Abstract
Introduction
Acute mesenteric ischaemia (AMI) is a life-threatening condition with short-term mortality of up to 80%. The diagnosis of AMI has remained troublesome due to the non-specific clinical presentation, symptoms and laboratory findings. Early unambiguous diagnosis of AMI is critical to prevent progression from reversible to irreversible transmural intestinal damage, thereby decreasing morbidity and improving survival. The present study aims to validate a panel of plasma biomarkers and investigate volatile organic compound (VOC) profiles in exhaled air as a tool to timely and accurately diagnose AMI.
Methods and analysis
In this international multicentre prospective observational study, 120 patients (>18 years of age) will be recruited with clinical suspicion of AMI. Clinical suspicion is based on: (1) clinical manifestation, (2) physical examination, (3) laboratory measurements and (4) the physician’s consideration to perform a CT scan. The patient’s characteristics, repetitive blood samples and exhaled air will be prospectively collected. Plasma levels of mucosal damage markers intestinal fatty acid-binding protein and villin-1, as well as transmural damage marker smooth muscle protein 22-alpha, will be assessed by ELISA. Analysis of VOCs in exhaled air will be performed by gas chromatography time-of-flight mass spectrometry. Diagnosis of AMI will be based on CT, endovascular and surgical reports, clinical findings, and (if applicable) verified by histopathological examination.
Ethics and dissemination
The study protocol was approved by the Medical Research Ethics Committee (METC) of Maastricht University Medical Centre+ and Maastricht University (METC azM/UM), the Netherlands (METC19-010) and the Ethics Committee Research UZ/KU Leuven, Belgium (S63500). Executive boards and local METCs of other Dutch participating centres Gelre Ziekenhuizen (Apeldoorn), Medisch Spectrum Twente (Enschede), and University Medical Centre Groningen have granted permission to carry out this study. Study results will be disseminated via open-access peer-reviewed scientific journals and national/international conferences.
Trial registration number
Keywords: SURGERY, GASTROENTEROLOGY, INTENSIVE & CRITICAL CARE
Strengths and limitations of this study
This is the first observational prospective clinical study that evaluates a panel of novel biomarkers for acute mesenteric ischaemia (AMI) in a multicentre international clinical cohort.
This study will provide the first data on breath analysis in patients suspected of AMI.
This study will rely on accurate clinical documentation and a high-quality biobank.
Patient inclusion is challenging due to acute condition of most patients and low incidence of AMI.
Introduction
Background
Acute mesenteric ischaemia (AMI) is a life-threatening condition caused by a sudden interruption of blood flow, resulting in decreased supply of oxygen and nutrients to a segment of the intestinal tract. Prolonged periods of AMI lead to cellular damage and, when left untreated, to necrosis of the intestinal wall, which may cause peritonitis.1 2 The occurrence of AMI is rare, with a reported incidence between 0.09% and 0.2% (for all admissions to emergency departments) in patients with an unknown cause of abdominal pain3–5 but strongly increases with age.6 It remains a highly underestimated clinical emergency with short-term mortality of up to 80%.7–11 The clinical presentation for AMI is marked by non-specific signs and symptoms, including abdominal pain, elevated white cell count and metabolic acidosis.7 9 10 12 The non-specific clinical presentation of AMI, combined with the absence of a specific serum/plasma marker, often leads to a delay in the diagnosis. Available conventional blood laboratory tests such as leucocytes, C reactive protein, lactate and D-dimer have a restricted specificity to aid in diagnosing AMI.13–17 Radiological imaging is one of the most commonly used non-invasive techniques for confirming AMI.12 18 19 CT can be performed quickly compared with standard laboratory tests, and when combined with contrast enhancement of the vessels, so-called CT angiography (CTA) provides a detailed visualisation of the intestines and mesenteric vasculature. CTA is the current gold standard imaging modality for diagnosing AMI, with an estimated sensitivity and specificity of around 89%–100%.12 13 However, this is probably an overestimation since the study cohort primarily consisted of patients with advanced mesenteric ischaemia and not early or progressive mesenteric ischaemia.6 Moreover, a considerable percentage of patients with AMI present without ischaemia-specific CT signs, which overlap with other acute abdominal complications.20–22 Therefore, an around-the-clock available, highly accurate, minimally invasive and rapid diagnostic test can increase the index of suspicion for early AMI, reducing the time to adequate treatment.
In recent years, several clinical studies investigated more specific serological markers for diagnosing AMI and determining the severity of ischaemic intestinal damage.23–25 One of these potential biomarkers for AMI is intestinal fatty acid-binding protein (I-FABP), a small cytosolic protein that is abundantly expressed in mature enterocytes.26 On a decrease in bowel perfusion and consequent loss of enterocyte cell membrane integrity, a rapid release of I-FABP within the circulation is observed.23 27 Another mucosal marker for detecting intestinal mucosal damage is villin-1 (VIL-1), which, similar to I-FABP, is detectable in the plasma of rat and human models of mesenteric ischaemia.24 As opposed to I-FABP, VIL-1 remains detectable in plasma for more extended periods after the onset of ischaemic damage in rats.23 24 These findings identify I-FABP as a potential marker for early intestinal mucosal injury and VIL-1 as a potential marker for persisting ischaemic mucosal damage. Sustained periods of mesenteric ischaemia can lead to ischaemia of the intestinal muscle layers and, when left untreated, result in transmural ischaemia. Currently, known markers of mesenteric ischaemic damage focus primarily on mucosal injury, but they provide no insight regarding the possible development of transmural ischaemia. An earlier study showed that plasma levels of smooth muscle protein 22-alpha (SM22) could differentiate between patients with transmural ischaemia and those with mesenteric ischaemia confined to the mucosal layer.25 SM22 is a small protein (22 kDa) with a high expression in intestinal smooth muscle tissue28 29 and is released on sustaining ischaemic damage. However, the SM22 protein is not exclusively expressed in the intestinal muscle tissue.29 Still, in combination with other specific markers for intestinal mucosal damage, such as I-FABP, it is expected to provide insight into the severity and progression of intestinal injury in patients with AMI. Unfortunately, none of the described markers have yet made their appearance into the clinic. Currently, there is limited knowledge of the I-FABP, VIL-1 and SM22 specificity in patients with AMI.
In recent years, analysis of volatile organic compounds (VOCs) in exhaled air to diagnose various pathologies has gained increasing interest. The exhaled air of humans consists of a broad spectrum of VOCs. The composition of these VOCs is influenced by exogenous (oral ingestion, smoking, air quality) and endogenous (activity, microbiome, hormonal) factors.30 The hundreds of VOCs in exhaled air can give valuable information about various (patho)physiological processes. The analysis of VOCs in exhaled air is a non-invasive technique that has already been demonstrated to differentiate between multiple clinical conditions and healthy subjects, including inflammatory bowel disease and non-alcoholic steatohepatitis.31 In 2011, a pilot study on the analysis of VOCs in rats following acute superior mesenteric artery (SMA) occlusion identified a small cluster of VOCs that increase during ischaemic bowel injury.32 Other studies have explored the possibility of monitoring exhaled methane (CH4) concentrations in order to detect SMA malperfusion.33 This is now being investigated in a prospective observational study in patients with trauma-related haemorrhage.34 Based on these findings we could speculate that CH4 concentrations could be relevant in our future analyses. As the pathophysiological processes of inflammatory bowel disease and AMI share common mechanisms, it is expected that VOC profiling could aid in diagnosing AMI in a rapid and non-invasive manner in the future.35
Methods and analysis
Objectives
This study aims to improve the diagnosis of patients with AMI. Our primary objective is to validate the diagnostic accuracy of a selected panel of plasma and serum biomarkers, I-FABP, SM22 and VIL-1, in patients with AMI. Furthermore, we will investigate if these markers can determine the severity of ischaemic intestinal damage. The secondary objective of this study is to identify a VOC profile in exhaled breath to identify AMI non-invasively.
Study design and eligibility criteria
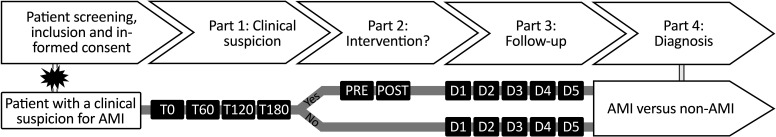
The current study is an international multicentre, prospective observational study aiming to include 120 patients with acute abdominal symptoms fitting to AMI. The main objective is to compare biomarker expression in 60 patients with confirmed mesenteric ischaemia and 60 patients with another clinical condition. We may include a higher percentage of patients without mesenteric ischaemia due to its non-specific clinical presentation and low overall incidence. Therefore, study inclusions will be finalised when 60 patients with confirmed AMI are included.3–5 Patients clinically suspected of AMI will be screened for inclusion at one of the participating centres. All study participants must fulfil the study inclusion criteria and will be excluded from participation if they cannot provide written informed consent or do not fulfil the inclusion criteria (box 1). Patients are eligible for study participation if they have a clinical suspicion of AMI, which is based on1 the clinical manifestation of the disease,2 physical examination by the local physician,3 laboratory measurements and4 the physician’s consideration to perform a CT(A)-scan. If all criteria are met, the physician will contact the local research team and the patient or legal representative will be asked for informed consent to participate in the study. Clinical study procedures are initiated when all criteria are met and informed consent is obtained from the patient or legal representative. After inclusion, blood and exhaled breath will be collected at consecutive time points (figure 1).
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
The ability to provide informed consent, either by themselves or by a legal representative.
-
The patients must be suspected of AMI, which is based on the following:
Clinical manifestation of the disease such as sudden abdominal pain, nausea, vomiting, abdominal distension, diarrhoea, haematochezia, haematemesis, tenderness and signs of peritonitis.
Physical examination by the local physician such as body temperature, heart rate, blood pressure.
Laboratory measurements such as white cell count, lactate, pH, CRP.
Physician’s consideration to perform CT(A) scan.
Exclusion criteria
Unable to provide informed consent.
<18 years of age.
AMI, acute mesenteric ischaemia; CRP, C reactive protein; CTA, CT angiography.
Figure 1.
Study outline. Patients with a clinical suspicion of acute mesenteric ischaemia (AMI) are considered applicable for participation if all criteria are met. Patients are screened and asked for study participation by informed consent. When consent has been received, study procedures will start. Part 1: blood and exhaled air are collected at inclusion (T0) and every 60 min, up to 180 min (T180) after inclusion; part 2 is initiated if the patient receives an intervention (endovascular or surgical), with a preoperative and postoperative sample collection. Patients that do not receive the intervention will directly move into part 3: follow-up. Daily samples up to 5 days (D1-D5) will be retrieved during routine blood collection. In the final stage of the study (part 4: diagnosis), each patient will be placed in one of the two study groups (AMI vs non-AMI) based on the collected data. POST, postoperative; PRE, preoperative; T, time point.
Study sponsor
The sponsor (Maastricht University, Maastricht, The Netherlands) is responsible for the study design and management and for obtaining all study authorisations (Clinical Trial Centre Maastricht and medical research ethics committee). Furthermore, the study sponsor also declares all information regarding the inclusion period, beginning and end, final study report, and study results to these authorities. Finally, all obtained study samples and study-related documents will be stored for at least 15 years after the study ends.
Study population and participating medical centres
The study population will consist of patients clinically suspected of AMI admitted at Maastricht University Medical Centre+ (MUMC+, Maastricht, The Netherlands), Amsterdam University Medical Centre (AUMC), location VUmc and AMC, Gelre Ziekenhuizen Apeldoorn (Apeldoorn, The Netherlands), Medisch Spectrum Twente, Enschede, The Netherlands, University Medical Centre Groningen, The Netherlands and University Hospitals Leuven, Belgium. The clinical course of all patients will be monitored throughout the study, and medical information will be collected, including medical history, medication, vital signs, medical imaging and information regarding clinical management during admission. The study has been open for inclusion since June 2020.
Clinical study procedures
Samples will be collected from included patients with a clinical suspicion of AMI at different time points with an in-hospital follow-up of a maximum of 5 days after inclusion (figure 1). Several baseline characteristics will be acquired at inclusion and during participation. After inclusion, blood and exhaled air samples will be collected every 60 min, up to 180 min. Re-establishing blood supply to the ischaemic bowel is the primary objective in patients with AMI. Therefore, patients may undergo endovascular revascularisation to restore mesenteric blood supply. Surgical resection of the necrotic bowel must occur if there are signs of non-viable tissue regions after revascularisation. If the patient undergoes an endovascular or surgical intervention, preoperative and postoperative samples will be taken. Postoperatively, the patient will be monitored for up to 5 days, and samples (blood and exhaled air) will be collected daily, parallel with the morning routine blood collections. In addition, patients without any treatment interventions will also be monitored for up to 5 days, and similar blood and air samples will be obtained identically to patients with a treatment intervention. At the end of the study, each participant will be allocated to one of the two study groups (AMI vs non-AMI). Diagnosis of mesenteric ischaemia will be based on CT, endovascular and surgical reports, clinical findings and (if applicable) verified by histopathological examination.
Blood collection and biomarker analysis
In this study, obtained blood samples will be analysed for serum and plasma biomarker analysis of I-FABP, SM22 and VIL-1. Blood samples will be collected via an arterial line, an intravenous needle or a central venous catheter. Occasionally a separate venapuncture can also be used to collect blood. Blood samples will be collected in vacutainer tubes treated with EDTA for plasma and SST II advance tubes for serum specimens. Whole blood samples will be centrifuged, and plasma/serum will be transferred to storage tubes. After processing, the samples are stored at −80°C until further analysis. I-FAPB, SM22 and VIL-1 concentrations will be determined in plasma/serum samples through ELISA. Highly specific I-FABP and SM22 ELISAs were developed and validated in our lab and selectively detect human I-FABP and human SM22 in plasma with a lower limit of detection of 12.5 pg/mL and 62.5 pg/mL, respectively.25 36 The intra-assay and inter-assay coefficient of variation is 4.1% and 6.2%, respectively, for I-FABP ranging from 6.2% to 14.8% and 4.9% to 16.3%, respectively, for SM22.25 36 VIL-1 ELISA was developed at PharmAbs (KU Leuven, Leuven, Belgium) and can detect human VIL-1 with a lower detection limit of 0.78 ng/mL. VIL-1 is detectable in plasma, however earlier studies showed a better detection in serum compared with plasma (Ceulemans et al data not shown).
Exhaled breath collection and analysis
This study’s second objective focuses on using VOCs in exhaled breath as a potential diagnostic tool for AMI. Exhaled breath is collected using resistance-free plastic bags (Tedlar bag, 3L, SKC Ltd, Dorset, UK) parallel to the blood samples. To collect breath samples, the patient must breathe into the valve of the Tedlar bag, which takes three to four exhalations to fill. Exhaled breath from an incapacitated patient will be collected from mechanical ventilation through a coaxial tubing system. Collected exhaled air containing VOCs is stabilised on carbon desorption tubes (SU60520-60-S, Camsco) with a flow air sampling pump (LFS-113, 360-041-01, Sensidyne) and stored at 4°C until further analysis by gas chromatography time of flight mass spectrometry (GC-tof-MS).35 The GC-tof-MS analysis was performed as described previously.37
Study outcomes
We hypothesise that with the use of serum/plasma biomarkers I-FABP, VIL-1 and SM22, a timely diagnosis of patients with AMI before irreversible transmural bowel damage occurs will be achieved. Through a multimodal diagnostic approach, we will be able to characterise each patient’s condition and correlate these biomarkers’ concentration to the disease’s corresponding aetiology. The primary outcome is plasma/serum concentrations of I-FABP, VIL-1 (markers for mucosal damage) and SM22 (a marker for transmural ischaemia) in patients with a clinical suspicion of AMI. The sensitivity and specificity of the described biomarkers will be determined and compared with the current gold standard.12 13 A receiver operating characteristic (ROC) curve analysis will be used to evaluate the diagnostic power of the biomarker (panel) test.
This study’s secondary outcome is identifying specific VOC profiles in exhaled air of patients suspected of AMI. Individual compounds of these profiles will be chemically identified to discover novel pathophysiological pathways involved in AMI. Furthermore, these VOC profiles will be used to investigate their potential use as a novel non-invasive diagnostic technique for AMI.
Data collection and management
Patients will receive a patient information folder and consent forms before study initiation, explaining the study procedures in detail and providing information on the study data collection, protection and pseudonymisation of their medical information. Data will be obtained by the local study teams and registered using study-specific case report forms and CASTOR38 electronic data capture system, which facilitates monitoring the study progress and outcomes in real-time. To ensure the privacy of all individuals in this study, blood and breath samples, data, and results of our research will be treated confidentially and encoded accordingly. The encoding of their personal data will ensure the patient’s anonymity. The source data and encoding key for the patient’s personal data will only be accessible to the principal and coordinating investigator. After the termination and publication, the patients can be informed about their study results, which can be explained to them if requested on their informed consent form. With the participants’ approval in this study, collected data, blood and exhaled air will be stored for 15 years for future research purposes. All samples will be transported to the Department of Surgery (Maastricht University, Maastricht, The Netherlands), where they will be stored and analysed. All data concerning participants or their participation in this study will be considered confidential and handled in compliance with all applicable regulations. Only members of the study team and local investigators have access to these data.
Safety considerations and withdrawal of participation
Patient safety and treatment is always prioritised and is not influenced by the study. The study will be suspended if there is sufficient ground that continuation of the study will jeopardise the subject health or safety. The sponsor will notify the accredited Medical Ethical Board without undue delay of a temporary halt, including the reason for such an action. The study will be suspended pending a further favourable decision by the accredited board. The coordinating researcher will ensure that all subjects and (if applicable) legal representatives are kept informed during study participation.
Patients participate in this research voluntarily. Any sign of patient resistance will lead to the discontinuation of research involving this patient. Patients or legal representative can withdraw their permission and leave the study at any time for any reason if they wish to do so without any consequences for their further treatment. For example, the investigator can withdraw a subject from the study for urgent medical reasons. Data obtained during participation can be used for future research purposes unless the patient or legal representative gives a written or verbal objection.
Sample size
Data from a previous study undertaken by our group was used to determine the sample size.25 Based on an effect size (medium to large) of 0.631 (determined by Cohen’s d formula based on the difference in mean I-FABP levels), with a power of 0.8 and alpha 0.05/3 (corrected for multiple testing due to analysis of three primary outcome parameters), 54 patients per group (AMI vs non-AMI) are needed for this cohort. By including 60 patients per group, possible dropouts (10%, n=6) are considered.
Statistical analysis
Statistical analysis will be performed with SPSS software (IBM) and GraphPad Prism 8 software. All the data obtained consists of continuous and categorical variables. The data will be tested for normality using the Kolmogorov-Smirnov test. Relative changes between the two groups will be tested using a Student’s t-test. Dichotomous variables will be compared using Pearson’s χ2 test. During the statistical analysis, numerical values will be reported as mean±SD or median. (IQR, ie, 25th to 75th percentile). Relevant variables with a p value <0.05 for univariate analysis will be introduced into a multiple logistic regression model using CI.) The area under the ROC curve will be calculated and is used to determine diagnostic utilities (sensitivity, specificity, positive predictive value and negative predictive value) of the biomarkers I-FABP, SM22 and VIL-1 to discriminate between AMI or non-AMI patients. Statistical analysis of VOCs expression profiles will be performed according to the published standards by Horváth et al for the exhaled breath analysis.39 Logistic regression analyses will be performed to investigate the most effective biomarker combination in patients with ischaemia or without ischaemia. Furthermore, we will assess the difference in mean I-FABP, SM22 and VIL1 plasma/serum levels between patients suffering from AMI and those diagnosed with other clinical conditions at different times. The severity of the mesenteric ischaemic damage will be determined (reversible vs non-reversible mesenteric ischaemic injury) by1 levels of plasma I-FABP, VIL1 and SM22 at baseline and2 an increase of I-FAPB, VIL1 and SM22 plasma levels over time as AMI progresses (until intervention).
Ethics and dissemination
The TACTIC study protocol was approved by the Medical Research Ethics Committee (METC) of Maastricht University Medical Centre+ and Maastricht University (METC azM/UM), the Netherlands (registration number METC19-010) and the Ethics Committee Research UZ/KU Leuven, Belgium (registration number S63500). Executive boards and local METCs of the Dutch participating centres Gelre Ziekenhuizen (Apeldoorn), Medisch Spectrum Twente, (Enschede), and University Medical Centre Groningen have granted permission to carry out this study according to the regulations of The Central Committee on Research Involving Human Subjects (CCMO, The Hague, The Netherlands). The study has been registered at ClinicalTrials.gov (NCT05194527).
This study will be conducted according to the principles of the Declaration of Helsinki (adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013) in accordance with the Dutch WMO Act. Study results will be disseminated via open-access peer-reviewed scientific journals and national and international conferences.
Clinical study protocol guidelines
The protocol has been reported according to the Strengthening the Reporting of Observational Studies in Epidemiology statement (http://www.strobe-statement.org/) and Standards for the Reporting of Diagnostic Accuracy Studies guidelines (https://www.equator-network.org/reporting-guidelines/stard/). The checklists are given as online supplemental materials 1 and 2.
bmjopen-2023-072875supp001.pdf (131.8KB, pdf)
bmjopen-2023-072875supp002.pdf (5.4MB, pdf)
Supplementary Material
Footnotes
Collaborators: Dutch Mesenteric Ischemia Study (DMIS) group: Ron Balm, Gert Jan de Borst, Juliette T Blauw, Marco J Bruno, Olaf J Bakker, Louisa J D van Dijk, Hessel C J L Buscher, Bram Fioole, Robert H Geelkerken, Jaap F Hamming, Jihan Harki, Daniel A F van den Heuvel, Eline S van Hattum, Jan Willem Hinnen, Jeroen J Kolkman, Maarten J van der Laan, Kaatje Lenaerts, Adriaan Moelker, Desirée van Noord, Maikel P Peppelenbosch, André S van Petersen, Pepijn Rijnja, Peter J van der Schaar, Luke G Terlouw, Hence J M Verhagen, Jean Paul P M de Vries, Dammis Vroegindeweij.
Contributors: KL and TL originated the study. AAMD, KL, LJC and TL, and DMIS were involved in the study design. AAMD, KL and TL drafted the manuscript. AAMD, RHG, MC, JPMD, J-PPMdV, HCJLB, SWMOD, FJvS, LJC, TL and KL are local investigators at the participating centres. The study is supervised and coordinated by AAMD, KL and TL. All authors provided essential feedback to the successive manuscript versions and approved the final version.
Funding: Dutch Digestive Foundation (Maag Lever Darm Stichting, MLDS), Amersfoort, The Netherlands (grant number D17-14).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Dutch Mesenteric Ischemia Study (DMIS) group:
Ron Balm, Gert Jan de Borst, Juliette T Blauw, Marco J Bruno, Olaf J Bakker, Louisa J D van Dijk, Hessel C J L Buscher, Bram Fioole, Robert H Geelkerken, Jaap F Hamming, Jihan Harki, Daniel A F van den Heuvel, Eline S van Hattum, Jan Willem Hinnen, Jeroen J Kolkman, Maarten J van der Laan, Kaatje Lenaerts, Adriaan Moelker, Desirée van Noord, Maikel P Peppelenbosch, André S van Petersen, Pepijn Rijnja, Peter J van der Schaar, Luke G Terlouw, Hence J M Verhagen, Jean-Paul P M de Vries, and Dammis Vroegindeweij
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology 1995;108:1566–81. 10.1016/0016-5085(95)90708-4 [DOI] [PubMed] [Google Scholar]
- 2. DeMeo MT, Mutlu EA, Keshavarzian A, et al. Intestinal Permeation and gastrointestinal disease. J Clin Gastroenterol 2002;34:385–96. 10.1097/00004836-200204000-00003 [DOI] [PubMed] [Google Scholar]
- 3. Acosta S, Björck M. Acute thrombo-Embolic occlusion of the superior mesenteric artery: a prospective study in a well defined population. Eur J Vasc Endovasc Surg 2003;26:179–83. 10.1053/ejvs.2002.1893 [DOI] [PubMed] [Google Scholar]
- 4. Stoney RJ, Cunningham CG. Acute mesenteric ischemia. Surgery 1993;114:489–90. [PubMed] [Google Scholar]
- 5. Duran M, Pohl E, Grabitz K, et al. The importance of open emergency surgery in the treatment of acute mesenteric ischemia. World J Emerg Surg 2015;10:45.:45. 10.1186/s13017-015-0041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kärkkäinen JM, Acosta S. Acute mesenteric ischemia (part I) - incidence, Etiologies, and how to improve early diagnosis. Best Pract Res Clin Gastroenterol 2017;31:15–25. 10.1016/j.bpg.2016.10.018 [DOI] [PubMed] [Google Scholar]
- 7. Oldenburg WA, Lau LL, Rodenberg TJ, et al. Acute mesenteric ischemia. Arch Intern Med 2004;164:1054. 10.1001/archinte.164.10.1054 [DOI] [PubMed] [Google Scholar]
- 8. Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg 2010;23:4–8. 10.1053/j.semvascsurg.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 9. Bradbury AW, Brittenden J, McBride K, et al. Mesenteric ischaemia: a Multidisciplinary approach. Br J Surg 1995;82:1446–59. 10.1002/bjs.1800821105 [DOI] [PubMed] [Google Scholar]
- 10. American Gastroenterological Association medical position statement: guidelines on intestinal ischemia. Gastroenterology 2000;118:951–3. 10.1016/s0016-5085(00)70182-x [DOI] [PubMed] [Google Scholar]
- 11. Howard TJ, Plaskon LA, Wiebke EA, et al. Nonocclusive mesenteric ischemia remains a diagnostic dilemma. Am J Surg 1996;171:405–8. 10.1016/S0002-9610(97)89619-5 [DOI] [PubMed] [Google Scholar]
- 12. Menke J. Diagnostic accuracy of Multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010;256:93–101. 10.1148/radiol.10091938 [DOI] [PubMed] [Google Scholar]
- 13. Cudnik MT, Darbha S, Jones J, et al. The diagnosis of acute mesenteric ischemia: A systematic review and meta-analysis. Acad Emerg Med 2013;20:1087–100. 10.1111/acem.12254 [DOI] [PubMed] [Google Scholar]
- 14. Björck M, Koelemay M, Acosta S, et al. Editor’s choice – management of the diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg 2017;53:460–510. 10.1016/j.ejvs.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 15. Nuzzo A, Maggiori L, Ronot M, et al. Predictive factors of intestinal necrosis in acute mesenteric ischemia: prospective study from an intestinal stroke center. Am J Gastroenterol 2017;112:597–605. 10.1038/ajg.2017.38 [DOI] [PubMed] [Google Scholar]
- 16. Acosta S, Nilsson TK, Björck M. D-Dimer testing in patients with suspected acute thromboembolic occlusion of the superior mesenteric artery. Br J Surg 2004;91:991–4. 10.1002/bjs.4645 [DOI] [PubMed] [Google Scholar]
- 17. Lemma A, Tolonen M, Vikatmaa P, et al. Editor's choice - epidemiology, diagnostics, and outcomes of acute occlusive arterial mesenteric ischaemia: A population based study. Eur J Vasc Endovasc Surg 2022;64:646–53. 10.1016/j.ejvs.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 18. Kirkpatrick IDC, Kroeker MA, Greenberg HM. Abbreviations: AMI acute mesenteric ischemia IMA inferior mesenteric artery SMA superior mesenteric artery Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience 1. Radiology 2003;229:91–8. 10.1148/radiol.2291020991 [DOI] [PubMed] [Google Scholar]
- 19. Angelelli G, Scardapane A, Memeo M, et al. Acute bowel ischemia: CT findings. Eur J Radiol 2004;50:37–47. 10.1016/j.ejrad.2003.11.013 [DOI] [PubMed] [Google Scholar]
- 20. Chou CK. CT manifestations of bowel ischemia. AJR Am J Roentgenol 2002;178:87–91. 10.2214/ajr.178.1.1780087 [DOI] [PubMed] [Google Scholar]
- 21. Chou CK, Mak CW, Tzeng WS, et al. CT of small bowel ischemia. Abdom Imaging 2004;29:18–22. 10.1007/s00261-003-0073-3 [DOI] [PubMed] [Google Scholar]
- 22. Florim S, Almeida A, Rocha D, et al. Acute mesenteric ischaemia: a pictorial review. Insights Imaging 2018;9:673–82. 10.1007/s13244-018-0641-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schellekens DHSM, Grootjans J, Dello SAWG, et al. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human Translational ischemia-reperfusion model. J Clin Gastroenterol 2014;48:253–60. 10.1097/MCG.0b013e3182a87e3e [DOI] [PubMed] [Google Scholar]
- 24. Ceulemans L, De Hertogh G, Farré R, et al. Villin-1 is a novel serological biomarker for intestinal ischemia and reperfusion injury in rats and humans. Transplantation 2017;101:6S2. 10.1097/01.tp.0000521418.83368.0a [DOI] [Google Scholar]
- 25. Schellekens D, Reisinger KW, Lenaerts K, et al. Sm22 a plasma biomarker for human Transmural intestinal ischemia. Ann Surg 2018;268:120–6. 10.1097/SLA.0000000000002278 [DOI] [PubMed] [Google Scholar]
- 26. Derikx JPM, Schellekens D, Acosta S. Serological markers for human intestinal ischemia: A systematic review. Best Pract Res Clin Gastroenterol 2017;31:69–74. 10.1016/j.bpg.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 27. Pelsers M, Namiot Z, Kisielewski W, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem 2003;36:529–35. 10.1016/s0009-9120(03)00096-1 [DOI] [PubMed] [Google Scholar]
- 28. Lees-Miller JP, Heeley DH, Smillie LB, et al. Isolation and characterization of an abundant and novel 22-kDa protein (Sm22) from chicken gizzard smooth muscle. J Biol Chem 1987;262:2988–93. [PubMed] [Google Scholar]
- 29. Chiavegato A, Roelofs M, Franch R, et al. Differential expression of Sm22 Isoforms in Myofibroblasts and smooth muscle cells from Rabbit bladder. J Muscle Res Cell Motil 1999;20:133–46. 10.1023/a:1005411201187 [DOI] [PubMed] [Google Scholar]
- 30. Sethi S, Nanda R, Chakraborty T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin Microbiol Rev 2013;26:462–75. 10.1128/CMR.00020-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Berkel JJBN, Dallinga JW, Möller GM, et al. Development of accurate classification method based on the analysis of volatile organic compounds from human exhaled air. J Chromatogr B Analyt Technol Biomed Life Sci 2008;861:101–7. 10.1016/j.jchromb.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 32. Jimenez JC, DeLano F, Wilson JM, et al. Analysis of exhaled volatile compounds following acute superior mesenteric artery occlusion in a pilot rat study. Ann Vasc Surg 2011;25:1113–7. 10.1016/j.avsg.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Szűcs S, Bari G, Ugocsai M, et al. Detection of intestinal tissue perfusion by real-time breath methane analysis in rat and pig models of mesenteric circulatory distress. Crit Care Med 2019;47:e403–11. 10.1097/CCM.0000000000003659 [DOI] [PubMed] [Google Scholar]
- 34. Jávor P, Rárosi F, Horváth T, et al. Detection of exhaled methane levels for monitoring trauma-related haemorrhage following blunt trauma: study protocol for a prospective observational study. BMJ Open 2022;12:e057872. 10.1136/bmjopen-2021-057872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bodelier AGL, Smolinska A, Baranska A, et al. Volatile organic compounds in exhaled air as novel marker for disease activity in Crohn's disease: A Metabolomic approach. Inflamm Bowel Dis 2015;21:1776–85. 10.1097/MIB.0000000000000436 [DOI] [PubMed] [Google Scholar]
- 36. van Wijck K, Wijnands KAP, Meesters DM, et al. L-Citrulline improves Splanchnic perfusion and reduces gut injury during exercise. Med Sci Sports Exerc 2014;46:2039–46. 10.1249/MSS.0000000000000332 [DOI] [PubMed] [Google Scholar]
- 37. Kienhorst S, van Aarle MHD, Jöbsis Q, et al. The Adem2 project: early pathogenic mechanisms of preschool wheeze and a randomised controlled trial assessing the gain in health and cost-effectiveness by application of the breath test for the diagnosis of asthma in wheezing preschool children. BMC Public Health 2023;23:629. 10.1186/s12889-023-15465-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Castor EDC. Castor electronic data capture. 2019. Available: https://castoredc.com [Accessed 27 Aug 2019].
- 39. Horváth I, Barnes PJ, Loukides S, et al. A European respiratory society technical standard: exhaled biomarkers in lung disease. Eur Respir J 2017;49:1600965. 10.1183/13993003.00965-2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-072875supp001.pdf (131.8KB, pdf)
bmjopen-2023-072875supp002.pdf (5.4MB, pdf)