ||||||||
| M1: Configural | 151.046 | 52 | 0.907 | 0.871 | 0.096[0.078,0.114] | 0.087 | ||
| M2: Metric | 162.278 | 59 | 0.902 | 0.882 | 0.092[0.075,0.109] | 0.095 | 0.005 | 0.004 |
| M3: Scalar | 181.592 | 66 | 0.892 | 0.881 | 0.092[0.076,0.108] | 0.099 | 0.010 | 0.000 |
Note:x2=chi square, df = degrees of freedom, RMSEA = root mean square error of approximation, SRMR = standardized root mean square residual,CFA = comparative fit index; TLI = tucker-lewis index.
3.5. Identification of patient clusters and characteristics associated with fear of progression
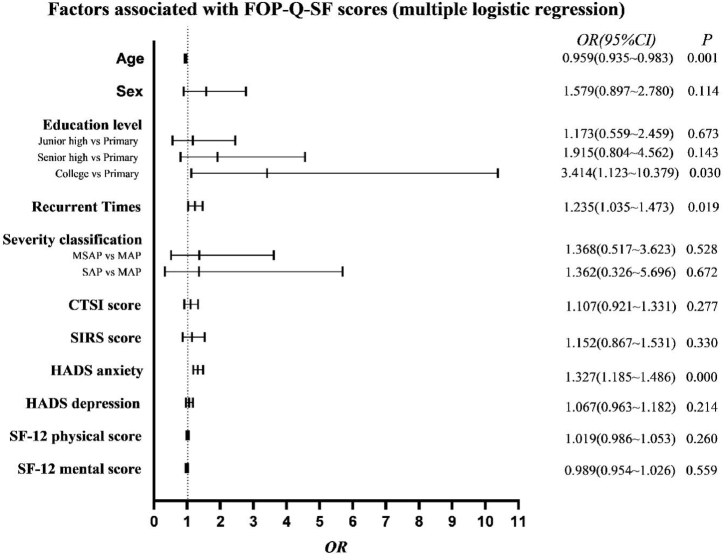
The total summary score on the AP-FoP-Q-SF was 23.506 (±6.581 SD, range 9–45). According to the ROC curve analysis, the best suited cutoff score for relevant FoP was 26 points, resulting in an area under the curve of 0.805 (95% CI: 0.747–0.863, p < 0.001). This provided a sensitivity of 74.0% and a specificity of 72.8%. A total of 147 patients (35.3%) reported FoP at or above the cutoff value of 26 points. Multiple logistic regression analysis indicated that age, education level, number of recurrences and HADS anxiety were independently associated with high FoP scores (Fig. 2).
Fig. 2.
Variables independently associated with a high FoP score.
3.6. The negative effects of high FoP
The patients with high FoP spent more costs and time to recover from AP than those who without high FoP (p = 0.031 and p = 0.046, respectively). (Table 8).
Table 8.
The different of the costs and the length of stay in hospital between AP patients with and without high FoP (N = 417).
| With high FoP median (IQR) | Without high FoP median (IQR) | Z value | p value | |
|---|---|---|---|---|
| Costs (¥) | 18044.27(21129.96) | 15428.51(15893.53) | −2.158 | 0.031 |
| Length of stay(days) | 10(8) | 9(6) | −1.991 | 0.046 |
Note: IQR = inter quartile range.
4. Discussions
The fear that the illness will progress is one of the main reasons for distress in patients with AP. In this study, we firstly developed the AP-FoP-Q-SF which demonstrated acceptable psychometric properties: high internal coherence, good discriminant validity, reliable measurement results and adequate stability. The AP-FoP-Q-SF is short (9 items), quick to use, simple to score and may be a useful tool in both routine practice and clinical trials. Secondly, we found almost one-third (35.3%) of evaluated patients had high fear scores, even though the disease is usually well managed, and these scores were associated with psychological distress. The result extends our knowledge that such a comparably high proportion is also rare even in different cancer populations [29]. One explanation of our finding regards the time of assessment in the disease trajectory. FoP is a consciously perceived fear that arises based on the real experience of a severe, potentially life-threatening or incapacitating illness. Specifically, the strong sense of life-threatening and hypervigilance to physical symptoms during hospitalization, which may account for the high prevalence. Furthermore, the high prevalence of FoP can be explained by the perceived threat and uncertainty of the disease. In addition, psychological distress interacts with the progress of the disease. The fears identified in this study need to be addressed even in patients whose disease is acute. Nurses should pay attention to this psychological problem, which is not only conducive to disease management, but also conducive to behavioral change according to various behavioral theories.
The AP-FoP-Q-SF is the first validated, reliable, and clinically feasible questionnaire developed for patients with AP to evaluate a specific fear. The original FoP-Q-SF was used only for chronic diseases. After expert discussion and qualitative interviews, the contents of the questionnaire were adjusted. According to the questionnaire data of patients with AP in multiple centers, scientific statistical analysis was carried out to exclude irrelevant and unimportant questionnaire items, making it more suitable for AP patients. Therefore, the AP-FoP-Q-SF was validated as a newly developed instrument. The single-factor and two-factors structure of FoP-Q-SF have been identified by the original and partner version [30]. Construct validity was identified by EFA, and the model was confirmed with CFA, concluding that physical health factors and social and family factors accounted for 57.4% of the total variance, which is considered a good result. The first component of our scale, physical health which is the foundation of FoP, assesses physical feelings and therapeutic thoughts. Those with higher scores are more worried about their physical condition compared with those with lower scores, concerning about the consequences of disease progression. The second component explained for 24.7% of the total variance, social and family, refers to fearing the impact of disease progression on the future, including family system and the role functions. Those with higher scores are more fear of losing value in the future compared with those with lower scores. Our findings suggested that it is an important part of FoP, which was different to that of previous studies [12,30]. This may due to the demographic factors of our patients. They are the breadwinners for the vast majority of families. A further test of criterion validity correlated the AP-FoP-Q-SF scores to the patients’ nonspecific psychological distress, such as their HADS anxiety and depression scores, and quality of life scores. The significant correlation indicated that quality of life decreased with increasing AP-FoP-Q-SF total scores. We observed a moderate correlational association among FoP, anxiety and depression, indicating that total AP-FoP-Q-SF scores are related to the associated psychological burden. The correlation coefficient was similar to that in other research [21]. The reliability of the questionnaire is acceptable.
Three patient variables were also associated with high fear scores, namely, age, education level, recurrence times and HADS anxiety. Our results showed that younger patients tended to report more fear. One possible reason is that they consider their disease to be more unexpected and experience higher levels of psychological distress. The other reason is that younger patients have more responsibilities in their family and society, so the disease makes them more fearful. Our result also suggested that patients with college education experience had a higher level of FoP than patients with primary education experience, which is different from the studies of Zheng Hu and Meissner et al. [31,32]. Consistent with us, the higher the educational level, the more fear of coronavirus disease 2019 [33]. Patients with high education level are more willing to learn disease-related knowledge. However, due to the limitation of access to relevantly correct knowledge, patients cannot comprehensively understand their own disease, and thus generate negative emotions of FoP. Furthermore, an association was found between HADS anxiety and FoP. Researchers found that anxious patients spend greater time thinking about the risk of recurrence [34]. There is an interesting result in this article that the more recurrences there were, the higher the FoP scores of patients with AP. The clinical symptoms and recurrence rate in recurrent AP are greater than those in patients experiencing a first attack [35,36]. Somatic cues trigger vulnerabilities and thereby elicit worry.
Patients with high FoP spent more costs and time, suggesting that FoP is associated with greater use of healthcare resources. As it in our study, Williams JTW et al. also found FoP may be treated cost-effectively [37]. Thus, appropriate FoP treatments may not only improve the individual quality of life, but also reduce the strain on the healthcare system.
4.1. Limitations
This study has certain limitations. Firstly, our sample was only from three tertiary centers and thus lacks representativeness and needs to be verified in more populations. Secondly, the scale dimensions were not extracted and determined after a qualitative interview, thereby lacking a theoretical basis. Thirdly, convenience sampling weakens the generalizability of the findings in our study. In addition, the cross-sectional study design did not enable us to assess test–retest reliability (i.e., stability over time). Hence, this aspect needs to be further evaluated.
5. Conclusions
The AP-FoP-Q-SF is the first valid and reliable, clinically feasible questionnaire for patients with AP in China. It assesses the most important aspects of FoP in AP patients, and the score reflects their quality of life. We recommend that it could be used in future research as a clinical instrument to evaluate and monitor FoP. The high FOP level of AP patients is related to age, recurrence times and anxiety, suggesting that these patients require more attention and close psychological guidance.
Author contribution statement
Shuli Ma; Xiaoxi Yang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Shengxiao Xiang; Guotao Lu; Weijuan Gong: Contributed reagents, materials, analysis tools or data; Analyzed and interpreted the data; Wrote the paper.
Weiwei Chen: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research was funded by National Natural Science Foundation (grant number: 82004291); 333 High-Level Talents Training Project of Jiangsu Province; Six Talent Peaks Project of Jiangsu Province (grant number: WSN-325) and Research Foundation of Affiliated Hospital of Nantong University (grant number: Tfh 2211).
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Arvanitakis M., et al. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis. Clin. Nutr. 2020;39(3):612–631. doi: 10.1016/j.clnu.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Petrov M.S., Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019;16(3):175–184. doi: 10.1038/s41575-018-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mokrowiecka A., et al. Clinical, emotional and social factors associated with quality of life in chronic pancreatitis. Pancreatology. 2010;10(1):39–46. doi: 10.1159/000225920. [DOI] [PubMed] [Google Scholar]
- 4.Shuli M., et al. Negative emotion in patients with acute pancreatitis and its risk factors:a mixed methods systematic review. Nurs. J. Chin. People's Liber. Army. 2021;38(7):6–9. doi: 10.3969/j.issn.1008-9993.2021.07.002. 15. [DOI] [Google Scholar]
- 5.Reja D., Weisberg I. Tu1602 Acute pancreatitis with comorbid anxiety and depression is associated with increased 30-day readmissions, length of stay, and costs of hospitalization: analysis of a nationwide inpatient cohort. Gastroenterology. 2020;158(6) doi: 10.1016/s0016-5085(20)33501-0. 1133. [DOI] [Google Scholar]
- 6.Bolourani S., et al. Risk factors for early readmission after acute pancreatitis: importance of timely interventions. J. Surg. Res. 2020;252:96–106. doi: 10.1016/j.jss.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Pezzilli R., et al. Evaluation of patient-reported outcome in subjects treated medically for acute pancreatitis: a follow-up study. Pancreatology. 2009;9(4):375–382. doi: 10.1159/000181171. [DOI] [PubMed] [Google Scholar]
- 8.Liu J., Zhang B. The lived experience of inpatients with acute recurrent pancreatitis: a qualitative research study from west China. Gastroenterol. Nurs. 2020;43(3):249–257. doi: 10.1097/sga.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 9.Herschbach P., Dinkel A. Fear of progression. Recent Results Cancer Res. 2014;197:11–29. doi: 10.1007/978-3-642-40187-9_2. [DOI] [PubMed] [Google Scholar]
- 10.Herschbach P., et al. Fear of progression in chronic diseases: psychometric properties of the fear of progression questionnaire. J. Psychosom. Res. 2005;58(6):505–511. doi: 10.1016/j.jpsychores.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Simard S., Savard J. Fear of cancer recurrence inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support. Care Cancer. 2008;17(3):241. doi: 10.1007/s00520-008-0444-y. [DOI] [PubMed] [Google Scholar]
- 12.Mehnert A., et al. Fear of progression in breast cancer patients--validation of the short form of the fear of progression questionnaire (FoP-Q-SF) Z. Psychosom. Med. Psychother. 2006;52(3):274–288. doi: 10.13109/zptm.2006.52.3.274. [DOI] [PubMed] [Google Scholar]
- 13.Ma S., et al. Psychological experience of inpatients with acute pancreatitis: a qualitative study. BMJ Open. 2022;12(6) doi: 10.1136/bmjopen-2021-060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks P.A., et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 15.Balthazar E.J., et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174(2):331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 16.Boije K., et al. Patients' perceptions of experiences of recovering from acute pancreatitis: an interview study. Gastroenterol. Nurs. 2019;42(3):233–241. doi: 10.1097/SGA.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 17.Theunis J., et al. Development and preliminary validation of the patient-reported chronic itch burden scale assessing health-related quality of life in chronic pruritus. Br. J. Dermatol. 2022;186(1):86–95. doi: 10.1111/bjd.20582. [DOI] [PubMed] [Google Scholar]
- 18.Bergman L., et al. Development and initial psychometric testing of the intrahospital transport safety scale in intensive care. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-038424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streiner D.L., Norman G.R., Cairney J. Health measurement scales: a practical guide to their development and use (5th edition) Aust. N. Z. J. Publ. Health. 2016;40(3):294–295. doi: 10.1111/1753-6405.12484. [DOI] [PubMed] [Google Scholar]
- 20.Li J., et al. Development and validation of the modified patient-reported outcome scale for chronic obstructive pulmonary disease (mCOPD-PRO) Int. J. Chronic Obstr. Pulm. Dis. 2020;15:661–669. doi: 10.2147/copd.S240842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner T., et al. Fear of cancer progression in patients with stage IA malignant melanoma. Eur. J. Cancer Care. 2018;27(5) doi: 10.1111/ecc.12901. [DOI] [PubMed] [Google Scholar]
- 22.Hu L.t., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives, structural equation modeling. A Multidiscip. J. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 23.Cheung G.W., Rensvold R.B. Evaluating goodness-of-fit indexes for testing measurement invariance. Struct. Equ. Model. 2002;9(2):233–255. doi: 10.1207/S15328007SEM0902_5. [DOI] [Google Scholar]
- 24.Chen F.F. Sensitivity of goodness of fit indexes to lack of measurement invariance. Struct. Equ. Model. 2007;14(3):464–504. doi: 10.1080/10705510701301834. [DOI] [Google Scholar]
- 25.Wu Q., et al. Vol. 50. 2015. pp. 1515–1519. (Reliability and validity of Chinese version of fear of progression questionnaire-short form for cancer patients). (12) [DOI] [Google Scholar]
- 26.Rababah J.A., Al-Hammouri M.M., Aldalaykeh M. Validation and measurement invariance of the Arabic health literacy questionnaire. Heliyon. 2022;8(4) doi: 10.1016/j.heliyon.2022.e09301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H., et al. Confirmatory and exploratory factor analysis for validating the phlegm pattern questionnaire for healthy subjects. Evid. Bas. Compl. Alter. Med. 2016 doi: 10.1155/2016/2696019. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabrigar L.R., et al. Vol. 4. 1999. (Evaluating the use of exploratory factor analysis in psychological research). (3) 272-229. [DOI] [Google Scholar]
- 29.Thiele S., et al. Fear of disease progression and relevant correlates in acute leukemia patients prior to allogeneic hematopoietic stem cell transplantation. Psycho Oncol. 2020;29(8):1248–1254. doi: 10.1002/pon.5397. [DOI] [PubMed] [Google Scholar]
- 30.Clever K., et al. Psychometric properties of the fear of progression questionnaire for parents of children with cancer (FoP-Q-SF/PR) J. Psychosom. Res. 2018;107:7–13. doi: 10.1016/j.jpsychores.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Zheng W., Hu M., Liu Y. Social support can alleviate the fear of cancer recurrence in postoperative patients with lung carcinoma. Am. J. Trans. Res. 2022;14(7):4804–4811. https://pubmed.ncbi.nlm.nih.gov/35958474/ [PMC free article] [PubMed] [Google Scholar]
- 32.Meissner V.H., et al. Fear of cancer recurrence and disease progression in long-term prostate cancer survivors after radical prostatectomy: a longitudinal study. Cancer. 2021;127(22):4287–4295. doi: 10.1002/cncr.33836. [DOI] [PubMed] [Google Scholar]
- 33.Zamanian M., et al. Fear and rumor associated with COVID-19 among Iranian adults, 2020. J. Educ. Health Promot. 2020;9:355. doi: 10.4103/jehp.jehp_589_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y., et al. Factors associated with fear of progression in Chinese cancer patients: sociodemographic, clinical and psychological variables. J. Psychosom. Res. 2018;114:18–24. doi: 10.1016/j.jpsychores.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Pai C.G., et al. Continuing episodes of pain in recurrent acute pancreatitis: prospective follow up on a standardised protocol with drugs and pancreatic endotherapy. World J. Gastroenterol. 2017;23(19):3538–3545. doi: 10.3748/wjg.v23.i19.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu B., et al. Progression to recurrent acute pancreatitis after a first attack of acute pancreatitis in adults. Pancreatology. 2020;20(7):1340–1346. doi: 10.1016/j.pan.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Williams J.T.W., Pearce A., Smith A. A systematic review of fear of cancer recurrence related healthcare use and intervention cost-effectiveness. Psycho Oncol. 2021;30(8):1185–1195. doi: 10.1002/pon.5673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.