Abstract
This study was performed to examine the effects of anti- lipopolysaccharide (LPS) of Escherichia coli chicken egg Yolk immunoglobulin (IgY) provided to calves for 7 weeks during the pre- and post-weaning periods on rumen LPS activity, plasma acute phase protein (APP) concentrations, and metabolic parameters. A total of 30 Holstein calves were randomly assigned to two groups of 15 each: an IgY group fed Anti-E. coli LPS IgY, and a control group fed whole egg powder as a placebo. The study was conducted on calves aged 3–10 weeks, weaned at 7 weeks. The ruminal LPS activity of the IgY group was approximately 60% lower than the control group at 10 weeks of age. Plasma APP and cytokine concentrations in the IgY group did not differ from those in the control group. The daily weight gain in the IgY group was significantly higher than the control group for the whole experimental period. Plasma albumin/globulin was lower (P<0.05), and plasma aspartate transferase concentration was higher (P<0.05) in the IgY group than in the control group during the experimental period. In conclusion, feeding Anti-E. coli LPS IgY for 7 weeks pre- and post-weaning remarkably reduced the rumen LPS activity and improved the daily weight gain. The impact of Anti-E. coli LPS IgY on LPS activities in the lower gastrointestinal tract, and elucidation as to the mechanism responsible for the improvement in daily weight gain require further investigation.
Keywords: acute phase proteins, anti-lipopolysaccharide of Escherichia coli antibody, immunoglobulin Y, lipopolysaccharide, weaning period
Maximizing starter food intake before weaning is important for dairy calves because they need to develop a rumen that will allow them to obtain adequate nutrition from solid feed before weaning [14]. Weaning with insufficient starter intake will result in a temporary lack of energy previously supplied by milk consumption [5]. Failure to wean may lead to a reduced growth rate and disease outbreaks.
It has been suggested that even at about five months, calf rumens cannot absorb and produce the same level of volatile fatty acids (VFA) as mature cows [22]. During the pre-and post-weaning periods, when starter is fed at a high proportion, rumen pH can quickly decrease and exceed the proposed threshold for subacute ruminal acidosis (SARA) in dairy cows (pH <5.6 more than 3/hr/day) [7, 14, 15]. Furthermore, if starch in the feed flows into the hindgut and ferments, the pH of the hindgut may also decrease [3].
In a dairy cow, SARA triggered by poor feed management, such as low forage-to-concentrate ratios and abrupt changes in the diet [10, 17], can lead to various symptoms, including inflammatory disorders [10]. Increased ruminal lipopolysaccharide (LPS) activities, translocation of LPS into the systemic circulation, and induction of an inflammatory response have been implicated as contributing factors to the disorders associated with SARA [7]. LPS in the gastrointestinal tract may affect metabolites in calves as well as mature cows [6], but the severity of its impact is unclear.
Recent study has shown that the forage intake during the weaning period is crucial as it affects ruminal pH levels [14]. However, to allow for early weaning, starter feed tends to be provided at a greater amount and proportion during weaning periods. We focused on using chicken egg yolk antibodies (IgY), which play a role in neutralizing particular immunogens. This specific IgY can be harvested from hens immunized with antigens and applied as passive immunization by treating animals orally [23]. The IgY targeting enteric infectious pathogens is commercially available as a dietary additive for suckling calves. Other specific IgY against rumen bacteria, such as Streptococcus bovis and Fusobacterium necrophorum, have been experimentally administered to cows [1, 2]. To date, there are no reports of feeding IgY to target gastrointestinal tract LPS and examining its effects on calves by assessing plasma acute phase proteins (APPs) and cytokine concentrations. Using a specific IgY against LPS of E. coli (Anti-E. coli LPS IgY) that binds to free LPS in the rumen fluid [16], this study aimed to determine the effects of feeding Anti-E. coli LPS IgY for 7 weeks during pre- and post-weaning periods on rumen fermentation, rumen LPS activity, and plasma metabolites and hormone concentrations.
MATERIALS AND METHODS
This experimental design was approved by the Animal Care and Use Committee of the Institute of Livestock and Grassland Science, NARO (NILGS) (1811C065), and the study was performed at the NILGS research facility (Tsukuba, Japan).
Animals and experimental design
A total of 30 Holstein calves (16 female and 14 male) were used during the 7-week experimental period. Calves at 3 weeks of age were housed in individual pens and randomly placed in a control or IgY group (8 females and 7 males per group) at the start of the experiment. The IgY group was fed Anti-E. coli LPS IgY (EW Nutrition Japan K.K., Gifu, Japan) produced for research use as described previously [16]. Each 1 g of Anti-E. coli LPS IgY binds 0.25 g of E. coli serotype O111:B4-derived LPS (Sigma-Aldrich Corp., St. Louis, MO, USA) in vitro [16]. Anti-E. coli LPS IgY 2 g/day was added to the morning feed, pre-weaning whole milk and post-weaning starter. The control group was fed 2 g/day of whole egg powder as a placebo instead of Anti-E. coli LPS IgY.
Whole milk was fed at 11% of body weight per day, specifically 5% at 9:00 and 6% at 16:00, with a maximum feeding limit of 8 L/day. The provision of whole milk was reduced at 6 weeks, and calves were weaned at 7 weeks. Feeding of starter (84% TDN, 21% CP; New Makestar, The National Federation of Dairy Co-Operative Associations, Tokyo, Japan) and cut timothy (8.1% CP, 63.5% NDF) was allowed from 1 week of age. The amount of starter and cut timothy were increased weekly to 600 g/day and 300 g/day, respectively, at 3 weeks, and 2,000 g/day and 800 g/day, respectively, at 10 weeks. Both feeds were divided into two equal portions and offered at 9:30 and 16:30.
Sampling
The rumen fluid sample was taken orally at 11:00 at 10 weeks of age. After immediately filtering through two layers of sterile gauze, 2 mL was centrifuged (9,000 × g, 30 min, 4°C), and the supernatant was collected for LPS activity analysis. To 10 mL of the filtered rumen fluid, 2 mL of 3N sulfuric acid with 25% metaphosphoric acid was added to prepare the sample for VFA measurement. Aliquots of approximately 2 mL were preserved at −80°C for ruminal ammonia nitrogen analysis.
Blood was collected from the jugular vein into heparin sodium-added evacuated tubes or EDTA2Na-added evacuated tubes (Thermo Corp., Tokyo, Japan) at 3, 4, 6, 7, 8, and 10 weeks of age prior to whole milk feeding at 9:00 am. The samples were immediately mixed with 500 kIU/mL of aprotinin (Sigma-Aldrich Corp., St. Louis, MO, USA) and centrifuged (2,000 × g, 20 min, at 4°C). The obtained plasma samples were stored at −80°C until analyzed.
The feed intake of the individual calves was recorded each day. Body weights were measured once a week at 13:00.
Measurements
Rumen LPS activity measurements were performed as described previously [8]. The rumen VFA concentration was quantified by gas chromatography (GC-2014; Shimadzu Corp., Kyoto, Japan) using a Thermon-3000 3% glass column (Shinwa Chemical Industries Ltd., Kyoto, Japan). Ammonia concentration in the rumen fluid was determined using a commercial kit (Lab Assay TM Ammonia; Fujifilm Wako Pure Chemicals Corp., Osaka, Japan).
Plasma LPS-binding protein concentration (HK503; Hycult Biotech, Uden, Netherlands), haptoglobin concentration (Cow Haptoglobin ELISA Kit; Life Diagnostics, Inc., West Chester, PA, USA), serum amyloid A concentration (Multispecies SAA ELISA Kit; Tridelta Development Ltd., Maynooth County Kildare, Ireland), and alpha-1 acidic glycoprotein concentration (Cow AGP ELISA Kit; Life Diagnostics Inc., West Chester, PA, USA) were measured with commercially available ELISA kits.
Plasma tumor necrosis factor-α, interleukin-1, interleukin-6, and interferon-γ concentrations were measured as previously reported [13]. Plasma concentrations of total protein, albumin, glucose, β-hydroxybutyric acid, blood urea nitrogen, triglycerides, inorganic phosphorus, calcium, iron, aspartate aminotransferase (AST), alkaline phosphatase (ALP), and non-esterified fatty acids were evaluated using an automated analyzer (7070; Hitachi Co., Ltd., Tokyo, Japan). Plasma growth hormone (GH) and insulin concentrations were determined via a radioimmunoassay [12]. Plasma insulin-like growth factor-1 (IGF-1) was measured using a competitive solid-phase immunoassay as previously described [19]. The plasma insulin concentration was measured using a commercial radioimmunoassay kit (Rat Insulin RIA Kit; EMD Millipore Corp., Burlington, MA, USA). The intra-assay coefficients of measurement variation were 7.2% for GH, 1.9% for insulin, and 5.1% for IGF-1.
Statistical analyses
All results are presented as the least-squares means with standard errors. Data analysis was conducted using SAS software (SAS University Edition; SAS Institute, Cary, NC, USA). The data of ruminal parameters were evaluated using the General Linear Model procedure. The following model was employed:
Yijk=μ + Ti + Sj + T ×Sij + eijk
where Yijk is the observation, μ is the overall mean, Ti is the effect of treatment, Sj is the effect of sex, T ×Sij is the interaction between dietary treatment and sex, and eijk represents the residual errors.
Dry matter intake, daily weight gain (DG), and measurements within blood plasma were evaluated with the MIXED procedure. The following model was used:
Yijkl=μ + Ti + Sj + C (TS)ijk + Wl + T ×Sij + T ×Wil + eijkl
where Yijkl is the observation, μ is the overall mean, Ti is the effect of treatment, Sj is the effect of sex, C (TS)ijk represents the random variable of a calf subjected to treatment, Wl is the effect of time (i.e., week), T ×Sij represents the interaction between dietary treatment and sex, T ×Wil represents the interaction between dietary treatment and week, and eijkl represents the residual errors. When significant interactions were detected between treatment and time, comparisons between treatments were evaluated using the Tukey–Kramer test.
Differences were considered significant at P<0.05, and tendencies were identified at P<0.1.
RESULTS
Calves in both groups received the entirety of their feed allowance according to the experimental design. Solid and liquid feed intake was equal to the total amount of provisions, and dry matter intake did not differ between the groups throughout the experimental period (data not shown). No clinical disorders were observed in the calves during the testing period.
The rumen LPS activity, VFA composition, and ammonia concentration at 10 weeks of age are shown in Table 1. The rumen LPS activity in the IgY group was less than half of the level in the control group. There were no differences in total VFA concentrations in the rumen fluid between the groups. The IgY group tended to have a higher proportion of rumen propionic acid (P<0.1) than the control group. Other rumen VFA compositions and ammonia nitrogen concentrations did not differ significantly between the two groups.
Table 1. Ruminal fermentation characteristics of calves fed egg yolk powder with or without specific chicken egg yolk immunoglobulins (IgY) after weaning.
| Item | Treatment | SEM | P-value | |
|---|---|---|---|---|
| Control | IgY | |||
| Total VFA, mmol/dL | 9.22 | 9.51 | 0.52 | 0.694 |
| Molor proportions | ||||
| Acetate | 65.15 | 62.96 | 1.34 | 0.261 |
| Propionate | 22.45 | 25.07 | 1.06 | 0.092 |
| Butyrate | 10.12 | 9.69 | 0.62 | 0.634 |
| Valerate | 2.28 | 2.28 | 0.23 | 0.989 |
| Acetate: Propionate | 3.00 | 2.60 | 0.17 | 0.118 |
| Rumen ammonia N, mg/dL | 11.80 | 11.36 | 2.56 | 0.905 |
| Rumen LPS, × 104 EU/mL | 13.34 | 5.40 | 1.28 | 0.001 |
Values are expressed as least squares mean ± SEM. Control group (n=15), supplemented with nonspecific yolk powder; IgY group (n=15), supplemented with specific IgY against the lipopolysaccharides of Escherichia coli. The rumen fluid samples were taken orally at 10 weeks of age (post-weaning period). VFA, volatile fatty acid; LPS, lipopolysaccharide.
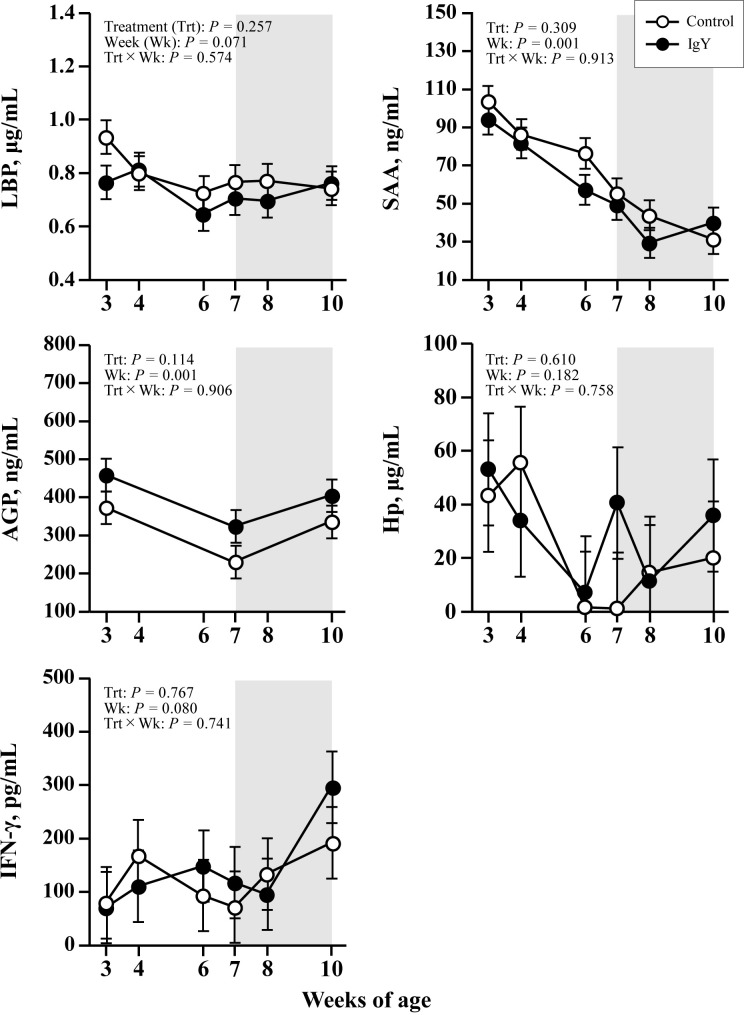
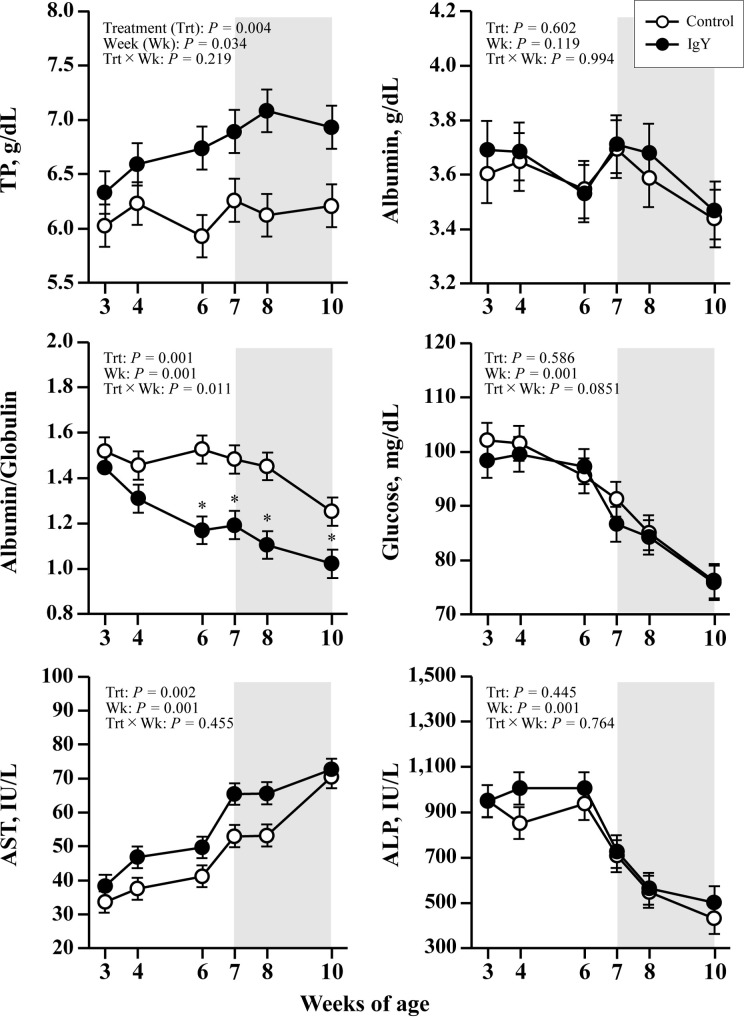
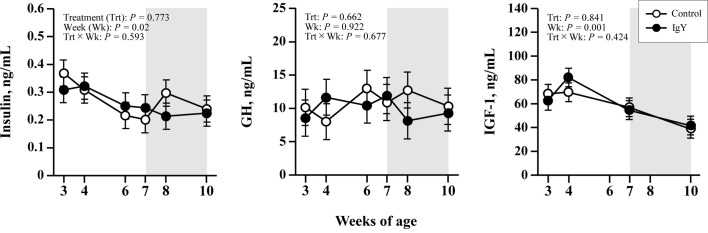
Neither group significantly differed in plasma APPs and cytokine concentrations (Fig. 1). Nor was any effect of time detected in either group for each parameter (Week P>0.1), and no high values were observed that could infer inflammation. The plasma metabolic components are provided in Fig. 2. Plasma total protein concentrations increased with weeks in the IgY group, whereas no increase was observed in the control group; thus, plasma total protein concentrations in the IgY group were significantly higher than in the control group (TRT P<0.05). Plasma A/G in both groups decreased with weeks (Week P<0.05), and after 6 weeks of age, plasma A/G in the IgY group was significantly lower than in the control group (P<0.05). Plasma AST concentrations elevated with weeks in both groups (Week P<0.05), and the AST concentration was higher in the IgY group than in the control group (TRT P<0.05). Figure 3illustrates the plasma hormone concentrations. There were no significant differences in plasma hormones, other metabolites, or mineral concentrations between the groups (data not shown except as indicated in the figures).
Fig. 1.
Plasma concentrations of lipopolysaccharide-binding protein, serum amyloid A (SAA), α1-acid glycoprotein (AGP), haptoglobin (Hp), and interferon-γ (IFN-γ) in calves fed egg yolk powder with (IgY group) or without (control group) specific chicken egg yolk immunoglobulins (IgY). Values are expressed as mean ± SEM (n=15). The shaded background shows the post-weaning period (7–10 weeks of age).
Fig. 2.
Plasma concentrations of total protein, albumin, albumin to globulin ratio, glucose, aspartate aminotransferase (AST), and alkaline phosphatase (ALP) in calves fed egg yolk powder with (IgY group) or without (control group) specific chicken egg yolk immunoglobulins (IgY). Values are expressed as mean ± SEM (n=15). The shaded background shows the post-weaning period (7–10 weeks of age). Asterisk (*) indicates significant difference (P<0.05) between two groups at the same point in the experimental period.
Fig. 3.
Plasma concentrations of insulin, growth hormone (GH), and insulin-like growth factor-1 (IGF-1) in calves fed egg yolk powder with (IgY group) or without (control group) specific chicken egg yolk immunoglobulins (IgY). Values are expressed as mean ± SEM (n=15). The shaded background shows the post-weaning period (7–10 weeks of age).
Growth performance during the experimental period is shown in Table 2. DG in the IgY group tended to be higher (P<0.1) than in the control group before weaning and was significantly higher than in the control group after weaning and during the whole experimental period.
Table 2. Growth performance of calves fed egg yolk powder with or without specific chicken egg yolk immunoglobulins (IgY).
| Item | Treatment | SEM | P-value | |
|---|---|---|---|---|
| Control | IgY | |||
| BW, kg | ||||
| Initial | 56.79 | 54.78 | 1.36 | 0.310 |
| Final | 91.72 | 96.68 | 2.16 | 0.117 |
| DG, kg/d | ||||
| Total | 0.71 | 0.86 | 0.03 | 0.001 |
| Pre-weaning | 0.71 | 0.81 | 0.04 | 0.084 |
| Post-weaning | 0.74 | 0.92 | 0.03 | 0.023 |
Values are expressed as least squares mean ± SEM. Control group (n=15), supplemented with nonspecific yolk powder; IgY group (n=15), supplemented with specific IgY against the lipopolysaccharides of Escherichia coli. Total, entire period of experiment (21–70 days of age); pre-weaning period, 21–48 days of age; Post-weaning period, 49–70 days of age. BW, body weight; DG, daily weight gain.
DISCUSSION
The pre-and post-weaning periods, when concentrated feeding is rapidly increased, is a high-risk time for rumen and hindgut acidosis [3, 14]. Furthermore, under acidotic conditions, the permeability of the gastrointestinal tract to low-molecular-weight carbohydrates, such as mannitol, increases in the presence of LPS [4]. The translocation of contents from the gastrointestinal lumen across the epithelium induces local and systemic inflammatory responses [18]. When inflammation becomes pathological, not only is there a substantially higher risk of developing metabolic disorders or disease [9], high energy and nutrient requirements can divert resources away from production and growth [18]. Hence, controlling LPS levels in the digestive tract is important to minimize stress and ease the weaning process while maintaining highly concentrated feeding.
Feeding Anti-E. coli LPS IgY for 7 weeks pre- and post-weaning resulted in a remarkable reduction in the rumen LPS activity. However, activities of LPS in the lower gastrointestinal tract were not clear in this study. The results are similar to a previous study [16] that experimentally induced SARA in 5-month-old Holstein bull cattle. The level of rumen LPS activity in the control group was as high as that of adult cattle [8] and consistent with earlier results in 10-week-old calves [20]. Plasma APPs and cytokine concentrations were normal throughout the experiment, suggesting no inflammatory response. This is the first report to examine the effect of feeding Anti-E. coli LPS IgY pre- and post-weaning on rumen LPS activities and related data.
Plasma A/G in the IgY group was lower than in the control group from 1 week before to 3 weeks after weaning. A report on feeding yeast and flavonoids to calves around weaning [11] found that their serum IgG levels had increased. The authors attributed this result to the interaction of probiotics as immunomodulators of the intestinal resident microflora, epithelial and immune cells, stimulating immune functions and leading to antibody production [11]. A similar mechanism may apply to this study. The plasma A/G in the IgY group at 10 weeks was lower than that in the control group, suggesting that active IgY reached the intestinal tract even after weaning [21]. Moreover, plasma AST concentrations in the IgY group increased within the normal range from before weaning. Thus, the increase in plasma AST concentrations may be related to IgY-induced immunostimulation of intestinal epithelial cells.
The addition of Anti-E. coli LPS IgY increased DG during the whole experimental period compared to the control group. There was no difference in feed intake between the two groups, suggesting that feed efficiency in the IgY group calves had improved. A study with anti-Streptococcus bovis IgY in feedlot cattle showed increased weight gain per feed, which was implicated to be related to changes in the rumen microflora provoked by IgY [2]. The rumen propionate proportion in the IgY group tended to be higher than that in the control group, which could be associated with an increased growth rate [11, 24].
In conclusion, feeding Anti-E. coli LPS IgY for 7 weeks during pre- and post-weaning periods remarkably reduced the rumen LPS activity at 3 weeks post-weaning and improved DG. The present study showed no differences between the groups in plasma APPs and cytokine concentrations. The anti-inflammatory effect of added IgY only appeared in the rumen LPS activity. The mechanism by which Anti-E. coli LPS IgY improves DG, including its effect on the lower gastrointestinal tract, needs further investigation.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this article.
Acknowledgments
ACKNOWLEDGMENT. The authors appreciate Dr. Fuminori Terada for statistical assistance.
REFERENCES
- 1.DiLorenzo N, Diez-Gonzalez F, DiCostanzo A. 2006. Effects of feeding polyclonal antibody preparations on ruminal bacterial populations and ruminal pH of steers fed high-grain diets. J Anim Sci 84: 2178–2185. doi: 10.2527/jas.2005-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiLorenzo N, Dahlen CR, Diez-Gonzalez F, Lamb GC, Larson JE, DiCostanzo A. 2008. Effects of feeding polyclonal antibody preparations on rumen fermentation patterns, performance, and carcass characteristics of feedlot steers. J Anim Sci 86: 3023–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert E, Brown HE, Leslie KE, DeVries TJ, Steele MA. 2015. Weaning age affects growth, feed intake, gastrointestinal development, and behavior in Holstein calves fed an elevated plane of nutrition during the preweaning stage. J Dairy Sci 98: 6315–6326. doi: 10.3168/jds.2014-9062 [DOI] [PubMed] [Google Scholar]
- 4.Emmanuel DGV, Madsen KL, Churchill TA, Dunn SM, Ametaj BN. 2007. Acidosis and lipopolysaccharide from Escherichia coli B:055 cause hyperpermeability of rumen and colon tissues. J Dairy Sci 90: 5552–5557. doi: 10.3168/jds.2007-0257 [DOI] [PubMed] [Google Scholar]
- 5.Fukumori R, Mita T, Sugino T, Hasegawa Y, Kojima M, Kangawa K, Obitsu T, Taniguchi K. 2012. Effects of glucose and volatile fatty acids on blood ghrelin concentrations in calves before and after weaning. J Anim Sci 90: 4839–4845. doi: 10.2527/jas.2012-5344 [DOI] [PubMed] [Google Scholar]
- 6.Gelsinger SL, Coblentz WK, Zanton GI, Ogden RK, Akins MS. 2020. Physiological effects of starter-induced ruminal acidosis in calves before, during, and after weaning. J Dairy Sci 103: 2762–2772. doi: 10.3168/jds.2019-17494 [DOI] [PubMed] [Google Scholar]
- 7.Gozho GN, Plaizier JC, Krause DO, Kennedy AD, Wittenberg KM. 2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J Dairy Sci 88: 1399–1403. doi: 10.3168/jds.S0022-0302(05)72807-1 [DOI] [PubMed] [Google Scholar]
- 8.Hirabayashi H, Kawashima K, Okimura T, Tateno A, Suzuki A, Asakuma S, Isobe N, Obitsu T, Kushibiki S, Sugino T. 2017. Effect of nutrient levels during the far-off period on postpartum productivity in dairy cows. Anim Sci J 88: 1162–1170. doi: 10.1111/asj.12743 [DOI] [PubMed] [Google Scholar]
- 9.Horst EA, Kvidera SK, Baumgard LH. 2021. Invited review: The influence of immune activation on transition cow health and performance-A critical evaluation of traditional dogmas. J Dairy Sci 104: 8380–8410. doi: 10.3168/jds.2021-20330 [DOI] [PubMed] [Google Scholar]
- 10.Kleen JL, Hooijer GA, Rehage J, Noordhuizen JPTM. 2003. Subacute ruminal acidosis (SARA): a review. J Vet Med A Physiol Pathol Clin Med 50: 406–414. doi: 10.1046/j.1439-0442.2003.00569.x [DOI] [PubMed] [Google Scholar]
- 11.Kong L, Yang C, Dong L, Diao Q, Si B, Ma J, Tu Y. 2019. Rumen fermentation characteristics in pre- and post-weaning calves upon feeding with mulberry leaf flavonoids and Candida tropicalis individually or in combination as a supplement. Animals (Basel) 9: 990. doi: 10.3390/ani9110990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kushibiki S, Hodate K, Shingu H, Obara Y, Touno E, Shinoda M, Yokomizo Y. 2003. Metabolic and lactational responses during recombinant bovine tumor necrosis factor-α treatment in lactating cows. J Dairy Sci 86: 819–827. doi: 10.3168/jds.S0022-0302(03)73664-9 [DOI] [PubMed] [Google Scholar]
- 13.Kushibiki S, Shingu H, Komatsu T, Itoh F, Kasuya E, Aso H, Hodate K. 2006. Effect of recombinant bovine tumor necrosis factor-α on hormone release in lactating cows. Anim Sci J 77: 603–612. doi: 10.1111/j.1740-0929.2006.00392.x [DOI] [Google Scholar]
- 14.Laarman AH, Oba M. 2011. Short communication: Effect of calf starter on rumen pH of Holstein dairy calves at weaning. J Dairy Sci 94: 5661–5664. doi: 10.3168/jds.2011-4273 [DOI] [PubMed] [Google Scholar]
- 15.Laarman AH, Sugino T, Oba M. 2012. Effects of starch content of calf starter on growth and rumen pH in Holstein calves during the weaning transition. J Dairy Sci 95: 4478–4487. doi: 10.3168/jds.2011-4822 [DOI] [PubMed] [Google Scholar]
- 16.Mizuguchi H, Ikeda T, Watanabe Y, Kushibiki S, Ikuta K, Kim YH, Sato S. 2021. Anti-lipopolysaccharide antibody administration mitigates ruminal lipopolysaccharide release and depression of ruminal pH during subacute ruminal acidosis challenge in Holstein bull cattle. J Vet Med Sci 83: 905–910. doi: 10.1292/jvms.21-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nocek JE. 1997. Bovine acidosis: implications on laminitis. J Dairy Sci 80: 1005–1028. doi: 10.3168/jds.S0022-0302(97)76026-0 [DOI] [PubMed] [Google Scholar]
- 18.Sanz-Fernandez MV, Daniel JB, Seymour DJ, Kvidera SK, Bester Z, Doelman J, Martín-Tereso J. 2020. Targeting the Hindgut to Improve Health and Performance in Cattle. Animals (Basel) 10: 1817. doi: 10.3390/ani10101817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugino T, Hasegawa Y, Kurose Y, Kojima M, Kangawa K, Terashima Y. 2004. Effects of ghrelin on food intake and neuroendocrine function in sheep. Anim Reprod Sci 82-83: 183–194. doi: 10.1016/j.anireprosci.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 20.Takemura K, Shingu H, Mizuguchi H, Kim YH, Sato S, Kushibiki S. 2019. Effects of forage feeding on rumen fermentation, plasma metabolites, and hormones in Holstein calves during pre- and postweaning periods1. J Anim Sci 97: 2220–2229. doi: 10.1093/jas/skz088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan X, Li J, Li Y, Li J, Wang Q, Fang L, Ding X, Huang P, Yang H, Yin Y. 2019. Effect of chicken egg yolk immunoglobulins on serum biochemical profiles and intestinal bacterial populations in early-weaned piglets. J Anim Physiol Anim Nutr (Berl) 103: 1503–1511. doi: 10.1111/jpn.13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe Y, Kim YH, Kushibiki S, Ikuta K, Ichijo T, Sato S. 2019. Effects of active dried Saccharomyces cerevisiae on ruminal fermentation and bacterial community during the short-term ruminal acidosis challenge model in Holstein calves. J Dairy Sci 102: 6518–6531. doi: 10.3168/jds.2018-15871 [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama H, Peralta RC, Sendo S, Ikemori Y, Kodama Y. 1993. Detection of passage and absorption of chicken egg yolk immunoglobulins in the gastrointestinal tract of pigs by use of enzyme-linked immunosorbent assay and fluorescent antibody testing. Am J Vet Res 54: 867–872. [PubMed] [Google Scholar]
- 24.Zhang Q, Koser SL, Donkin SS. 2016. Propionate induces mRNA expression of gluconeogenic genes in bovine calf hepatocytes. J Dairy Sci 99: 3908–3915. doi: 10.3168/jds.2015-10312 [DOI] [PubMed] [Google Scholar]