Abstract
Background
Non-suicidal self-injury (NSSI) is a frequent and prominent phenomenon in major depressive disorder (MDD). Even though its prevalence and risk factors are relatively well understood, the potential mechanisms of NSSI in MDD remain elusive.
Aims
To review present evidence related to the potential mechanisms of NSSI in MDD.
Methods
According to Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines, articles for this systematic review were searched on Medline (through PubMed), Embase (through Elsevier), PsycINFO (through OVID) and Web of Science databases for English articles, as well as China National Knowledge Infrastructure (CNKI), SinoMed, Wanfang Data, and the Chongqing VIP Chinese Science and Technology Periodical (VIP) Databases for Chinese articles published from the date of inception to 2 August 2022. Two researchers (BW, HZ) independently screened studies based on inclusion and exclusion criteria and assessed their quality.
Results
A total of 25 157 studies were searched. Only 25 of them were ultimately included, containing 3336 subjects (1535 patients with MDD and NSSI, 1403 patients with MDD without NSSI and 398 HCs). Included studies were divided into 6 categories: psychosocial factors (11 studies), neuroimaging (8 studies), stress and hypothalamic-pituitary-adrenal (HPA) axis (2 studies), pain perception (1 study), electroencephalogram (EEG) (2 studies) and epigenetics (1 study).
Conclusions
This systematic review indicates that patients with MDD and NSSI might have specific psychosocial factors, aberrant brain functions and neurochemical metabolisms, HPA axis dysfunctions, abnormal pain perceptions and epigenetic alterations.
Keywords: self-injurious behavior; depressive disorder, major
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Relevant risk factors and prevalence of non-suicidal self-injury (NSSI) have been frequently investigated; however, the potential mechanisms of NSSI remain unclear, especially in major depressive disorder (MDD).
WHAT THIS STUDY ADDS
This systematic review summarised 25 case-controlled original studies, indicating the possible mechanisms of psychosocial factors, aberrant brain functions and neurochemical metabolisms, hypothalamic-pituitary-adrenal (HPA) axis dysfunctions, abnormal pain perceptions and epigenetic alterations in MDD with NSSI.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Because of the high heterogeneity of included studies, more original research concerning the mechanisms of NSSI in subjects with MDD should be conducted to tailor personalised treatments of NSSI.
Introduction
Non-suicidal self-injury (NSSI) refers to the behaviour of deliberate self-injury to the surface of the body without any suicidal intention, including but not limited to cutting, burning, striking, needling, excessive friction, etc.1 It is a prominent phenomenon with a worldwide prevalence of around 17.2% in adolescents, 13.4% in young adults and 5.5% in adults.2 Moreover, the prevalence of NSSI in adolescents raised to 40.9% during the coronavirus disease 2019 (COVID-19) pandemic.3 Not surprisingly, NSSI is one of the strong predictors of future suicidal behaviours.4–6
In the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fourth Edition,7 NSSI was regarded as one of the symptoms of borderline personality disorder (BPD). Virtually, NSSI can occur with any psychiatric disorder (eg, major depression disorder (MDD), BPD and substance use disorders).6 8–10 Considering its high prevalence and increased risk for mortality, NSSI has been counted as a potential discrete diagnostic entity in the DSM, Fifth Edition.1 Because of its heterogeneity in psychiatric disorders, it is better to stratify individuals with NSSI to understand further the mechanisms underlying NSSI.
MDD is one of the main contributors to the global burden of disease,11 12 with a weighted lifetime prevalence of 3.4% in China.13 However, 34.2% of patients with MDD reported having a history of NSSI,14 and individuals with NSSI were more likely to be diagnosed as MDD.15–17 Supported by robust evidence, NSSI is related to emotional dysregulation18–21 and depressive symptoms,22 23 which are more strongly associated with MDD.24 25 It was demonstrated that MDD and NSSI share an interactive effect on the risk of suicide, which is higher than with either MDD or NSSI alone.26 Furthermore, NSSI may play a mediating role between emotional reactivity and suicide risk in patients with MDD.27 Given its high comorbidity and increased mortality risk, we focused on the subgroup of NSSI in MDD to tailor personalised treatment for patients with MDD and NSSI. However, because of the absence of obviously effective treatment for NSSI,28 further exploration of the mechanisms of NSSI behaviour is needed. In recent years, research on the mechanisms has progressed, including the development of a four-function model,29 a theory of endorphin and child trauma,30 a hypothesis of abnormal pain perception31 and a model of addiction.32 Nevertheless, the theories were all based on mixed samples; thus, for lack of scientific rigour, they could not be indiscriminately applied to MDD. To better clarify this pathological behaviour, this systematic review aimed to summarise the potential mechanisms of NSSI in patients with MDD.
Method
Search strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. For studies from the date of inception to 2 August 2022, we searched Medline (through PubMed), Embase (through Elsevier), PsycINFO (through OVID), and Web of Science databases for published English articles and the China National Knowledge Infrastructure (CNKI), SinoMed, Wanfang Data, and the Chongqing VIP Chinese Science and Technology Periodical (VIP) Databases for articles published in Chinese. For English databases, keywords were the following: (“Self-Injurious Behavior”[Mesh] OR self injurious behavior*[tiab] OR intentional self injur*[tiab] OR intentional self harm*[tiab] OR nonsuicidal self injur*[tiab] OR deliberate self-harm*[tiab] OR deliberate self harm*[tiab] OR self-injur*[tiab] OR self injur*[tiab] OR non-suicidal self injur*[tiab] OR non suicidal self injur*[tiab] OR self harm*[tiab] OR self-destructive behavior*[tiab] OR self destructive behavior*[tiab] OR NSSI[tiab]) AND (“Depressive Disorder”[Mesh] OR Depressive Disorder*[tiab] OR Depressive Neuros*[tiab] OR Endogenous Depression*[tiab] OR Depressive Syndrome*[tiab] OR Neurotic Depression*[tiab] OR Melancholia*[tiab] OR Unipolar Depression*[tiab]). Similar search strategies were also performed in Chinese databases. More specifically, the main Chinese search terms were: (“Self-injurious behavior” OR “Self-injury” OR “Self-mutilation” OR “Self-destruction” OR “NSSI”) AND (“Depression” OR “Melancholia” OR “Depressive Disorder” OR “Depressive syndrome”). The retrieval strategy was modified accordingly for different databases. A more detailed research strategy can be seen in the online supplemental file. In addition, the reference lists of the included studies were manually searched to find further relevant research.
gpsych-2022-100946supp003.pdf (80.7KB, pdf)
Eligibility criteria
We selected original articles on the mechanisms of NSSI in patients with MDD, including psychosocial and biological dimensions. Animal models can only imitate self-injurious behaviours, not replicate actual NSSI. So even using the popular primate model, the rhesus macaque, it is challenging to imitate NSSI when accompanied by an affective disorder.33 Therefore, we only selected the potential mechanisms research in humans.
The inclusion criteria were as follows: (1) original studies evaluating the mechanisms of NSSI in patients with MDD; (2) peer-reviewed journal articles written in English or Chinese (for Chinese articles, titles and abstracts in English were required); (3) case-control studies comparing patients with MDD and NSSI (MDD+NSSI) and patients with MDD without NSSI (MDD−NSSI) or healthy controls (HCs); (4) having a strict definition of NSSI, including a clear distinction from suicidal behaviour and suicide attempt; (5)the diagnosis of MDD was made by specialised psychiatrists or based on the International Statistical Classification of Diseases and Related Health Problems or DSM system. We excluded studies if they met the following criteria: (1) animal studies, reviews, case reports, meeting abstracts and editorials; (2) articles written in other languages or without peer review.
Study selection and data collection
Two researchers (BW, HZ) independently screened the titles and abstracts of the retrieved studies to assess their eligibility for recruitment into this systematic review. Full texts of eligible studies were further assessed to identify additional studies for inclusion. Any potential conflicts or disputes were resolved by discussion. Using standardised Excel sheets, the following data were extracted and recorded for all included studies: authors, year of publication, sample size, methods and main results (difference in descriptions between patients with MDD and NSSI and those without NSSI).
Quality evaluation of studies
According to the research type demanded by the eligibility criteria, the Newcastle-Ottawa Quality Assessment Scale (NOS) for case-control studies, commonly used in systematic reviews, was applied to evaluate the risk of bias of the selected studies.34 The scale is composed of three dimensions: selection, comparability and exposure. Judgement was based on the definition and representativeness of the subjects, the selection and definition of the controls, the main and additional factors controlled between groups, the ascertainment of exposure and the methods used for ascertaining the exposure between groups, and the non-response rate. A study could be awarded a maximum of nine stars to reflect the quality.35 Two researchers (BW, HZ) independently completed the quality assessment and the differences were resolved by discussion.
Statistical method
Because of the high heterogeneity of the included evidence that had completely different experimental designs and domains interested in (online supplemental table 1), we only performed qualitative research. Thus, effect measures, synthesis methods and certainty assessment which could be found in a meta-analysis were not used in this systematic review.
gpsych-2022-100946supp001.pdf (113.4KB, pdf)
Results
Study characteristics
The initial retrieval search yielded 25 157 records in English and Chinese, with a total of 21 177 studies remaining after removing duplicates. Then, an additional 21 100 were excluded from this systematic review by screening titles and abstracts. After assessing full texts, 17 English and 8 Chinese studies were included in this analysis. The included literature had no research that was repeated in the two languages. The research processes for the English and Chinese databases are shown in figures 1 and 2, separately. The 25 included studies containing 3336 subjects (1535 MDD+NSSI patients, 1403 MDD−NSSI patients and 398 HCs), are shown in the online supplemental table 1. They consisted of 13 MDD+NSSI versus MDD−NSSI studies, 2 MDD+NSSI versus HC studies and 10 MDD+NSSI versus MDD−NSSI versus HC studies. As reviewed below, the included studies were divided into 6 categories: psychosocial factors14 20 36–44 (11 studies), neuroimaging45–52 (8 studies), stress and hypothalamic-pituitary-adrenal axis (HPA axis)53 54 (2 studies), pain perception55 (1 study), electroencephalogram (EEG)56 57 (2 studies) and epigenetics58 (1 study). The details of the 25 studies included are shown in online supplemental table 1.
Figure 1.
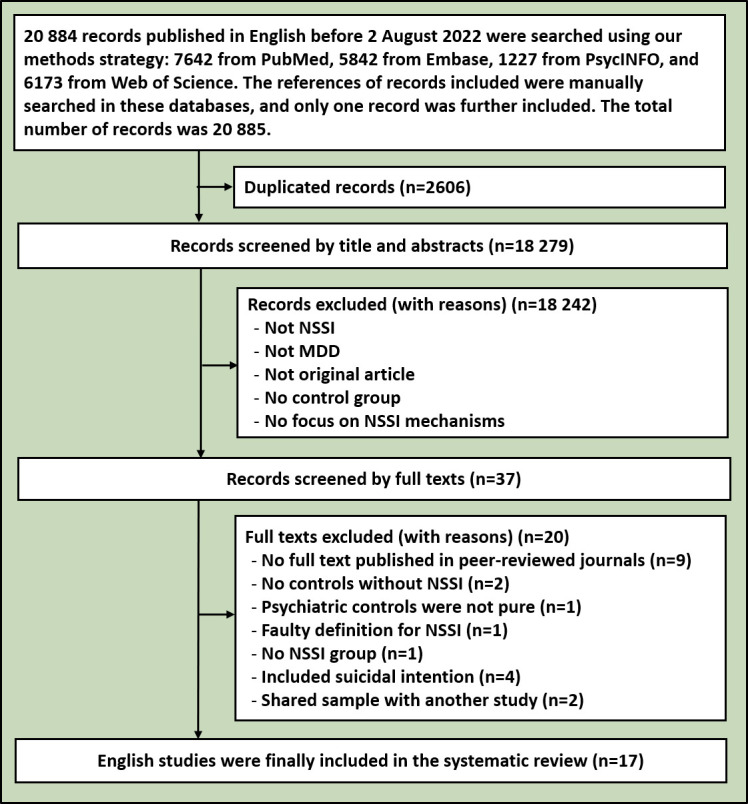
Flowchart of the search for relevant English references. MDD, major depressive disorder; NSSI, non-suicidal self-injury.
Figure 2.
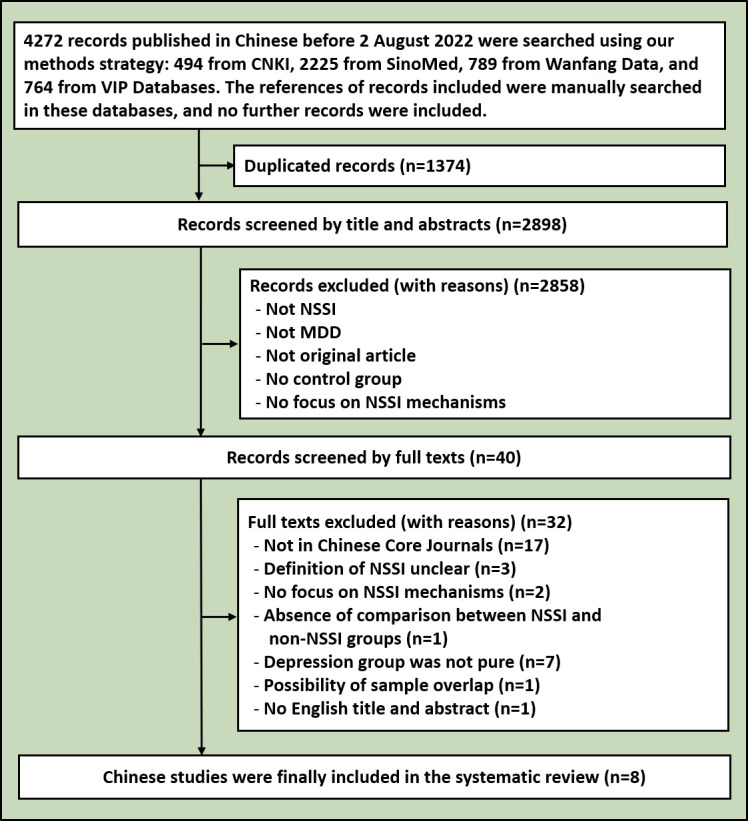
Flowchart of the search for relevant Chinese references. MDD, major depressive disorder; NSSI, non-suicidal self-injury.
Research quality
The results of the quality evaluation are displayed in online supplemental table 2. The total quality-rating scores of most of the included literature varied from 4 to 7, except for the study by Taş Torun et al which did not adjust for age or other demographic factors.44 The overall quality of the 25 studies was low because more than half of the included studies did not establish an HC group, and nearly all the studies identified the exposures without blinding. Only Xu et al grouped the depressive participants after completing the experiment.55 Nevertheless, all the studies applied the same method for ascertaining cases and controls and clarified the definition of controls.
gpsych-2022-100946supp002.pdf (107.5KB, pdf)
Psychosocial factors
As of the retrieval date, 11 studies had investigated psychosocial profiles of NSSI in patients with MDD, including goal-directed control, emotional regulation, personality traits, childhood maltreatment, impulsivity, alexithymia and interpersonal relationships. Chen et al used the Pavlovian‐to‐Instrumental Transfer (PIT) paradigm to investigate the dysfunction of goal‐directed control in patients with MDD and NSSI.36 The results showed that compared with HCs, the MDD+NSSI group had significantly poorer performance on PIT, which was negatively associated with NSSI frequency. This study figured out goal‐directed control deficits and a correlation with compulsivity in patients with MDD and NSSI.
The remaining studies only adopted clinical interviews and questionnaires to identify psychosocial risks and protective factors for the occurrence of NSSI in patients with MDD, and they omitted HCs. Specifically, nearly half of the literature mentioned that child abuse, especially emotional abuse and emotional neglect, might contribute to the generation of NSSI in patients with MDD.14 20 37–39 Additionally, compared to patients with MDD−NSSI, significant differences showed that patients with MDD+NSSI usually suffered from worse interpersonal and family relationships14 37 40 41 and more negative and stressful life events.20 41 Qian et al revealed that childhood maltreatment and stressful life events had an indirect effect on NSSI via adaptive cognitive emotional regulation instead of maladaptive strategies.20 Furthermore, Zuo et al enrolled a relatively large sample of 573 patients with MDD and figured out a diagnostic model in which childhood trauma and peer rejection might predict the development of NSSI in adolescents with MDD.39
Apart from child abuse and interpersonal relationships, Kang et al also found that compared to patients with MDD−NSSI, those with MDD+NSSI showed significant differences in personality traits (ie, psychoticism and neuroticism).14 By comparison, a higher level of impulsivity and a lower level of self-consciousness were found in adolescents with MDD+NSSI.42 Moreover, Shen et al discovered different parenting styles and alexithymia in patients with MDD+NSSI when opposed to depressed patients without NSSI.43 These results may provide potential evidence that these psychosocial factors may affect the occurrence of NSSI through chain mediation.
Considering that NSSI has motivational factors and multiple functions, Taş Torun et al focused on two types of its functions: interpersonal functions (eg, to influence others’ behaviour, to hurt/punish others) and intrapersonal functions (eg, emotional regulation, anti-dissociation, anti-suicide, and self-punishment).44 The results showed that the most common intrapersonal functions associated with NSSI were emotional regulation and marking distress, and the most frequent interpersonal functions were interpersonal boundaries and toughness. Interestingly, childhood trauma, alexithymia and emotional regulation abilities were also associated with the interpersonal or intrapersonal functions of NSSI.
Neuroimaging
Many of the included studies focused on neuroimaging, advancing our understanding of the neural substrates underlying NSSI. According to neuroimaging approaches, we divided the eight neuroimaging studies into task-state functional magnetic resonance imaging (task-fMRI) studies, resting-state fMRI (rs-fMRI) studies and magnetic resonance spectroscopy (MRS) studies.
Two studies used the task-fMRI technique to reveal brain activation patterns in patients with MDD+NSSI. Using the ‘Cyberball’ paradigm, a well-established experimental tool to arouse feelings of social exclusion, Groschwitz et al aimed to identify distinct neural processing of social rejection in MDD+NSSI versus MDD−NSSI versus HC.45 This study reported that compared to patients with MDD−NSSI and HCs, patients with MDD+NSSI had enhanced brain activation in the ventrolateral prefrontal cortex, and the medial prefrontal cortex, indicating specific neurophysiological responses of social exclusion in patients with MDD and NSSI. The others used an interpersonal self-processing task, including direct (self) and indirect (best friends’, mothers’ or classmates’) perspectives to reflect self-characteristics.46 Across all perspectives of self-processing, patients with MDD+NSSI showed higher brain activation in the superior frontal gyrus and less deactivation in the limbic structures, superior parietal lobule and middle temporal gyrus compared to patients with MMD−NSSI and HCs. Engaging in self-reflection, the NSSI group, from their mother’s perspective, showed more enhanced activation in the left and right amygdala, parahippocampus, hippocampus and fusiform than the other two groups.
In addition, 5 of the included studies also identified alterations of neural activity using resting state approaches, including amplitude of low-frequency fluctuation (ALFF) analysis, fractional amplitude of low-frequency fluctuation, regional homogeneity, functional connectivity (FC) and the brain network. In the research of Xin et al, only ALFF was applied; higher ALFF values were discovered in the left thalamus and right caudate nucleus and lower ALFF values were found in the right precuneus of patients with MDD+NSSI when contrasted with subjects with MMD−NSSI and HCs.47 Nevertheless, consistent results did not reappear in the other research. Specifically, Yan et al adopted FC and ALFF measures to explore the NSSI-related neural circuits and suggested that aberrant ALFFs were observed in the right middle occipital gyrus, right lingual gyrus and right superior frontal gyrus as well as altered FCs in these brain circuits in subjects with MDD+NSSI compared to subjects with MDD−NSSI.48 Additional inconsistent findings were reported in another study using FC and ALFF measures: significant neural activity alterations were observed in the right fusiform gyrus, the right median cingulate and the paracingulate gyri.49 Critically, Zhou et al also confirmed the above-mentioned neural activity alterations, especially those located in the default mode network (DMN).50 To address the question of how intrinsic brain networks communicate with each other in patients with MDD and NSSI, Ho et al compared groups in network coherence (ie, within-network connectivity) of the DMN, the salience network (SN) and the central executive network (CEN).51 This study demonstrated that patients with MDD+NSSI showed lower coherence in the insula-SN and anterior DMN and higher DMN-CEN connectivity compared to patients with MDD−NSSI and HCs. Interestingly, NSSI was specifically related to lower network coherence in insula-SN and all DMN subnetworks which were implicated with disruptions in interoceptive awareness.
Additionally, Zhang et al adopted an MRS technique to detect the neurobiochemical metabolic changes and executive dysfunction of NSSI in adolescents with MDD.52 This study suggested that patients with MDD+NSSI may suffer executive dysfunction and choline-containing compound metabolic alterations in the thalamus. Furthermore, the executive dysfunction may be implicated with the abnormal N-acetyl aspartate metabolism in the left thalamus and anterior cingulate cortex.
Stress and HPA axis
Only two studies concerning psychosocial stress were included; they were focused on the association between stress and HPA axis dysregulation. Klimes-Dougan et al used the Trier Social Stress Test and collected salivary cortisol during laboratory testing.53 The results showed that compared to subjects with MDD−NSSI and HCs, subjects with MDD+NSSI had the lowest level of salivary cortisol and the highest ratings of observed stress, suggesting blunted reactivity and recovery from psychosocial stress in patients with MDD+NSSI. The other study explored the association between childhood trauma and cortisol levels instead of measuring cortisol response during stress tests.54 Peng et al reported that in comparison to subjects with MDD−NSSI, the resting level of serum cortisol was lower in those with MDD+NSSI after controlling for interference factors. Moreover, a significantly negative correlation between serum cortisol levels and emotional neglect was only found in the subjects with MDD+NSSI.
Pain perception
Only one study focused on the pain perception of patients with NSSI. Manipulating an electronic pain measuring instrument, Xu et al measured the pressure pain threshold in the forearm to explore the potential pain mechanism.55 A higher pressure pain threshold with significant differences was found in adolescents with MDD+NSSI when compared with the MDD−NSSI group. The researchers also considered skin pressure pain threshold as an independent risk factor for NSSI behaviour in MDD.
Electroencephalogram
Only two EEG studies were included in the systematic review, both of which identified group effects among the subjects with MDD+NSSI versus those with MDD−NSSI versus HCs. Event-related potentials (ERPs) were used to assess the cognitive function of adolescents with NSSI in the study by Wen et al, where P50, P300, N400 and N170 were applied to evaluate the ability to selectively process stimuli, executive function and memory, language function as well as facial recognition ability, respectively.56 However, no statistically significant difference was found between the MDD+NSSI versus the MDD−NSSI subjects. Notable prolongation of the latency of the P3a, P3b, P50 and N1, N2 components, as well as a decrease of the amplitude of P50 and increasing inhibition of P50 (S1/S2), were observed in MDD+NSSI subjects compared with HCs. Moreover, the other EEG study concerned addictive perspectives of NSSI.57 Providing neutral pictures and self-injury-related pictures, researchers used a two-choice Oddball paradigm to examine the neural reactivity of NSSI. The amplitude of P3d, reflecting the process of response inhibition, showed a significant main effect of cue as well as a significant group×cue interaction. When exposed to the self-injury-related cues, the MDD+NSSI subjects showed a larger amplitude of P3d than the HCs. Only in the MDD+NSSI group were significant differences observed between the P3d amplitude with self-injury cues and with neutral cues.
Epigenetics
Only one included study addressed epigenetics in MDD+NSSI patients.58 Epigenetic alterations, especially those in the expression of the pro-opiomelanocortin (POMC) gene, which encodes the precursor of the adrenocorticotropic hormone, have been implicated with the occurrence and progression of MDD;59 Zheng et al aimed to assess the relationship between the DNA methylation of the POMC gene and NSSI in MDD. Compared with HCs, a higher methylation level of the POMC gene promoter region was displayed at the cytosine-guanine dinucleotide 1 site in subjects with NSSI.
Discussion
Main findings
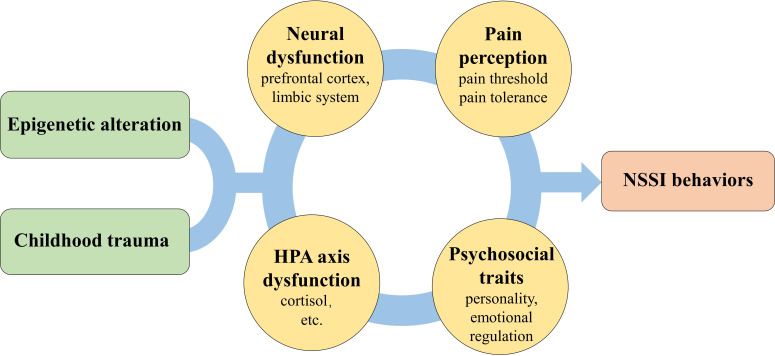
This systematic review identified the psychosocial and biological mechanisms underlying NSSI in patients with MDD, providing a preliminary step for understanding this clinical issue. Unfortunately, limited to the heterogeneity of the methodology and research design of the included studies, this review is unable to specify a rigorous model of NSSI in MDD. Thus, we have only discussed the potential mechanisms of NSSI in MDD in this section and briefly expanded upon NSSI in other disorders. Herein, we display a joint hypothesis theoretically incorporating childhood trauma, epigenetics and other biological factors which may, to a degree, underlie the behaviours of NSSI (figure 3).
Figure 3.
Potential mechanisms of NSSI in MDD. In this figure, we included 6 domains of 25 studies and hypothesised a potential model. Epigenetic alterations as well as childhood maltreatment might play a role in the dysfunction of neural activity, pain perception, HPA axis and psychosocial problems, which were associated with the behaviours of NSSI in patients with MDD. HPA, hypothalamic-pituitary-adrenal; MDD, major depressive disorder; NSSI, non-suicidal self-injury.
Some of the included studies in our review14 20 36–44 53 demonstrated that psychosocial factors may play an important role in the occurrence of NSSI in MDD, especially childhood trauma, which was comparably crucial in patients with other disorders,60–63. The relationship between NSSI and childhood trauma has also been pointed out in a high-level meta-analysis.64 Moreover, the relationship between NSSI and impulsivity,65 rumination66 and alexithymia67–69 has also been explored in mixed samples that had not been limited to patients with MDD.
Exposure to childhood trauma may trigger psychiatric disorders by altering the function of the HPA axis.70 71 As the correlation between the hyperactivation of the HPA axis and chronic stress has been indicated,72 childhood maltreatment could lead to higher cortisol levels.73 Our included studies demonstrated the dysfunction of the HPA axis and relevant epigenetic alteration in patients with MDD+NSSI.53 54 58 Moreover, an elevated cortisol awakening response was discovered in patients with NSSI and MDD74 or with non-specific disorders.75 In addition, a sibling study of adolescents reported more severe childhood adversity and showed higher hair cortisol levels in those with NSSI than their healthy siblings.76 Interestingly, as for resting-state cortisol, there was little difference between our study54 and self-injurious behaviour animal models, which had lower plasma cortisol levels than HCs.77 There may be an essential mechawas excluded because ofnism between NSSI and the HPA axis, but it is too early to draw a conclusion regarding the complex relationship among MDD, childhood trauma, NSSI and the HPA axis.
Notably, there was only one study focusing on the pain perception of NSSI in MDD. However, in mixed samples, relevant research has been repeated constantly. Higher hot pain and cold pain thresholds have been discovered in adolescents with NSSI when compared with HCs.78 79 A higher pain tolerance of patients with NSSI was also revealed in a systematic review.80 Interestingly, in the theory of childhood trauma, pain and endorphins, early psychological trauma may influence the system of the HPA axis and endogenous opioids, which ultimately alter the pain perception and trigger NSSI.81 Combining the above evidence, there might be a potential association between childhood trauma, pain perception and NSSI, but more research is needed, especially regarding MDD.
Over the past decade, an exciting discovery has been that the psychosocial environment can affect gene expression and even trigger epigenetic modifications of DNA.82 83 As discussed in the reviewed studies,14 20 37–39 58 childhood trauma may play a pivotal role in NSSI involved in hereditary mechanisms. An Australian study conducted multivariate biometric modelling and showed the correlation between high-risk trauma exposure and NSSI, regulated to some degree by the heritable factors in a mixed sample.84 Dopaminergic genes also have been investigated adequately. Additionally, a remarkable three-way interaction between the monoamine oxidase A gene, the catechol-O-methyltransferase gene, and childhood maltreatment was found in a sample of Chinese male teenagers with NSSI who had no major diseases.85 Furthermore, the brain-derived neurotrophic factor Val66Met polymorphism was found to regulate the relationship between NSSI and childhood emotional environments.86 Also, by detecting DNA methylation and mRNA expression,87 Wang et al found a higher methylation level of silent information regulator 2 related enzyme 1 (SIRT1) gene promoter region and a lower expression of Sirt1 protein, related to MDD in some manner, in subjects with MDD+NSSI compared with HCs. However, this study was excluded because of the possibility that the samples were shared with the research of Zheng et al.58
To this point, we have discussed the potential correlation between psychosocial traits, hereditary factors, HPA axis dysfunction, pain perception alteration and NSSI, where childhood trauma might play a role, according to the studies focused on MDD and other disorders. Aside from these domains, researchers have been exploring the brain function alteration of NSSI by neuroimaging and EEG, providing new angles to understand this behaviour. Addictive models, proposed to better tailor treatment approaches and strategies for NSSI,88 89 have elucidated the development of this behaviour. Although only one included study focused on it,57 cue reactivity, a crucial characteristic of addiction, has been examined repeatedly in the field of NSSI without the restriction of disease. Using a dot probe task (ie, NSSI-related cues, neutral cues and negatively valenced cues), Riquino et al measured the mechanism of attentional bias in young adults with NSSI who had no specific disorders.90 They showed significant attentional bias and experienced the torture of NSSI urges when exposed to NSSI-related cues rather than the other two cues. Moreover, Hooley et al used the task-fMRI technique and anNSSI-related experimental paradigm (namely, consisting of NSSI-related images, positive images, neutral images and negative images); they showed that when exposed to NSSI-related images and negative images, the NSSI group, who had no specific diagnosis, had significantly decreased amygdala activation and increased cingulate cortex and orbitofrontal cortex (OFC) activation compared with HCs.91 Although no similar study in our retrieval was conducted in patients with MDD and NSSI, aberrant limbic regions and frontal cortexes may be involved in the development of NSSI in MDD. Coincidentally, despite the difference in study design, aberrant limbic regions and frontal cortexes were also observed in our included studies,45 46 50 suggesting potential neural alteration in NSSI patients.
Close to the model of addiction, reward circuits have received widespread attention. Using monetary and social reward tasks, researchers also found reward process dysfunction in youth with MDD and NSSI.92 Disrupted connectivity between the bilateral caudate and putamen, insula, ventromedial prefrontal cortex and parietal operculum cortex was associated with NSSI when depressive symptoms were controlled.92 This study was excluded because it lacked diagnostic criteria for MDD. To our knowledge, by the final retrieval date, no additional related studies of MDD and NSSI have been reported. Thus, the following describes the results in NSSI patients without a specific diagnosis. Sauder et al conducted task-state fMRI with a monetary incentive delay task and found that NSSI participants showed less activation in the striatum, OFC and bilateral amygdala during reward anticipation compared with those without NSSI.93 Similarly, an ERP study, which revealed a heightened neural initial reward responsiveness to loss versus reward task in children with NSSI, provided further evidence for reward response alterations.94
Limitations
Although our review may shed insights into a deeper understanding of potential mechanisms of NSSI in MDD, there are still several limitations when interpreting our findings. First, to reduce the impact of disease heterogeneity, we only reviewed those studies that focused on the mechanisms of NSSI in MDD and included control groups. Hence, only a limited number of studies were included in this systematic review, and these have yielded inconsistent findings. Moreover, domain heterogeneity (ie, neuroimaging, HPA axis, pain perception, EEG, epigenetics and psychosocial factors) among the reviewed studies hinders drawing overall conclusions and applying quantitative methods. Also, all the studies included and discussed were observational studies from which we could not draw causal relationships. And there also existed limitations on methodology. We did not register on PROSPERO and did not publish a protocol in advance. Finally, limited by language, we could not search and include all published original articles on the mechanisms of NSSI.
Implications
Even though NSSI has become an increasingly serious clinical problem,95 effective interventions remain in the development phase.96 Thus, there is an urgency for more exploration of the mechanisms of NSSI. As this review pointed out, the mechanisms in psychosocial factors, aberrant brain functions, HPA axis dysfunctions, abnormal pain perceptions and epigenetic alterations may, to some extent, play an important role in the behaviours of NSSI. However, limited by the available evidence, we could not draw a scientific and linked hypothesis. To date, research on the mechanisms of NSSI in MDD has been insufficient (eg, in this systematic review we could not find even one article focusing on the theory of the endogenous opioid system in MDD and NSSI, which is so popular in the domain of NSSI97–99). Researchers have preferred to investigate the mechanisms in mixed samples of NSSI, so further work is required that specifically addresses NSSI and MDD or other specific disorders. Furthermore, research covering multiple dimensions is needed to delve deeper into the integrated mechanisms of NSSI.
Conclusions
Focusing on patients with MDD and NSSI, our systematic review included 25 original studies that involved various domains of mechanisms underlying NSSI. In summary, the above-mentioned findings indicated that patients with MDD and NSSI might have specific psychosocial factors, aberrant brain functions and neurochemical metabolisms, HPA axis dysfunctions, abnormal pain perception and epigenetic alterations. Although a large number of studies have focused on the mechanisms of NSSI regardless of disease diagnosis, limited research on combined MDD and NSSI impede our ability to draw overall conclusions. To address this gap, future research should incorporate relevant methodological and clinical covariates and pay more attention to the clinical implications of the mechanisms of NSSI in MDD.
Acknowledgments
The authors would like to acknowledge the reviewers of this article.
Biography
Baichuan Wu graduated from Shanghai Jiao Tong University School of Medicine, China in 2021. He is currently studying on a Doctorate’s program at Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, China. Since 2021, he has been engaged in research activities in the Division of Mood Disorders of Shanghai Mental Health Center as a student. His main research interests include the neurobiological mechanisms of non-suicidal self-injury, and he is participating in several cohort studies on depression.

Footnotes
Twitter: @CJH_gp
Contributors: BW: retrieval, screening and quality assessment of studies and writing the paper. HZ: retrieval, screening and quality assessment of studies and writing the paper. JH C: guidance of topic selection and revising the paper. JY C: revising the paper. ZL: revising the paper. YC: revising the paper. TY: guidance of topic selection and revising the paper. DP: guidance of topic selection, paper modification and the guarantor of the whole work.
Funding: This study was funded by Shanghai Science and Technology Committee (grant no. 20ZR1448500, YDZX20213100001003, 22YF1439100) and the National Natural Science Foundation of China (grant no. 82201678).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th edn. Arlington, VA: American Psychiatric Association, 2013. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 2. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav 2014;44:273–303. 10.1111/sltb.12070 [DOI] [PubMed] [Google Scholar]
- 3. Tang W-C, Lin M-P, You J, et al. Prevalence and psychosocial risk factors of nonsuicidal self-injury among adolescents during the COVID-19 outbreak. Curr Psychol 2021;2021:1–10. 10.1007/s12144-021-01931-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ribeiro JD, Franklin JC, Fox KR, et al. Self-injurious thoughts and behaviors as risk factors for future suicide ideation, attempts, and death: a meta-analysis of longitudinal studies. Psychol Med 2016;46:225–36. 10.1017/S0033291715001804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bryan CJ, Rudd MD, Wertenberger E, et al. Nonsuicidal self-injury as a prospective predictor of suicide attempts in a clinical sample of military personnel. Compr Psychiatry 2015;59:1–7. 10.1016/j.comppsych.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res 2006;144:65–72. 10.1016/j.psychres.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 7. American Psychiatric Association . Diagnostic and Statistical Manual of Mental disorders. 4th edn. Arlington, VA: American Psychiatric Association, 1994. [Google Scholar]
- 8. Ghinea D, Edinger A, Parzer P, et al. Non-suicidal self-injury disorder as a stand-alone diagnosis in a consecutive help-seeking sample of adolescents. J Affect Disord 2020;274:1122–5. 10.1016/j.jad.2020.06.009 [DOI] [PubMed] [Google Scholar]
- 9. Glenn CR, Klonsky ED. Nonsuicidal self-injury disorder: an empirical investigation in adolescent psychiatric patients. J Clin Child Adolesc Psychol 2013;42:496–507. 10.1080/15374416.2013.794699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nitkowski D, Petermann F. Non-suicidal self-injury and comorbid mental disorders: a review. Fortschr Neurol Psychiatr 2011;79:9–20. 10.1055/s-0029-1245772 [DOI] [PubMed] [Google Scholar]
- 11. Malhi GS, Mann JJ. Depression. Lancet 2018;392:2299–312. 10.1016/S0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- 12. Lu J, Xu X, Huang Y, et al. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry 2021;8:981–90. 10.1016/S2215-0366(21)00251-0 [DOI] [PubMed] [Google Scholar]
- 13. Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. The Lancet Psychiatry 2019;6:211–24. 10.1016/S2215-0366(18)30511-X [DOI] [PubMed] [Google Scholar]
- 14. Kang L, Li R, Liu H, et al. Nonsuicidal self-injury in undergraduate students with major depressive disorder: the role of psychosocial factors. J Affect Disord 2021;290:102–8. 10.1016/j.jad.2021.04.083 [DOI] [PubMed] [Google Scholar]
- 15. In-Albon T, Ruf C, Schmid M. Proposed diagnostic criteria for the DSM-5 of nonsuicidal self-injury in female adolescents: diagnostic and clinical correlates. Psychiatry J 2013;2013:159208. 10.1155/2013/159208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kiekens G, Hasking P, Claes L, et al. The DSM-5 nonsuicidal self-injury disorder among incoming college students: prevalence and associations with 12-month mental disorders and suicidal thoughts and behaviors. Depress Anxiety 2018;35:629–37. 10.1002/da.22754 [DOI] [PubMed] [Google Scholar]
- 17. Groschwitz RC, Kaess M, Fischer G, et al. The association of non-suicidal self-injury and suicidal behavior according to DSM-5 in adolescent psychiatric inpatients. Psychiatry Research 2015;228:454–61. 10.1016/j.psychres.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 18. Andover MS, Morris BW. Expanding and clarifying the role of emotion regulation in nonsuicidal self-injury. Can J Psychiatry 2014;59:569–75. 10.1177/070674371405901102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mettler J, Stern M, Lewis SP, et al. Perceived vs actual emotion reactivity and regulation in individuals with and without a history of NSSI. Front Psychol 2021;12:612792. 10.3389/fpsyg.2021.612792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qian H, Shu C, Feng L, et al. Childhood Maltreatment, stressful life events, cognitive emotion regulation strategies, and non-suicidal self-injury in adolescents and young adults with first-episode depressive disorder: direct and indirect pathways. Front Psychiatry 2022;13:838693. 10.3389/fpsyt.2022.838693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry 2019;59:25–36. 10.1016/j.eurpsy.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burke TA, Hamilton JL, Abramson LY, et al. Non-suicidal self-injury prospectively predicts interpersonal stressful life events and depressive symptoms among adolescent girls. Psychiatry Research 2015;228:416–24. 10.1016/j.psychres.2015.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burke TA, Fox K, Zelkowitz RL, et al. Does Nonsuicidal self-injury prospectively predict change in depression and self-criticism. 2019;43:345–53. 10.1007/s10608-018-9984-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park C, Rosenblat JD, Lee Y, et al. The neural systems of emotion regulation and abnormalities in major depressive disorder. Behav Brain Res 2019;367:181–8. 10.1016/j.bbr.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 25. Rive MM, van Rooijen G, Veltman DJ, et al. n.d. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev;37:2529–53. 10.1016/j.neubiorev.2013.07.018 [DOI] [PubMed] [Google Scholar]
- 26. Knorr AC, Tull MT, Anestis MD, et al. The interactive effect of major depression and nonsuicidal self-injury on current suicide risk and lifetime suicide attempts. Arch Suicide Res 2016;20:539–52. 10.1080/13811118.2016.1158679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang L, Cui Q, Liu J, et al. Emotion reactivity and suicide risk in patients with depression: the mediating role of non-suicidal self-injury and moderating role of childhood neglect. Front Psychiatry 2021;12:707181. 10.3389/fpsyt.2021.707181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plener PL, Kaess M, Schmahl C, et al. Nonsuicidal self-injury in adolescents. Dtsch Arztebl Int 2018;115:23–30. 10.3238/arztebl.2018.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nock MK. Why do people hurt themselves? New insights into the nature and functions of self-injury. Curr Dir Psychol Sci 2009;18:78–83. 10.1111/j.1467-8721.2009.01613.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord 2010;124:134–40. 10.1016/j.jad.2009.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lalouni M, Fust J, Bjureberg J, et al. Augmented pain inhibition and higher integration of pain modulatory brain networks in women with self-injury behavior. Mol Psychiatry 2022;27:3452–9. 10.1038/s41380-022-01639-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Victor SE, Glenn CR, Klonsky ED. Is non-suicidal self-injury an "addiction"? A comparison of craving in substance use and non-suicidal self-injury. Psychiatry Res 2012;197:73–7. 10.1016/j.psychres.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Novak MA, Meyer JS. A Rhesus monkey model of non-suicidal self-injury. Front Behav Neurosci 2021;15:674127. 10.3389/fnbeh.2021.674127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 35. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses, Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm [Accessed 7 Apr 2023].
- 36. Chen Q, Liu M, Wen R, et al. Dysfunction of goal-directed control in patients with depression and nonsuicidal self-injury. Brain Behav 2022;12:e2607. 10.1002/brb3.2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shao C, Wang X, Ma Q, et al. Analysis of risk factors of non-suicidal self-harm behavior in adolescents with depression. Ann Palliat Med 2021;10:9607–13. 10.21037/apm-21-1951 [DOI] [PubMed] [Google Scholar]
- 38. Zhong Y, Yang Y, Zhang Y, et al. Childhood trauma experiences and their impact on non-suicidal self-injury in adolescents with first episode depressive disorder. Chin J Psychiatry 2020;53:520–6. [Google Scholar]
- 39. Zuo T, Wang K, Hu Y, et al. The predictive role of childhood trauma and peer environment on non-suicidal self-injurious behavior in adolescents with depression. Chin J Psychiatry 2022;55:272–80. [Google Scholar]
- 40. Xiang J, Jin L, Qian H, et al. The influence of interpersonal relationship on non-suicidal self-injury behavior in adolescents with depression. Neural Injury Funct Reconstr 2022;17:444–8. [Google Scholar]
- 41. Xu M, Liu S, Chen J, et al. Relationships among life events, emotional symptoms and non-suicidal self-injury behaviors in adolescents with depression. J Psychiatry 2020;33:420–3. [Google Scholar]
- 42. Lu X, Zhu F, Liu Y, et al. A comparative study of impulsivity and self-consciousness between depressive adolescents with and without nonsuicidal self-injury. J Psychiatry 2018;31:325–7. [Google Scholar]
- 43. Shen X, Dong Z, Luo S, et al. A study on parenting style and alexithymia of depressive adolescent with non-suicidal self-injury behavior. J Neurosci Ment Health 2020;20:101–4. [Google Scholar]
- 44. Taş Torun Y, Gul H, Yaylali FH, et al. Intra/interpersonal functions of non-suicidal self-injury in adolescents with major depressive disorder: the role of emotion regulation, alexithymia, and childhood traumas. Psychiatry 2022;85:86–99. 10.1080/00332747.2021.1989854 [DOI] [PubMed] [Google Scholar]
- 45. Groschwitz RC, Plener PL, Groen G, et al. Differential neural processing of social exclusion in adolescents with non-suicidal self-injury: an fMRI study. Psychiatry Res Neuroimaging 2016;255:43–9. 10.1016/j.pscychresns.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 46. Quevedo K, Martin J, Scott H, et al. The neurobiology of self-knowledge in depressed and self-injurious youth. Psychiatry Res Neuroimaging 2016;254:145–55. 10.1016/j.pscychresns.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xin B, Wang C, Li N, et al. Study of spontaneous neural activities in first-episode of childhood and adolescent depressive disorder with non-suicidal self-injury. J Neurosci Ment Health 2022;22:13–7. [Google Scholar]
- 48. Yan R, Huang Y, Shi J, et al. Alterations of regional spontaneous neuronal activity and corresponding brain circuits related to non-suicidal self-injury in young adults with major depressive disorder. J Affect Disord 2022;305:8–18. 10.1016/j.jad.2022.02.040 [DOI] [PubMed] [Google Scholar]
- 49. Huang Q, Xiao M, Ai M, et al. Disruption of neural activity and functional connectivity in adolescents with major depressive disorder who engage in non-suicidal self-injury: a resting-state fMRI study. Front Psychiatry 2021;12:571532. 10.3389/fpsyt.2021.571532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou Y, Yu R, Ai M, et al. A resting state functional magnetic resonance imaging study of unmedicated adolescents with non-suicidal self-injury behaviors: evidence from the amplitude of low-frequency fluctuation and regional homogeneity indicator. Front Psychiatry 2022;13:925672. 10.3389/fpsyt.2022.925672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ho T, Walker J, Teresi G, et al. Default mode and salience network alterations in suicidal and non-suicidal self-injurious thoughts and behaviors in adolescents with depression. Neuropsychopharmacology 2020;45:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Y, Lai S, Wu W, et al. Associations between executive function impairment and biochemical abnormalities in depressed adolescents with non-suicidal self-injury. J Affect Disord 2022;298:492–9. 10.1016/j.jad.2021.10.132 [DOI] [PubMed] [Google Scholar]
- 53. Klimes-Dougan B, Begnel E, Almy B, et al. Hypothalamic-pituitary-adrenal axis dysregulation in depressed adolescents with non-suicidal self-injury. Psychoneuroendocrinology 2019;102:216–24. 10.1016/j.psyneuen.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 54. Peng B, Li J, Liu H, et al. Childhood maltreatment, low serum cortisol levels, and non-suicidal self-injury in young adults with major depressive disorders. Front Pediatr 2022;10:822046. 10.3389/fped.2022.822046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu L, Zhao J, Jin Y, et al. Studies on the related factors of non-suicidal self-injury in adolescent patients with depression. J Kunming Med University 2022;43:58–64. [Google Scholar]
- 56. Wen Y, Zhang X, Xu Y, et al. Cognitive impairment in adolescent major depressive disorder with nonsuicidal self-injury: evidence based on multi-indicator ERPs. Front Hum Neurosci 2021;15:637407. 10.3389/fnhum.2021.637407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou D-D, Zhao L, Ma L-L, et al. Altered neural reactivity in adolescents with nonsuicidal self-injury during exposure to self-injury related cues: electrophysiological evidence from a two-choice oddball paradigm. Front Psychiatry 2022;13:827480. 10.3389/fpsyt.2022.827480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zheng D, Bi X, Zhang T, et al. Epigenetic alterations of the promoter region of the POMC gene in adolescent depressive disorder patients with nonsuicidal self-injury behaviors. Psychol Res Behav Manag 2020;13:997–1008. 10.2147/PRBM.S272445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chang HS, Won ES, Lee H-Y, et al. The Association of proopiomelanocortin polymorphisms with the risk of major depressive disorder and the response to antidepressants via interactions with stressful life events. J Neural Transm (Vienna) 2015;122:59–68. 10.1007/s00702-014-1333-9 [DOI] [PubMed] [Google Scholar]
- 60. Brown RC, Heines S, Witt A, et al. The impact of child maltreatment on non-suicidal self-injury: data from a representative sample of the general population. BMC Psychiatry 2018;18:181. 10.1186/s12888-018-1754-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Franzke I, Wabnitz P, Catani C. Dissociation as a mediator of the relationship between childhood trauma and nonsuicidal self-injury in females: a path analytic approach. J Trauma Dissociation 2015;16:286–302. 10.1080/15299732.2015.989646 [DOI] [PubMed] [Google Scholar]
- 62. Lüdtke J, In-Albon T, Michel C, et al. Predictors for DSM-5 nonsuicidal self-injury in female adolescent inpatients: the role of childhood maltreatment, alexithymia, and dissociation. Psychiatry Res 2016;239:346–52. 10.1016/j.psychres.2016.02.026 [DOI] [PubMed] [Google Scholar]
- 63. Titelius EN, Cook E, Spas J, et al. Emotion dysregulation mediates the relationship between child maltreatment and non-suicidal self-injury. J Aggress Maltreat Trauma 2018;27:323–31. 10.1080/10926771.2017.1338814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry 2018;5:51–64. 10.1016/S2215-0366(17)30469-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hamza CA, Willoughby T, Heffer T. Impulsivity and nonsuicidal self-injury: a review and meta-analysis. Clin Psychol Rev 2015;38:13–24. 10.1016/j.cpr.2015.02.010 [DOI] [PubMed] [Google Scholar]
- 66. Coleman SE, Dunlop BJ, Hartley S, et al. The relationship between rumination and NSSI: a systematic review and meta-analysis. Br J Clin Psychol 2022;61:405–43. 10.1111/bjc.12350 [DOI] [PubMed] [Google Scholar]
- 67. Gatta M, Dal Santo F, Rago A, et al. Alexithymia, impulsiveness, and psychopathology in nonsuicidal self-injured adolescents. Neuropsychiatr Dis Treat 2016;12:2307–17. 10.2147/NDT.S106433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sleuwaegen E, Houben M, Claes L, et al. The relationship between non-suicidal self-injury and Alexithymia in borderline personality disorder: "actions instead of words". Compr Psychiatry 2017;77:80–8. 10.1016/j.comppsych.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 69. Tang W-C, Lin M-P, Wu JY-W, et al. Mediating role of depression in the association between alexithymia and nonsuicidal self-injury in a representative sample of adolescents in Taiwan. Child Adolesc Psychiatry Ment Health 2022;16:43. 10.1186/s13034-022-00477-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kuhlman KR, Chiang JJ, Horn S, et al. Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neurosci Biobehav Rev 2017;80:166–84. 10.1016/j.neubiorev.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gu W, Zhao Q, Yuan C, et al. Impact of adverse childhood experiences on the symptom severity of different mental disorders: a cross-diagnostic study. Gen Psychiatr 2022;35:e100741. 10.1136/gpsych-2021-100741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stalder T, Steudte-Schmiedgen S, Alexander N, et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 2017;77:261–74. 10.1016/j.psyneuen.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 73. Lu S, Gao W, Huang M, et al. In search of the HPA axis activity in unipolar depression patients with childhood trauma: combined cortisol awakening response and dexamethasone suppression test. J Psychiatr Res 2016;78:24–30. 10.1016/j.jpsychires.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 74. Klimes-Dougan B, Thai M, Schreiner MW, et al. Elevations in cortisol awakening response in depressed adolescents with a history of non-suicidal self injury. Biological Psychiatry 2018;83:S184. 10.1016/j.biopsych.2018.02.481 [DOI] [Google Scholar]
- 75. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology 2016;74:203–11. 10.1016/j.psyneuen.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 76. Reichl C, Brunner R, Bender N, et al. Adolescent nonsuicidal self-injury and cortisol response to the retrieval of adversity: a sibling study. Psychoneuroendocrinology 2019;110:104460. 10.1016/j.psyneuen.2019.104460 [DOI] [PubMed] [Google Scholar]
- 77. Tiefenbacher S, Novak MA, Jorgensen MJ, et al. Physiological correlates of self-injurious behavior in captive, socially-reared Rhesus monkeys. Psychoneuroendocrinology 2000;25:799–817. 10.1016/s0306-4530(00)00027-5 [DOI] [PubMed] [Google Scholar]
- 78. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord 2021;278:199–208. 10.1016/j.jad.2020.09.036 [DOI] [PubMed] [Google Scholar]
- 79. Miglani M, Chavan BS, Gupta N. Pain threshold and pain tolerance as a predictor of deliberate self-harm among adolescents and young adults. Indian J Psychiatry 2021;63:142–5. 10.4103/psychiatry.IndianJPsychiatry_348_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kirtley OJ, O’Carroll RE, O’Connor RC. Pain and self-harm: a systematic review. J Affect Disord 2016;203:347–63. 10.1016/j.jad.2016.05.068 [DOI] [PubMed] [Google Scholar]
- 81. Johnson BN, McKernan LC, Bruehl S. A theoretical endogenous opioid Neurobiological framework for co-occurring pain, trauma, and non-suicidal self-injury. Curr Pain Headache Rep 2022;26:405–14. 10.1007/s11916-022-01043-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thumfart KM, Jawaid A, Bright K, et al. Epigenetics of childhood trauma: long term sequelae and potential for treatment. Neurosci Biobehav Rev 2022;132:1049–66. 10.1016/j.neubiorev.2021.10.042 [DOI] [PubMed] [Google Scholar]
- 83. Jawaid A, Roszkowski M, Mansuy IM. Transgenerational epigenetics of traumatic stress. Prog Mol Biol Transl Sci 2018;158:273–98. 10.1016/bs.pmbts.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 84. Richmond-Rakerd LS, Trull TJ, Gizer IR, et al. Common genetic contributions to high-risk trauma exposure and self-injurious thoughts and behaviors. Psychol Med 2019;49:421–30. 10.1017/S0033291718001034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gao Y, Xiong Y, Liu X, et al. The effects of childhood maltreatment on non-suicidal self-injury in male adolescents: the moderating roles of the monoamine oxidase A (MAOA) gene and the catechol-O-methyltransferase (COMT) gene. IJERPH 2021;18:2598. 10.3390/ijerph18052598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bresin K, Sima Finy M, Verona E. Childhood emotional environment and self-injurious behaviors: the moderating role of the BDNF Val66Met polymorphism. Journal of Affective Disorders 2013;150:594–600. 10.1016/j.jad.2013.01.050 [DOI] [PubMed] [Google Scholar]
- 87. Wang L, Zheng D, Liu L, et al. Relationship between SIRT1 gene and adolescent depressive disorder with nonsuicidal self-injury behavior: based on gene methylation and mRNA expression. Medicine 2021;100:e26747. 10.1097/MD.0000000000026747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Blasco-Fontecilla H, Fernández-Fernández R, Colino L, et al. The addictive model of self-harming (non-suicidal and suicidal). Front Psychiatry 2016;7:8. 10.3389/fpsyt.2016.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pritchard TR, Fedchenko CA, Lewis SP. Self-injury is my drug: the functions of describing nonsuicidal self-injury as an addiction. J Nerv Ment Dis 2021;209:628–35. 10.1097/NMD.0000000000001359 [DOI] [PubMed] [Google Scholar]
- 90. Riquino MR, Reese SE, Garland EL. Assessing attentional bias toward nonsuicidal self-injury cues in young adults with histories of engaging in self-harm. Child Adolesc Soc Work J 2021;38:641–50. 10.1007/s10560-020-00692-2 [DOI] [Google Scholar]
- 91. Hooley JM, Dahlgren MK, Best SG, et al. Decreased amygdalar activation to NSSI-stimuli in people who engage in NSSI: a neuroimaging pilot study. Front Psychiatry 2020;11:238. 10.3389/fpsyt.2020.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Case JAC, Mattoni M, Olino TM. Examining the neurobiology of non-suicidal self-injury in children and adolescents: the role of reward responsivity. J Clin Med 2021;10:3561. 10.3390/jcm10163561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sauder CL, Derbidge CM, Beauchaine TP. Neural responses to monetary incentives among self-injuring adolescent girls. Dev Psychopathol 2016;28:277–91. 10.1017/S0954579415000449 [DOI] [PubMed] [Google Scholar]
- 94. Tsypes A, Owens M, Hajcak G, et al. Neural reward responsiveness in children who engage in nonsuicidal self-injury: an ERP study. J Child Psychol Psychiatry 2018;59:1289–97. 10.1111/jcpp.12919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Geoffroy M-C, Bouchard S, Per M, et al. Prevalence of suicidal ideation and self-harm behaviours in children aged 12 years and younger: a systematic review and meta-analysis. The Lancet Psychiatry 2022;9:703–14. 10.1016/S2215-0366(22)00193-6 [DOI] [PubMed] [Google Scholar]
- 96. Duan S, Wang H, Wilson A, et al. Developing a text messaging intervention to reduce deliberate self-harm in Chinese adolescents: qualitative study. JMIR Mhealth Uhealth 2020;8:e16963. 10.2196/16963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bresin K, Gordon KH. Endogenous opioids and nonsuicidal self-injury: a mechanism of affect regulation. Neurosci Biobehav Rev 2013;37:374–83. 10.1016/j.neubiorev.2013.01.020 [DOI] [PubMed] [Google Scholar]
- 98. Kirtley OJ, O’Carroll RE, O’Connor RC. The role of endogenous opioids in non-suicidal self-injurious behavior: methodological challenges. Neurosci Biobehav Rev 2015;48:186–9. 10.1016/j.neubiorev.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 99. Störkel LM, Karabatsiakis A, Hepp J, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology 2021;46:1357–63. 10.1038/s41386-020-00914-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gpsych-2022-100946supp003.pdf (80.7KB, pdf)
gpsych-2022-100946supp001.pdf (113.4KB, pdf)
gpsych-2022-100946supp002.pdf (107.5KB, pdf)